Back to Journals » Cancer Management and Research » Volume 17
Treatment and Outcomes of Primary Tracheal Adenoid Cystic Carcinoma: A Systematic Review
Received 24 October 2024
Accepted for publication 17 January 2025
Published 5 April 2025 Volume 2025:17 Pages 767—778
DOI https://doi.org/10.2147/CMAR.S501202
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Yiping Shi,1 Shan Xue,2 Jing Zou2
1Department of Nuclear Medicine, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Respirology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Jing Zou, Department of Respirology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, No. 160 Pujian Road, Shanghai, 200127, People’s Republic of China, Tel +86-021-68383093, Email [email protected]
Abstract: Primary tracheal adenoid cystic carcinoma (TACC) is a rare malignant tumor. This systematic review describes the treatment strategies and oncological outcomes in patients with TACC. Full-text English manuscripts published from January 1st 1960, to December 31th 2023, were included. Data on demographics, treatment, and outcomes were also collected. A pooled analysis of 5-year overall survival (OS) was performed. Nineteen articles and 1488 patients met the inclusion criteria. The first-choice treatment strategy was surgery (74.5%), whereas adjuvant radiotherapy was administered to 56.7% of the patients. The follow-up range was 6– 390 months. A total of 552 deaths were recorded, 98.7% of which were cancer related. The 5-year OS rate ranged from 28.6% to 100.0%; in the pooled analysis, it was 65% (range, 32%– 96%). Surgery remains the mainstay of treatment for TACC. Adjuvant radiotherapy is commonly administered to patients with positive margins, and survival estimates are good overall but heterogeneous.
Keywords: adenoid cystic carcinoma, trachea, treatment, survival
Introduction
Adenoid cystic carcinoma (ACC) is a kind of primarily salivary gland tumor, which may also occur in other sites such as the paranasal sinuses, larynx, and trachea.1 ACC is the second-most common primary malignant tracheal tumor, ranked after squamous cell carcinoma (SCC), with an incidence of 0.04–0.2%.2 It is a rare slow growing tumor that arises from secretory glands in the trachea, with late distant metastasis. It has not been found to be associated with smoking or alcohol consumption.3 The sex incidence of tracheal adenoid carcinoma (TACC) is fairly even, and the age incidence predominates in the fourth and fifth decades. The management of TACC involves surgical resection and reconstruction, with the recommendation of adjuvant radiotherapy for controlling microscopic disease or as salvage treatment for unresectable disease.
In recent decades, oncological treatment modalities have considerably improved. However, the infrequency of this tumor impedes the establishment of treatment standards, and survival analyses may be biased by the small size of published series. In addition, larger cohorts occasionally gather tumors from different sites, with limited detailed data for the trachea site origin. Therefore, we performed a systematic review of TACC to describe the treatment strategies and outcomes of this rare tumor.
Materials and Methods
Objectives
The primary objective was to define the survival outcomes in patients diagnosed with TACC. The secondary objective was to describe the treatment modality used.
Search Strategy
This systematic review was performed and reported in accordance with the Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist and statement recommendations.4 A comprehensive search on PubMed, Web of Science and MEDLINE was conducted on February 28th, 2024, using the following queries: “(adenoid cystic carcinoma) AND (tumor*OR neoplasm*OR malignancy) AND (Tracheal)”.
Selection Criteria
Only original papers published in English in a peer-reviewed journal from January 1st, 1960, to December 31th, 2023, were included. Exclusion criteria were: 1) impossible to extrapolate specific data on treatments and survival of patients with TACC; 2) pediatric cohorts; 3) series with less than or equal to 5 patients; 4) non-human studies.
Two authors (YS and SX) independently screened all titles and abstracts. Full text was obtained for publications that fulfilled the inclusion criteria. The references of all the selected articles were reviewed to identify additional relevant articles. Disagreements were resolved by discussion with the senior author (JZ).
Data Extraction
The relevant features of each study (publication year, country, study design, and period of observation) were collected along with demographics (age at presentation, sex), pre-operative imaging, tumor features (site and subsite, TNM classification, perineural invasion [PNI], lymphovascular invasion [LVI], grading, and margin status), treatment modality and post-operative complications, mean follow-up, death, recurrence rate (local or distant), 5- and 10-year overall survival (OS), disease-free survival (DFS), local recurrence free survival (LRFS), and distant recurrence free survival (DRFS). Two authors (YS and SX) independently extracted data from the eligible articles and created a dedicated database. Data analyses were performed using Microsoft Excel. A pooled analysis of the 5-year OS extracted from the selected articles was performed to obtain a general estimate of the outcome.
We have not used any generative AI tools in this research.
Results
General Features
The initial search returned 1888 articles. The identification, screening, inclusion, and exclusion criteria are shown in Figure 1. Nineteen articles, all based on a retrospective series of 1488 patients, were included in the present systematic review (Table 1). The age range of the patients was 18–93 years old.
 |
Table 1 General Features of the Articles Included in the Systematic Review |
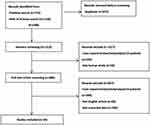 |
Figure 1 Identification of studies via database and registers. |
Demographics and Tumor Characteristics
The demographic and tumor characteristics are summarized in Table 2. Clinical features were reported for all 1488 patients. The male-to-female ratio was 1:1.38. There were 63 patients with T stage disease, 275 with negative lymph node invasion, 7 with distant metastasis, and 37 with perineural invasion. Excluded 1056 (71.0%) of patients with no specific subsite documented, the most common anatomical region of origin was distal/carinal trachea (149/10.0%), followed by cervical trachea (130/8.7%) and middle trachea (100/6.7%). The TNM classification has been reported in a few articles,2,13,17–19,21,22 and tumor grading has rarely been reported (Bhattacharyya’s staging system was adopted23). Lymph node involvement was reported in seventy-two cases, and metastasis status was reported in seven cases. PNI was reported in 2.5% (37/1488) of the cases, and LVI has not been reported. The resection margin status was detailed for 690 patients who were surgically treated, with 178 cases with negative margins, 255 with positive margins (not otherwise specified), 249 with microscopically positive margins (R1), and 8 with macroscopic positive margins (R2). Among 690 cases, 36.1% (249/690) presented with a confirmed R1 margin status and only 1.2% (8/690) with a confirmed R2 margin status and 25.8% (178/690) with negative margin.
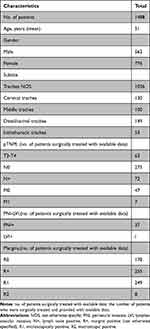 |
Table 2 Demographics and Tumor Features |
Treatment
The treatment modalities are described in all studies and are detailed in Table 3. Surgery was the most common treatment option. One thousand one hundred and nine patients (74.5%) were treated with different surgical procedures according to the tumor site. Tracheal surgery was performed in 416 patients, and when the distal trachea was involved, tracheal resection was associated with lobectomy or pulmonary resection in two and 55 patients, respectively. About 57.6% (639/1109) was managed with adjuvant radiotherapy (RT), 4.1% (46/1109), and 0.3% (3/1109) were managed by adjuvant chemoradiotherapy and chemotherapy, respectively. Almost all patients who received adjuvant radiotherapy were treated with photons, whereas only one patient was treated with carbon-ion radiotherapy (CIRT). Neoadjuvant therapy was administered to 2 patients.22
 |
Table 3 Treatment Strategies in the Reported Series of Patients |
Post-operative complications were described in 40 patients who underwent surgery, including 6.4% of non-fatal complications and 11 deaths due to anatomical problems (Table 4).
 |
Table 4 Post-Operative Complications |
Follow-up and Outcomes
The follow-up time was available for 1416 patients (range, 6–390 months). Patient status was available for 1364 patients. Five-hundred fifty-two patients died, with the most frequent cause of death being disease-related (98.7%). During the follow-up period, 139 patients reported recurrence. Survival estimates for 5-years and 10-years DFS, LRFS, and DRFS were frequently unavailable (Table 5). According to the data collected, 5-year OS ranged from 28.6% to 100.0%.2,5–15,17–22 The better survival rate (5-year OS 100.0%) was reported by Hazama et al,7 while the worse 5-year OS (28.6%) was described by Yang et al.9 Due to the relative lack of available data in the published documents, we aimed to obtain an average of 5-year OS was 65%. The 95% CI for 5-year OS was calculated by a weighted mean based on the inverse of the standard errors derived from individual study proportions, resulting in an estimated range of 32%–96%.
 |
Table 5 Oncologic Outcomes |
The 5-year DFS was described in four articles12,14,15,17 and ranged from 28.4% to 67.0%. The 5-year DRFS was reported in only two articles and ranged from 52.6% to 69.0%.2,17
The 10-year survival outcomes were available in 11 studies and ranged from 14.3% to 100.0%. The 10-year LRFS and DRFS rates were reported in two studies, ranging from 70.2% to 83.0% and 33.5% to 53.0%, respectively.2,17
In most studies, patients with surgery treatment resulted in a better survival rate than in patients without surgical treatment. However, Högerle et al found that patients treated with radiotherapy alone seemed to present with better outcome of 5-year OS (100%) than patients treated with surgery (with/without RT) (90.0%).2 In the subgroup of patients who underwent surgery combined with adjuvant RT, seemed to present with better outcome of 5-year OS, ranged from 76.2% to 100.0%.
Discussion
Due to the rare prevalence of TACC, the published literatures are mostly retrospective, in the form of relatively small cohort studies or even case reports. This leads to evidence in the literature that is of low quantity and quality. Consequently, data on treatment and outcomes are biased by the features of the reviewed studies, resulting in poor generality. In this systematic review, we aimed to present an overview of TACC with a focus on treatment strategies and oncological outcomes.
Our review included 1488 patients with a slightly higher proportion of females (1:1.38) and peak diagnosis in adulthood. It is seen that the sex distribution presents with an equal distribution, and the incidence increases with age and peaks in the fourth decade.24 Meanwhile, Mallick et al reported that TACC is seen more commonly in females.25 This is consistent with the findings of our review. Patients with TACC typically have a delay in diagnosis, one reason is that the nature of the indolent nature and slow growth and the other is that symptoms usually mimic other respiratory diseases, such as asthma, chronic obstructive pulmonary disease, or pneumonia. Radiological imaging, particularly computed tomography (CT), can assess the primary tumor location, extraluminal involvement, and regional/distant metastasis.26 Irrespective of anatomical origin, one of the most outstanding features of ACC is its tendency to invade nerves. For tumors in the paranasal sinuses or oral cavity, perineural spread is usually along the second and/or third branch of the trigeminal nerve.27 Here, only 49 cases were reported with PNI status (among them, 37 cases were positive), and rare reports of PNI in TACC have been described.14 Hornings et al also found that the presence of perineural growth decreased both long-term survival and DFS.14 Considering its importance, researchers recommended to include a statement regarding perineural growth in the pathology reports.
Excluded for 1056 cases with no specific subsite, the distal/carinal trachea was the most common region. Montenegro et al reported that distal/carinal trachea was the most involved subsites, as well.28 TNM stage was reported in a small portion, with 7 distant metastasis cases.
In our review, the most common intervention was surgery (74.5%) with or without post-operative RT. Surgical procedures differed according to the site of involvement. A positive resection margin status was reported in 72.2% of the patients. Owing to the delay in diagnosis and its growth pattern with submucosal and perineural spread along the airways, performing a complete resection of TACC and achieving negative surgical margins can be difficult.29–31
A series of case reports and small single-center studies suggest that a combination of surgery and adjuvant (post-operative) RT is beneficial for patients with TACC.16,30,32–36 However, there is limited evidence to support this treatment strategy. The efficiency of adjuvant RT is still debated and is generally recommended in the case of incomplete resection.37 Chen et al, followed 37 patients with incomplete resection, found that patients with adjuvant RT were associated with improved DFS and OS.16 Behbahani et al reviewed 394 patients, and they found that the 5-year OS and 10-year OS in patients with surgery alone were 77.5% and 65.9%, respectively, but 76.2% and 52.5%, respectively, in patients underwent surgery with RT.21 Meanwhile, Yang et al reported that patients with negative margin did not present with a better 5-year survival compared with patients with positive margin, and adjuvant radiation therapy to patients with positive margins was not associated with improved 5-year overall survival.19 Surgery treatment and surgical resection of small tumor size were the favorable factors to the OS.2,21
According to our data, more than half of patients who underwent surgery were treated with adjuvant RT. Conventional photon therapy was mostly used, with only one patient treated with CIRT. Therefore, reliable data on the effectiveness of RT as an adjuvant therapy for TACC need to be further researched.
Intervention with RT alone or CRT without surgery is rare and is usually managed in patients with unresectable tumors or distant metastases at diagnosis. The clinic value of systemic treatment in patients with recurrent or metastatic tracheal ACC has not been well demonstrated owing to the limited number of clinical trials published to date. ACC has been regarded as a histotype that presents with poor sensitivity to chemotherapy. Combined therapy with cisplatin may improve the response rate, but not more than 33% of patients.38 In our review, very few patients were treated with chemotherapy. With an improved understanding of the molecular mechanism of carcinogenesis in ACC, opening up new therapeutic perspectives, a series of trials have been designed to evaluate the efficiency of molecular therapeutic targets. According to the results of studies on the inhibition of the c-KIT pathway39–41 and the EGFR pathway,42–44 these two pathways are not able to modify the natural history of ACC. Dovitinib, a small molecular inhibitor of fibroblast growth factor receptor 1(FGFR1), produced few objective responses but suppressed the tumor growth rate with progression-free survival (8.2 months) and OS (21 months) compared favorably with other targeted agents.45 Other agents targeting the NOTCH signaling pathway have been studied, including Brontictuzumab and AL101. Brontictuzumab, a monoclonal antibody targeted Nothch1, showed partial response and prolonged disease stabilization.46 ACCURACY trial demonstrated AL101 was the potential anti-tumor activity of Notch pathway inhibition, based on clinical benefit response rate of 67.5% in patients with ACC-bearing activating NOTCH mutations.47 The NISCAHN trial observed the 8.7% partial response and 56% of stable disease in ACC patients treated with nivolumab.48
The follow-up range was 6–390 months. The natural history of ACC features slow tumor progression yet carries a substantial risk of late recurrence, potentially occurring 10 to 20 years after initial therapy. These distinctive biological characteristics warrant cautious interpretation of the presented survival outcomes.
There is a consensus that radical surgical resection is associated with better survival outcomes in patients with TACC. Based on the data collected, surgery had a better impact on the outcomes (Table 5), mainly in terms of the 5- and 10-year OS. Interestingly, Högerle et al retrospectively analyzed the outcomes of 38 patients and found that patients who received radiotherapy alone seemed to have better survival outcomes than those who underwent surgery.2
Given the limited scope of tracheal resection and the tendency of TACC to spread in submucosal tissue, complete resection is not always achievable, with a 59–63% positive resection margin.49 So, adjuvant RT is usually recommended for TACC with positive resection margins. In this review, surgery associated with adjuvant RT presented a good outcome, mainly in terms of 5- and 10-year LRFS (100%), which indicated that adjuvant RT may help in delaying local recurrence.2,17 However, Behbahani et al found that surgical treatment was an independent predictor of OS, rather than surgery combined with adjuvant RT.21 Therefore, whether adjuvant RT or alternative options should be managed upfront should be studied further for those with positive margins.
In order to obtain an estimate of the 5-year OS rate, we performed a pooled analysis, yielding a value of 65% (ranging from 32% to 96%). The same statistical analysis was not applicable to other outcomes because of the lack of precise data.
This systematic review has some limitations. Considering all the articles included, due to the rarity of this type of malignancy, and the time span in which these series studies were frequently collected, we were not able to acquire complete oncologic outcomes. The pooled analysis was applicable only to the 5-year OS, as very little data were available for other statistical analyses. The results should be interpreted with caution, as the majority of the literature reported OS at 5-and 10-years, which is not long enough to conclude an indisputable answer to TACC outcomes.
Conclusion
TACC is a very rare malignancy, often present in the fourth decade of life, and its diagnosis is often delayed. To date, no guidelines have been established for the treatment of TACC. Surgery is the most common first-choice treatment; adjuvant RT is frequently managed for patients with positive margins, while survival still presents with heterogeneity. Distant metastases may occur for more than 5 years, even after treatment. To improve oncological outcomes, large-scale prospective studies with extended longitudinal follow-up and more detailed data including OS, DFS, LRFS, and DRFS are required to better understand the nature of the disease and to better define treatment strategies.
Abbreviations
TACC, tracheal adenoid cystic carcinoma; OS, overall survival; SCC, squamous cell carcinoma; PNI, perineural invasion; LVI, lymphovascular invasion; DFS, disease free survival; LRFS, local recurrence free survival; DRFS, distant recurrence free survival; R1, microscopically positive; R2, macroscopic positive margins; RT, radiotherapy; CIRT, carbon-ion radiotherapy; CI, confidence interval; CRT, chemoradiotherapy; TS, tracheal surgery; C, chemotherapy.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck–an update. Oral Oncol. 2015;51(7):652–661. doi:10.1016/j.oraloncology.2015.04.005
2. Högerle BA, Lasitschka F, Muley T, et al. Primary adenoid cystic carcinoma of the trachea: clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat Oncol. 2019;14(1):117. doi:10.1186/s13014-019-1323-z
3. Maziak DE. Biology of adenoid cystic carcinoma of the tracheobronchial tree and principles of management. Thoracic Surgery Clinics. 2018;28(2):145–148. doi:10.1016/j.thorsurg.2018.01.002
4. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700. doi:10.1136/bmj.b2700
5. Azar T, Abdul-Karim FW, Tucker HM. Adenoid cystic carcinoma of the trachea. Laryngoscope. 1998;108(9):1297–1300. doi:10.1097/00005537-199809000-00006
6. Harms W, Latz D, Becker H, et al. Treatment of primary tracheal carcinoma. The role of external and endoluminal radiotherapy. Strahlenther Onkol. 2000;176(1):22–27. doi:10.1007/PL00002300
7. Hazama K, Miyoshi S, Akashi A, et al. Clinicopathological investigation of 20 cases of primary tracheal cancer. Eur J Cardiothorac Surg. 2003;23(1):1–5. doi:10.1016/S1010-7940(02)00728-5
8. Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. 2004;78(6):1889–1896. doi:10.1016/j.athoracsur.2004.05.064
9. Yang PY, Liu MS, Chen CH, et al. Adenoid cystic carcinoma of the trachea: a report of seven cases and literature review. Chang Gung Med J. 2005;28(5):357–363.
10. Honings J, van Dijck JA, Verhagen AF, et al. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol. 2007;14(2):968–976. doi:10.1245/s10434-006-9229-z
11. Zhengjaiang L, Pingzhang T, Dechao Z, et al. Primary tracheal tumours: 21 years of experience at Peking Union Medical College, Beijing, China. J Laryngol Otol. 2008;122(11):1235–1240. doi:10.1017/S0022215108001710
12. Bittner N, Koh WJ, Laramore GE, et al. Treatment of locally advanced adenoid cystic carcinoma of the trachea with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(2):410–414. doi:10.1016/j.ijrobp.2008.01.016
13. Ahn Y, Chang H, Lim YS, et al. Primary tracheal tumors: review of 37 cases. J Thorac Oncol. 2009;4(5):635–638. doi:10.1097/JTO.0b013e31819d18f9
14. Honings J, Gaissert HA, Weinberg AC, et al. Prognostic value of pathologic characteristics and resection margins in tracheal adenoid cystic carcinoma. Eur J Cardiothorac Surg. 2010;37(6):1438–1444. doi:10.1016/j.ejcts.2010.01.005
15. Yang H, Yao F, Tantai J, et al. Resected tracheal adenoid cystic carcinoma: improvements in outcome at a single institution. Ann Thorac Surg. 2016;101(1):294–300. doi:10.1016/j.athoracsur.2015.06.073
16. Chen F, Huang M, Xu Y, et al. Primary tracheal adenoid cystic carcinoma: adjuvant treatment outcome. Int J Clin Oncol. 2015;20(4):686–692. doi:10.1007/s10147-014-0771-6
17. Je HU, Song SY, Kim DK, et al. A 10-year clinical outcome of radiotherapy as an adjuvant or definitive treatment for primary tracheal adenoid cystic carcinoma. Radiat Oncol. 2017;12(1):196. doi:10.1186/s13014-017-0933-6
18. Benissan-Messan DZ, Merritt RE, Bazan JG, et al. National utilization of surgery and outcomes for primary tracheal cancer in the United States. Ann Thorac Surg. 2020;110(3):1012–1022. doi:10.1016/j.athoracsur.2020.03.048
19. Yang CJ, Shah SA, Ramakrishnan D, et al. Impact of positive margins and radiation after tracheal adenoid cystic carcinoma resection on survival. Ann Thorac Surg. 2020;109(4):1026–1032. doi:10.1016/j.athoracsur.2019.08.094
20. Baig MZ, Weber JF, Connery CP, Bhora FY. A SEER database cohort of 868 patients with primary tracheal cancers: characteristics and outcomes and the role of bronchoscopic interventions. Inter J Surg Oncol. 2020;5(4):e90. doi:10.1097/IJ9.0000000000000090
21. Behbahani S, Barinsky GL, Wassef D, et al. Patterns of care and outcomes of primary adenoid cystic carcinoma of the trachea. Ann Otol Rhinol Laryngol. 2022;131(1):78–85. doi:10.1177/00034894211008101
22. Li J, Tan F, Wang Y, et al. Clinical characteristics, surgical treatments, prognosis, and prognostic factors of primary tracheal cancer patients: 20-year data of the national cancer center, China. Transl Lung Cancer Res. 2022;11(5):735–743. doi:10.21037/tlcr-22-258
23. Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg. 2004;131(5):639–642. doi:10.1016/j.otohns.2004.05.018
24. Madariaga MLL, Gaissert HA. Overview of malignant tracheal tumors. Ann Cardiothorac Surg. 2018;7(2):244–254. doi:10.21037/acs.2018.03.04
25. Mallick S, Benson R, Giridhar P, et al. Demography, patterns of care and survival outcomes in patients with malignant tumors of trachea: a systematic review and individual patient data analysis of 733 patients. Lung Cancer. 2019;132:87–93. doi:10.1016/j.lungcan.2019.04.017
26. Zvrko E, Golubović M. Laryngeal adenoid cystic carcinoma. Acta Otorhinolaryngol Ital. 2009;29(5):279–282.
27. Cantù G. Adenoid cystic carcinoma. An indolent but aggressive tumour. Part A: from aetiopathogenesis to diagnosis. Acta Otorhinolaryngol Ital. 2021;41(3):206–214. doi:10.14639/0392-100X-N1379
28. Montenegro C, Mattavelli D, Lancini D, et al. Treatment and outcomes of minor salivary gland cancers of the larynx and trachea: a systematic review. Acta Otorhinolaryngologica Italica. 2023;43(6):365–374. doi:10.14639/0392-100X-N2635
29. Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg. 1996;112(6):1522–1531. doi:10.1016/S0022-5223(96)70011-9
30. Charlton P, Pitkin L. Airway compromise due to adenoid cystic carcinoma obstructing the distal trachea: a review of current management and clinical trials. BMJ Case Rep. 2015;bcr2014204063. doi:10.1136/bcr-2014-204063
31. Choudhury BK, Barman G, Singh S, et al. Adenoid cystic carcinoma of the upper trachea: a rare neoplasm. J Clin Imaging Sci. 2013;3:39. doi:10.4103/2156-7514.119021
32. Bonner Millar LP, Stripp D, Cooper JD, et al. Definitive radiotherapy for unresected adenoid cystic carcinoma of the trachea. Chest. 2012;141(5):1323–1326. doi:10.1378/chest.11-0925
33. Bradley P. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):127–132. doi:10.1097/00020840-200404000-00013
34. Cheung AY. Radiotherapy for primary carcinoma of the trachea. Radiother Oncol. 1989;14(4):279–285. doi:10.1016/0167-8140(89)90139-4
35. Shimizu J, Oda M, Matsumoto I, et al. Clinicopathological study of surgically treated cases of tracheobronchial adenoid cystic carcinoma. Gen Thorac Cardiovasc Surg. 2010;58(2):82–86. doi:10.1007/s11748-009-0467-4
36. Calzada AP, Miller M, Lai CK, et al. Adenoid cystic carcinoma of the airway: a 30-year review at one institution. Am J Otolaryngol. 2012;33(2):226–231. doi:10.1016/j.amjoto.2011.07.003
37. Marchioni A, Tonelli R, Samarelli AV, et al. Molecular biology and therapeutic targets of primitive tracheal tumors: focus on tumors derived by salivary glands and squamous cell carcinoma. Int J mol Sci. 2023;24(14):11370. doi:10.3390/ijms241411370
38. Sheikh E, Tran T, Vranic S, et al. Role and significance of c-KIT receptor tyrosine kinase in cancer: a review. Bosn J Basic Med Sci. 2022;22(5):683–698. doi:10.17305/bjbms.2021.7399
39. Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23(3):585–590. doi:10.1200/JCO.2005.06.125
40. Ghosal N, Mais K, Shenjere P, et al. Phase II study of cisplatin and imatinib in advanced salivary adenoid cystic carcinoma. Br J Oral Maxillofac Surg. 2011;49(7):510–515. doi:10.1016/j.bjoms.2010.09.013
41. Wong SJ, Karrison T, Hayes DN, et al. Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors. Ann Oncol. 2016;27(2):318–323. doi:10.1093/annonc/mdv537
42. Jakob JA, Kies MS, Glisson BS, et al. Phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck. 2015;37(5):644–649. doi:10.1002/hed.23647
43. Locati LD, Bossi P, Perrone F, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol. 2009;45(7):574–578. doi:10.1016/j.oraloncology.2008.07.010
44. Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbb2 expressing adenoid cystic carcinoma and non–adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25(25):3978–3984. doi:10.1200/JCO.2007.11.8612
45. Dillon PM, Petroni GR, Horton BJ, et al. A phase II study of dovitinib in patients with recurrent or metastatic adenoid cystic carcinoma. Clin Cancer Res. 2017;23(15):4138–4145. doi:10.1158/1078-0432.CCR-16-2942
46. Ferrarotto R, Eckhardt G, Patnaik A, et al. A Phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann Oncol. 2018;29(7):1561–1568. doi:10.1093/annonc/mdy171
47. Ayala Pharmaceuticals I. A study of AL101 in patients with adenoid cystic carcinoma (ACC) bearing activating notch mutations (ACCURACY). Available from: https://clinicaltrials.gov/ct2/show/NCT03691207b.
48. Fayette J, Even C, Digue L, et al. NISCAHN: a Phase II, multicenter nonrandomized trial aiming at evaluating nivolumab (N) in two cohorts of patients (pts) with recurrent/metastatic (R/M) salivary gland carcinoma of the head and neck (SGCHN), on behalf of the unicancer head & neck group. J Clin Oncol. 2019;37(15_suppl):6083. doi:10.1200/JCO.2019.37.15_suppl.6083
49. Ran J, Qu G, Chen X, et al. Clinical features, treatment and outcomes in patients with tracheal adenoid cystic carcinoma: a systematic literature review. Radiat Oncol. 2021;16(1):38. doi:10.1186/s13014-021-01770-0
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

