Back to Journals » Patient Preference and Adherence » Volume 18
Treatment Preferences of Adult Patients with Attention-Deficit/Hyperactivity Disorder – A Discrete Choice Experiment
Authors Schein J, Cloutier M, Gauthier-Loiselle M , Catillon M , Meng Y, Libchaber B, Jiang F, Childress A
Received 7 March 2024
Accepted for publication 18 July 2024
Published 6 August 2024 Volume 2024:18 Pages 1651—1664
DOI https://doi.org/10.2147/PPA.S467724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jeff Schein,1 Martin Cloutier,2 Marjolaine Gauthier-Loiselle,2 Maryaline Catillon,3 Yan Meng,4 Beatrice Libchaber,2 Fanny Jiang,2 Ann Childress5
1Otsuka Pharmaceutical Development & Commercialization, Inc, Princeton, NJ, USA; 2Analysis Group, Inc, Montréal, QC, Canada; 3Analysis Group, Inc, New York, NY, USA; 4Analysis Group, Inc, London, UK; 5Center for Psychiatry and Behavioral Medicine, Las Vegas, NV, USA
Correspondence: Maryaline Catillon, Analysis Group, Inc, 151 West 42nd Street, 23rd Floor, New York, NY, 10036, USA, Tel +1 857 222 6863, Email [email protected]
Background: Understanding patient preferences for treatments may facilitate shared decision-making. This study assessed adult patient preferences for attention-deficit/hyperactivity disorder (ADHD) treatments in a sample of 600 patients in the United States (US).
Methods: A web-based discrete choice experiment (DCE) survey was conducted among treated adults with ADHD. Participants were recruited from Dynata’s US panel (06/22/2023-07/06/2023). Attributes and levels, identified based on clinical inputs and published data, included efficacy and safety. Participants’ preferences were estimated using conditional logistic regression. Willingness to trade-off and attributes’ relative importance were calculated. Overall preferences for treatment profiles approximating centanafadine, lisdexamfetamine, atomoxetine, and viloxazine were estimated using adjusted total utilities. Results were stratified by current treatment status. Sensitivity analyses including participants who passed validity tests were conducted.
Results: Among the 600 participants (mean age 37.9 years; 66.2% female; 50.8% treated), all attributes had a statistically significant impact on preferences for ADHD treatments (p < 0.001); the most important attribute was improvement in ADHD symptoms (36%), followed by risks of nausea (25%), insomnia (20%), anxiety (8%), dry mouth (6%), and feeling jittery (5%). Together, safety attributes accounted for > 60% of relative importance in decision-making. Participants were willing to forgo 0.59, 0.57, 0.49, 0.32, and 0.17 percentage points of symptom improvement to achieve one-percentage-point reduced risk of insomnia, nausea, anxiety, feeling jittery, and dry mouth, respectively. Centanafadine profile had consistently higher adjusted total utilities than its comparators. Similar results were obtained in the subgroup and sensitivity analyses.
Conclusion: Efficacy was the most important attribute for patients when making treatment decision, but taken together, AEs had greater relative importance than efficacy alone. Accordingly, a profile resembling that of centanafadine would be preferred by an average patient compared to key competitors due to its favorable safety profile. These findings may help improve treatment decision-making, enhance treatment satisfaction, and foster adherence.
Keywords: ADHD, decision-making, discrete choice experiment, patient-centered care, patient satisfaction, patient preference, utility
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders and can persist throughout a patient’s lifetime.1,2 The disorder interferes with functioning and affects patient’s social and emotional behaviors, which can impair work performance, interpersonal relationships, and typical activities of daily living such as sleeping and driving.1,3 In the United States (US), an estimated 4.4% of adults have ADHD.4
The treatment landscape for ADHD in adults includes traditional stimulants, selective norepinephrine reuptake inhibitors (SNRIs), and alpha-adrenergic agonists.5–8 Traditional stimulants have been shown to effectively treat core ADHD symptoms;9 however, they have been associated with adverse events (AEs) such as decreased appetite, insomnia, and mood lability.8,10 SNRIs and alpha-adrenergic agonists exhibit less robust short-term efficacy than that of stimulants10,11 and have been associated with AEs such as liver toxicity, increased blood pressure, and somnolence.7,12 A recent matching-adjusted indirect comparison (MAIC) has suggested that the investigational ADHD treatment centanafadine, a norepinephrine/dopamine/serotonin triple reuptake inhibitor, may have a better safety profile than some currently approved treatments.13
Treatment decision-making involves balancing of benefits and risks, and the importance of shared decision-making and patient-centered care has been increasingly recognized.14 In addition to efficacy, patients may have other considerations (eg, impact of AEs on daily activities) that may influence their treatment decisions.14 Understanding patient preferences for treatments in relation to treatment attributes and identifying treatment trade-offs may help facilitate effective shared decision-making, which may in turn enhance treatment satisfaction and improve quality of care.14–16 While several real-world studies have examined treatment patterns among adult patients with ADHD in the US,17–19 there is limited information on the factors influencing treatment decisions in this population.16,20 One prior US study assessed treatment preferences among adult patients with ADHD using discrete choice experiment (DCE), focusing only on long-acting stimulants.21 To fill the literature gap, an online DCE survey was conducted to assess and quantify the extent to which different attributes (ie, efficacy and safety) of ADHD treatments impact adult patient preferences in the US. The overall patient preferences for treatment profiles resembling centanafadine and three common treatments for adults with ADHD (ie, lisdexamfetamine [Vyvanse], atomoxetine [Strattera], and viloxazine extended release [ER; Qelbree]) were also estimated.
Methods
Data Source and Study Population
Participants were recruited from the panel of a well-established market research firm, Dynata, through Email invitations. Data collection for the online DCE survey spanned from June 22 to July 6, 2023. To be eligible, participants must have been 1) 18 years or older; 2) diagnosed with ADHD and had been treated with at least one pharmacological treatment for ADHD at any time; 3) residing in the US at the time of the survey; and 4) at minimum somewhat comfortable reading and understanding English. Participants who completed the survey were compensated with panel points/rewards according to their membership.
This study was exempt by the Western Copernicus Group Institutional Review Board (WCG IRB) under 45 CFR § 46.104(d)(2), because the research only included interactions involving educational tests, survey procedures, interview procedures, or observations of public behavior; and the information obtained was recorded by the investigator in such a manner that the identity of the human participants cannot readily be ascertained, directly or through identifiers linked to the participants. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Study Design and Measures
A web-based DCE survey was conducted among adults with ADHD to assess and quantify their preferences for ADHD treatment attributes, in accordance with the recommendations of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Research Practices for Conjoint Analysis Task Force.22,23 The survey involved a screener to confirm participant’s eligibility and willingness to participate in the study, an assessment of participant’s characteristics and treatment experience and perspectives, and a DCE to assess participants’ preferences for different ADHD treatment options. Prior to data collection, pilot tests were conducted with four eligible participants to review the survey content and ensure comprehension; questions were refined as needed.
Identification of Treatment Attributes and Levels
Treatment attributes and levels included in the DCE were identified based on clinical inputs and a review of published clinical trial data of centanafadine (NCT03605680, NCT03605836),24 lisdexamfetamine (NCT00334880),25 atomoxetine (NCT00190736),26 and viloxazine ER (NCT04016779).27 The efficacy attribute identified was the improvement in ADHD symptoms, in which the levels for improvement were based on the change in Adult ADHD Investigator Symptom Rating Scale score from baseline reported in each active arm of the respective clinical trials. Safety attributes were related to the risk of experiencing AEs from a treatment. The AE selection criteria included 1) an incidence of ≥5% and twice that of the placebo rate in the active arm of the respective clinical trials and 2) statistically significant differences in risk difference between centanafadine and other treatments in a previously published MAIC analysis.13 AEs that were not frequently associated with treatment changes or did not apply to the entire population (eg, erectile dysfunction) were excluded based on clinical inputs. Additionally, when AEs were strongly correlated (eg, fatigue and insomnia), only one of them was considered. For each safety attribute, the levels were selected to cover the range of AEs rates reported in each active arm of the respective clinical trials.
The final list of attributes included improvement in ADHD symptoms, risk of anxiety, risk of dry mouth, risk of feeling jittery, risk of insomnia, and risk of nausea; the levels for each attribute are listed in Supplementary Table 1.
DCE Design
Based on the attributes and levels identified, a total of 36 choice cards were generated using a D-efficient design in Ngene (software specifically developed to design DCE experiments).28 To limit response burden, the choice cards were administered in four blocks with nine different choice cards in each block. Each participant was assigned randomly to one of the blocks and hence answered nine choice cards. Four additional choice cards were presented to assess the internal validity of the responses (see Sensitivity analyses below). Therefore, each participant answered a total of 13 choice cards. Figure 1 shows an example of a choice card that contains two hypothetical ADHD treatment profiles with combinations of attributes at various levels. For each choice card, participants were asked to choose their preferred treatment option.
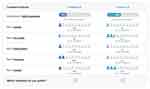 |
Figure 1 Example choice card. Abbreviation: ADHD, attention-deficit/hyperactivity disorder. |
Statistical Analyses
Participants’ demographic and clinical characteristics as well as treatment experience and perspectives were descriptively summarized. Means, medians, and standard deviations were reported for continuous variables; frequency counts and percentages were reported for categorical variables.
Data collected from the DCE were analyzed using a conditional logistic regression model, in which the dependent variable was the participant’s preference data for a given choice card (ie, a binary variable indicating if a given treatment option was selected), and the independent variables were the levels of each attribute evaluated as continuous variables. Regression coefficients (ie, preference weights), 95% confidence interval, and p-values were reported for each attribute. The coefficients were used to calculate the willingness to trade-off (WTT) using the formula − (βrisk of AE/βimprovement in ADHD symptoms); the analysis was anchored to efficacy to estimate how much efficacy (ie, percentage points of improvement in ADHD symptoms) a participant would be willing to forgo in favor of a better safety profile (ie, one-percentage-point reduction in the risk of a particular AE).
Part-worth utility, which measures the utility associated with each attribute level, was calculated by multiplying the coefficients with each potential level of a given attribute. To allow for comparisons across treatment attributes, attributes’ relative importance was calculated by multiplying the coefficients with the difference between the best and worst levels of the corresponding attribute.29
Subgroup Analyses
To understand potential variations in treatment preferences by current treatment status, analyses were also conducted in two subgroups (ie, currently treated and currently untreated) based on whether participants had a pharmacological treatment for ADHD within the last month of the survey. The target sample size for each subgroup was approximately half of that of the overall sample.
Sensitivity Analyses
Sensitivity analyses were conducted to evaluate the internal validity of the preference data. Based on the results of the four additional choice cards designed to assess data quality, only participants who passed the validity tests (ie, stability and transitivity tests) were included in the sensitivity analyses.
Reconstruction of Treatment Profiles and Estimation of Overall Preferences
Treatment profiles approximating centanafadine and three comparators (ie, lisdexamfetamine, atomoxetine, and viloxazine ER) were reconstructed based on published clinical trials and MAIC results.13,24–27 The total utility for each treatment profile was obtained by summing the individual utility from each efficacy and safety attribute in the corresponding treatment profile. As there were significant placebo effects in each trial, incremental utility of each treatment compared to placebo was calculated to tease out the placebo effect. The resulting adjusted total utility represents the incremental utility between each treatment profile and its corresponding placebo profile; a higher value indicates preferred treatment profiles. The adjusted total utility of centanafadine could vary across comparisons, as the underlying efficacy and AE rates of centanafadine were measured at different time points across MAICs.13
All analyses were performed using the SAS Enterprise Guide statistical software version 7.1 (SAS Institute, Cary, NC).
Results
Participant Characteristics
A total of 600 participants completed the survey and were included in the overall sample. Approximately half (n = 305; 50.8%) of the participants were currently treated and half (n = 276; 46.0%) were currently untreated at the time of the survey, in accord with the target subgroup sample sizes; 19 (3.2%) participants reported “unknown” or “prefer not to answer” to their current pharmacological treatment status and were excluded from the subgroup analyses.
Overall, participants had a mean age of 37.9 years, and 66.2% were female. Most participants (85.5%) were White, 9.3% were Hispanic or Latino, and 6.8% were African American or Black. The majority of participants (78.0%) had a college or more advanced degree. Approximately two-thirds (59.5%) of participants were diagnosed with ADHD as adults (Table 1).
 |
Table 1 Participant Demographic and Clinical Characteristics |
Treatment Experience and Perspectives
Among the 305 currently treated participants, 272 (89.2%) were receiving stimulants. Stimulants were also the most common previous ADHD treatment received by 518 of 600 (86.3%) participants overall (Table 2).
 |
Table 2 Participant Treatment Experience and Perspectives |
When currently treated participants were asked how their ADHD treatment was selected, 65.6% reported that the health provider presented them with several options, whereas 23.0% reported that they were presented with only one option; 9.8% of participants reported suggesting the treatment option to the health provider; and the remaining 1.6% of participants preferred not to answer or the response was unknown. Approximately two-thirds of currently treated participants reported discussing treatment efficacy and risk of side effects (both 65.9%) with their health provider regarding the selected treatment.
Overall, a vast majority (93.8%) of participants reported ≥1 side effect while on an ADHD treatment, and the most frequently reported side effects included decreased appetite (48.7%), dry mouth (41.2%), anxiety (39.8%), and insomnia (39.7%). There were 90.8% of participants who had ever stopped or switched an ADHD pharmacological treatment. The most common reason for stopping or switching a treatment was experience of side effects, reported in 133 of 384 (34.6%) and 97 of 226 (42.9%) participants who previously stopped or switched a treatment, respectively (Figure 2). Among those who stopped or switched treatment because of side effects, 42.9% and 48.5% reported anxiety as the side effect leading to treatment stop or switch, respectively, while 38.3% and 38.1% reported feeling jittery, 36.1% and 30.9% reported insomnia, 23.3% and 18.6% reported dry mouth, and 15.8% and 23.7% reported nausea as the side effect leading to treatment stop or switch, respectively (Supplementary Figure 1).
 |
Figure 2 Main reasons to treatment stop and switch.a (A) Main reasons for treatment stop (N=384). (B) Main reasons for treatment switch (N=226). aResponses were not mutually exclusive. |
DCE Results
All six attributes included in the DCE had a statistically significant impact on participants’ preferences for ADHD treatments (Table 3). Improvement in ADHD symptoms was found to positively impact treatment preferences (p < 0.001), meaning participants would prefer treatments providing a larger improvement of ADHD symptoms. All safety attributes negatively impacted participants’ preferences (all p < 0.001).
 |
Table 3 Preference Weights and WTT |
WTT represents the improvement in ADHD symptoms (in percentage points) that an average participant would be willing to forgo to achieve a one-percentage-point reduction in the risk of an AE (Table 3). On average, participants were willing to forgo the most efficacy points for insomnia, with a WTT of 0.59 (ie, an average participant was willing to forgo 0.59 percentage points of improvement in ADHD symptoms to reduce the risk of insomnia by one percentage point). The WTT to avoid one percentage point of risks of nausea, anxiety, feeling jittery, and dry mouth were 0.57, 0.49, 0.32, 0.17 percentage points of improvement in ADHD symptoms, respectively. The estimated preference weights of the attributes and WTT were similar in the sensitivity analysis among the 518 participants (86.3%) who passed the validity tests, which demonstrated the robustness of our main analysis (Table 3).
In the overall sample, part-worth utilities increased with greater levels of improvement in ADHD symptoms (ie, the utilities associated with 25%, 35%, and 45% of ADHD symptom improvements were 2.53, 3.54, and 4.55, respectively), whereas higher risks of AEs were associated with disutilities (eg, disutilities associated with 1%, 5%, and 10% risk of anxiety were −0.05, −0.25, and −0.50, respectively; Figure 3). Of all attributes, the improvement in ADHD symptoms had the highest relative importance (36%), followed by the risk of nausea (25%), risk of insomnia (20%), risk of anxiety (8%), risk of dry mouth (6%), and risk of feeling jittery (5%). Although the improvement in ADHD symptoms had the highest relative importance among all attributes, the combined relative importance of all safety attributes (ie, 64%) was higher than that of efficacy (Figure 4).
 |
Figure 3 Part-worth utilities – Overall sample. Abbreviation: ADHD, attention-deficit/hyperactivity disorder. |
 |
Figure 4 Relative importance of treatment attributes – Overall sample. Abbreviation: ADHD, attention-deficit/hyperactivity disorder. |
Similar results were obtained in the currently treated and currently untreated subgroups, although currently treated participants had slightly stronger preference for improvement in ADHD symptoms than currently untreated participants, as shown by the relative importance (Figure 5) and part-worth utilities (Supplementary Figure 2).
Reconstruction of Treatment Profiles and Participant Preferences
The adjusted total utilities of the reconstructed treatment profiles resembling centanafadine were higher relative to each of its three comparators (ie, lisdexamfetamine, atomoxetine, and viloxazine ER profiles), indicating that the centanafadine profile was consistently the preferred treatment option (Figure 6). For example, the higher adjusted total utility between centanafadine vs placebo (0.4) than that between lisdexamfetamine vs placebo (0.1) indicated that the centanafadine profile was more preferred than the lisdexamfetamine profile by an average participant. The preference for centanafadine was due to its more favorable AE profiles, with similar results obtained in the overall sample and subgroups (Supplementary Table 2). These results were also robust in sensitivity analyses among participants who passed the validity tests (data not shown).
Discussion
Based on an online DCE, this study evaluated adult patient preferences for ADHD pharmacological treatments and found that efficacy, measured as improvement in ADHD symptoms, was the most important treatment attribute; nonetheless, safety attributes together accounted for more than 60% of relative importance in treatment decision-making. These preference results were consistent across currently treated and untreated subgroups. In addition, participants were willing to trade off efficacy for a lower risk of AE, particularly for insomnia, nausea, and anxiety. For instance, participants were willing to forgo 0.59 percentage point of improvement in ADHD symptoms to avoid the risk of insomnia by one percentage point. To contextualize this result with an example, a previous MAIC has found that the incremental risk of insomnia with lisdexamfetamine relative to that with centanafadine was fifteen percentage points;13 in this case, an average patient would be willing to forgo almost nine percentage points of improvement in ADHD symptoms to avoid the incremental risk of insomnia with lisdexamfetamine relative to centanafadine. The current study also found that a profile resembling that of centanafadine was consistently the preferred option by an average patient over profiles resembling that of its key comparators (ie, lisdexamfetamine, atomoxetine, and viloxazine ER) due to a better safety profile. Findings were similar irrespective of participants’ current treatment status.
Our finding on the importance of efficacy on patient preferences for ADHD treatment is consistent with a prior DCE evaluating patient preferences for long-acting stimulants among adults with ADHD in the US, which reported that an efficacy attribute, speed of onset, was the most valued by patients; this was followed by the risk of headache (eg, a reduction from 31% to <1%), risk of insomnia (eg, a reduction from 31% to 5%), duration of effect (eg, an increase from 8 to 14 hours), and risk of anxiety (eg, a reduction from 13% to <1%).21 DCEs outside of the US have reported that attributes with statistically significant importance on adult patient preferences for ADHD treatments included efficacy (eg, improvement in the ability to concentrate, control of impulses); changes in social, emotional, learning, and other long-term behaviors; as well as safety such as AE severity.30–32 Two related Australian studies combined several safety endpoints into a single attribute (ie, side effects: none, mild, moderate, and severe) and found that AEs were the most important factor for adult patients with ADHD when making treatment decisions;30,31 this aligns with the current finding on the high combined relative importance of safety attributes for patients when selecting ADHD treatments. The current study additionally quantified the extent to which individual AEs commonly reported in clinical trials could impact patient preferences, thereby providing insights on the importance of each AE on patients’ treatment decisions. Further research is warranted to explore the preference heterogeneity across countries.
This study helps better understand patients’ preferences and willingness to trade off efficacy and safety when selecting ADHD treatments. Indeed, even though efficacy is the most valued attribute, adult patients with ADHD are willing to trade it off in exchange for reduced risks of AEs. Therefore, it is imperative for physicians and patients to discuss potential AEs along with efficacy associated with various treatments when considering optimal ADHD management and making treatment decisions to improve patients’ quality of life.14 Shared decision-making may help physicians offer more tailored treatment options and in turn improve patients’ treatment satisfaction and adherence.16 Notably, the current study found that one in four patients were only offered a single treatment option from their physician, and one-third did not receive information about efficacy or AEs from their physician when making a treatment decision, suggesting that there may still be room for improvement regarding shared decision-making in routine ADHD management. Future research should investigate whether certain groups of patients are less likely to be provided with relevant treatment information and various treatment options.
Findings from the current study also suggest that an average patient may prefer a placebo over a treatment profile resembling certain existing ADHD treatments, as demonstrated by the negative adjusted total utilities in some reconstructed treatment profiles (ie, atomoxetine, viloxazine ER). These findings are consistent with the notion that ADHD is a largely under-treated disorder.4,33 In addition to the challenges in diagnosing ADHD that lead to nontreatment, frequent discontinuation and low adherence to treatment have also been observed in real-world ADHD populations.18,34,35 While common reasons for treatment discontinuation and nonadherence include suboptimal symptom improvement and treatment-related complications,36 the lack of patient involvement during the treatment-selection process may also contribute to treatment dissatisfaction and hence poor persistence and adherence.37 In this regard, selecting a treatment option with attributes valued by patients may potentially improve patient satisfaction. Future research should investigate the potential impact of patient characteristics such as gender, race, ADHD subtype, and health insurance type on patients’ perceptions of AEs and treatment preferences. A future US DCE study among physicians is also warranted to understand potential discrepancies in the preferences of ADHD treatment attributes between physicians and patients, which may further facilitate the discussions during shared decision-making.
The findings of the study should be considered in light of limitations. First, the current study included only individuals accessible through the Dynata panel who wished to participate in the study. The survey was completed by participants who had access to computers and had the desire to serve on an online panel. Participants who complete this type of survey generally tend to be more educated, younger, and with better health status than the general population of patients with ADHD. The current sample also comprised a higher percentage of female participants, whereas ADHD is more predominant among males.4,38 As a result, the sample may not be representative of the US population of adults with ADHD. Second, this study relied on participants’ recollection of past events; thus, recall bias, or errors in the accuracy or completeness of recalled experiences, could be an issue particularly if past memories were influenced by more recent events. Nonetheless, the study attempted to minimize recall bias by asking participants to recall events that occurred in the recent past. Third, to be considerate of response burden, only a limited number of key attributes were included in the DCE; additional attributes may have been important for patients’ treatment preferences.
Conclusions
In selecting ADHD pharmacological treatment, efficacy, as measured by improvement in ADHD symptoms, was the most important attribute for adult patients, but all treatment attributes impacted patient preferences, with safety attributes accounting for more than 60% of relative importance in treatment decision-making. Patients were willing to trade off varying degree of efficacy for a lower risk of AE, particularly for insomnia, nausea, and anxiety. A profile similar to that of centanafadine would be a preferred option for an average patient compared with profiles similar to those of lisdexamfetamine, atomoxetine, and viloxazine ER, due to its better safety profile. Results were similar irrespective of current treatment status. Findings from this study help better understand attributes of ADHD pharmacological treatments valued by patients and the extent to which they are willing to trade off efficacy for safety, which have the potential to improve treatment decision-making, enhance treatment satisfaction, and foster adherence to treatment. Future research is warranted to assess the potential impact of patient characteristics on treatment preferences.
Abbreviations
ADHD, Attention-deficit/hyperactivity disorder; AE, Adverse events; DCE, Discrete choice experiment; ER, Extended release; MAIC, Matching-adjusted indirect comparison; SNRI, Selective norepinephrine reuptake inhibitors; US, United States; WTT, Willingness to trade-off.
Data Sharing Statement
The data that support the findings of this study are available on reasonable request. The data are not publicly available and cannot be deposited into a public repository, primarily due to the fact that study participants did not consent to this. The project team was responsible for not compromising research participant privacy/consent.
Ethics Approval and Informed Consent
This study was exempt by the WCG IRB under 45 CFR § 46.104(d)(2), because the research only included interactions involving educational tests, survey procedures, interview procedures, or observations of public behavior; and the information obtained was recorded by the investigator in such a manner that the identity of the human participants cannot readily be ascertained, directly or through identifiers linked to the participants. Participants provided informed consent prior to completing the survey.
Acknowledgments
Medical writing assistance was provided by professional medical writer, Flora Chik, PhD, MWC, an employee of Analysis Group, Inc., and was funded by Otsuka Pharmaceutical Development & Commercialization, Inc.
Part of the material in this paper was presented at ISPOR 2024 as a poster presentation. The poster’s abstract was published in Value in Health 27, no. 6 (2024): S310; https://doi.org/10.1016/j.jval.2024.03.1957.
Author Contributions
All authors have made substantial contributions to the conception or design of the study, or the acquisition, analysis, or interpretation of data, drafting the manuscript and revising it critically for important intellectual content, and have provided final approval of this version to be published and agree to be accountable for all aspects of the work.
Funding
This study was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
Disclosure
Jeff Schein is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. Martin Cloutier, Marjolaine Gauthier-Loiselle, Maryaline Catillon, Yan Meng, Beatrice Libchaber, and Fanny Jiang are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Otsuka Pharmaceutical Development & Commercialization, Inc. Ann Childress received research support from Aardvark, Allergan, Axsome, Emalex, Akili, Cingulate, Corium, Ironshore, Les Laboratoires Servier, Lumos, Neurocentria, Otsuka, Purdue, Adlon, Sunovion, Tris, KemPharm, and Supernus; was on the advisory board of Corium, Otsuka, Tris, and Supernus; received consulting fees from Aardvark, Alora, Axsome, Aytu, Cingulate, Corium, Lumos, Medison Pharma, Neurocentria, Noven, Otsuka, Sky, Tris, KemPharm, Supernus, and Tulex; received speaker fees from Takeda, Corium, Ironshore, Tris, and Supernus; and received writing support from Otsuka, Takeda, Corium, Ironshore, Purdue, and Tris. The authors report no other conflicts of interest in this work.
References
1. American Psychiatric Association. Attention-Deficit/ Hyperactivity Disorder. Diagnostic and Statistical Manual of Mental Disorders.
2. Cherkasova MV, Roy A, Molina BSG, et al. Review: adult outcome as seen through controlled prospective follow-up studies of children with attention-deficit/hyperactivity disorder followed into adulthood. J Am Acad Child Adolesc Psychiatry. 2022;61(3):378–391. doi:10.1016/j.jaac.2021.05.019
3. Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. doi:10.1038/nrdp.2015.20
4. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006;163(4):716–723. doi:10.1176/ajp.2006.163.4.716
5. Caye A, Swanson JM, Coghill D, Rohde LA. Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Mol Psychiatry. 2019;24(3):390–408. doi:10.1038/s41380-018-0116-3
6. Qelbree (viloxazine extended-release capsules) Prescribing Information. Rockville, MD: Supernus Pharmaceuticals, Inc;2022.
7. Strattera (atomoxetine) Prescribing Information. Indianapolis, IN: Indianapolis;2020.
8. Vyvanse (lisdexamfetamine dimesylate) Prescribing Information. Lexington, MA: Takeda Pharmaceuticals America, Inc; 2022.
9. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–738. doi:10.1016/S2215-0366(18)30269-4
10. De Crescenzo F, Cortese S, Adamo N, Janiri L. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4–11. doi:10.1136/eb-2016-102415
11. Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4). doi:10.1542/peds.2019-2528
12. Cinnamon Bidwell L, Dew RE, Kollins SH. Alpha-2 adrenergic receptors and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2010;12(5):366–373. doi:10.1007/s11920-010-0136-4
13. Schein J, Cloutier M, Gauthier-Loiselle M, Catillon M, Childress A. Assessment of centanafadine in adults with ADHD: a matching adjusted indirect comparison vs lisdexamfetamine dimesylate, atomoxetine hydrochloride, and viloxazine extended release. Curr Med Res Opin. 2024;27:1–6. doi:10.1080/03007995.2024.2373883
14. Keirns CC, Goold SD. Patient-centered care and preference-sensitive decision making. JAMA. 2009;302(16):1805–1806. doi:10.1001/jama.2009.1550
15. Kon AA. The shared decision-making continuum. JAMA. 2010;304(8):903–904. doi:10.1001/jama.2010.1208
16. Van Brunt K, Matza LS, Classi PM, Johnston JA. Preferences related to attention-deficit/hyperactivity disorder and its treatment. Patient Prefer Adherence. 2011;5:33–43. doi:10.2147/PPA.S6389
17. Christensen L, Sasane R, Hodgkins P, Harley C, Tetali S. Pharmacological treatment patterns among patients with attention-deficit/hyperactivity disorder: retrospective claims-based analysis of a managed care population. Curr Med Res Opin. 2010;26(4):977–989. doi:10.1185/03007991003673617
18. Schein J, Childress A, Adams J, et al. Treatment patterns among adults with attention-deficit/hyperactivity disorder in the United States: a retrospective claims study. Curr Med Res Opin. 2021;37(11):2007–2014. doi:10.1080/03007995.2021.1968814
19. Perwien A, Hall J, Swensen A, Swindle R. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122–129. doi:10.18553/jmcp.2004.10.2.122
20. Schatz NK, Fabiano GA, Cunningham CE, et al. Systematic review of patients’ and parents’ preferences for ADHD treatment options and processes of care. Patient. 2015;8(6):483–497. doi:10.1007/s40271-015-0112-5
21. Cambron-Mellott MJ, Mikl J, Matos JE, et al. Adult patient preferences for long-acting ADHD treatments: a discrete choice experiment. Patient Prefer Adherence. 2021;15:1061–1073. doi:10.2147/PPA.S311836
22. Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13. doi:10.1016/j.jval.2012.08.2223
23. Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
24. Adler LA, Adams J, Madera-McDonough J, et al. Efficacy, safety, and tolerability of centanafadine sustained-release tablets in adults with attention-deficit/hyperactivity disorder: results of 2 Phase 3, randomized, double-blind, multicenter, placebo-controlled trials. J Clin Psychopharmacol. 2022;42(5):429–439. doi:10.1097/jcp.0000000000001575
25. Adler LA, Goodman DW, Kollins SH, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–1373. doi:10.4088/jcp.v69n0903
26. Adler LA, Spencer T, Brown TE, et al. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29(1):44–50. doi:10.1097/JCP.0b013e318192e4a0
27. Nasser A, Hull JT, Chaturvedi SA, et al. A Phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897–915. doi:10.1007/s40263-022-00938-w
28. Bliemer MC, Rose JM. Designing and conducting stated choice experiments. In: Handbook of Choice Modelling. Cheltenham: Edward Elgar Publishing; 2014.
29. Gonzalez JM. A guide to measuring and interpreting attribute importance. Patient. 2019;12(3):287–295. doi:10.1007/s40271-019-00360-3
30. Khan MU, Balbontin C, Bliemer M, Aslani P. Using discrete choice experiment to investigate patients’ and parents’ preferences for initiating ADHD medication. J Ment Health. 2023;32(2):373–385. doi:10.1080/09638237.2021.1979495
31. Khan MU, Balbontin C, Bliemer MCJ, Aslani P. Eliciting preferences for continuing medication among adult patients and parents of children with attention-deficit hyperactivity disorder. Health Expect. 2022;25(3):1094–1107. doi:10.1111/hex.13462
32. Muhlbacher AC, Nubling M. Analysis of patients’ preferences: direct assessment and discrete-choice experiment in therapy of adults with attention-deficit hyperactivity disorder. Patient. 2010;3(4):285–294. doi:10.2165/11584640-000000000-000009
33. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960–966. doi:10.1176/appi.ajp.2016.15091207
34. Frank E, Ozon C, Nair V, Othee K. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459–68. doi:10.4088/JCP.14r09478
35. Gajria K, Lu M, Sikirica V, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543–1569. doi:10.2147/NDT.S65721
36. Schein J, Childress A, Cloutier M, et al. Reasons for treatment changes in adults with attention-deficit/hyperactivity disorder: a chart review study. BMC Psychiatry. 2022;22(1):377. doi:10.1186/s12888-022-04016-9
37. Guadagnoli E, Ward P. Patient participation in decision-making. Soc Sci Med. 1998;47(3):329–339. doi:10.1016/s0277-9536(98)00059-8
38. Fayyad J, Sampson NA, Hwang I, et al. The descriptive epidemiology of DSM-IV adult ADHD in the world health organization world mental health surveys. Atten Defic Hyperact Disord. 2017;9(1):47–65. doi:10.1007/s12402-016-0208-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



