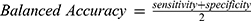Back to Journals » International Journal of General Medicine » Volume 18
Ultrasound and Clinicopathological Characteristics of Papillary Thyroid Carcinoma Predict the Coexistence of TERT Promoter and BRAFV600E Mutations
Authors Yu M, Zhang C, Wang Z, Lv S, Sun Y, Zhao W, Li L, Kong Q, Liu K, Wang S
Received 10 January 2025
Accepted for publication 15 March 2025
Published 24 March 2025 Volume 2025:18 Pages 1643—1656
DOI https://doi.org/10.2147/IJGM.S513319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ching-Hsien Chen
Mei Yu,1,* ChengZheng Zhang,1,* ZhengTong Wang,2,* ShaoWen Lv,3 YiFang Sun,1 WenWen Zhao,4 Lu Li,5 QingFeng Kong,3 Kun Liu,1 Shuanglong Wang3
1Department of Ultrasound Medicine, Affiliated Hospital of Jining Medical University, Jining, Shandong Province, 272000, People’s Republic of China; 2Department of Radiology, Affiliated Hospital of Jining Medical University, Jining, Shandong Province, 272000, People’s Republic of China; 3Department of Ultrasound Medicine, Jining NO.1 People’s Hospital, Jining, Shandong Province, 272000, People’s Republic of China; 4Department of Cardiology, Affiliated Hospital of Jining Medical University, Jining, Shandong Province, 272000, People’s Republic of China; 5Department of Pathology, Affiliated Hospital of Jining Medical University, Jining, Shandong Province, 272000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuanglong Wang, Department of Ultrasound Medicine, Jining NO.1 People’s Hospital, No. 6 Jiankang Road, Jining, Shandong Province, 272000, People’s Republic of China, Tel +86 15153740602, Email [email protected] Kun Liu, Department of Ultrasound Medicine, Affiliated Hospital of Jining Medical University, No. 89 Guhuai Road, Jining, Shandong Province, 272000, People’s Republic of China, Tel +86 15264766616, Email [email protected]
Purpose: The coexistence of TERT promoter and BRAFV600E mutations is strongly linked to aggressive behavior and poor prognosis in papillary thyroid carcinoma (PTC). This study aimed to develop preoperative and postoperative predictive models for coexisting mutations based on ultrasound and clinicopathological characteristics to stratify prognostic risks and guide clinical decision-making.
Methods: Retrospective analysis of the ultrasound and clinicopathological characteristics of 120 patients with a surgical pathology of PTC with TERT promoter and BRAFV600E gene testing results in the Affiliated Hospital of Jining Medical University from December 2020 to December 2023. Univariate and multivariate logistic regression identified independent predictors, and nomograms were constructed. Model performance was evaluated using ROC curves, calibration curves, and decision curves, with internal validation via Bootstrap resampling.
Results: Age (OR: 1.24; 95% CI 1.12– 1.37, P< 0.001), tumor size (OR: 5.51; 95% CI 2.26– 13.46, P< 0.001), lateral lymph node metastasis (OR: 7.36; 95% CI 1.48– 36.48, P=0.015), and irregular/ill-defined margins (OR: 6.06; 95% CI 1.19– 30.75, P=0.030) were independent predictors of coexisting mutations. The cutoff values for age and tumor size were 44.5 years and 1.55 cm, respectively. Two models incorporating the four independent predictors were established to predict coexisting mutations in the preoperative and postoperative periods, achieving AUCs of 0.95 and 0.96, respectively, with both models demonstrating good calibration ability and clinical practicability through calibration and decision curve analyses.
Conclusion: The predictive models enable clinicians to identify high-risk patients with coexisting mutations both preoperatively and postoperatively, supporting the development of individualized treatment strategies and potentially improving patient outcomes. However, the study is limited by its single-center design, and further external validation is needed to confirm the generalizability of the model.
Keywords: ultrasound and clinicopathological characteristics, coexistence of TERT promoter and BRAFV600E mutations, papillary thyroid carcinoma, predictive model, risk stratification
Introduction
Papillary thyroid carcinoma (PTC) is the most common histological subtype of thyroid cancer, and its incidence has increased worldwide in recent decades.1–3
Although it has a generally indolent clinical course and favorable prognosis, 10–20% of PTC cases exhibit aggressive features and may cause frequent recurrence, distant metastasis and mortality.4,5 Consequently, accurate risk stratification is essential for optimizing individualized treatment in these patients, including guiding surgical decisions and postoperative management.
In recent years, a growing body of evidence has suggested that gene mutations are involved in the development and progression of thyroid cancer.6 Several molecular markers have been investigated as adjunct diagnostic markers of thyroid cancer and accurate predictors of disease prognosis.7
Recent studies have demonstrated that the coexistence of TERT promoter and BRAFV600E mutations is associated with aggressive features of PTC, such as older age, larger tumor size, extrathyroidal extension, lateral lymph node metastasis, advanced tumor stage, recurrence and death.8,9 These studies suggest that the coexistence of TERT promoter and BRAFV600E mutations can be used to predict the aggressiveness and prognosis of PTC. In addition, PTC with both mutations exhibits greater aggressiveness, and patients experience a worse prognosis compared to those with a single mutation, including higher rates of recurrence, distant metastasis, and mortality.10,11 Therefore, it is crucial to develop a simpler, more cost-effective, and non-invasive method to predict BRAFV600E and TERT promoter co-mutations as an alternative to fine-needle aspiration or surgical specimens. Such a method would guide the selection of treatment strategies, the choice of surgical approach, and the formulation of postoperative management plans.
Previous studies have demonstrated that individual BRAF mutations, TERT mutations, and their co-mutation are associated with certain ultrasound and clinicopathological features. In addition, predictive models have been developed for single mutations of BRAF or TERT; however, existing predictive models primarily focus on single mutations and fail to address the complexity of dual mutations, limiting their clinical applicability in predicting the aggressiveness and prognosis of PTC.12–14
The purpose of our study was to fill the gaps by integrating ultrasound and clinicopathological characteristics into a predictive model specifically designed to predict coexisting mutations in PTC. This approach offers a more accurate and clinically relevant tool, addressing the limitations of existing models that fail to predict dual mutations, which are crucial for determining the aggressiveness and prognosis of PTC. This predictive model has the potential to guide surgical decisions, select the most appropriate treatment strategy, and improve postoperative care, including follow-up monitoring to predict recurrence. By identifying patients with a higher risk of aggressive features before surgery, clinicians can tailor treatment plans to achieve better outcomes. Moreover, the model can help predict recurrence and guide follow-up care after surgery.
Materials and Methods
Population
This study protocol was approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (Ethics ID: 2022–12-C009). The need for consent to participate was waived by the Ethics Committee of Affiliated Hospital of Jining Medical University because this retrospective study was conducted using only data with identifiable information and the study did not involve personal information or commercial interests. All participant information has been anonymized, and the data were maintained with strict confidentiality throughout the study to ensure the privacy of the participants. In this study, patients who underwent thyroid surgery due to suspected PTC at the Affiliated Hospital of Jining Medical College from December 2020 to December 2023 were retrospectively analyzed. All patients were confirmed to have PTC by postoperative pathology. A total of 120 patients were included based on the following criteria:(1)Postoperative genetic analysis: BRAFV600E and TERT promoter mutations were analyzed in postoperative pathological specimens.(2)Preoperative ultrasound examination: Ultrasound examination was performed in the ultrasound department of our hospital within one month before surgery, with a complete ultrasound description and clear images available.(3)Tumor characteristics: At least one PTC with a maximum diameter ≥1 cm, confirmed by pathological examination.(4)Surgical history: No prior history of thyroid surgery.(5)For patients with multifocal PTCs, ultrasound images of the largest nodule were analyzed.
Clinicopathological Data and US Imaging Analysis
All clinicopathologic data were retrieved from the electronic medical record system of our hospital, including the patient’s age, gender, tumor size, number, bilateral lobe distribution, Hashimoto presence, capsular invasion, central lymph node metastasis, lateral cervical lymph node metastasis, and tumor-node-metastasis (TNM) stage. The American Joint Committee on Cancer (AJCC) staging system (8th edition, 2017) for thyroid cancer was used in this paper.15
An Affiniti 70 (Philips Medical Systems, Netherlands), ACUSON OXANA2 (Siemens Medical Solutions, Germany) and UGEO WS80A (Samsung, Korea) with a 4 to 18 MHz linear-array transducer were used for neck US examinations.
Preoperative ultrasound examinations were performed by three radiologists who specialized in thyroid imaging and six senior residents with more than 5–10 years of experience. All ultrasound images were retrospectively reviewed by two radiologists with more than 10 years of experience in thyroid ultrasound diagnosis by consensus, and the two radiologists were blinded to the clinicopathological features and mutational analysis results. Inter-observer reliability was assessed using Weighted Kappa statistics to evaluate the consistency between the two radiologists’ interpretations. Weighted Kappa values are interpreted according to the following criteria: < 0.20: inconsistent, 0.21–0.40:generally consistent, 0.41–0.60:moderately consistent, 0.61–0.80:mostly consistent,>0.80:highly consistent. A high degree of agreement was observed between the radiologists, indicating strong inter-observer reliability in the image evaluation process.
The ultrasound images were analyzed based on the following characteristics:
Tumor location:PTC were categorized based on their anatomical location within the thyroid gland as follows. Left Lobe: Nodules located in the left thyroid lobe. Right Lobe: Nodules located in the right thyroid lobe. Isthmus: Nodules located in the thyroid isthmus.
Composition: The composition of thyroid nodules was defined based on the proportion of solid and cystic components as observed on ultrasound. Solid: PTC composed entirely or almost entirely (>90%) of solid components. Cyst-Solid: PTC containing both solid and cystic components, with the solid portion constituting 10–90% of the nodule.
Echogenicity: Echogenicity refers to the reflectivity of the noncalcified solid components of a nodule when compared with reference structures. Hypoechoic: PTC are less reflective than the thyroid. Marked hypoechoic is echogenicity less than or equal to that of the anterior neck muscles. Hyperechoic or isoechoic: PTC exhibits echogenicity that is greater than or equal to that of the adjacent thyroid tissue.
Orientation: The orientation of PTC was classified based on the direction of growth relative to the thyroid capsule as either nonparallel or parallel.
Margin: The margin refers to the boundary between the thyroid nodule and the surrounding thyroid parenchyma. Smooth: PTC that exhibit a sharp margin, without projections into the adjacent tissue.
Irregular or ill-defined: The irregular or ill-defined margins of PTC include small, rounded projections (microlobulated) as well as jagged, spike-like extensions (spiculated).
Calcification: Calcification refers to focal areas within or around the nodule that exhibit significantly hyperechoic characteristics compared to the rest of the nodule and the surrounding normal tissue. Calcifications are categorized into microcalcifications and macrocalcifications based on whether they are larger than 1 mm.
Capsule contactor involvement: Capsule contact involvement refers to the interaction or relationship between the thyroid nodule and the surrounding thyroid capsule, which is the outer layer of the thyroid gland. Specifically, it indicates whether the nodule touches or invades the capsule. In clinical and radiological practice, when a nodule exhibits “capsule contact involvement”, it means that the boundary of the nodule is in close proximity to, or in some cases, has penetrated the thyroid capsule. This feature can be significant, as involvement of the capsule may suggest more aggressive tumor behavior, such as extrathyroidal extension, which can be associated with an increased risk of recurrence and metastasis, especially in cases of thyroid cancer like papillary thyroid carcinoma (PTC).
Presence of vascularity: Presence of vascularity refers to the detection of blood vessels within the PTC during an ultrasound examination, usually through Doppler imaging.
TERT Promoter and BRAFV600E Mutation Analysis
Paraffin-embedded tumor tissue was sliced at a thickness of 10 μm and placed into a centrifuge tube for DNA extraction. DNA was extracted strictly in accordance with the instructions of the nucleic acid extraction kit (Amoydx, Fujian, China). Human BRAFV600E and TERT promoter mutation detection kits (Amoydx, Fujian, China) were used to detect mutations by real-time amplification refractory mutation system-quantitative PCR (RT-ARMS-qPCR). A TL988-IV real-time fluorescence quantitative PCR instrument (Biobase, Shandong, China) was used for amplification of 10 ng of total DNA per sample. All experiments were conducted in strict accordance with the human BRAFV600E and TERT gene promoter mutation detection kit instructions.
Statistical Analysis
Values are presented as the mean±standard deviation for data that were normally distributed or median and interquartile range for data that were not normally distributed for continuous variables and number (%) for categorical variables. To compare the clinicopathologic and US imaging characteristics between patients with coexisting BRAF and TERT promoter mutations and those without coexisting mutations, the chi-square test or Fisher’s exact probability method was used for categorical variables, and the t test was used for continuous variables.
Multivariate logistic regression analysis was used to determine if clinicopathologic and US imaging characteristics were independent variables for predicting coexisting mutations. These screened independent variables construct predictive models and are presented as nomograms.
The receiver operating characteristic curve (ROC curve) was plotted with independent variables to evaluate diagnostic effectiveness and determine the optimal cutoff value for predicting coexisting mutations.
In this study, internal validation is carried out using Bootstrap resampling method. The calibration curves and the Hosmer‒Lemeshow goodness of fit test was used to verify the calibration ability of the prediction model.
To assess the stability and robustness of the predictive model, we performed 10-fold cross-validation. The dataset was randomly divided into 10 subsets, and the model was trained and validated iteratively, with each subset serving as the validation set once. The Balanced Accuracy and AUC were calculated to evaluate the model’s performance. We employed Balanced Accuracy as a primary metric, particularly due to the imbalanced nature of our dataset. Balanced Accuracy is defined as the average of sensitivity (true positive rate) and specificity (true negative rate), calculated as follows:
Here, Sensitivity measures the proportion of correctly identified positive samples, while Specificity measures the proportion of correctly identified negative samples. This metric is particularly useful for imbalanced datasets, as it provides a more robust evaluation by equally considering the performance on both majority and minority classes, unlike traditional accuracy which can be biased towards the majority class.
Data processing and analysis were performed using R version 4.3.0 (2023–04-21), along with Zstats 1.0 (www.zstats.net). All statistical tests were two-sided, and P < 0.05 was considered significant.
Results
Clinicopathological Characteristics
A total of 120 PTC patients were enrolled in this study, which included 39 with coexisting mutations and 81 without coexisting mutations. The noncoexisting mutation group was defined as the group of patients who did not have both TERT promoter and BRAFV600Emutations concurrently. This group included the following patients:
Patients with TERT promoter mutations only (TERT+ only: 1 patients); Patients with BRAFV600E mutations only (BRAF+ only:73 patients); Patients with neither TERT promoter mutations nor BRAFV600E mutations (TERT-/BRAF-:7 patients). As shown in Table 1, the mean age of the patients and lateral lymph node metastasis were significantly higher in the coexisting mutation group than in the noncoexisting mutation group (P<0.001 a). (Table 1).
 |
Table 1 Association of BRAF V600E mutations+TERT Promoter Mutations With Clinical and Histopathologic Characteristics in Patients With PTC |
US Imaging Characteristics
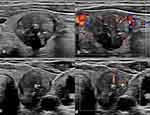
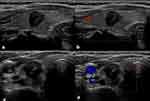
As shown in Figures 1 and 2 and Table 2, Irregular or ill-defined margins, capsule contact or involvement and the presence of vascularity were more common in the coexisting mutation group than in the noncoexisting mutation group (P<0.001, P<0.001 and P=0.026, respectively). (Figures 1 and 2 and Table 2). As shown in Table S1, there was a high degree of consistency in the interpretation of ultrasound images by the two radiologists.
 |
Table 2 Association of BRAFV600E Mutations + TERT Promoter Mutations With US Imaging Characteristics in Patients With PTC |
Multivariate Logistic Regression Analysis
Multivariate logistic regression analysis was performed for all clinicopathological and US imaging characteristics with statistically significant differences. The multivariate logistic regression analyses showed that age (OR: 1.24; 95% CI 1.12–1.37, P<0.001), tumor size (OR: 5.51; 95% CI 2.26–13.46, P<0.001), lateral lymph node metastasis (OR: 7.36; 95% CI 1.48–36.48, P=0.015) and irregular or ill-defined margins (OR: 6.06; 95% CI 1.19–30.75, P=0.030) were all independent predictors for the coexistence of BRAF and TERT promoter mutations (Table 3). The cutoff values for age and tumor size were 44.5 years and 1.55 cm, respectively (Table 4).
 |
Table 3 Multivariate Logistic Regression Analysis for Predicting BRAFV600E Mutations + TERT Promoter Mutations in PTC |
 |
Table 4 Performance of the Independent Predictors and Prediction Models |
Two models were established using the above four independent predictors to predict coexisting mutations. A preoperative prediction model was used to identify high-risk groups before FNA or surgery, develop corresponding treatment plans, and achieve more accurate treatment. A postoperative predictive model was used to assess prognosis and determine whether more active management and monitoring were needed (Figure 3a and b).
We carried out ROC curve, calibration curve, and decision curve analyses to evaluate the performance of the risk prediction models.
As shown in Figure 3c and d, the preoperative and postoperative prediction models achieved AUCs of 0.95 (95% confidence interval, 0.92–0.99) and 0.96 (95% confidence interval, 0.93–1.000), respectively. At their ideal cutoffs (0.425 points for the preoperative model and 0.566 points for the postoperative model), the preoperative model demonstrated an accuracy of 91.0%, sensitivity of 94.0%, specificity of 85.0%, PPV of 93.0%, and NPV of 87.0%, while the postoperative model showed an accuracy of 95.0%, sensitivity of 99.0%, specificity of 87.0%, PPV of 84.0%, and NPV of 97.0% (Table 4).
The Bootstrap resampling method was internally validated with 1000 resamples for both the preoperative and postoperative prediction models. The preoperative model showed a mean absolute error of 0.025, while the postoperative model had a mean absolute error of 0.029, both indicating good accuracy. The calibration curves demonstrated high agreement between model predictions and actual observations for coexisting mutations (Figure 4a and b). The Hosmer–Lemeshow goodness of fit test further confirmed the calibration ability, with results for the preoperative model (χ²=2.21, P=0.974) and the postoperative model (χ²=15.07, P=0.058) both exceeding the significance threshold (P>0.05), indicating good calibration ability for both models.
In addition, the decision curves in Figure 4c and d were located above the None and All lines, indicating that both the preoperative and postoperative prediction models demonstrated good clinical practicability.
The performance measures of the predictive models, including AUC, accuracy, sensitivity, specificity, PPV, and NPV, have significant clinical relevance. The high AUC values (0.95 for the preoperative model and 0.96 for the postoperative model) indicate excellent discriminatory ability, enabling the models to effectively distinguish between patients with and without coexisting mutations. The high accuracy and sensitivity ensures that most high-risk patients are correctly identified, minimizing the risk of false negatives, while the high specificity reduces unnecessary interventions for low-risk patients. The high PPV and NPV further support the models’ reliability in clinical practice. In practical terms, the preoperative model can be used to identify high-risk patients before FNA or surgery, guiding the choice of surgical extent and adjuvant therapies. The postoperative model can assess prognosis and guide follow-up management, such as more frequent monitoring or additional imaging studies. These applications have the potential to significantly improve patient outcomes by ensuring that high-risk patients receive appropriate treatment and surveillance.
10- Fold Cross-Validation
The preoperative model achieved a Balanced Accuracy of 0.841 and an AUC of 0.933 (Figure 4e), indicating strong classification performance and excellent discriminative ability. The postoperative model demonstrated further improvement, with a Balanced Accuracy of 0.867 and an AUC of 0.945 (Figure 4f).
Discussion
Previous studies have demonstrated that compared with PTCs without coexisting TERT promoter and BRAFV600E mutations, PTCs with coexisting TERT promoter and BRAFV600E mutations are more aggressive and have a worse prognosis.10 There is also a synergistic effect of BRAFV600E and TERT promoter mutations on exacerbating the clinicopathologic features of PTC.6,11,16 The synergistic effect is initiated by BRAF-induced activation of the mitogen-activated protein kinase (MAPK) pathway, which upregulates ETS transcription factors. These transcription factors bind to the increased ETS-binding sites on the mutant TERT promoter, resulting in escalated TERT mRNA expression. The transcription factor GABPA and phosphorylated Sp1 synergistically activate the mutant TERT promoter, contributing to tumorigenesis and cancer progression.17,18
Multiple studies have shown that the coexistence of TERT promoter and BRAFV600E mutations is significantly associated with older age, male sex, larger tumor size, multifocal tumors, extrathyroid invasion, distant metastasis, more advanced TNM stage, and a higher risk of recurrence.6,10,11,16,19–21 This study showed that age, tumor size, peripheral muscle invasion, lateral lymph node metastasis, and advanced TNM stage (stage III/IV) were significantly higher in the coexisting mutation group than in the noncoexisting mutation group, which was closely related to invasiveness and prognosis. There was no difference in central lymph node metastasis between the two groups, thus confirming that preventive central lymph node dissection is not the best choice for PTC. In contrast to previous studies, advanced TNM stage (stage III/IV) was not an independent risk factor. We considered that this was related to the narrowing of the advanced TNM stage (stage III/IV) of the AJCC 8th edition TNM staging system for thyroid carcinoma, the adjustment of the age cutoff point from 45 years to 55 years, and the absence of lymph node metastasis as a reference factor for advanced TNM stage (stage III/IV).8,9,15
Age and tumor size have long been recognized as important factors in determining the prognosis of various cancers, including PTC. In our study, age and tumor size were found to be significant contributors to the predictive accuracy of the models, which is consistent with previous research showing their association with tumor aggressiveness. Tumors in older patients tend to exhibit more aggressive features, and larger tumor size is often linked to an increased likelihood of extrathyroidal extension and lymph node metastasis. These factors, therefore, directly influence the risk of recurrence and overall prognosis.
The inclusion of age and tumor size in our predictive models enhances the ability to identify high-risk patients earlier in the clinical decision-making process. Specifically, age can reflect a patient’s overall health status, which can impact the selection of appropriate treatment strategies, such as the extent of surgery or the decision to use adjuvant therapies. Similarly, tumor size provides crucial information about the likelihood of extrathyroidal extension and the risk of lymph node involvement, which are critical considerations for surgical planning.
Of the US imaging characteristics, irregular or ill-defined margin was an independent risk factor. We considered that because tumors with the coexistence of TERT promoter and BRAFV600E mutations were more aggressive, with different growth rates when invading peripheral thyroid tissue, the margins showed differential foliation or irregularity. In addition, a larger tumor size with coexisting TERT promoter and BRAFV600E mutations can make irregular or ill-defined margins easier to see on US images.
Several other studies in the past have had similar results. Shi et al’s study suggested that a shape that is taller than it is wide, irregular margins and capsule contact or involvement were independent risk predictors for TERT promoter mutations.12 Kim et al’s study suggested that nonparallel orientation and irregular margins were independent ultrasonographic findings for predicting telomerase reverse transcriptase promoter-mutated papillary thyroid cancer in patients over 50 years.13 Hu et al’s study suggested that older age, maximum diameter of ≥ 10 mm, unilateral, multifocal, adjacent to the thyroid capsule, and accompanied by other benign nodules were independent risk factors for TERT promoter mutations in PTC.22 Unlike their study, our study examined the coexistence of TERT promoter and BRAFV600E mutations.
In this study, irregular or ill-defined margins, capsule contact or involvement and the presence of vascularity were more common in the coexisting mutation group than in the noncoexisting mutation group. Hahn et al’s study suggested that as the number of genetic mutations increased, from no mutation to BRAF mutation alone to both BRAF and TERT mutations, the proportions of hypoechogenicity, nonparallel orientation, spiculated/irregular margins, microcalcifications, and high suspicion category increased.14 Hahn et al14 studied PTCs with a maximum diameter greater than or equal to 0.5 cm, while the PTCs in our study had a maximum diameter greater than or equal to 1 cm.14 In addition, the number of coexisting mutations in our study was 39, which was significantly higher than the 11 coexisting mutations in the previous study.
In this study, a postoperative prediction model was established with four independent predictors, including age, tumor size, lateral lymph node metastasis and irregular or ill-defined margins, to predict coexisting mutations. The ROC curve showed that the prediction model established using the above four independent characteristics had higher accuracies, sensitivities, specificities, PPV and NPV in predicting coexisting mutations. The calibration curve showed that the postoperative prediction model had good calibration ability, and there was relatively high agreement between the predictions made by the model and the actual observations. In addition, the decision curve showed that the postoperative prediction model had good clinical practicability. Therefore, we suggest that PTC patients with a high probability of coexisting mutations have the following characteristics: age greater than 44.5 years old, maximum tumor diameter greater than 1.55 cm, lateral cervical lymph node metastasis and ultrasound features of irregular or ill-defined margins. A more active treatment strategy and stronger postoperative follow-up is suggested for these patients.
In addition to their prognostic value, the coexisting mutations are helpful for determining the extent of surgery, the extent of lymph node dissection, the dose of radioactive iodine, etc.23,24 Therefore, predicting coexisting mutations will significantly improve the risk stratification of PTC. If the coexisting mutations can be predicted and a corresponding treatment plan can be established before surgery, more accurate treatment will be achieved.
To find the best method to predict coexisting mutations before FNA or surgery, a preoperative prediction model was established with three independent predictors that can be obtained before FNA or surgery, including age, tumor size and irregular or ill-defined margins. ROC curves showed that the preoperative prediction model had higher accuracies, sensitivities, specificities, PPV and NPV in predicting coexisting mutations. The calibration curve showed that the preoperative prediction model had good calibration ability, and there was relatively high agreement between the predictions made by the model and the actual observations. In addition, the decision curve showed that the preoperative prediction model also had good clinical practicability.
Therefore, we believe that PTC patients with a high probability of coexisting mutations who have not undergone FNA or surgery have the following characteristics: age greater than 44.5 years old, maximum tumor diameter greater than 1.55 cm and ultrasound features of irregular or ill-defined margins. In these patients, more aggressive strategies, such as FNA or surgery, rather than observational follow-up are suggested.
When comparing our study with Hahn et al’s research, we observed similar correlations between TERT/BRAF mutations and various clinicopathological features. This finding further supports the robustness of our model, suggesting that it could be applicable across diverse clinical settings and populations. The consistent patterns observed in both studies indicate the potential for our predictive model to be used as a reliable tool for identifying high-risk patients in different clinical environments.
The Balanced Accuracy and AUC are crucial metrics for evaluating the stability of both preoperative and postoperative models, especially in the context of class imbalance. Unlike traditional accuracy, Balanced Accuracy accounts for the performance of both the positive and negative classes, mitigating the bias that may arise from class imbalance. By incorporating AUC, the results of this study further validated the overall performance of the model across different decision thresholds, which is essential for assessing the model’s reliability in real-world applications.
In addition, several studies have shown that combined detection of the TERT promoter and BRAFV600E mutations can be performed and is considered to be a reliable method for the preoperative diagnosis of high-risk thyroid nodules.25–27 Therefore, we believe that our conclusions are feasible and of great significance.
This study introduces an innovative approach by utilizing clinicopathological and ultrasound features to develop preoperative and postoperative prediction models for identifying concurrent mutations in papillary thyroid carcinoma (PTC). This method provides a more comprehensive strategy for patient management, surpassing traditional risk stratification models that rely solely on individual clinicopathological factors. The new model makes a significant contribution to clinical practice by offering the potential to identify high-risk patients at an early stage, facilitating more personalized treatment plans and improving prognostic predictions. The preoperative model can assist clinicians in making early decisions regarding surgical approaches and adjuvant therapies, while the postoperative model can guide follow-up care and monitoring to ensure better management of high-risk patients. By refining risk stratification and integrating these models into clinical practice, clinicians can enhance their decision-making processes, potentially leading to more accurate and efficient management of PTC patients. Furthermore, the findings of this study may aid in counseling patients about their prognosis, including the likelihood of recurrence or metastasis.
There were several limitations in this study. First, the group without coexisting mutations was not subdivided into patients with no mutations, TERT promoter mutations alone or BRAFV600E mutations alone. Therefore, this study did not evaluate the impact of the number of different types of mutations on the study after subdivision. Second, the correlation between clinicopathologic and ultrasonic features of PTCs smaller than 1 cm was not discussed. Third, although our predictive model demonstrated strong performance in the internal validation cohort, this study was conducted at a single center. Therefore, external validation in an independent cohort is essential to confirm the model’s generalizability and robustness. We plan to validate the model using data from a different institution in future work. This step will ensure that the model is applicable to diverse patient populations and can be reliably integrated into clinical practice for guiding treatment decisions in PTC. Fourth, there may be potential selection bias in our study. The higher proportion of patients with coexisting TERT promoter and BRAFV600E mutations (TERT+/BRAF+) in our cohort could introduce selection bias, potentially limiting the generalizability of our findings. This may be attributed to the unique characteristics of our study population, which included a higher proportion of patients with aggressive disease features, such as older age, larger tumor size, and advanced tumor stage. Additionally, our selection criteria, which required comprehensive clinicopathological, ultrasound, and genetic data, may have resulted in a higher prevalence of TERT+/BRAF+ patients, as these patients are more likely to undergo extensive diagnostic and genetic testing. To address the potential for selection bias and enhance the generalizability of our findings, we plan to collaborate with multiple centers to validate our predictive models in independent, multi-center cohort. This external validation will include a more diverse patient population and help confirm whether the high proportion of TERT+/BRAF+ patients in our study is reproducible in other settings. Finally, our predictive models include four key predictors (age, tumor size, lateral lymph node metastasis, and ill-defined margins). While these predictors were selected based on their clinical relevance and statistical significance, the models may not capture all potential factors associated with coexisting mutations. Future studies could explore the inclusion of additional predictors, such as molecular markers or advanced imaging features, to further improve model performance. This will be the focus of our future research.
In conclusion, ultrasound and clinicopathological characteristics can predict coexisting mutations. The establishment of a prediction model using ultrasound and clinicopathological characteristics can predict coexisting mutations before or after surgery, stratify prognostic risks and guide the choice of treatment. Although this study has limitations, such as a relatively small sample size and the lack of external validation, these aspects will be the focus of future research. The next step will involve multi-center collaborative studies, as well as incorporating additional molecular markers or multi-omics data to further enhance the model’s performance and generalizability.
Data Sharing Statement
The datasets generated/analysed during the current study are available.
Ethics Approval and Consent to Participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Affiliated Hospital of Jining Medical University (January 6, 2023/2022C320).
Consent for Publication
Consent for publication was obtained from the participants.
Acknowledgments
This manuscript has been uploaded to the preprint server ResearchSquare: https://www.researchsquare.com/article/rs-3388941/v1. We would like to acknowledge Lei Dong PhD for technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Key R&D Program of Jining (Grant number 2024YXNS174 and 2017SMNS011).
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Zhang J, Xu S. High aggressiveness of papillary thyroid cancer: from clinical evidence to regulatory cellular networks. Cell Death Discov. 2024;10(1):378. doi:10.1038/s41420-024-02157-2
2. Cao M, Yu T, Miao X, Wu Z, Wang W. The preferred surgical choice for intermediate-risk papillary thyroid cancer: total thyroidectomy or lobectomy? A systematic review and meta-analysis. Int J Surg. 2024;110(8):5087–5100. doi:10.1097/JS9.0000000000001556
3. Ghai S, Goldstein DP, Sawka AM. Ultrasound imaging in active surveillance of small, low-risk papillary thyroid cancer. Korean J Radiol. 2024;25(8):749–755. doi:10.3348/kjr.2024.0148
4. Coca-Pelaz A, Shah JP, Hernandez-Prera JC, et al. Papillary thyroid cancer-aggressive variants and impact on management: a narrative review. Adv Ther. 2020;37(7):3112–3128. doi:10.1007/s12325-020-01391-1
5. Ito Y, Miyauchi A, Kihara M, Fukushima M, Higashiyama T, Miya A. Overall survival of papillary thyroid carcinoma patients: a single-institution long-term follow-up of 5897 patients. World J Surg. 2018;42(3):615–622. doi:10.1007/s00268-018-4479-z
6. Zhao L, Wang L, Jia X, et al. The coexistence of genetic mutations in thyroid carcinoma predicts histopathological factors associated with a poor prognosis: a systematic review and network meta-analysis. Front Oncol. 2020;10:540238. doi:10.3389/fonc.2020.540238
7. Romei C, Elisei R. A narrative review of genetic alterations in primary thyroid epithelial cancer. Int J Mol Sci. 2021;22(4):1726. doi:10.3390/ijms22041726
8. Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32(25):2718–2726. doi:10.1200/JCO.2014.55.5094
9. Ren H, Shen Y, Hu D, et al. Co-existence of BRAF(V600E) and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag Res. 2018;10:1005–1013. doi:10.2147/CMAR.S159583
10. Chen B, Shi Y, Xu Y, Zhang J. The predictive value of coexisting BRAFV600E and TERT promoter mutations on poor outcomes and high tumour aggressiveness in papillary thyroid carcinoma: a systematic review and meta-analysis. Clin Endocrinol. 2021;94(5):731–742. doi:10.1111/cen.14316
11. Jin L, Chen E, Dong S, et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: a study of 653 patients. Oncotarget. 2016;7(14):18346–18355. doi:10.18632/oncotarget.7811
12. Shi H, Guo LH, Zhang YF, et al. Suspicious ultrasound and clinicopathological features of papillary thyroid carcinoma predict the status of TERT promoter. Endocrine. 2020;68(2):349–357. doi:10.1007/s12020-020-02214-7
13. Kim TH, Ki CS, Hahn SY, et al. Ultrasonographic prediction of highly aggressive telomerase reverse transcriptase (TERT) promoter-mutated papillary thyroid cancer. Endocrine. 2017;57(2):234–240. doi:10.1007/s12020-017-1340-3
14. Hahn SY, Kim TH, Ki CS, et al. Ultrasound and clinicopathological features of papillary thyroid carcinomas with BRAF and TERT promoter mutations. Oncotarget. 2017;8(65):108946–108957. doi:10.18632/oncotarget.22430
15. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
16. Huang M, Yan C, Xiao J, Wang T, Ling R. Relevance and clinicopathologic relationship of BRAF V600E, TERT and NRAS mutations for papillary thyroid carcinoma patients in Northwest China. Diagn Pathol. 2019;14(1):74. doi:10.1186/s13000-019-0849-6
17. Song YS, Yoo SK, Kim HH, et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr Relat Cancer. 2019;26(6):629–641. doi:10.1530/ERC-17-0562
18. Wu Y, Shi L, Zhao Y, et al. Synergistic activation of mutant TERT promoter by Sp1 and GABPA in BRAF(V600E)-driven human cancers. NPJ Precis Oncol. 2021;5(1):3. doi:10.1038/s41698-020-00140-5
19. Xu B, David J, Dogan S, et al. Primary high-grade non-anaplastic thyroid carcinoma: a retrospective study of 364 cases. Histopathology. 2022;80(2):322–337. doi:10.1111/his.14550
20. Wang JR, Montierth M, Xu L, et al. Impact of somatic mutations on survival outcomes in patients with anaplastic thyroid carcinoma. JCO Precis Oncol. 2022;6:e2100504. doi:10.1200/PO.21.00504
21. Chen Z, Wang W, Xu J, et al. Tumor mutation burden-assisted risk stratification for papillary thyroid cancer. Endocrine. 2022;78(2):296–305. doi:10.1007/s12020-022-03154-0
22. Hu Y, Xu S, Dong L, Pan Z, Zhang L, Zhan W. Clinical features combined with ultrasound characteristics to predict TERT promoter mutations in papillary thyroid carcinoma: a single-center study over the past 5 years. Front Endocrinol (Lausanne). 2024;15:1322731. doi:10.3389/fendo.2024.1322731
23. Krasner JR, Alyouha N, Pusztaszeri M, et al. Molecular mutations as a possible factor for determining extent of thyroid surgery. J Otolaryngol Head Neck Surg. 2019;48(1):51. doi:10.1186/s40463-019-0372-5
24. Meng Z, Matsuse M, Saenko V, et al. TERT promoter mutation in primary papillary thyroid carcinoma lesions predicts absent or lower (131) i uptake in metastases. IUBMB Life. 2019;71(7):1030–1040. doi:10.1002/iub.2056
25. Liu R, Xing M. Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr Relat Cancer. 2014;21(5):825–830. doi:10.1530/ERC-14-0359
26. Giorgenon TMV, Carrijo FT, Arruda MA, et al. Preoperative detection of TERT promoter and BRAFV600E mutations in papillary thyroid carcinoma in high-risk thyroid nodules. Arch Endocrinol Metab. 2019;63(2):107–112. doi:10.20945/2359-3997000000116
27. Dell’Aquila M, Fiorentino V, Martini M, et al. How limited molecular testing can also offer diagnostic and prognostic evaluation of thyroid nodules processed with liquid-based cytology: role of TERT promoter and BRAF V600E mutation analysis. Cancer Cytopathol. 2021;129(10):819–829. doi:10.1002/cncy.22454
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Preoperative Prediction of Metastatic Lymph Nodes Posterior to the Right Recurrent Laryngeal Nerve in cN0 Papillary Thyroid Carcinoma
Shao J, Wang X, Yu H, Ding W, Xu B, Ma D, Huang X, Yin H
Cancer Management and Research 2024, 16:421-429
Published Date: 6 May 2024