Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials: Antidepressants for Restricted and Repetitive Behaviors in Autism Spectrum Disorder
Authors Maneeton P, Maneeton B , Winichaikul Y, Kawilapat S, Kienngam N, Maneeton N
Received 22 March 2024
Accepted for publication 20 August 2024
Published 2 October 2024 Volume 2024:20 Pages 1711—1723
DOI https://doi.org/10.2147/NDT.S465611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Prapinpatch Maneeton,1 Benchalak Maneeton,2 Yanisa Winichaikul,3 Suttipong Kawilapat,2 Nongluck Kienngam,4 Narong Maneeton2
1Chiang Mai University Demonstration School, Faculty of Education, Chiang Mai University, Chiang Mai, Thailand; 2Department of Psychiatry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 3Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 4Department of Education Psychology and Guidance Program, Faculty of Education, Chiang Mai University, Chiang Mai, Thailand
Correspondence: Narong Maneeton, Department of Psychiatry, Faculty of Medicine, Chiang Mai University, 110 Intawaroros Road, Sriphum, Muang, Chiang Mai, 50200, Thailand, Tel +66 53 93 5422, Fax +66 53 5426, Email [email protected]
Abstract: In previously randomized controlled trials (RCTs) on the efficacy of antidepressants in restricted and repetitive behaviors (RRBs) in autism spectrum disorder (ASD), outcomes overwhelmingly showed no benefits of antidepressants studied in the larger multisite RCTs over placebo. However, the positive effect of antidepressants in the RRBs found in the small preliminary studies requires confirmation in larger trials. We aimed to systematically review the efficacy of antidepressants in the treatment of RRBs in ASD by including RCTs from the SCOPUS, PubMed, Embase, Cochrane Controlled Trials Register, Clinical Trials.gov, and other databases in January 2024. Analyzing data from 609 participants across nine RCTs showed no significant difference in the overall pooled mean-end score for RRBs between antidepressant- and placebo-treated groups [SMD (95% CI) of − 0.25 (− 0.53, 0.02), I2 = 54%, Tau2 = 0.10, prediction interval = − 1.03, 0.53]. In small preliminary studies by one group, the clomipramine-treated group’s pooled mean endpoint for obsessive-compulsive symptoms in ASD individuals showed a significantly better outcome than the desipramine-treated group, but in unconfirmed studies. Of the individual antidepressants investigated only clomipramine, and fluvoxamine illustrated some efficacy over placebo in small preliminary studies. These findings need confirmation in larger, multisite randomized controlled trials. There were no significant differences in the overall discontinuation rates or discontinuation due to adverse events between the antidepressant- and placebo-treated groups [RR (95% CI) of 1.30 (0.95, 1.78), I2 = 0%, and 1.33 (0.71, 2.47), I2=0%, respectively]. Common side effects included agitation, appetite disturbance, anorexia, gastrointestinal issues, and sleep disturbance, with no significant differences between the antidepressant and placebo groups. In conclusion, the results regarding the efficacy of antidepressants in the treatment of RRBs in ASD are inconsistent. Since previous evidence found a correlation between attention-deficit hyperactivity disorder (ADHD) symptoms including overactivity and impulsivity, and RRBs, further trials including the use of non-stimulants such as atomoxetine could be conducted.
Keywords: antidepressant, restricted and repetitive behavior, CY-BOCS, Y-BOCS, ASD
Introduction
Autism spectrum disorder (ASD), the early onset of a neurodevelopmental disorder, is characterized by deficits in social interaction and social communication and restrictive behavior, interest, or activity.1 Although the prevalence of ASD varies between studies (ranging from 0.3 to 2.5%),2–5 all evidence suggests that it is increasing worldwide.2,4 Core symptoms of ASD tend to be lifelong disabilities, significantly affecting individuals, families, and society.6
Restrictive and repetitive behaviors (RRBs), including repetitive motor movements and highly circumscribed interests, are one of the main symptoms of ASD,1 frequently interfering with learning and social adaptation. The pathophysiology of RBBs is not adequately known. A previous study found that proactive control deficits in ASD can lead to difficulties in delaying responses and inappropriate behaviors, such as RRBs.7 Another research study found that repetitive behaviors, impulsivity, and hyperactivity in intellectual disability overlap with ADHD.8 This overlap has led to growing interest in the use of ADHD medications to manage ASD symptoms such as repetitive behaviors. The outcomes of one study suggest that ADHD medications, such as atomoxetine,9 may be beneficial due to their effect on the norepinephrine transporter (NET).
Comparison of the mechanisms of RRBs in ASD and obsessive-compulsive disorder (OCD) reveals distinct neurobiological and cognitive processes. In ASD, RRBs like hand-flapping, rocking, and insistence on sameness stem from sensory sensitivities, difficulty in adapting to change, and social communication challenges. These behaviors involve abnormal neural circuits in relation to cognitive flexibility, executive function, and sensory processing, along with serotonin and glutamate system alterations. They often serve as coping mechanisms for sensory overload and anxiety reduction. In contrast, RRBs in OCD arise from obsessions and compulsions aimed at reducing distress or preventing harm. This disorder involves dysfunction in cortico-striatal-thalamo-cortical circuits and neurotransmitter imbalances, particularly serotonin and dopamine. While both disorders feature repetitive behaviors, RRBs associated with ASD are tied to sensory sensitivities, cognitive inflexibility, and social challenges, while those associated with OCD are rooted in circuit dysfunction and neurotransmitter dysregulation.10,11 The drugs related to serotonin transporter (SERT) regulation and expression, especially selective serotonin reuptake inhibitors (SSRIs), which can be treated in OCD, have been shown to have positive effects in treatment of RRBs in ASD individuals.12,13
Several antidepressants, including tricyclic antidepressants (TCAs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and SSRIs been shown to have an effect on SERT.14,15 Although several large randomized controlled trials have illustrated the efficacy of some antidepressants in the RRBs in autism spectrum disorder (ASD), the outcomes are not consistent since some RCTs did not find such efficacy.12,13,16–21 Since some studies related to antidepressants in RRBs have been published and the previous review did not include clomipramine treatment for RRBs,16,17 we planned to perform a systematic review and meta-analysis to determine and clarify the effect of antidepressants on RRBs in ASD.
Therefore, we aimed to review the evidence using meta-analysis by comparing the efficacy, acceptability, and tolerability of antidepressants in RRBs in ASD patients. The primary outcome was the mean endpoint in RRBs measured by any standardized rating scale. The response and discontinuation rates were also evaluated.
Material and Methods
Study Protocol
This systematic review was designed in accordance with the PRISMA 2020 checklist, and the protocol was registered at the PROSPERO 2022 CRD42023457683. Two authors individually completed each task of the review.
Inclusion and Exclusion Criteria
We included all RCTs fulfilling the following inclusion criteria: i) a study in ASD patients diagnosed by any set of criteria, ii) antidepressants were administered, iii) a controlled, blinded trial iv) treatment compared with placebo or other medication, and v) reporting the RRBs using a standardized rating scale.
Information Sources
The authors searched for relevant RCTs in the SCOPUS, PubMed, Embase, Cochrane Controlled Trials Register (CCTR), Clinical Trials.gov (CT.gov), and other databases from inception to January 2024. No language restrictions were imposed. In addition, the reference lists of the relevant studies were searched. If important outcomes of the included study were unclear or unavailable, we contacted the author of the original study by email.
Search Strategy
Standard search terms, including (autism spectrum disorder) AND (antidepressants), were applied to all databases. The specific strategic search for a specific database is illustrated in the index.
Data Collection Process
We gathered the overall search records from the databases and removed duplicate records. Two reviewers (NM and BM) evaluated the remaining records individually by considering their titles and abstracts. Following evaluation, the full-text version of relevant records was collected. Two reviewers (NM and BM) independently examined the full-text version of the studies for eligibility in this review. The reviewers reached a consensus in the case of disagreement.
Data Extraction
Two reviewers, NM and BM independently reviewed the eligible studies and extracted data using a standardized form. They worked separately to ensure the data-gathering process was consistent and accurate, following the form’s predefined guidelines. In case of any disagreements, the reviewers discussed the issue and reached a consensus.
Data Items
Data collection consisted of: i) the data related to the inclusion criteria, ii) first author and year of publication, iii) study duration, iv) participant characteristics and number of randomized participants, v) antidepressants and their doses, vi) a placebo and other comparators with its dose, vii) the mean scores of a standardized rating scale of each treatment, viii) dropout rates of each treatment.
Quality Assessment
NM and BM individually examined the risk of bias using the Cochrane Handbook for Systematic Reviews of Interventions. The risk of bias included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcomes assessment, incomplete outcome data, selective reporting, and other biases.22
Summary Measures
The primary outcomes of this review were efficacy, acceptability, and tolerability. The difference in the pooled mean endpoint scores of standardized rating scales for RRBs in ASD was estimated to evaluate the efficacious outcomes. Similar to a previous systematic review, acceptability was calculated using the relative risk (RR) of the overall discontinuation rate.23 Tolerability was estimated using the RR of the rate of discontinuation due to adverse events.24 In the case of a crossover RCT, only the data of Phase I were included in the synthesis.
Statistical Analysis
An inverse variance, weighing the effect of each individual RCT, was applied to estimate the pooled mean endpoint scores with 95% confidence intervals (CIs).22 The weighted mean differences (WMDs) or standardized mean differences (SMDs) were applied based on the use of the same or various measure rating scales across the studies. The relative risk (RRs) with a 95% confidence interval (95% CI) was applied consistently for discontinuation rates. All pooled RRs with 95% CIs were calculated using Mantel-Haenszel.25 Since the true effect may differ among the examined studies, this review for all outcomes was applied using a random-effect model for the synthesis.26 However, we also examine the heterogeneity across the included studies. Instead of considering only using I2 and Tau2 statistics, the prediction interval for overall effect was also calculated and reported in this study.27–30 The prediction intervals are different from the confidence intervals which provide the range of the true effect across all populations rather than the correctness of the estimated overall effect.31 The synthesis of all outcomes was carried out using the RevMan 5.4.1.
Risk of Bias Across Studies
According to the PRISMA checklist, risk of bias across studies consists of an assessment of the risk of bias that may affect the cumulative outcomes. Thus, the risk of bias across studies was determined by assessing the selective reporting within studies. Additionally, in cases in which the number of included RCTs was ten or more, the publication bias using Begg’s funnel plots test was conducted to determine such risk of bias.22 A funnel plot is a simple scatter plot to show the intervention effect estimated from each study against a measure of the individual study’s size. There is no significant publication bias if the plot resembles a symmetrical inverted funnel.22
Results
Study Selection
The search identified 852 citations from the following electronic searches (SCOPUS = 496, PubMed = 53, Embase = 237, Cochrane Controlled Trials Register = 24, ClinicalTrials. Gov = 42 (Figure 1). After removing 116 duplicated citations, 736 citations were further assessed for eligibility based on their title and abstracts. Out of these articles, 29 citations were evaluated again from the full-text version. Of these, fourteen citations were eliminated from this review, eleven citations did not fulfill the validated rating scale of RRBs,32–42 three citations did not publish the outcomes.43–45 Hence, nine studies of fifteen citations were included for qualitative and quantitative syntheses in this review.12,13,16–21,46–52
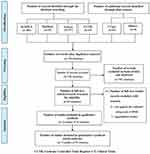 |
Figure 1 Flow diagram of study. |
Characteristics of Included Studies
All basic characteristics of the included trials are listed in Table 1. A total of 609 randomized participants from 9 RCTs were included in this review. Five studies12,16,18,19,52 were conducted in child and adolescent groups, three13,20,50 were carried out in adults and one17 was performed in 6–23 year olds. All patients were treated with antidepressants in comparison to placebo or other antidepressants for 10 to 20 weeks. The RRBs in ASD of included studies were determined by the modified National Institute of Mental Health (NIMH), Global Obsessive-Compulsive Disorder (OCD) and Anxiety scales, Modified NIMH OCD Scale, Modified Comprehensive Psychopathological Rating Scale (CPRS) OCD Subscale, Children’s Yale-Brown Obsessive-Compulsive Scale,(CY-BOCS), Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). The diagnostic criteria for ASD were mainly reliant on the Diagnostic and Statistical Manual of Mental Disorders (DSM), International Classification of Diseases (ICD), Autism Diagnostic Interview (ADI), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
 |
Table 1 Characteristics of the Randomized Clinical Trials of Antidepressants in the Treatment of Repetitive Restricted Behaviors in Autism Spectrum Disorders |
Risk of Bias Within Studies
Figure 2 displays the risks of bias in the included studies. The included studies had a low risk of bias or unclear risk of bias. An intention-to-treat analysis was conducted in three studies.12,18,19
 |
Figure 2 Risk of bias summary of antidepressants in ASD. |
Synthesis of Results
Efficacy
The pooled mean endpoint score for RRBs measured by the CY-BOCS or Y-BOCS and response rate in ASD patients showed significant heterogeneity between the antidepressant- and placebo-treated groups. The pooled mean-end score of the RRBs between the antidepressant- and placebo-treated groups was not significantly different [SMD (95% CI) of −0.25 (−0.53, 0.02), I2=54%, Tau2=0.10, prediction interval = −1.03, 0.53] (Figure 3). The pooled response rate of RRBs between the antidepressant- and placebo-treated groups was not significantly different (RR [95% CI) of 1.03 (0.58, 1.82), I2=59%).
 |
Figure 3 Mean-end scores of RRBs antidepressants vs placebo in ASD. |
Citalopram
Based on the included RCT,19 the mean endpoint score for CY-BOCS and response rate of the Citalopram-treated group was not significantly different from the placebo-treated group in the treatment of repetitive behavior in ASD patients.
Clomipramine and Desipramine
Two RCTs of clomipramine were included in this review.16,17 The pooled mean endpoint for obsessive-compulsive symptoms in ASD patients of the clomipramine-treated group was significantly less than in the desipramine-treated group [SMD (95% CI) of −1.11 (−1.80, −0.41), I2=0%]. Clomipramine, but not desipramine, was superior to placebo in treating obsessive-compulsive symptoms in ASD patients, measured by the modified NIMH OCD Scale and modified NIMH Global OCD and Anxiety scales.
Fluoxetine
The pooled mean-end score for RRBs measured by the CY-BOCS or Y-BOCS in ASD patients showed significant heterogeneity between the Fluoxetine-treated and placebo-treated groups. The overall pooled mean-end score of the RRBs between the fluoxetine-treated and placebo-treated groups was not significantly different [SMD (95% CI) of −0.12 (−0.53, 0.29), I2=67%] (Figure 3).
Fluvoxamine
According to the included RCTs, the findings showed that the reduction of Y-BOCS score from baseline and response rate in the Fluvoxamine-treated group was significantly better than the placebo-treated group. Similarly, its response in the fluvoxamine-treated was also superior to the placebo-treated group.
Milnacipran
A previous RCT illustrated that Milnacipran, compared to placebo, did not significantly decrease the Y-BOCS score from baseline.
Discontinuation Rates
Overall Discontinuation Rate (Acceptability)
No significant heterogeneity for overall discontinuation rate was found. The overall discontinuation rate of the antidepressant-treated groups [RR (95% CI) of 1.30 (0.95, 1.78), I2=0%] did not show significant differences from the placebo-treated group.
Discontinuation Rate Due to Adverse Events (Tolerability)
Heterogeneity was not significantly different in the pooled discontinuation rates. The discontinuation rates due to adverse events did not differ between the antidepressant- and placebo-treated groups [RR (95% CI) of 1.33 (0.71, 2.47), I2=0%].
Main Adverse Events
The main adverse events, including agitation, appetite disturbance, anorexia, gastrointestinal disturbance, and sleep disturbance were not different between the antidepressant- and placebo-treated groups.
Risk of Bias Across Studies
In cases where there are fewer than ten studies, a funnel plot evaluating the publication bias in a systematic review may not have sufficient power to estimate the chances of real asymmetry occurring due to the included results.53 For this reason, the funnel plot could not be tested because this review gathered nine RCTs. We therefore presented the risk of bias assessments in table format in the evidence synthesis, showing each included study and its strength across several quality criteria for that particular study type (Figure 2). From Figure 2, the selective reporting within studies, two studies showed a low risk of bias, in all domains.
Discussion
This meta-analysis updates the evidence on antidepressants in treating RRBs in patients with autism spectrum disorder. As a consequence of the methodological diversity of individual RCTs, it is challenging to interpret outcome data regarding the overall efficacy and safety of antidepressants. The synthesis of results shows the various outcomes of antidepressants in the treatment of RRB symptoms. Based on the non-duplicated studies, clomipramine and fluvoxamine appear to confer some level of efficacy in the reduction of RRBs in ASD, but this is not found in other antidepressants. According to some small included studies, the adverse effects including behavioral activation, irritability, appetite disturbance, anorexia, gastrointestinal disturbance, and sleep disturbance may be found, but they are not significantly different from placebo. The acceptability and tolerability of the drug were not different from that of the placebo.
The outcomes of treatment of RRBs in autism spectrum disorders with antidepressants including SSRIs remain inconclusive. Although previous meta-analysis of the studies54–56 demonstrated that serotonin reuptake inhibitors could improve RRBs in ASD, those trials have several limitations including being small and preliminary studies, with possibly selective reporting of publication outcomes. One meta-analysis conducted in 2020, showed no significant difference between SSRIs and placebo in treating RRBs in ASD.57 A previous review did not include clomipramine studies of RRBs in ASD. Therefore, this systematic review, including clomipramine and recent studies, could lead to a more comprehensive study in identifying the effect of antidepressants in RRBs treatment in ASD.
Inconsistent outcomes of antidepressants in the treatment of RRBs were also found in this review. Although fluvoxamine and clomipramine have shown some efficacy in small preliminary trials, citalopram and fluoxetine were not found to have any effects. These findings underscore the importance of recognizing the preliminary nature of these results, which require validation through larger, multisite RCTs. However, the present study suggests a potential relationship between antidepressant dosage and clinical response to RRBs in ASD, warranting further investigation for confirmation.
Previous reviews have not estimated the tolerability of antidepressants in treating RRBs in ASD.54,57 However, our outcome has illustrated that tolerability, measured by discontinuation rate due to adverse events such as irritability, activation, and mood, gastro-intestinal and sleep disturbances, was comparable to placebo. Based on our findings, physicians should consider the risk and benefit of prescribing the medication for those with ASD based on their own judgment.
The present outcomes of this systematic review have some limitations. First, the number of included RCTs is limited. Additionally, those included RCTs varied with regard to several factors including diagnostic criteria, age groups, and outcome measurement. Of the four fluoxetine RCTs, doses varied across studies. In children aged 5–17 years, doses ranged from 2.4 to 20 mg/day, with a mean maximum dose of 0.38 mg/kg/day and a mean final dose of 0.36 mg/kg/day. In adults aged 18–60 years, doses ranged from 20 to 80 mg/day, with a mean dose of 64.76 mg/day. Another study involving children and adolescents used flexible dosing of fluoxetine up to 20 mg/day for those weighing less than 40 kg and up to 30 mg/day for those weighing 40 kg or more. A final study in children and adolescents used flexible dosing of fluoxetine up to 18 mg/day. Second, significant heterogeneity among the included studies was found, which may be related to the diversity of eligible RCTs in terms of clinical, methodological, and statistical aspects. Third, since the included trials were limited, the funnel plot could not estimate the publication bias, hence, the risk of bias across studies was only determined by assessing selective reporting within studies. Based on these limitations, the present findings should be applied cautiously. Next, the large confidence interval was observed for milnacipran treatment. It might be due to a very small sample in the examined study which included only five participants. The examination of milnacipran with a larger sample size should be further conducted. Finally, previous evidence suggests that non-stimulant such as atomoxetine has some efficacy in RRBs for ASD/ID patients, but this review did not include the non-stimulants. Further studies including such non-stimulant ADHD medications are warranted.
Conclusions
The effects of antidepressants on the treatment of RRBs in ASD are inconsistent. According to the small, non-replicated clinical trials, clomipramine and fluvoxamine illustrate some efficacy for treatment of RRBs in ASD. Even though common side effects, including behavioral activation and irritability, occurred, they were no different from those experienced with a placebo. However, due to the impact of the limited number of studies, range of doses, intervention periods, and baseline characteristics of patients, in combine with heterogeneity in the combined effect of the studies, generalization of the evidence should be carefully applied in clinical practice. Since there is significant evidence showing an association between RRBs and symptoms of ADHD in Autism Spectrum Disorder and Intellectual and Developmental Disabilities, further research through clinical trials is necessary to explore the potential benefits of non-stimulant ADHD medications for these conditions.
Data Sharing Statement
Data sharing is not applicable as no datasets were generated and/or analyzed for this study.
Ethical Approval
This study waived the ethics requirements because it was not involved in the human subjects and the included data was only retrieved to synthesize from published studies.
Acknowledgments
The authors would like to thank the Faculty of Medicine, Chiang Mai University, Thailand, for their immense support and assistance during this project and for their help with English language editing. This study was partially supported by a grant from Chiang Mai University, Chiang Mai, Thailand (grant number RG 38/2566).
Disclosure
The authors report no conflicts of interest in this work.
References
1. American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Publishing; 2013.
2. Qiu S, Lu Y, Li Y, et al. Prevalence of autism spectrum disorder in Asia: a systematic review and meta-analysis. Psychiatry Res. 2020;284:112679. doi:10.1016/j.psychres.2019.112679
3. Zhou H, Xu X, Yan W, et al. Prevalence of Autism Spectrum Disorder in China: a Nationwide Multi-center Population-based Study Among Children Aged 6 to 12 Years. Neurosci Bull. 2020;36(9):961–971. doi:10.1007/s12264-020-00530-6
4. Zhou J, Liu A, He F, et al. High prevalence of serum folate receptor autoantibodies in children with autism spectrum disorders. Biomarkers. 2018;23(7):622–624. doi:10.1080/1354750X.2018.1458152
5. Kogan MD, Vladutiu CJ, Schieve LA, et al. The Prevalence of Parent-Reported Autism Spectrum Disorder Among US Children. Pediatrics. 2018;142(6). doi:10.1542/peds.2017-4161.
6. Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721–728. doi:10.1001/jamapediatrics.2014.210
7. Kelly SE, Schmitt LM, Sweeney JA, Mosconi MW. Reduced proactive control processes associated with behavioral response inhibition deficits in autism spectrum disorder. Autism Res. 2021;14(2):389–399. doi:10.1002/aur.2415
8. Burbidge C, Oliver C, Moss J, et al. The association between repetitive behaviours, impulsivity and hyperactivity in people with intellectual disability. J Intellect Disabil Res. 2010;54(12):1078–1092. doi:10.1111/j.1365-2788.2010.01338.x
9. Harfterkamp M, Buitelaar JK, Minderaa RB, van de Loo-Neus G, van der Gaag RJ, Hoekstra PJ. Atomoxetine in autism spectrum disorder: no effects on social functioning; some beneficial effects on stereotyped behaviors, inappropriate speech, and fear of change. J Child Adolesc Psychopharmacol. 2014;24(9):481–485. doi:10.1089/cap.2014.0026
10. Hendren RL. Editorial: what to Do About Rigid, Repetitive Behaviors in Autism Spectrum Disorder? J Am Acad Child Adolesc Psychiatry. 2021;60(1):22–23. doi:10.1016/j.jaac.2020.11.009
11. Jiujias M, Kelley E, Hall L. Restricted, repetitive behaviors in autism spectrum disorder and obsessive–compulsive disorder: a comparative review. Child Psychiatry & Human Development. 2017;48(6):944–959. doi:10.1007/s10578-017-0717-0
12. Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582–589. doi:10.1038/sj.npp.1300627
13. Hollander E, Soorya L, Chaplin W, et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am J Psychiatry. 2012;169(3):292–299. doi:10.1176/appi.ajp.2011.10050764
14. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(7):581–601. doi:10.1016/S2215-0366(20)30137-1
15. Maneeton N, Maneeton B, Karawekpanyawong N, Woottiluk P, Putthisri S, Srisurapanon M. Fluoxetine in acute treatment of children and adolescents with obsessive-compulsive disorder: a systematic review and meta-analysis. Nord J Psychiatry. 2020;74(7):461–469. doi:10.1080/08039488.2020.1744037
16. Gordon CT, Rapoport JL, Hamburger SD, State RC, Mannheim GB. Differential response of seven subjects with autistic disorder to clomipramine and desipramine. Am J Psychiatry. 1992;149(3):363–366.
17. Gordon CT, State RC, Nelson JE, Hamburger SD, Rapoport JL. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry. 1993;50(6):441–447. doi:10.1001/archpsyc.1993.01820180039004
18. Herscu P, Handen BL, Arnold LE, et al. The Sofia Study: negative Multi-center Study of Low Dose Fluoxetine on Repetitive Behaviors in Children and Adolescents with Autistic Disorder. J Autism Dev Disord. 2020;50(9):3233–3244. doi:10.1007/s10803-019-04120-y
19. King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583–590. doi:10.1001/archgenpsychiatry.2009.30
20. McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996;53(11):1001–1008. doi:10.1001/archpsyc.1996.01830110037005
21. Reddihough DS, Marraffa C, Mouti A, et al. Effect of Fluoxetine on Obsessive-Compulsive Behaviors in Children and Adolescents with Autism Spectrum Disorders: a Randomized Clinical Trial. JAMA. 2019;322(16):1561–1569. doi:10.1001/jama.2019.14685
22. H JPT, Thomas K, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022. Available from: www.training.cochrane.org/handbook.
23. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. doi:10.1016/S0140-6736(09)60046-5
24. Papakostas GI. Tolerability of modern antidepressants. J Clini Psych. 2008;69(Suppl E1):8–13.
25. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration; 2011.
26. Dettori JR, Norvell DC, Chapman JR. Fixed-Effect vs Random-Effects Models for Meta-Analysis: 3 Points to Consider. Global Spine J. 2022;12(7):1624–1626. doi:10.1177/21925682221110527
27. Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: i(2) is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5–18. doi:10.1002/jrsm.1230
28. Borenstein M. In a meta-analysis, the I-squared statistic does not tell us how much the effect size varies. J Clin Epidemiol. 2022;152:281–284. doi:10.1016/j.jclinepi.2022.10.003
29. Borenstein M. How to understand and report heterogeneity in a meta-analysis: the difference between I-squared and prediction intervals. Integr Med Res. 2023;12(4):101014. doi:10.1016/j.imr.2023.101014
30. Shamim MA, Dwivedi P, Akhtar N, et al. The missing piece: why clinicians, epidemiologists, and policymakers need prediction intervals in a meta-analysis. Obes Rev. 2024;25(6):e13732. doi:10.1111/obr.13732
31. Al Amer FM, Lin L. Empirical assessment of prediction intervals in Cochrane meta-analyses. Eur J Clin Invest. 2021;51(7):e13524. doi:10.1111/eci.13524
32. Carminati GG, Gerber F, Darbellay B, et al. Using venlafaxine to treat behavioral disorders in patients with autism spectrum disorder. Prog Neuro Psychopharmacol Biol Psychiatry. 2016;65:85–95. doi:10.1016/j.pnpbp.2015.09.002
33. Chantiluke K, Barrett N, Giampietro V, et al. Inverse fluoxetine effects on inhibitory brain activation in non-comorbid boys with ADHD and with ASD. Psychopharmacology). 2015;232(12):2071–2082. doi:10.1007/s00213-014-3837-2
34. ClinicalTrial.gov. Early Intervention With Fluoxetine in Autism. 2005. Available from: https://clinicaltrials.gov/study/NCT00183339.
35. ClinicalTrial.gov. Fluoxetine Essay in Children With Autism. Availble from: 2009. https://clinicaltrials.gov/study/NCT00873834.
36. ClinicalTrial.gov. Mirtazapine Treatment of Anxiety in Children and Adolescents With Pervasive Developmental Disorders. 2010. Available from: https://clinicaltrials.gov/study/NCT01302964.
37. ClinicalTrial.gov. Trial of Sertraline to Treat Children With Fragile X Syndrome. 2011. Available from: https://clinicaltrials.gov/study/NCT01474746.
38. Greiss Hess L, Fitzpatrick SE, Nguyen DV, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Low-Dose Sertraline in Young Children With Fragile X Syndrome. Journal of Developmental and Behavioral Pediatrics: JDBP. 2016;37(8):619–628. doi:10.1097/DBP.0000000000000334
39. Niederhofer H. Venlafaxine has modest effects in autistic children. Therapy. 2004;1(1):87–90. doi:10.2217/14750708.1.1.87
40. Potter LA, Scholze DA, Biag HMB, et al. A Randomized Controlled Trial of Sertraline in Young Children With Autism Spectrum Disorder. Front Psychiat. 2019;10:10. doi:10.3389/fpsyt.2019.00010
41. Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: a double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol. 2001;21(4):440–444. doi:10.1097/00004714-200108000-00012
42. Sugie Y, Sugie H, Fukuda T, et al. Clinical efficacy of fluvoxamine and functional polymorphism in a serotonin transporter gene on childhood autism. J Autism Dev Disord. 2005;35(3):377–385. doi:10.1007/s10803-005-3305-2
43. ClinicalTrial.gov. Fluvoxamine and Sertraline in Childhood Autism - Does SSRI Therapy Improve Behaviour and/or Mood? 2008. Available from: https://clinicaltrials.gov/study/NCT00655174.
44. ClinicalTrial.gov. Study of Fluoxetine in Adults With Autistic Disorder. 2001. Available from: https://clinicaltrials.gov/study/NCT00027404.
45. ClinicalTrial.gov. Functional MRI Evaluation of the Effect of Citalopram in Autism Spectrum Disorders. 2008. Available from: https://clinicaltrials.gov/study/NCT00609531.
46. Citalopram ineffective for reducing repetitive behavior in autism spectrum disorders. J Natl Med Assoc. 2009;101(9):976. doi:10.1016/S0027-9684(15)31055-5
47. ClinicalTrial.gov. Randomized Study of Fluoxetine in Children and Adolescents With Autism. 1999. Available from: https://clinicaltrials.gov/study/NCT00004486.
48. ClinicalTrial.gov. Citalopram for Children With Autism and Repetitive Behavior (STAART Study 1). 2004. Available from: https://clinicaltrials.gov/study/NCT00086645.
49. ClinicalTrial.gov. Study of Fluoxetine in Autism. 2007. Available from: https://clinicaltrials.gov/study/NCT00515320.
50. ClinicalTrial.gov. Milnacipran in Autism and the Functional Locus Coeruleus and Noradrenergic Model of Autism. 2010. Available from: https://clinicaltrials.gov/study/NCT01337700.
51. Mouti A, Reddihough D, Marraffa C, et al. Fluoxetine for Autistic Behaviors (FAB trial): study protocol for a randomized controlled trial in children and adolescents with autism. Trials. 2014;15(1). doi:10.1186/1745-6215-15-230.
52. Reddihough D, Marraffa C, Mouti A, et al. A randomised placebo-controlled trial to determine if fluoxetine is effective for improving autistic behaviours. J Paediatr Child Health. 2019;55:8–9.
53. Sterne JAC, Egger M, Moher D. Addressing Reporting Biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (Updated March 2011). The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org.
54. Carrasco M, Volkmar FR, Bloch MH. Pharmacologic treatment of repetitive behaviors in autism spectrum disorders: evidence of publication bias. Pediatrics. 2012;129(5):e1301–1310. doi:10.1542/peds.2011-3285
55. Soorya L, Kiarashi J, Hollander E. Psychopharmacologic interventions for repetitive behaviors in autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2008;17(4):753–771. doi:10.1016/j.chc.2008.06.003
56. Siafis S, Çıray O, Wu H, et al. Pharmacological and dietary-supplement treatments for autism spectrum disorder: a systematic review and network meta-analysis. Mol Autism. 2022;13(1):10. doi:10.1186/s13229-022-00488-4
57. Yu Y, Chaulagain A, Pedersen SA, et al. Pharmacotherapy of restricted/repetitive behavior in autism spectrum disorder: a systematic review and meta-analysis. BMC Psychiatry. 2020;20(1):121. doi:10.1186/s12888-020-2477-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

