Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Usefulness of Serum NOX4 as a Potential Biomarker to Predict Early Neurological Deterioration and Poor Outcome of Spontaneous Intracerebral Hemorrhage: A Prospective Observational Study
Authors Wu X, He H, Shen D, Ye X, Chen Z, Zou S, Zhou K, Ye X, Zhang Z, Li H, Liu J
Received 17 December 2024
Accepted for publication 13 February 2025
Published 19 February 2025 Volume 2025:21 Pages 295—307
DOI https://doi.org/10.2147/NDT.S512801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Taro Kishi
Xiaoyu Wu,1 Heng He,1 Dapu Shen,1 Xiaohui Ye,2 Ziyin Chen,3,4 Shengdong Zou,3,4 Kechen Zhou,5 Xiufeng Ye,6 Zhixing Zhang,7 Huguang Li,1 Jin Liu1
1Department of Neurosurgery, Lishui Hospital of Wenzhou Medical University, Lishui City People’s Hospital, Lishui, Zhejiang Province, People’s Republic of China; 2Department of Nursing, Lishui Hospital of Wenzhou Medical University, Lishui City People’s Hospital, Lishui, Zhejiang Province, People’s Republic of China; 3Department of Neurosurgery, Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 4The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, People’s Republic of China; 5Jiangxi Medical College, Nanchang University, Nangchang, Jiangxi Province, People’s Republic of China; 6Department of Neurosurgery, Longquan City People’s Hospital, Lishui, Zhejiang Province, People’s Republic of China; 7Department of Neurosurgery, Jinyun County Hospital of Traditional Chinese Medicine, Lishui, Zhejiang Province, People’s Republic of China
Correspondence: Jin Liu, Department of Neurosurgery, Lishui Hospital of Wenzhou Medical University, Lishui City People’s Hospital, Lishui, Zhejiang Province, People’s Republic of China, Email [email protected]
Background: NADPH oxidase 4 (NOX4) may play a critical role for inducing oxidative stress and inflammation after spontaneous intracerebral hemorrhage (sICH). This study was performed to assess associations of serum NOX4 levels with sICH severity, early neurological deterioration (END) and neurological outcomes.
Methods: In this prospective cohort study, serum of 161 sICH patients and 161 controls were collected for quantifying NOX4 levels. END was defined as a decrease of ≥ 2 points in Glasgow coma scale (GCS) score within 24 hours of admission. Poor outcome was referred to as Glasgow Outcome Scale (GOS) scores of 1– 3 at 90 days post-stroke.
Results: As compared to controls, a significant increase in serum NOX4 levels was observed among patients. NOX4 levels were independently associated with GCS scores and hematoma volumes (all P< 0.05). The levels were significantly higher in patients with END than in those without, and in patients with poor outcome than in those with good outcome, as well as independently predicted both END (OR=3.166, 95% CI 1.237– 8.105, P=0.016) and 90-day poor prognosis (OR=3.031, 95% CI 1.111– 8.269, P=0.030). Serum NOX4 significantly differentiated patients at risk of END (area under ROC curve (AUC), 0.768; 95% confidence interval (CI), 0.695– 0.831) and poor prognosis (AUC, 0.777; 95% CI, 0.705– 0.839), which had similar prognostic ability, as compared to GCS scores and hematoma volumes (all P> 0.05).
Conclusion: Elevated serum NOX4 levels during the early period of sICH are closely related to stroke severity, END and poor neurological outcome. Hypothetically, serum NOX4 may serve as a potential prognostic biomarker in sICH.
Keywords: spontaneous intracerebral hemorrhage, NADPH oxidase 4, severity, early neurological deterioration, outcome, biomarkers
Introduction
Spontaneous intracerebral hemorrhage (sICH) represents a notable subtype of stroke characterized by a high disability and lethality, and constitutes approximately 15% of all stroke cases.1 Early neurologic deterioration (END) is one of the common complications in the early stage of the disease in patients with sICH.2 Thus, END prediction holds paramount importance for optimizing clinical management strategies in sICH cases.3 In recent years, concerted research efforts have aimed at identifying biomarkers associated with brain injury following sICH. These endeavors seek to fulfill multiple objectives, including accurate assessment of hemorrhage severity, early prediction of END, quick identification of poor prognosis, and effective guidance of clinical interventions.
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family stands out as a prominent source of reactive oxygen species (ROS) within the central nervous system.4 Among its isoforms, NADPH oxidase 4 (NOX4) holds particular importance, boasting high expression levels in the central nervous system, particularly in cerebrovascular and brain tissues. This characteristic has garnered increasing attention from researchers, who recognize NOX4 as a pivotal contributor to ROS production following brain injury.5 The imbalance between ROS generation and elimination within the organism precipitates excessive ROS accumulation, thereby triggering oxidative stress and exacerbating tissue damage. Reportedly, there was a notable elevation in NOX4 expressions within brain tissues of rats after ICH.6 Similarly, following ischemic stroke, heightened NOX4 expressions have been observed in neurons and endothelial cells, coinciding with increased neuronal apoptosis.7 Furthermore, after traumatic brain injury, both NOX4 mRNA and protein expressions show significant upregulation in the damaged cerebral cortices, with parallel findings in peripheral blood samples.8 Overall, we hypothesized that NOX4 may be a potential biomarker for acute brain injury diseases. And this study was aimed to determine whether serum NOX4 levels are associated with hemorrhage severity, END and poor outcomes at 90 days after sICH.
Materials and Methods
Study Design and Populations
This prospective observational cohort study was conducted according to the STROBE statement. Here, we recruited patients admitted between August 2020 and May 2022, who presented with a first diagnosis of sICH. All enrolled patients were required to be admitted to the hospital within 24 hours of symptom onset and be aged 18 years or older, and undergo non-surgical treatments.
Our study excluded patients based on the following criteria: (1) the presence of previous brain-injurious diseases, such as intracerebral hemorrhage, aneurysmal subarachnoid hemorrhage, severe craniocerebral trauma, and cerebral infarction; (2) a history of previous neurological diseases, such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and craniocerebral tumors; and (3) secondary intracerebral hemorrhages, such as ruptured intracranial aneurysms, craniocerebral trauma, arteriovenous malformations and ICH caused by post-infarction hemorrhagic transformation; (4) other specific conditions, such as severe systemic diseases (uremia, heart failure, cirrhosis, etc), pregnancies, infections in the last month, and surgeries; (5) refusal to participate in the study; (6) unavailable blood samples, incomplete information or loss to follow-up. The healthy controls group was prospectively recruited in the physical examination center of Lishui People’s Hospital during the same period. Some controls were excluded based on the following criteria: (1) age <18 years; (2) the presence of previous underlying diseases such as hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, cirrhosis, and heart failure; (3) a history of previous neurological disorders; (4) malignant neoplasms; (5) pregnancies; and (6) infections in the last month, and surgeries.
This study is completed based on the principles of the Declaration of Helsinki and local ethical laws, and its protocol was approved by the Ethics Committee at the Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine (Opinion No: Medical Ethics Review No. 058–01) and Lishui People’s Hospital (Opinion No: Medical Ethics Review No. 2020–001). Because patients with sICH were at state of consciousness disturbances or fluctuations, their legal representatives were informed of study details and authorized to sign written informed consent forms. And controls themselves provided written informed consent for willingness to participate in this study.
Data Collection and Clinical Assessment
We comprehensively collected patient data, including age, gender, smoking status, alcohol consumption habits, and previous underlying diseases such as hypertension, diabetes, hyperlipidemia, etc. Additionally, we recorded the time intervals from stroke onset to hospital admission and from stroke onset to blood-sampling collection. Vital signs upon admission, Glasgow Coma Scale (GCS) scores at admission, imaging data, and laboratory findings were also meticulously documented. Noteworthily, GCS scores and hematoma volumes are believably accepted as a conventional severity indicator of sICH.9 Upon arrival at emergency department, GCS scores were recorded and a head CT scan was performed, and hematoma volumes were calculated according to the ABC/2 method.10 END is defined as a decrease of ≥2 points in GCS score within post-admission 24 hours.11 Glasgow Outcome Scale (GOS) scores were utilized to evaluate the neurological prognosis of patients at 90 days post-stroke.12 Functional assessment was completed via telephone by the trained personnel. GOS scores ranging from 1 to 3 was classified as indicating poor outcomes, while scores of 4 to 5 signified good outcomes.13
Immune Analysis
Venous blood samples from patients were collected upon admission to the hospital, while those from healthy controls were obtained during routine physical examinations. Biochemical data, such as blood leukocyte counts, blood C-reactive protein (CRP) levels, blood glucose levels, etc., were routinely tested at admission. Subsequently, for determinations of serum NOX4 levels, blood samples underwent centrifugation at 3000 ×g for 15 minutes. The resulting serum samples were then stored at −80°C for subsequent testing. Serum NOX4 levels were measured using enzyme-linked immunosorbent assay. This kit was purchased from Shanghai FANKEL Industrial Co., Ltd (Shanghai, China). This process was repeated twice for all samples to ensure accuracy and reliability of results. The average of the two measurements was calculated for each sample and further utilized for statistical analysis. The technician was unaware of the clinical information, which was pertinent to the patients, for ensuring unbiased testing and analysis.
Statistical Analysis
Data were statistically analyzed using the SPSS 25.0 (IBM Corp. Armonk, NY, USA) and graphs were plotted using the GraphPad Prism 8.0 (GraphPad Software Inc. La Jolla, CA, USA). Normality distribution of quantitative data was assessed using the Kolmogorov–Smirnov test or Shapiro–Wilk test, with normally distributed data expressed as mean ± standard deviation, and non-normally distributed data as median (upper-lower quartiles). Qualitative data were expressed as counts (proportions). For intergroup comparisons, the χ2 test or Fisher exact test was employed for qualitative data, and the Mann–Whitney U-test or t test for quantitative data. The Kruskal–Wallis test was used for multiple-intergroup comparisons of serum NOX4 levels, and the Mann–Whitney U-test was applied for two-by-two comparisons between groups. Bivariate correlations were analyzed using the Spearman correlation test. Multivariate linear regression models were constructed to identify variables which were independently associated with serum NOX4 levels. For the sake of comparing the differences in each variable between END and non-END patients as well as between patients with a poor 90-day prognosis and a good prognosis, univariate logistic regression models were established to analyze the relationship between each variable and END or 90-day poor outcome in patients with sICH. Binary logistic regression models were configured to identify variables, which were independently associated with the occurrence of END or 90-day adverse outcome after stroke. Odds ratios (ORs) and the corresponding 95% confidence intervals (95% CI) values were calculated for showing associations. Subsequently, a receiver operating characteristic curve (ROC) curve was constructed to investigate the predictive value of serum NOX4 levels for the occurrence of END or adverse outcomes in sICH patients, and the Area under curve (AUC) was estimated, and the Z-test was used to compare the AUCs. Nomogram models were built to predict the risk of END and poor outcomes. The calibration curves were drawn to validate the stability of the predictive models. Two-tailed P<0.05 signifies statistical difference.
Results
Study Populations
A total of 214 patients, who were admitted to our hospital within 24 hours of the first-onset symptom of sICH, were initially included in the study. Subsequently, 53 patients were excluded from this study, who comprised 6 patients with a recent history of infection or surgery within the past month, 15 with secondary cerebral hemorrhage, 10 with other neurological disorders, 10 with known malignancies or other specific diseases or conditions, 5 with unavailable blood samples, 3 because of refusal to participation, and 4 owing to loss to follow-up. Ultimately, 161 patients were enrolled in the study, all of whom received conservative treatment for hematoma. Baseline features and laboratory assessments of initially enrolled patients (n=214) and finally eligible patients (n=161) were shown in Supplemental Table 1. Additionally, 161 healthy controls were recruited for comparison purposes. No statistically significant differences were observed in terms of age, gender, smoking, and alcohol consumption between the patients and healthy controls (all P>0.05; Supplemental Table 1).
Change of Serum NOX4 Levels After sICH
Among this cohort of sICH patients, the median value of time between symptom onset and blood sample-collection was 7.6 h (upper and lower quartiles, 5.2–12.8 h). Serum NOX4 levels in sICH patients ranged from 2.1–17.6 ng/mL (median, 7.6 ng/mL; upper and lower quartiles, 6.2–9.7 ng/mL). In contrast, serum NOX4 levels ranged from 0.3–2.5 ng/mL (median, 0.5 ng/mL; upper and lower quartiles, 0.4–0.6 ng/mL) in controls. sICH patients had significantly higher serum NOX4 levels as compared with controls (P<0.001; Figure 1).
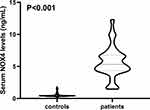 |
Figure 1 Boxplot illustrating admission serum NADPH oxidase 4 levels between patients with spontaneous intracerebral hemorrhage and controls. Abbreviation: NOX4, NADPH oxidase 4. |
Serum NOX4 Levels and Disease Severity After sICH
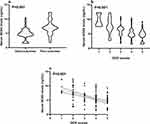
As shown in Table 1, serum NOX4 levels significantly correlated with GCS scores (P<0.001) and hematoma volumes (P<0.001). Close correlations were also found with age (P<0.001), intraventricular bleedings (P<0.001), blood CRP levels (P<0.01), blood leukocyte count (P<0.01) and blood glucose levels (P<0.01). Consistent findings were obtained through univariate binary linear regression analysis (Table 1). Following the incorporation of the variables significantly correlated with serum NOX4 levels into multifactorial linear regression analysis, notable distinctions persisted between GCS scores and serum NOX4 levels, as well as hematoma volumes and serum NOX4 levels (Table 1, Figure 2a and b). In Figure 2c and d, patients were categorized based on GCS scores or hematoma volumes, namely, GCS scores of 3–8, 9–12, and 13–15, or hematoma volumes <30 mL and ≥30 mL. It was observed that serum NOX4 levels exhibited significant associations with both GCS scores and hematoma volume following sICH, irrespective of whether they were analyzed as categorical or continuous variables (Figure 2, all P<0.001).
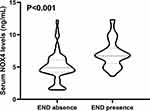
Relationship Between Serum NOX4 Levels and END
A total of 51 patients (31.7%) experienced END after stroke. Serum NOX4 levels were notably higher in patients who suffered from END than those who did not (Figure 3). In Figure 4a, serum NOX4 levels efficiently predicted the occurrence of END after sICH, with AUC at 0.768 (95% CI, 0.695–0.831). Using the Youden method, a cutoff value of 9.0 ng/mL was determined, which predicted END with sensitivity of 70.6% and specificity of 77.3%, with the maximal Jordon index J at 0.479. Interestingly, our study demonstrated that serum NOX4 levels had an efficient predictive ability for END, which was comparable to that of GCS scores (AUC=0.774, 95% CI 0.701–0.836, P=0.881) and hematoma volumes (AUC=0.828, 95% CI 0.761–0.883, P=0.097) (Figure 4b). Subsequently, we constructed a combined binary Logistic regression model using serum NOX4 levels, GCS scores and hematoma volumes. The model (AUC=0.868, 95% CI 0.805–0.916) exhibited significantly higher predictive capability than those of serum NOX4 levels, GCS scores and hematoma volumes for END (all P<0.05).
Additionally, as opposed to patients not experiencing END, those with development of END exhibited significantly lower GCS scores, older age, higher blood leukocyte counts, serum CRP levels, blood glucose levels, hematoma volumes, and serum NOX4 levels, as well as higher proportion of intraventricular hemorrhage (all P<0.05; Supplemental Table 2). Using univariate logistic regression analysis, those substantial findings were corroborated (all P<0.05; Supplemental Table 3). Moreover, following the inclusion of the aforementioned significant variables on univariate analysis into multivariate model, hematoma volumes and serum NOX4 levels exceeding 9.0 ng/mL were identified as independent predictors of END in sICH patients (Table 2).
 |
Table 2 Multivariate Logistic Regression Analysis of Predictors for Early Neurological Deterioration After Spontaneous Intracerebral Hemorrhage |
As shown in Supplemental Figure 1, the variables with significant differences on the multifactorial logistic regression model were included in the nomogram model to predict the associated risks. And the scores corresponding to the above two variables were summed to calculate the total scores, and different scores corresponded to different levels of risk, which were 0.1=17.9, 0.3=45.5, 0.5=62.8, 0.7=80.1, and 0.9=107.7, respectively. Under the calibration curve, the prediction model was relatively stable (Supplemental Figure 2).
Relationship Between Serum NOX4 Levels and 90-Day Poor Outcomes After sICH
A total of 58 patients experienced poor outcomes (GOS scores 1–3) at 90 days after sICH. Notably, serum NOX4 levels were significantly elevated in patients with poor outcomes, as compared to those with good outcomes (median, 9.8 ng/mL vs 7.0 ng/mL, P<0.001; Figure 5a). Subsequently, patients were categorized based on GOS scores, namely, GOS scores of 1, 2, 3, 4 and 5. It was observed that serum NOX4 levels exhibited significant associations with GOS scores following sICH, whether it was analyzed as a categorical or continuous variable (Figure 5b and c) (all P<0.001).
In Figure 6a, serum NOX4 emerged as a significant discriminator of poor outcomes following sICH, with AUC of 0.777 (95% CI, 0.705–0.839). Using a cutoff value of 9.1 ng/mL, serum NOX4 levels distinguished poor outcomes with a sensitivity of 70.7% and a specificity of 84.5%, with a maximal Jordon’s index of 0.552. Markedly, serum NOX4 levels displayed significant predictive ability for poor outcomes, which was similar to those of GCS scores (AUC=0.852, 95% CI 0.787–0.903, P=0.053) and hematoma volumes (AUC=0.854, 95% CI 0.790–0.905, P=0.080) (Figure 6b). Subsequently, we constructed a combined binary Logistic regression model using serum NOX4 levels, GCS scores and hematoma volumes. The model (AUC=0.896, 95% CI 0.839–0.939) exhibited significantly higher predictive capability than those of serum NOX4 levels, GCS scores and hematoma volumes for poor outcome (all P<0.05).
Furthermore, as compared to patients with good outcomes, those with poor outcomes exhibited significantly lower GCS scores, were significantly older, displayed substantially higher blood leukocyte count, blood CRP levels, blood glucose levels, hematoma volumes and serum NOX4 levels, as well as had dramatically higher proportion of intraventricular hemorrhage (all P<0.05; Supplemental Table 4). Using univariate logistic regression analysis, such findings still existed (P<0.05; Supplemental Table 5). Moreover, as depicted in Table 3, after incorporation of the aforementioned variables with significant differences into a multivariate logistic regression model, hematoma volumes, GCS scores and serum NOX4 levels exceeding 9.1 ng/mL appeared as the three independent predictors of poor outcomes at 90 days after stroke.
 |
Table 3 Multivariate Logistic Regression Analysis of Predictors for 90-Day Poor Outcomes After Spontaneous Intracerebral Hemorrhage |
As shown in Supplemental Figure 3, we included the variables with significant differences in the multifactorial logistic regression model in the nomogram model to predict the associated risks. And the scores corresponding to the above three variables were summed to calculate the total scores, and different scores corresponded to different levels of risk, which were 0.1=36.9, 0.3=63.2, 0.5=80.9, 0.7=98.7, and 0.9=127.0, respectively. We found that serum NOX4 levels >9.1 ng/mL, GCS scores, and hematoma volumes effectively predicted the risk of poor prognosis in patients with sICH. Under calibration curve, the prediction model was relatively stable (Supplemental Figure 4).
Discussion
sICH represents a grave neurological condition, which has significantly increased the burden of mortality and disability worldwide.14 END emerges as a frequent and severe complication at the initial phase during sICH.15 In clinical practice, GCS scores and hematoma volumes serve as pivotal indicators for evaluating disease severity following sICH and predicting both END and subsequent neurological sequelae.16 Over the years, researchers have directed their attention towards elucidating the prognostic predictive capacity of peripheral blood biomarkers in the context of sICH. The application of biomarkers such as S100B, interleukin-12 and matrix metalloproteinase-1 in the assessment of disease severity and prognosis prediction of ICH has received extensive attentions from scholars in the past decades.17–19 And it was found that peripheral blood biomarkers may be able to improve prognostic predictability and help to guide clinical treatment in patients with sICH.
In our present study, we found that (1) serum NOX4 levels were significantly elevated in sICH patients in the early stage of the disease; (2) serum NOX4 levels showed a significant positive correlation with hematoma volumes on admission and a significant negative correlation with GCS scores; (3) serum NOX4 levels > 9.0 ng/mL were significantly effective in differentiating the risk between patients who experienced END and those who did not, and serum NOX4 levels > 9.1 ng/mL could effectively differentiated patients with poor outcomes from those with good outcomes after sICH; (4) the predictive ability of serum NOX4 levels for END and poor outcomes were similar to those of GCS scores and hematoma volumes. In summary, serum NOX4 levels were strongly associated with disease severity, development of END, and neurological function outcomes, suggesting that serum NOX4 may be a potential prognostic biomarker for sICH patients.
NOX4 is widely present in the central nervous system and is one of the major sources of ROS in brain tissues.20 It has been found that NOX4 is involved in the development of a variety of acute brain injury diseases. After ischemic stroke, the expressions of NOX4 protein in neurons as well as endothelial cells were significantly elevated.21 In addition, after experimental brain injury, including ICH, traumatic brain injury and subarachnoid hemorrhage the expressions of NOX4 in brain tissues were actually increased.6,22,23 Further studies showed that NOX4-/- mouse had a better neurological prognosis after traumatic brain injury, as compared to controls.22 Its neuroprotective mechanisms were related to reductions of lesion size, oxidative damage, neurodegeneration and apoptosis in injured brain tissues.23 In the ischemic stroke rat model, NOX4 inhibitor significantly diminished infarct area, effectively suppressed elevated ROS levels and neuronal apoptotic degeneration, and strongly reduced blood brain barrier damage and subsequently obviously improved neurological function.24 These findings underscore the potential therapeutic significance of targeting NOX4 in mitigating the detrimental consequences of acute brain injury.
In several clinical studies, researchers have observed elevated expression levels of NOX4 protein in peripheral neurons and astrocytes within injured brain tissues among patients with acute brain injury diseases.22,25,26 Moreover, there was a significant elevation in NOX4 levels in the peripheral blood of patients during the early stages of traumatic brain injury and aneurysmal arachnoid hemorrhage.27,28 Such a level elevation may be attributed to the substantial releasing of NOX4 from central nervous system to peripheral blood via disrupted blood-brain barrier following acute brain injury. Also, NOX4 levels in both brain tissue and peripheral blood were tremendously correlated with disease severity and prognosis of such patients. For instance, a study involving 165 aneurysmal subarachnoid hemorrhage patients and 165 controls demonstrated that serum NOX4 levels upon admission strongly correlated with World Federation of Neurosurgical Societies scores and modified Fisher scores. Additionally, elevated serum NOX4 levels effectively predicted the occurrence of delayed cerebral ischemia and poor prognosis in those patients.28 Similarly, another study of 105 patients with traumatic brain injury showed a strong correlation between serum NOX4 levels and GCS scores in addition to GOS scores at 90 days post-injury; and serum NOX4 was identified as an independent predictor of death and poor prognosis in such patients.29 These findings are extremely indicative of the assumption that serum NOX4 may be potentially used as a prognostic biomarker of acute brain injury.
Here, we sought to investigate the relationship between peripheral blood NOX4 levels, disease severity and prognosis in patients with sICH. Through multifactorial analysis, we discovered that serum NOX4 levels in sICH patients were independently associated with GCS scores, hematoma volumes, and GOS scores at 90 days post-stroke. Serum NOX4 levels exceeding 9.0 ng/mL effectively differentiated the risk of END, while levels exceeding 9.1 ng/mL effectively differentiated patients with poor outcomes (GOS score 1–3). By multifactorial logistic regression models, serum NOX4 was confirmed as an independent predictor of END and poor outcomes, exhibiting a predictive ability, which was comparable to those of GCS scores and hematoma volumes, and demonstrating strong model stability. In summary, elevated serum NOX4 levels exhibited significant correlations with disease severity and prognosis in patients after stroke. Additionally, serum NOX4 levels demonstrated high predictive value for 90-day poor outcomes and the occurrence of END. Also, the joint models showed exhibited significantly higher predictive capability than those of serum NOX4 levels, GCS scores and hematoma volumes for END occurrence and poor outcome, which can help clinicians grasp the risk stratification and prognostic predictions after sICH better. Alternatively, determinations of serum NOX4 may change clinical practice, eg decision about DNR / DNO. Therefore, NOX4 may hold promise as a potential biomarker for assessing brain injury in the context of sICH.
Several limitations warrant to be considered. First, the sample size was relatively small, encompassing only 161 patients with sICH. Consequently, it is imperative to carry out a large cohort study in future to validate the current findings and enhance the generalizability of our results. Second, serum NOX4 levels were measured only upon admission in sICH patients. It may be of benefit if serum NOX4 levels could be assessed at multiple time-points post-admission (eg, days 1, 2, 3, 5, 7, and 10). This would facilitate the observation of the dynamic changes in serum NOX4 levels throughout the course of sICH, thus providing valuable insights into the temporal evolution of NOX4 expression in relation to disease progression. Such an approach holds potential for offering more clinically relevant information regarding the role of NOX4 in the pathophysiology of sICH and its implications for patient management. Moreover, considering that NOX4 is one of the markers of oxidative stress, its specific mechanisms in secondary brain injury post-sICH should be further studied so as to aid in exploration of a new therapeutic agent of sICH. Third, it may be unnecessary to select a total of 161 healthy controls for clinical investigation based on statistical power analysis. As a fact, for the sake of better heightening research efficiency and lowering potential risks to study subjects, reducing sample size can be an alternative suitable modality. Fourth, National Institute of Health stroke scale, in comparison to GCS, displays higher advantages for neurological assessment in minor stroke, modified Rankin scale may be more suitable for assessing neurological outcome than GOS and hematoma expansion may be related to END. So, utilizations of GCS and GOS for neurological and outcome evaluation and non-observation of hematoma expansion in this study may cause bias of results. Fifth, blood samples of patients were measured for the first time after admission. However, a part of blood samples were collected after 24 hours after symptom onset. In the follow-up study, we will collect the blood samples of patients within 24 hours of symptom onset. Sixth, the impact of wake up-stroke patients on study outcomes should also be considered, although they accounted for a small proportion of patients with sICH. Seventh, GOS scores at 3 months after stroke could not be recorded for patients lost to follow-up, these patients were not included in the 3-month outcome and END analysis. The exclusion of patients with missed visits may have an impact on the results of the experiment. Finally, we will pay more attention to the relationship between cardiovascular risk factors and serum NOX4 levels.
Conclusion
In this study, the association of serum NOX4 levels with disease severity, END, and 90-day functional outcomes in sICH patients were analyzed for the first time using multivariate analysis. Notably, elevated serum NOX4 levels were independently associated with GCS scores and hematoma volumes. Furthermore, serum NOX4 levels exceeding 9.0 ng/mL and 9.1 ng/mL took possession of high efficacy in predicting the risk of END and poor outcomes, respectively. Importantly, serum NOX4 levels exhibited similar predictive abilities for END and prognosis, as compared to GCS scores and hematoma volumes. In conclusion, our findings suggest that serum NOX4 holds promise as a potential biomarker for assessing the severity of sICH, predicting the occurrence of END, and forecasting long-term functional prognosis. Further research and validation studies are warranted to corroborate these findings and elucidate the clinical utility of serum NOX4 in the management of sICH patients.
Abbreviations
sICH, spontaneous intracerebral hemorrhage; ICH, intracerebral hemorrhage, GCS, Glasgow coma scale; GOS, Glasgow outcome scale; ROC, receiver operating characteristic; AUC, area under curve; 95% CI, 95% confidence interval; ROS, reactive oxygen species.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical Statement
This study was carried out in accordance with the ethical guidelines of the Helsinki Declaration, and its protocol was approved by the Institutional Review Committee of the Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine (Opinion number: Medical Ethics Review No. (058)-01) and Lishui People’s Hospital (Opinion No: Medical Ethics Review No. 2020-001, 2020-002). Legal representatives of patients and controls themselves gave written informed consent to participate in this study.
Acknowledgments
The authors thank all staffs in Department of Neurosurgery, the Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine (Hangzhou, China) and Department of Neurosurgery, Lishui Hospital of Wenzhou Medical University (Lishui, China) for their technical support.
Author Contributions
Xiaoyu Wu, Heng He and Dapu Shen contributed equally to this work. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is financially supported by Medical and Health Research Project of Zhejiang province (2023XY260, 2025KY1969, 2025KY1970), City-level public welfare technology application research project of Lishui (2021SJZC086, 2021SJZC080, 2023SJZC078 and 2023SJZC101) and Public Welfare Technology Research Program of Lishui (2022GYX24, 2024GYX69).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. JAMA. 2019;321(13):1295–1303. doi:10.1001/jama.2019.2413
2. Zhu W, Zhou J, Ma B, Fan C. Predictors of early neurological deterioration in patients with intracerebral hemorrhage: a systematic review and meta-analysis. J Neurol. 2024;271(6):2980–2991. doi:10.1007/s00415-024-12230-6
3. Lv XN, Cheng J, Liu XY, et al. Ultraearly intraventricular hemorrhage growth predicts early neurologic deterioration and poor functional outcome after acute intracerebral hemorrhage. J Am Heart Assoc. 2023;12(21):e031214. doi:10.1161/JAHA.123.031214
4. Kovac S, Angelova PR, Holmström KM, Zhang Y, Dinkova-Kostova AT, Abramov AY. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850(4):794–801. doi:10.1016/j.bbagen.2014.11.021
5. Nishimura A, Ago T, Kuroda J, et al. Detrimental role of pericyte Nox4 in the acute phase of brain ischemia. J Cereb Blood Flow Metab. 2016;36(6):1143–1154. doi:10.1177/0271678X15606456
6. Xie J, Hong E, Ding B, et al. Inhibition of NOX4/ROS suppresses neuronal and blood-brain barrier injury by attenuating oxidative stress after intracerebral hemorrhage. Front Cell Neurosci. 2020;14:578060. doi:10.3389/fncel.2020.578060
7. Li G, Ye C, Zhu Y, et al. Oxidative injury in ischemic stroke: a focus on NADPH oxidase 4. Oxid Med Cell Longev. 2022;2022:1148874. doi:10.1155/2022/1148874
8. Li Z, Tian F, Shao Z, et al. Expression and clinical significance of non-phagocytic cell oxidase 2 and 4 after human traumatic brain injury. Neurol Sci. 2015;36(1):61–71. doi:10.1007/s10072-014-1909-z
9. You S, Zheng D, Delcourt C, et al. Determinants of early versus delayed neurological deterioration in intracerebral hemorrhage. Stroke. 2019;50(6):1409–1414. doi:10.1161/STROKEAHA.118.024403
10. Gebel JM, Sila CA, Sloan MA, et al. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998;29(9):1799–1801. doi:10.1161/01.str.29.9.1799
11. Yan T, Wang ZF, Wu XY, et al. Plasma SIRT3 as a biomarker of severity and prognosis after acute intracerebral hemorrhage: a prospective cohort study. Neuropsychiatr Dis Treat. 2022;18:2199–2210. doi:10.2147/NDT.S376717
12. Sreekrishnan A, Dearborn JL, Greer DM, et al. Intracerebral hemorrhage location and functional outcomes of patients: a systematic literature review and meta-analysis. Neurocrit Care. 2016;25(3):384–391. doi:10.1007/s12028-016-0276-4
13. Wang S, Xu X, Yu Q, Hu H, Han C, Wang R. Combining modified Graeb score and intracerebral hemorrhage score to predict poor outcome in patients with spontaneous intracerebral hemorrhage undergoing surgical treatment. Front Neurol. 2022;13:915370. doi:10.3389/fneur.2022.915370
14. Garg R, Biller J. Recent advances in spontaneous intracerebral hemorrhage. F1000Res. 2019;8:F1000FacultyRev–302. doi:10.12688/f1000research.16357.1
15. Kuohn LR, Witsch J, Steiner T, et al. Early deterioration, hematoma expansion, and outcomes in deep versus lobar intracerebral hemorrhage: the FAST trial. Stroke. 2022;53(8):2441–2448. doi:10.1161/STROKEAHA.121.037974
16. Lin F, He Q, Tong Y, et al. Early deterioration and long-term prognosis of patients with intracerebral hemorrhage along with hematoma volume more than 20 mL: who needs surgery? Front Neurol. 2022;12:789060. doi:10.3389/fneur.2021.789060
17. Honegger T, Schweizer J, Bicvic A, et al. Serum S-100B adds incremental value for the prediction of symptomatic intracranial hemorrhage and brain edema after acute ischemic stroke. Eur Stroke J. 2023;8(1):309–319. doi:10.1177/23969873221145391
18. Zhang Y, Tian Y, Wei J, Xiang Y. Relationship of serum IL-12 to inflammation, hematoma volume, and prognosis in patients with intracerebral hemorrhage. Emerg Med Int. 2022;2022:8688413. doi:10.1155/2022/8688413
19. Lorente L, Martín MM, Ramos L, et al. High serum tissue inhibitor of matrix metalloproteinase-1 levels and mortality in patients with spontaneous intracerebral Hemorrhage. World Neurosurg. 2020;134:e476–e480. doi:10.1016/j.wneu.2019.10.106
20. Boonpraman N, Yoon S, Kim CY, Moon JS, Yi SS. NOX4 as a critical effector mediating neuroinflammatory cytokines, myeloperoxidase and osteopontin, specifically in astrocytes in the hippocampus in Parkinson’s disease. Redox Biol. 2023;62:102698. doi:10.1016/j.redox.2023.102698
21. Radermacher KA, Wingler K, Langhauser F, et al. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal. 2013;18(12):1418–1427. doi:10.1089/ars.2012.4797
22. Ma MW, Wang J, Dhandapani KM, Wang R, Brann DW. NADPH oxidases in traumatic brain injury - Promising therapeutic targets? Redox Biol. 2018;16:285–293. doi:10.1016/j.redox.2018.03.005
23. Zhang L, Li Z, Feng D, et al. Involvement of Nox2 and Nox4 NADPH oxidases in early brain injury after subarachnoid hemorrhage. Free Radic Res. 2017;51(3):316–328. doi:10.1080/10715762.2017.1311015
24. Ma MW, Wang J, Dhandapani KM, Brann DW. Deletion of NADPH oxidase 4 reduces severity of traumatic brain injury. Free Radic Biol Med. 2018;117:66–75. doi:10.1016/j.freeradbiomed.2018.01.031
25. He R, Jiang Y, Shi Y, Liang J, Zhao L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater Sci Eng C Mater Biol Appl. 2020;117:111314. doi:10.1016/j.msec.2020.111314
26. Sorokina EG, Semenova ZB, Reutov VP, et al. Brain biomarkers in children after mild and severe traumatic brain injury. Acta Neurochir Suppl. 2021;131:103–107. doi:10.1007/978-3-030-59436-7_22
27. Lucke-Wold BP, Naser ZJ, Logsdon AF, et al. Amelioration of nicotinamide adenine dinucleotide phosphate-oxidase mediated stress reduces cell death after blast-induced traumatic brain injury. Transl Res. 2015;166(6):509–528.e1. doi:10.1016/j.trsl.2015.08.005
28. Pan J, Lao L, Shen J, et al. Utility of serum NOX4 as a potential prognostic biomarker for aneurysmal subarachnoid hemorrhage. Clin Chim Acta. 2021;517:9–14. doi:10.1016/j.cca.2021.02.007
29. Jiang F, Chen Z, Hu J, Liu Q. Serum NOX4 as a promising prognostic biomarker in association with 90-day outcome of severe traumatic brain injury. Int J Gen Med. 2022;15:5307–5317. doi:10.2147/IJGM.S366170
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Prospective Longitudinal Cohort Study of Serum Stanniocalcin-1 as a Potential Prognostic Biomarker of Severe Traumatic Brain Injury
Jin C, Huang X, Hu Y, Xu B, Ma J
Therapeutics and Clinical Risk Management 2024, 20:341-361
Published Date: 11 June 2024
Alteration of Serum MLKL Levels and Their Association with Severity and Clinical Outcomes in Human Severe Traumatic Brain Injury: A Prospective Cohort Study
Jin Y, Zhang H, Zhou M, Zhang S, Guo M
International Journal of General Medicine 2024, 17:5069-5084
Published Date: 6 November 2024
Prognostic Significance of Serum NLRP3 in Spontaneous Intracerebral Hemorrhage
Cai Y, Ma Y, Tang C, Li W, Lv X, Xie Z, Wang J
International Journal of General Medicine 2025, 18:745-757
Published Date: 12 February 2025