Back to Journals » International Journal of Nanomedicine » Volume 20
Using siRNA-Based Anti-Inflammatory Lipid Nanoparticles for Gene Regulation in Psoriasis
Authors Zeng A, Liu Y, Wang P, Cao Y, Guo W
Received 6 December 2024
Accepted for publication 20 March 2025
Published 12 April 2025 Volume 2025:20 Pages 4519—4533
DOI https://doi.org/10.2147/IJN.S504639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Aizhong Zeng,* Yuanyuan Liu,* Ping Wang, Yufei Cao, Wei Guo
School of Life Science and Technology, China Pharmaceutical University, Nanjing, 211112, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Guo, School of Life Science and Technology, China Pharmaceutical University, Nanjing, 211112, People’s Republic of China, Email [email protected]
Background: Psoriasis is a chronic inflammatory autoimmune disease, yet it affects hundreds of millions of people. Long-term effective intervention of the disease by targeting the causative genes via RNAi (RNA interference) has become a reality. However, its further application is hindered by inflammatory side effects caused by delivery systems such as LNP (lipid nanoparticles).
Purpose: This study aimed to develop a novel anti-inflammatory LNP rationally tailored for topical application in psoriasis and to validate its potential to deliver Stat3 (signal transducer and activator of transcription 3) siRNA for the treatment of psoriasis.
Methods: To assess the transfection efficiency, anti-inflammatory capacity of LNPs. The therapeutic effect of modified anti-inflammatory LNP delivery of Stat3 siRNA on psoriasis was evaluated both in vitro and in an imiquimod-induced mice.
Results: LNPs exhibit both superior transfection efficiency and significant anti-inflammatory effects. In vitro functional studies showed that in an inflammatory DC model, anti-inflammatory LNP (C8B2) inhibited inflammatory mediators much better than classical LNPs by delivering Stat3 siRNA; in pathological HaCat cells, Stat3 siRNA reduced cell proliferation and promoted apoptosis. In the imiquimod-induced mouse model, the C8B2-si-Stat3 group demonstrated a clear reduction in psoriasis progression, whereas the C8B2 carrier group also exhibited a notable decrease in inflammation.
Conclusion: In this study, we successfully developed a novel anti-inflammatory LNP, which demonstrated notable advantages in delivery capacity, anti-inflammatory effect, and targeting therapy against STAT3, providing new ideas and strategies for nucleic acid therapy of psoriasis. This LNP platform could be broadly applicable to various inflammatory conditions, offering a versatile tool for targeted gene modulation and inflammation control.
Keywords: psoriasis, lipid nanoparticle, RNA interference, STAT3
Graphical Abstract:

Introduction
Psoriasis is a chronic, relapsing, inflammatory skin disease driven by autoimmune mechanisms. Globally, approximately 125 million individuals suffer from psoriasis, exhibiting significant geographical variability in prevalence, with equal incidence rates among men and women, and onset at any age.1,2
Psoriasis is characterized by clinical manifestations such as localized or extensive skin thickening, scaling, and erythema. Pathologically, it is marked by features like epidermal keratosis with acanthosis, a diminished or absent stratum granulosum, an expanded stratum spinosum, and vascular changes in the form of elongated and dilated capillaries. Additionally, there is an infiltration of immune lymphocytes in both the dermis and epidermis.3 Due to autoimmune overactivity, patients with psoriasis frequently experience a range of comorbid conditions. About one-third of patients with psoriasis progress to psoriatic arthritis, and other high-risk comorbidities include cardiovascular disease, inflammatory bowel disease, anxiety disorders, and depression.4 In addition, the absence of timely and correct diagnosis and treatment, coupled with social prejudice and other factors, significantly impacts the quality of life for patients, while also imposing a substantial economic burden and psychological strain.5
While significant advancements have been achieved in the management and treatment of psoriasis through modern medical research and clinical practice, existing traditional drug therapies are fraught with limitations.6–8 These impediments hinder the attainment of a disease cure, mitigation of toxic side effects, and the facilitation of personalized treatment regimens.9,10 Signal transducer and activator of transcription 3 (STAT3) represents a crucial class of transcription factors.11 Activated STAT3 facilitates the transcription of several key genes involved in the differentiation, activation, and proliferation of Th17 cells.12,13 Moreover, STAT3 plays a significant role in the signaling pathways of IL-6, IL-21, and IL-23 receptors.14–16 In recent years, several genome-wide association studies have revealed a serious association between psoriasis and STAT3 gene mutations.17 The expression level of STAT3 is markedly elevated in the skin lesion tissues of psoriasis patients.18 Inhibitors targeting STAT3 have a broad therapeutic prospect theoretically, but STAT3 is a typical non-catalytic transcription factor, and small molecules are difficult to be drug-formulated.19 To date, a limited number of STAT3-targeting inhibitors have advanced to clinical trials, yet they face numerous challenges regarding in vivo efficacy, specificity, formulation, and safety.20 Therefore, exploring a novel therapeutic strategy that can effectively target STAT3 directly and overcome the shortcomings of existing drugs is crucial for improving the outcome of psoriasis patients. In 2018, the first siRNA-LNP therapeutic drug was approved, marking a significant milestone in the transition of RNAi therapies from theory to clinical practice. Subsequently, five more highly specific, effective, and long-lasting siRNA drugs have been approved, paving the way for a broad range of chronic conditions.21 Direct siRNA targeting of STAT3 mRNA effectively inhibits the aberrant transmission of the STAT3 signaling pathway, offering a novel approach for the long-term control of psoriasis. LNP has emerged one of the preferred technologies in the field of nucleic acid drug delivery, due to its efficient delivery capacity, good biocompatibility, scalable production, and clinically proven effectiveness.22 However, the strong immunogenicity of LNP has limited the further application of LNP drugs.23 Relevant evidence indicates that in vivo application of LNP leads to a swift and robust inflammatory response, including massive neutrophil infiltration, activation of multiple inflammatory pathways, and production of a variety of inflammatory cytokines and chemokines.24 Onpattro, an intravenously administered siRNA medication necessitates pretreatment with glucocorticosteroids and antihistamines prior to administration to minimize the incidence of adverse reactions.25 In some cases, a moderate inflammatory response may be beneficial, as it can temporarily increase vascular permeability, aid drug penetration, and serve as a vaccine adjuvant to elicit a more robust immune response.26,27 However, excessive inflammation can be detrimental, particularly in the management of chronic or inflammatory diseases, and even exacerbate the condition.28 The challenge lies in ensuring that LNP maintains high transfection efficiency while effectively managing its inflammatory side effects, making this a critical focus for ongoing LNP research and optimization. Indeed, other nucleic acid systems such as polymers or inorganic metal nanoparticles have the advantage of simple preparation, but suffer from the disadvantages of low delivery efficiency and poor safety; extracellular vesicles have good biocompatibility, but their production and quality control are still challenging. The development of novel potentially ionizable lipid molecules for LNP is a long and difficult task. In this study, we innovatively incorporated a fifth anti-inflammatory lipid into the formulation, drawing from the tetralipid composition of conventional lipid nanoparticles, to mitigate inflammation in immune cells through RNAi technology. Rationally designed anti-inflammatory LNPs for topical application in psoriasis were synthesized and characterized. The delivery efficiency, anti-inflammatory effects, and therapeutic potential of these LNPs for the treatment of psoriasis were subsequently validated in vitro and in vivo.
Materials and Methods
Lipid Nanoparticle Preparation
LNPs were prepared either by vortex mixing or microfluidic mixing as previously described. Briefly, an aqueous solution of the siRNA and an ethanolic solution of the lipid components were mixed at a ratio of 3:1, respectively. The ethanol phase consists of ionizable lipids ALC-0315 (Avanti, China) or cationic lipids 1,2-dioleoyl-3-trimethylammonium propane (DOTAP, Avanti, China), 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC, Avanti, China), cholesterol (Avanti, China) and 1,2-dimyristoyl-sn-glycerol, methoxypolyethylene glycol (DMG-PEG2000, Avanti, China), budesonide (MedChemExpress, USA). The aqueous phase was prepared in 10 mm citrate buffer pH 4 containing siRNA (GenePharma, China). After mixing, ethanol, free siRNA and lipids were removed by centrifugal ultrafiltration (MWCO 30 kDa; Amicon, MilliporeSigma, USA) at 3000 g for 30 min, and the buffer was replaced with RNase-free PBS. Finally, the LNPs were filtered using a 220 nm filter membrane.
LNP Formulation Design
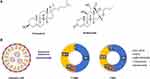
Based on previous research base formulations containing 50% ionizable lipids (ALC-0315) or 50% cationic lipids (DOTAP), 10% DSPC, 1.5% DMG-PEG2000, and 38.5% cholesterol by molar ratio were used as standard formulations for LNP. Keeping the molar ratios of the remaining lipid components unchanged, cholesterol was replaced by budesonide at various molar percentages (20%, 40%, 60%, 80%, and 100%) such that the total amount of cholesterol and budesonide combined in any given formulation was still 38.5% of the total lipid molar amount. Each candidate formulation is named by the cholesterol, budesonide and their proportions. For example, a formulation containing 80% cholesterol and 20% budesonide (ie, 20% replacement) is named C8B2. The preferred formulation is C8B2, because C8B2 has similar physical characteristics and significant anti-inflammatory effects as the baseline formulation, taking into account the principle of the lowest effective dose of glucocorticoids.
LNP Characterization
The prepared LNP was diluted 40 times with PBS buffer. After stabilization at room temperature, the z-average diameter (size) and polydispersity index (PDI) and zeta potential of the LNPs were determined using Zetasizer Nano (Malvern Instruments, Malvern).
The siRNA encapsulation efficiency of LNP was determined using Quant-it RiboGreen RNA assay (Invitrogen). For the determination of free RNA in LNP- siRNA samples, 1×TE buffer was used at a certain ratio, and for the determination of total RNA, 2% TE-Triton buffer was used at a certain ratio, 100 μL of RiboGreen and 100 μL of sample were added to each well, and the samples were incubated for 5–10 min. Data were recorded using a multifunctional enzyme marker Spark (Tecan Group Ltd) with excitation light 485 nm and emission light 528 nm. Encapsulation efficiencies are reported as calculated values: 1 - Rf / Rt, where Rf is the amount of free siRNA and Rt is the amount of total siRNA.
Cells Culture
Cell lines were maintained in DMEM or RPMI-1640 medium (KeyGEN, China) supplemented with 10% fetal bovine serum (FBS, Gibco Life Technologies). The source of cell lines: DC2.4 cell (Procell, china); HaCat cell (Procell, china).
In Vitro Transfection
Cells were seeded in 12-well plates at 20,000 DC2.4 cells or HaCat cells per well. The following day, siRNA-LNPs were added to the cells at a concentration of 30 nM siRNA per well. Stat3 mRNA level was detected by qRT-PCR 24 h after transfection; STAT3 and Phospho-STAT3 protein level was detected by Western Blot 72 h after transfection. For the inflammation model, DC2.4 cells were treated with R848 (1.5 μg/mL, MedChemExpress) to stimulate the production of inflammatory factors. qRT-PCR was performed to detect the relative levels of TNF-α, IL-1β and IL-23 mRNA after transfection for 48 h.
Quantitative Real-Time PCR
Total cellular RNA was extracted using Trizol reagent (Invitrogen) in accordance with the manufacturer’s instructions. Total RNA in each sample was reverse transcribed into cDNA, The resulting cDNA (40 ng/μL) was used as a template to quantify the relative levels of mRNA using a Hieff qPCR SYBR Green Master Mix (Yeasen Biotechnology Co., Ltd) with QuantStudioTM 3 System (Applied Biosystems Inc). The relative expression of the target gene was calculated using the 2^(−ΔΔCT) method. The relevant primers used were listed in Table S1, Supporting Information.
CCK-8 Assay
HaCat and DC2.4 cells and inoculate the cells in 96-well plates according to 5000 cells/well, after 12 h of incubation in the incubator, transfected with 30 nM si-NC, respectively, the cells continued to be cultured for 24 h and 48 h. 10 μL CCK-8 solution was added to each well, and the cells were incubated in the incubator at 37°C for 2 h. Absorbance was detected by multifunctional enzyme marker Spark at a wavelength of 450 nm and measured. Data processing: calculate cell viability (%) = A (experimental group)/A (control group) × 100% A: absorbance of CCK8 solution at 450 nm.
Western Blot Analysis
Cells were rinsed twice in pre-cooled PBS, lysed in RIPA Lysis Buffer (Beyotime, China) containing protease and phosphatase inhibitors, and protein concentration was determined using the Pierce BCA Protein Assay (Thermo Fisher Scientific). All samples were heat denatured in SDS-PAGE sample buffer prior to sampling. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane and sealed with primary antibodies (STAT3 Rabbit mAb, 1:1000; Phospho-STAT3 Rabbit mAb, 1:1000; GAPDH Rabbit mAb, 1:1000; Cyclin A2 Rabbit mAb, 1:1000; CDK1 Rabbit mAb, 1:1000, Cell Signaling Technology), secondary antibody (Goat Anti-Rabbit IgG H&L [HRP], 1:4000, Abcam) were incubated together. Images were visualized by BeyoECL Plus (Beyotime, China) and captured by Tanon 5500 (Tanon, China) imaging system.
Flow Cytometry
The apoptosis of HaCaT cells was quantified using the Annexin V-FITC/PI Apoptosis Detection Kit (MultiSciences, China), in accordance with the manufacturer’s instructions. In brief, 20,000 haCaT cells were inoculated into each well of a 12-well plate. Following transfection with siRNA LNP 48 hours later, the cells were harvested, washed with PBS, and then incubated with membrane-bound protein-V fluorescein isothiocyanate (AnnexinV-FITC) and PI for 15 minutes at room temperature in the dark. Thereafter, the percentage of apoptotic cells was measured by flow cytometry (BD Biosciences). The apoptosis rate was defined as the sum of the early apoptosis rate and the late apoptosis rate.
Enzyme-Linked Immunosorbent Assay (ELISA)
The expression levels of TNF-α and IL-23 were quantified using ELISA kits (MultiSciences, China) in accordance with the manufacturer’s instructions. Prior to testing the samples, the kits were removed from the refrigerator and allowed to reach room temperature. For cell supernatant samples, the samples should be centrifuged at 300×g for 10 minutes in order to remove any precipitates that may have formed. The mouse blood samples were collected for ELISA analysis by excising the eyeballs under deep anaesthesia, the samples should be left at room temperature for 30 minutes, after which they should be centrifuged at 1.000×g for 10 minutes, aspirate the serum samples, and then test the concentrations of TNF-α and IL-23 according to the instructions of the TNF-α and IL-23 ELISA kits, respectively. Subsequently, the optical density (OD) value was determined at a wavelength of 450 nm using a multifunctional enzyme marker (Spark). The OD value was then read at 450 nm, and a standard curve was constructed with the OD value as the vertical coordinate and the concentration as the horizontal coordinate. The value was subsequently substituted into the aforementioned regression equation to determine the concentration of each specimen.
Animal Studies
All animal studies were approved by China Pharmaceutical University (approval number 2023–08-022). The procedures and studies were conducted in accordance with the standards set forth by the China Pharmaceutical University Animal Care and Use Committee and in compliance with all applicable regulations. Eight-week-old BALB/c female mice were selected and housed in an SPF-grade animal room. After one week of acclimatisation, the mice were randomly divided into four groups: the normal group (Normal), the model group (Model), the C8B2-si-NC administration group, and the C8B2-si-Stat3 administration group. Each group consisted of five mice. The model and drug-dosing groups applied 30 mg of 5% imiquimod cream in a uniform manner to the right ear on a daily basis from day 0, with the aim of inducing a psoriasis model. The normal group, in contrast, applied an equal amount of medical petroleum jelly cream. In the drug administration group, 0.5 nmol of siRNA was injected intra-auricularly into the right ear of each mouse on day −1 and day 1.
Statistical Analysis
All data are presented as the mean ± standard deviation (SD), with n values representing the number of individuals in each experimental group. Statistical analyses were performed using GraphPad Prism 8 software, employing one-way ANOVA or Student’s t-test where appropriate. A p-value < 0.05 was considered indicative of statistical significance. Flow cytometry data were analyzed using FlowJo version 10.6.2.
Results
LNPs Design, Formulation, and Characterization
The objective of the present study was to investigate and analyse the impact of the adulteration of the steroidal lipid budesonide on the function of LNP. Budesonide is a synthetic steroid hormone drug with anti-inflammatory activity that belongs to the same steroid family as cholesterol and shares a similar cyclic structural basis (Figure 1A). It was hypothesised that doping budesonide to replace part of the cholesterol could preserve the delivery capacity of LNP while mitigating its inflammatory side effects. Firstly, a series of LNPs were prepared by the addition of budesonide and the reduction of cholesterol to the tetralipid fraction of the classical LNP, without any alteration to the molar ratios of the remaining lipid components (Figure 1B). The characterisation of the LNPs was conducted through the evaluation of their size, polydispersity (PDI), mRNA encapsulation efficiency and zeta potential (Table 1). The results demonstrated that following the doping of budesonide to replace a portion of the cholesterol, the LNPs could still form stable nanoparticles. The particle sizes of the LNPs were observed to range from 150 to 250 nm, with zeta potentials within the range of −10 to 0 mV, PDIs below 0.2, and encapsulation rates exceeding 85.00%.
 |
Table 1 Characterization of LNPs |
Effectiveness of LNPs Transfection in Vitro
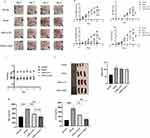
LNPs were prepared using ALC-0315, and 30 nM si-GAPDH was used to transfect HaCat cells (Figure 2A). LNPs were prepared using ALC-0315, and 30 nM si-Stat3 was used to transfect HaCat cells (Figure 2B). LNPs were prepared using ALC-0315, and 30 nM si-GAPDH was used to transfect DC 2.4 cells (Figure 2C). LNPs were prepared using DOTAP, and 30 nM si-GAPDH was used to transfect HaCat cells (Figure 2D). Subsequently, the relative expression of the target mRNAs in the cells was detected by qRT-PCR after 24 hours. The results demonstrated that all 30 nM siRNAs were capable of effectively silencing the target genes, and the budesonide-doped LNPs exhibited comparable silencing efficiencies to the standard LNP prescription (ALC0315, C10B0). Notably, even in LNP(DOTAP), budesonide-doped LNPs demonstrated a higher silencing efficiency. Independent of the amount of budesonide incorporation, there was no significant difference in transfection efficiency between C8B2 and other LNP batches.
Anti-Inflammatory Potential of Budesonide-Doped LNPs
LNPs were prepared using ALC-0315. Twenty-four hours after transfection of DC2.4 cells with 30 nM si-NC, the relative levels of cellular inflammatory factors IL-1β, IL-6, and TNF-α mRNA were detected using quantitative reverse transcription polymerase chain reaction (qRT-PCR). The standard LNP prescription (ALC-0315, C10B0) induced the expression of IL-1β, IL-6, and TNF-α as compared to the PBS group. Furthermore, groups of budesonide-adulterated LNPs demonstrated a significant inhibitory effect on the production of these inflammatory mediators (Figure 3A). Replacing 20% cholesterol with budesonide was effective in reducing the LNP inflammatory response, and C8B2 showed the strongest anti-inflammatory effect of all batches. Similar results were obtained in HaCat cells (Figure 3B). 0.5 nmol si-NC LNPs were administered intradermally into C57BL/6 mice, and the skin tissues were harvested 24 h later. The relative levels of IL-1β, IL-6, and TNF-α mRNA were then detected using quantitative reverse transcription polymerase chain reaction (qRT-PCR). The C10B0 group was observed to induce a significant production of TNF-α, IL-1β, and IL-6 in mouse skin tissues when compared to the PBS control group (Figure 3C). In contrast, the C8B2 group demonstrated an inhibitory effect on TNF-α, IL-1β, and IL-6 production.
C57BL/6 mice were intravenously injected with 1.0 nmol of si-NC LNPs, and serum samples were collected 24 hours later. An ELISA assay was conducted to ascertain the concentration of TNF-α. The C10B0 group elicited an elevation in TNF-α levels in mouse serum compared to the PBS group, whereas the C8B2 group demonstrated a near-neutralization of TNF-α production (Figure 3D). These findings indicate that budesonide-doped LNPs can effectively mitigate the inflammatory response triggered by standard LNPs in vitro and in vivo.
In Vitro Delivery of Stat3 siRNA by C8B2 Inhibits Psoriasis
To assess the safety of LNPs, a CCK-8 assay was conducted to evaluate the impact of si-NC at 30 nM and 60 nM on the viability of HaCat and DC2.4 cells (Figure 4A). Neither C10B0 nor C8B2 exhibited notable cytotoxicity (Figure 4A). The impact of LNPs delivering si-Stat3 on HaCat cell apoptosis was evaluated using flow cytometry. Compared to the si-NC group, both C10B0-si-Stat3 and C8B2-si-Stat3 at 60 nM effectively induced apoptosis in HaCat cells. However, the pro-apoptotic effect of C10B0-si-STAT3 was pronounced (Figure 4B). HaCat cells were stimulated with IL-17A and transfected with 60 nM si-Stat3 LNPs for 48 hours. Western blot analysis was then performed to detect the expression levels of Cyclin-A2 and CDK1. IL-17A stimulation resulted in STAT3 up-regulation, which was inhibited by both C10B0-si-STAT3 and C8B2-si-STAT3, as well as by the inhibition of cell proliferation-related protein expression, namely Cyclin-A2 and CDK1 (Figure 4C). DC2.4 cells were treated with R848 (1.5 μg/mL), and the relative levels of TNF-α, IL-1β, and IL-23 mRNA were assessed by qRT-PCR after transfection of DC2.4 with 30 nM C10B0-si-NC, C10B0-si-STAT3, and C8B2-si-Stat3 for 48 h.
Stimulation of DC 2.4 with R848 induces an increase in psoriasis-associated inflammatory factors. Both C10B0-si-Stat3 and C8B2-si-Stat3 were observed to reduce the production of inflammatory factors in comparison to C10B0-si-NC. However, the inflammation-suppressing effect of C8B2-si-Stat3 was notably more pronounced than that of C10B0-si-Stat3 (Figure 4D). These findings suggest that C10B0 and C8B2 exhibit a favourable safety profile for normal cells. The in vitro delivery of Stat3 siRNA by LNPs effectively inhibited the expression of proliferative proteins associated with pathological keratinocytes and promoted their apoptosis. Furthermore, both Stat3 siRNA LNPs demonstrated the capacity to inhibit the expression of psoriasis-associated inflammatory mediators. In contrast, C8B2-si-Stat3 was observed to induce an appropriate pro-apoptotic response in keratinocytes, while also exhibiting a superior anti-inflammatory effect.
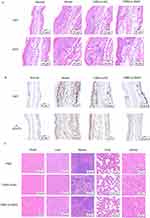
Topical Application of C8B2-Si-Stat3 Inhibits Imiquimod-Induced Psoriasis
Following the induction of psoriasis and the topical application of C8B2-si-Stat3, the skin tissues were harvested and subjected to analysis. A macroscopic examination revealed that both C8B2-si-NC and C8B2-si-Stat3 exhibited a reduction in imiquimod-induced inflammation in comparison to the model. As a consequence of the inhibitory effect of Stat3 siRNA, C8B2-si-Stat3 displayed a more pronounced degree of cutaneous inflammation than C8B2-si-NC (Figure 5A). The application of the topical treatment resulted in a notable reduction in erythema, scaling and PASI scores, with the C8B2-si-Stat3 group exhibiting the lowest clinical scores (Figure 5B). As anticipated, mice in the imiquimod-exposed group exhibited transient weight loss and an elevated splenic index. However, the topical application of C8B2 did not elicit a systemic response (Figure 5C and D). The concentration of inflammatory factors in the mice serum was determined using an ELISA kit. The serum levels of TNF-α and IL-23 were elevated in the model group in comparison with the normal group, and a meaningful reduction was observed following treatment with C8B2-si-NC and C8B2-si-Stat3. In comparison to the C8B2-si-NC group, the levels of TNF-α and IL-23 also demonstrated a tendency to decrease in the C8B2-si-Stat3 group (Figure 5E). Similarly, H&E staining and IHC analysis demonstrated a high proliferation of keratinocytes, accompanied by strong positive Ki67 and p-STAT3 staining, in the untreated group. In contrast, the C8B2-si-NC-treated group exhibited insufficient proliferation of keratinocytes and weakly positive Ki67 and p-STAT3 staining. The C8B2-si-stat3-treated group displayed the most pronounced treatment effect (Figure 6A and B). Furthermore, the topical application of C8B2 did not result in any discernible toxic effects on the major organs of the system (Figure 6C). In conclusion, the topical application of C8B2-si-Stat3 was effective in alleviating psoriasis-related symptoms and reducing levels of psoriasis-related inflammatory mediators without any adverse systemic effects.
Discussion
Psoriasis is a highly complex autoimmune disease whose pathogenesis remains incompletely understood.29 The limited understanding of the role of key inflammatory signalling pathways in psoriasis in relation to the regulation of immune cells presents a challenge to the development of effective treatments.30 Recent findings indicate that STAT3 activation is of particular significance with regard to the development of psoriasis. Hyperactivation of STAT3 affects virtually all cell types involved in the initiation and maintenance of psoriasis. These include the regulation of the differentiation and function of T cells and other immune cells, promotion of hyperproliferation of keratinocytes and the phenomenon of aberrant keratinization, and involvement in the survival and activation of tissue-resident memory T cells.31,32 Consequently, the targeting of STAT3 in order to inhibit pro-inflammatory signalling pathways and the production of psoriasis-related gene products represents a viable strategy for the treatment of psoriasis.33,34 In clinical trials, small molecule inhibitors targeting Janus kinase (JAK), an upstream molecule of STAT3, have demonstrated promising efficacy in meeting primary endpoints and improving disease status.35,36 However, no JAK inhibitor has been approved for the treatment of psoriasis. Furthermore, JAK family kinases phosphorylate multiple proteins simultaneously, often with unavoidable toxic side effects.37 Therefore, there is a need to develop inhibitors that directly target STAT3 without affecting JAKs.
It is anticipated that the inhibition of STAT3 gene activity through RNAi will result in a more precise and sustained effect than would otherwise be achievable. In recent years, significant advances have been made in the development of siRNA drugs, with the advent of chemical modification technology and novel delivery systems.38 Lipid nanoparticle-based delivery systems have emerged as the most widely used and intensively studied nucleic acid delivery platforms.39 Nevertheless, the considerable immunogenicity of LNP represents a significant obstacle to further applications. A substantial body of evidence indicates that the lipid component of LNP can elicit a robust inflammatory response in immune cells.40,41 LNP has been demonstrated to activate the immune system by interacting with pattern-recognition receptors on antigen-presenting cells, resulting in the release of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, and others. In a murine model, Ndeupen et al demonstrated that intradermal injection of LNP lacking ionizable cationic components was efficacious in abrogating the skin inflammatory response. Conversely, flow cytometry revealed a substantial and expeditious leukocyte infiltration in skin samples injected intradermally with empty-shell LNP prepared from ionizable cationic lipids. The results of these studies indicate that the ionizable cationic lipid component of LNP preparations plays an indispensable role in mediating inflammatory responses.24 These inflammatory responses reflect, on the one hand, the potential self-adjuvant effect of LNP as a vaccine composition and, on the other hand, excessive or persistent inflammation that may result in unavoidable toxic side effects. Indeed, other delivery systems, including polymers and inorganic materials, have also been reported to elicit a substantial immunological response42,43. In order to optimise the safety and efficacy of nucleic acid drugs, researchers have continued to work on improving LNP formulations. This has involved the development of novel ionizable lipids, the adjustment of the ratio of lipid components, the improvement of the tissue specificity and intracellular delivery efficiency of LNPs, and the aim of enhancing the delivery efficiency and reducing unwanted inflammatory side effects. For example, Tang et al developed mLNPs based on mildronate with only 1% cationic lipids, which were demonstrated to have high in vivo transfection efficiency and an extremely low inflammatory response.44 The development of new delivery materials or new ionizable lipids is a complex, challenging, and long-term endeavour, and is subject to a new wave of clinical trials.
This study proposes a novel yet straightforward approach to developing an anti-inflammatory lipid nanoparticle (LNP) by modifying the conventional LNP carrier. The addition of a fifth anti-inflammatory lipid, budesonide, to the tetralipid component of the LNP aims to mitigate the inflammatory response that may be induced by conventional LNPs, while providing a safer and more efficient delivery platform. The experimental results demonstrated that the modified LNP not only exhibited sustained delivery efficacy but also enhanced anti-inflammatory activity. This was evidenced by the preparation, physical characterisation, detection of delivery ability, and comparison of anti-inflammatory ability of LNPs. In the inflammatory DC model, the inhibitory effect of C8B2-si-Stat3 on inflammatory mediators was superior to that of the classical LNP. In the experiments on pathological HaCat cells, although C8B2-si-STAT3 exhibited slightly inferior inhibitory effects on cell proliferation and promotion of apoptosis compared to C10B0-si-STAT3, its regulatory effects on cell proliferation and apoptosis remained significant. This may be attributed to the addition of budesonide, which attenuated the cytotoxicity of cationic lipids and optimized the therapeutic effect. Subsequently, the study further validated the therapeutic effect of C8B2-si-Stat3 in a mouse model of psoriasis. The psoriasis model induced by imiquimod was observed to successfully mimic human psoriasis symptoms, including erythema, scaling, and skin thickening, with overexpression of p-STAT3. The administration of C8B2-si-Stat3 resulted in the complete inhibition of skin thickening, a reduction in clinical scores, and an improvement in dermatopathological damage in the animal model. Furthermore, the C8B2-si-NC group, which was loaded with nonsense siRNA, also demonstrated some therapeutic benefits. This provides additional evidence that the C8B2 vector itself possesses anti-inflammatory properties and suggests that it may have potential for use in the treatment of inflammatory diseases. Furthermore, the biocompatibility assessment of C8B2 demonstrated that local application did not result in any toxic effects on major organs, thereby enhancing the safety profile for clinical applications. In conclusion, the novel anti-inflammatory LNP formulation, C8B2, not only enhances the treatment of psoriasis but also holds promise for addressing other inflammatory conditions such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis by modulating STAT3 signaling. Its optimized composition reduces immune activation and toxicity risks, making it suitable for long-term RNAi therapies in chronic diseases. Additionally, C8B2’s robust manufacturing process ensures scalability for clinical applications, while its compatibility with existing drug delivery systems opens avenues for combination therapies, enhancing overall treatment efficacy. To guarantee the clinical efficacy of C8B2, its long-term stability and scalability are critical. We have initiated efforts to optimize these key attributes, focusing on enhancing stability through improved formulations and supporting large-scale production while maintaining product quality consistency.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant numbers 82473841).
Disclosure
Dr Aizhong Zeng reports a patent Application of anti-inflammatory steroids in lipid nanoparticles pending to Aizhong Zeng. Dr Yuanyuan Liu reports a patent Application of anti-inflammatory steroids in lipid nanoparticles pending to Zeng Aizhong, Liu Yuanyuan, Guo Wei. Dr Wei Guo reports a patent Application of anti-inflammatory steroids in lipid nanoparticles pending to Zeng AIzhong, Liu Yuanyuan, Guo Wei. The authors report no conflicts of interest in this work.
References
1. Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi:10.1136/bmj.m1590
2. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
3. Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
4. Gelfand JM. Psoriasis - more progress but more questions. N Engl J Med. 2024;390(6):561–562.
5. Griffiths CE, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/S0140-6736(20)32549-6
6. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi:10.3390/ijms20061475
7. Boehncke WH, Brembilla NC. Unmet needs in the field of psoriasis: pathogenesis and treatment. Clin Rev Allergy Immunol. 2018;55(3):295–311. doi:10.1007/s12016-017-8634-3
8. Sharma A, Upadhyay DK, Gupta GD, et al. IL-23/Th17 axis: a potential therapeutic target of psoriasis. Curr Drug Res Rev. 2022;14(1):24–36. doi:10.2174/2589977513666210707114520
9. Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63(4):278–285.
10. Lee HJ, Kim M. Challenges and future trends in the treatment of psoriasis. Int J Mol Sci. 2023;24(17):13313. doi:10.3390/ijms241713313
11. Xue C, Yao Q, Gu X, et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. 2023;8(1):204. doi:10.1038/s41392-023-01468-7
12. Banerjee S, Biehl A, Gadina M, et al. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(8):939. doi:10.1007/s40265-017-0736-y
13. Howell MD, Kuo FI, Smith PA. Targeting the janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi:10.3389/fimmu.2019.02342
14. Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847. doi:10.3389/fimmu.2019.02847
15. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390. doi:10.1007/s12016-018-8702-3
16. Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 2: applications in psoriasis, atopic dermatitis, and other dermatoses. Actas Dermosifiliogr. 2021;112(7):586–600. doi:10.1016/j.ad.2020.12.006
17. Tsoi LC, Spain SL, Knight J, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44(12):1341–1348. doi:10.1038/ng.2467
18. Zhou J, Bian H, Wu N. Protein inhibitor of activated STAT3 (PIAS3) attenuates psoriasis and associated inflammation. J Dermatol. 2023;50(10):1262–1271. doi:10.1111/1346-8138.16874
19. Shiah JV, Grandis JR, Johnson DE. Targeting STAT3 with proteolysis targeting chimeras and next-generation antisense oligonucleotides. Mol Cancer Ther. 2021;20(2):219–228. doi:10.1158/1535-7163.MCT-20-0599
20. Shi W, Yan D, Zhao C, et al. Inhibition of IL-6/STAT3 signaling in human cancer cells using Evista. Biochem Biophys Res Commun. 2017;491(1):159–165. doi:10.1016/j.bbrc.2017.07.067
21. Ahn I, Kang CS, Han J. Where should siRNAs go: applicable organs for siRNA drugs. Exp Mol Med. 2023;55(7):1283–1292. doi:10.1038/s12276-023-00998-y
22. Jia Y, Wang X, Li L, et al. Lipid nanoparticles optimized for targeting and release of nucleic acid. Adv Mater. 2024;36(4):e2305300. doi:10.1002/adma.202305300
23. Vlatkovic I. Non-immunotherapy application of LNP-mRNA: maximizing efficacy and safety. Biomedicines. 2021;9(5):530. doi:10.3390/biomedicines9050530
24. Ndeupen S, Qin Z, Jacobsen S, et al. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24(12):103479. doi:10.1016/j.isci.2021.103479
25. Davies N, Hovdal D, Edmunds N, et al. Functionalized lipid nanoparticles for subcutaneous administration of mRNA to achieve systemic exposures of a therapeutic protein. Mol Ther Nucleic Acids. 2021;24:369–384. doi:10.1016/j.omtn.2021.03.008
26. Suzuki Y, Miyazaki T, Muto H, et al. Design and lyophilization of lipid nanoparticles for mRNA vaccine and its robust immune response in mice and nonhuman primates. Mol Ther Nucleic Acids. 2022;30:226–240. doi:10.1016/j.omtn.2022.09.017
27. Alameh MG, Tombácz I, Bettini E, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2022;55(6):1136–1138. doi:10.1016/j.immuni.2022.05.007
28. Parhiz H, Brenner JS, Patel PN, et al. Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE). J Control Release. 2022;344:50–61. doi:10.1016/j.jconrel.2021.12.027
29. Liang Y, Sarkar MK, Tsoi LC, et al. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1–8. doi:10.1016/j.coi.2017.07.007
30. Xu X, Zhang HY. The immunogenetics of psoriasis and implications for drug repositioning. Int J Mol Sci. 2017;18(12):2650. doi:10.3390/ijms18122650
31. Calautti E, Avalle L, Poli V. Psoriasis: a STAT3-centric view. Int J Mol Sci. 2018;19(1):171. doi:10.3390/ijms19010171
32. Kishimoto M, Komine M, Sashikawa-Kimura M, et al. STAT3 activation in psoriasis and cancers. Diagnostics. 2021;11(10):1903. doi:10.3390/diagnostics11101903
33. Welsch K, Holstein J, Laurence A, et al. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol. 2017;47(7):1096–1107. doi:10.1002/eji.201646680
34. Ti C, Chen H, Zhou W, et al. WB518, a novel STAT3 inhibitor, effectively alleviates IMQ and TPA-induced animal psoriasis by inhibiting STAT3 phosphorylation and Keratin 17. Int Immunopharmacol. 2024;127:111344. doi:10.1016/j.intimp.2023.111344
35. Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561. doi:10.1016/S0140-6736(14)62113-9
36. Asahina A, Etoh T, Igarashi A, et al. Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double-blind, phase 3 study. J Dermatol. 2016;43(8):869–880. doi:10.1111/1346-8138.13258
37. Plens-Galaska M, Szelag M, Collado A, et al. Genome-wide inhibition of pro-atherogenic gene expression by multi-STAT targeting compounds as a novel treatment strategy of CVDs. Front Immunol. 2018;9:2141. doi:10.3389/fimmu.2018.02141
38. Hu B, Zhong L, Weng Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5(1):101. doi:10.1038/s41392-020-0207-x
39. Hald Albertsen C, Kulkarni JA, Witzigmann D, et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416. doi:10.1016/j.addr.2022.114416
40. Liu Y, Hardie J, Zhang X, et al. Effects of engineered nanoparticles on the innate immune system. Semin Immunol. 2017;34:25–32. doi:10.1016/j.smim.2017.09.011
41. Alameh MG, Tombácz I, Bettini E, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54(12):2877–2892.
42. Mandal A, Kumbhojkar N, Reilly C, et al. Treatment of psoriasis with NFKBIZ siRNA using topical ionic liquid formulations. Sci Adv. 2020;6(30):eabb6049.
43. Shi W, Fuad ARM, Li Y, et al. Biodegradable polymeric nanoparticles increase risk of cardiovascular diseases by inducing endothelium dysfunction and inflammation. J Nanobiotechnol. 2023;21(1):92. doi:10.1186/s12951-023-01839-w
44. Liu J, Xiao B, Yang Y, et al. Low-dose mildronate-derived lipidoids for efficient mRNA vaccine delivery with minimal inflammation side effects. ACS Nano. 2024;18(34):23289–23300. doi:10.1021/acsnano.4c06160
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.