Back to Journals » International Journal of Nanomedicine » Volume 19
Unraveling the Connection: Extracellular Vesicles and Non-Small Cell Lung Cancer
Received 11 May 2024
Accepted for publication 4 August 2024
Published 9 August 2024 Volume 2024:19 Pages 8139—8157
DOI https://doi.org/10.2147/IJN.S477851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Jiankang Wu,1– 4 Yan Chen1– 4
1Department of Pulmonary and Critical Care Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2Research Unit of Respiratory Disease, Central South University, Changsha, Hunan, People’s Republic of China; 3Clinical Medical Research Center for Pulmonary and Critical Care Medicine, Changsha, Hunan, People’s Republic of China; 4Diagnosis and Treatment Center of Respiratory Disease, Central South University, Changsha, Hunan, People’s Republic of China
Correspondence: Yan Chen, Email [email protected]
Abstract: Extracellular vesicles (EVs) are nanoscale lipid bilayer vesicles released during cell activation, cellular damage, or apoptosis. They carry nucleic acids, proteins, and lipids facilitating intercellular communication and activate signaling pathways in target cells. In non-small cell lung cancer (NSCLC), EVs may contribute to tumor growth and metastasis by modulating immune responses, facilitating epithelial-mesenchymal transition, and promoting angiogenesis, while potentially contributing to resistance to chemotherapy drugs. EVs in liquid biopsies serve as non-invasive biomarkers for early cancer detection and diagnosis. Due to their small size, inherent molecular transport properties, and excellent biocompatibility, EVs also act as natural drug delivery vehicles in NSCLC therapy.
Keywords: extracellular vesicles, non-small cell lung cancer, immunity, metastasis, drug, biomarkers
Graphical Abstract:

Introduction
With increasing population and global aging, cancer has become a leading cause of premature death and reduced life expectancy in many countries, with lung cancer as one of the primary contributors to cancer-related mortality worldwide. According to 2020 statistics from the World Health Organization, there were approximately 2.2 million new cases of lung cancer worldwide, resulting in about 1.8 million deaths, accounting for 11.4% and 18.0% of the total cancer cases and cancer-related deaths, respectively.1 Lung cancer incidence and mortality rates vary among countries, influenced by factors such as population demographics and economic development. Globally, China has the highest number of new lung cancer cases and mortality rates, accounting for 37.0% of total cases and 39.8% of deaths, followed by the United States and Japan.2 Smoking, both active and passive, remains a major risk factor for lung cancer.3 The lack of specific clinical symptoms in early-stage lung cancer makes early diagnosis challenging, causing most patients being diagnosed at advanced stages or with metastases, resulting in a 5-year survival rate of less than 5% for advanced lung cancer patients.4,5 Non-small cell lung cancer (NSCLC), accounting for approximately 85% of all lung cancer histological types, includes three subtypes: adenocarcinoma (including bronchioloalveolar carcinoma), squamous cell carcinoma, and large cell carcinoma.6,7 Despite pathological differences among these subtypes, treatment approaches are generally similar. The treatment of NSCLC is typically determined by tumor staging and histological type and may include a combination of surgical resection, radiotherapy, chemotherapy, targeted therapy, and immunotherapy.8–10 Therefore, alleviating the disease burden caused by NSCLC, understanding its pathogenesis in depth, and exploring diagnostic and therapeutic modalities applicable to clinical practice have become urgent priorities in the medical community.
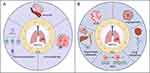
Communication between newly emerged tumor precursor or malignant cells and other cells within the tumor, as well as with local tissues and host cells throughout the body, facilitates cancer initiation and progression. Intercellular communication can induce microenvironmental changes, thereby influencing tumor growth and the dissemination of cancer cells. This signaling can occur through the secretion of soluble factors or the exchange of extracellular vesicles (EVs). EVs, which are nanoscale lipid bilayer vesicles released by nearly all cells upon activation, injury, or apoptosis, have increasingly been recognized as key participants in the tumor microenvironment in recent years.11–13 At the pan-cancer level, EVs play several critical roles in cancer biology. They are involved in tumor progression, metastasis, and immune regulation, and they serve as biomarkers and therapeutic delivery systems, reflecting their common mechanisms across various types of cancer. Various cargo molecules carried by EVs are believed to contribute to metastasis and organotropism.14 EVs are abundant in various human biological fluids, including blood and urine to cerebrospinal fluid and semen.14–17 Protected by a lipid bilayer membrane structure, EVs carry a variety of bioactive molecules on their membranes and within their vesicles, including proteins, nucleic acids, and lipids.18 Depending on their biogenesis pathways, EVs are generally classified into three major types: exosomes, microvesicles, and apoptotic bodies, as shown in Figure 1A. Exosomes are derived from endosomes and form when multivesicular bodies (MVBs) fuse with the plasma membrane, releasing intraluminal vesicles into the extracellular space.19 In contrast, microvesicles are usually larger and bud directly from the plasma membrane. These vesicles, also known as shedding vesicles or ectosomes, form when molecular cargo is transported to the cell surface. The release of microvesicles is often triggered by an increase in intracellular calcium levels, activating the plasma membrane.20 Microvesicles, like exosomes, possess unique lipid compositions and are enriched in phosphatidylserine.21 Many protein markers are expressed on exosomes and microvesicles, including tetraspanins (eg, CD9, CD63, and CD81), membrane transport proteins, fusion proteins (eg, annexins), EV synthesis proteins (eg, TSG101), and other EV-related proteins.22 Apoptotic bodies are primarily secreted by cells undergoing apoptosis. Generally larger than exosomes and microvesicles, they contain DNA fragments and organelles from apoptotic cells and form during plasma membrane budding processes.23 In our review, we summarize the key roles of EVs in the occurrence, progression, and metastasis of NSCLC, as well as their impact on treatment responses, their potential as biomarkers, and their role in the development of cancer therapies.
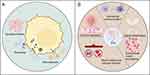 |
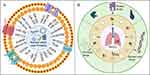
Figure 1 (A) Biogenesis of the three main groups of EVs. (B) The principal physiological functions of EVs. Created with BioRender.com. |
Physiological Functions of EVs
EVs play several important roles in physiology, including intercellular communication, signal transduction, immune regulation, blood clotting, vascular function, and extracellular matrix remodeling, as shown in Figure 1B. In terms of intercellular communication, EVs facilitate this process by transporting essential biomolecules such as proteins and nucleic acids, contributing to the maintenance of cellular homeostasis and coordination of functions both locally and distantly. This information transfer is not confined to neighboring cells but can extend across tissue and organ boundaries, exerting a broader regulatory influence.24 Regarding signal transduction, EVs transport biomolecules, including cytokines, growth factors, and nucleic acids, which influence the signaling pathways of recipient cells, thereby regulating their physiological functions.25,26 EVs in the bloodstream also play roles in blood clotting and vascular function by influencing thrombus formation, vascular permeability, and angiogenesis. They also carry antigens and immune-regulatory factors that influence immune cell function and balance. Additionally, EVs modulate extracellular matrix remodeling, affecting tissue growth, development, and tumor invasion.27–30
The Functional Roles and Mechanisms of EVs in NSCLC
EVs and Immunity
A substantial body of research has underscored the increasing focus on EVs in cancer research, particularly within the tumor microenvironment. EVs play a pivotal role in regulating tumor cell behavior and influencing the tumor microenvironment.31,32 They transmit information to cancer, immune, and stromal cells by carrying molecules that can impact cancer growth, metastasis, and immune responses. EVs assist cancer cells in evading immune surveillance by carrying immunosuppressive factors such as PD-L1 and TGF-β, and can also promote immune tolerance, thereby aiding cancer cell survival.33 However, EVs can also carry tumor-associated antigens that may trigger immune responses. Despite this, the presence of immunosuppressive factors frequently suppresses these responses, thereby promoting tumor growth and evasion.34 Additionally, EVs from immune cells can exert both inhibitory and stimulatory effects, carrying immune-stimulating molecules such as interferon and tumor necrosis factor, which promote immune activation and cancer cell apoptosis.35 Thus, EVs play a dual role in cancer immunity, influencing both tumor progression and immune responses, as shown in Figure 2A.
EVs-Mediated NSCLC Immunosuppression
Numerous studies have demonstrated that tumor-derived EVs play a significant role in immune suppression.36,37 Ligands on the surface of EVs can bind to homologous receptors on immune cells, transmitting inhibitory signals, which promote tumor progression. Specifically, EVs transfer immunosuppressive molecules to immune cells via direct contact or paracrine signaling, resulting in their functional inhibition.38 Extracellular vesicle PD-L1 plays a crucial role in mediating immune evasion by cancer cells. When EVs carrying PD-L1 enter the lymphatic system, they can inhibit T cell activity, thereby preventing immune cells from recognizing and killing tumor cells.39,40 Additionally, EVs can further promote tumor progression and metastasis by inhibiting T cell activation and proliferation, inducing regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs), and suppressing natural killer cells and CD8+ T cells.37,41 A study demonstrated that EVs from NSCLC cells expressing PD-L1 contribute to immune evasion by reducing T cell activity and promoting tumor growth.42 Furthermore, EVs isolated from lung cancer cells contain abundant epidermal growth factor receptor (EGFR), which can induce tolerogenic dendritic cells, thereby suppressing the function of tumor antigen-specific CD8+ T cells.43 Additionally, EVs derived from NSCLC can also promote CD8+ T cell dysfunction and immune suppression by secreting substances such as circUSP7 (Circular Ubiquitin-Specific Peptidase 7) and S100A4 (S100 Calcium-Binding Protein A4), exacerbating tumor development.44,45 Thus, these research findings collectively indicate that EVs from NSCLC evade immune surveillance through various mechanisms, promoting tumor cell survival and offering important therapeutic targets for the development of cancer immunotherapy strategies.
EVs and NSCLC Immunotherapy
The primary goal of tumor immunotherapy is to activate the host immune system to recognize and overcome the immune suppression induced by cancer cells. EVs are regarded as potential immunotherapeutic agents, widely used in immunotherapy, antigen delivery, and gene transfer systems.46 Dendritic cells (DCs) are considered important sources of EVs. Both immature and mature dendritic cells can produce EVs. EVs produced by mature DCs contain higher levels of Major Histocompatibility Complex (MHC) I, MHC II, and co-stimulatory molecules, thus exhibiting stronger immune-stimulatory effects. EVs derived from DCs carry MHC/peptide complexes on their surface, facilitating the proliferation and activation of NK cells dependent on Interleukin-15 Receptor Alpha (IL-15Ra) and Natural Killer Group 2 Member D (NKG2D), while also enhancing the anti-tumor activity of T cells.47,48 In the treatment of NSCLC, the feasibility and potential efficacy of using DC vaccines carrying the melanoma antigen gene MAGE (Melanoma Antigen Gene) have been demonstrated.49 Studies have shown that DCs can enhance the anti-tumor immune capability of NK cells in patients with late-stage NSCLC.50 Furthermore, compared to EVs from other sources, those derived from NK cells exhibit higher stability, greater potential for modification, and lower immunogenicity.51 Chimeric antigen receptor T (CAR-T) cells are genetically engineered T cells designed to recognize and attack cancer cells expressing specific antigens on their surface, thereby enhancing the immune system’s ability to target cancer cells. EVs derived from CAR-T cells represent an alternative approach to tumor immunotherapy. EVs secreted by antigen-presenting cells (APCs) can stimulate T cell proliferation in vitro and induce anti-tumor immune responses in vivo.52 A study demonstrated that EVs derived from NSCLC containing CD39 can effectively reduce ATP levels in targeted T cells, inducing the activation of AMP-Activated Protein Kinase (AMPK) and the inactivation of the Mechanistic Target of Rapamycin (mTOR). Therefore, targeting tumor CD39 may correct the aberrant differentiation of CD4+ T cells in human NSCLC.53 In conclusion, EVs, as a potential immunotherapeutic agents, have broad prospects in NSCLC immunotherapy. They can stimulate host immune system responses and promote the immune recognition and clearance of NSCLC cells, thereby offering new strategies and possibilities for NSCLC immunotherapy.
EVs and NSCLC Metastasis
Tumor metastasis is a major cause of cancer-related deaths. Over the years, researchers have focused on unraveling the mechanisms of tumor metastasis, emphasizing the interactions between tumors and host biology. Recent studies show that EVs play a crucial role in tumor metastasis by transmitting specific information between cells. Additionally, EVs may regulate epithelial-mesenchymal transition (EMT) and remodel the extracellular matrix (ECM).54,55 In NSCLC, the role of EVs is especially prominent. By carrying tumor-related signaling molecules such as miRNA, proteins, and DNA fragments, EVs promote the growth, migration, and invasion of tumor cells. Moreover, EVs can modulate the tumor microenvironment by promoting angiogenesis and suppressing immune responses, thereby facilitating the establishment of pre-metastatic niches. In NSCLC, the role of EVs in regulating tumor angiogenesis and immune evasion has garnered particular attention. Furthermore, EVs play a key role in determining organ metastasis. By carrying organ metastasis-related molecules such as miRNA and transcription factors, EVs regulate the microenvironment of target organs, providing favorable conditions for tumor cell migration and settlement, as shown in Figure 2B.56–58
Regulation of EMT
EMT is a biological process in which epithelial cells acquire mesenchymal characteristics. During this transition, cells lose epithelial traits, such as cell polarity and cell-cell junctions, while gaining mesenchymal traits, including enhanced migratory and invasive capabilities. EMT is crucial for various physiological and pathological processes, such as embryonic development, tissue repair, and tumor metastasis.59,60 In this process, tumor cells are influenced by cancer-associated fibroblasts (CAFs) in the tumor microenvironment, leading to the acquisition of mesenchymal properties and the loss of cell polarity and cell-cell connections, ultimately enhancing their migratory and invasive potential.61,62 Research has demonstrated the significant role of EVs in EMT in NSCLC, particularly involving oncogenic miRNAs. For instance, miRNAs like miR-23a, miR-193a-3p, miR-210-3p, and miR-5100 are implicated in regulating cell migration.63,64 Additionally, EVs in the serum of patients with EGFR-mutant NSCLC can stimulate cell invasion by promoting a mixed EMT.65 Moreover, the intracellular expression of FAM3C (Family with Sequence Similarity 3 Member C) in NSCLC cells promotes cancer cell growth and invasion, with EVs containing FAM3C inducing invasive phenotypes in recipient cells.66 Studies have shown that EV-derived miRNAs transferred by tumor cells can affect sensitivity to EGFR-TKI (Tyrosine Kinase Inhibitor) and serve as prognostic biomarkers in EGFR-mutant NSCLC.67 Conversely, miRNAs like miR-4739 and miR-224-5p activate the Wnt/β-catenin signaling pathway, promoting EMT and angiogenesis in NSCLC, thereby contributing to drug resistance and metastasis regulation.68–70 Additionally, EVs secreted by M2-type tumor-associated macrophages (TAMs) are enriched with miR-155 and miR-196a-5p, enhancing cell viability, migration, invasion, and EMT in NSCLC.71 c-Src (cellular-src tyrosine kinase), a non-receptor tyrosine kinase, is linked to EMT promotion. Recent studies show that EVs with high c-Src expression in metastatic NSCLC cells elevate c-Src levels in primary NSCLC cells, promoting EMT through the TGF-β1 pathway.72 In summary, EVs play a pivotal role in modulating the EMT process by influencing the expression of miRNAs and other molecules, impacting tumor cell invasion, metastasis, and drug sensitivity, and offering novel avenues for research and potential therapeutic interventions in NSCLC.
Regulation of NSCLC Angiogenesis
Angiogenesis within tumor environments is a multifaceted and crucial process governed by factors such as cytokines, cellular signaling cascades, and microenvironmental cues. Hypoxic conditions in tumor tissues, resulting from rapid proliferation and metabolic demands, prompt tumor cells to release pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). These factors stimulate the proliferation, migration, and formation of new blood vessels by surrounding vascular endothelial cells, ensuring a sustained supply of nutrients and oxygen to the tumor and facilitating cancer cell metastasis.73,74 In NSCLC, the hypoxic microenvironment triggers the release of EVs by tumor cells. EVs serve as crucial messengers, mediating communication between tumor cells and endothelial cells. These vesicles contain a myriad of molecular signals, including cytokines, growth factors, and miRNAs, which, upon transfer between cells, activate endothelial cell proliferation and migration, thereby fostering the formation of new blood vessels.75 Notably, EVs released by NSCLC harbor specific molecules such as CCL18 (Chemokine Ligand 18) and MFI2-AS1 (MFI2 Antisense RNA 1), which bolster angiogenesis by modulating signaling pathways, including the CCL18-mediated pathway and the miR-107/NFAT5/AKT axis.76,77 Additionally, molecules such as angiopoietin-like 4 (ANGPTL4) EV-derived protein and miR-1260b contribute to promoting angiogenesis through distinct mechanisms.78,79 Furthermore, certain miRNAs, such as miR-619-5p, exert regulatory control over target genes like RCAN1.4 (Regulator of Calcineurin 1, Isoform 4), influencing the progression of angiogenesis.80 Investigations have unveiled the involvement of other molecules abundant in NSCLC-derived EVs, such as SYT7 (Synaptotagmin VII) and miR-3157-3p, in facilitating endothelial cell migration, proliferation, and tube formation, while also modulating gene expression to augment vascular permeability.81,82 Moreover, LRG1 (Leucine-Rich Alpha-2-Glycoprotein 1) enriched in EVs derived from NSCLC tissues and cells is implicated in activating the TGF-β pathway to orchestrate angiogenesis, underscoring its potential as a therapeutic target for NSCLC.83 These cumulative findings provide invaluable insights into the mechanisms underpinning NSCLC angiogenesis, paving the way for the identification of novel therapeutic targets.
Regulation of ECM Remodeling
The ECM consists of diverse components, including collagen, fibronectin, glycosaminoglycans, and proteoglycans. These constituents intricately influence the phenotype and function of both tumor cells and surrounding stromal cells in the tumor microenvironment. Serving as both a structural scaffold and a regulatory milieu, the ECM profoundly affects tumor cell behavior. Alterations in ECM composition and structure play crucial roles in tumor growth, invasion, and metastasis.84,85 EVs emerge as crucial mediators in modulating the tumor microenvironment by orchestrating ECM remodeling, ultimately fostering tumor metastasis. Of particular interest in lung adenocarcinoma is the expression pattern and functional significance of SPON2 (Spondin 2), a specific ECM protein secreted by CAFs. Remarkably, elevated SPON2 expression levels significantly correlate with the invasiveness and metastatic potential of lung adenocarcinoma. Furthermore, SPON2 enhances the pro-invasive effects of CAFs on lung adenocarcinoma cells. Recent investigations have revealed that EVs released by lung adenocarcinoma cells carry SPON2 and HOTAIRM1 (HOXA Transcript Antisense RNA, Myeloid-Specific 1). Notably, HOTAIRM1 modulates SPON2 expression by sequestering miR-328-5p, augmenting the invasive and metastatic capacities of lung adenocarcinoma.86 Moreover, in patients with NSCLC, serum miR-17 and miR-20a correlate with significant downregulation of tissue inhibitor of metalloproteinase 3 (TIMP3) expression. This downregulation may contribute to ECM remodeling within the cancer microenvironment.87 Alterations in ECM composition are closely linked with changes in the tumor microenvironment, with EVs emerging as key players in ECM remodeling. These findings provide valuable insights into the mechanistic underpinnings of ECM remodeling in the tumor microenvironment.
Regulation of Organotropic Metastasis
Tumor metastatic target organs are specific tissues or organs where cancer cells tend to migrate and establish secondary growths during metastasis. These organs typically offer a microenvironment conducive to cancer cell survival, proliferation, and dissemination. The selection of target organs for metastasis is often influenced by interactions between these organs and the primary tumor, possibly mediated by cell surface receptor-ligand interactions, activation of cell factors and signaling pathways, and unique local microenvironmental properties.88,89 Studies have elucidated the role of EVs in shaping the pre-metastatic microenvironment, thus impacting the outcome of metastasis. Proteomic analyses have revealed distinct integrin expression profiles in tumor cell-derived EVs from different organs. For instance, integrins α6β4 and α6β1 are associated with lung metastasis, while integrin αvβ5 is linked to liver metastasis. Downregulation of integrin α6β4 and αvβ5 expression can reduce EV uptake by target organ cells, thereby mitigating lung and liver metastasis, respectively.90 In NSCLC, brain and bone metastases are prevalent, posing significant challenges to patient survival. Various gene mutations are implicated in NSCLC metastasis.91–93 However, emerging evidence highlights the crucial role of the tumor microenvironment (TME) in metastasis, encompassing invasion at the primary site and colonization at distant sites.94–96 Studies show that EVs induce M2 polarization of microglia by delivering LINC00482, which regulates the miR-142-3p/TGF-β1 axis, modulating the pre-metastatic niche and promoting NSCLC brain metastasis.97 Moreover, SUMOylated hnRNPA2B1 interacts with the SIM motif in ALIX to activate ALIX, facilitating the packaging of circTLCD4-RWDD3 into EVs, thereby inducing lymphangiogenesis and lymph node metastasis in NSCLC.98 Conversely, a novel ceRNA regulatory pathway shows that lnc-MMP2-2 upregulates EPB41L5 expression by sequestering miR-1207-5p, promoting Endothelial-to-Mesenchymal Transition (EndoMT), disrupting tight junctions, increasing Blood-Brain Barrier permeability, and ultimately facilitating NSCLC brain metastasis.99 M2-exos significantly enhance the migration and invasion of NSCLC cells by delivering integrin αVβ3.100 Additionally, the transfer of lung cancer EV-miR-122-5p promotes hepatocyte migration, potentially contributing to the pre-metastatic microenvironment and liver metastasis.101 Studies have also found that NSCLC EVs containing Amphiregulin (AREG) activate the EGFR pathway in preosteoclasts, leading to increased Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) expression. RANKL can induce protease expression, a hallmark of osteoclastogenesis, initiating a vicious cycle of osteolytic metastasis.102 Another study found that NSCLC cell-derived EVs containing lncRNA-SOX2OT regulate osteoclast differentiation and stimulate bone metastasis by targeting the miRNA-194-5p/RAC1 signaling axis and the TGF-β/pTHrP/RANKL signaling pathway in osteoclasts.103 These findings underscore the pivotal role of EVs in NSCLC metastasis, providing crucial insights into metastatic mechanisms and the exploration of novel therapeutic strategies.
EVs and NSCLC Drug Resistance
NSCLC is a prevalent malignant tumor type, and chemotherapy is a common treatment option for advanced-stage patients. Cisplatin is a widely used platinum-based chemotherapy drug in NSCLC treatment that inhibits cancer cell proliferation by interfering with DNA replication and transcription. It is typically used in combination with other chemotherapy agents such as paclitaxel or gemcitabine. Gefitinib and erlotinib are EGFR tyrosine kinase inhibitors used to treat NSCLC patients with tumors that are EGFR mutation-positive. They suppress tumor cell proliferation and survival by inhibiting the activation of the EGFR signaling pathway. Osimertinib is a third-generation EGFR tyrosine kinase inhibitor that selectively targets tumor cells with EGFR mutations and is used to treat late-stage NSCLC patients with EGFR T790M mutation-positive tumors. Anlotinib is a multi-targeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumor activities, suitable for advanced NSCLC patients who have progressed after receiving at least two chemotherapy agents and targeted therapies.104,105 Tumor drug resistance is a frequent cause of treatment failure, and increasing evidence suggests that EVs can promote drug resistance through various mechanisms. EVs can transport miRNAs, lncRNAs, and proteins to target cells, facilitating signal transduction between drug-resistant cells, sensitive cells, stromal cells, and tumor cells, thereby inducing drug resistance in tumor cells.106–108
EVs from irradiated cell lines promote radioresistance in previously unexposed NSCLC cells by transferring miR-23a. Additionally, EVs derived from CAFs containing lncRNA SNHG12 enhance resistance to cisplatin in NSCLC cells by binding with RNA-binding protein HuR, thereby promoting RNA stability and XIAP (X-linked Inhibitor of Apoptosis Protein)-dependent apoptosis suppression.109 EV-mediated circVMP1 targets the miR-524-5p-METTL3/SOX2 axis to promote NSCLC progression and cisplatin resistance.110 Moreover, hypoxia-induced EVs confer cisplatin resistance to sensitive NSCLC cells by delivering PKM2.111 Furthermore, EV-derived miR-4443 facilitates cisplatin resistance in NSCLC by regulating ferroptosis mediated by FSP1 (Ferroptosis Suppressor Protein 1) m6A modification.112 EVs derived from CAFs confer cisplatin resistance to NSCLC cells by transferring miRNA-130a, with PUM2 being a critical factor in packaging miRNA-130a into EVs.113 In other chemotherapy contexts, EVs isolated from gefitinib-resistant NSCLC cell line cultures contain miR-21, which can confer resistance to gefitinib-sensitive cells by activating p-Akt.114 Similarly, EVs from erlotinib-resistant NSCLC cell lines contain lncRNA H19, which induces resistance in erlotinib-sensitive cells by targeting miR-615-3p.115 Conversely, studies have shown that wild-type EGFR protein can confer osimertinib resistance to mutant EGFR NSCLC cells via EVs, followed by activation of the PI3K/AKT and MAPK signaling pathways.102 MiR-522-3p, transmitted by EVs shed from EGFR-TKI-resistant cells with the T790M mutation, can induce gefitinib resistance in sensitive cells by activating the PI3K/AKT signaling pathway.116 EVs derived from M2 tumor-associated macrophages promote osimertinib resistance in NSCLC via the MSTRG.292666.16-miR-6836-5p-MAPK8IP3 axis.117 Additionally, miR-7 delivered by EVs can reverse gefitinib resistance in NSCLC by targeting YAP.118 Moreover, the expression levels of miR-184 and miR-3913-5p from EVs in peripheral blood of NSCLC patients serve as biomarkers for osimertinib resistance.119 In NSCLC, EVs carrying circKIF20B inhibit gefitinib resistance and cell proliferation via the circKIF20B/miR-615-3p/MEF2A axis, thereby suppressing the cell cycle, promoting apoptosis, and reducing OXPHOS.120 Lastly, EV-derived miR-136-5p from anlotinib-resistant NSCLC cells can promote NSCLC cell proliferation and anlotinib resistance by targeting PPP2R2A. Conversely, miR-136-5p antagonist derived from anlotinib-resistant NSCLC cell EVs can restore anlotinib responsiveness in NSCLC cells.121
Furthermore, studies have revealed that plasma-derived EVs from NSCLC patients who develop resistance to immunotherapies such as nivolumab and pembrolizumab exhibit elevated levels of EV-bound PD-L1.122 Patients with higher baseline level of PD-L1+ EVs in their bloodstream demonstrate significantly better responses to immunotherapy and longer survival periods. This is particularly evident in subsets of NSCLC patients with low or absent tumor PD-L1 expression, identifying PD-L1-positive circulating EVs as novel predictive and prognostic markers for immunotherapy.123 In summary, EVs play a crucial role in NSCLC by mediating the generation and dissemination of drug resistance through various mechanisms, thereby providing new insights and therapeutic strategies for combating drug resistance.
EV and NSCLC Diagnosis
Compared to traditional tumor biomarker assays, the identification of EV content offers a more comprehensive source of information, as EVs contain various biomolecules from the source cells, including miRNAs, lncRNAs, and proteins. Monitoring specific biomarkers within tumor EV content offers a convenient non-invasive diagnostic method and is expected to become a standard for monitoring disease progression and treatment efficacy.124 In NSCLC, despite efforts in various clinical trials, improved supportive care, novel drugs and targeted therapies, and modern diagnostic technologies, the prognosis remains challenging. Therefore, there is an urgent need to discover new biomarkers for the early diagnosis of NSCLC. Compared to traditional tissue biopsies, liquid biopsies are non-invasive and more easily implemented, making them highly feasible for clinical testing.125 In this regard, EVs as biomarkers have indispensable advantages: they are easy to obtain, isolate, and store. Furthermore, the biomolecules within EVs can reflect the status of the source cells, offering important insights into NSCLC biology. Thus, research utilizing EVs as biomarkers holds great potential and is expected to lead to breakthroughs in the early diagnosis and treatment of NSCLC.
Nucleic Acids in EVs
EVs contain various nucleic acids, including mRNA, miRNA, lncRNA, circRNA, and DNA. MiRNAs are short, endogenous non-coding RNAs. They are typically transcribed from DNA sequences into primary miRNAs, which are then processed into mature miRNAs from precursor miRNAs. MiRNAs typically interact with the 3’ untranslated region (3’ UTR) of target mRNAs, leading to mRNA degradation and translational inhibition. However, studies have revealed that miRNAs also interact with other regions, including the 5’ UTR, coding sequences, and promoters.126–128 These miRNAs can be transported to target cells either through protein binding or encapsulation in EVs.129,130 EV-derived miRNAs play crucial roles in regulating cellular functions and gene expression across various cancers, as illustrated in Figure 3A.131,132 In histological studies, Poroyko et al identified 13 miRNAs in EVs from patients with small cell lung cancer (SCLC) and NSCLC, which are suitable for distinguishing between these two types of cancer. Among these, miR-331-5p, miR-451a, and miR-363-3p demonstrated the highest specificity and sensitivity in distinguishing SCLC from NSCLC.133 Rabinowits et al previously reported that miR-203 is useful for identifying NSCLC and SCLC cases, with a sensitivity of 80% and a specificity of 100%.134 Another study suggested that EV miR-126 could serve as a potential diagnostic biomarker for NSCLC.135 Furthermore, a panel of three miRNAs (miR-21, miR-205, and miR-155) has been proposed for the early detection of NSCLC.136 Similarly, Jin et al identified four EV miRNAs (miR-let-7b-5p, miR-let-7e-5p, miR-23a-3p, and miR-486-5p) as potential diagnostic biomarkers for stage I NSCLC patients, with a sensitivity of 80.5% and a specificity of 92.31%.137 EVs containing MALAT-1 exhibit high specificity (96%) and acceptable sensitivity (56%) and can be used to differentiate NSCLC patients from healthy individuals, supporting its potential as a blood-based microRNA biomarker for NSCLC diagnosis.138 Additionally, EVs containing circSHKBP1, which is associated with NSCLC progression via the miR-1294/PKM2 axis, may serve as diagnostic and therapeutic biomarkers for NSCLC.139 Furthermore, circSATB2, involved in NSCLC progression, exhibited differential expression in lung cancer tissues and serum EVs, potentially serving as a diagnostic biomarker for NSCLC.140 The study also indicated that circEML4 from TAM-derived EVs could serve as a diagnostic biomarker for NSCLC, particularly in patients with a history of smoking.141
EVs are present not only in blood but also in other fluids such as bronchoalveolar lavage (BAL) fluid, pleural effusion, urine, and cerebrospinal fluid, with extensive studies aiming to identify novel diagnostic biomarkers. A study confirmed elevated levels of EV-derived miRNAs in both plasma and BAL samples from NSCLC patients compared to non-tumor individuals.142 Additionally, some studies identified nine EV-derived miRNAs (miR-141-3p, miR-200a-3p, miR-200b-3p, miR-200c-3p, miR-205-5p, miR-375, miR-483-5p, miR-429, miR-203a-3p) that are primarily present in lung cancer but absent in pulmonary tuberculosis and other benign lesions in pleural effusion.143,144 Furthermore, detection of EV-derived miR-200 in pleural effusion has been proposed to distinguish lung adenocarcinoma from benign effusion.145 Recently, research found elevated expression of miR-182 and miR-210 in malignant adenocarcinoma pleural effusion compared to benign effusion.146 Differential levels of lncRNA in urine EVs are considered potential diagnostic biomarkers for NSCLC, with enriched lncRNA potentially associated with tumor cell proliferation, apoptosis, and the pathogenesis of NSCLC.147 EV-derived miRNAs in cerebrospinal fluid can also serve as diagnostic or monitoring biomarkers for NSCLC brain metastases.148 A related study demonstrated high-accuracy diagnosis using EV-derived DNA collected from BALF from lung cancer patients, establishing a rapid and reliable method for EV DNA-targeted gene identification and overcoming the low sensitivity and instability issues associated with circulating free DNA detection.149
In summary, nucleic acids carried by EVs, including mRNA, miRNA, lncRNA, circRNA, and DNA, have promising potential for the diagnosis and treatment of NSCLC. Monitoring specific biomarkers in EVs allows for convenient, non-invasive diagnosis and tracking of disease progression, offering new insights and strategies for personalized therapy. Therefore, nucleic acids in EVs have significant potential for the diagnosis, treatment, and prognosis assessment of NSCLC, potentially becoming indispensable tools in future clinical practice.
Proteins in EVs
In recent years, the widespread use of proteomic techniques has revealed the composition and function of proteins in EVs, attracting significant attention for their roles in predicting, diagnosing, and understanding tumor progression.150 For instance, serum EVs from NSCLC patients contain overexpressed proteins such as nyeso-1, EGFR, PLAP (Placental Alkaline Phosphatase), EpCam (Epithelial Cell Adhesion Molecule), and Alix (ALG-2-interacting Protein X), which are closely associated with poorer overall survival rates.151 Sun et al systematically compared the protein profiles of saliva and serum EVs from healthy volunteers and lung cancer patients using liquid chromatography-mass spectrometry, identifying differentially expressed proteins that could serve as biomarkers for lung cancer diagnosis.152 Additionally, EV-derived EGFR has been proposed as a diagnostic biomarker to distinguish between NSCLC and chronic lung inflammation.153 Huang et al found that approximately 80% of EVs from cancer biopsies were EGFR-positive, while only 2% of EVs isolated from chronic inflammatory lung tissue were EGFR-positive. DCs that take up EGFR-containing cancer-derived EVs produce indoleamine 2.3-dioxygenase, which suppresses the function of tumor-specific CD8+ T cells.43 CD91, derived from EVs, acts as a transmembrane receptor, regulating ligand internalization and molecular trafficking to lysosomes. Furthermore, combining ELISA with cancer embryonic antigen detection of EVs CD91 significantly improves the sensitivity (71.4%) and specificity (91.8%) for lung adenocarcinoma diagnosis.154 LRG1, associated with protein interactions, signal transduction, and cell adhesion, is highly expressed in urine-derived EVs and lung tissues of NSCLC patients. Thus, urinary LRG1 derived from EVs may serve as a potential biomarker for detecting NSCLC.155 Lipopolysaccharide-binding protein in EVs can distinguish healthy donors from NSCLC patients with an area under the curve of 0.713, sensitivity of 65%, and specificity of 75.6%. Additionally, significant differences in LBP (Lipopolysaccharide-Binding Protein) levels exist between metastatic and non-metastatic NSCLC patients, with an AUC of 0.803, sensitivity of 83.1%, and specificity of 67%.156 One study identified 1220 proteins in EVs, with an initial set of biomarkers showing potential for early NSCLC diagnosis and correlating directly with patient survival time. Another set of biomarkers was identified for assessing NSCLC metastasis, with CFHR5 (Complement Factor H-Related Protein 5) alone significantly correlating with the overall survival rate of NSCLC patients.157 Another study identified 302 differentially secreted proteins from EV-activated lung fibroblasts, confirming that these proteins may alter ECM composition and promote cancer cell growth.158
Collectively, these research findings demonstrate the significant potential of EV proteins for early diagnosis, treatment selection, and prognosis evaluation of lung cancer. Studying EV proteins allows for more accurate identification of lung cancer patients for early diagnosis and treatment initiation, as shown in Figure 3A.157,159 Moreover, these studies offer new directions for personalized therapy, including targeted treatments based on specific proteins and adjustments to treatment regimens according to protein composition. Future research should further explore the functions and mechanisms of EV proteins, as well as their relationships with lung cancer development and treatment responses, to better guide clinical practice and improve patient outcomes.
Diagnosis of New Technology
In the field of NSCLC diagnosis and treatment, the continuous emergence of new technologies and methods presents opportunities to enhance accuracy and treatment outcomes. One study employed a Tannic Acid-Iron (III) three-dimensional network-coated mesoporous silica beads strategy, achieving an accuracy of 87.1% in distinguishing between NSCLC and SCLC. The Tannic Acid-Iron (III) three-dimensional network-coated mesoporous silica beads feature label-free, universal, low-cost, and scalable characteristics, offering an effective liquid biopsy technique for lung cancer diagnosis and classification based on serum EVs.160 Another study utilized an on-demand EV isolation chip to achieve rapid and specific separation of extracellular vesicles via catalyst-free click chemistry. Subsequently, the isolated extracellular vesicles released dithiothreitol for downstream functional analyses. This combined isolation and release process provides a powerful tool for selecting and quantifying target extracellular vesicles. The platform was tested for selective isolation and release of EVs in NSCLC patient samples, showing 76% higher selectivity for the EGFR-assisted platform compared to healthy donors.161 Additionally, an immunogold surface-enhanced Raman scattering biosensor chip was proposed for quantifying single extracellular vesicle RNA and proteins as a non-invasive alternative method. Using just 20 μL of purified serum, the biosensor detected surface PD-1/PD-L1 proteins and PD-1/PD-L1 mRNA at single-vesicle resolution, with sensitivity exceeding conventional batch analysis methods like ELISA and qRT-PCR by 1000-fold. By detecting dual single extracellular vesicle PD-1/PD-L1 mRNA, the method distinguished responders from non-responders with an accuracy of 72.2%, and the diagnostic accuracy for NSCLC reached 93.2%.162 The emergence of these innovative technologies offers new possibilities for lung cancer diagnosis and treatment, with the potential to enhance patient survival rates and quality of life. However, despite these encouraging results, further research and clinical validation are needed to ensure the effectiveness and reliability of these technologies in clinical practice.
EVs as Drug Carriers for Antitumor Therapy
Most drugs exert therapeutic effects only when delivered to the diseased site in sufficient quantities; otherwise, their efficacy may be reduced, potentially leading to toxicity and adverse effects in patients. EVs, natural carriers of intercellular information that facilitate biomolecule exchange between cells, are promising candidates for novel drug delivery vehicles, as illustrated in Figure 3B for NSCLC. EVs offer advantages such as small size, natural molecular transport properties, and excellent biocompatibility, making them more suitable than synthetic lipid carriers for drug delivery systems. As drug carriers, EVs can preserve drug activity within their membranes and release the drugs without inducing immune reactions. EV-based tumor therapy may become a crucial component of personalized medicine, as numerous studies highlight the potential of EVs in cancer treatment.163,164
In NSCLC, one study developed a novel therapeutic strategy using EV technology to modify T cells, targeting mesothelin with a single-chain variable fragment-directed chimeric antigen receptor against Lewis lung carcinoma.165 Another study developed and evaluated a therapeutic approach that involved encapsulating miR-497 in EVs using a 3D microfluidic device in an NSCLC model. This study demonstrated the multifunctionality of EV therapy, including stable protection, effective delivery of miR-497, and inhibition of angiogenesis in NSCLC tumor endothelial cells.166 Another study found that EVs derived from cancer stem cells, loaded with APE1 (Apurinic/Apyrimidinic Endonuclease 1) shRNA, could reverse erlotinib resistance in NSCLC through IL-6/STAT3 signaling.167 Researchers also demonstrated that EVs from breast cancer cells could specifically internalize into NSCLC cells by interacting with overexpressed integrin β4 and surfactant protein C on the surface of these cells. These 231-Exo could recognize A549 cells in the bloodstream and effectively evade immune surveillance in vitro.168 Furthermore, a study engineered extracellular vesicles (miR-449a Exo) that actively deliver miR-449a and are specifically taken up by A549 cells. Moreover, miR-449a Exo demonstrated high efficiency in delivering miR-449a both in vitro and in vivo, effectively inhibiting A549 cell proliferation and promoting apoptosis.169
In summary, these studies highlight the potential of EVs in cancer therapy, including enhancing drug delivery efficiency through specific targeting and internalization mechanisms, and inhibiting tumor cell proliferation, migration, and invasion through various pathways. Furthermore, the successful engineering of EVs and advancements in their size and functionality provide new possibilities for cancer treatment. Furthermore, the successful engineering of EVs and advancements in their size and functionality provide new possibilities for cancer treatment.
Additional Studies on EVs
Due to their endogenous nature, EVs must be derived from parental cells to achieve high yields. Currently, EVs used for therapeutic applications are typically sourced from MSCs, which are well-suited for large-scale production.170 MSCs, or multipotent mesenchymal stromal cells, are a type of adult stem cell that can be isolated from various tissues, including bone, umbilical cord tissue, placental tissue, and adipose tissue.171–174 These cells have the potential to differentiate into both mesodermal and non-mesodermal lineage tissues in vitro and in vivo.174 Consequently, they are widely regarded as having regenerative and reparative capabilities, making them suitable for treating various tissue injuries.175 Recent research has increasingly focused on the potential role of MSCs in cancer therapy. Research indicates that MSCs preferentially migrate to tumor sites and integrate into the tumor stroma.176,177 Furthermore, MSCs can regulate the fate of tumor cells through paracrine pathways rather than direct cell-to-cell interactions. In this process, MSC-derived EVs are considered major paracrine effectors.178 Excitingly, MSC-derived EVs, with their strong migratory capacity toward tumor sites, are considered to have significant bioengineering potential as carriers for targeted anti-cancer drug delivery.179,180 This discovery opens up new prospects and possibilities for cancer therapy.
In NSCLC treatment, MSCs have been extensively researched due to their low immunogenicity, multipotent differentiation capabilities, and tissue regeneration-promoting properties. Studies have demonstrated that MSCs and their secreted EVs, known as MSC-exosomes, significantly promote EMT, cell migration, anti-apoptosis, and autophagy in polyploid A549 and H1299 lung cancer cells by activating the AMPK signaling pathway.181 During this process, overexpression of miR-204 inhibits the activity of KLF7 (Krüppel-like Factor 7) and the AKT/HIF-1α pathway, leading to impaired cell migration, invasion, and EMT.182 Additionally, research has shown that EVs derived from MSCs are rich in miR-598, which targets Thrombospondin-2, inhibiting the proliferation and migration of NSCLC cells both in vitro and in vivo.183 Adipose-derived MSCs have sparked widespread interest in cancer therapy. Studies have shown that adipose-derived MSCs have potential in treating gliomas, increasing the sensitivity of hepatocellular carcinoma cells to chemotherapy, and inhibiting the malignant transformation of NSCLC cells via the miR-141-3p-LATS2 axis carried by CircRNA_100395.184 Furthermore, bone marrow stromal cell-derived exosomes engineered with miR-193a can inhibit colony formation, invasion, proliferation, and migration of NSCLC cells, and promote apoptosis of cisplatin-resistant cells by downregulating LRRC1 (Leucine-Rich Repeat Containing 1).185 EVs carrying microRNA-144 from bone marrow mesenchymal stem cells inhibit NSCLC progression by targeting CCNE1 (Cyclin E) and CCNE2.186
These studies suggest that MSCs and their EVs can influence lung cancer cell behavior through various pathways, including promoting epithelial-mesenchymal transition, cell migration, apoptosis inhibition, and autophagy suppression. Moreover, by regulating specific miRNAs, EVs derived from MSCs can inhibit tumor cell proliferation and migration, and hold potential for reducing tumor cell resistance to chemotherapy. These findings offer new prospects for the application of MSCs and their EVs in NSCLC treatment, providing valuable theoretical and practical foundations for future therapies.
Conclusions and Future Prospects
In recent years, growing interest in EVs and their role in intercellular and intracellular communication has led to an increasing number of studies focusing on their involvement in cancer, particularly in NSCLC development. As crucial mediators of information exchange, EVs play a pivotal role in understanding their influence on NSCLC progression. Primarily, EVs play a significant role in the immune response associated with NSCLC. Research indicates that EVs can transmit signaling molecules between immune cells and NSCLC cells, thereby influencing the immune system’s recognition and attack on cancer cells. Specifically, EVs contribute to NSCLC cells evading immune surveillance and developing immune tolerance, which enhances cancer cell survival. Conversely, EVs from immune cells can exert inhibitory effects on NSCLC cells. Studies suggest that these EVs carry immunostimulatory molecules, such as interferons and tumor necrosis factors, which promote immune cell activation and apoptosis of NSCLC cells, thereby inhibiting cancer cell proliferation and metastasis. Additionally, EVs are involved in crucial processes of NSCLC metastasis, including epithelial-mesenchymal transition, angiogenesis, and extracellular matrix remodeling. These processes are essential for cancer cell dissemination and invasion, and EVs influence these processes by carrying signaling molecules or modulating gene expression. Furthermore, EVs can promote NSCLC drug resistance through various mechanisms, including the transmission of drug-resistant genes and modulation of cell signaling pathways. In summary, EVs regulate NSCLC signaling pathways by participating in processes related to its occurrence, development, and metastasis, thus impacting cancer progression. This capability offers new insights and approaches for the diagnosis and treatment of NSCLC. In terms of treatment, harnessing EVs to modulate the immune response and antigen presentation within the NSCLC microenvironment could lead to the development of safe and effective vaccines. Additionally, as natural drug carriers, EVs have targeting potential and can be utilized to deliver anticancer drugs for targeted therapy.
Despite the promising role of EVs in cancer therapy, several key challenges must be addressed before their clinical application. One challenge is the limitations of current EV isolation and purification methods. Current isolation techniques are often constrained by sample sources, and the purification process may result in loss or contamination of EVs, impacting their stability and reliability in clinical settings. Additionally, the heterogeneity of EVs is a significant issue that needs to be addressed. Since EVs can originate from various cell types and their composition can be influenced by cellular states and environmental factors, this heterogeneity must be considered in clinical applications. Another challenge is the susceptibility of EVs to immune system clearance in vivo. Since the immune system clears extraneous substances, efforts are needed to enhance the stability and survival of EVs in vivo, potentially through surface modifications or packaging to improve their ability to evade the immune system. Despite these challenges, EVs still hold significant potential for NSCLC therapy. Future research should focus on addressing these key issues to enable more effective clinical applications of EVs.
Abbreviations
EVs, Extracellular vesicles; NSCLC, non-small cell lung cancer; MVBs, multivesicular bodies; PD-L1, Programmed Death-Ligand 1; TGF-β, Transforming Growth Factor Beta; Treg, regulatory T cells; MDSC, myeloid-derived suppressor cells; EGFR, epidermal growth factor receptor; circUSP7, Circular Ubiquitin-Specific Peptidase 7; S100A4, S100 Calcium-Binding Protein A4; DCs, Dendritic cells; MHC, Major Histocompatibility Complex; NK cells, natural killer cells; IL-15Ra, Interleukin-15 Receptor Alpha; NKG2D, Natural Killer Group 2 Member D; MAGE, Melanoma Antigen Gene; CAR-T, Chimeric antigen receptor T; APCs, antigen-presenting cells; AMPK, AMP-Activated Protein Kinase; mTOR, Mechanistic Target of Rapamycin; EMT, epithelial-mesenchymal transition; ECM, remodeling the extracellular matrix; CAFs, cancer-associated fibroblasts; FAM3C, Family with Sequence Similarity 3 Member C; TKI, Tyrosine Kinase Inhibitor; TAMs, tumor-associated macrophages; c-Src, cellular-src tyrosine kinase; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; CCL18, Chemokine Ligand 18; MFI2-AS1, MFI2 Antisense RNA 1; ANGPTL4, angiopoietin-like 4; RCAN1.4, Regulator of Calcineurin 1, Isoform 4; SYT7, Synaptotagmin VII; LRG1, Leucine-Rich Alpha-2-Glycoprotein 1; SPON2, Spondin 2; HOTAIRM1, HOXA Transcript Antisense RNA, Myeloid-Specific 1; TIMP3, tissue inhibitor of metalloproteinase 3; TME, tumor microenvironment; EndoMT, Endothelial-to-Mesenchymal Transition; AREG, Amphiregulin; RANKL, Receptor Activator of Nuclear Factor Kappa-B Ligand; XIAP, X-linked Inhibitor of Apoptosis Protein; FSP1, Ferroptosis Suppressor Protein 1; BAL, bronchoalveolar lavage; PLAP, Placental Alkaline Phosphatase; EpCam, Epithelial Cell Adhesion Molecule; Alix, ALG-2-interacting Protein X; LBP, Lipopolysaccharide-Binding Protein; CFHR5, Complement Factor H-Related Protein 5; APE1, Apurinic/Apyrimidinic Endonuclease 1; KLF7, Krüppel-like Factor 7; LRRC1, Leucine-Rich Repeat Containing 1; CCNE, Cyclin E.
Acknowledgement
Graphical abstract was created with BioRender.com.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82370054, 82070049 and 81873410), the Fundamental Research Funds for the Central Universities of Central South University (No. 2024ZZTS0875), the Natural Science Foundation of Hunan Province (No. 2022JJ30060), the Beijing Bethune Charitable Foundation (BJ-RW2020011J) and the National Key Clinical Specialty Construction Projects of China.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Cao W, Chen H-D, Yu Y-W, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–791. doi:10.1097/CM9.0000000000001474
3. Zhou W, Liu G, Hung RJ, et al. Causal relationships between body mass index, smoking and lung cancer: univariable and multivariable Mendelian randomization. Int J Cancer. 2021;148(5):1077–1086. doi:10.1002/ijc.33292
4. Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624–639. doi:10.1038/s41571-023-00798-3
5. Wang Q, Gümüş ZH, Colarossi C, et al. SCLC: epidemiology, risk factors, genetic susceptibility, molecular pathology, screening, and early detection. J Thorac Oncol. 2023;18(1):31–46. doi:10.1016/j.jtho.2022.10.002
6. Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol. 2021;16(2):205–215. doi:10.1016/j.jtho.2020.10.006
7. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300. doi:10.21037/tlcr.2016.06.07
8. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi:10.1016/j.mayocp.2019.01.013
9. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198(6):897–907. doi:10.1007/s00408-020-00407-5
10. Halliday PR, Blakely CM, Bivona TG. Emerging targeted therapies for the treatment of non-small cell lung cancer. Curr Oncol Rep. 2019;21(3):21. doi:10.1007/s11912-019-0770-x
11. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):6478. doi:10.1126/science.aau6977
12. Xu R, Rai A, Chen M, et al. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–638. doi:10.1038/s41571-018-0036-9
13. Veerman RE, Güçlüler Akpinar G, Eldh M, et al. Immune cell-derived extracellular vesicles - functions and therapeutic applications. Trends Mol Med. 2019;25(5):382–394. doi:10.1016/j.molmed.2019.02.003
14. Goberdhan DCI. Large tumour-derived extracellular vesicles as prognostic indicators of metastatic cancer patient survival. Br J Cancer. 2023;128(3):471–473. doi:10.1038/s41416-022-02055-3
15. Linxweiler J, Junker K. Extracellular vesicles in urological malignancies: an update. Nat Rev Urol. 2020;17(1):11–27. doi:10.1038/s41585-019-0261-8
16. Welton JL, Loveless S, Stone T, et al. Cerebrospinal fluid extracellular vesicle enrichment for protein biomarker discovery in neurological disease; multiple sclerosis. J Extracell Vesicles. 2017;6(1):1369805. doi:10.1080/20013078.2017.1369805
17. Welch JL, Kaddour H, Schlievert PM, et al. Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-κB/Sp1/Tat circuitry. J Virol. 2018;92(21). doi:10.1128/JVI.00731-18
18. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
19. Fujita Y, Yoshioka Y, Ochiya T. Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci. 2016;107(4):385–390. doi:10.1111/cas.12896
20. Palmisano G, Jensen SS, Le Bihan M-C, et al. Characterization of membrane-shed microvesicles from cytokine-stimulated β-cells using proteomics strategies. Mol Cell Proteomics. 2012;11(8):230–243. doi:10.1074/mcp.M111.012732
21. Muralidharan-Chari V, Clancy JW, Sedgwick A, et al. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603–1611. doi:10.1242/jcs.064386
22. Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2(1):20424.
23. Latifkar A, Hur YH, Sanchez JC, et al. New insights into extracellular vesicle biogenesis and function. J Cell Sci. 2019;132(13). doi:10.1242/jcs.222406
24. Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
25. Xu Z, Chen Y, Ma L, et al. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol Ther. 2022;30(10):3133–3154. doi:10.1016/j.ymthe.2022.01.046
26. Li X, Li C, Zhang L, et al. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol Cancer. 2020;19(1):1. doi:10.1186/s12943-019-1085-0
27. Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–570. doi:10.1038/s41590-021-00899-0
28. Zarà M, Guidetti GF, Camera M, et al. Biology and role of Extracellular Vesicles (EVs) in the pathogenesis of thrombosis. Int J Mol Sci. 2019;20(11):2840. doi:10.3390/ijms20112840
29. Genschmer KR, Russell DW, Lal C, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176(1–2):113–126.e15. doi:10.1016/j.cell.2018.12.002
30. Al Halawani A, Mithieux SM, Yeo GC, et al. Extracellular vesicles: interplay with the extracellular matrix and modulated cell responses. Int J Mol Sci. 2022;23(6):3389. doi:10.3390/ijms23063389
31. LeBleu VS, Kalluri R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer. 2020;6(9):767–774. doi:10.1016/j.trecan.2020.03.007
32. Kim H, Kim DW, Cho JY. Exploring the key communicator role of exosomes in cancer microenvironment through proteomics. Proteome Sci. 2019;17:5. doi:10.1186/s12953-019-0154-z
33. Serratì S, Guida M, Di Fonte R, et al. Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol Cancer. 2022;21(1):20. doi:10.1186/s12943-021-01490-9
34. Mahaweni NM, Kaijen-Lambers ME, Dekkers J, Aerts JG, Hegmans JP. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J Extracell Vesicles. 2013;2(1):22492.
35. Liu H, Chen L, Liu J, et al. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017;411:182–190. doi:10.1016/j.canlet.2017.09.022
36. Greening DW, Gopal SK, Xu R, et al. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi:10.1016/j.semcdb.2015.02.009
37. Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126(4):1216–1223. doi:10.1172/JCI81136
38. Chen R, Xu X, Qian Z, et al. The biological functions and clinical applications of exosomes in lung cancer. Cell Mol Life Sci. 2019;76(23):4613–4633. doi:10.1007/s00018-019-03233-y
39. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi:10.1038/s41586-018-0392-8
40. Poggio M, Hu T, Pai -C-C, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–427.e13. doi:10.1016/j.cell.2019.02.016
41. Tian X, Shen H, Li Z, et al. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol. 2019;12(1):84. doi:10.1186/s13045-019-0772-z
42. Kim DH, Kim H, Choi YJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. 2019;51(8):1–13.
43. Huang SH, Li Y, Zhang J, et al. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest. 2013;31(5):330–335. doi:10.3109/07357907.2013.789905
44. Chen SW, Zhu S-Q, Pei X, et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer. 2021;20(1):144. doi:10.1186/s12943-021-01448-x
45. Wu X, Zhang H, Jiang G, et al. Exosome-transmitted S100A4 induces immunosuppression and non-small cell lung cancer development by activating STAT3. Clin Exp Immunol. 2022;210(3):309–320. doi:10.1093/cei/uxac102
46. Jella KK, Nasti TH, Li Z, et al. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines. 2018;6(4):69. doi:10.3390/vaccines6040069
47. Shen M, Ren X. New insights into the biological impacts of immune cell-derived exosomes within the tumor environment. Cancer Lett. 2018;431:115–122. doi:10.1016/j.canlet.2018.05.040
48. Viaud S, Terme M, Flament C, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4(3):e4942. doi:10.1371/journal.pone.0004942
49. Morse MA, Garst J, Osada T, et al. A Phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi:10.1186/1479-5876-3-9
50. Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4):e1071008. doi:10.1080/2162402X.2015.1071008
51. Kang YT, Niu Z, Hadlock T, et al. On-chip biogenesis of circulating NK cell-derived exosomes in non-small cell lung cancer exhibits antitumoral activity. Adv Sci. 2021;8(6):2003747. doi:10.1002/advs.202003747
52. Tang XJ, Sun X-Y, Huang K-M, et al. Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget. 2015;6(42):44179–44190. doi:10.18632/oncotarget.6175
53. Wang Y, Liu M, Zhang L, et al. Cancer CD39 drives metabolic adaption and mal-differentiation of CD4(+) T cells in patients with non-small-cell lung cancer. Cell Death Dis. 2023;14(12):804. doi:10.1038/s41419-023-06336-4
54. Jerabkova-Roda K, Dupas A, Osmani N, et al. Circulating extracellular vesicles and tumor cells: sticky partners in metastasis. Trends Cancer. 2022;8(10):799–805. doi:10.1016/j.trecan.2022.05.002
55. Menck K, Sivaloganathan S, Bleckmann A, et al. Microvesicles in cancer: small size, large potential. Int J Mol Sci. 2020;21(15):5373. doi:10.3390/ijms21155373
56. Liang X, Wu Q, Wang Y, Li S. MicroRNAs as early diagnostic biomarkers for non‑small cell lung cancer (Review). Oncol Rep. 2023;49(1):8.
57. Tasso R, Marconi S, Rossi G, et al. Platelets and their derived extracellular vesicles: the new generation of markers in non-small cell lung cancer management. Drug Discov Today. 2023;28(7):103616. doi:10.1016/j.drudis.2023.103616
58. Lin H, Li J, Wang M, et al. Exosomal long noncoding RNAs in NSCLC: dysfunctions and clinical potential. J Cancer. 2023;14(10):1736–1750. doi:10.7150/jca.84506
59. Bakir B, Chiarella AM, Pitarresi JR, et al. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30(10):764–776. doi:10.1016/j.tcb.2020.07.003
60. Brabletz S, Schuhwerk H, Brabletz T, et al. Dynamic EMT: a multi-tool for tumor progression. EMBO j. 2021;40(18):e108647. doi:10.15252/embj.2021108647
61. Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi:10.1016/j.ceb.2016.06.002
62. Lobb RJ, Visan KS, Wu L-Y, et al. An epithelial-to-mesenchymal transition induced extracellular vesicle prognostic signature in non-small cell lung cancer. Commun Biol. 2023;6(1):68. doi:10.1038/s42003-022-04350-4
63. Gross JC, Chaudhary V, Bartscherer K, et al. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036–1045. doi:10.1038/ncb2574
64. Zhang X, Sai B, Wang F, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18(1):40. doi:10.1186/s12943-019-0959-5
65. Jouida A, O’Callaghan M, Mc Carthy C, et al. Exosomes from EGFR-mutated adenocarcinoma induce a hybrid EMT and MMP9-dependant tumor invasion. Cancers. 2022;14(15):3776. doi:10.3390/cancers14153776
66. Thuya WL, Kong LR, Syn NL, et al. FAM3C in circulating tumor-derived extracellular vesicles promotes non-small cell lung cancer growth in secondary sites. Theranostics. 2023;13(2):621–638. doi:10.7150/thno.72297
67. Lin CC, Wu C-Y, Tseng JT-C, et al. Extracellular vesicle miR-200c enhances gefitinib sensitivity in heterogeneous EGFR-mutant NSCLC. Biomedicines. 2021;9(3):243. doi:10.3390/biomedicines9030243
68. Cen W, Yan Q, Zhou W, et al. miR-4739 promotes epithelial-mesenchymal transition and angiogenesis in ”driver gene-negative” non-small cell lung cancer via activating the Wnt/β-catenin signaling. Cell Oncol. 2023;46(6):1821–1835. doi:10.1007/s13402-023-00848-z
69. Zhou J, Wang H, Sun Q, et al. miR-224-5p-enriched exosomes promote tumorigenesis by directly targeting androgen receptor in non-small cell lung cancer. Mol Ther Nucleic Acids. 2021;23:1217–1228. doi:10.1016/j.omtn.2021.01.028
70. Hisakane K, Seike M, Sugano T, et al. Exosome-derived miR-210 involved in resistance to osimertinib and epithelial–mesenchymal transition in EGFR mutant non-small cell lung cancer cells. Thorac Cancer. 2021;12(11):1690–1698. doi:10.1111/1759-7714.13943
71. Li X, Chen Z, Ni Y, et al. Tumor-associated macrophages secret exosomal miR-155 and miR-196a-5p to promote metastasis of non-small-cell lung cancer. Transl Lung Cancer Res. 2021;10(3):1338–1354. doi:10.21037/tlcr-20-1255
72. Shen Y, TanTai J. Exosomes secreted by metastatic cancer cells promotes epithelial mesenchymal transition in small cell lung carcinoma: the key role of Src/TGF-β1 axis. Gene. 2024;892:147873. doi:10.1016/j.gene.2023.147873
73. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745–1770. doi:10.1007/s00018-019-03351-7
74. Jiang X, Wang J, Deng X, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39(1):204. doi:10.1186/s13046-020-01709-5
75. Hsu YL, Hung J-Y, Chang W-A, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–4942. doi:10.1038/onc.2017.105
76. Shefler I, Salamon P, Zitman-Gal T, et al. Tumor-derived extracellular vesicles induce CCL18 production by mast cells: a possible link to angiogenesis. Cells. 2022;11(3):353. doi:10.3390/cells11030353
77. Xu J, Wang H, Shi B, et al. Exosomal MFI2-AS1 sponge miR-107 promotes non-small cell lung cancer progression through NFAT5. Cancer Cell Int. 2023;23(1):51. doi:10.1186/s12935-023-02886-x
78. Mo F, Xu Y, Zhang J, et al. Effects of hypoxia and radiation-induced exosomes on migration of lung cancer cells and angiogenesis of umbilical vein endothelial cells. Radiat Res. 2020;194(1):71–80. doi:10.1667/RR15555.1
79. Kim DH, Park H, Choi YJ, et al. Exosomal miR-1260b derived from non-small cell lung cancer promotes tumor metastasis through the inhibition of HIPK2. Cell Death Dis. 2021;12(8):747. doi:10.1038/s41419-021-04024-9
80. Kim DH, Park S, Kim H, et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020;475:2–13. doi:10.1016/j.canlet.2020.01.023
81. Liu X, Li R, Chen X, et al. SYT7 is a key player in increasing exosome secretion and promoting angiogenesis in non-small-cell lung cancer. Cancer Lett. 2023;577:216400. doi:10.1016/j.canlet.2023.216400
82. Ma Z, Wei K, Yang F, et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis. 2021;12(9):840. doi:10.1038/s41419-021-04037-4
83. Li Z, Zeng C, Nong Q, et al. Exosomal leucine-rich-Alpha2-glycoprotein 1 derived from non-small-cell lung cancer cells promotes angiogenesis via TGF-β signal pathway. Mol Ther Oncolytics. 2019;14:313–322. doi:10.1016/j.omto.2019.08.001
84. Wu Q, Zhou L, Lv D, et al. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12(1):53. doi:10.1186/s13045-019-0739-0
85. Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192–200. doi:10.1016/j.semcancer.2019.09.004
86. Chen Z, Bian C, Huang J, et al. Tumor-derived exosomal HOTAIRM1 regulates SPON2 in CAFs to promote progression of lung adenocarcinoma. Discov Oncol. 2022;13(1):92. doi:10.1007/s12672-022-00553-7
87. Czarnecka KH, Szmyd B, Barańska M, et al. A strong decrease in TIMP3 expression mediated by the presence of miR-17 and 20a enables extracellular matrix remodeling in the NSCLC lesion surroundings. Front Oncol. 2019;9:1372. doi:10.3389/fonc.2019.01372
88. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101.
89. Mo Z, Cheong JYA, Xiang L, et al. Extracellular vesicle-associated organotropic metastasis. Cell Prolif. 2021;54(1):e12948. doi:10.1111/cpr.12948
90. Hoshino A, Costa-Silva B, Shen T-L, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi:10.1038/nature15756
91. Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12(1):134. doi:10.1186/s13045-019-0818-2
92. Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-Mutated Non–small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi:10.1056/NEJMoa2027071
93. Kim J, Lee HM, Cai F, et al. The hexosamine biosynthesis pathway is a targetable liability in KRAS/LKB1 mutant lung cancer. Nat Metab. 2020;2(12):1401–1412. doi:10.1038/s42255-020-00316-0
94. Wu Y, Yang S, Ma J, et al. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 2022;12(1):134–153. doi:10.1158/2159-8290.CD-21-0316
95. Dai J, Cimino PJ, Gouin KH, et al. Astrocytic laminin-211 drives disseminated breast tumor cell dormancy in brain. Nat Cancer. 2022;3(1):25–42. doi:10.1038/s43018-021-00297-3
96. Kfoury Y, Baryawno N, Severe N, et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell. 2021;39(11):1464–1478.e8. doi:10.1016/j.ccell.2021.09.005
97. Xu W, Patel N, Deng Y, et al. Extracellular vesicle-derived LINC00482 induces microglial M2 polarization to facilitate brain metastasis of NSCLC. Cancer Lett. 2023;561:216146. doi:10.1016/j.canlet.2023.216146
98. Diao X, Guo C, Zheng H, et al. SUMOylation-triggered ALIX activation modulates extracellular vesicles circTLCD4-RWDD3 to promote lymphatic metastasis of non-small cell lung cancer. Signal Transduct Target Ther. 2023;8(1):426. doi:10.1038/s41392-023-01685-0
99. Wu D, Deng S, Li L, et al. TGF-β1-mediated exosomal lnc-MMP2-2 increases blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis. 2021;12(8):721. doi:10.1038/s41419-021-04004-z
100. Huang L, Wang F, Wang X, et al. M2-like macrophage-derived exosomes facilitate metastasis in non-small-cell lung cancer by delivering integrin αVβ3. MedComm. 2023;4(1):e191. doi:10.1002/mco2.191
101. Li C, Qin F, Wang W, et al. hnRNPA2B1-mediated extracellular vesicles sorting of miR-122-5p potentially promotes lung cancer progression. Int J Mol Sci. 2021;22(23):12866. doi:10.3390/ijms222312866
102. Taverna S, Pucci M, Giallombardo M, et al. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep. 2017;7(1):3170. doi:10.1038/s41598-017-03460-y
103. Ni J, Zhang X, Li J, et al. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis. 2021;12(7):662. doi:10.1038/s41419-021-03928-w
104. Herrera-Juárez M, Serrano‐Gómez C, Bote‐de‐Cabo H, et al. Targeted therapy for lung cancer: beyond EGFR and ALK. Cancer. 2023;129(12):1803–1820. doi:10.1002/cncr.34757
105. Rodak O, Peris-Díaz MD, Olbromski M, et al. Current landscape of non-small cell lung cancer: epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers. 2021;13(18):4705. doi:10.3390/cancers13184705
106. Shedden K, Xie XT, Chandaroy P, et al. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63(15):4331–4337.
107. Bach D-H, Hong J-Y, Park HJ, et al. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int, J, Cancer. 2017;141(2):220–230. doi:10.1002/ijc.30669
108. Yu DD, Wu Y, Shen H-Y, et al. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 2015;106(8):959–964. doi:10.1111/cas.12715
109. Zheng Y, Liu L, Chen C, et al. The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a. PeerJ. 2017;5:e3627. doi:10.7717/peerj.3627
110. Xie H, Yao J, Wang Y, et al. Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 2022;29(1):1257–1271. doi:10.1080/10717544.2022.2057617
111. Wang D, Zhao C, Xu F, et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics. 2021;11(6):2860–2875. doi:10.7150/thno.51797
112. Song Z, Jia G, Ma P, et al. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276:119399. doi:10.1016/j.lfs.2021.119399
113. Zhang T, Zhang P, Li HX. CAFs-derived exosomal miRNA-130a confers cisplatin resistance of NSCLC cells through PUM2-dependent packaging. Int J Nanomed. 2021;16:561–577. doi:10.2147/IJN.S271976
114. Jing C, Cao H, Qin X, et al. Exosome-mediated gefitinib resistance in lung cancer HCC827 cells via delivery of miR-21. Oncol Lett. 2018;15(6):9811–9817. doi:10.3892/ol.2018.8604
115. Pan R, Zhou H. Exosomal transfer of lncRNA H19 promotes erlotinib resistance in non-small cell lung cancer via miR-615-3p/ATG7 axis. Cancer Manag Res. 2020;12:4283–4297. doi:10.2147/CMAR.S241095
116. Liu X, Jiang T, Li X, et al. Exosomes transmit T790M mutation-induced resistance in EGFR-mutant NSCLC by activating PI3K/AKT signalling pathway. J Cell Mol Med. 2020;24(2):1529–1540. doi:10.1111/jcmm.14838
117. Wan X, Xie B, Sun H, et al. Exosomes derived from M2 type tumor-associated macrophages promote osimertinib resistance in non-small cell lung cancer through MSTRG.292666.16-miR-6836-5p-MAPK8IP3 axis. Cancer Cell Int. 2022;22(1):83. doi:10.1186/s12935-022-02509-x
118. Chen R, Qian Z, Xu X, et al. Exosomes-transmitted miR-7 reverses gefitinib resistance by targeting YAP in non-small-cell lung cancer. Pharmacol Res. 2021;165:105442. doi:10.1016/j.phrs.2021.105442
119. Li X, Chen C, Wang Z, et al. Elevated exosome-derived miRNAs predict osimertinib resistance in non-small cell lung cancer. Cancer Cell Int. 2021;21(1):428. doi:10.1186/s12935-021-02075-8
120. Wei SL, Ye -J-J, Sun L, et al. Exosome-derived circKIF20B suppresses gefitinib resistance and cell proliferation in non-small cell lung cancer. Cancer Cell Int. 2023;23(1):129. doi:10.1186/s12935-023-02974-y
121. Gu G, Hu C, Hui K, et al. Exosomal miR-136-5p derived from anlotinib-resistant NSCLC cells confers anlotinib resistance in non-small cell lung cancer through targeting PPP2R2A. Int J Nanomed. 2021;16:6329–6343. doi:10.2147/IJN.S321720
122. Del Re M, Marconcini R, Pasquini G, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. 2018;118(6):820–824. doi:10.1038/bjc.2018.9
123. Schöne N, Kemper M, Menck K, et al. PD-L1 on large extracellular vesicles is a predictive biomarker for therapy response in tissue PD-L1-low and -negative patients with non-small cell lung cancer. J Extracell Vesicles. 2024;13(3):e12418. doi:10.1002/jev2.12418
124. Carreca AP, Tinnirello R, Miceli V, et al. Extracellular vesicles in lung cancer: implementation in diagnosis and therapeutic perspectives. Cancers. 2024;16(11):1967. doi:10.3390/cancers16111967
125. Tomasik B, Skrzypski M, Bienkowski M, et al. Current and future applications of liquid biopsy in non-small-cell lung cancer-a narrative review. Transl Lung Cancer Res. 2023;12(3):594–614. doi:10.21037/tlcr-22-742
126. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524. doi:10.1038/nrm3838
127. Broughton JP, Lovci M, Huang J, et al. Pairing beyond the seed supports MicroRNA targeting specificity. Mol Cell. 2016;64(2):320–333. doi:10.1016/j.molcel.2016.09.004
128. Gallach S, Calabuig-Fariñas S, Jantus-Lewintre E, et al. MicroRNAs: promising new antiangiogenic targets in cancer. Biomed Res Int. 2014;2014:878450. doi:10.1155/2014/878450
129. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi:10.1038/cr.2008.282
130. Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–5008. doi:10.1073/pnas.1019055108
131. Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. doi:10.3402/jev.v5.31292
132. Wang N, Yao C, Luo C, et al. Integrated plasma and exosome long noncoding RNA profiling is promising for diagnosing non-small cell lung cancer. Clin Chem Lab Med. 2023;61(12):2216–2228. doi:10.1515/cclm-2023-0291
133. Poroyko V, Mirzapoiazova T, Nam A, et al. Exosomal miRNAs species in the blood of small cell and non-small cell lung cancer patients. Oncotarget. 2018;9(28):19793–19806. doi:10.18632/oncotarget.24857
134. Rabinowits G, Gerçel-Taylor C, Day JM, et al. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10(1):42–46. doi:10.3816/CLC.2009.n.006
135. Grimolizzi F, Monaco F, Leoni F, et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci Rep. 2017;7(1):15277. doi:10.1038/s41598-017-15475-6
136. Lai X, Friedman A. Exosomal miRs in lung cancer: a mathematical model. PLoS One. 2016;11(12):e0167706. doi:10.1371/journal.pone.0167706
137. Jin X, Chen Y, Chen H, et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non-small cell lung cancer using next-generation sequencing. Clin Cancer Res. 2017;23(17):5311–5319. doi:10.1158/1078-0432.CCR-17-0577
138. Weber DG, Johnen G, Casjens S, et al. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Res Notes. 2013;6:518. doi:10.1186/1756-0500-6-518
139. Chen W, Tang D, Lin J, et al. Exosomal circSHKBP1 participates in non-small cell lung cancer progression through PKM2-mediated glycolysis. Mol Ther Oncolytics. 2022;24:470–485. doi:10.1016/j.omto.2022.01.012
140. Zhang N, Nan A, Chen L, et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. 2020;19(1):101. doi:10.1186/s12943-020-01221-6
141. Cheng C, Wang P, Yang Y, et al. Smoking-induced M2-TAMs, via circEML4 in EVs, promote the progression of NSCLC through ALKBH5-regulated m6A modification of SOCS2 in NSCLC cells. Adv Sci. 2023;10(22):e2300953. doi:10.1002/advs.202300953
142. Rodríguez M, Silva J, López‐Alfonso A, et al. Different exosome cargo from plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes Chromosomes Cancer. 2014;53(9):713–724. doi:10.1002/gcc.22181
143. Wang Y, Xu Y-M, Zou Y-Q, et al. Identification of differential expressed PE exosomal miRNA in lung adenocarcinoma, tuberculosis, and other benign lesions. Medicine. 2017;96(44):e8361. doi:10.1097/MD.0000000000008361
144. Lin J, Wang Y, Zou Y-Q, et al. Differential miRNA expression in pleural effusions derived from extracellular vesicles of patients with lung cancer, pulmonary tuberculosis, or pneumonia. Tumour Biol. 2016;37:15835–15845. doi:10.1007/s13277-016-5410-6
145. Hydbring P, De Petris L, Zhang Y, et al. Exosomal RNA-profiling of pleural effusions identifies adenocarcinoma patients through elevated miR-200 and LCN2 expression. Lung Cancer. 2018;124:45–52. doi:10.1016/j.lungcan.2018.07.018
146. Tamiya H, Mitani A, Saito A, et al. Exosomal MicroRNA expression profiling in patients with lung adenocarcinoma-associated malignant pleural effusion. Anticancer Res. 2018;38(12):6707–6714. doi:10.21873/anticanres.13039
147. Lin Q, Xie D, Pan L, et al. Urinary exosomal long noncoding RNAs serve as biomarkers for early detection of non-small cell lung cancer. Biosci Rep. 2021;41(10). doi:10.1042/BSR20210908
148. Li H, Xia M, Zheng S, et al. Cerebrospinal fluid exosomal microRNAs as biomarkers for diagnosing or monitoring the progression of non-small cell lung cancer with leptomeningeal metastases. Biotechnol Genet Eng Rev. 2023;1–22. doi:10.1080/02648725.2023.2183613
149. Hur JY, Lee JS, Kim IA, et al. Extracellular vesicle-based EGFR genotyping in bronchoalveolar lavage fluid from treatment-naive non-small cell lung cancer patients. Transl Lung Cancer Res. 2019;8(6):1051–1060. doi:10.21037/tlcr.2019.12.16
150. Baran K, Waśko J, Kryczka J, et al. The comparison of serum exosome protein profile in diagnosis of NSCLC patients. Int J Mol Sci. 2023;24(18):13669. doi:10.3390/ijms241813669
151. Sandfeld-Paulsen B, Aggerholm-Pedersen N, Bæk R, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol. 2016;10(10):1595–1602. doi:10.1016/j.molonc.2016.10.003
152. Sun Y, Liu S, Qiao Z, et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal Chim Acta. 2017;982:84–95. doi:10.1016/j.aca.2017.06.005
153. Yamashita T, Kamada H, Kanasaki S, et al. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Pharmazie. 2013;68(12):969–973.
154. Ueda K, Ishikawa N, Tatsuguchi A, et al. Antibody-coupled monolithic silica microtips for highthroughput molecular profiling of circulating exosomes. Sci Rep. 2014;4:6232. doi:10.1038/srep06232
155. Li Y, Zhang Y, Qiu F, et al. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32(15):1976–1983. doi:10.1002/elps.201000598
156. Wang N, Song X, Liu L, et al. Circulating exosomes contain protein biomarkers of metastatic non-small-cell lung cancer. Cancer Sci. 2018;109(5):1701–1709. doi:10.1111/cas.13581
157. Luo B, Que Z, Lu X, et al. Identification of exosome protein panels as predictive biomarkers for non-small cell lung cancer. Biol Proced Online. 2023;25(1):29. doi:10.1186/s12575-023-00223-0
158. Zhang J, Fu B, Li M, et al. Secretome of activated fibroblasts induced by exosomes for the discovery of biomarkers in non-small cell lung cancer. Small. 2021;17(4):e2004750. doi:10.1002/smll.202004750
159. Brocco D, Lanuti P, Pieragostino D, et al. Phenotypic and proteomic analysis identifies hallmarks of blood circulating extracellular vesicles in NSCLC responders to immune checkpoint inhibitors. Cancers. 2021;13(4):585. doi:10.3390/cancers13040585
160. Wang H, Liu D, Zhang L, et al. Label-free small extracellular vesicles capturing strategy for lung cancer diagnosis and typing based on a natural polyphenol-metal three-dimensional network. Anal Chem. 2022;94(46):16103–16112. doi:10.1021/acs.analchem.2c03283
161. Kang YT, Hadlock T, Jolly S, et al. Extracellular vesicles on demand (EVOD) chip for screening and quantification of cancer-associated extracellular vesicles. Biosens Bioelectron. 2020;168:112535. doi:10.1016/j.bios.2020.112535
162. Nguyen LTH, Zhang J, Rima XY, et al. An immunogold single extracellular vesicular RNA and protein (Au SERP) biochip to predict responses to immunotherapy in non-small cell lung cancer patients. J Extracell Vesicles. 2022;11(9):e12258. doi:10.1002/jev2.12258
163. Kim H, Kim EH, Kwak G, et al. Exosomes: cell-derived nanoplatforms for the delivery of cancer therapeutics. Int J Mol Sci. 2020;22(1):14. doi:10.3390/ijms22010014
164. Chinnappan M, Srivastava A, Amreddy N, et al. Exosomes as drug delivery vehicle and contributor of resistance to anticancer drugs. Cancer Lett. 2020;486:18–28. doi:10.1016/j.canlet.2020.05.004
165. Zheng W, Zhu T, Tang L, et al. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer. J Transl Med. 2023;21(1):383. doi:10.1186/s12967-023-04206-3
166. Jeong K, Yu YJ, You JY, et al. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip. 2020;20(3):548–557. doi:10.1039/C9LC00958B
167. Tang CH, Qin L, Gao Y-C, et al. APE1 shRNA-loaded cancer stem cell-derived extracellular vesicles reverse Erlotinib resistance in non-small cell lung cancer via the IL-6/STAT3 signalling. Clin Transl Med. 2022;12(5):e876. doi:10.1002/ctm2.876
168. Nie H, Xie X, Zhang D, et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale. 2020;12(2):877–887. doi:10.1039/C9NR09011H
169. Zhou W, Xu M, Wang Z, et al. Engineered exosomes loaded with miR-449a selectively inhibit the growth of homologous non-small cell lung cancer. Cancer Cell Int. 2021;21(1):485. doi:10.1186/s12935-021-02157-7
170. Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–341. doi:10.1016/j.addr.2012.07.001
171. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi:10.1126/science.276.5309.71
172. In’t Anker PS, Scherjon SA, Kleijburg‐van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. doi:10.1634/stemcells.2004-0058
173. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi:10.1091/mbc.e02-02-0105
174. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. doi:10.1046/j.1365-2141.2000.01986.x
175. Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4(1):22. doi:10.1038/s41536-019-0083-6
176. Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–1603. doi:10.1093/jnci/djh299
177. Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614–2623. doi:10.1002/stem.187
178. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi:10.1186/s13287-016-0363-7
179. Baek G, Choi H, Kim Y, et al. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019;8(9):880–886. doi:10.1002/sctm.18-0226
180. Massa M, Croce S, Campanelli R, et al. Clinical applications of mesenchymal stem/stromal cell derived extracellular vesicles: therapeutic potential of an acellular product. Diagnostics. 2020;10(12):999. doi:10.3390/diagnostics10120999
181. Wang L, Ouyang M, Xing S, et al. Mesenchymal stem cells and their derived exosomes promote malignant phenotype of polyploid non-small-cell lung cancer cells through AMPK signaling pathway. Anal Cell Pathol. 2022;2022:8708202. doi:10.1155/2022/8708202
182. Liu XN, Zhang C-B, Lin H, et al. microRNA-204 shuttled by mesenchymal stem cell-derived exosomes inhibits the migration and invasion of non-small-cell lung cancer cells via the KLF7/AKT/HIF-1α axis. Neoplasma. 2021;68(4):719–731. doi:10.4149/neo_2021_201208N1328
183. Li X, Wu F. Mesenchymal stem cell-derived extracellular vesicles transfer miR-598 to inhibit the growth and metastasis of non-small-cell lung cancer by targeting THBS2. Cell Death Discov. 2023;9(1):3. doi:10.1038/s41420-022-01283-z
184. Zhang C, Cao J, Lv W, et al. CircRNA_100395 carried by exosomes from adipose-derived mesenchymal stem cells inhibits the malignant transformation of non-small cell lung carcinoma through the miR-141-3p-LATS2 axis. Front Cell Dev Biol. 2021;9:663147. doi:10.3389/fcell.2021.663147
185. Wu H, Mu X, Liu L, et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11(9):801. doi:10.1038/s41419-020-02962-4
186. Liang Y, Zhang D, Li L, et al. Exosomal microRNA-144 from bone marrow-derived mesenchymal stem cells inhibits the progression of non-small cell lung cancer by targeting CCNE1 and CCNE2. Stem Cell Res Ther. 2020;11(1):87. doi:10.1186/s13287-020-1580-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.