Back to Journals » Journal of Inflammation Research » Volume 18
Urine Neutrophil Elastase: A Novel Predictor of ICU Admission for Patients with COVID-19 Infection
Authors Song Y, Zeng K, Zhang LK, Zhang JN , Zhang KL, Xin Y, Wang XR, Zhou YX, Li HX, Wang CS, Yu KJ
Received 28 October 2024
Accepted for publication 5 April 2025
Published 24 April 2025 Volume 2025:18 Pages 5545—5553
DOI https://doi.org/10.2147/JIR.S503276
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yu Song, Kai Zeng, Li-Kun Zhang, Jian-Nan Zhang, Kai-Li Zhang, Yu Xin, Xin-Ran Wang, Yu-Xin Zhou, Hong-Xu Li, Chang-Song Wang, Kai-Jiang Yu
Key Laboratory of Critical Care Medicine of Heilongjiang Province; Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China
Correspondence: Chang-Song Wang, Email [email protected] Kai-Jiang Yu, Email [email protected]
Introduction: We aimed to explore the differences of neutrophil elastase (NE) levels between intensive care unit (ICU) and non-ICU patients with COVID-19 infection, as well as its predictive value for COVID-19 progression.
Methods: We enrolled the patients admitted with a primary diagnosis of COVID-19. All patients in ICU were diagnosed with the critical type upon admission. Blood was taken within 24 hours, followed by examination of the blood NE level and urine NE level. Other clinical features were recorded. A logistic regression model was used to predict ICU admission.
Results: A total of 83 patients were diagnosed, including 52 non-ICU cases and 31 ICU cases. The ICU group showed significantly elevated levels of Neutrophil%, Cr, D-dimer (DD), Procalcitonin (PCT), and C-reactive protein (CRP). Meanwhile, the CD3-cell, T4-cell, and Lymphocyte% levels were lower in the ICU group. Notably, the blood NE levels were similar between groups, whereas the urine NE level was highly significantly higher in the ICU group vs the non-ICU group. After dimension reduction, we constructed a logistic model (UD) using only two factors: the urine NE level and the blood DD level. The overall accuracy of was 86.1%. The urine NE has a strong efficacy in ICU prediction (AUC = 0.893), and the performance of the UD model was even better (AUC = 0.933).
Conclusion: Urine NE level is a useful predictor of COVID-19 progression, particularly in patients requiring ICU care. Urine NE has a significantly positive correlation with neutrophil%, DD, and PCT, as well as a negative correlation with lymphocyte levels.
Keywords: COVID-19, neutrophil elastase, intensive care unit, urine, acute respiratory distress syndrome, ARDS
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused substantial death worldwide, and it is currently still without any specific drug treatment. After infection, more than 20% of patients admitted to the hospital require intensive care unit (ICU) admission, with an in-hospital mortality between 5% and 20%.1,2 Generally, patients admitted to the ICU are the critical type of COVID-19 with a more intense inflammatory response and more severe organ injuries. Over the course of more than three years of the epidemic, many biomarkers indicative of COVID-19 criticality, organ injury, and inflammation have been explored, such as D-Dimer, TnI, IL-6, KIM-1, MCP-1, NGAL, miR-21, miR-146a, miR-155, miR-let-7, and miR-223, in urine, serum, and nasopharyngeal samples.3–5 In particular, compared with blood indicators, urine indicators have the unique advantages of less invasive, easy to repeat sampling, easy to observe continuously, and reflecting the metabolic capacity of the liver and kidneys, which are clinical features worth emphasizing. Although the outbreak of COVID-19 has been widely controlled, there is still a need to explore more valuable urinary markers for early warning in at-risk populations.
Neutrophils play a pathogenic role in COVID-19 by releasing neutrophils extracellular traps (NETs) and neutrophil elastase (NE).6,7 NE is a serine protease mainly expressed in primary neutrophils. NE cleaves neutral, non-aromatic dipeptides, and so on.8,9 It has a broad array of substrates, and its typical substances include elastin, collagen, and fibronectin. NE induces the disruption of the gingival epithelial barrier and bacterial invasion in periodontal tissues, aggravating periodontitis.10 NE hydrolysis plays a role in inflammation and infection progression, and the level/activity of NE can reflect disease state and severity, as well as cancer progression and inflammation resistance.11–16 In lung diseases, NE facilitates neutrophil transmigration to the site of infection, and it is involved in clearance of Gram-negative bacteria.17 NE activates inflammation when released into the airway milieu. For example, in chronic obstructive pulmonary disease (COPD), NE may trigger the airway neutrophil extracellular traps (NETs) formation and activates airway inflammation through different mechanisms.16 Plasma NE and elafin imbalance are associated with acute respiratory distress syndrome (ARDS) development.18 High levels of NE have been found in both nasopharyngeal swabs and in the peripheral blood of patients infected by COVID-19.19,20 Meanwhile, this protease may be involved in SARS-CoV-2 virulence, that it may be necessary during the cleavage of the spike protein to enter host cells.19–21 In COVID-19 treatment, NE inhibition may be a feasible strategy. In China, Sivelestat, a specific inhibitor of NE with several clinical applications, has been approved for using in patients with COVID-19.22,23 Therefore, NE may play a pivotal role in COVID-19 infection and progression.
To date, the association between NE and COVID-19 has been confirmedly observed. However, specifically, some questions remain to be clarified, such as: how is NE (at the blood level and at the urine level) related to COVID-19 progression? Can NE serve as a predictive marker for COVID-19 progression or adverse outcomes? And how does NE correlate with COVID-19-related inflammatory markers? In this study, we enrolled patients with moderate to critical COVID-19 infection and grouped them by whether or not they were admitted to the ICU due to progression of the critically ill type. We focused on the differences and predictive value of NE (as well as other relevant characteristics) between ICU and non-ICU patients.
Methods
Participants
The ethical approval of this study was obtained from our hospital. All participants have signed the informed consent prior to recruitment. We enrolled the patients admitted with a primary diagnosis of COVID-19 in the Department of Intensive Care Medicine of the First Hospital of Harbin Medical University from December 2022 to September 2023. According to whether admitted in the intensive care unit (ICU), the patients were divided into two groups: the non-ICU group and the ICU group. For the ICU group, all patients were confirmed the critical type when admitted in the ICU. The inclusion criteria were: (1) microbiologically confirmed COVID-19 infection (SARS-CoV-2 PCR positivity in nasopharyngeal swab samples), and (2) typical of viral pneumonia in lung imaging, (3) from the moderate type to the critical type; and (4) age ≥18 years. Blood was taken within 24 hours after admission to the hospital, and following examinations were conducted: the blood routine test, liver and kidney function indices, examination of the blood NE level and urine NE level. The exclusion criteria were: (1) refusal to sign informed consent or (2) lacking the NE level of the blood sample.
Information Collection
For each patient, the following clinical features were recorded: gender, age, body height, body weight, with hypertension or not, with diabetes or not, the total white blood cell level in the peripheral blood routine (WBC), the neutrophil ratio in the peripheral blood routine (Neutrophil%), the lymphocyte ratio in the peripheral blood routine (Lymphocyte%), the platelet level in the peripheral blood routine (PLT), the CD3 Cell level in the peripheral blood, the T4 Cell level in the peripheral blood, the alanine aminotransferase (ALT) level, the aspartate aminotransferase (AST) level, the creatinine (Cr) level, the albumin (ALB) level, the total bilirubin level, the direct bilirubin level, the indirect bilirubin level, the D-dimer (DD) level, the procalcitonin (PCT) level, the Cystatin C level, the C-reactive protein (CRP) level, the IL-6 level. Besides, the NE levels in blood and urine were examined. For the ICU group, following Features were recorded: whether Shock occurred, Death in 28 days, the SOFA Score at admission, and the APACHE-II Score at admission.
Statistical Analysis
The SPSS software (25.0) was used for statistical analysis. Categorical data were described by percentages, and numeric variables were expressed as (1) Median with (25–75% interquartile range), and (2) mean ± standard deviation (SD). For the numeric variables, t-test and Mann–Whitney U were used for comparison of two groups. For categorical data, Chi-square analysis was used for comparison of the incidence of two groups. A P value <0.05 was considered statistically significant. The logistic regression model was used to predict the admission to the ICU. The AUC of operating characteristic curve (ROC) was calculated for each import feature regarding the ICU admission.
Results
The Clinical Characteristics and Differences Between Non-ICU and ICU Groups
As Table 1 shows, a total of 83 patients were diagnosed, including 52 in the non-ICU group and 31 in the ICU group. The proportion of females (19.35%) admitted to the ICU was significantly lower than that of males (48.08%) (P = 0.0089). The median ages of two groups were 75 (67–79.5) and 73 (61–85), respectively (P > 0.05). No significant differences were found in the ratio of hypertension and diabetes. Also, two groups had the comparable levels of BMI, WBC, PLT, ALT, ALB, bilirubin, IL-6, Cystatin C, and the blood NE. The ICU group had significantly higher levels of Neutrophil% (86.8 vs 80.2), Cr (79.2 umol/L vs 68.75 umol/L), D-dimer (1.9 mg/l vs 0.74 mg/l), PCT (0.4 ng/mL vs 0.11 ng/mL), and CRP (119 mg/L vs 64.25 mg/L). Meanwhile, the CD3-cell, T4-cell, and Lymphocyte% levels were lower in the ICU group. These results suggest that more severe neutrophil activation may be present in the ICU group. Notably, the blood NE levels were similar between groups, whereas the urine NE level was highly significantly higher in the ICU group vs the non-ICU group. Using the t-test, we confirmed that the blood NE levels were similar between groups, while the urine NE was dramatically higher in the ICU group vs the non-ICU group (215.4 ng/mL vs 6.27 ng/mL) (Table 2). This result suggests, on the one hand, that urinary NE can be used as an early and non-invasive warning indicator of the ICU outcome, and on the other hand, that ICU patients may have a fragile ability to regulate neutrophil activation (which is especially represented by NE expression).
 |
Table 1 Clinical Characteristics of Two Groups of Patients |
 |
Table 2 Differences in NE Levels Between NON-ICU and ICU Patients (t-Test) |
Logistic Regression Models for ICU Prediction
Next, the associated factors were applied for the logistic regression models for ICU prediction. After dimension reduction, we constructed a logistic model using only two factors: the Urine NE level and the blood DD level. The details of this two-parameter model are presented in Table 3. Besides, in combination of the PCT level, the performance (accuracy) can be enhanced slightly, however, there were many lack values in PCT, and the three-parameter had a higher requirement towards the samples (and accordingly a smaller sample size). The details of the three-parameter model are shown in Table 3. The confusion matrix of the two-parameter model (Urine-NE and DD, or UD) and the three-parameter model (Urine-NE, DD, and PCT, or UDP) was presented in Table 4. The overall accuracy of the UD model was 86.1%, and that of the UDP model was 87.8%. Considering the performance of the UDP model hardly outperformed the UD model while the sample-size loss was obvious, the UD model was proposed as a practical model for ICU prediction. The ROC curves of the UD model, Urine NE, DD and PCT with AUC values were shown in Figure 1. The urine NE has a strong efficacy in ICU prediction (AUC = 0.893), and the performance of the UD model was even better (AUC = 0.933). Together, it is reasonable to assume that this UD model can be used in the treatment of COVID-19 infection, and the detection of urine NE and blood DD should be performed as early as possible for the hospitalized COVID-19 patients.
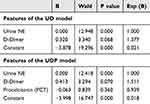 |
Table 3 Logistic Regression for ICU Prediction by the UD Two-Parameter Model |
 |
Table 4 The Performance of the UD Two-Parameter Model and UDP Three-Parameter Model for ICU Prediction |
The Correlation Between NE Levels and Other Inflammation Factors
For the ICU patients, we have explored the association between blood/urine NE and the outcomes (including shock occurrence, death in 28 days or 2 months), but no links have been observed. Finally, to deepen the understanding of the impact of NE on COVID-19 progression, we analyzed the correlation between NE levels and other blood parameters or inflammation factors (Table 5). The blood NE showed a significantly positive correlation with CRP and IL-6. And the urine NE had a significantly positive correlation with Neutrophil%, DD, and PCT, which is in line with the results mentioned above, that the urine NE is a superior indicator of the inflammation and severity condition in COVID-19 patients (moderate, severe, and critical types). And the urine NE is negatively correlated with lymphocyte levels (including Lymphocyte%, CD3 Cells, and T4 cells). Taken together, NE may reflect an imbalance between neutrophils and lymphocytes, and it may be involved in neutrophil-associated amplification of inflammatory signals that drives the progression of COVID-19.
 |
Table 5 The Correlation Between NE Levels and Other Inflammation Factors |
Discussion
The main findings of this study are as follows. The ICU patients had significantly higher levels of urine NE and neutrophil percentage, as well as other classic inflammatory factors. Urine NE has a significantly positive correlation with neutrophil%, DD, and PCT. Urine NE and DD can be used to construct a two-parameter model (the UD model) for prediction of ICU admission.
Elastase for viral pathogenesis (especially for SARS-CoV-2 activation) has been proposed and reported by different scholars.24 NE is generally considered the main contributor of neutrophil protease activity.25 During viral infection, NE attacks the proteins of invading microorganisms, but it also enables the hydrolyzation of proteins in the host extracellular matrix. Therefore, it drives degenerative pathology and inflammatory signals. Theoretically, NE, at least in part, is a risk factor of COVID-19 infection/progression. Since 2021, Tehran scholars have proposed a possible pathogenic correlation between NE and inflammation in the pathogenesis of COVID-19, and they have examined clinical samples at different time points.26 In that study, serum levels of NE, IL-6, IL-8, and CRP in ICU and non-ICU patients were significantly higher than healthy controls in three-time points; and levels of NE, IL-6, IL-8, and CRP in ICU patients were significantly higher than non-ICU patients; moreover, the levels of NE, CRP, IL-6, IL-8 were gradually decreased from the day of admission to later time. There are very few in-vivo studies of NE in the lungs of COVID-19 patients. In 2023, the PET tracer 11C-NES has been made for detection of NE in the clinical setting.27 Recently, targeting NE has been regarded as a promising strategy in COVI-19 treatment. The NE inhibitor sivelestat may be a new option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19.22 Besides, scholars have previously proposed that oxidation of α1-antitrypsin (AAT) may prevent the binding of NE and play a therapeutic role in COVID-19, for that in healthy individuals, NE activity is balanced by AAT.28 However, the actual effect of targeting AAT is limited.6,28,29
Although previous studies have implied the relationships between NE and COVID-19, and group differences in blood NE have been reported as mentioned above, the role of urine NE in COVID-19 is poorly understood so far. We here, for the first time, show that the urine NE is an indicator with better performance than blood NE. The blood NE levels were similar between groups in our results, while the median urine NE of the ICU group was 6.58 times that of the non-ICU group (P < 0.0001), and the mean value of NE vs non-ICU was as high as 34.35 times. This result is very novel and interesting. However, at present we cannot confirm the mechanism of this phenomenon. One possible reason is that patients in the ICU group may have had worse renal function. However, this hypothesis awaits support from more detailed test indicators.
Moreover, the AUC of the ROC curve using only one variable, urine NE, was as high as 0.893, and that using urine NE plus DD was 0.933. In comparison, with previous studies,26,30 our results support the role for NE for COVID-19 infection and progression, however, we found the correlation is not significant at the blood level, instead, for the first time, we here report an indicative role for NE in urine. Higher urinary NE levels in ICU patients suggest that this group may be poorer to metabolize NE, which may be one of the mechanisms for the progression of COVID-19. However, it remains to be explored regarding the exact mechanism as to why there was no change in blood NE but a significant increase in urinary NE levels in high-risk patients. According to Table 5, urine NE had a significantly positive correlation with Neutrophil%, DD, and PCT, while it is negatively correlated with lymphocyte levels, which suggests that the high expression of NE may be due to continuous activation of neutrophils with a decreased proportion of lymphocytes.
Still, this study has some limitations. First, although we reported for the first time the important role of urinary NE, there was no difference in blood NE levels between the two groups, which is inconsistent with previous studies. This remains to be explained by more follow-up studies. One of the possible reasons is that our sample size is still small, and the difference is not yet significant. Besides, the performance of our models (UD and UDP) was satisfactory for the non-ICU population (89.6–90.7%). But the accuracy in the ICU cohort was lower than 90% (80.6–83.9%). Considering the consequences of ICU admission, the prevention and treatment of this population is even more important, and, therefore, the efficacy of our model still needs to be optimized by additional clinical indicators.
Conclusion
The ICU patients had significantly higher levels of urine NE and neutrophil percentage. Urine NE has a significantly positive correlation with neutrophil%, DD, and PCT, as well as a negative correlation with lymphocyte levels. Urine NE and DD can be used to construct a two-parameter model for prediction of ICU admission.
Data Sharing Statement
The data are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study complies with the Declaration of Helsinki, and the ethical approval of this study was obtained from the ethics committee of the First Affiliated Hospital of Harbin Medical University (ethical approval number: 2023IIT223).
Informed Consent
All participants have signed the informed consent prior to recruitment.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Outstanding Youth Project of Heilongjiang Provincial Natural Science Foundation (No. JQ2021H003) and the Emergency Diagnosis and Treatment Technology Research Project for Novel Coronavirus Pneumonia funded by Heilongjiang Provincial Department of Science and Technology.
Disclosure
We declare no competing interests exist.
References
1. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
2. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi:10.1001/jama.2020.5394
3. Soltane R, Almulla N, Alasiri A, et al. A comparative analysis of MicroRNA expression in mild, moderate, and severe COVID-19: insights from urine, serum, and nasopharyngeal samples. Biomolecules. 2023;13(12):1681. doi:10.3390/biom13121681
4. Schmidt W, Jóźwiak B, Czabajska Z, et al. On-admission laboratory predictors for developing critical COVID-19 during hospitalization—a multivariable logistic regression model. Ann Agric Environ Med. 2022;29(2):274–280. doi:10.26444/aaem/145376
5. Menez S, Moledina DG, Thiessen-Philbrook H, et al. Prognostic significance of urinary biomarkers in patients hospitalized with COVID-19. Am J Kidney Dis. 2022;79(2):257–267.e1. doi:10.1053/j.ajkd.2021.09.008
6. D’Amato M, Vertui V, Pandolfi L, et al. Investigating the link between Alpha-1 antitrypsin and human neutrophil elastase in bronchoalveolar lavage fluid of COVID-19 patients. Curr Issues Mol Biol. 2022;44(5):2122–2138. doi:10.3390/cimb44050143
7. Zhang R, Sun C, Han Y, et al. Neutrophil autophagy and NETosis in COVID-19: perspectives. Autophagy. 2023;19(3):758–767. doi:10.1080/15548627.2022.2099206
8. Kelly E, Greene CM, McElvaney NG. Targeting neutrophil elastase in cystic fibrosis. Expert Opin Ther Targets. 2008;12(2):145–157. doi:10.1517/14728222.12.2.145
9. Voynow JA, Fischer BM, Zheng S. Proteases and cystic fibrosis. Int J Biochem Cell Biol. 2008;40(6–7):1238–1245. doi:10.1016/j.biocel.2008.03.003
10. Hiyoshi T, Domon H, Maekawa T, et al. Neutrophil elastase aggravates periodontitis by disrupting gingival epithelial barrier via cleaving cell adhesion molecules. Sci Rep. 2022;12(1):8159. doi:10.1038/s41598-022-12358-3
11. Hansen AH, Mortensen JH, Rønnow SR, et al. A serological neoepitope biomarker of neutrophil elastase-degraded calprotectin, associated with neutrophil activity, identifies idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease more effectively than total calprotectin. J Clin Med. 2023;12(24):7589. doi:10.3390/jcm12247589
12. Lee H, Kim T-S, Gu J-Y, et al. Value of circulating neutrophil elastase for detecting recurrence of differentiated thyroid cancer. Endocr Connect. 2023;12(12). doi:10.1530/EC-23-0400
13. Lulla AR, Akli S, Karakas C, et al. Neutrophil elastase remodels mammary tumors to facilitate lung metastasis. Mol Cancer Ther. 2023;23(4):492–506.
14. Torzyk-Jurowska K, Ciekot J, Winiarski L. Targeted library of phosphonic-type inhibitors of human neutrophil elastase. Molecules. 2024;29(5):1120. doi:10.3390/molecules29051120
15. Wang L, Zhou Z, Xu X, et al. Elevated first-trimester neutrophil elastase and proteinase 3 increase the risk of gestational diabetes mellitus and adverse fetal outcomes. Reprod Biol Endocrinol. 2024;22(1):2. doi:10.1186/s12958-023-01170-x
16. Zheng S, Kummarapurugu AB, Bulut GB, et al. Neutrophil elastase activates the release of extracellular traps from COPD blood monocyte-derived macrophages. Clin Transl Sci. 2023;16(12):2765–2778. doi:10.1111/cts.13671
17. Voynow JA, Shinbashi M. Neutrophil elastase and chronic lung disease. Biomolecules. 2021;11(8):1065. doi:10.3390/biom11081065
18. Wang Z, Chen F, Zhai R, et al. Plasma neutrophil elastase and elafin imbalance is associated with acute respiratory distress syndrome (ARDS) development. PLoS One. 2009;4(2):e4380. doi:10.1371/journal.pone.0004380
19. Guéant JL, Guéant‐Rodriguez R-M, Fromonot J, et al. Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19. Allergy. 2021;76(6):1846–1858. doi:10.1111/all.14746
20. Akgun E, Tuzuner MB, Sahin B, et al. Proteins associated with neutrophil degranulation are upregulated in nasopharyngeal swabs from SARS-CoV-2 patients. PLoS One. 2020;15(10):e0240012. doi:10.1371/journal.pone.0240012
21. Pandolfi L, Fossali T, Frangipane V, et al. Broncho-alveolar inflammation in COVID-19 patients: a correlation with clinical outcome. BMC Pulm Med. 2020;20(1):301. doi:10.1186/s12890-020-01343-z
22. Sahebnasagh A, Saghafi F, Safdari M, et al. Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J Clin Pharm Ther. 2020;45(6):1515–1519. doi:10.1111/jcpt.13251
23. Zeng W, Song Y, Wang R, et al. Neutrophil elastase: from mechanisms to therapeutic potential. J Pharm Anal. 2023;13(4):355–366. doi:10.1016/j.jpha.2022.12.003
24. Watanabe R, Matsuyama S, Shirato K, et al. Entry from the cell surface of severe acute respiratory syndrome coronavirus with cleaved S protein as revealed by pseudotype virus bearing cleaved S protein. J Virol. 2008;82(23):11985–11991. doi:10.1128/JVI.01412-08
25. Chu X, Sun Z, Baek D-S, et al. Human antibody domains and fragments targeting neutrophil elastase as candidate therapeutics for cancer and inflammation-related diseases. Int J Mol Sci. 2021;22(20):11136. doi:10.3390/ijms222011136
26. Karampoor S, Hesamizadeh K, Maleki F, et al. A possible pathogenic correlation between neutrophil elastase (NE) enzyme and inflammation in the pathogenesis of coronavirus disease 2019 (COVID-19). Int Immunopharmacol. 2021;100:108137. doi:10.1016/j.intimp.2021.108137
27. Antoni G, Lubberink M, Sörensen J, et al. In vivo visualization and quantification of neutrophil elastase in lungs of COVID-19 patients: a first-in-humans PET study with (11)C-NES. J Nucl Med. 2023;64(1):145–148. doi:10.2967/jnumed.122.263974
28. D’Amato M, Campagnoli M, Iadarola P, et al. Could the oxidation of α1-antitrypsin prevent the binding of human neutrophil elastase in COVID-19 patients? Int J Mol Sci. 2023;24(17):13533. doi:10.3390/ijms241713533
29. Lascano J, Oshins R, Eagan C, et al. Correlation of alpha-1 antitrypsin levels and exosome associated neutrophil elastase endothelial injury in subjects with SARS-CoV2 infection. PLoS One. 2022;17(9):e0274427. doi:10.1371/journal.pone.0274427
30. Mustafa Z, Zhanapiya A, Kalbacher H, et al. Neutrophil elastase and proteinase 3 cleavage sites are adjacent to the polybasic sequence within the proteolytic sensitive activation loop of the SARS-CoV-2 spike protein. ACS Omega. 2021;6(10):7181–7185. doi:10.1021/acsomega.1c00363
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Cold Plasmamed Beam as a Supporting Treatment of Soft Tissue Injuries in Severe Covid-19 Patients: A Preliminary Report
Nguyen TX, Nguyen DH, Ho-Man TP, Bui VDA, Phan PN
Medical Devices: Evidence and Research 2022, 15:277-283
Published Date: 18 August 2022
Pneumothorax in Critically Ill COVID-19 Patients: Prevalence, Analysis of Risk Factors and Clinical Outcomes
AlGhamdi Z, Alqahtani SY, AlDajani K, Alsaedi A, Al-Rubaish O, Alharbi A, Elbawab H
International Journal of General Medicine 2022, 15:8249-8256
Published Date: 21 November 2022
Value of Laboratory Indicators in Predicting Pneumonia in Symptomatic COVID-19 Patients Infected with the SARS-CoV-2 Omicron Variant
Zhu K, Ma S, Chen H, Xie J, Huang D, Fu C, Ma G, Huang Y
Infection and Drug Resistance 2023, 16:1159-1170
Published Date: 28 February 2023
Determinants of Pneumothorax Among Mechanically Ventilated COVID-19 Intensive Care Unit Patients, a Single Centre Study
Hundie TG, Alemu ZA, Getachew LZ, Abera LA, Seyoum AB, Mogus LS, Admasu NM, Regassa GB, Tilahun YB, Bareamichael PI, Tessema AG, Derese TN
Journal of Multidisciplinary Healthcare 2023, 16:3977-3989
Published Date: 11 December 2023


