Back to Journals » Infection and Drug Resistance » Volume 18
Unexpected Efficacy of Albumin-Bound Glycerol Monolaurate Against Multidrug-Resistant Bacterial Isolates: A Time-Kill Assay Study
Authors Alrabiah MA, Hassan AA, Mubaraki MA, Albarrag AM, Dkhil MA , Somily AM , Delic D, Hafiz TA
Received 10 December 2024
Accepted for publication 12 March 2025
Published 24 March 2025 Volume 2025:18 Pages 1581—1593
DOI https://doi.org/10.2147/IDR.S502165
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Mona A Alrabiah,1,* Amina A Hassan,2 Murad A Mubaraki,3 Ahmed M Albarrag,4 Mohamed A Dkhil,5 Ali M Somily,4 Denis Delic,6 Taghreed A Hafiz3,*
1Microbiology and Immunology Department, King Khaled University Hospital, Riyadh, 12372, Saudi Arabia; 2National Center for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority (EAEA), Cairo, Egypt; 3Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, 12372, Saudi Arabia; 4Pathology Department, College of Medicine, King Saud University, Riyadh, 12372, Saudi Arabia; 5Department of Zoology and Entomology, Faculty of Science, Helwan University, Cairo, Egypt; 6Department of Translational Medicine & Clinical Pharmacology, Boehringer Ingelheim Pharma, Biberach, Germany
*These authors contributed equally to this work
Correspondence: Taghreed A Hafiz, Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, 12372, Saudi Arabia, Email [email protected]
Background: The rise of antibiotic resistance is a significant threat to global health, necessitating the exploration of novel antimicrobial agents. Glycerol monolaurate (GML) is known for its antimicrobial properties, primarily against Gram-positive bacteria, with limited evidence of efficacy against Gram-negative pathogens.
Methods: This study evaluated the antibacterial activity of GML alone and in combination with human serum albumin (HSA) against clinical isolates of carbapenem-resistant and vancomycin-resistant bacteria using MIC and time-kill assays.
Results: Contrary to previous reports, we demonstrate that GML exhibits significant antibacterial activity against Gram-negative bacteria, including strains resistant to conventional antibiotics. It inhibited carbapenem-resistant isolates with MIC values ranging from 25 to 100 μg/mL for E. coli, K. pneumoniae, and E. cloacae and showed bacteriostatic and bactericidal activity. The combination of HSA and GML enhanced this effect, showing potent bactericidal properties across all tested concentrations.
Conclusion: Current findings suggest that HSA-bound GML could be developed as a novel broad-spectrum antimicrobial agent targeting multidrug-resistant pathogens. Future research should focus on formulation optimization, in vivo efficacy studies, and preclinical evaluations to determine its therapeutic potential in clinical settings.
Keywords: Carbapenem-resistant, human serum albumin, MIC, time-kill assay, clinical isolates
Introduction
The emergence of antibiotic resistance in both Gram-positive and Gram-negative bacteria poses a significant threat to public health. Carbapenem-resistant and vancomycin-resistant pathogens are of particular concern, resulting in substantial health, environmental, and economic costs to the global healthcare system.1,2 The World Health Organization has developed an international policy to combat antibiotic resistance by restricting antibiotic usage in animals, encouraging better hand hygiene in hospitals, and prohibiting antibiotic prescriptions for viral infections. Importantly, in the last decade, Saudi Arabia’s Ministry of Health has set antibiotic use and prescribing restrictions.3,4 Furthermore, one of the current actions in addressing this growing issue is the investigation of alternative compounds and substances. Pharmaceutical companies and healthcare institutions have tried to generate novel antibiotics or modify natural antimicrobial medicines.5–8
Glycerol monolaurate (GML) is a naturally occurring surfactant molecule abundant in human milk. It possesses antimicrobial activity against bacteria, fungi, and viruses. It has been utilized as a therapeutic agent in various clinical applications, including wound dressing, to inhibit the growth of exotoxin-producing bacteria.9,10
Human serum albumin (HSA) is a macromolecular monomeric plasma protein with antioxidant and antimicrobial activity used as a therapeutic agent to treat several diseases.11,12 HSA has a high ligand-binding capacity and can affect the pharmacokinetic properties of many drugs, including warfarin, chlorpromazine, naproxen, and ibuprofen.13–16 As reported in later research, HSA tended to reduce the action of GML when linked together.13 This study aims to evaluate the antibacterial activity of HSA coupled with GML against carbapenem-resistant, vancomycin-resistant, and methicillin-resistant isolates. The isolates were collected from immunocompromised patients with cancer and diabetes, patients with wound infections, and ICU patients with pulmonary edema.
Materials and Methods
Bacterial Strains
This investigation employed eleven bacterial isolates, including American Type Culture Collection (ATCC) strains. The two types of bacteria used were Gram-negative bacteria strains susceptible to Gentamicin and strains resistant to it and Gram-positive bacteria strains sensitive to Vancomycin and resistant to it. All bacteria strains have been collected from King Khalid University Hospital in Riyadh in 2019 (Table 1). The isolates were cultured and identified by the Micro Scan Walk-Away® system (Beckman Coulter Inc). For antibiotic susceptibility, Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 29213 were used to confirm the quality of MIC values according to the Clinical Laboratory Standards Institute (CLSI) and to check the growth condition and sterility of the media.17
 |
Table 1 The Clinical Details of the Bacterial Isolate’s Origin |
Preparation for Bacterial Suspension
The strains were sub-cultured and revitalized by streaking on blood agar plates and incubated overnight at 37°C. Then, direct saline suspension of isolated colonies was prepared from an 18–24-hour incubated blood agar plate. The turbidity of the suspension was adjusted to a 0.5 McFarland standard (1x108 CFU/mL). To prepare the colony count to approximately 5 × 106 CFU/mL, the suspension was diluted 1:20 times in Muller Hinton Broth (MHB).
Preparation of Stock and Working Solutions for Antibiotics, GML and HSA
Two different antibiotics were used: Gentamicin and Vancomycin (Sigma–Aldrich, St. Louis, USA). The pure antibiotic powder was dissolved in water to the desired concentration.17 The stock solution of this antibiotic was prepared with a concentration of 1600 µg/mL. Then, it was diluted to the desired starting concentration, 64 μg/mL. Glycerol Monolaurate (purity ≥ 99%) and Human serum Albumin were purchased from (Sigma–Aldrich, St. Louis, USA). Pure powder of GML was dissolved in absolute ethanol. The stock solution of GML was prepared by dissolving 1 mg of GML in 1 mL of ethanol to get a concentration of 1000 µg/mL.18 Then, the stock solution was diluted in MHB to get the desired 400 μg/mL concentration. However, HSA was dissolved in Muller Hinton broth at 5 mg/mL in a water bath at 37°C for 30 min to allow protein binding. The HSA bound to GML stock was prepared using a stock solution of GML and HSA in equal volume (1:1) and allowed to stand overnight to form the complex.
Minimum Inhibitory Concentration (MIC)
The Minimum inhibitory concentration was determined with standard antibiotics (Vancomycin and Gentamicin), GML alone, and HSA linked with GML by broth micro dilution method using sterile 96-well Polystyrene Cell Culture plates. The micro dilution plate was prepared by adding 100 μL MHB from well two to well twelve, the first well containing 200 μL of standard antibiotic or GML or HSA linked to GML, and serial double dilution from well one to tenth was performed by transferring 100 μL from each well. Finally, ten μL of bacterial suspension was added to all the wells except well 12. Eleven was kept as a positive control containing MHB and bacterial suspension. At the same time, well 12 was marked as a negative control containing only MHB. Next, the plates were incubated at 37°C for 18–24 h. Similarly, we repeat the above mentioned process for HSA bound to GML (HSA+GML).19 After incubation, a freshly prepared MTT reagent in sterile water at 0.5 mg/mL stock solution of volume 40 μL was added to each well and incubated for 30 min. Plates were measured at an absorbance of 600 nm in a microplate reader (SpectraMax plus 384). The experiment was done in triplicate.19,20 The IC50 has been calculated using the Quest Graph™ IC50 Calculator, which is based on the four-parameter logistic regression model; MLA“Quest Graph™ IC50 Calculator” (AAT Bioquest, Inc., https://www.aatbio.com/tools/ic50-calculator.21
Time-Kill Assay
The time-kill kinetics assay was analyzed using the MIC values evaluated in the above assay. The test bacterial strain in the late logarithmic growth phase was diluted 1.5 × 108 cfu/mL. One hundred microliters of the antibiotics, GML, HSA, and HSA bound to GML samples containing the highest MIC at 1xMIC, 2xMIC, and 4xMIC concentration was put into each well. These wells have 100 μL of NB and 20 μL of each diluted bacterial cell suspension. Incubation was done at 37°C. Then, 10 µL was aspirated at different time intervals (0, 2, 4, 8, 16, and 24 hours) to identify the effects of antibiotics, GML, and HSA bound to GML of different concentrations on the bacterial population. The colonies were counted and compared with the control regarding cfu/mL. After incubation, colonies were counted and recorded on an Excel sheet to plot the Time-Kill curve. Bactericidal activity is defined as a decrease in colony-forming units (surviving bacteria) greater than three log10-fold, equivalent to 99.9% death of the inoculum.19
Statistical Analysis
Statistical analysis has been performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). The paired t-test was performed to determine the difference in the antibacterial pattern of GML alone and HSA bound to GML on the selected bacterial strains.
Ethical Consideration
The study was designed and conducted in accordance with the Helsinki Declaration and approved by the Institutional Review Board of the College of Medicine (Ref. No. 19/0473/RB). The data was anonymized properly before it was accessed.
Results
Minimum Inhibitory Concentration of Vancomycin and Gentamicin for Clinical Isolates
The MIC of Vancomycin and Gentamicin against clinical bacterial isolates (MRSA, E. faecalis, E. coli, K. pneumoniae, and E. cloacae), S. aureus ATCC 29213 and P. aeruginosa ATCC 27813 were determined. The antibiotic sensitivity pattern has been assessed as sensitive, moderate, or resistant based on CLSI recommendations. S. aureus ATCC 29213 had a MIC of 2 μg/mL for Vancomycin, while P. aeruginosa ATCC 27853 had a MIC of 0.5 μg/mL. Three strains of MRSA had MICs of 4 μg/mL, indicating a moderate sensitivity to vancomycin, in contrast to E. faecalis, which was found to be resistant and had MICs of 64 μg/Whereaseras the MIC of Gentamicin was determined to be 2 μg/mL for E. coli, 64 μg/mL for K. pneumoniae, and E. cloacae found resistant to Gentamicin (Table 2).
 |
Table 2 The Minimum Inhibitory Concentration of Vancomycin and Gentamicin for Clinical Isolates |
Minimum Inhibitory Concentration of GML Alone and HSA Bound to GML (HSA+GML)
The MIC of GML against S. aureus ATCC 29213 and P. aeruginosa ATCC 27853 was found to be 25 μg/mL and 50 μg/mL, respectively. The MIC for MRSA strains was found to be between 12.5 and 25 μg/mL, which was similar to the MIC for E. faecalis samples at 12.5 μg/mL and for strains of K. pneumoniae, E. cloacae, and E. coli showed MIC at 100 μg/mL. Exceptionally, the E. coli (KPC-19-03) isolate showed MIC at 25 μg/mL.
The minimum inhibitory concentration for HSA bound to GML (HSA+GML) showed less difference than GML alone. The standard bacterial strains S. aureus ATCC 29213 and P. aeruginosa ATCC 27853 showed similar MIC of 50 μg/mL. The MIC for clinical isolates of MRSA varied between 12.5 and 100 μg/mL, whereas E. faecalis showed the highest concentration, 100 μg/mL, compared with GML alone. Moreover, E. coli strains showed the highest concentration of MIC values, 50 to 200 μg/mL. In contrast, gentamicin-resistant strains K. pneumoniae and E. cloacae were inhibited at higher concentrations of 200 μg/mL and 400 μg/mL, respectively (Table 3). Based on the statistical analysis, the effects of GML alone and HSA bound to GML were found to be significant with a p-value of 0.021, which means the HSA bound to GML showed a highly significant difference in antibacterial activity in comparison with GML alone among all the bacterial isolates.
 |
Table 3 Minimum Inhibitory Concentration of GML and HSA Bound to GML for Clinical Isolates |
IC50 Values of GML Alone and HSA Bound to GML (HSA+GML)
E. faecalis (VRE-19-01) and E. coli (KPC-19-03) showed the lowest IC50 value, 3.82 and 8.59 (µg/mL) with GML, whereas MRSA (19–86) exhibited IC50 value of 11.91 (µg/mL) with HSA bound to GML (Table 4). E. coli (KPC-19-02) exhibited the highest IC50 value, 215.07 (µg/mL) with GML, whereas E. faecalis (VRE-19-01) showed the highest IC50 value, 195.72 (µg/mL) with HSA bound to GML (Table 4). The statistical analysis results showed a significant p-value of 0.026, which means the effects of GML alone and HSA bound to GML were significantly different in the antimicrobial effects on all the bacterial isolates.
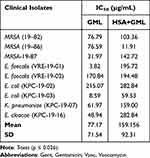 |
Table 4 IC50 Values of GML and HSA Bound to GML for Clinical Isolates |
Time-Kill Assay
MRSA (MRSA-19-82)
The isolate was intermediate susceptible to vancomycin with a MIC of 4 μg/mL. Using different concentrations equal to 1xMIC, 2xMIC, and 4xMIC (4 μg/mL, 8 μg/mL and 16 μg/mL) showed a bactericidal effect, a decline in the number of colonies over time until there were none (Figure 1A). GML had a similar impact on MRSA and exhibited a bactericidal effect, reducing the initial log CFU/mL by more than three logs over time (Figure 1B). Furthermore, HSA bound to GML (HSA+GML) had similar effects (Figure 1C). Importantly, HSA alone had no antibacterial effect against MRSA (Figure 1D).
E. faecalis (VRE-19-02)
The isolate showed resistance towards vancomycin with different concentrations (1xMIC, 0.25xMIC, and 0.5xMIC) as the log CFU/mL remained unchanged across time (Figure 2A). However, GML exhibited a bactericidal effect, reducing the starting log CFU/mL over time by greater than 3 logs (Figure 2B). HSA+GML had a similar effect to GML but was quicker than GML alone (Figure 2C). HSA had no antibacterial effect (1x, 2x, and 4xMIC) (Figure 2D).
E. coli (KPC-19-02)
The isolate was killed by Gentamicin with a MIC of 2 μg/mL. The number of colonies decreased sharply after two h of incubation and showed no growth after four h with different concentrations (4xMIC, 2xMIC, and 1xMIC) (Figure 3A). However, GML killing activity varied depending on the concentration. GML (1xMIC) displayed a bacteriostatic effect, as the log CFU/mL over time remained roughly the same as the starting point. Notably, GML (2xMIC and 4xMIC) showed a bactericidal effect with the initial log CFU/mL reduction by more than three logs over time (Figure 3B). The bactericidal effect of HSA+GML was remarkable; after 2 hours of incubation with different concentrations (4xMIC, 2xMIC, and 1xMIC), HSA bound to GML was able to suppress bacterial growth to zero colonies (Figure 3C). HSA alone had no antibacterial effect against E. coli (Figure 3D).
K. pneumoniae (KPC-19-07)
The isolate was highly resistant to Gentamicin with an MIC of 64 μg/mL (Figure 4A). In comparison, GML at concentrations of 1xMIC and 2xMIC displayed bacteriostatic activity, as the log CFU/mL declined and remained relatively constant across time. However, the higher concentration of GML (4xMIC) showed a bactericidal effect with a significant reduction of the initial log CFU/mL by more than three logs over time (Figure 4B). Comparison to HSA alone had no antibacterial effect against K. pneumoniae at different concentrations (Figure 4D). Remarkably, HSA bound to GML (1xMIC, 2xMIC, and 4xMIC) was found to be bactericidal with a significant reduction of the starting log CFU/mL over time by greater than 3 logs (Figure 4C).
Discussion
Since the last few decades, an ever-increasing number of research has been conducted on antibiotic resistance, and many organizations and nations have made it a major priority in their agendas.22 Against bacteria that are resistant to many drugs, natural substances are effective in several scientific investigations as antibacterial agents.23,24 A recent Australian study examined the antibacterial action of twenty natural compounds and showed that vancomycin and these compounds had a synergistic effect.25
To address the problem of antibiotic resistance in Gram-positive and Gram-negative bacterial strains, which is causing severe nosocomial infections in immunocompromised patients, our study has evaluated the efficacy of HSA linked to GML against clinically isolated Gram-negative and Gram-positive bacterial isolates. HSA is the most abundant protein in the blood and can bind a variety of endogenous chemical compounds. In contrast, GML is a natural chemical product that has been deemed safe, is used in foods and cosmetics, and has antibacterial activity against Gram-positive bacterial strains.
GML was found to inhibit S. aureus ATCC 29213 with a MIC of 25 µg/mL, while MRSA strains were also found to be inhibited by GML with a MIC of 12.5 µg/mL, but HSA+GML was shown to be equally effective with a MIC of 12.5 µg/mL. The antibacterial effects of GML alone and HSA bound to GML showed high significance with p ≤ 0.021 when paired t-test was applied. In contrast to other studies, GML and MIC values for different Gram-positive bacterial strains such as Clostridium perfringens, Streptococcus pneumoniae, and Streptococcus suis were observed to be 300 µg/mL, ten µg/mL, and 400 µg/mL, respectively,26 whereas, clinical isolate of Staphylococcus epidermidis required a higher concentration of GML, with MIC and MBC values of 1000 µg/mL,27 In literature, GML found to inhibit the production of exotoxins in Gram-positive bacteria such as S. aureus, by inhibiting transcription of S. aureus msrA1 genes.28 Our findings indicate that vancomycin, GML alone, and HSA+GML at 1xMIC, 2xMIC, and 4xMIC were found to exhibit bactericidal effect, reducing the initial log CFU/mL of MRSA (MRSA-19-82) isolate by more than three logs over time. Correspondingly, Singh et al 2009 reported that vancomycin at a dose equal to 2xMIC suppressed MRSA after 4 and 8 hours.
Additionally, GML was found to be a potent inhibitor of E. faecalis (VRE-19-01) in the planktonic form (MIC 12.5 µg/mL), which was isolated from body fluids of an immunocompromised patient diagnosed with recto-sigmoid cancer metastasis to lung and liver. Moreover, the Time-kill assay of E. faecalis (VRE-19-02) showed resistance towards vancomycin with different concentrations. However, GML alone was found to be bactericidal, while notably, HSA bound to GML showed an earlier killing effect. E. faecalis (VRE-19-02) was isolated from a urine sample of a patient diagnosed with breast cancer. As reported in the previous findings, the immunocompromised patients suffering from cancer would be given prophylactic antibiotic treatments, which would create an optimal condition for the opportunistic Enterococci strains to grow and Enterococci strains are adapted to survival on abiotic and biotic surfaces,29 and also, in comparisons to earlier results, indicated efficacy of GML against the biofilm form of E. faecalis.30
The MIC of GML for P. aeruginosa (ATCC 27853) was 50 μg/mL, while the MICs of GML for clinical isolates E. coli (KPC-19-02) and E. coli (KPC-19-03) were 100 μg/mL and 25 μg/mL, respectively. Furthermore, K. pneumoniae (KPC-19-07) and E. cloacae (KPC-19-16) were found to be inhibited at MIC100 μg/mL concentration with GML. In contrast to Wang et al's findings, that GML had no antibacterial effect against E. coli and P. aeruginosa with MICs greater than 10 mg/mL,31 however, GML in gel form was found to be effective and had a bactericidal effect against A. baumannii, E. coli, and P. aeruginosa with MICs greater than 10 mg/mL.10,32 Importantly, our findings showed successful results with lower MIC values than previous research findings. In the Time-Kill assay results, E. coli (KPC-19-02), isolated from a cholangiocarcinoma patient, showed bactericidal activity with Gentamicin, GML alone, and HSA+GML reducing the starting log CFU/mL over time by greater than 3 logs. Regarding K. pneumoniae (KPC-19-07), it showed resistance to Gentamicin, but GML exhibited bacteriostatic activity with this isolate at 1xMIC and 2xMIC and bactericidal effect at 4xMIC. Remarkably, HSA bound to GML showed a significant bactericidal effect with this Gram-negative isolate, which had been isolated from diabetic patients. The unexpected findings of GML alone and HSA bound to GML against Gram-negative isolates, which may impact their ability to resist, may be attributed to the bacterial fitness that colonizes immunocompromised and chronically ill individuals. It is worth noting that the antibacterial activity of HSA bound to GML may be attributed to HSA’s composition as a single polypeptide chain of 585 amino acid residues with multiple metal-binding sites, which is known to play a key role in drug delivery.33 Thus, the binding of GML to HSA could enhance its stability and bioavailability, thereby amplifying its antimicrobial properties. Further investigation is needed to clarify the nature of this interaction and to assess whether the effectiveness of the HSA-GML complex is primarily due to the improved delivery and stability of GML facilitated by HSA.
A key strength of this study is the exploration of HSA bound to GML as a novel therapeutic approach against antibiotic-resistant bacteria. It demonstrates broad-spectrum antibacterial activity against both Gram-positive and Gram-negative clinical isolates, including multidrug-resistant pathogens such as MRSA, VRE, and carbapenem-resistant Enterobacterales (CRE). The bactericidal effects of HSA+GML were confirmed through Time-Kill assays. Additionally, a comparative analysis with standard antibiotics like vancomycin and gentamicin revealed a relatively stronger efficacy of HSA+GML, with lower MIC values for GML against certain strains. This study emphasizes its relevance for treating hospital-acquired infections by focusing on clinical isolates from immunocompromised patients.
Conversely, a primary limitation of this study is the small number of clinical isolates, which may not represent the full diversity of resistant bacterial strains. A larger sample size from various healthcare settings would improve the applicability of the findings. Moreover, including a wider variety of significant multidrug-resistant isolates like Acinetobacter baumannii and Pseudomonas aeruginosa and comparing the results with other last-line antibiotics, such as daptomycin, linezolid, or colistin, would strengthen the conclusions.
Conclusion
Our findings showed that GML alone and HSA+GML had bactericidal efficacy against Gram-negative and Gram-positive isolates. GML alone, on the other hand, was found to be concentration-dependent on Gram-negative isolates. Our results demonstrate that GML alone and the HSA+GML compound could be effectively used in therapeutic medication formulations, potentially assisting in creating a new therapeutic drug target utilizing HSA connected to GML as a protein and fatty acid antibacterial agent.
Acknowledgments
The authors thank the Researchers Supporting Project number (RSPD2025R655) King Saud University, Riyadh, Saudi Arabia, for funding this project. The authors thank Dr Ayesha Mateen and Dr Syed Rabani for supporting this project.
Funding
The Researchers Supporting Project number (RSPD2025R655) King Saud University, Riyadh, Saudi Arabia.
Disclosure
All authors declare that they have no competing interests.
References
1. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T Peer-Rev J Formul Manag. 2015;40:277–283.
2. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi:10.1128/MMBR.00016-10
3.. Almeleebia TM, Alhifany AA, Almutairi F, Alshibani M, Alhossan AM. Regulating antimicrobial sales in Saudi Arabia: achievements and challenges. Int J Clin Pract. 2021;75. doi:10.1111/ijcp.13833.
4. Alrasheedy AA, Alsalloum MA, Almuqbil FA, et al. The impact of law enforcement on dispensing antibiotics without prescription: a multi-methods study from Saudi Arabia. Expert Rev Anti Infect Ther. 2020;18:87–97. doi:10.1080/14787210.2020.1705156
5. Miethke M, Pieroni M, Weber T, et al. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem. 2021;5:726–749. doi:10.1038/s41570-021-00313-1
6. Stan D, Enciu A-M, Mateescu AL, et al. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front Pharmacol. 2021;12:723233. doi:10.3389/fphar.2021.723233
7. Dutescu IA, Hillier SA. Encouraging the development of new antibiotics: are financial incentives the right way forward? A systematic review and case study. Infect Drug Resist. 2021;14:415–434. doi:10.2147/IDR.S287792
8. Jackson N, Czaplewski L, Piddock LJV. Discovery and development of new antibacterial drugs: learning from experience? J Antimicrob Chemother. 2018;73:1452–1459. doi:10.1093/jac/dky019
9. Lin Y-C, Peterson ML. New insights into the prevention of staphylococcal infections and toxic shock syndrome. Expert Rev Clin Pharmacol. 2010;3:753–767. doi:10.1586/ecp.10.121
10. Vetter SM, Schlievert PM. Glycerol monolaurate inhibits virulence factor production in Bacillus anthracis. Antimicrob Agents Chemother. 2005;49:1302–1305. doi:10.1128/AAC.49.4.1302-1305.2005
11. Arzumanyan VG, Ozhovan IM, Svitich OA. Antimicrobial effect of albumin on bacteria and yeast cells. Bull Exp Biol Med. 2019;167:763–766. doi:10.1007/s10517-019-04618-6
12. Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. Structural basis of the drug-binding specificity of human serum albumin. J mol Biol. 2005;353:38–52. doi:10.1016/j.jmb.2005.07.075
13. Zhang MS, Houtman JCD. Human serum albumin (HSA) suppresses the effects of glycerol monolaurate (GML) on human T cell activation and function. PLoS One. 2016;11:e0165083. doi:10.1371/journal.pone.0165083
14. James TJ, Hughes MA, Cherry GW, Taylor RP. Simple biochemical markers to assess chronic wounds. Wound Repair Regen. 2000;8:264–269. doi:10.1046/j.1524-475x.2000.00264.x
15. Li L, Hitchcock AP, Cornelius R, Brash JL, Scholl A, Doran A. X-Ray microscopy studies of protein adsorption on a phase segregated polystyrene/polymethylmethacrylate surface. 2. effect of PH on site preference. J Phys Chem B. 2008;112:2150–2158. doi:10.1021/jp076583h
16. Evans TW. Review article: albumin as a drug-biological effects of albumin unrelated to oncotic pressure: REVIEW: ALBUMIN AS A DRUG. Aliment Pharmacol Ther. 2002;16:6–11. doi:10.1046/j.1365-2036.16.s5.2.x
17. Cockerill F. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard.
18. Schlievert PM, Kilgore SH, Seo KS, Leung DYM. Glycerol monolaurate contributes to the antimicrobial and anti-inflammatory activity of human milk. Sci Rep. 2019;9:14550. doi:10.1038/s41598-019-51130-y
19. Barry AL. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline. Wayne, PA: National Committee for Clinical Laboratory Standards; 1999. ISBN 978-1-56238-384-8.
20. Zarai Z, Kadri A, Ben Chobba I, et al. The In-vitro evaluation of antibacterial, antifungal and cytotoxic properties of marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011;10:161. doi:10.1186/1476-511X-10-161
21. IC50 Calculator | AAT Bioquest. Available from: https://www.aatbio.com/tools/ic50-calculator. (
22. Liu X, Wu X, Tang J, Zhang L, Jia X. Trends and development in the antibiotic-resistance of Acinetobacter baumannii: a scientometric research study (1991–2019). Infect Drug Resist. 2020;13:3195–3208. doi:10.2147/IDR.S264391
23. Zhou Y-X, Cao X-Y, Peng C. Antimicrobial activity of natural products against MDR bacteria: a scientometric visualization analysis. Front Pharmacol. 2022;13:1000974. doi:10.3389/fphar.2022.1000974
24. Porras G, Chassagne F, Lyles JT, et al. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem Rev. 2021;121:3495–3560. doi:10.1021/acs.chemrev.0c00922
25. Roshan N, Riley TV, Hammer KA. Antimicrobial activity of natural products against Clostridium difficile in vitro. J Appl Microbiol. 2017;123:92–103. doi:10.1111/jam.13486
26. Kovanda L, Zhang W, Wei X, et al. In vitro antimicrobial activities of organic acids and their derivatives on several species of gram-negative and gram-positive bacteria. Molecules. 2019;24(3770):3770. doi:10.3390/molecules24203770
27. Krislee A, Fadly C, Nugrahaningsih DAA. The 1-monolaurin inhibit growth and eradicate the biofilm formed by clinical isolates of staphylococcus epidermidis. BMC Proc. 2019;13:19. doi:10.1186/s12919-019-0174-9
28. Pechous R, Ledala N, Wilkinson BJ, Jayaswal RK. Regulation of the expression of cell wall stress stimulon member gene MsrA1 in methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:3057–3063. doi:10.1128/AAC.48.8.3057-3063.2004
29. Patel R, Gallagher JC. Vancomycin-resistant enterococcal bacteremia pharmacotherapy. Ann Pharmacother. 2015;49:69–85. doi:10.1177/1060028014556879
30. Hess DJ, Henry-Stanley MJ, Wells CL. The natural surfactant glycerol monolaurate significantly reduces development of Staphylococcus aureus and Enterococcus faecalis biofilms. Surg Infect. 2015;16:538–542. doi:10.1089/sur.2014.162
31. Wang W, Wang R, Zhang G, Chen F, Xu B. In vitro antibacterial activities and mechanisms of action of fatty acid monoglycerides against four foodborne bacteria. J Food Prot. 2020;83:331–337. doi:10.4315/0362-028X.JFP-19-259
32. Mueller EA, Schlievert PM. Non-aqueous glycerol monolaurate gel exhibits antibacterial and anti-biofilm activity against gram-positive and gram-negative pathogens. PLoS One. 2015;10:e0120280. doi:10.1371/journal.pone.0120280
33. Tao H-Y, Wang R-Q, Sheng W-J, Zhen Y-S. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. Int J Biol Macromol. 2021;187:24–34. doi:10.1016/j.ijbiomac.2021.07.080
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





