Back to Journals » Cancer Management and Research » Volume 17
Viral Involvement in Oral Potentially Malignant Disorders: A Scoping Review
Authors Zahid KS , Hidayat W , Zakiawati D
Received 3 July 2024
Accepted for publication 27 January 2025
Published 18 February 2025 Volume 2025:17 Pages 309—330
DOI https://doi.org/10.2147/CMAR.S485418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Antonella D'Anneo
Khalid Sulthoni Zahid,1 Wahyu Hidayat,2 Dewi Zakiawati2
1Undergraduate Program in Dental Medicine, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Oral Medicine, Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Wahyu Hidayat, Department of Oral Medicine, Faculty of Dentistry, Universitas Padjadjaran, Jl. Sekeloa Selatan No. 1, Bandung, West Java, 40132, Indonesia, Tel +6287822404343, Email [email protected]
Purpose: The purpose of this scoping review was to investigate which viruses other than HPV and EBV-associated with OPMDs and investigate whether viruses are linked solely to the etiology of OPMDs, their malignant transformation (MT), or both.
Methods: A scoping review following PRISMA-ScR methodological framework was used during the process. We conducted thorough searches in the EBSCOhost and PubMed databases. The inclusion criteria were publications that described viruses in OPMDs and identified pertinent research published between 2014 and 2023. The articles included underwent a thorough analysis and synthesis process to map out viruses in OPMDs. Pertinent characteristics such as research domains, publication dates, authors, type of research studies, sample sizes, gender ratios, types of OPMDs lesions, detected viruses, and methodological detection approaches were incorporated into the analysis.
Results: A total of twenty-eight articles were eligible for inclusion. The prevalence of viruses detected in OPMDs was found to be 78.57%. Viruses detected in this study, including HPV (0% to 86.6%), EBV (8% to 95.7%), hepatitis B virus (HBV) (6.71%) and herpes simplex virus (HSV) (1%). The biggest risk factor for OPMDs found in this study was tobacco use.
Conclusion: Given that 90% of oral cancers worldwide are attributable to OSCC, it is crucial to understand the role of viruses such as HPV, EBV, HBV, and HSV, along with unhealthy risk factors like tobacco and alcohol, which may contribute to the etiology and progression of lesions into OPMDs. Global data indicate that these viruses play varying roles in the etiology of OPMDs, with significant geographic variability, co-infections, and interactions with lifestyle factors influencing their oncogenic potential. Although this study found that virus positivity rates were higher in the malignant stage (OSCC) than in OPMDs and that there is a high prevalence of viruses in OPMDs, further research is needed to clarify the direct causality of virus-induced malignant transformation in OPMDs.
Plain Language Summary: Some conditions in the mouth are more likely to become cancerous. These conditions are called Oral Potentially Malignant Disorders (OPMDs). Most cases of mouth cancer are of a serious type called oral squamous cell carcinoma (OSCC), which carries a high risk of death. Recent studies suggest that certain viruses, such as human papillomavirus (HPV) and Epstein-Barr virus (EBV), may increase the risk of OPMDs developing into cancer. This study looks at whether these viruses might cause OPMDs, make them more likely to turn into cancer, or both.
Keywords: Epstein-Barr Virus, human papillomavirus, malignant transformation, OPMDs, oral squamous cell carcinoma, oral cancer
Introduction
Oral cancer is the eighth most common cause of cancer-related death worldwide.1 There is a significant death rate from oral cancer and significant morbidity rate, making it a global health challenge, especially in developing countries.2,3 A survival rate of approximately 50% underscores the urgent need to improve strategies for prevention, early detection and treatment.4,5 Oral Squamous Cell Carcinoma (OSCC) has a prevalence of 90% of all oral cancer cases.6 A peculiar kind of cancer, OSCC, develops in the tissues of the oral mucosa and widely acknowledged as one of the most prevalent cancers in the world.7 This high prevalence highlights the critical need for focused research and interventions aimed at mitigating the burden of OSCC, which significantly impacts patient outcomes and healthcare systems. Currently, the main therapeutic benefit of OSCC is surgical removal of the tumour, with total resection being the main goal. With poor Positive resection margin success rates range from 30 to 40%, while local recurrence rates range from 25 to 50%, total resection is still challenging.7 These challenges underscore the importance of developing complementary strategies, such as early detection and targeted prevention, to enhance patient prognosis. These features make OSCC a significant threat to both life and quality of life for affected individuals.8 Oral Potentially Malignant Disorders (OPMDs) are defined as a group of oral mucosal lesions with an increased risk of malignant transformation.9 A significant portion of OSCC cases originates from OPMDs.10 OPMDs lesions make for 17–35% of all new occurrences of oral cancer, and between 0.7 and 2.9% of them develop into malignant transformation each year.11 OPMDs encompass a variety of diseases, which present with a multitude of clinical manifestations, histologic subtypes, risk factors and etiologies, and which are classified into a diverse array of entities.9 The overall prevalence of OPMDs worldwide is currently 4.47%, and men are more commonly affected.12 Leukoplakia, erythroplakia, oral lichen planus (OLP), and oral submucous fibrosis (OSMF) are some of the most common types of OPMDs. Research shows leukoplakia has a recorded global incidence of 2–4%.13 The global prevalence of leukoplakia is estimated to be 4.11%, with the highest incidence in Asian populations (7.77%).12 The rate of malignant transformation of leukoplakia is reported to be 20% and 9.5%. Malignant transformation rates vary across studies due to differing criteria, follow-up periods, and geographic regions. Leukoplakia lesions are most commonly seen in middle-aged and older men. The rate of dysplastic or malignant change in leukoplakia has been reported to be between 15.6–39.2%. Leukoplakia typically manifests approximately five years prior to the onset of OSCC.14,15 OLP and OSMF can also develop into oral cancer, with a global prevalence of 1.01%, 4.47%.16 Another OPMDs lesion is erythroplakia with a prevalence between 0.02–0.83%, but a higher MT probability, of 14–50%.17 These statistics emphasize the heterogeneity of OPMDs and the need for targeted approaches to identify high-risk lesions for timely intervention.
Risk factors in OPMDs cases are related to lifestyle patterns, such as tobacco use, whether smoking, chewing tobacco, or smokeless tobacco, alcohol abuse, betel nut chewing and many hypothesise that viral infections such as human papillomavirus (HPV) and Epstein-Barr virus (EBV) may also play a role in OPMDs.9,18,19 According to a study by de la Cour et al the prevalence of HPV in OPMDs was 22.5%, with HPV-16 as the most common genotype.20 HPV virus infection is related to the era of globalisation which causes changes in lifestyle and sexual behaviour patterns that are prone to spreading various kinds of viral infections, as discussed in a study by Itarat et al regarding sexual behaviour is linked to a higher chance of contracting HPV 16/18.21 The chance of contracting HPV 16 is increased by early sexual contact, while the risk of contracting HPV 18 is increased by having several sexual partners. Research conducted by Pierangeli et al found 52/116 samples (44.8%) were positive for one of the HPV genotypes they tested.18 Then a research study conducted by Mahalingam et al which states the involvement of EBV viruses that are closely related in OPMDs cases.19 However, this is still a matter of debate because there are also journals that state that the potential implications of viruses such as HPV and EBV in OPMDs manifestations such as leukoplakia is still unclear.22 This conflicting evidence necessitates further investigation into the exact role of viral infections in OPMDs, particularly in populations with high-risk lifestyle patterns.
OPMDs can progress to OSCC through a process called malignant transformation.23 Malignant transformation (MT) is the term given to the process by which normal, metaplastic, or benign neoplastic tissue transforms into cancerous tissue. This process usually occurs in a series of steps, and the affected tissue gradually accumulates genetic mutations that express a malignancy phenotype.9 Among the OPMDs lesions identified with a high risk of MT, four types stand out including leukoplakia, erythroplakia, oral lichen planus (OLP), and oral submucous fibrosis (OSMF). These are the most common and frequently discussed lesions due to their elevated potential for progression to malignancy.24,25 Chronic inflammation plays a part in the slow and intricate process of genetic mutation accumulation that leads to the malignant transformation of OPMDs.26,27 Similar to OPMDs, MT is associated with risk factors related to lifestyle choices such alcoholism, tobacco use, and viral infections. OPMDs that has gone through MT can end up becoming malignant such as OSCC which can result in increased mortality, increased organ dysfunction so that patients cannot perform normal activities, which will greatly affect the quality of life of the patients.9 Therefore, early diagnosis and appropriate treatment are very important to prevent the development of oral cancer. Integrating comprehensive education on risk factors, including viral infections, into public health strategies is pivotal for mitigating the progression of OPMDs to malignancy. However, most cases of oral cancer, especially OSCC, are diagnosed at an advanced stage, emphasising the need for early diagnosis and timely treatment is paramount in treating patients and preventing oral cancer at an early stage, through evaluation and management of OPMDs, as well as massive education on risk factors, especially viral infections and understanding of the mechanisms involved in the malignancy of the disease.25
To gain a deeper insight into the role of viral infections in oral potentially malignant disorders (OPMDs) and their malignant transformation, as well as to elucidate other viruses implicated and facilitate the prevention of oral cancer, further research on viral infections in OPMDs from a range of study sources is imperative. Consequently, the objective of this scoping review was to identify the viruses, in addition to HPV and EBV, that are associated with OPMDs and to ascertain whether these viruses are accountable for the aetiology of OPMDs, their malignant transformation (MT), or both.
Methods
Protocol
The research procedure was carried out using the Joanna Briggs Institute (JBI) scoping review protocol guidelines.28 This research was conducted using a framework of Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR).29
Sample Criteria and Study Selection
Studies were included with the following criteria, namely: 1) Articles that included research related to OPMDs diseases associated with viral infections; 2) Cross-sectional, cohort, case control, and pilot studies; 3) Articles written or available in English; 4) Published and accessible full-text articles; 5) Articles published in the last 10 years (2014–2023). The eligibility study conformed to the exclusion criteria, namely: 1) Articles in the form of case study, case series, case report; 2) Review articles.
Search Strategy
The Snowballing approach, Boolean operators, and manual searching were used to search the literature. Searching for scientific articles on PubMed and EBSCOhost database search engines using keywords
((OPMD) OR (OPMDs) OR (oral premalignant disorders) OR (oral potentially malignant disorder) OR (leukoplakia) OR (erythroplakia) OR (oral lichen planus) OR (OLP) OR (oral submucous fibrosis) OR (OSF) OR (OSMF)) AND ((virus) OR (virus infection) OR (HPV) OR (Human Papillomavirus) OR (EBV) OR (Epstein-Barr Virus) OR (HSV) OR Herpes Simplex Virus)).
Data Extraction
Data extraction was conducted in order to provide a summary of the characteristics of the included studies. Data extraction was carried out by retrieving data from each literature including author’s name, title, year of publication, type of study design, study objectives, sample, methods, results, and conclusions Tables 1–2.
Results
Literature Search and Papers Selection
A literature search using PubMed and EBSCOhost databases and other sources identified the total number of 590 studies. The first screening was done by checking for duplicates from all database search engines, resulting in a total of 549 studies. The second screening was done by reading the titles and abstracts of articles and then selecting those that were not relevant, resulting in data for 87 studies. Following the third screening, the article’s content was read through in its entirety to assess its fit with the study topic, the remaining 28 studies were included in the review (Figure 1). Table 1 shows the demographic characteristics of the article sample including the code number representing the journal of the article, researcher’s name, year of publication, title, location, type of article design, sample and sex ratio (male:female), age range, and virus detection method used in the study.
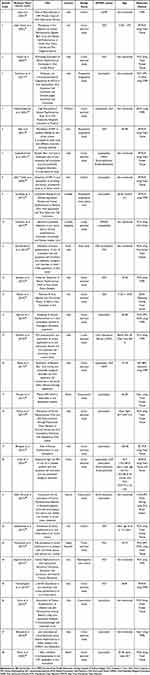 |
Table 1 Article Characteristics |
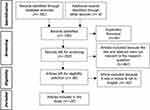 |
Figure 1 Flow Diagram of Study Selection Based on PRISMA-ScR. |
Characteristics of Studies
The study design variations in the articles used in this study vary. There were 17 cross-sectional studies;19,22,31,32,34,38,40–44,46,47,50,52 three cohort study;30,36,49 three prospective longitudinal study;33,35,37 three case control study;45,48,53 one retrospective case control study;51 and one pilot study39(Table 1).
There are several groups of OPMDs lesion types detected in this study, including OLP in 13 articles;31,32,34,35,39–41,44,47,49–52 leukoplakia in 16 articles;19,22,33,34,36,37,39,42,44–48,53 oral submucous fibrosis (OSMF) in three articles;30,43,44 erythroplakia in three articles;19,47,48 OPMDs-non specified in one article38(Table 1).
The sample articles used in this study were conducted in different countries, 13 articles (42.86%) were conducted in India;19,22,30,32,33,39,40,42–44,46,53,54 four articles were conducted in Iran;31,41,49,51 three articles were conducted in Italy;35,36,52 one article were conducted in Thailand;34 one article were conducted in China;47 two article were conducted in Brazil;45 one article were conducted in Austria;48 one article were conducted in Czech Republic;50 one article were conducted in Sweden;37 and one article were conducted in Cordoba, Argentina38(Table 1).
There were variations in virus detection methods in the articles used in the study. There were 13 articles using PCR method;32,33,38–40,42,43,46,49–51,53,55 eight articles using RT-PCR method;19,22,31,35–37,47,52 one article using qPCR method;34 two article with nPCR method;45,54 one article using HC2-HPV-DNA-Testing method;48 one article using Biopsy-Paraffin-Embedded-Tissue Biopsy method;30 one article with IHC-EBV-detection method;44 while one other article did not mention the detection method34(Table 1).
Study Results
A total of twenty-eight articles met the inclusion criteria that discussed viral infections in OPMDs. The types of viruses detected in OPMDs cases vary widely around the world where in this scoping review study positive viruses were detected in a variety of OPMDs cases including leukoplakia, erythroplakia, OLP, and OSMF. Viruses were detected positively in 22 of the 28 journals studied, making the prevalence of viruses associated with OPMDs cases in this article 78.57%. The viruses detected in this study, including human papillomavirus or HPV ranged from 0% to 86.6% (mean 24.65%), Epstein-Barr virus ranged from 8% to 95.7% (mean 38.13%), hepatitis B virus (HBV) by 6.71% and HSV by 1%.(Table 1) When calculating the percentage of viruses detected positively from each type of virus, the prevalence of HPV was 79.2%, EBV by 12.5%, HSV by 4.2%, and HBV by 4.2%(Figure 2).
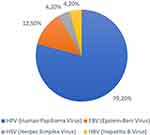 |
Figure 2 Type of virus detected positively in percent (%). |
In this study, there were several groupings based on viral involvement in OPMDs, namely possible involvement in 5 articles, which indicates the possibility of HPV involvement in OPMDs lesions, but further study is needed or there are explanations that indicate doubt about the role of HPV in the condition.22,31,46,47,50 Furthermore, there are articles that have confirmed viral involvement in OPMDs, namely 9 articles stating that HPV is confirmed being a part of the pathophysiology of OPMDs lesions and oral cancer.30,32,39,40,42,43,48,49,53 Several articles also mentioned prevalence, types of HPV involved, and association with malignant transformation, the article that stated the virus was not involved was article 17, which stated that there was no evidence to suggest a role for HPV in the development of malignancy in oral lesions, although the overall prevalence in OPMDs lesions such as leukoplakia surpassing that of the control group.45(Table 2) Factors associated with risk include tobacco chewing, smoking, and alcohol consumption allow for malignant transformation of OPMDs. Many articles in the sample in this study stated the possibility of the virus being involved in OPMDs and/or MT. However, one journal in the sample, sample number 17 by Ferreira et al did not establish HPV’s role in MT due to limited follow-up data. Instead, their study highlights blood plasma and saliva as reliable sources for HPV detection and encourages further research into HPV’s influence on MT.45(Table 2) Next is the group that discusses HPV involvement in MT, namely in sample number 18, the article mentions the role of HPV and p53 polymorphisms as important markers to indicate malignant transformation in oral lesions.46 The next group was those who stated that HPV was not involved with OPMDs and its MT, namely samples on the article number 20 and 23, both samples stated that HPV did not play a role in OPMDs MT, or there was no positive evidence indicating the presence of HPV in the samples tested.47,50 The last group was those requiring further epidemiological investigation, sample 19, stating the need for further investigation, especially in the Indian population, to ascertain the role of HPV, especially in OPMDs lesions such as leukoplakia22(Table 2).
 |
Table 2 Data Extraction Table |
According to the results of the three articles used in this study, OSCC had a greater prevalence of viral infection than OPMDs, the percentage positive rate of virus detected in OSCC was much higher than OPMDs31,33,37(Figure 3).
 |
Figure 3 Comparison of positive detected viruses in percent (%). |
Discussion
The association of the role of viral infection in OPMDs is still controversial, most of these research articles found HR-HPV and viral DNA in OPMDs, but on the other hand HPV is also widely reported to be detected in healthy oral mucosa. In this study, there was a viral positive prevalence of 78.57% from a total of 26 journal articles examining OPMDs including leukoplakia, erythroplakia, OLP, and OSMF in relation to viral infections including HPV, EBV, HSV and HBV.(Table 2) The HPV prevalence of 79.2% (Figure 2) found in the results of this study is in line with a previous study conducted by Pierangeli et al who found 44.8% of their OPMDs samples were positive for one of the HPV viral genotypes they tested.18 The ability of HPV to initiate malignant transformation is largely attributed to its E6 and E7 oncoproteins, which disrupt p53 and Rb tumor suppressor pathways.56 This suggests that HPV could have a significant role in the progression of OPMDs. Where in the research of Pierangeli et al found a high positivity rate of HPV in OLP lesions, namely 75%, which is similar to the findings in this study where there are 3 journal articles that also state the high positivity rate of HPV in OLP cases in articles 2, 12, and 23, namely 25%, 86.5%, and 53.3% by Saber Amoli et al, 2022; Sameera et al, 2019; Radochova et al, 2015. In addition, the study by Pierangeli et al also found a high positivity rate of HPV in leukoplakia of 33.3%, which is similar to the findings in this study where there is one article (Agarwal et al, 2018) which also detected HPV in leukoplakia and the results were the same at 33.3% as well. A study by Gilligan et al (2023) reported a low frequency of positive HPV infection in their study, where only 15.15% were detected positive for HPV virus infection in their leukoplakia samples. This is in contrast to the overall results of this study, which found a viral positive prevalence of 78.57%.57 Study by Gilligan et al, however, is consistent with a number of this study’s papers that discovered low percentages of positive HPV virus detection in their leukoplakia samples, specifically articles 4, 5, 8, 9, 18, and 19. (Table 2) Interestingly, two articles utilized in this study, Tomo et al (2020) and Bhosale et al (2016), provide valuable insights into oral leukoplakia and its relationship with HPV. Notably, both studies reported 0% detection of HPV in their research. Furthermore, article 28 by Tomo et al highlighted that immunohistochemical overexpression of p16 is frequently observed in HPV-negative oral leukoplakia, suggesting the involvement of alternative mechanisms driving p16 expression and lesion pathogenesis in the absence of the virus. This underscores the importance of considering non-viral factors in the oncogenesis of oral lesions, broadening the understanding of variability in HPV detection rates. Article 27 by Bhosale et al emphasized that the mere presence of HPV DNA does not confirm its biological activity in head and neck squamous cell carcinoma (HNSCC) or leukoplakia. They stressed the necessity of evaluating transcriptional activity, particularly E6/E7 mRNA, to establish the virus’s active role in pathogenesis. Without such confirmation, HPV’s contribution to tumorigenesis remains ambiguous. These findings collectively highlight the complexity of linking HPV detection to disease progression and reinforce the critical need to assess transcriptional activity, not just DNA presence, to better understand HPV’s role in oral potentially malignant disorders (OPMD). Both studies also advocate for broader epidemiological investigations to address geographic and methodological variability in HPV detection.
Furthermore, in article 2 of this study found EBV, resulting in an EBV prevalence of 12.5%. (Figure 2) Article 2 shows a greater prevalence in the malignant group compared to non-malignant. EBV is thought to contribute to oncogenesis by expressing latent membrane proteins (LMP1 and LMP2), which activate oncogenic pathways and inhibit apoptosis.58 This lends credence to the idea that EBV can contribute indirectly to the development of oral lesions. A similar percentage difference was also discovered in a study carried out by Shariati et al, who in their study found EBV infection in their OLP group samples showing a significantly higher percentage of positive viruses compared to their control group samples.59 In the study of Shariati et al also stated that in fact, the results of their study explained that EBV might be involved in the pathogenesis of OLP because in the study the EBV virus was detected positively in OLP cases as much as 15.8% while in the control subjects 0%. However, in this study there are also 2 articles that contradict the research of Shariati et al where in 2 articles of this study, namely articles 13 and 16 (Table 2), the level of virus positivity in OLP is very low, namely 6.7% and 8% only, which raises doubts from researchers whether EBV may not be involved in OLP cases.
Other viruses besides HPV and EBV found in this study were HSV in one sample where HSV was found positive in OPMDs in sample article 10 (Table 2), resulting in a prevalence of HSV positivity of 4.2% in this study.(Figure 2) HSV is known to establish latent infections, which can reactivate under immunosuppressive conditions. This reactivation might exacerbate pre-existing lesions in OPMDs.60 This is supported by the research of Jalouli et al (2015) who found HSV-1 positivity in 36% of their leukoplakia samples.61 However, the study concluded that there was no specific correlation between HSV-1 and the development of OSCC or malignant transformation in OPMDs. This is because there is a high incidence of HSV-1, both in healthy oral mucosa, as well as in OPMDs leukoplakia, and OSCC tissue. Furthermore, another type of virus that is rarely discussed and studied, but associated with OPMDs cases is hepatitis-B virus (HBV), with a prevalence of HBV in this study of 4.2%.(Figure 2) Article 13 (Table 2), found a percentage of HBV virus infection in their OLP samples of 6.71%. HBV-related chronic liver disease is hypothesized to induce systemic immunosuppression, increasing susceptibility to oral lesions such as OPMDs. This supports the notion that HBV may influence OPMDs development indirectly through systemic effects on host immunity.62 Although the percentage is small, the article concluded that the risk of OLP in individuals with a background of chronic liver disease is two to five times higher than in normal individuals. The reason for this is that individuals with chronic liver disease are likely to be caused by HBV. However, the results in article 13 contradict with Nosratzahi et al (2018), the study concluded that there is no association between OLP and hepatitis B virus.63
Viruses detected in this study include HPV, EBV, HBV and HSV. Several journals included in this study confirmed or suggested that viruses may be associated with the aetiology of OPMDs and may also be involved in malignant transformation. High-risk HPV (HR-HPV) is known to have a strong potential to induce malignant transformation in mucosal epithelial cells.34 This study shown in articles 2, 4, and 6 (Table 2), there was a significant difference in the positivity rate of detected viruses between OPMDs and OSCC.(Figure 3) In this study, the virus positivity rate in OSCC was much higher than that in OPMDs. This suggests that the virus has a potential role in malignant transformation (MT) in OPMDs. The mechanism by which viruses may contribute to carcinogenesis, as identified by Parada et al, involves promoting the activation of proto-oncogenes or inhibiting tumor suppressor genes such as p53 and Rb, leading to uncontrolled cell proliferation and increased survival of malignant cells.64 However, further studies are needed to elucidate the direct causal relationship between the virus and the induction of malignant transformation in OPMDs. Another hypothesis obtained from this study (Figure 3) is that the virus infects more cells after carcinogenesis (already at the OSCC stage). This hypothesis is aligned with the research conducted by Budhy (2018), which revealed that viruses, particularly EBV, play an important role in the carcinogenesis of OSCC. These findings suggest that viral infections could intensify after carcinogenesis, indicating their potential role in the progression of OPMDs to malignancy.65 It is crucial to comprehend the part played by viral infections in the development of oral potentially malignant disorders OPMDs into OSCC in order to developing targeted interventions in order to detect, prevent and treat oral cancer early. However, viral infections are not the only contributors to OPMDs development. Lifestyle factors, particularly the high prevalence of tobacco use, also play a significant role.
To ensure the validity of our findings, potential confounding factors were identified, including alcohol consumption and usage of tobacco. These factors are well-established as independent risk factors for oral OPMDs and OSCC and could influence the relationship between the detected virus and the disease outcome. Detailed information regarding lifestyle habits (smoking frequency, and type of tobacco used) was obtained. This study shows the high percentage of OPMDs cases in the country, which is due to the habitual usage of tobacco products, such as cigarettes, tobacco chewing, gutka, and other forms of smokeless tobacco. Tobacco carcinogens, such as nitrosamines, cause DNA damage and epigenetic alterations in oral mucosa, promoting the development of OPMDs. The role of tobacco in OPMDs is particularly significant, as it facilitates genetic mutations that predispose cells to malignant transformation.1,66 Of the 12 articles from India used in this study, nine recorded the percentage of tobacco use.(Figure 4) In their sample of articles, all nine articles found high tobacco use in the form of smoking, chewing, hookah, gutka, and other forms of smokeless tobacco with 100% in seven articles and around 90% in two articles. The explanation in these articles regarding the form of tobacco use, of the 12 articles there are 6 articles that mention the form of tobacco use, namely cigarette tobacco (smoking) and smokeless (chewing), 4 articles (7, 11, 14, 19) of the 6 articles mention that the form of use of chewing tobacco (betel nut) is more than smoking. (Figure 4) The finding of a high prevalence of tobacco use in OPMDs cases in India in this study is in line with the study of Singh et al who found 45% tobacco use from a total of 1280 OPMDs and oral cancer samples that they studied.67 In the study of Singh et al also stated that tobacco use both in the form of smoking and chewing is a significant risk factor associated with OPMDs, especially leukoplakia and OSMF. The high use of tobacco in OPMDs patients, especially leukoplakia in India, has also been studied by Venkat et al comparable to this study’s findings, in the Venkat et al study found the percentage of tobacco use in leukoplakia patients by 80%.68 The overall tobacco risk factor in this study was shown in 16 of the 28 articles used. (Figure 4) Of the 16 articles, the average tobacco use was 74.30%, indicating that tobacco use is significant for the occurrence of OPMDs. This is also supported by the research of Kusiak et al who examined the relationship between tobacco use and OPMDs, namely leukoplakia, he found tobacco use in his study of > 80%.69 Interestingly, the highest prevalence of smoking in the Kusiak et al study was found in the youngest age group, namely 21–40 years with a smoking prevalence of 86.8%, with a predominance of female gender.
 |
Figure 4 Comparison of tobacco and alcohol risk factors in percent (%). |
Furthermore, there were 11 articles in this study that examined alcohol use in their studies, 10 of the 11 articles found alcohol use in the OPMDs patients they studied, resulting in an alcohol positivity of 90.91%. (Figure 4) Alcohol contributes to OPMDs by increasing the permeability of the oral epithelium to carcinogens and generating reactive oxygen species (ROS) that damage cellular DNA. This dual effect of alcohol enhances the carcinogenic potential of co-factors such as tobacco in OPMDs progression.70 In line with the results of this study, the results of Worakhajit et al also found an association between alcohol consumption and the incidence of OPMDs.71 The study examined a sample with alcohol consumption habits in individuals with OPMDs, including hard liquor and combined with beer consumption.
The sex ratio in the articles of this study had different variations, most of them showed more male OPMDs lesions than female with a ratio of 14:8.(Table 2) In line with this study, Kumar et al found that male sex was more dominant in their study.72 Of the 375 cases of OPMDs that Kumar et al studied there were 247 (65.87%) male patients and 128 (34.13%) female patients. Another study by Singh et al also found results that support this study, the Singh et al study concluded that men have a higher association with OPMDs, especially leukoplakia, with a 12.8% higher prevalence difference than women.67
There were 13 articles (40.6%) out of a total of 28 articles studied using the PCR detection method, PCR was chosen as a detection method due to its sensitivity and specificity in detecting viral DNA sequences.(Table 2) In article number 14 mentioned PCR was used because it has sensitivity in detecting genetic material even in very small amounts. Article 10 mentions PCR has good specificity in selectively amplifying target DNA sequences. This is in line with the research of Benevolo et al which states that PCR has good specificity and sensitivity in detecting viruses.73 On the other hand, article 16 mentions that viral genomes detected by PCR can give false results, because transcriptionally inactive viral genomes can also be detected by PCR. Research by Healy et al also said PCR can produce false virus detection, so several strategies are needed to minimise this.74 The solution to this problem according to Zhang et al is by using RT-qPCR and qPCR with internal amplification controls (IACs) can reduce false positives by minimizing the impact of PCR inhibitors and ensuring results are accurate. The inclusion of IACs enhances assay robustness and minimizes the risk of false positives.75
Detection of viral DNA and transcriptional activity highlights the intricate interplay of viruses such as human papillomavirus (HPV), Epstein-Barr virus (EBV), and Merkel Cell Polyomavirus (MCPyV) in oral lesions and their potential oncogenic roles. Iranian studies identified HPV DNA in approximately 27% of malignant lesions and slightly less in non-malignant ones, often involving genotypes other than HPV-16 and 18.(article 2 and 18) Similarly, EBV DNA was present in 30% of malignant lesions, and MCPyV DNA was found in comparable proportions across lesion types.(article 2 and 16) Co-infections were observed in 21.1% of cases, with triple infections involving HPV, EBV, and MCPyV found in 20.8% of co-infected samples(article 2, 20, and 23).
Carcinogenesis is significantly influenced by HPV oncogenes E6 and E7 by inactivating tumor suppressors p53 and pRb, promoting cell cycle dysregulation.(article 9, 13, and 25) EBV latency proteins, such as LMP1, contribute to immune evasion and cellular proliferation, while MCPyV LT-Ag targets similar pathways. However, MCPyV’s role in oral lesions remains less established compared to its recognized involvement in Merkel cell carcinoma.(article 7, 2, and 25) Interestingly, p16 overexpression has been linked to HPV-positive lesions, yet its presence in oral lichen planus (OLP) often occurs independently of HPV infection, suggesting alternative pathways of oncogenesis(article 9 and 25).
The prevalence of viral DNA and the interplay between these viruses highlight significant geographic variations and emphasize the complexity of oral lesion pathogenesis. Studies from regions like the Czech Republic reported 53% HPV positivity in OLP, with significant variations compared to other populations.(article 23) Conversely, Italian studies noted a limited association between p16 overexpression and HPV in oral lesions, reinforcing the need for region-specific diagnostic approaches.(article 25) Advanced molecular diagnostics, including RT-qPCR and qPCR, with internal amplification controls, can enhance the accuracy of detecting viral contributions to malignant transformation(article 10, 2, and 25).
While PCR offers high sensitivity and specificity, improvements such as internal amplification controls in RT-qPCR and qPCR are needed to increase detection accuracy and minimise false positives, thereby aiding in the identification of key viral contributions to malignant transformation. A fascinating field of study with significant implications for oral cancer prevention and management techniques is the intricate link between viral infections and possibly malignant abnormalities of the mouth. To decrease the worldwide oral cancer burden and improve our understanding of viral aetiology in oral carcinogenesis, collaborative initiatives including multidisciplinary research and clinical practice are crucial.
Major limitations of this review include the limited geographic representation, with a heavy focus on studies from India, which reduces the generalizability of the findings, and the lack of data availability of articles discussing HSV and HBV in relation to OPMDs and OSCC, which limits understanding of their specific roles in OPMDs and OSCC. Methodological variability, particularly in viral detection techniques such as PCR without consistent internal controls, also undermines the reliability of comparisons between studies. The lack of quantitative meta-analysis and incomplete assessment of cofactors such as tobacco and alcohol further limit the ability to evaluate synergistic effects contributing to malignant transformation. Finally, inadequate investigation of molecular mechanisms and temporal associations weakens the causal links between viral infections and malignant progression.
Conclusion
Given that 90% of oral cancers worldwide are attributable to OSCC, it is crucial to understand the role of viruses such as HPV, EBV, HBV, and HSV, along with unhealthy risk factors like tobacco and alcohol, which may contribute to the etiology and progression of lesions into OPMDs. Global data indicate that these viruses play varying roles in the etiology of OPMDs, with significant geographic variability, co-infections, and interactions with lifestyle factors influencing their oncogenic potential. Although this study found that virus positivity rates were higher in the malignant stage (OSCC) than in OPMDs and that there is a high prevalence of viruses in OPMDs, further research is needed to clarify the direct causality of virus-induced malignant transformation in OPMDs.
Recommendations
More studies need to be conducted using a longitudinal methodological approach with multidisciplinary evaluation to correlate clinical conditions with virus detection to determine the clarity of the relationship between viral infection and OPMDs conditions and their malignant transformation. The evaluation can be carried out by involving health workers such as dentists, especially dentists specialising in oral disease science who are competent experts for the detection of OPMDs and malignant lesions. Thus, researchers can gain a more comprehensive understanding of the role of viral infections found in individuals with OPMDs conditions.
Acknowledgments
The authors would like to express their gratitude to Universitas Padjadjaran for their financial support in covering the article processing charge (APC) for this publication. Additionally, the authors would like to thank the Department of Dental Medicine, Faculty of Dentistry, Universitas Padjadjaran, Bandung, West Java, Indonesia, for their invaluable support in the preparation of this manuscript. Furthermore, the authors extend their gratitude to all the authors of the included studies.
Funding
This study was supported by Universitas Padjadjaran for the payment of the article processing charge (APC) only, as the research itself was not funded by Universitas Padjadjaran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jiang* X, Wu* J, Wang J, Huang R. Tobacco and oral squamous cell carcinoma: a review of carcinogenic pathways. Tob Induc Dis. 2019;17(April). doi:10.18332/tid/105844
2. Cosetti-Olivera ML, da CAR, Prass TS, Martins MAT, Hugo FN, Martins MD. Mortality due to oral and oropharyngeal cancer in Uruguay from 1997 to 2014. J Appl Oral Sci. 2020;28. doi:10.1590/1678-7757-2019-0166
3. González-Ruiz I, Ramos-García P, Ruiz-ávila I, González-Moles MÁ. Early diagnosis of oral cancer: a complex polyhedral problem with a difficult solution. Cancers. 2023;15(13):3270. doi:10.3390/cancers15133270
4. Kim MJ, Ahn KM. Prognostic factors of oral squamous cell carcinoma: the importance of recurrence and pTNM stage. Maxillofac Plast Reconstr Surg. 2024;46(1):8. doi:10.1186/s40902-024-00410-3
5. Ahmad P, Nawaz R, Qurban M, et al. Risk factors associated with the mortality rate of oral squamous cell carcinoma patients. Medicine. 2021;100(36):e27127. doi:10.1097/MD.0000000000027127
6. Tandon P, Dadhich A, Saluja H, Bawane S, Sachdeva S. The prevalence of squamous cell carcinoma in different sites of oral cavity at our rural health care centre in loni, Maharashtra – a retrospective 10-year study. Wspólczesna Onkol. 2017;2:178–183. doi:10.5114/wo.2017.68628
7. Zhou K, Zheng K, Huang L, et al. Discrimination of healthy oral tissue from oral cancer based on the mean grey value determined by optical coherence tomography. BMC Oral Health. 2024;24(1):1004. doi:10.1186/s12903-024-04741-5
8. Yang Z, Du W, Zhang X, et al. Nonsmoking and nondrinking oral squamous cell carcinoma patients: a different entity. Front Oncol. 2021;11:558320. doi:10.3389/fonc.2021.558320
9. Lorini L, Bescós Atín C, Thavaraj S, et al. Overview of oral potentially malignant disorders: from risk factors to specific therapies. Cancers. 2021;13(15):3696. doi:10.3390/cancers13153696
10. Zhang X, Yang M, Liu Y, et al. A novel 4-gene signature model simultaneously predicting malignant risk of oral potentially malignant disorders and oral squamous cell carcinoma prognosis. Arch Oral Biol. 2021;129:105203. doi:10.1016/j.archoralbio.2021.105203
11. Kansara S, Sivam S. Premalignant lesions of the oral Mucosa. 2023.
12. Mello FW, Miguel AFP, Dutra KL, et al. Prevalence of oral potentially malignant disorders: a systematic review and meta‐analysis. J Oral Pathol Med. 2018;47(7):633–640. doi:10.1111/jop.12726
13. Ramôa Pires F, Barreto ME, Nunes JG, Carneiro NS, Azevedo AB, Dos Santos TC. Oral potentially malignant disorders: clinical-pathological study of 684 cases diagnosed in a Brazilian population. Med Oral Patol Oral Cir Bucal. 2019:e84–e88. doi:10.4317/medoral.23197
14. Ray JG. Oral potentially malignant disorders: revisited. J Oral Maxillofac Pathol. 2017;21(3):326–327. doi:10.4103/jomfp.JOMFP_224_17
15. Mortazavi H, Baharvand M, Mehdipour M. Oral potentially malignant disorders: an overview of more than 20 entities. J Dent Res Dent Clin Dent Prospects. 2014;8(1):6–14. doi:10.5681/joddd.2014.002
16. González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27(4):813–828. doi:10.1111/odi.13323
17. Anggarista KAN, Datau MA, Mahdani FY, Radithia D, Ernawati DS, Surboyo MDC. Knowledge of dental students about erythroplakia as an oral potentially malignant disorder. J Allied Health Sci. 2023. doi:10.1055/s-0043-1774299
18. Pierangeli A, Cannella F, Scagnolari C, et al. Frequent detection of high human papillomavirus DNA loads in oral potentially malignant disorders. Clin Microbiol Infect. 2016;22(1):95.e9–95.e15. doi:10.1016/j.cmi.2015.09.011
19. Gopalakrishnan Mahalingam KK, Sankar LS, Masthan KMK, Mahalakshmi K, Naveen Kumar VE. Barr viral load in exfoliated cells of oral squamous cell carcinoma and oral potentially malignant disorders - A cross-sectional study. J Clin Virol. 2021;1(4):100051. doi:10.1016/j.jcvp.2021.100051
20. de la Cour CD, Sperling CD, Belmonte F, Syrjänen S, Kjaer SK. Human papillomavirus prevalence in oral potentially malignant disorders: systematic review and meta‐analysis. Oral Dis. 2021;27(3):431–438. doi:10.1111/odi.13322
21. Itarat Y, Kietpeerakool C, Jampathong N, et al. Sexual behavior and infection with cervical human papillomavirus types 16 and 18. Int J Womens Health. 2019;11:489–494. doi:10.2147/IJWH.S218441
22. Bhargava A, Shakeel M, Srivastava A, Raza T, Rizvi S, Varshney P. Role of human papilloma virus in oral leukoplakia. Indian J Cancer. 2016;53(1):206. doi:10.4103/0019-509X.180812
23. Tan Y, Wang Z, Xu M, et al. Oral squamous cell carcinomas: state of the field and emerging directions. Int J Oral Sci. 2023;15(1):44. doi:10.1038/s41368-023-00249-w
24. Warnakulasuriya S. Oral potentially malignant disorders: a comprehensive review on clinical aspects and management. Oral Oncol. 2020;102:104550. doi:10.1016/j.oraloncology.2019.104550
25. Kumari P, Debta P, Dixit A. Oral potentially malignant disorders: etiology, pathogenesis, and transformation into oral cancer. Front Pharmacol. 2022;13. doi:10.3389/fphar.2022.825266
26. Irani S, Barati I, Badiei M. Periodontitis and oral cancer - current concepts of the etiopathogenesis. Oncol Rev. 2020;14(1). doi:10.4081/oncol.2020.465
27. Hibino S, Kawazoe T, Kasahara H, et al. Inflammation-induced tumorigenesis and metastasis. Int J mol Sci. 2021;22(11):5421. doi:10.3390/ijms22115421
28. Peters MDJ, Godfrey C, McInerney P, et al. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20(4):953–968. doi:10.11124/JBIES-21-00242
29. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
30. Syed F, Wani HUI, Jha SK, Banerjee K, Singh S, Kumar AP. Role of physiotherapy in HPV proven cases of oral submucous fibrosis. J Pharm Bioallied Sci. 2023;15(Suppl 2):S837–S839. doi:10.4103/jpbs.jpbs_273_23
31. Saber Amoli S, Hasanzadeh A, Sadeghi F, et al. Prevalence of co-infection by human papillomavirus, Epstein- Barr virus and Merkel cell polyomavirus in Iranian oral cavity cancer and pre-malignant lesions. Int J mol Cell Med. 2022;11(1):64–77. doi:10.22088/IJMCM.BUMS.11.1.64
32. Vijayan AK, Muthukrishnan A, Nair AM, Fathima S, Nair PV, Roshan J. PCR-based evaluation of human papillomavirus genotypes in oral lichen planus. J Pharm Bioallied Sci. 2022;14(Suppl 1):S449–S453. doi:10.4103/jpbs.jpbs_147_22
33. Sivakumar N, Narwal A, Kamboj M, Devi A, Kumar S, Bhardwaj R. Molecular and immunohistochemical cognizance of HPV16 in oral leukoplakia, oral squamous cell carcinoma and oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2021;15(3):882–892. doi:10.1007/s12105-021-01309-5
34. Kaewmaneenuan N, Lekawanvijit S, Pongsiriwet S, Chatupos V, Iamaroon A. High prevalence of human papillomavirus type 18 in oral potentially malignant disorders in Thailand. Asian Pac J Cancer Prev. 2021;22(6):1875–1881. doi:10.31557/APJCP.2021.22.6.1875
35. Della Vella F, Lauritano D, Pannone G, et al. Prevalence of HPV in patients affected by oral Lichen planus: a prospective study using two different chair‐side sampling methods. J Oral Pathol Med. 2021;50(7):716–722. doi:10.1111/jop.13164
36. Della Vella F, Pannone G, Patano A, et al. Detection of HPV in oral leukoplakia by brushing and biopsy: prospective study in an Italian cohort. Clin Oral Investig. 2020;24(5):1845–1851. doi:10.1007/s00784-019-03048-y
37. Sundberg J, Korytowska M, Burgos PM, et al. Combined testing of p16 tumour-suppressor protein and human papillomavirus in patients with oral leukoplakia and oral squamous cell carcinoma. Anticancer Res. 2019;39(3):1293–1300. doi:10.21873/anticanres.13241
38. Mosmann JP, Talavera AD, Criscuolo MI, et al. Sexually transmitted infections in oral cavity lesions: human papillomavirus, Chlamydia trachomatis, and Herpes simplex virus. J Oral Microbiol. 2019;11(1):1632129. doi:10.1080/20002297.2019.1632129
39. Panneerselvam K, Rameshkumar A, Rajkumar K, Ramadoss R. Detection of human papillomavirus 16 and 18 in patients with oral squamous cell carcinoma and potentially malignant oral disorders in South Indian population. J Cancer Res Ther. 2019;15(3):571–575. doi:10.4103/jcrt.JCRT_1012_17
40. Sameera A, Kotikalpudi R, Patel RK, Reddy KK, Prasanna M, Erugula SR. Molecular detection of human papillomavirus DNA in oral lichen planus patients. J Clin Diagn Res. 2016;10(10):ZC20–ZC23. doi:10.7860/JCDR/2019/32397.12466
41. Khajavi MA, Meshkat Z, Pasdar A, et al. Hepatitis B virus infection and oral lichen planus: a report from Northeast of Iran. Dent Mater Tech, 2018. doi:10.22038/jdmt.2018.10508
42. Agarwal SK, Bharani S, Lakshmi S, Jajodia N. Screening of human papilloma virus-16 in Oral leukoplakia patients in Davangere, Karnataka Population. JIDA. 2018;Vol 12.
43. Hallikeri K, Burde K, Anehosur V, Kulkarni BB, Hiremath SV. p53 polymorphism and association of human papillomavirus in oral submucous fibrosis and oral squamous cell carcinoma: a case-control study. J Oral Maxillofac Pathol. 2019;23(1):97–103. doi:10.4103/jomfp.JOMFP_180_18
44. Reddy SS, Sharma S, Mysorekar V. Expression of Epstein-Barr virus among oral potentially malignant disorders and oral squamous cell carcinomas in the South Indian tobacco-chewing population. J Oral Pathol Med. 2017;46(6):454–459. doi:10.1111/jop.12508
45. Ferreira LL, Biasoli ÉR, Bernabé DG, Nunes CM, Miyahara GI. Plasma HPV DNA is detectable in oral leukoplakia patients. Pathol Res Pract. 2017;213(7):759–765. doi:10.1016/j.prp.2017.04.005
46. Ramya AS, Majumdar S, Babu TM, Uppala D, Srinivas B, Rao AK. Expression of human papillomavirus DNA and p53 polymorphisms through polymerase chain reaction in normal mucosa and oral leukoplakia individuals with deleterious oral habits. Int J Appl Basic Med Res. 2017;7(2):134–138. doi:10.4103/ijabmr.IJABMR_57_16
47. Chen XJ, Sun K, Jiang WW. Absence of high-risk HPV 16 and 18 in Chinese patients with oral squamous cell carcinoma and oral potentially malignant disorders. Virol J. 2016;13(1):81. doi:10.1186/s12985-016-0526-2
48. Dalla Torre D, Burtscher D, Edlinger M, et al. Comparison of the prevalence of human papilloma virus infection in histopathologically confirmed premalignant oral lesions and healthy oral mucosa by brush smear detection. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(3):333–339. doi:10.1016/j.oooo.2014.11.013
49. Sahebjamiee M, Sand L, Karimi S, Biettolahi JM, Jabalameli F, Jalouli J. Prevalence of human papillomavirus in oral lichen planus in an Iranian cohort. J Oral Maxillofac Pathol. 2015;19(2):170–174. doi:10.4103/0973-029X.164528
50. Radochová V, Plíšková L, Slezák R. The prevalence of human papillomavirus in patients with oral lichen planus and normal oral mucosa. Acta Virol. 2015;59(4):434–436. doi:10.4149/av_2015_04_434
51. Saghravanian N, Ghazi N, Meshkat Z, Mohtasham N. Human papillomavirus in oral leukoplakia, verrucous carcinoma, squamous cell carcinoma, and normal mucous membrane. Oman Med J. 2015;30(6):455–460. doi:10.5001/omj.2015.89
52. Montebugnoli L, Gissi DB, Scapoli L, et al. p16(INK4) expression is not associated with human papillomavirus in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(6):694–702. doi:10.1016/j.oooo.2014.09.004
53. Sikka S, Sikka P. Association of human papilloma virus 16 infection and p53 polymorphism among tobacco using oral leukoplakia patients: a clinicopathologic and genotypic study. Int J Prev Med. 2014;5(4):430–438.
54. Bhosale PG, Pandey M, Desai RS, et al. Low prevalence of transcriptionally active human papilloma virus in Indian patients with HNSCC and leukoplakia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(5):609–618.e7. doi:10.1016/j.oooo.2016.06.006
55. Tomo S, Biss SP, Crivelini MM, et al. High p16INK4a immunoexpression is not HPV dependent in oral leukoplakia. Arch Oral Biol. 2020;115:104738. doi:10.1016/j.archoralbio.2020.104738
56. Ferreira DA, McMillan NAJ, Idris A. Genetic deletion of HPV E7 oncogene effectively regresses HPV driven oral squamous carcinoma tumour growth. Biomed Pharmacother. 2022;155:113782. doi:10.1016/j.biopha.2022.113782
57. Gilligan G, Panico R, Di Tada C, Lucca A, Brunotto M, Piemonte E. HPV frequency, p16 expression and risk factors for oral leukoplakia from Córdoba, Argentina. Infectio. 2023:36–43. doi:10.22354/24223794.1117
58. Raab-Traub N. EBV-induced oncogenesis. Human herpesvirus 2007.
59. Shariati M, Mokhtari M, Masoudifar A. Association between oral lichen planus and Epstein-Barr virus in Iranian patients. J Res Med Sci. 2018;23:24. doi:10.4103/jrms.JRMS_438_17
60. Canova PN, Charron AJ, Leib DA. Models of herpes simplex virus latency. Viruses. 2024;16(5):747. doi:10.3390/v16050747
61. Jalouli MM, Jalouli J, Hasséus B, Öhman J, Hirsch JM, Sand L. Nested PCR for detection of HSV-1 in oral mucosa. Med Oral Patol Oral Cir Bucal. 2015;20(6):e664–9. doi:10.4317/medoral.20630
62. Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: a review. World J Gastroenterol. 2019;25(27):3537. doi:10.3748/wjg.v25.i27.3527
63. Nosratzahi T, Raiesi M, Shahryari B. Lack of association between oral lichen planus and hepatitis B and C virus infection - a report from Southeast Iran. Asian Pac J Cancer Prev. 2018;19(6):1633–1637. doi:10.22034/APJCP.2018.19.6.1633
64. Parada LF, Tabin CJ, Shih C, Weinberg RA. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982;297(5866):474–478. doi:10.1038/297474a0
65. Budhy TI. Molecular grading of oral squamous cell carcinomas infected with EBV. Asian Pac J Cancer Prev. 2018;19(7):1793–1796. doi:10.22034/APJCP.2018.19.7.1793
66. Eloranta R, Vilén ST, Keinänen A, et al. Oral squamous cell carcinoma: effect of tobacco and alcohol on cancer location. Tob Induc Dis. 2024;22:1–9. doi:10.18332/tid/189303
67. Singh AK, Chauhan R, Anand K, Singh M, Das SR, Sinha AK. Prevalence and risk factors for oral potentially Malignant Disorders in Indian Population. J Pharm Bioallied Sci. 2021;13(Suppl 1):S398–S401. doi:10.4103/jpbs.JPBS_751_20
68. Venkat A, Sk M, A R, M KT, S A. Analysis of oral leukoplakia and tobacco-related habits in population of chengalpattu district- an institution-based retrospective study. Cureus. 2022;14(6):e25936. doi:10.7759/cureus.25936
69. Kusiak A, Maj A, Cichońska D, Kochańska B, Cydejko A, Świetlik D. The analysis of the frequency of leukoplakia in reference of Tobacco Smoking among Northern Polish Population. Int J Environ Res Public Health. 2020;17(18):6919. doi:10.3390/ijerph17186919
70. Mello FW, Melo G, Pasetto JJ, Silva CAB, Warnakulasuriya S, Rivero ERC. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: a systematic review and meta-analysis. Clin Oral Investig. 2019;23(7):2849–2859. doi:10.1007/s00784-019-02958-1
71. Worakhajit P, Fuangtharnthip P, Khovidhunkit SOP, Chiewwit P, Klongnoi B. The Relationship of Tobacco, alcohol, and betel quid with the formation of oral potentially malignant disorders: a community-based Study from Northeastern Thailand. Int J Environ Res Public Health. 2021;18(16):8738. doi:10.3390/ijerph18168738
72. Kumar GK, Abidullah M, Elbadawi L, Dakhil S, Mawardi H. Epidemiological profile and clinical characteristics of oral potentially malignant disorders and oral squamous cell carcinoma: a pilot study in Bidar and Gulbarga Districts, Karnataka, India. J Oral Maxillofac Pathol. 2019;23(1):90–96. doi:10.4103/jomfp.JOMFP_116_18
73. Benevolo M, Vocaturo A, Caraceni D, et al. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol. 2011;49(7):2643–2650. doi:10.1128/JCM.02570-10
74. Healy B, Khan A, Metezai H, Blyth I, Asad H. The impact of false positive COVID-19 results in an area of low prevalence. Clin Med. 2021;21(1):e54–e56. doi:10.7861/clinmed.2020-0839
75. Zhang G, Brown EW, González-Escalona N. Comparison of real-time PCR, reverse transcriptase real-time PCR, loop-mediated isothermal amplification, and the FDA conventional microbiological method for the detection of Salmonella spp. in produce. Appl Environ Microbiol. 2011;77(18):6495–6501. doi:10.1128/AEM.00520-11
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Anti-PD-1 Therapy is Beneficial for the Survival of Patients with Oral Squamous Cell Carcinoma
Feng L, Yin K, Zhang S, Chen Z, Bao Y, Li T
Cancer Management and Research 2022, 14:2723-2731
Published Date: 14 September 2022

