Back to Journals » International Journal of Nanomedicine » Volume 20
Tumor Microenvironment-Responsive Nanoparticles: Promising Cancer PTT Carriers
Authors Sun H, Li Y, Xue M, Feng D
Received 6 March 2025
Accepted for publication 12 June 2025
Published 23 June 2025 Volume 2025:20 Pages 7987—8001
DOI https://doi.org/10.2147/IJN.S526497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Heming Sun,1 Yuebo Li,2,3 Ming Xue,2,3 Dingqing Feng3
1Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, 100029, People’s Republic of China; 2Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100029, People’s Republic of China; 3Department of Obstetrics and Gynecology, China-Japan Friendship Hospital, Beijing, 100029, People’s Republic of China
Correspondence: Dingqing Feng, Department of Obstetrics and Gynecology, China-Japan Friendship Hospital (Institute of Clinical Medical Sciences), Beijing, 100029, People’s Republic of China, Email [email protected]
Abstract: The tumor microenvironment (TME) is often characterized by distinctive features such as hypoxia, low pH, the overexpression of extracellular matrix-degrading enzymes, and increased redox reactions. These attributes create a specialized internal environment that promotes tumor cell survival and proliferation, thereby facilitating tumor development, metastasis, and the emergence of drug resistance. These challenging aspects pose significant hurdles to the efficacy of traditional cancer therapies. However, they also offer unique opportunities for the development of responsive nanomedicines that specifically target the TME to improve treatment outcomes for cancer patients when combined with photothermal therapy (PTT). This review provides an overview of the predominant features of the TME and delves into recent advancements in the field of nanomedicine, with a special focus on TME-responsive nanomedicines. Each type of TME-responsive nanomedicine is reviewed for its potential value in drug delivery in combination with PTT and chemotherapy, which may enable effective multimodal antitumor therapy. Finally, the review discusses the challenges and opportunities associated with the use of TME-responsive nanomaterials in PTT, highlighting the potential for these innovative strategies to overcome current therapeutic limitations and improve patient outcomes.
Keywords: tumor microenvironment, photothermal therapy, cancer therapy, nanoparticle
Introduction
Cancer remains one of three leading causes of death worldwide,1 particularly since many patients are diagnosed with advanced, metastatic disease, which is still considered to be incurable.2 Over the past decade or so, there has been a continuous search for new strategies and methods for tumor treatment, and nanomaterials have gradually been used for the diagnosis and treatment of cancer3 to reduce systemic toxicity and increase the targeting of tumors and metastatic cancer cells.4
In the early stages of nanotechnology development, scientists discovered that some metal nanoparticles, owing to their unique optical properties, possess excellent photothermal conversion abilities.5 They can exert thermal effects upon irradiation with a laser or another light source.6 This provides more options and potential applications for photothermal therapy (PTT). Therefore, in recent years, PTT with nanomaterials has become a hot topic in both research and application.7 With the advancement of nanotechnology, scientists have begun to develop various types of nanomaterials for PTT, such as semiconductor nanocrystals,8 multiwalled carbon nanotubes,9 graphene,10 iron oxide nanoparticles,11 and nanoparticles based on organic materials.12 PTT, which converts light energy into heat to kill tumor cells, has attracted widespread attention. Furthermore, there is a new understanding of cancer, which is now considered an evolutionary and ecological process involving continuous, dynamic, and interactive relationships between tumor cells and the tumor microenvironment (TME).13 Therefore, TME-responsive nanoparticles are promising carriers for cancer PTT, which is an effective tumor treatment modality; the combination of these strategies has advantages such as noninvasive application, strong targeting ability, low systemic toxicity, and fewer side effects.14
This review mainly summarizes the TME and discusses the application of TME-responsive nanomaterials in PTT.
Characteristics of the Tumor Microenvironment
The TME is a crucial component in the process of tumor growth and metastasis.15 It consists not only of the tumor cells themselves but also of the surrounding nontumor cells, the extracellular matrix (ECM), the vascular system, immune cells, and various cytokines.16 Within the TME, different components interact with each other in complex ways that affect tumor development and response to treatment.13 The characteristics of the TME include hypoxia,17 an acidic pH value (pH 6.0–7.0),18 the overexpression of certain enzymes,19 a shift in redox potential toward more reductive conditions,20 and high ATP levels,21 among others (Figure 1).
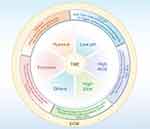 |
Figure 1 Illustrated Guide to the Features of the TME and Corresponding Response Strategies. |
Hypoxia
The TME is hypoxic, where tumor cells often proliferate and expand rapidly, leading to a swift increase in oxygen demand. Hypoxia may also be associated with angiogenesis.22 Tumor-induced angiogenesis (the formation of new blood vessels) is usually disordered and abnormal. These newly formed vessels are often twisted and immature, and their blood flow may be unstable or insufficient, leading to problems with oxygen delivery and thus preventing sufficient oxygen from reaching the tumor regions.23 Although hypoxia can damage both normal and cancerous cells, cancer cells are able to adapt, survive and proliferate under hypoxic conditions.24 Moreover, hypoxia also participates in the regulation of factors such as glycolysis, growth factor signaling, immortality, genetic instability, tissue invasion and metastasis, and apoptosis, among others, enhancing tumor cell resistance to radiotherapy and chemotherapy.25,26
Low pH
One of the prominent features of the TME is that its pH value is lower than that of normal tissues (pH 7.2–7.4).27 This may be attributed to the preference of the tumor tissue for anaerobic respiration and its state of high metabolism, which leads to increased production of lactic acid and other acidic metabolic byproducts, resulting in a local decrease in pH.28 Additionally, cancer cells normally exhibit rapid proliferation and metabolic activity far exceeding that of normal cells.29 These conditions increase the demand for energy and metabolic substrates, which could also result in the generation of excessive amounts of acidic metabolic byproducts. Hypoxia can further cause cellular acidosis, which inactivates proton pumps on the cell membrane, impedes the extrusion of protons, and thereby further reduces the pH of the extracellular fluid.30 The infiltration of inflammatory cells31 and the activity of immune cells32 can also affect the local pH value.
High GSH
Glutathione (GSH), a thiol-containing organic molecule abundantly found in the human body, plays a significant role in facilitating the maintenance of the cellular redox equilibrium and providing antioxidant protection.32 It maintains the static potential of various cellular organelles and sustains a difference in surface potential.33 GSH, primarily in the form of small, free sulfonyl groups, is highly reactive at low concentrations. In cancer cells, increased GSH levels are associated with the overexpression of γ-glutamyl cysteine synthetase and γ-glutamyl transferase. These enzymes are key in the GSH biosynthetic pathway, and their active expression in tumor cells leads to a significant increase in the GSH concentration, endowing the tumor cells with a stronger antioxidant capacity and greater potential to eliminate reactive oxygen species (ROS).34 GSH levels in cells can reach up to four times the normal level, and GSH effectively helps tumor cells evade programmed cell death triggered by ROS.35 GSH can maintain redox homeostasis, protect cells from oxidative damage, and reduce the toxicity induced by chemotherapy drugs, thereby safeguarding tumor cells from the impacts of most oxidative cancer treatment strategies.36
Overexpressed Enzymes
Enzyme overexpression is one of the key characteristics of the TME and may be caused by the upregulation of genes within tumor cells. These cells may harbor mutated proteins that increase the production of enzymes.37 In particular, oncogenes with the potential to induce cancer may become hyperactive, whereas tumor suppressor genes, which typically regulate cell division, may be inactivated. This results in an imbalance of cellular regulatory mechanisms. These genetic alterations are often the drivers of increased enzyme production.38 Abnormal expression and dysregulation of enzymes in the human body are the pathological bases of many diseases. Dysregulated enzymes are very valuable tumor diagnostic markers and treatment targets.
Overexpression of matrix metalloproteinases (MMPs) can promote tumor cell migration and invasion by promoting degradation of the ECM; this provides a physical pathway by which tumor cells can more easily penetrate the basement membrane and surrounding tissues, thereby facilitating the invasion and spread of the tumor.39 Cyclooxygenase-2 (COX-2) is an enzyme that is also overexpressed in many types of tumors, where it participates in the synthesis of prostaglandins. These prostaglandins can promote inflammatory responses and contribute to the growth and apoptosis resistance of tumors.40
ROS
In the TME, the level of ROS production is usually high, mainly due to high metabolic activity, oxidative stress, impaired mitochondrial function, and a lack of antioxidant capacity. Tumor cells often exhibit high aerobic glycolysis to meet their demands for rapid growth and proliferation, which results in increased ROS production.41 Moreover, tumor cells are affected by various stimuli, such as the hypoxic state in the TME and inflammatory responses. These factors can cause oxidative stress, prompting the cells to produce excess ROS to maintain survival.42 Furthermore, the mitochondrial function of tumor cells may be compromised, leading to leakage in the mitochondrial electron transport chain and increased instability of electrons, thereby promoting ROS generation.43 Tumor cells also lack a robust antioxidant defense system to effectively clear or neutralize ROS.
ECM
The ECM in the TME undergoes pathological remodeling, which is characterized by abnormal composition and structure, altered physical properties, and dysregulated biochemical functions.44 This pathological transformation stems from multifactorial interactions: cancer-associated fibroblasts (CAFs)45 and immune cells actively remodel the ECM by secreting proteases and profibrotic factors,46 whereas tumor cell genetic mutations drive abnormal ECM protein expression.47 Hypoxia and acidosis activate collagen-synthesizing enzymes and accelerate ECM degradation, creating a vicious cycle.48 Additionally, posttranslational modifications of ECM components alter their functionality, promoting immune evasion and therapy resistance.49 In summary, the aberrant evolution of the tumor-associated ECM arises from bidirectional crosstalk between tumor cells and the microenvironment, ultimately promoting the formation of a protumor fibrotic matrix that profoundly influences tumor progression and therapeutic outcomes.
TME-Responsive Nanoparticles for PTT
Nanoparticles can circulate within the body and eventually accumulate near tumor tissues, where they utilize the unique characteristics of the TME to achieve precise and efficient drug release. This targeted drug delivery enables accurate and effective attacks on tumor cells. In this process, PTT plays a crucial role. When nanoparticles release photosensitizers at the tumor site and are exposed to light, the local temperature rapidly increases. Elevated temperatures can directly kill tumor cells or increase the efficacy of drugs, thereby inhibiting tumor growth (Figure 2).
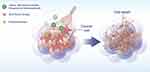 |
Figure 2 TME-responsive nanodelivery system for multimodal cancer therapy. |
Photosensitizers can precisely target the tumor microenvironment through nanomaterials to trigger localized heating for PTT and synergize with chemotherapy via a multimodal approach to increase efficacy, achieving TME-responsive drug release for improved therapeutic outcomes.
Hypoxia-Responsive Nanomaterials
Hypoxia-responsive nanomaterials change their structure under low-oxygen conditions by breaking specific chemical bonds such as disulfide50 or azo bonds,51 thus causing drug release from the nanoparticles (Figure 3). They can also alter optical properties,52 such as fluorescence, thereby serving as tools for monitoring the hypoxic state of tumors.53
 |
Figure 3 A graphical illustration of the mechanism of hypoxia-responsive nanoparticles. |
YB1, a particularly attractive genetically modified safe Salmonella Typhimurium strain, shows self-propelling properties to penetrate the hypoxic tumor core while minimizing damage to normal tissue. Chen et al developed a biohybrid/non-biohybrid system, YB1-INPs, by linking indocyanine green (ICG), a fluorescent dye widely used in medical imaging and diagnostics, loaded nanoparticles to YB1 via amide bonds for precise tumor targeting. This system showed specific targeting of hypoxic areas in solid tumors, excellent photothermal conversion, and efficient fluorescence imaging.54 Following photothermal treatment, YB1-INP bioaccumulation increased 14-fold due to the synergistic effects of disrupting tumor tissue and attracting nutrients.
Shi et al developed a hemoglobin (Hb)-based biomimetic synthesis method to create gadolinium (Gd) nanostructures coloaded with chlorin e6 (Ce6) and oxygen, aiming to alleviate tumor hypoxia and increase the efficacy of PTT under MRI guidance.55 The Gd@Hb-Ce6-PEG nanoparticles effectively maintained tumor oxygenation, delivered oxygen and Ce6 to tumor tissues, and exhibited excellent tumor-targeted accumulation for precise MRI-guided PTT. Photoacoustic imaging confirmed their ability to alleviate tumor hypoxia, whereas post-PTT diffusion-weighted imaging quantified their therapeutic efficacy. The biomimetic synthesis of ultrasmall paramagnetic Gd nanostructures using endogenous Hb represents a novel approach for MRI-guided, oxygen-supplying PTT.
Hypoxia-responsive nanotherapeutics offer promise in targeting tumor cells, but challenges in accurately identifying hypoxic regions within tumors can affect their efficacy. Due to tumor heterogeneity., oxygen levels can differ due to factors such as the tumor type and local conditions. This can result in uneven drug distribution across the tumor, causing overmedication in some areas while leaving others with insufficient drug exposure.56 Overdosing can harm nearby healthy tissue, and underdosing can reduce the efficacy and potentially lead to drug resistance in cancer cells. Advancements in imaging techniques, hypoxia indicators, and nanocarrier design are needed to improve the precision of hypoxia detection and drug delivery, increasing the overall success of nanotherapeutic treatments for cancer.57
pH-Responsive Nanomaterials
pH-responsive nanomaterials, which are composed of copolymers58 or monomers59 with functional groups, react to environmental pH variations by undergoing protonation or deprotonation. This molecular adjustment alters their hydrophilicity or hydrophobicity, triggering conformational changes that affect their stability and functionality.60 These properties are crucial for applications such as targeted drug delivery, where drugs are released in response to the acidic pH of tumors, and in biosensing technologies used to monitor pH-related changes61 (Figure 4).
 |
Figure 4 A graphical illustration of the mechanism of pH-responsive nanoparticles. |
Polymer nanoparticles were synthesized with a pH-sensitive polyethylene glycol (PEG) coating on their surface and were successfully loaded with the drug ICG. The results demonstrated that these nanoparticles possess excellent drug loading efficiency and pH-triggered drug release properties. Upon exposure to near-infrared (NIR) laser irradiation, the nanoparticles generated a significant photothermal effect, enhancing the thermotherapeutic effect on cancer cells.62
Additionally, Wang et al developed a smart polymer drug carrier that delivers docetaxel (DTX) and the photosensitizer IR825 to tumor cells through a pH-responsive charge reversal mechanism, achieving combined chemotherapy and PTT.63 The polymer nanoparticles are capable of charge reversal in a slightly acidic environment, which enhances their cellular uptake and drug release. Research results indicate that these polymer nanoparticles increase the efficacy of antitumor therapy in vitro and in vivo with good biocompatibility and safety.
Although the concept of using a decreased pH in the TME to facilitate the action of responsive nanomaterials is innovative, there are still many limitations to its current application. Within the human body, there are numerous other locations where a decrease in pH occurs, not just within the tumor area. Lysosomes, for example, can internalize nanomaterials and cause them to degrade within the body, which might lead to off-target effects and undermine the specificity of the therapy, potentially resulting in undesirable side effects.64
Therefore, the development of nanomaterials that can precisely identify and effectively respond to the unique microenvironment of tumors, as well as improve the stability and responsiveness of these materials, are key issues that need to be addressed in current research.
Enzyme-Responsive Nanomaterials
Nanomaterials with specificity for the enzymatic overexpression characteristic of pathologic tissues—predominantly cancerous anomalies as opposed to their normal physiological counterparts—are engineered to exploit this dysregulated expression. These constructs encompass moieties that are reactive to the aberrant enzymatic activity, incorporating peptides,65 synthetic polymers66 or alternative small-molecule substrates that undergo enzymatic cleavage or modification.67 These functionalities act as conjugative linkers tethering therapeutic modalities to the nanomaterial framework, thereby localizing agent deployment until enzymatic mediation occurs. Upon infiltration of the neoplastic milieu, pathological enzyme activity facilitates the severance of these linkers, instigating site-specific liberation of the chemotherapeutic payload (Figure 5). This ensures an augmented concentration gradient within the neoplasm while mitigating the propensity for systemic toxicity.68
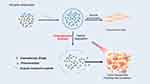 |
Figure 5 A graphical illustration of the mechanism of enzyme-responsive nanoparticles. |
MMPs
MMPs are a prime example of enzymes that are often overexpressed in tumors.69 MMPs play a pivotal role in the degradation of the ECM, which is a necessary step for cancer cells to invade neighboring tissues and form metastatic lesions in distant organs. By breaking down the ECM, MMPs facilitate the formation of new blood vessels (angiogenesis), thereby supplying the tumor with the necessary oxygen and nutrients for continued growth.70 Furthermore, MMPs contribute to the modulation of the immune response, as well as signal transduction processes that further increase tumor progression.71
MMP-2
An innovative nanocarrier system comprising gold nanorods (AuNRs)-doxorubicin (DOX)/mPEG10K-peptide/P(AAm-co-AN) (APP-DOX) has been presented for drug delivery. In this system, the AuNRs act as potent agents for PTT. The APP-DOX nanoparticles are optimally sized to enable targeted localization within tumor tissue following intravenous circulation. High concentrations of MMP-2 in the tumor milieu capture and sever the peptide chains conjugated to the surface of APP-DOX. Concurrently, shedding of the PEG chains results in an increase in the hydrophobic nature of the nanoparticles, leading to their aggregation into larger particles that are retained within the tumor for enhanced accumulation. Under irradiation with an 808 nm NIR laser, the APP-DOX system undergoes a progressive heating process. The generated heat can efficiently ablate tumor cells while triggering a phase transition in the upper critical-solution-temperature (UCST) polymer that facilitates the release of the encapsulated anticancer drug DOX, which then efficiently eradicates cancer cells.72
MMP-9
A self-assembled spherical nucleic acid liposome was ingeniously designed by coupling 1,2-diacyl-sn-glycero-3-phosphoethanolamine (DOPE) with the chemotherapeutic agent DOX and conjugating DOPE with MMP-9 and an immunostimulatory CpG oligodeoxynucleotide. This innovative spherical nucleic acid liposome (SNA) was specifically designed for targeted delivery, enabling the efficient cotransport of DOX and CpG directly to tumor sites. Upon reaching the TME, this system leverages the enzymatic activity of MMP-9, which is prevalent in many tumors, to trigger the release of both therapeutic agents.73
The strategic release of these drugs not only maximizes their therapeutic efficacy but also limits off-target effects, enhancing safety. Extensive research into the impact of liposomal SNAs on the immune system has revealed significant immunomodulatory benefits. Notably, it amplifies the activation of dendritic cells (DCs), which are critical players in initiating and regulating the immune response. Additionally, it promotes the proliferation of CD8+ and CD4+ T cells both within the tumor and the spleen, which are essential for mounting an effective antitumor response. The culmination of these effects is a pronounced inhibition of tumor growth and a notable extension of survival times in experimental models, highlighting the potential of this approach in advancing cancer treatment paradigms.
HAase
A hyaluronidase-responsive nanoplatform based on oxygen-deficient titanium dioxide (TiO2-x) is used for mild NIR phototherapy. Within the TME, overexpressed hyaluronidase (HAase) can easily lead to dysregulation of hyaluronic acid (HA) levels and the release of Cas9/sgRNA targeting stress alleviation regulators, namely, nuclear factor E2-related factor 2 and heat shock protein 90α, thus reducing the stress tolerance of tumor cells. Under subsequent NIR light irradiation, TiO2-x displays enhanced anticancer effects both in vitro and in vivo.74
Importantly, enzymes present at nonpathological sites can also trigger the release of enzyme-responsive nanomedicines. Many enzymes abnormally present in tumor sites are also widely found in normal tissues, and enzymes within the same family often have similar substrates, which can cause the release of the drug at nontarget sites. To ensure that enzyme-responsive drugs act only at the pathological site, it is necessary to identify more ideal tumor-specific enzymes. Moreover, the design of enzyme-responsive nanomedicines should focus on the kinetics of enzyme-catalyzed reactions. The time of the enzyme reaction and the extent of the reaction affect both the rate of drug release and the therapeutic effect and can be optimized on the basis of enzyme catalytic kinetics to adjust the dosage used for drug delivery.
Redox-Responsive Nanomaterials
Redox potential is one of the primary physiological differences between tumor and normal tissues and is advantageous for designing redox-reactive nanostructures for the delivery of chemotherapy drugs.75 The realization of redox-triggered drug release is primarily based on the presence of redox-sensitive components within the carriers, such as disulfide and selenide bonds. These compounds can maintain stability in the mildly oxidized extracellular space, where there is a relatively low concentration of GSH, yet they become disrupted by the significantly higher concentration of GSH present within cells. Consequently, carriers can deliver their payload intact to target cells and then rapidly disintegrate following endocytosis, thereby triggering the swift and complete release of the loaded drug within the target cells76 (Figure 6). Redox-reactive nanocarriers achieve nearly zero premature drug release, thus avoiding off-target toxicity associated with free drugs. Thus, these nanocarriers have considerable potential in the development of more effective drug delivery systems with better pharmacokinetics and pharmacodynamics.77
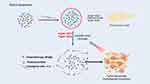 |
Figure 6 A graphical illustration of the mechanism of redox-responsive nanoparticles. |
ROS-Responsive Nanomaterials
Nanomaterials are designed to contain chemical bonds that can be oxidized or reduced by ROS, such as thioether bonds, aryl thioether bonds, disulfide bonds, and silex bonds. When these nanoparticles are exposed to high concentrations of ROS, they undergo chemical changes leading to the disaggregation or disintegration of the nanomaterial.78 Active drug molecules can be encapsulated within ROS-responsive nanomaterials.79 Once these nanocarriers enter cells or tissues with high levels of ROS, they respond to the ROS signals by undergoing structural changes, which in turn lead to the release of the encapsulated drugs.80
Zhang et al developed a cluster-bomb-like nanoplatform (CPIM). For the first time, this platform combines variable size and cellular internalization strategies to enhance both passive and active transport. For passive diffusion, to address the high ROS concentration in the TME, the cluster bomb CPIM releases drug-loaded “bomblets” (PAMAM loaded with IR780/1-methyl-D-tryptophan (1-MT), <10 nm) to promote diffusion within the tumor. Additionally, IR780 generates ROS under NIR irradiation and enhances this responsiveness; thus, NIR-triggered autodestruction behavior is induced, which endows CPIM with spatiotemporal regulatory function. In terms of anticancer performance, CPIM enhances both the PDT/PTT activity of IR780 and the indoleamine 2,3-dioxygenase (IDO) pathway inhibition effect of 1-MT, thereby enhancing tumor penetration and achieving desirable phototherapy efficiency.81
GSH-Responsive Nanomaterials
GSH-responsive nanomaterials are generated by incorporating GSH-sensitive chemical bonds into the structure of the nanomaterials. These chemical bonds or crosslinkers can be selectively reduced by high concentrations of GSH within cells, leading to structural changes in the nanomaterials. Inside targeted cells, GSH penetrates the nanomaterials and reacts with the GSH-sensitive bonds, which results in structural changes in the nanomaterials and the subsequent release of encapsulated drugs. This release mechanism enables efficient drug delivery targeting tumor cells.82
Yang et al discussed a GSH-responsive PTT nanoparticle. The study revealed that the nanoparticle undergoes fluorescence signal restoration in the presence of GSH because GSH can cleave the disulfide bonds within it, leading to the collapse of its hydrophobic core and thus the release of the ultraviolet fluorescence dye DiR and the anticancer drug paclitaxel (PTX). Additionally, the study revealed that the fluorescence signal restoration of DiR is related to the release and therapeutic action of PTX. Therefore, by monitoring the fluorescence signal restoration of DiR, one can track the release and therapeutic efficacy of PTX. These GSH-responsive PTT nanoparticles hold potential for future clinical applications. Furthermore, researchers discovered that the concentration of GSH affects the fluorescence signal restoration of this kind of nanoparticle and its cytotoxicity. Higher concentrations of GSH result in stronger fluorescence signal restoration and greater toxicity to cancer cells. Conversely, lower concentrations of GSH lead to weaker fluorescence signal restoration and reduced toxicity to cancer cells.63,83
Zhang et al utilized monodispersed gold nanoparticles (GNPs), which were conjugated to hepatocellular carcinoma cell-specific targeting oligonucleotides (TLS11a) via Au‒S bonds. The targeting oligonucleotide featured Ce6 labeling at the 5′ end, which was linked through (GA)10 repetitive bases to Cu(II) to load AQ4N at the 3′ end. In cancer cells, the fluorescence and ROS-generating capabilities of Ce6 were specifically restored by strategic cleavage of the Au–S bond, which was facilitated by elevated levels of intracellular GSH.84
Furthermore, the interaction between cellular GSH levels and the efficacy of cancer therapies highlights a significant challenge in oncological treatments. When GSH in cancer cells is depleted, these cells activate their internal GSH synthesis systems. This response serves as a defense mechanism, potentially diminishing the long-term effectiveness of treatments that depend on GSH depletion to impair cancer cell viability.85 Therefore, therapeutic approaches that integrate multiple strategies, not only targeting GSH depletion but also inhibiting the compensatory synthesis of GSH, may be a more robust solution. Such multifaceted therapies may include oxidative stress-inducing agents or inhibitors of key enzymes in the GSH synthesis pathway, with the goal of disrupting the cellular redox balance thoroughly and preventing cancer cells from adapting to and resisting treatment.
Multimodal Strategies
Due to the complexity of the TME, therapeutic approaches predicated solely on reactive measures are limited by the specificity of pharmaceuticals to singular pathological conditions.86 However, an integrated pharmacological approach in which multiple agents are modulated in a synergistic manner can induce concomitant biochemical responses, thereby facilitating holistic and efficacious modulation of the TME. This approach effectively mitigates the inherent qualitative heterogeneity and dynamic attributes of the microenvironment. Empirical evidence suggests that the use of multiple modalities that are responsive to different stimuli not only increase precision in neoplastic targeting but also substantially attenuate collateral damage to healthy human tissues.87 Moreover, the orchestrated release of therapeutic agents from nanoscale delivery systems, triggered by distinct stimuli at respective loci, surpasses the limitations of traditional pharmacotherapy—characterized by suboptimal solubility, pronounced toxicity and compromised stability—culminating in superior drug bioavailability and administration fidelity.88
Zheng et al developed a highly biocompatible nanocarrier called BMP NTs, which achieve precise antitumor therapy via a two-stage delivery mechanism. First, BMP NTs utilize the enhanced permeation and retention (EPR) effect and selectively shed their surface cleavable layer under the action of overexpressed MMP-2 in the TME, exposing hydrophobic sections and thereby achieving significantly increased tumor accumulation. Once inside cancer cells, which have an excess of GSH and H2O2, BMP NTs undergo a second-stage “unloading process”, releasing Mn2+ ions and ultrasmall Bi2S3@BSA nanoparticles. The resulting Mn2+ can act as a Fenton-like catalyst, continuously catalyzing endogenous H2O2 to produce highly toxic hydroxyl radicals (•OH) for chemodynamic therapy (CDT). The depletion of GSH, in turn, improved the Mn2±H2O2 reaction, further increasing the efficiency of CDT. Moreover, the ultrasmall Bi2S3@BSA endows the BMP NTs with excellent photothermal conversion capability, generating local high temperatures to expedite the internal Fenton reactions within the tumor, thereby achieving effective tumor treatment under the synergistic effect of CDT/PTT. Furthermore, BMP NTs can also perform in situ spontaneous MRI and photoacoustic imaging (PA) for the guidance of precise cancer therapy.89
Combining different stimulus-responsive strategies can overcome the drawbacks of single stimulus responses, providing mutual assistance and complementarity. Such integrated, more advanced, and intelligent strategies are the direction of development in tumor therapy and hold immense potential. However, the preparation and therapeutic mechanisms of multi-stimulus-responsive nanodrugs are quite complex, and the absence of a single stimulus might lead to the failure of drug therapy. It is necessary to continuously observe the effects over an extended period under regular medication conditions in live animal models and to assess the efficacy and toxicity when a single stimulus is lacking.90
TME-Responsive Nanoparticles for Drug Delivery and Imaging
A novel carrier-free nanoconjugate that functions as a direct-acting antiviral (DAA) agent was created and featured near-infrared activity, synergistic antivascular properties, and pH-responsive photodynamic and photothermal capabilities to increase the effectiveness of cancer therapy.91 DAA nanoparticles, developed via self-assembly, are biocompatible and have enhanced photodynamic and photothermal effects in acidic tumor environments due to diethylaminophenyl protonation. The antivascular agent 5,6-dimethylxanthenone-4-acetic acid is released by ester bond cleavage in the acidic vesicles of endothelial cells, where it targets vascular endothelial growth factor and disrupts the tumor vasculature. This precise delivery to vascular endothelial cells and tumor lysosomes enables effective tumor elimination in vitro and in vivo, suggesting a promising cancer therapy that combines antivascular effects with dual photodynamic and photothermal treatments.
Liu et al developed a new type of targeted polymeric micelle that can sense hypoxia in tumors, thereby activating cytotoxic and immunogenic responses and effectively eradicating advanced breast cancer.92 These low-activation polymer micelles can also effectively deliver anticancer drugs and photosensitizers to cancer cells, inducing synergistic cytotoxicity and immunogenic cell death through chemotherapy and PTT. In addition, the micelles can activate a systemic antitumor immune response, effectively suppressing tumor recurrence and significantly inhibiting distant metastases.
TME-responsive nanoparticles enable targeted drug delivery and imaging by responding to tumor microenvironment cues such as pH and enzymes. They minimize off-target effects, improve treatment efficacy, and allow real-time monitoring via fluorescence imaging. This approach improves the accuracy of diagnosis, personalized therapy, and cancer treatment.
Conclusion
Responsive nanomaterials have emerged as pivotal carriers in PTT, offering transformative potential in tumor treatment. Their unique advantages stem from their ability to achieve precise targeting93 and synergistic therapeutic effects.94 By harnessing TME-specific response mechanisms, these materials enable intelligent, on-demand drug release while significantly increasing photothermal conversion efficiency and prolonging retention at the lesion site.95 Furthermore, the photothermal effect can indirectly modulate the TME by suppressing stromal components, thereby improving nanomaterial penetration and therapeutic efficacy.96
The future of the use of responsive nanomaterials in PTT lies in the following key directions. First, NIR-II-responsive materials need to be developed to increase tissue penetration and therapeutic depth.97 Second, multimodal synergistic platforms that integrate immune modulation and other therapeutic mechanisms are needed to amplify efficacy while minimizing toxicity.98 Finally, new personalized nanomedicine strategies, in which material response thresholds are dynamically tailored to patient-specific microenvironmental features,99 are needed.
Despite these promising attributes, there are several critical challenges in the clinical translation of responsive nanomaterials. Due to tumor heterogeneity, a delicate balance between universality and specificity needs to be achieved in material design, yet current research models often fall short of capturing the complexity of real-world pathologies.100 Additionally, the photothermal ablation of deep-seated or large tumors remains constrained by limited tissue penetration depth and uneven thermal diffusion, underscoring the urgent need to expand the spectral response range of conventional photothermal agents.101 Biosafety concerns further complicate their clinical application, as the long-term in vivo metabolic behavior and stability of these materials require comprehensive evaluation.
By addressing these challenges and embracing these innovations, responsive nanomedicines have the potential to bridge the gap between groundbreaking research and transformative clinical applications, ultimately redefining the landscape of tumor therapy.
Author Contributions
Heming Sun: Design, conceptualization, writing, and draft; Yuebo Li and Ming Xue: data mining, revision, supervision, and figure preparation; Dingqing Feng: design, review and editing. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National High-Level Hospital Clinical Research Funding (2024-NHLHCRF-PYI-06, 2024-NHLHCRF-YXHZ-MS-06) and the National Natural Science Foundation of China (82273052, 82374227, and 82472653).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi:10.1016/s0140-6736(12)61728-0
3. Li Y, Zhou P, Wang Z, et al. Sea anemone-like nanomachine based on DNA strand displacement composed of three boolean logic gates: diversified input for intracellular multitarget detection. Anal Chem. 2024;96(10):4120–4128. doi:10.1021/acs.analchem.3c05059
4. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1). doi:10.1186/s12951-018-0392-8
5. Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J hematol oncol. 2019;12(1). doi:10.1186/s13045-019-0833-3
6. Kamada T, Sato N, Kakubari N, et al. Noninvasive assessment of microcirculation of living organs and tissues using laser. Lasers Surg Med. 1983;2(3):275–280. doi:10.1002/lsm.1900020311
7. Cheng K, Guo Q, Shen Z, et al. Bibliometric analysis of global research on cancer photodynamic therapy: focus on nano-related research. Front Pharmacol. 2022;13. doi:10.3389/fphar.2022.927219
8. Reiss P, Protière M, Li L. Core/Shell semiconductor nanocrystals. Small. 2009;5(2):154–168. doi:10.1002/smll.200800841
9. Dineshkumar B, Krishnakumar K, Bhatt AR, et al. Single-walled and multi-walled carbon nanotubes based drug delivery system: cancer therapy: a review. Indian J Cancer. 2015;52(3):262. doi:10.4103/0019-509x.176720
10. Huang X, Qi X, Boey F, Zhang H. Graphene-based composites. Chem Soc Rev. 2012;41(2):666–686. doi:10.1039/c1cs15078b
11. Vangijzegem T, Stanicki D, Laurent S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv. 2018;16(1):69–78. doi:10.1080/17425247.2019.1554647
12. Cai Y, Si W, Huang W, Chen P, Shao J, Dong X. Organic dye based nanoparticles for cancer phototheranostics. Small. 2018;14(25). doi:10.1002/smll.201704247
13. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi:10.1016/j.pharmthera.2020.107753
14. Xu P, Liang F. Nanomaterial-Based Tumor Photothermal Immunotherapy Int J Nanomed. 2020;Volume 15:9159–9180. doi:10.2147/ijn.S249252
15. Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discover. 2021;11(4):933–959. doi:10.1158/2159-8290.Cd-20-1808
16. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature Med. 2013;19(11):1423–1437. doi:10.1038/nm.3394
17. Jing X, Yang F, Shao C, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1). doi:10.1186/s12943-019-1089-9
18. Wang JX, Choi SYC, Niu X, et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. 2020;21(21):8363. doi:10.3390/ijms21218363
19. Peng S, Xiao F, Chen M, Gao H. Tumor‐microenvironment‐responsive nanomedicine for enhanced cancer immunotherapy. Adv Sci. 2021;9(1). doi:10.1002/advs.202103836
20. Wang L, Huo M, Chen Y, Shi J. Tumor microenvironment‐enabled nanotherapy. Adv Healthcare Mater. 2017;7(8). doi:10.1002/adhm.201701156
21. Vultaggio-Poma V, Sarti AC, Di Virgilio F. Extracellular ATP: a feasible target for cancer therapy. Cells. 2020;9(11):2496. doi:10.3390/cells9112496
22. Wicks EE, Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Investig. 2022;132(11). doi:10.1172/jci159839
23. Metheny-Barlow LJ, Li LY. The enigmatic role of angiopoietin-1 in tumor angiogenesis. Cell Res. 2003;13(5):309–317. doi:10.1038/sj.cr.7290176
24. Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia‐inducible factor (HIF): implications for cellular physiology. J Physiol. 2020;599(1):23–37. doi:10.1113/jp280572
25. Korbecki J, Simińska D, Gąssowska-Dobrowolska M, et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci. 2021;22(19). doi:10.3390/ijms221910701
26. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi:10.1085/jgp.8.6.519
27. Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharmaceut. 2011;8(6):2032–2038. doi:10.1021/mp200292c
28. Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11. doi:10.1016/j.drup.2018.03.001
29. Xia L, Oyang L, Lin J, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20(1):28. doi:10.1186/s12943-021-01316-8
30. Kobliakov VA. Role of proton pumps in tumorigenesis. Biochem Biokhimiia. 2017;82(4):401–412. doi:10.1134/s0006297917040010
31. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
32. DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21(12):785–797. doi:10.1038/s41577-021-00541-y
33. Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30(1–2):1–12. doi:10.1016/j.mam.2008.08.006
34. Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217(7):2291–2298. doi:10.1083/jcb.201804161
35. Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180–2198. doi:10.1016/j.biomaterials.2009.01.026
36. Kennedy L, Sandhu JK, Harper M-E, Cuperlovic-Culf M. Role of glutathione in cancer: from mechanisms to therapies. Biomolecules. 2020;10(10):1429. doi:10.3390/biom10101429
37. Fujita K, Kamiya M, Urano Y. Rapid and sensitive detection of cancer cells with activatable fluorescent probes for enzyme activity. Methods Mol Biol. 2021;2274:193–206. doi:10.1007/978-1-0716-1258-3_17
38. de la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Delivery Rev. 2012;64(11):967–978. doi:10.1016/j.addr.2012.01.002
39. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73. doi:10.1016/bs.pmbts.2017.02.005
40. Misra S, Sharma K. COX-2 signaling and cancer: new players in old arena. Curr Drug Targets. 2014;15(3):347–359. doi:10.2174/1389450115666140127102915
41. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi:10.1016/j.semcdb.2017.05.023
42. Wang L, Kuang Z, Zhang D, Gao Y, Ying M, Wang T. Reactive oxygen species in immune cells: a new antitumor target. Biomed Pharmacother. 2021;133:110978. doi:10.1016/j.biopha.2020.110978
43. Tao X, Go V, Xiao GG. Progress on the role of reactive oxygen species-mediated tumor microenvironment in pancreatic cancer. Sheng Li Xue Bao. 2021;73(2):197–207.
44. Huang J, Zhang L, Wan D, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther. 2021;6(1):153. doi:10.1038/s41392-021-00544-0
45. Jurj A, Ionescu C, Berindan-Neagoe I, Braicu C. The extracellular matrix alteration, implication in modulation of drug resistance mechanism: friends or foes? J Exp Clin Cancer Res. 2022;41(1):276. doi:10.1186/s13046-022-02484-1
46. Yu S, Wang S, Wang X, Xu X. The axis of tumor-associated macrophages, extracellular matrix proteins, and cancer-associated fibroblasts in oncogenesis. Can Cell Inter. 2024;24(1):335. doi:10.1186/s12935-024-03518-8
47. Stankevicius V, Vasauskas G, Bulotiene D, et al. Gene and miRNA expression signature of Lewis lung carcinoma LLC1 cells in extracellular matrix enriched microenvironment. BMC Cancer. 2016;16(1):789. doi:10.1186/s12885-016-2825-9
48. Rainu SK, Singh N. Dual-sensitive fluorescent nanoprobes for simultaneously monitoring in situ changes in pH and matrix metalloproteinase expression in stiffness-tunable three-dimensional in vitro scaffolds. ACS Appl Mater Interfaces. 2024;16(10):12175–12187. doi:10.1021/acsami.3c16334
49. Darvishi B, Eisavand MR, Majidzadeh AK, Farahmand L. Matrix stiffening and acquired resistance to chemotherapy: concepts and clinical significance. Br J Cancer. 2022;126(9):1253–1263. doi:10.1038/s41416-021-01680-8
50. Ashrafi K, Heaysman CL, Phillips GJ, Lloyd AW, Lewis AL. Towards hypoxia-responsive drug-eluting embolization beads. Int J Pharm. 2017;524(1–2):226–237. doi:10.1016/j.ijpharm.2017.03.084
51. Zhou T, Xiong H, Yao SY, et al. Hypoxia and matrix metalloproteinase 13-responsive hydrogel microspheres alleviate osteoarthritis progression in vivo. Small. 2024;20(19):e2308599. doi:10.1002/smll.202308599
52. Hu B, Song N, Cao Y, et al. Noncanonical amino acids for hypoxia-responsive peptide self-assembly and fluorescence. J Am Chem Soc. 2021;143(34):13854–13864. doi:10.1021/jacs.1c06435
53. Karan S, Cho MY, Lee H, et al. Hypoxia-responsive luminescent CEST MRI agent for in vitro and in vivo tumor detection and imaging. J Med Chem. 2022;65(10):7106–7117. doi:10.1021/acs.jmedchem.1c01745
54. Chen F, Zang Z, Chen Z, et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials. 2019;214:119226. doi:10.1016/j.biomaterials.2019.119226
55. Shi X, Yang W, Ma Q, et al. Hemoglobin-mediated biomimetic synthesis of paramagnetic O(2)-evolving theranostic nanoprobes for MR imaging-guided enhanced photodynamic therapy of tumor. Theranostics. 2020;10(25):11607–11621. doi:10.7150/thno.46228
56. Li Y, Jeon J, Park JH. Hypoxia-responsive nanoparticles for tumor-targeted drug delivery. Cancer Lett. 2020;490:31–43. doi:10.1016/j.canlet.2020.05.032
57. Kumari R, Sunil D, Ningthoujam RS. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review. J Control Release. 2020;319:135–156. doi:10.1016/j.jconrel.2019.12.041
58. Du J, Tang Y, Lewis AL, Armes SP. pH-sensitive vesicles based on a biocompatible zwitterionic diblock copolymer. J Am Chem Soc. 2005;127(51):17982–17983. doi:10.1021/ja056514l
59. El-Sayed ME, Hoffman AS, Stayton PS. Rational design of composition and activity correlations for pH-sensitive and glutathione-reactive polymer therapeutics. J Control Release. 2005;101(1–3):47–58. doi:10.1016/j.jconrel.2004.08.032
60. Zhao S, Tan S, Guo Y, et al. pH-sensitive docetaxel-loaded D-α-tocopheryl polyethylene glycol succinate-poly(β-amino ester) copolymer nanoparticles for overcoming multidrug resistance. Biomacromolecules. 2013;14(8):2636–2646. doi:10.1021/bm4005113
61. Chen J, Dong X, Feng T, et al. Charge-conversional zwitterionic copolymer as pH-sensitive shielding system for effective tumor treatment. Acta Biomater. 2015;26:45–53. doi:10.1016/j.actbio.2015.08.018
62. Ting CW, Chou YH, Huang SY, Chiang WH. Indocyanine green-carrying polymeric nanoparticles with acid-triggered detachable PEG coating and drug release for boosting cancer photothermal therapy. Colloids Surf B. 2021;208:112048. doi:10.1016/j.colsurfb.2021.112048
63. Wang X, Gu Y, Li Q, et al. Synergistic chemo-photothermal cancer therapy of pH-responsive polymeric nanoparticles loaded IR825 and DTX with charge-reversal property. Colloids Surf B. 2022;209(Pt 2):112164. doi:10.1016/j.colsurfb.2021.112164
64. Ghaznavi H, Shirvaliloo M, Zarebkohan A, et al. An updated review on implications of autophagy and apoptosis in tumorigenesis: possible alterations in autophagy through engineered nanomaterials and their importance in cancer therapy. Mol Pharmacol. 2021;100(2):119–143. doi:10.1124/molpharm.121.000234
65. Gao J, Zhan J, Yang Z. Enzyme-Instructed Self-Assembly (EISA) and hydrogelation of peptides. Adv Mater. 2020;32(3):e1805798. doi:10.1002/adma.201805798
66. Tse Sum Bui B, Haupt K. Molecularly imprinted polymer hydrogel nanoparticles: synthetic antibodies for cancer diagnosis and therapy. Chembiochem. 2022;23(8):e202100598. doi:10.1002/cbic.202100598
67. Barve A, Jain A, Liu H, Zhao Z, Cheng K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020;113:501–511. doi:10.1016/j.actbio.2020.06.019
68. Vellard M. The enzyme as drug: application of enzymes as pharmaceuticals. Curr Opin Biotechnol. 2003;14(4):444–450. doi:10.1016/s0958-1669(03)00092-2
69. Kalafatovic D, Nobis M, Son J, Anderson KI, Ulijn RV. MMP-9 triggered self-assembly of doxorubicin nanofiber depots halts tumor growth. Biomaterials. 2016;98:192–202. doi:10.1016/j.biomaterials.2016.04.039
70. Quintero-Fabián S, Arreola R, Becerril-Villanueva E, et al. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol. 2019;9:1370. doi:10.3389/fonc.2019.01370
71. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi:10.1016/j.cell.2010.03.015
72. Lin Q, Jia M, Fu Y, et al. Upper-critical-solution-temperature polymer modified gold nanorods for laser controlled drug release and enhanced anti-tumour therapy. Front Pharmacol. 2021;12:738630. doi:10.3389/fphar.2021.738630
73. Deng B, Ma B, Ma Y, et al. Doxorubicin and CpG loaded liposomal spherical nucleic acid for enhanced Cancer treatment. J Nanobiotechnology. 2022;20(1):140. doi:10.1186/s12951-022-01353-5
74. Li Z, Pan Y, Du S, et al. Tumor-microenvironment activated duplex genome-editing nanoprodrug for sensitized near-infrared titania phototherapy. Acta pharmaceutica Sinica B. 2022;12(11):4224–4234. doi:10.1016/j.apsb.2022.06.016
75. Mahdaviani P, Bahadorikhalili S, Navaei-Nigjeh M, et al. Peptide functionalized poly ethylene glycol-poly caprolactone nanomicelles for specific cabazitaxel delivery to metastatic breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2017;80:301–312. doi:10.1016/j.msec.2017.05.126
76. Yang X, Cai X, Yu A, Xi Y, Zhai G. Redox-sensitive self-assembled nanoparticles based on alpha-tocopherol succinate-modified heparin for intracellular delivery of paclitaxel. J Colloid Interface Sci. 2017;496:311–326. doi:10.1016/j.jcis.2017.02.033
77. Sufi SA, Pajaniradje S, Mukherjee V, Rajagopalan R. Redox nano-architectures: perspectives and implications in diagnosis and treatment of human diseases. Antioxid Redox Signaling. 2019;30(5):762–785. doi:10.1089/ars.2017.7412
78. Yue W, Chen L, Yu L, et al. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat Commun. 2019;10(1):2025. doi:10.1038/s41467-019-09760-3
79. Zhou P, Wang Z, Chen H, et al. Oxygen vacancy-enhanced catalytic activity of hyaluronic acid covered-biomineralization nanozyme for reactive oxygen species-augmented antitumor therapy. Int J Biol Macromol. 2023;236:124003. doi:10.1016/j.ijbiomac.2023.124003
80. Ranji-Burachaloo H, Gurr PA, Dunstan DE, Qiao GG. Cancer treatment through nanoparticle-facilitated fenton reaction. ACS nano. 2018;12(12):11819–11837. doi:10.1021/acsnano.8b07635
81. Zhang Y, Du X, Liu S, et al. NIR-triggerable ROS-responsive cluster-bomb-like nanoplatform for enhanced tumor penetration, phototherapy efficiency and antitumor immunity. Biomaterials. 2021;278:121135. doi:10.1016/j.biomaterials.2021.121135
82. Chen M, Liu D, Liu F, Wu Y, Peng X, Song F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J Control Release. 2021;332:269–284. doi:10.1016/j.jconrel.2021.02.030
83. Li Y, Wu Y, Chen J, et al. A simple glutathione-responsive turn-on theranostic nanoparticle for dual-modal imaging and chemo-photothermal combination therapy. Nano Lett. 2019;19(8):5806–5817. doi:10.1021/acs.nanolett.9b02769
84. Zhang D, Zheng A, Li J, et al. Smart Cu(II)-aptamer complexes based gold nanoplatform for tumor micro-environment triggered programmable intracellular prodrug release, photodynamic treatment and aggregation induced photothermal therapy of hepatocellular carcinoma. Theranostics. 2017;7(1):164–179. doi:10.7150/thno.17099
85. Niu B, Liao K, Zhou Y, et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110. doi:10.1016/j.biomaterials.2021.121110
86. Chen Z, Wen D, Gu Z. Cargo-encapsulated cells for drug delivery. Sci China Life Sci. 2020;63(4):599–601. doi:10.1007/s11427-020-1653-y
87. Tang X, Sheng Q, Xu C, et al. pH/ATP cascade-responsive nano-courier with efficient tumor targeting and siRNA unloading for photothermal-immunotherapy. Nano Today. 2021;37:101083. doi:10.1016/j.nantod.2021.101083
88. Li M, Tang Z, Lv S, et al. Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials. 2014;35(12):3851–3864. doi:10.1016/j.biomaterials.2014.01.018
89. Zheng Z, Chen Q, Rong S, et al. Two-stage activated nano-truck enhanced specific aggregation and deep delivery for synergistic tumor ablation. Nanoscale. 2020;12(29):15845–15856. doi:10.1039/d0nr03661g
90. Zhang J, Lin Y, Lin Z, et al. Stimuli-responsive nanoparticles for controlled drug delivery in synergistic cancer immunotherapy. Adv Sci. 2022;9(5):e2103444. doi:10.1002/advs.202103444
91. Liang P, Huang X, Wang Y, et al. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS nano. 2018;12(11):11446–11457. doi:10.1021/acsnano.8b06478
92. Liu J, Ai X, Cabral H, Liu J, Huang Y, Mi P. Tumor hypoxia-activated combinatorial nanomedicine triggers systemic antitumor immunity to effectively eradicate advanced breast cancer. Biomaterials. 2021;273:120847. doi:10.1016/j.biomaterials.2021.120847
93. Du T, Shi Z, Mou X, Zhu Y. Axial assembly of AuNR for tumor theranostics via Zn(2+)-GSH chelation induced degradation of AuNR@ZIF-8 heterostructures. Colloids Surf B. 2024;234:113706. doi:10.1016/j.colsurfb.2023.113706
94. Feng Y, Li Z, Song L, et al. A ZIF-8-based dual-modal smart responsive nanoplatform for overcoming radiotherapy resistance in advanced tumors. Nanoscale. 2025;17(19):12134–12148. doi:10.1039/d5nr01093d
95. Pu XQ, Ju XJ, Zhang L, et al. Novel multifunctional stimuli-responsive nanoparticles for synergetic chemo-photothermal therapy of tumors. ACS Appl Mater Interfaces. 2021;13(24):28802–28817. doi:10.1021/acsami.1c05330
96. Zheng D, Wan C, Yang H, et al. Her2-targeted multifunctional nano-theranostic platform mediates tumor microenvironment remodeling and immune activation for breast cancer treatment. Int J Nanomed. 2020;15:10007–10028. doi:10.2147/ijn.S271213
97. Yin C, Li X, Wen G, et al. Organic semiconducting polymer amphiphile for near-infrared-II light-triggered phototheranostics. Biomaterials. 2020;232:119684. doi:10.1016/j.biomaterials.2019.119684
98. Kv R, Liu TI, Lu IL, et al. Tumor microenvironment-responsive and oxygen self-sufficient oil droplet nanoparticles for enhanced photothermal/photodynamic combination therapy against hypoxic tumors. J Control Release. 2020;328:87–99. doi:10.1016/j.jconrel.2020.08.038
99. Chen X, Cao Q, Liang Z, Huang L, Wang J, Hu Y. Hollow magnetic nanocarrier-based microrobot swarms for nir-responsive targeted drug delivery and synergistic therapy. ACS Appl Mater Interfaces. 2024;16(44):60874–60883. doi:10.1021/acsami.4c14062
100. Yu S, Xia G, Yang N, et al. Noble metal nanoparticle-based photothermal therapy: development and application in effective cancer therapy. Int J Mol Sci. 2024;25(11). doi:10.3390/ijms25115632
101. Zhang H, Yang M, Wu Q, Xue J, Liu H. Engineering two-dimensional nanomaterials for photothermal therapy. Angew Chem. 2025;64(12):e202424768. doi:10.1002/anie.202424768
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

