Back to Journals » Cancer Management and Research » Volume 16
Ultrasound Diagnosis of Bilateral Primary Breast Burkitt Lymphoma in a 28-Year-Old Lactating Patient: A Case Report
Authors Jiang QX , Shi LJ, Hong XY, Zhu YQ, Guo QL, Xie W, Lyu GR
Received 20 June 2024
Accepted for publication 4 September 2024
Published 11 September 2024 Volume 2024:16 Pages 1247—1252
DOI https://doi.org/10.2147/CMAR.S483592
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Qiu-Xia Jiang,1 Li-Jing Shi,1 Xiu-Yang Hong,2 Yu-Qin Zhu,3 Qiu-Ling Guo,1 Wen Xie,1 Guo-Rong Lyu4
1Department of Ultrasound, Quan-Zhou Women’s and Children’s Hospital, Quanzhou, 362000, People’s Republic of China; 2Department of Women Health Care, Quan-Zhou Women’s and Children’s Hospital, Quanzhou, 362000, People’s Republic of China; 3Department of Pathology, Quan-Zhou Women’s and Children’s Hospital, Quanzhou, 362000, People’s Republic of China; 4Department of Ultrasound, the Second Affiliated Hospital of Fujian Medical University, Quanzhou, 362000, People’s Republic of China
Correspondence: Guo-Rong Lyu, Email [email protected]
Abstract: Primary breast Burkitt lymphoma (PB-BL) is an exceedingly rare form of primary breast lymphoma. Ultrasonography is the preferred modality for diagnosing breast diseases; however, the ultrasonic features of Burkitt lymphoma have rarely been reported. Herein, we report a case of ultrasonically diagnosed bilateral PB-BL in a lactating patient and present a literature review. A 28-year-old female patient experienced bilateral breast engorgement starting more than a month after childbirth. At three months postpartum, the patient experienced extreme bilateral breast engorgement, with the skin appearing dark purple and jaundiced. Based on the imaging diagnosis, pathological, immunohistochemical, and molecular biological findings, she was diagnosed with Burkitt lymphoma involves bilateral breasts, right adrenal glands, uterus, and multiple bones. After 4 cycles of combination chemotherapy, the tumor basically disappeared, and then after autologous stem cell transplantation and one cycle of combination chemotherapy, the patient is generally in good condition and is under follow-up. We found that the ultrasonic characteristics of PB-BL are different from those of common breast cancer or lactation mastitis. PB-BL lesions are often multiple, large masses, and even involve the whole breast. The characteristic reticular structures are common in lesions, and irregular hyperechoic masses can be seen around it. The mass has abundant peripheral and internal blood flow signals, but internal calcification and attenuated posterior echoes of masses are rarely observed. Thus, the ultrasonic features of breast Burkitt lymphoma are somewhat specific and understanding these features is conducive to its early identification.
Keywords: Burkitt lymphoma, ultrasonography, case report
Introduction
Primary breast lymphoma is a rare malignancy originating in breast lymphoid tissue. It is derived from B or T lymphocytes, representing only 0.04–1% of breast malignancies and <1% of non-Hodgkin’s lymphoma.1 While <90% of primary breast lymphoma cases are diffuse large B-cell lymphomas, primary breast Burkitt lymphoma (BL) (PB-BL) is an exceedingly rare form of primary breast lymphoma.2 However, differentiating breast BL (BBL) from breast cancer is challenging due to their similar clinical manifestations, although they have different treatment modalities and prognoses.
At present, the treatment of PB-BL is mainly multi-drug combination chemotherapy, including intrathecal injection of MTX to prevent or treat central nervous system involvement. In addition, performing autologous stem cell transplantation (ASCT) at the time of initial treatment remission may help improve 5-year survival. At the same time, multiple CD19 chimeric antigen receptor T cells (CAR T cell therapy) have been approved by the US FDA for the second-line treatment of relapsed/refractory BP-PL. Although PB-BL is sensitive to chemotherapy, the incidence of treatment failure in adult patients may be as high as 35%, patients with lymphoma may succumb to the disease shortly because of a poor prognosis after a distant metastasis. An analysis of data from a large retrospective series of studies identified four factors independently associated with clinical outcomes in adults treated with standard regiments: Age ≥40 years, ECOG performance-status score ≥2, LDH ≥ 3 X ULN, and CNS involvement.3 Therefore, identifying BL and providing symptomatic treatment in the early stage significantly contribute to improving the prognosis of patients with BL. Ultrasonography is the preferred modality for diagnosing breast diseases; however, the ultrasonic features of BL have rarely been reported in the literature. Herein, we report a case of ultrasonically diagnosed bilateral PB-BL in a lactating patient and present a literature review to improve the understanding of this disease.
Case Report
A 28-year-old female patient experienced bilateral breast engorgement more than a month after childbirth, yet she did not seek attention. Consequently, the breast engorgement symptoms worsened over time. At three months postpartum, the bilateral breast engorgement was extreme, with the skin appearing dark purple and jaundiced.
Physical Examination
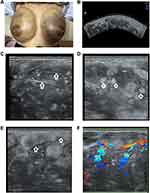
The entire body was jaundiced, and the skin of the bilateral breasts appeared dark purple. Both breasts had palpable large solid masses involving the entire breasts, about 30cm × 25cm. The local skin temperature was elevated, and superficial veins were engorged (Figure 1A). However, superficial lymphadenectasis was not observed in the preauricular, postauricular, neck, supraclavicular, axillary, and groin regions.
Ultrasound Examination
We used a GE E8 colour Doppler ultrasound machine (GE Healthcare, Austria) with 5-MHz convex array probe and 13-MHz linear array probe. As the lesions were too large, a 5-MHz convex array probe was used to observe the overall view of the lesions (Figure 1B), and a 13-MHz linear probe was used to observe the internal echoes and blood flow of the lesions (Figure 1C–F). Hypoechoic and mixed-echogenicity nodules of varying sizes were found in both breasts, with unclear boundaries and irregular margins; some lesions fused with each other, involving the entire breasts (Figure 1B). Multiple reticular echogenic bands were noted in the lesions (Figure 1C), with multiple isoechoic and hyperechoic spots and masses around the lesions (Figure 1D). The breast skin and subcutaneous tissue was swollen along with echo enhancement (Figure 1E).
Imageological Examination
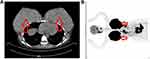
PET CT (Figure 2B): Right maxillary sinus, left sphenoid sinus, right nasopharyngeal posterior wall, right parapharyngeal space, left posterior neck subcutaneous, bilateral breasts, right adrenal gland, hilar space, uterus, left groin region, right buttock subcutaneous, right vertical spinal muscle, multiple high metabolic mass; The increased metabolism of the left margin of the T3 vertebra, the left femoral head, and the left femoral bone marrow cavity were all considered as lymphoma infiltration. Chest CT: bilateral breasts diffuse distribution medium density imaging (Figure 2A).
Color Doppler Flow Imaging
Abundant blood flow signals in the lesions revealed a resistance index of 0.61 (Figure 1F). Ultrasound examination signaled the presence of multiple space-occupying lesions in both breasts, which were graded as category 5 according to the Breast Imaging Reporting and Data System (BI-RADS).
Serum Chemistry Panel
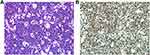
Blood test results were as follows: total bilirubin, 524.1 µmol/L; indirect bilirubin, 332.5 µmol/L; alanine aminotransferase, 326 U/L; aspartate aminotransferase, 184 U/L; and lactate dehydrogenase, 776.6 U/L. Anti-human immunodeficiency virus (HIV) and Epstein–Barr virus (EBV) antibodies were negative in sera. A breast aspiration biopsy was performed and the pathological findings revealed diffuse infiltration of uniformly heterogeneous lymphoid cells observed microscopically. The cells were medium-sized and basophilic, with a moderate amount of cytoplasm and round nuclei. Nucleolar division and karyokinesis, karyorrhexis, and apoptosis were readily observed. The “starry sky pattern” was evident in the background (Figure 3).
Immunohistochemical Testing
Immunohistochemical test results were as follows: CD21 (-), CD20 (+), CD3 (-), ki-67 (100%+), CD10 (+), Bcl-2 (-), Bcl-6 (+), c-myc (95%+), CD5 (-), CyclinD-1 (-), CD30 (-), TdT (-), P53 (90%+), MUM-1 (+), CD38 (+), CD48 (+), and LM0-2 (-). EBV-encoded RNA via in-situ hybridization was negative.
Molecular Detection Results
The patient was negative for LF22-00556:11q23.3 and 11q24.3 deletion and LF22-00558: BLC2 gene isolation. However, the patient was positive for LF22-00557: MYC gene isolation.
Treatment
Based on the pathological, immunohistochemical, and molecular biological findings, the patient was diagnosed with Burkitt lymphoma involves bilateral breasts, right adrenal glands, uterus, and multiple bones. The patients received one cycle of R-CHOPE chemotherapy (VCR 2 mg, etoposide 400 mg, cyclophosphamide 800mg, epirubicin 60mg, rituximab 600mg, and DXM100 mg). Add dexamethasone 5mg and MTX 15mg intravenously once. Subsequently, the tumor shrank from 30cm × 25cm to 8cm × 7cm. Then, the patients received three cycles of R-DAEPOCG chemotherapy (rituximab 600mg, vincristine 2.0mg, epirubicin 80mg, etoposide 320mg, prednisolone acetate 500mg, cyclophosphamide 1200mg). Add dexamethasone 5mg and MTX 15mg intravenously once. Subsequently, the tumors shrank to 6cm × 7cm and 3cm x 4cm, respectively, and finally almost disappeared. The patient was generally in good condition and stem cells were collected for the patient. Then, R-MTX (rituximab 600mg, MTX 5.0mg) regimen was given for one cycle, followed by R-DAEPOCG regimen for one cycle. Tumor lesions of the patients all subsided, and she underwent autologous stem cell transplantation. At present, the patient is generally in good condition and is under follow-up.
Discussion
PB-BL, which is exceedingly rare, is a highly invasive non-Hodgkin’s B-cell lymphoma with an extremely short doubling time. It was first reported by Denis Burkitt in 1958. Before 2010, 48 cases of PB-BL were reported in the literature: 13 were diagnosed during pregnancy, 15 during lactation, and the remaining during an unknown period. BL can be categorized into three subtypes: endemic, sporadic, and immunodeficiency-related. Endemic BL, associated with EBV infection and common in Africa, typically involves the jaw and other facial skeletal structures. Sporadic BL, reported worldwide, largely manifests as abdominal masses, and typically involves the ileocecal are, however, this case involves both breasts, which is very rare. Immunodeficiency-related BL occurs in immunodeficient patients (eg, patients with acquired immunodeficiency syndrome and organ transplant) and typically involves lymph nodes and bone marrow. The present patient was HIV- and EBV-negative and thus was considered to have sporadic PB-BL.
Ultrasonography is the primary method for diagnosing breast diseases, with a diagnostic accuracy for breast cancer as high as 80%; however, correct PB-BL diagnosis is challenging. Ultrasound findings of breast Burkitt lymphoma are rarely reported in the current literature. However, the ultrasound features of this patient are similar to those reported in the literature for other types of lymphoma of the breast. Based on existing reports4–6 and the ultrasound images pertinent to this case, the ultrasonic features of PB-BL can be summarized as follows: 1) multiple lesions commonly seen in a single breast or both breasts; 2) most masses are large (>5 cm), and even involve the entire breasts; 3) presence of unclear boundaries and irregular margins, often accompanied by hydrops and thickening of the skin and subcutaneous fat layer and echo enhancement; 4) the internal echoes, which are mostly hypoechoic or extremely hypoechoic, are cluttered, irregular iso- to hyperechoic masses are present around the lesions, and reticular structures are often seen in the lesions (these changes are the specific signs of PB-BL, which are similar to the reticular changes in primary thyroid lymphoma), and posterior echo enhancement; 5) presence of abundant peripheral and internal blood flow signals; and 6) internal calcification and attenuated posterior echoes of masses are rarely observed. If a high-frequency (>15MHz) probe is available, it should be used in a targeted way to image breasts. Very fine scans can be obtained, with optimal spatial resolution and anatomic detail.7
The above-mentioned signs are conducive to differentiating PB-BL from breast cancer. Breast cancer often presents as low echo, irregular shape, aspect ratio greater than 1, strong echo ring can be seen around, and clusters of microcalcification can be seen inside, and some masses decay behind. Moreover, as PB-BL is prevalent in lactating women, it is readily misdiagnosed as lactational mastitis. However, reticular structures and abundant blood flow signals present in the lesions facilitate differentiation. In addition, mastitis is often accompanied by abscess formation, abscess can be seen in the fine spot echo, can also be distinguished from it. At the same time, it is also necessary to exclude lesions from the superficial fat layer of the breast or the deep pectoralis major muscle. Microscopically, a diffuse distribution of uniform lymphoid cells is found in the lesions, which may result in extremely hypoechoic nodules in patients with BL with the reticular structures in the lesions being fibrous tissues. Therefore, a sonographer must routinely check for the presence of internal blood flow signals in the presence of extremely hypoechoic homogeneous cystic nodules.
Additionally, the clinical features of PB-BL are specific: 1) PB-BL is commonly prevalent in young women of childbearing age, particularly pregnant or lactating women;8 2) it progresses rapidly, and often manifests as rapid diffuse engorgement of bilateral breasts;9 3) serum lactate dehydrogenase levels in some patients are markedly elevated; 4) it histopathologically manifests as diffuse infiltration of uniformly heterogeneous B lymphocytes, causing the formation of a characteristic “starry sky” appearance;10 and 5) is immunohistochemically positive for CD20 and the germinal center markers, CD10 and BCL6 27, 30, and 31, and is almost 100% positive for Ki67, highlighting its high proliferation capacity.11 The clinical and pathological features of the present patient corresponded to those in the previous reports leading to a diagnosis of BL. A confirmed diagnosis of BBL should be based on clinical, histomorphological, immunophenotypic, and molecular genetic features because the cause of BBL remains unknown. Moreover, hormones are considered to play a pivotal role in its pathogenesis because BBL is frequently prevalent in pregnant or lactating women as evidenced by a report of a male patient with BBL12 with a history of basal cell carcinoma who was administered spironolactone (anti-androgen therapy). In addition, genomic studies have shown that mutations in the p53 and phosphatidylinositol 3-kinase (PI3K) signaling pathways contribute to tumorigenesis. The scientists also identified mutual translocations of the MYC oncogene on chromosome 8 and immunoglobulin loci on chromosomes 2, 14, or 22, which led to dysregulation of MYC expression and uncontrolled tumor proliferation.
Limitations
Since PB-BL is rare, this article is only a case report, and more cases are needed to summarize its ultrasonic characteristics. In addition, if a probe with a higher frequency (> 15MHz) can be used, the internal echoes and blood flow signals of lesions can be more clearly displayed, which is more conducive to the diagnosis and differential diagnosis of breast diseases.
Conclusion
The ultrasonic features of BBL are somewhat specific. Therefore, understanding these features is conducive to an early identification of the lesions. Furthermore, an early diagnosis and treatment can be achieved based on clinical features and pathological and molecular biological changes, improving the BBL prognosis.
Ethics Approval and Informed Consent
Ethical approval was acquired from Quan-Zhou Women’s and Children’s Hospital Ethics Committee (Decision No: 2024/59). Informed consent was obtained from the patient for the publication of her case details included publication of the images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Moura C, Leite MI, Parreira R, Medeiros A. Primary breast lymphoma. J Surg Case Rep. 2020;2020(1):rjz405. doi:10.1093/jscr/rjz405
2. Thomas A, Link BK, Altekruse S, Romitti PA, Schroeder MC. Primary breast lymphoma in the United States: 1975–2013. J Natl Cancer Inst. 2017;109(6):djw294. doi:10.1093/jnci/djw294
3. Mark R, Louis M, Wilson WH. Burkitt’s Lymphoma. New Engl J Med. 2022;387:1111–1122. doi:10.1056/NEJMra2025746
4. Jing JG, Peng YL, Li JJ. Sonographic characteristics of breast lymphoma. West China Med J. 2008;23:572–573.
5. Jia ZY, Jiang DH, Zhang LH. Analysis of sonographic features of primary thyroid lymphoma. Chin J Ultrason. 2010;19:457–458.
6. Du ZS, Tang LN, Wang YQ, Chen Y, Ke X. Diagnosis and differential diagnosis of breast lymphoma by color Doppler ultrasound. J Leuk Lymphoma. 2021;30:87–90.
7. Corvino A, Varelli C, Catalano F, et al. Use of high-frequency transducers in breast. Sonography. J Pers Med. 2022;12:1960. doi:10.3390/jpm12121960
8. James ER, Miranda RN, Turner SD. Primary lymphomas of the breast: a review. JPRAS Open. 2022;32:127–143. doi:10.1016/j.jpra.2022.01.004
9. Testa AC, De Blasis I, Di Legge A, Belli P, Hohaus S, Ferrandina G. Burkitt’s lymphoma of the breast metastatic to the ovary diagnosed during pregnancy. Ultrasound Obstet Gynecol. 2013;42(3):364–366. doi:10.1002/uog.12533
10. Wei J, Lin C, Xu C, Xi Q, Wang C. Primary Burkitt’s lymphoma of the breast without Epstein-Barr virus infection: a case report and literature review. Indian J Pathol Microbiol. 2015;58(4):546–549. doi:10.4103/0377-4929.168857
11. Negahban S, Ahmadi N, Oryan A, et al. Primary bilateral Burkitt lymphoma of the lactating breast: a case report and review of the literature. Mol Diagn Ther. 2010;14(4):243–250. doi:10.1007/BF03256380
12. Elgaafary S, Nagel I, López C, et al. Double-hit lymphoma of the male breast: a case report. J Med Case Rep. 2020;14(1):245. doi:10.1186/s13256-020-02526-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.