Back to Journals » Risk Management and Healthcare Policy » Volume 18
Using the Task-Oriented Quality Control Circle to Build the Central Sterile Supply Department Quality Control System for Foreign Objects Remaining in Sterile Packages
Authors Yao S, Yi L , Hu R, Chen Y, Pan W, Zhang J, Zhao X
Received 26 December 2024
Accepted for publication 2 April 2025
Published 27 April 2025 Volume 2025:18 Pages 1441—1454
DOI https://doi.org/10.2147/RMHP.S514458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jongwha Chang
Shunyu Yao,1,2 Liangying Yi,1,2 Ruixue Hu,1,2 Yanhua Chen,1,2 Wei Pan,1,2 Jinhui Zhang,1,2 Xiaochun Zhao1,2
1Department of Sterile Processing Nursing, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, People’s Republic of China
Correspondence: Liangying Yi, Department of Sterile Processing Nursing, West China Second University Hospital, Sichuan University, No. 20 section 3, South Renmin Road, Chengdu, Sichuan, 610041, People’s Republic of China, Email [email protected]
Background: The occurrence of foreign objects in sterile packages may delay surgeries and affect the quality of medical treatment.
Purpose: This study aimed to use the task-oriented quality control circle (QCC) to build the central sterile supply department (CSSD) quality control system for foreign objects remaining in sterile packages.
Methods: A task-oriented QCC was created under the leadership of the CSSD within our hospital. The following were investigated by determining a topic and a task, setting targets, formulating strategies and determining optimal strategies: incidence of foreign objects remaining in sorted and folded textiles; incidence of foreign objects remaining in contaminated instrument packages; incidence of foreign objects remaining in clean textiles; incidence of complaints for foreign objects remaining in sterile packages; and satisfaction among the CSSD and operating room staff.
Results: The incidence of foreign objects remaining in contaminated textiles decreased from 7.1% to 0.96%. The incidence of foreign objects remaining in contaminated instrument packages decreased from 0.27% to 0.04%. The incidence of foreign objects remaining in clean textiles decreased from 2.91% to 0.21%. The incidence of complaints received from operating room staff for foreign objects remaining in sterile packages decreased from 41.69% to 0. Satisfaction among the operating room and CSSD staff was improved from 53.7% to 100%.
Conclusion: The task-oriented QCC could reduce the incidence of foreign objects remaining in sterile packages, thereby reducing the quality defect rates in instrument packaging.
Keywords: task oriented, quality control circle, sterile packages, foreign objects, central sterile supply department
Introduction
The Central Sterile Supply Department (CSSD) is responsible for the cleaning, disinfection, sterilization and sterile supply of all reusable instruments, utensils and articles for diagnosis and treatment in the hospital. It plays a significant role in the prevention and control of nosocomial infections. Packaging is a key step in instrument reprocessing in the CSSD. It provides a barrier against microorganisms, ensuring that instruments remain sterile during storage, transportation and until clinical use. Previous studies have shown that the rates of quality defects in packaging of sterile packages are between 1.43‰ - 1.67‰.1–3 Quality defects in packaging have always been the focus of CSSD quality management.4 Foreign objects remaining in sterile packages refer to the unexpected substances (such as suture needles, blades, dressings, fragments of packaging materials, hair, and environmental particles) remaining in the sterile packages after being reprocessed by the CSSD.5 Previous studies have shown that the occurrence of foreign objects remaining in sterile packages are associated with various factors, such as inadequate cleaning of instruments and wraps, damage to packaging materials, and secondary contamination during sterilization.6–8 If sterile packages with foreign objects are used for surgery, this will violate the requirements of the operating room safety management rules.9,10 They can lead to counting errors of dressings, and a foreign object being left in a patient’s body could cause infections or the death of the patient.11,12 Therefore, when a foreign object is found in a sterile package, this sterile package must be regarded as a failure. It should be sent back to the CSSD for reprocessing according to the CSSD’s relevant guidelines,13–16 and the guidelines for operating room safety management.17,18 This will delay emergency surgeries and affect the quality of medical treatment.
Most studies on quality control focus on using cleaning and packaging optimization to reduce the incidence of defects in the CSSD,19–22 lacking the studies of cross-departmental collaboration.23 Our study went beyond conventional single-department quality control frameworks by adopting a multi-disciplinary collaboration approach. In our study, a process quality control system was developed based on a multi-disciplinary team consisting of staff representatives from the CSSD, operating room, Laundry Department and Logistics Department. Standardized operating procedures and cross-departmental training and quality control measures were implemented to reduce the incidence of foreign objects remaining in sterile packages.
A quality control circle (QCC) is a quality management group consisting of frontline employees who collaborate to continuously improve work quality and efficiency through teamwork and the Plan-Do-Check-Act cycle. The QCCs can be classified into problem-solving-oriented and task-oriented, which are applied differently in healthcare quality management.24
The problem-solving-oriented QCC is usually used to solve specific quality problems that have already occurred, focusing on analyzing causes and taking improvement measures to improve the overall quality through local optimization. It is applicable in routine management in hospitals,25 such as reducing documentation errors in nursing records, minimizing medical equipment malfunctions, and optimizing surgical instrument counting procedures.8,26 Its theoretical basis relies on failure analysis and root cause analysis (such as fishbone diagrams and the 5W1H method (what, why, where, when, who, and how)).
The task-oriented QCC aims to complete a task that contribute to achieving long-term quality improvement. It is often used for healthcare quality management problems involving multiple departments and complex processes, such as optimizing nosocomial infection control systems, improving aseptic techniques in the operating rooms, and building multi-disciplinary diagnosis and treatment models. The work procedures of the task-oriented QCC usually contain 9 steps (Figure 1): QCC formation → topic determination → task determination (problem investigation and breakthrough point identification) → target setting → strategy formulation and optimal strategy determination → implementation of optimal strategies → effect confirmation → standardization and dissemination.
 |
Figure 1 Steps of task-oriented quality control circle. |
The task-oriented QCC is usually suitable for a high-challenge, long-term task requiring cross-departmental collaboration. It focuses on determination of a clear task, a detailed implementation plan, and continuous evaluation and improvement. This is different from other quality improvement tools. For example, Lean management focuses on minimizing waste, optimizing processes and improving efficiency. It can improve productivity by reducing unnecessary processes or steps. It is ideal for improving the operational efficiency of hospitals.27 Six Sigma focuses on reducing defects, improving quality and reducing variation. It employs statistical analysis to systematically analyze problems. It is suitable for identified recurring problems.28 Total quality management emphasizes organization-wide involvement and continuous improvement of employees, management and service processes, ensuring the permeation of quality in every aspect of an organization.29
This study used a task-oriented QCC to build a quality control system for foreign objects remaining in sterile packages. Through cross-departmental collaboration and process control based on a multi-disciplinary team, this study integrated expertise and resources across departments to collaboratively solve complex quality management problems and effectively reduce the incidence of foreign objects remaining in sterile packages. This study fostered closer cooperation across departments and could provide a new theoretical basis and practical experience for the continuous improvement of quality management in hospitals.
Materials and Methods
Ethical Approval
Ethical approval was waived by the Medical Research Ethics Committee of West China Second University Hospital, Sichuan University [2024 Medical Scientific Research for Ethical Approval No. (053)].
General Information
This was a quasi-experimental study. A total of 32718 sterile packages distributed by our hospital’s CSSD from April 1, 2023 to April 30, 2023 were classified into the control group. A total of 35899 sterile packages distributed by our hospital’s CSSD from December 1, 2023 to December 31, 2023 were classified into the experimental group.
Inclusion and Exclusion Criteria
All reusable medical devices cleaned, disinfected and packaged by our CSSD were included in this study. The exclusion criteria were as follows: (1) disposable medical devices; (2) medical devices sterilized by our CSSD after being cleaned, disinfected and packaged by other hospitals’ CSSDs; or (3) loaner instrumentation reprocessed by medical device companies.
If unexpected substances (such as suture needles, blades, dressings, fragments of packaging materials, hair, and environmental particles) remained in sterile packages after being reprocessed by the CSSD, these packages would be considered the sterile packages with foreign objects.
All sterile packages which met the inclusion criteria but did not met the exclusion criteria during the study period were the study samples.
QCC Formation
Led by the CSSD, the CSSD, Laundry Department, operating room and Logistics Department jointly established a QCC. The circle was named Jingxin Circle. The meaning of the circle badge was “ten work procedures ensure women’s and children’s safety”. The circle was composed of 7 people, aged 28–45 years, with an average age of 37 years. Of them, 1 was a chief nurse, 2 were co-chief nurses, 3 were supervisor nurses, and 1 was a senior nurse. The head nurse, who was engaged in cross-departmental nursing amongst the operating room, CSSD, Anesthesiology Department, Emergency Department and Ambulatory Surgery Department, served as the facilitator. The CSSD head nurse served as the circle leader. Among the remaining 5 members, 2 came from the CSSD, 1 came from Laundry Department, 1 came from Logistics Department, and 1 came from the operating room.
Topic Determination and Activity Planning
A total of 12 topics were proposed by the QCC members after brainstorming and were scored using the 5/3/1 scoring method and the weighted point system. Of the 12 topics, “building a prevention system for foreign objects remaining in sterile packages” received the highest score and was determined as being the topic of the QCC activities according to the urgency of the topic, feasibility, leader’s attention, and circle capability.30 The topic of the QCC activities was considered a task-oriented topic according to the Decision Table for Types of QC Story (Table 1).31 The task was considered innovative after information retrieval. An implementation plan was formulated and a gantt chart was design to clarify the content, time, place, person in charge and method of each step and carry out the QCC activities in strict accordance with the plan.
 |
Table 1 Decision Table for Types of QC Story |
Task Determination
In order to make the task clear, the circle members implemented the following steps:
- Problem investigation. The circle members designed an investigation form to investigate the details of sterile packages with foreign objects based on the 5 components (man, machine, material, method, and environment) within Laundry Department, operating room, and the CSSD, so as to identify the demands and problems at work.
- Determination of breakthrough points. The data collected in the problem investigation was analyzed to identify the gap between the current state and the expected results. The breakthrough points were nominated, and were scored for the feasibility, economy, and the circle capability using the 5/3/1 scoring method. The 80/20 rule was also applied in the selection of breakthrough points. The following 6 breakthrough points were finally determined (Table 2): (1) the textile sorting procedures of the Laundry Department should be improved; (2) the textile organizing procedures of Laundry Department should be improved; (3) standard occupational exposure risk prevention systems should be created in Laundry Department; (4) standard operating procedures for intraoperative reprocessing of surgical items (such as sharp instruments and dressings) should be created in the operating room; (5) standard operating procedures for foreign object inspection for surgical instruments and utensils should be created within the CSSD; (6) the textile organizing procedures of the CSSD should be improved.
 |
Table 2 Determination and Consolidation of Breakthrough Points |
Target Setting
According to the relevant guidelines14,32–34 expert opinions, and situation of our hospital, the following 5 indicators and the target values were determined: incidence rate of foreign objects remaining in sorted and folded textiles; incidence rate of foreign objects remaining in contaminated instrument packages; incidence rate of foreign objects remaining in clean textiles; incidence rate of complaints for foreign objects remaining in sterile packages (the number of complaints received from operating room staff for foreign objects remaining in sterile packages / the number of complaints received from operating room staff for packaging defects of sterile packages × 100%); and satisfaction among the CSSD and operating room staff (Table 3).
 |
Table 3 Task Indicators and Target Values |
Strategy Formulation and Optimal Strategies
In view of the finally determined 6 breakthrough points, all circle members brainstormed to carry out expansion of the strategy. A total of 10 strategies were drafted. Evaluation was performed on the 6 breakthrough points according to the feasibility, economy and circle capability. Then, 6 strategies were selected (Table 4).
 |
Table 4 Strategy Formulation |
The process decision program chart35 was used to determine the optimal strategies through the determination of obstacles and side effects and the comprehensive assessment of gains and losses. The following three strategies were finally determined: (1) Build a prevention system for foreign objects remaining in sterile packages within Laundry Department; (2) Build a prevention system for foreign objects remaining in sterile packages within the operating room; and (3) Build a prevention system for foreign objects remaining in sterile packages within the CSSD.
Implementation of Strategies
Build a Prevention System for Foreign Objects Remaining in Sterile Packages Within Laundry Department
- The sorting and organizing procedures for textiles in Laundry Department was improved. During the problem investigation, it was found that textile sorting and organizing in the Laundry Department was too arbitrary. Although there were procedures for textile sorting and organizing in the Laundry Department, they did not include inspection of foreign objects. Therefore, the circle members drew on the procedure development experience of leading Chinese hospitals to revise the sorting and organizing procedures of textiles in the Laundry Department and to determine the collection process of contaminated textiles. Shaking and moving the textiles should be minimized during the collection of the contaminated textiles.36 All medical waste attached to the contaminated textiles should be removed from the contaminated textiles. The bloody gauze, cotton swabs, scalpel blades and stitches should be disposed of in time as infectious and hazardous waste.16,37,38
- Standard operating procedures for occupational exposure risk prevention was created for textile organizing personnel in Laundry Department. During the problem investigation, it was found that the Laundry Department staff lacked the awareness of occupational protection and did not take protective measures. In addition, the CSSD staff found foreign objects such as hair on the textiles. Therefore, standard operating procedures for occupational exposure risk prevention were created for textile organizing personnel in Laundry Department according to the relevant guidelines.16 Training on prevention of infection and injury due to occupational exposure was provided for Laundry Department textile organizing personnel so that they could perform self-protection according to the standard operating procedures and wash hands according to the 7 steps of hand washing. They were required to wear masks, gloves and hair coverings when organizing the textiles.13 Quality controllers supervised the textile organizing personnel. A reward and punishment system was created to strengthen occupational protection.
Build a Prevention System for Foreign Objects Remaining in Sterile Packages Within the Operating Room
During the problem investigation, it was found that the operating room nurses roughly knew the general reprocessing procedures, but no standard operating procedures were formulated in the operating room. Therefore, the intraoperative reprocessing procedures of common surgical items (such as sharp instruments and dressings) were created within the operating room according to relevant guidelines,14 and relevant training was provided for the operating room staff. After surgery, the scrub nurse should dispose the bloody gauze, cotton swabs, scalpel blades and stitches in time as infectious and hazardous waste.
Build a Prevention System for Foreign Objects Remaining in Sterile Packages Within the CSSD
- The foreign object inspection procedures were created within the CSSD. During the problem investigation, it was found that the CSSD only had a rough procedure for instrument reprocessing, and no standard operating procedures for foreign object inspection were formulated in the CSSD. Therefore, the circle members formulated inspection procedures for surgical instruments and foreign objects for the CSSD according to relevant guidelines,14,39 and the relevant training was provided for the CSSD workers and nurses. The labels outside the sterile packages were taken as the reference for inspection. The CSSD staff were required to check the items one by one in strict accordance with the label details when counting, cleaning and packaging, and were required to strictly implement the standard operating procedures for foreign object inspection.
- The CSSD textile organizing procedures was improved. During the problem investigation, it was found that the CSSD’s current textile organizing procedures did not include the inspection of foreign objects, resulting in occasional complaints of foreign objects being found in dressings. Therefore, the CSSD improved the textile organizing procedures according to relevant guidelines, and relevant training was provided for the CSSD workers and nurses. The CSSD packaging personnel were required to organize the dressings in strict accordance with the standard operating procedures for textile organizing, and a two-person verification approach to check was adopted.
Statistical Analysis
SPSS 20.0 was used for data analysis. The Chi-square test was performed with the enumeration data for the two groups. A statistical significant difference was identified by P < 0.05.
Results
The Incidence Rates of Foreign Objects Before and After the Implementation of the QCC Activities
Table 5 and Table 6 show that the incidence of foreign objects remaining in contaminated textiles decreased from 7.1% to 0.96% (χ2 = 78.348, P < 0.001). The incidence of foreign objects remaining in contaminated instrument packages decreased from 0.27% to 0.04% (χ2 = 20.195, P < 0.001). The incidence of foreign objects remaining in the clean textiles decreased from 2.91% to 0.21% (χ2 = 48.678, P < 0.001). The incidence of complaints for foreign objects remaining in sterile packages reprocessed by the CSSD decreased from 41.69% to 0 (χ2 = 52.668, P < 0.001). Satisfaction among operating room and CSSD staff was significantly improved.
 |
Table 5 The Incidence Rates of Foreign Objects Before and After the Implementation of the Quality Control Circle Activities |
 |
Table 6 Satisfaction Among Operation Room and CSSD Staff |
Sense of Responsibility, Ability to Use the QCC, Enthusiasm, Communication and Coordinational Skills, and Self-Confidence of the QCC Members
The Sense of responsibility, ability to use the QCC, enthusiasm, communication and coordinational skills, and self-confidence of the QCC members before and after implementation of the QCC activities is shown in Figure 2.
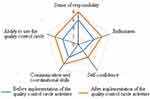 |
Figure 2 Sense of responsibility, ability to use the quality control circle, enthusiasm, communication and coordinational skills, and self-confidence of the quality control circle members. |
It can be seen from Figure 2 that the circle members’ sense of responsibility, ability to use the quality control circle, communication and coordination skills, and self-confidence, were improved to varying degrees after the implementation of the QCC activities. In particular, the circle members’ sense of responsibility, ability to use the quality control circle, and communication and coordination ability, were significantly improved.
Continuous Monitoring
The results of continuous monitoring after completion of the study are shown in Table 7.
 |
Table 7 The Incidence Rates of Foreign Objects During January 2024 to June 2024 (Continuous Monitoring) |
Discussion
Improvement of Hospital Management Quality
In this study, quality control systems for foreign objects remaining in sterile packages were created through the implementation of the task-oriented QCC. The results of the experimental group were better than those of the control group (P < 0.001). The incidence of foreign objects remaining in sterile packages significantly decreased in this study. This can provide strong support for the improvement of the overall quality management system of the hospital. The more appropriate workflows and standard operating procedures were created based on cross-departmental collaboration to ensure the quality control for all steps of the work procedures. This can reduce the incidences of surgical site infections and medical disputes and improve the management efficiency of the hospital. In terms of patient safety, the reduction of incidence of foreign objects remaining in sterile packages could help reduce the risks of infections and surgical accidents, thereby building patient trust and increasing patient satisfaction with the hospital. This quality-oriented management model can improve the overall quality of hospital services and is reproducible in other healthcare institutions. This can help healthcare institutions improve management quality continuously, thereby ensuring patient safety.
Improvement of CSSD Management System
The implementation of the task-oriented QCC activities built a new quality control system within the CSSD, clarified the work responsibilities and criteria of the CSSD staff at each job position, created appropriate standard operating procedures, optimized work flows, improved the work efficiency of the CSSD staff, and improved the quality of medical device disinfection. This could lay a foundation for continuous improvement of future CSSD work.
Improvement of Problem-Solving Skills
The task-oriented QCC activities improved the ability of hospital staff to use research to solve clinical practical problems. In the quality control circle activities, the awareness of staff to find problems was gradually improved, and the ability to comprehensively analyze problems was gradually enhanced. Through the cooperation between the circle members, all the circle members participated in building quality control system. Their knowledge and skills in evidence selection and use, data collection, statistical analysis and research were improved to varying degrees. The research made clinical nursing work more closely combined with research, promoting the output of research results.
Improvement of Packaging Quality Based on QCC and Cross-Departmental Collaboration
The studies by Blackmore et al1 and Liu et al3 have shown that the incidence of packaging quality defects can be effectively reduced by optimizing the work procedures and improving the quality awareness of staff. The studies by Su et al8 and Yu39 have shown that QCCs can be used to improve the quality of packaging in the CSSD. The studies focus on the internal management of the CSSD,6,8,40 lacking systematic and multi-disciplinary collaboration. Our study implemented management across the CSSD, operating room, Laundry Department and Logistics Department, to achieve more comprehensive quality control. Our study provided detailed descriptions for quality management measures.
Improvement of Sterile Package Quality Management Supported by Multi-Disciplinary Collaboration and Process Quality Control
This study innovatively applied the task-oriented QCC and cross-departmental collaboration to achieve systematic control for foreign objects remaining in sterile packages. The main contributions of this study are as follows: (1) Innovation in methodology. The management mode based on the task-oriented QCC increased scientific rigor and effectiveness; (2) Process quality control system. The process management across the Laundry Department, the CSSD and operating room was implement to ensure the quality of every step; (3) Improvement of healthcare quality and safety. In this study, the incidence of foreign objects remaining in sterile packages significantly decreased and satisfaction among operating room staff increased. This can provide a new quality control method for management of nosocomial infections.
Improvement of Hospital Management and Staff Training Supported by Multi-Disciplinary Collaboration and Process Quality Control
This study provides a new idea for the quality management policy of the hospital and promotes the hospital management to pay more attention to cross-departmental collaboration and process quality control. This study encourages the hospital to pay more attention to systematic and multi-disciplinary collaboration for formulating future quality management policies, especially in the quality control in sterile supply and nosocomial infection management. In addition, this study provides a practical basis for hospitals to add more training content concerning multi-disciplinary collaboration and quality control to staff training, further strengthening the sense of responsibility and professionalism of hospital staff in quality management. The results of this study provide a useful reference for the CSSDs around the world. Hospitals with sufficient resources can fully implement cross-departmental collaboration, while hospitals with limited resources can start from key steps, such as optimizing the cleaning, disinfection and packaging processes for surgical instruments. The quality management can be improved through teamwork, process optimization and staff training.
Continuous Monitoring After Completion of the Study
This study achieved significant short-term success. The CSSD and relevant departments continued to follow the standard operating procedures and provided continuous quality management training for relevant personnel after the completion of this study. We conducted continuous monitoring of relevant indicators (the incidence of foreign objects remaining in contaminated textiles, the incidence of foreign objects remaining in contaminated instrument packages, the incidence of foreign objects remaining in the clean textiles, and the incidence of complaints for foreign objects remaining in sterile packages reprocessed by the CSSD). The incidences remained low, achieving continuous improvement.
Limitations
Despite the remarkable results, this study still has some limitations. First, the research time of this study was short. The duration of the intervention in this study was 1 month. Significant improvements have been observed, but the effects of long-term sustainability were not assessed. Second, this was a single-center study. The limitations on the external validity might exist. Third, there are few standardized evaluation systems for the quality control of foreign body remaining in sterile packages worldwide. This might lead to difficulties in comparison of research data. Fourth, confounding variables might exist.
The following measures can be taken to address the limitations: (1) Extension of the study period. The study period should be extended to more than 2 years to observe the stability of the intervention effect over the long term. (2) Extension of the scope of the study. The study method should be popularized in different hospitals to verify its applicability in different healthcare settings. (3) Creation of a standardized evaluation system. The hospital should cooperate with relevant academic institutions or quality control organizations to develop more authoritative monitoring criteria for foreign objects remaining in sterile packages, so as to facilitate comparison between different studies.
Directions for Future Research
Sterile item packaging is one of the key steps of the CSSD work. With the development of science and technology, artificial intelligence and digital management may be applied in the quality control of sterile packages. For example, computer vision technology can be used to automatically detect foreign objects remaining in sterile packages. This can improve the accuracy and efficiency of the detection. The cross-departmental collaboration can be optimized to refine the roles and responsibilities of each department in the quality control system for sterile packages. This can improve the efficiency of collaboration.
Conclusions
In this study, a quality control system for foreign objects remaining in sterile packages was built through the task-oriented QCC and was successfully implemented in the CSSD. The incidence of foreign objects remaining in sterile packages decreased from 41.69% to 0%. The incidence of foreign objects remaining in contaminated textiles, the incidence of foreign objects remaining in contaminated instrument packages, the incidence of foreign objects remaining in the clean textiles, and the incidence of complaints for foreign objects remaining in sterile packages reprocessed by the CSSD also decreased significantly. This shows that the task-oriented QCC can effectively optimize the quality management process, promote cross-departmental collaboration, improve the quality management awareness of employees, and ensure health care quality.
The results of this study are consistent with quality management theories, such as Total Quality Management (TQM) and Lean Six Sigma. TQM emphasizes full participation and continuous improvement. Our study strengthened the collaboration between the CSSD, operating room, Laundry Department and the Logistics Department by the implementation of the task-oriented QCC. The process quality management was implemented to achieve continuous optimization. Lean Six Sigma focuses on reducing variations and optimizing processes. In this study, standard operating procedures were created and continuous quality monitoring was conducted, thereby effectively reducing the incidence of quality defects of sterile packages and improving the overall work efficiency.40 This shows that the task-oriented QCC is suitable to the existing quality management framework and can provide a more flexible and adaptable implementation path. It is particularly suitable for cross-department collaboration within a hospital.
This study not only focused on the quality improvement in a single department, but it also extended the task-oriented QCC to multi-disciplinary collaboration to achieve cross-departmental management. This can ensure quality control throughout the entire supply chain for sterile items. Compared with previous studies on CSSD management that focused on equipment improvement or optimization of a single department, this study innovatively adopted a systematic management method to integrate cross-departmental resources and build a process tracking and control mechanism. This can achieve more refined quality management.
The success of this study provides a feasible quality improvement model for other hospitals, especially for healthcare institutions hoping to optimize the CSSD management and reduce the incidence of foreign objects remaining in sterile packages.41
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Ethical approval was waived by the Medical Research Ethics Committee of West China Second University Hospital, Sichuan University [2024 Medical Scientific Research for Ethical Approval No. (053)].
Funding
No funding was obtained for this study.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Blackmore CC, Bishop R, Luker S, Williams BL. Applying lean methods to improve quality and safety in surgical sterile instrument processing. Jt Comm J Qual Patient Saf. 2013;39(3):99–105. doi:10.1016/s1553-7250(13)39014-x
2. Liu B. The cause analysis and countermeasure for quality defects of sterile packs after integration of operating and supply room. Practical Journal of Clinical Medicine. 2014;11(2):122–123.
3. Liu L, He C, Zhang S, Dong P. Implementation of improvement measures in quality management and control of sterile packages in the central sterile supply department. Shanghai Nursing Journal. 2017;17(3):80–82. doi:10.3969/j.issn.1009-8399.2017.03.023
4. Chang S, Yao Z, Li M, Wang Y, Ye W. Effects of quality control circle in reducing the defect rate of surgical power devices. J Nurs Train. 2015;30(14):1271–1273.
5. Fang Y. Strength safety management in the operating room to prevent the retention of foreign objects. Jiangsu Med J. 2022;37(4):495–496.
6. Zhang C, Zhang Q, Liu F. The influence of process control management on the quality of instrument sterilization and packaging and satisfaction among the central sterile supply department staff. J Qilu Nursing. 2021;27(21):170–172. doi:10.3969/j.issn.1006-7256.2021.21.061
7. Li J. Cause analysis of common quality defects of packaging in the central sterile supply department and countermeasures.
8. Su M, Ruan W. The application of quality control circle activities in quality control of surgical instrument packaging in disinfection supply center. Med Equip. 2024;37(17):53–56. doi:10.3969/j.issn.1002-2376.2024.17.012
9. Ryckman FC, Schoettker PJ, Hays KR, et al. Reducing surgical site infections at a pediatric academic medical center. Jt. Comm J Qual Patient Saf. 2009; 35(4):192–198. doi:10.1016/S1553-7250(09)35026-6
10. Copeland EM. A surgical safety checklist to reduce morbidity and mortality in a global population. Year Book of Surg. 2010;2010:1–2. doi:10.1016/S0090-3671(09)79568-0
11. Gawande AA, Thomas EJ, Zinner MJ, Brennan TA. The incidence and nature of surgical adverse events in Colorado and Utah in 1992. Surgery. 1999;126(1):66–75. doi:10.1067/msy.1999.98664
12. Kable AK, Gibberd RW, Spigelman AD. Adverse events in surgical patients in Australia. Int J Qual Health Care. 2002;14(4):269–276. doi:10.1093/intqhc/14.4.269
13. National Health and Family Planning Commission of the People’s Republic of China. Guidelines (WS 310.1-2016) for healthcare sector of the People’s Republic of China - Central sterile supply department (CSSD) - Part 1: Management standard. 2016. Available from: http://www.nhc.gov.cn/ewebeditor/uploadfile/2017/01/20170105090443523.pdf.
14. National Health and Family Planning Commission of the People’s Republic of China. Guidelines (WS 310.2-2016) for healthcare sector of the People’s Republic of China - Central sterile supply department (CSSD) - Part 2: standard for operating procedure of cleaning, disinfection and sterilization. 2016. Available from: http://www.nhc.gov.cn/ewebeditor/uploadfile/2017/01/20170105090606684.pdf.
15. National Health and Family Planning Commission of the People’s Republic of China. Guidelines (WS 310.3-2016) for healthcare sector of the People’s Republic of China - Central sterile supply department (CSSD) - Part 3: surveillance standard for cleaning, disinfection and sterilization. 2016. Available from: http://www.nhc.gov.cn/ewebeditor/uploadfile/2017/01/20170105090648964.pdf.
16. National Health and Family Planning Commission of the People’s Republic of China. Guidelines (WS/T 508-2016) for healthcare sector of the People’s Republic of China - Central sterile supply department (CSSD) - Regulation for washing and disinfection technique of medical textiles in healthcare facilities. Available from: http://www.nhc.gov.cn/ewebeditor/uploadfile/2017/01/20170105092028826.pdf.
17. Croke L. Guideline for care and cleaning of surgical instruments. AORN J. 2020;112(3):9–11. doi:10.1002/aorn.13187
18. Guo L. Guide to Operating Room Nursing Practice. Beijing: People’s Medical Publishing House; 2014.
19. Ye J, Fu Y. Impact of quality control circle on reducing the defect rate of instrument packaging. Int J Nurs. 2018;37(16):2279–2281. doi:10.3760/cma.j.issn.1673-4351.2018.16.040
20. Ding L.Analysis on quality management of packaging link in disinfection supply center. Smart Healthcare. 2018;4(25):3–4.
21. Chen R. Effect of tracking supply room on improving packaging quality. China Health Standard Management. 2018;9(22):125–127. doi:10.3969/j.issn.1674-9316.2018.22.056
22. Zhang X. Impact of process control and management on quality of cleaning and packaging of instruments in the central sterile supply department. Capital Food Med. 2019;26(6):85. doi:10.3969/j.issn.1005-8257.2019.06.071
23. Zhu X, Yuan L, Li T, Cheng P. Errors in packaging surgical instruments based on a surgical instrument tracking system: an observational study. BMC Health Serv Res. 2019;19(1):176. doi:10.1186/s12913-019-4007-3
24. Li Q. Application of quality control circle in management of outpatient infusion center. J Trad Chin Med Manag. 2014;22(8):1270–1271.
25. Zhao Q, Xiao M, Liu J, Wei H. Research progress on quality control circle in nursing quality management. J Nurs Sci. 2014;29(6):94–96. doi:10.3870/hlxzz.2014.06.094
26. Rui T, Zhang C. Application effect of quality control circle activities in cleaning and disinfection management of otolaryngology head and neck surgical instruments in hospital. China Modern Med. 2024;31(30):126–129. doi:10.3969/j.issn.1674-4721.2024.30.030
27. Zhou J, Xia P, Lin D, et al. Lean healthcare management in optimizing foreign hospital operating room service process. West China Med J. 2024;39(12):1860–1866. doi:10.7507/1002-0179.202411115
28. Zhang R, Lu G. Application of Six Sigma in safety management of diagnosis and treatment of traditional Chinese medicine. J Trad Chin Med Manag. 2025;2025:1.
29. Ci L, De Y, Huang Y, et al. Construction and application of Total Quality Management (TQM) concept in hospital nursing management system. Tibetan Med. 2024;175(4):12–14.
30. Gong Y, Zhang P, Hong X, Chen R, Xu W, Zhu J. Application of subject-achieving quality control circle in improving pharmaceutical care for patients with chronic hepatitis B. J Pharmaceut Res. 2022;41(2):131–135,140. doi:10.13506/j.cnki.jpr.2022.02.014
31. Zhang L. Application of task-oriented quality control circle activities in the interhospital transport of critically ill patients. Chin Gen Pract Nurs. 2021;19(32):4600–4604,4608. doi:10.12104/j.issn.1674-4748.2021.32.039
32. Li Y. Study on standard and adaptability of final sterilization and packaging for medical devices. Capital Med. 2008;2008(8):9–10. doi:10.3969/j.issn.1005-8257.2008.08.004
33. Tang S, Ma N, Qi J, Liu G, Ye G. Interpretation of key points of EN 868 “packaging Materials for terminally sterilized medical devices”. Packaging Eng. 2010;31(19):124–127.
34. Yao X, Gong Y, Zhang Y, et al. Implementation of Regulation of disinfection technique in healthcare settings. Chinese J Infection Control. 2020;19(8):728–732. doi:10.12138/j.issn.1671-9638.20205761
35. Zhou D, Wu H, Ge H, Yan Y, Yang T, Zhu W. The practice and effect of using the subject-achieving quality control circle to improve the accuracy of the detection of unqualified residual amounts of intravenous drug preparations. Chin Hospitals. 2021;25(11):82–84. doi:10.19660/j.issn.1671-0592.2021.11.26
36. Shan P. Infection management, cleaning and disinfection in hospital laundry department. World Latest Med Info. 2016;16(35):184–184,187. doi:10.3969/j.issn.1671-3141.2016.35.136
37. Wu G. Situation analysis of infection management in hospital laundry department. Shenzhen J Integrated Trad Chin Western Med. 2017;27(3):185–186. doi:10.16458/j.cnki.1007-0893.2017.03.095
38. Huang H, Liu X, Chen H. Discussion on the development of laundry in public hospital under new circumstances - reform exploitation of washing center of West China Hospital, Sichuan University. Chinese Hospital Architecture Equipment. 2017;2017(5):36–38.
39. Yu Y. Analysis of the influence of quality control circle on reducing the defect rate of instrument packaging. Chinese Community Doctors. 2019;35(24):181,183. doi:10.3969/j.issn.1007-614x.2019.24.132
40. Wang F, Yao Z, Geng J, Zhan M, Zhao Y. Application of quality control circle in reducing the defect rate of sterile packages containing precision surgical instruments. Clinical Res. 2020;28(9):57–58.
41. Lu Q, Xu X, He Z, et al. Application of QCC quality control nursing management measures in nursing management of Disinfection Supply Center. Hainan Med J. 2023;34(2):268–271. doi:10.3969/j.issn.1003-6350.2023.02.027
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

