Back to Journals » Infection and Drug Resistance » Volume 18
Vaccination Coverage Covid-19 and Self-Reported Adverse Post-Vaccination Events in Mbuji-Mayi, DR Congo
Authors Musuamba Tshipata R, Kabeya Diyoka C , Nwakama CN, Ngongo Mwanvua L, Ndjibu mpoji F, Muamba Mubalamata C, Mbelu Kanyinda S, Bilonda Kasekelayi D, Mutombo Munyangama B, Kashala Snr JM, Koba Mjumbe C
Received 10 November 2024
Accepted for publication 28 March 2025
Published 3 April 2025 Volume 2025:18 Pages 1687—1697
DOI https://doi.org/10.2147/IDR.S504760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Rebecca Musuamba Tshipata,1 Chadrack Kabeya Diyoka,2 Chijindu N Nwakama,3 Laetitia Ngongo Mwanvua,2 Faustin Ndjibu Mpoji,1 Claude Muamba Mubalamata,1 Stéphanie Mbelu Kanyinda,4 Davina Bilonda Kasekelayi,5 Barry Mutombo Munyangama,1 Justine Mbelu Kashala Snr,6 Criss Koba Mjumbe7
1Department of Public Health, Faculty of Medicine, Pharmacy and Public Health, University of Mbujimayi, Mbujimayi, Democratic Republic of Congo; 2School of Public Health, University of Lubumbashi, Lubumbashi, Democratic Republic of Congo; 3Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; 4Department of Gynecology, Faculty of Medicine, Pharmacy and Public Health, University of Mbujimayi, Mbujimayi, Democratic Republic of Congo; 5Department of Pediatry, Faculty of Medicine, Pharmacy and Public Health, University of Mbujimayi, Mbujimayi, Democratic Republic of Congo; 6Mbuji-Mayi Higher Technical Medical Institute, Mbujimayi, Democratic Republic of Congo; 7Department of Public Health, Faculty of Medicine, University of Lubumbashi, Lubumbashi, Democratic Republic of Congo
Correspondence: Criss Koba Mjumbe, Department of Public Health, Faculty of Medicine, University of Lubumbashi, Lubumbashi, Democratic Republic of Congo, Email [email protected] Chadrack Kabeya Diyoka, School of Public Health, University of Lubumbashi, Lubumbashi, Democratic Republic of Congo, Email [email protected]; [email protected]
Purpose: This study examines covid-19 vaccine coverage among adults and describes self-reported post-vaccine adverse events and associated risk factors in Mbuji-Mayi.
Patients and Methods: A cross-sectional study was conducted, with the study population including adults (18 years and older) who received at least one dose of vaccine in the Bonzola health zone in Mbuji-Mayi between 27 March 2023 and 27 June 2023. The Pearson chi-square test (χ 2) and multinomial logistic regression analyses were performed using IBM SPSS Statistics 29 and Epi Info 7.2.4.0 software.
Results: A survey of 422 people showed that only 164 (38.86%) had been vaccinated, and 83.54% of these people had had bad reactions to the vaccine. Respondents were mainly men (50.4%) aged between 18 and 25 (52.44%). The most common vaccine was the Johnson & Johnson vaccine (43.8%), followed by the AstraZeneca vaccine (40.1%). The most frequent side effects after vaccination were moderate (fever and vomiting) and least frequent (cough and headache). The results of the multivariate analysis showed that age and sex (respectively 2,946 times (95% CI: 2,946– 197,279), 10,019 times (95% CI: 1,214– 82,660) and 55,489 times (95% CI: 5,742– 536,248) higher than that of men) were associated with the occurrence of self-reported post-vaccination side effects, as well as fever, headache and cough.
Conclusion: In view of the sub-optimal rate of vaccination coverage, it is evident that the prevailing circumstances have not been immune to the occurrence of adverse events reported by stakeholders following vaccination against the new coronavirus (Covid-19). This underscores the necessity for rigorous safety monitoring. The dissemination of these findings, in conjunction with the results of vaccine clinical trials, has the potential to contribute to the reduction of mistrust, which is a persistent challenge in the context of vaccine hesitancy.
Keywords: COVID-19 vaccine, vaccination coverages, adverse events following, self reports
Introduction
Coronavirus disease 2019 (Covid-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In January 2020, the SARS-CoV-2 virus was identified as the causative agent of an outbreak in China. The disease rapidly disseminated globally, prompting the World Health Organization (WHO) to declare it a pandemic in March 2020.1
As of March 2022, the global tally of cases stands at over 445 million, with 6 million deaths reported. Of these, over 327 million cases (74%) have been reported in the WHO surveillance database, of which 255 million (57%) are disaggregated by age and sex.1,2
The initial case of coronavirus disease 2019 (Covid-19) in the Democratic Republic of Congo (DRC) was identified on 10 March 2020, involving an individual who had recently travelled from Europe. By 2 January 2022, the Democratic Republic of Congo had recorded a total of 72,108 positive cases of the disease, including 9,641 cases (17.0%) during the first wave, 16,643 cases (25.0%) during the second, 24,172 cases (15.3%) during the third and 21,652 cases (13.2%) during the fourth. There was a notable decline in the case fatality rate, from 5.1% to 0.9%, between the first and fourth waves.3
The ongoing global health crisis, caused by the novel coronavirus (SARS-CoV-2), has resulted in the implementation of a range of preventive measures on a global scale.3 These measures have been implemented to varying degrees across the globe and have demonstrated varying degrees of efficacy in controlling disease transmission, due to the presence of a number of challenges.4–6
In consequence of the urgent public health need, the development of vaccines against the novel coronavirus (2019-nCoV) has been achieved in an exceptionally short period of time. As of 13 August 2021, only 21 vaccines out of 138 candidates have been approved worldwide for emergency use.
The vaccine is regarded as the sole means of returning to a state of pre-pandemic normality in the context of the ongoing global pandemic caused by the SARS-CoV-2 virus.7 The initial administration of a vaccine for the novel coronavirus (SARS-CoV-2) outside of a clinical trial occurred on 8 December 2020. The primary objective was to achieve a vaccination coverage of 20% by the end of 2021, as defined by the Access Mechanism Vaccines against CoronaVirus Disease (COVAX) and the WHO.8 In order to achieve the aforementioned goal, the WHO has established a global strategy with the objective of reaching 70% coverage in all countries by mid-2022. The interim target is 40% coverage by the end of 2021.9
Their mode of action relies on the immune response to their integral parts (DNA, RNA or protein.10,11 The despite the efficacy and numerous advantages conferred by the latter, namely the reduction in rates of hospitalisation and mortality,12 they are also likely to induce mild to moderate adverse events following vaccination. These events are expected to be moderate, transient and short-lived.4 As is the case with all other vaccines, the potential adverse effects include flu-like symptoms (eg headache, fatigue and myalgia) and injection site reactions.13–15 It is worth noting that, although rare, some more serious symptoms have been reported, including anaphylaxis, coagulation, myocarditis, thyroiditis and even death.16,17
Nevertheless, a number of myths, fears, rumours and misconceptions persist, particularly in relation to adverse post-vaccination events.18,19 It is imperative that the safety profiles of vaccines are monitored in order to enhance public confidence and improve the safety of the vaccines in question. The WHO International Pharmacovigilance Program offers a vaccine for the novel coronavirus.18,20 The safety of the COVD-19 vaccine has been demonstrated in clinical trials, yet there is a paucity of evidence comparing post-vaccination events in the Congolese context. This study examines covid-19 vaccine coverage among adults and describes self-reported post-vaccine adverse events and associated risk factors in Mbuji-Mayi.
Materials and Methods
The present descriptive cross-sectional study was conducted via a pre-tested questionnaire with the aim of assessing vaccination coverage and adverse effects of the SARS-CoV-2 vaccines in individuals who have received at least one dose of a vaccine approved by the Ministry of Health and Public Hygiene. The study was conducted in the health zone (HZ) of Bonzola.
The Bonzola HZ (code in the normative framework: 11020201) is an urban health zone situated to the south of the city of Mbuji-Mayi in the Democratic Republic of Congo (DRC), within the commune of KANSHI (Figure 1).
 |
Figure 1 Mapping of the Bonzola health zone (Source Bonzola HS). |
In order to achieve this objective, a survey was conducted between March and June 2023. A probability sampling technique was employed in order to obtain a representative sample from the target population, which consisted of individuals residing in HS Bonzola who had received either a full course of vaccinations or a single dose. The sample size was calculated using the following formula:21
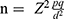 ; with the sample size of 422 participants. Only subjects over the age of 18, residing in the study health zone were included in the study.
; with the sample size of 422 participants. Only subjects over the age of 18, residing in the study health zone were included in the study.
All of our respondents were submitted to a questionnaire.
The data were entered into an MS Excel 2016 spreadsheet and subsequently analysed using IBM SPSS Statistics 29 and Epi Info 7.2.4.0 software.
The comparison of the proportions of adverse post-vaccination events according to demographic data and the type of vaccines administered against SARS-CoV-2 was conducted using the Pearson chi-square (χ²) test. A multinomial logistic regression model was employed for the purpose of evaluating the suggested risk factors for self-reported adverse post-vaccination events. A confidence level of 95% and a significance level of ≤0.05 were employed.
The quality of the logistic regression model is determined by following the classification proposed by Landis and Koch:22 < 0 Disagreement; 0–0.20 Very poor agreement; 0.21–0.40 Weak (fair) agreement; 0.41–0.60 Moderate agreement; 0.60–0.80 Strong agreement (good) and 0.81–1.00 Almost perfect agreement (very good).
Ethical Considerations
In order to safeguard the privacy of respondents, they were approached and provided with comprehensive and transparent information regarding the purpose of the study and the intended use of the data collected. The respondents were informed of their right to decline participation in the survey. All participants gave their informed consent. In accordance with the fifth chapter of the Declaration of Helsinki, which stipulates that “every precaution must be taken to protect the privacy and confidentiality of personal information concerning the persons involved in research in the research”, the individual data of all those who agreed to take part in the study remained completely anonymous and were treated in the same way throughout the study. The requisite research authorisation was obtained from the management of the University of Mbuji-Mayi (reference number 089/F.MED.PH.SP/UM/KLN/2022).
Results
Vaccination Coverage Covid-19 and Undesirable Post-Vaccination Events
As illustrated in Table 1, In the sample of 422 participants, only 164 people (38.86%) were vaccinated. The self-reported adverse post-vaccination events were observed in 83.54% of participants, predominantly manifested as moderate adverse events following immunization, including fever and vomiting, which were reported by 32.12% and 32.12% of participants, respectively. Additionally, less common AEFIs, such as cough and headache, were observed in 16.06% and 14.60% of participants, respectively. The majority of participants (96.95) indicated a willingness to receive the covid-19 vaccine, despite the potential for adverse effects.
 |
Table 1 Covid-19 Vaccination Coverage, Self-Reported Post-Vaccination Adverse Events and Willingness to Be Vaccinated in the Near Future Among Those Vaccinated |
Risk Factors Associated With COVID-19 Self-Reported Adverse Post-Vaccination Events
Table 2 presents an illustration of the predictive factors of adverse post-vaccination events. Of the participants, 50.4% were male, resulting in a sex ratio of 1.01. The majority of participants (46.6%) identified as members of revival churches, with the largest proportion of respondents falling within the 18–25 age range (52.44%). With regard to the level of education, 49.6% were enrolled in tertiary education, while 45.3% had completed secondary studies. The majority of participants were single (49.6%), followed by those who were married (39.02%). The most prevalent type of the vaccine administered for the prevention of the effects of the virus known as SARS-CoV-2, also known as the Coronavirus, was the Johnson & Johnson vaccine (43.8%), followed by the AstraZeneca vaccine (40.1% versus the Moderna vaccine at 16.1%).
 |
Table 2 Bivariate Analyzes Between Self-Reported Adverse Post-Vaccination Events and Sociodemographic Parameters |
Table 2 presents the distribution of self-reported adverse post-vaccination events according to the sociodemographic factors of the study. Significant variations were observed in the distribution of participants according to sex, age group, religion, profession, marital status and type of vaccine administered (p < 0.001).
A multinomial logistic regression model was constructed in order to ascertain the existence of self-reported adverse post-vaccination events. The model was expanded to incorporate the variables of the vaccines administered, the subjects’ sex, and their age (Table 3).
 |
Table 3 Final Results of Multinomial Logistic Regression of Self-Reported Adverse Post-Vaccination Events According to Vaccines Administered, Sex and Age |
The independent variables yielded a substantial amount of information, as indicated by a likelihood ratio χ2 value of 251.57 and a p-value less than 0.001. The results of the tests based on the three predictive variables could account for 78.4% of the variance in the presence of self-reported adverse post-vaccination events, as indicated by the Nagelkerke R² statistic of 0.814.
The results of the multivariate analysis indicated that age and sex were associated with the occurrence of self-reported adverse post-vaccination events. For fever, the values χ² = 24.820, p < 0.001 and χ² = 8.805, p = 0.003 were observed; for headache, χ² = 11.626, p < 0. For cough, the results were χ2 = 6.957, p = 0.008 and χ2 = 12.042, p < 0.001 for sex. The likelihood of females presenting with fever, headache, and cough was, respectively, 24.107 times, 10.019 times, and 55.489 times higher than that of males.
The occurrence of self-reported adverse post-vaccination events was found to be negatively associated with age, with coefficients of −0.117 for cough, −0.252 for fever, and −0.151 for headaches. Furthermore, the Astrazeneca and Johnson & Johnson vaccines exhibited adverse effects when compared to the Moderna vaccine, with coefficients of −16,301 and −20,749 for cough and −17,595 and −21,972 for fever, respectively.
Table 4 presents the confusion matrix, which allows for an evaluation of the capacity to accurately categorise the data. It can be observed that the observations of self-reported adverse post-vaccination events vomiting were correctly classified at 100% (44 observations well predicted out of 44), and then cough at 45.5% (10 observations well predicted out of 22). This indicates that the logistic regression model has an overall accuracy of 63.4%, with a Kappa index of 0.499 (95% CI: 0.495–0.504), which can be considered to represent moderate agreement (Table 4).
 |
Table 4 Confusion Matrix |
Discussion
The objective of this study was to examine the extent of vaccination coverage against the virus in adults and to describe self-reported adverse events following vaccination, along with the associated risk factors in Mbuji-Mayi.Vaccination has been widely regarded as a key preventive measure in controlling infection, eradicating the disease and reducing mortality and morbidity rates.23–25Vaccination against the virus has been the main strategy employed by most countries to limit the spread of the virus. In the context of the global pandemic of the Coronavirus, the COVAX initiative was established with the initial objective of achieving 20% vaccination coverage by the end of 2021. This target was subsequently revised to 70% for all countries by mid-2022. However, the uptake of the vaccine has been low in some European countries and particularly in Africa.26,27 In the present study, the vaccination coverage among adults was found to be 38.86% during the specified study period (in 2023). This figure is almost half the proportion reported on a global scale (69%) at the conclusion of 2022, yet it approaches the figure reported in low- and middle-income countries (25%).28
The most common reasons cited for non-compliance included perceived risks versus benefits, religious beliefs, lack of knowledge and awareness, fear of the effects of the vaccine, and cultural factors.29–35
The ongoing risk of severe acute respiratory syndrome (SARS) due to the highly infectious nature of the virus, the probability of resurgence and the appearance of new variants, even in regions where vaccination programmes have been implemented, is a salient concern.36 The decline in immunity and the emergence of new variants will shape the burden and long-term dynamics of the disease. Improving vaccination coverage is necessary for public health in the Democratic Republic of the Congo, particularly in Mbuji-Mayi, and its introduction into the Expanded Programme on Immunisation is not yet effective.
The safety of the COVD-19 vaccine has been demonstrated in clinical trials, yet there is a paucity of evidence comparing post-vaccination events in the Congolese context. The results of this study demonstrate that 83.54% of respondents experienced self-reported post-vaccination manifestations of Covid-19 in the community. This result is consistent with those reported in other studies conducted among healthcare workers in Ghana (80.7%),37 Nigeria (90.0%)38 and Germany (88.1%).39 In the Polish community, the prevalence was 96.5%.40 Conversely, the prevalence of self-reported adverse post-vaccination events reported was higher than that reported in studies conducted in India (65.9%)41 and in the Ivory Coast (57.4%).42 The discrepancy in prevalence can be attributed to a number of factors, including methodological approaches, such as data collection, methodology, study environment and sample size. Furthermore, the aforementioned studies on satisfaction rates were conducted in disparate settings, including healthcare workers37–39 and the community.40
The primary mechanism underlying the development of systemic symptoms following vaccination is the presence of inflammatory markers in the bloodstream. These markers signal at the blood-brain barrier, thereby inducing symptoms that are characteristic of influenza.43 The occurrence of symptoms subsequent to vaccination may result in apprehension regarding injections, the formation of adverse attitudes and non-compliance, which ultimately undermines the efficacy of vaccination programmes.43 The results of this study demonstrated that the most prevalent adverse effects following vaccination against SARS-CoV-2 were fever and vomiting, occurring in 32.12% and 32.12% of participants, respectively. Less common adverse effects included cough (16.06%) and headaches (14.60%). Additionally, other authors16,44 have observed that headaches, 38.8%,45 and 21.1%,38 while fever44 was reported in 19.1% of cases.37
Nevertheless, the occurrence of localised oedema at the vaccination site was infrequently documented (5.11%), which contrasts with the findings of several researchers who identified pain at the injection site as the most prevalent adverse post-vaccination events.37,38,44,45 It is a common phenomenon that vaccines, irrespective of their composition, induce a degree of inflammation at the injection site during the initial hours following administration. This is postulated to contribute to the symptoms of pain, redness and swelling. The release of pyrogenic factors into the systemic circulation stimulates a cascade of crosstalk between the immune system and the nervous system, which can result in the onset of systemic flu-like symptoms, including an increase in body temperature. There is mounting evidence to suggest a correlation between systemic inflammatory mediators and the onset of systemic symptoms following vaccination.43
It can be observed that both extrinsic and intrinsic factors have the potential to impact the self-reported adverse post-vaccination events profile of a given individual. Host characteristics, such as age (including both older and younger individuals, due to low immunity or a lack of a well-established immune system, respectively), sex, general health status, and pre-existing immunity, as well as vaccine administration and composition factors are among the extrinsic and intrinsic factors that can impact the self-reported adverse post-vaccination events profile in a given individual.43 In our study, all sociodemographic characteristics, including gender, age, occupation, marital status, and type of vaccine administered, were identified as risk factors associated with post-vaccination events (p < 0.001). This contrasts with the findings, who reported age as the only risk factor.46
The multinomial logistic regression model revealed that age and gender are the two factors associated with the occurrence of self-reported adverse post-vaccination events, including fever, headache and cough, in Mbuji-Mayi town, as reported.47 However, this was not influenced by the type of vaccine, contrary to the results reported.12,48 Indeed, women tend to have a higher incidence of injection site reactions compared to men, but not systemic symptoms after vaccination.49–52 In our study, women were more likely to report the occurrence of side effects than men, a finding that is consistent with those reported.47,48
It is possible that the observed differences are attributable to genetic or hormonal factors.50 For example, anatomical differences in skin thickness, blood flow, and nervous system structure between men and women may contribute to the development of injection site inflammation in women.53 Moreover, sex hormones have been demonstrated to modulate immune responses and cytokine levels, with androgens and high doses of estrogens exhibiting immunosuppressive properties.54,55
The experience of pain and distress at the time of vaccination represents a significant clinical issue for individuals of all age groups who undergo an injection. The failure to treat pain at the time of vaccination may result in vaccine hesitancy and potentially influence future health-seeking behaviours and healthcare decisions.43 The physiological functions of the immune and nervous systems undergo changes throughout the lifespan. These changes have implications for the ability to defend against infectious diseases at different ages and may also influence susceptibility to adverse reactions to vaccination. Although infants and toddlers exhibit a reduced incidence of injection site reactions following vaccination in comparison to adults, they are more susceptible to episodes of fever resulting from vaccination or other incidental infections.43
The incidence of adverse events following immunization increases during childhood and adolescence as the immune system matures. The incidence of self-reported adverse post-vaccination events declines during adulthood, potentially reflecting greater tolerance to pain and disease symptoms acquired through life experience and/or a decline in innate immune defence mechanisms. This is corroborated by the observation that older adults exhibit reduced systemic levels of IL-6, IL-10 and CRP following vaccination,56 which may contribute to their proclivity to report fewer systemic AEs, particularly fever.
The utilisation of this model as part of the research process resulted in the attainment of a commendable overall precision for the variables, with a range of 63.4% and moderate Kappa Fleiss indices based on the classification proposed.22,57 While the majority of the explanatory variables, namely the type of vaccines administered, were not found to be significant in these models, this differs from the results reported,12 which were associated with the types of vaccines. The marginal probabilities associated with each variable and each modality exhibited an intriguing trend that appeared to align with the logic of post-vaccination events in our study environment.
Conclusion
The low vaccination coverage rate has resulted in a high incidence of adverse reactions following vaccination against the new coronavirus (Covid-19), highlighting the crucial need for vigilant safety monitoring. In order to develop evidence-based intervention strategies that can be adapted to local and national contexts, it is essential to understand the factors associated with the occurrence of self-reported post-vaccination adverse events. Adverse events following vaccination (AEFI) linked to the new coronavirus (Covid-19) have been reported during the vaccine clinical trials, and the dissemination of this information could assist in the reduction of vaccine hesitancy, a persistent challenge, particularly among the Congolese. The enhancement of surveillance systems and the refinement of policy frameworks to ensure optimal post-vaccination follow-up are also imperative. The enhancement of such surveillance systems and the refinement of policy frameworks will facilitate the deployment of other vaccines that are required to manage both current and future emerging health problems. Furthermore, it will allow for consideration to be given to the introduction of the COVD-19 vaccine into routine immunisation programmes.
Data Sharing Statement
The original contributions to this study are presented in the article, and the database supporting this manuscript is provided in (DOI: 10.6084/m9.figshare.28474052).
Acknowledgments
We would like to express our gratitude to all those who took part in the project, as well as to the investigators, authors and authorities of the University of Mbuji-Mayi. We would like to extend our gratitude to all those who contributed to the production of this manuscript.
Disclosure
No competing interests have been declared by the author.
References
1. Allan M, Lièvre M, Laurenson-Schafer H, et al. The World Health Organization COVID-19 surveillance database. Int J Equity Health. 2022;21(3):167. doi:10.1186/s12939-022-01767-5
2. Akiba DB, Madabali EA, Fataki RB, et al. Acceptability of immunization against COVID-19 by the populations of the Kasenga State Health Area in the Uvira Health Zone, DR Congo. J Immune Based Ther Vaccines Antimicrob. 2024;13(03):33–46. doi:10.4236/jibtva.2024.133003
3. Otshudiema JO, Folefack GLT, Nsio JM, et al. Epidemiological comparison of four COVID-19 waves in the Democratic Republic of the Congo, March 2020–January 2022. J Epidemiol Glob Health. 2022;12(3):316–327. doi:10.1007/s44197-022-00052-6
4. Mallhi TH, Khan AH, Sarriff A, Adnan AS, Khan YH. Determinants of mortality and prolonged hospital stay among dengue patients attending tertiary care hospital: a cross-sectional retrospective analysis. BMJ Open. 2017;7(7):e016805. doi:10.1136/bmjopen-2017-016805
5. Butt MH, Ahmad A, Misbah S, et al. Ensuring the quality and appropriate use of hand sanitizers during the COVID-19 pandemic: suggestions and recommendations with the role of the pharmacist. Disaster Med Public Health Prep. 2022;16(5):1708–1709. doi:10.1017/dmp.2021.55
6. Mallhi TH, Khan YH, Butt MH, et al. Risks of Zoonotic transmission of COVID-19 during Eid-Ul-Adha in Pakistan. Disaster Med Public Health Prep. 2020;14(4):e40–e41. doi:10.1017/dmp.2020.278
7. Ullah I, Lin C, Malik NI, et al. Factors affecting Pakistani young adults’ intentions to uptake COVID‐19 vaccination: an extension of the theory of planned behavior. Brain Behav. 2021;11(11):e2370. doi:10.1002/brb3.2370
8. Hung M, Lauren E, Hon ES, et al. Social network analysis of COVID-19 sentiments: application of artificial intelligence. J Med Internet Res. 2020;22(8):e22590. doi:10.2196/22590
9. Cinelli M, Quattrociocchi W, Galeazzi A, et al. The COVID-19 social media infodemic. Sci Rep. 2020;10(1):16598. doi:10.1038/s41598-020-73510-5
10. Shrotri M, Swinnen T, Kampmann B, Parker EP. An interactive website tracking COVID-19 vaccine development. Lancet Glob Health. 2021;9(5):e590–e592. doi:10.1016/S2214-109X(21)00043-7
11. Funk C, Marques da Silveira E, Santos D, Ott M, Raschbichler V, Bailer SM. The HSV1 tail-anchored membrane protein pUL34 contains a basic Motif that supports active transport to the inner nuclear membrane prior to formation of the nuclear Egress complex. Viruses. 2021;13(8):1544. doi:10.3390/v13081544
12. Alzarea AI, Khan YH, Alatawi AD, et al. Surveillance of post-vaccination side effects of COVID-19 vaccines among Saudi population: a real-world estimation of safety profile. Vaccines. 2022;10(6):924. doi:10.3390/vaccines10060924
13. El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi Residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi:10.2147/IJGM.S310497
14. Harry AM, Edet CK, Ekanem NE, Kemdirim CJ, Uduak AE. Adverse events following COVID-19 vaccination in rivers State, Nigeria: a cross-sectional study. Niger Postgrad Med J. 2022;29(2):89–95. doi:10.4103/npmj.npmj_11_22
15. Lounis M, Aouissi HA, Abdelhadi S, Rais MA, Belkessa S, Bencherit D. Short-term adverse effects following booster dose of inactivated-virus vs. adenoviral-vector COVID-19 vaccines in Algeria: a cross-sectional study of the general population. Vaccines. 2022;10(11):1781. doi:10.3390/vaccines10111781
16. Alghamdi AA, Alkazemi A, Alissa A, Alghamdi I, Alwarafi G, Waggas HA. Adverse events following AstraZeneca COVID-19 vaccine in Saudi Arabia: a cross-sectional study among healthcare and nonhealthcare workers. Intervirology. 2021;65(2):104–109. doi:10.1159/000519456
17. Rahman MM, Masum MHU, Wajed S, Talukder A. A comprehensive review on COVID-19 vaccines: development, effectiveness, adverse effects, distribution and challenges. VirusDisease. 2022;33(1):1–22. doi:10.1007/s13337-022-00755-1
18. Shrestha S, Khatri J, Shakya S, et al. Adverse events related to COVID-19 vaccines: the need to strengthen pharmacovigilance monitoring systems. Drugs Ther Perspect. 2021;37(8):376–382. doi:10.1007/s40267-021-00852-z
19. Medeiros KS, Costa APF, Sarmento ACA, Freitas CL, Gonçalves AK. Side effects of COVID-19 vaccines: a systematic review and meta-analysis protocol of randomised trials. BMJ Open. 2022;12(2):e050278. doi:10.1136/bmjopen-2021-050278
20. Organization WH. COVID-19 vaccines: safety surveillance manual; 2021. https://apps.who.int/iris/bitstream/handle/10665/345178/9789240032781-eng.pdf.
21. Del Águila MR, González-Ramírez AR. Sample size calculation. Allergol Immunopathol. 2014;42(5):485–492. doi:10.1016/j.aller.2013.03.008
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi:10.2307/2529310
23. Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11. doi:10.3389/fmicb.2020.01526
24. Hajj Hussein I, Chams N, Chams S, et al. Vaccines through centuries: major cornerstones of global health. Front Public Health. 2015;3:3. doi:10.3389/fpubh.2015.00269
25. Andre FE, Booy R, Bock HL, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2007;86(2):140. doi:10.2471/BLT.07.040089
26. Harapan H, Wagner AL, Yufika A, et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Front Public Health. 2020;8:8. doi:10.3389/fpubh.2020.00381
27. Akilimali PZ, Egbende L, Kayembe DM, et al. COVID-19 vaccine coverage and factors associated with vaccine hesitancy: a cross-sectional survey in the City of Kinshasa, Democratic Republic of Congo. Vaccines. 2024;12(2):188. doi:10.3390/vaccines12020188
28. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our World Data. 2020; 2022. http://www.barkerstats.com/PDFs/Vaccines/GlobalDistribution/Vaccinations-WorldWide.pdf.
29. Karafillakis E, Larson HJ. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine. 2017;35(37):4840–4850. doi:10.1016/j.vaccine.2017.07.061
30. Pelčić G, Karačić S, Mikirtichan GL, et al. Religious exception for vaccination or religious excuses for avoiding vaccination. Croat Med J. 2016;57(5):516. doi:10.3325/cmj.2016.57.516
31. Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: a critical review. Soc Sci Med. 2014;112:1–11. doi:10.1016/j.socscimed.2014.04.018
32. Al-Qerem WA, Jarab AS. COVID-19 vaccination acceptance and its associated factors among a Middle Eastern population. Front Public Health. 2021;9. doi:10.3389/fpubh.2021.632914
33. Kaplan RM, Milstein A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci. 2021;118(10):e2021726118. doi:10.1073/pnas.2021726118
34. Nguyen KH, Chen Y, Huang J, Allen JD, Beninger P, Corlin L. Who has not been vaccinated, fully vaccinated, or boosted for COVID-19? Am J Infect Control. 2022;50(10):1185–1189. doi:10.1016/j.ajic.2022.05.024
35. García LY, Cerda AA. Acceptance of a COVID-19 vaccine: a multifactorial consideration. Vaccine. 2020;38(48):7587. doi:10.1016/j.vaccine.2020.10.026
36. Are EB, Song Y, Stockdale JE, Tupper P, Colijn C. COVID-19 endgame: from pandemic to endemic? Vaccination, reopening and evolution in low- and high-vaccinated populations. J Theor Biol. 2023;559:111368. doi:10.1016/j.jtbi.2022.111368
37. Serwaa D, Osei-Boakye F, Nkansah C, et al. Non-life-threatening adverse reactions from COVID-19 vaccine; a cross-sectional study with self-reported symptoms among Ghanaian healthcare workers. Hum Vaccines Immunother. 2021;17(11):3881–3886. doi:10.1080/21645515.2021.1963600
38. Alao MA, Ogunbosi BO, Ibrahim OR, Oladokun RE, Lagunju IA. Adverse events following COVID-19 vaccination: a cross-sectional study in Ibadan, Nigeria: adverse events following COVID-19 vaccination. Niger Med J. 2022;63(3):248–258. doi:10.60787/NMJ-63-3-46
39. Klugar M, Riad A, Mekhemar M, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology. 2021;10(8):752. doi:10.3390/biology10080752
40. Andrzejczak-Grzadko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25:4418–4421. doi:10.26355/eurrev_202106_26153
41. Jayadevan R, Shenoy R, A TS. Survey of symptoms following COVID-19 vaccination in India. Medrxiv. 2021;2021–2022.
42. Aka LBN, Noufe S, Douba A, et al. Manifestations post-vaccinales indésirables dans le cadre de la vaccination contre la COVID-19 à Abidjan, Côte d’Ivoire, 2022. Rev DÉpidémiologie Santé Publique. 2023;71:101533. doi:10.1016/j.respe.2023.101533
43. Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. Npj Vaccines. 2019;4(1):39. doi:10.1038/s41541-019-0132-6
44. Mohamed MS, Mohamed AO, Alenazy R, et al. A first report on side-effects of COVID-19 vaccines among general population in Sudan: a cross-sectional analysis. Vaccines. 2023;11(2):315. doi:10.3390/vaccines11020315
45. Desalegn M, Garoma G, Tamrat H, Desta A, Prakash A. The prevalence of AstraZeneca COVID-19 vaccine side effects among Nigist Eleni Mohammed memorial comprehensive specialized hospital health workers. Cross sectional survey. PLoS One. 2022;17(6):e0265140. doi:10.1371/journal.pone.0265140
46. Zahid MN. Unfolding the mild to moderate short-term side effects of four COVID-19 vaccines used in Bahrain: a cross-sectional study. Vaccines. 2021;9(11):1369. doi:10.3390/vaccines9111369
47. Alfaleh A, Alkattan A, Radwan N, et al. Adverse drug reactions from two COVID-19 vaccines reported in Saudi Arabia. Drugs Ther Perspect. 2022;38(2):84–92. doi:10.1007/s40267-022-00893-y
48. Ahsan W, Syed NK, Alsraeya AA, et al. Post-vaccination survey for monitoring the side effects associated with COVID-19 vaccines among healthcare professionals of Jazan province, Saudi Arabia. Saudi Med J. 2021;42(12):1341–1352. doi:10.15537/smj.2021.42.12.20210576
49. Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5(7):441–449. doi:10.4161/hv.8476
50. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–349. doi:10.1016/S1473-3099(10)70049-9
51. Pittman PR. Aluminum-containing vaccine associated adverse events: role of route of administration and gender. Vaccine. 2002;
52. Weber SK, Schlagenhauf P. Childhood vaccination associated adverse events by sex: a literature review. Travel Med Infect Dis. 2014;12(5):459–480. doi:10.1016/j.tmaid.2014.01.008
53. McCarthy MM, Nugent BM, Lenz KM. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat Rev Neurosci. 2017;18(8):471–484. doi:10.1038/nrn.2017.61
54. Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294(2):87–94. doi:10.1016/j.cellimm.2015.02.004
55. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. doi:10.1016/j.cellimm.2015.01.018
56. El Yousfi M, Mercier S, Breuillé D, et al. The inflammatory response to vaccination is altered in the elderly. Mech Ageing Dev. 2005;126(8):874–881. doi:10.1016/j.mad.2005.03.008
57. Branger B. Accord entre observateurs: indice kappa de Cohen. Réseau «Sécurité Naiss–Naître Ensemble» Pays Loire. 2009;1–7.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

