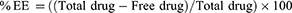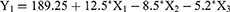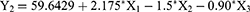Back to Journals » International Journal of Nanomedicine » Volume 19
Virgin Coconut Oil-based Nanostructured Lipid Carrier Improves the Hypolipidemic Effect of Rosuvastatin
Authors Shehata TM , Aldhubiab B , Elsewedy HS
Received 10 February 2024
Accepted for publication 25 July 2024
Published 5 August 2024 Volume 2024:19 Pages 7945—7961
DOI https://doi.org/10.2147/IJN.S463750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Tamer M Shehata,1,2 Bandar Aldhubiab,1 Heba S Elsewedy3
1Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, Alhofuf, Al-Ahsa, 36362, Saudi Arabia; 2Department of Pharmaceutics, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt; 3Department of Pharmaceutical Sciences, College of Pharmacy, AlMaarefa University, Riyadh, 11597, Saudi Arabia
Correspondence: Heba S Elsewedy; Tamer M Shehata, Email [email protected]; [email protected]
Background: Monitoring noncommunicable diseases is regarded as a critical concern that has to be managed in order to avoid a wide variety of complications such as increasing blood lipid levels known as dyslipidemia. Statin drugs, mostly, Rosuvastatin (RSV) was investigated for its effectiveness in treating dyslipidemia. However, reaching the most efficient treatment is essential and improving the effect of RSV is crucial. Therefore, a combination therapy was a good approach for achieving significant benefit. Although RSV is hydrophobic, which would affect its absorption and bioavailability following oral administration, overcoming this obstacle was important.
Purpose: To that end, the purpose of the present investigation was to incorporate RSV into certain lipid-based nanocarriers, namely, nanostructured lipid carrier (NLC) prepared with virgin coconut oil (CCO).
Methods: The optimized RSV-NLC formula was selected, characterized and examined for its in vitro, kinetic, and stability profiles. Eventually, the formula was investigated for its in vivo hypolipidemic action.
Results: The optimized NLC formulation showed a suitable particle size (279.3± 5.03 nm) with PDI 0.237 and displayed good entrapment efficiency (75.6± 1.9%). Regarding in vitro release, it was efficiently prolonged for 24 h providing 93.7± 1.47%. The optimized formula was established to be stable after 3 months storage at two different conditions; 4°C and 25°C. Importantly, including CCO in the development of RSV-NLC could impressively enhance lowering total cholesterol level in obese rat models, which endorse the potential synergistic action between RSV and CCO.
Conclusion: The study could elucidate the impact of developing NLC using CCO for improving RSV anti-hyperlipidemic activity.
Keywords: Rosuvastatin, natural compounds, coconut oil, nanostructured lipid carrier, optimization, hypolipidemic activity
Introduction
Noncommunicable diseases including; cancer, stroke, diabetes and coronary heart disease should be managed at their early stages in order to lower the chance of their risk factors.1 Dyslipidemia is a common risk factor of such diseases with overall prevalence that could reach 35%.2 Investigations revealed that dyslipidemia could be either primary or secondary, where primary dyslipidemia is frequently due to genetic causes, however, secondary dyslipidemia could be due to other consequences.3 These consequences mostly happen as a result of unhealthy lifestyle. The most common causes include excessive alcohol intake, smoking, obesity, and some underlying diseases such as uncontrolled diabetes, hypothyroidism, and kidney disorders.4,5 A variety of consequences could arise from dyslipidemia, which would greatly influence the human health issues.6 Therefore, controlling this condition and working for monitoring blood lipid levels is considered essential.
Dyslipidemia is characterized by imbalance in blood lipids, mostly higher levels of cholesterol and triglycerides, which is termed as hyperlipidemia.7 Two types of cholesterols are available, where high density lipoprotein (HDL) is good cholesterol while low density lipoprotein (LDL) is the harmful one.8 Therefore, treating dyslipidemia and lowering the harmful cholesterol levels is a critical task.
Statin drugs are first line medication for efficient lowering of cholesterol levels.9 Rosuvastatin (RSV) is the latest synthetic statin drug that reported to have a great influence in lowering blood cholesterol levels and accordingly lower the risk of noncommunicable diseases especially heart diseases.10 It is highly potent and recommended for lowering triglycerides, improving cholesterol levels, and protecting against heart diseases.11,12 It works by decreasing cholesterol production in the liver via inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase.13 Among statins, RSV exhibited poor water solubility and consequently resulted in very low oral bioavailability that could be less than 20%.14 One way of solving the problem is to find a suitable drug delivery system to incorporate the drug and improve its properties exploiting the nanotechnology approach.
Nanotechnology is a science that applies technology to produce smart matters in a nanoscale. It could be applied widely in various fields especially in the medical field to help in developing formulations that could deliver drugs efficiently with the least complications.15–17 Nanosystems and nanocarriers as drug delivery systems were used in order to circumvent the drawbacks of conventional systems.17,18 They could enhance drug solubility, bioavailability, provide controlled drug release properties and offer long-term stability for the formulation.19 Nanolipid carriers are lipid-based systems which are used greatly to include hydrophobic medications. Several lipidic systems considered being lipid-based nanocarriers such as liposomes, nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers (NLCs).20 NLC considered a remarkable type of lipid carrier since its lipid phase contains both liquid lipid, represented by oil, and solid lipid represented by fat.21 Generally, it keeps the advantages of most lipid nanocarriers and avoids their weaknesses. The most important advantage of NLCs are their ability to incorporate hydrophobic compounds, provide enhanced loading for the drug and improve the physical stability of the formulation.22 Consequently, NLCs are regarded as the nanolipid system of choice instead of other carriers. Various drugs could be extensively entrapped within NLCs such as anticancer,23 anti-inflammatory,24 antibacterial25 and anti-hyperlipidemic drugs.26
Right now, great concern has been driven toward natural compounds owing to their safety in treating different disorders with greater efficacy and least drawbacks.27 Virgin coconut oil (CCO) is natural plant oil found mainly in tropical regions and obtained from Cocos nucifera.28 It was documented that CCO exhibited various activities such as analgesic, anti-inflammatory, and antibacterial effect.29,30 In addition, it has a role in controlling blood lipid levels and subsequently, it could treat hyperlipidemia.31 CCO has been proven excellent in lowering bad cholesterol (LDL) and triglyceride levels and increasing levels of good one (HDL).32–34 It was verified via an in vivo study performed by Caesario et al, that virgin CCO was effective in lowering blood total glycerides levels same like conventional treatment with simvastatin hypolipidemic agent.35 Other studies found that daily intake of virgin CCO could raise the HDL levels in healthy individuals and another study showed that daily intake of CCO could lower the total cholesterol and triglycerides levels.36,37 The reason behind that biological influence could be attributable to the presence of great polyphenol content including ferulic acid, p-coumaric acid, vanillic acid, and caffeic acid. It has been observed that these polyphenols improve lipid profile by increasing the rate of fatty acid metabolism and decreasing lipogenesis.38
In the foregoing and in order to achieve efficient treatment and greater outcomes, natural compounds could be used in combination with pharmaceutical products for expected synergistic effect.39 Therefore, the aim of the present study was to develop NLC containing RSV for augmenting its oral bioavailability. While NLCs need two lipid phases, solid and liquid lipid, CCO would represent the liquid lipid phase in the formulation. Quality by design approach, mainly; Box–Behnken Design (BBD) was operated for selecting best optimized formula. The optimized formula was investigated for its physicochemical properties, in vitro studies and stability tests. Eventually, certain parameters including triglycerides (TG), total cholesterol (TC), LDL, and HDL levels were measured to validate the hypolipidemic effect.
Materials and Methods
Materials
Virgin CCO was purchased from NOW® Essential Oils (NOW Foods, Bloomingdale, IL, USA). RSV-Calcium and Tween 80 was bought from Sigma-Aldrich Co. (St Louis, MO, USA). Lauroyl polyoxyl-32-glycerides (Gelucire 44/14®) and MC60 glycerol monocaprylocaprate (Labrafac™) were procured from Gattefosse SAS (Saint-priest Cedex France). Total cholesterol, LDL, HDL and triglycerides kits were obtained from United Diagnostic Industry (Dammam, KSA). All other chemicals were of analytical grade.
Designing the Experiment
In order to develop and optimize NLC formulations containing RSV, BBD was operated using three independent factors that were examined at three distinct levels as shown in Table 1. Lipid phase (X1), Tween 80 surfactant (X2) and Labrafac™ cosurfactant concentration (X3) were considered the selected independent factors that were studied against two responses: particle size and entrapment efficiency (EE) referred as Y1 and Y2, respectively. This examination was assembled using Design-Expert version 12.0 software (Stat-Ease, Minneapolis, MN, USA). The constructed design was validated using analysis of variance (ANOVA) test in addition to other statistical tools that were functioned to select the best fit model. Besides, the outcomes were emphasized by some modeling graphs created by the design along with mathematical equations.17
 |
Table 1 Box–Behnken Design Plan Showing Three Independent Factors Studied on Three Levels for Two Responses |
Manufacturing Process of RSV-NLC
Numbers of RSV-NLC formulations were developed using previously reported melt emulsification-ultrasonication method.40 According to the performed preliminary study, the ratio between solid lipid and liquid lipid phase that being most suitable and applicable was 2:8. Certain amount of solid lipid (Gelucire 44/14®), according to that proposed by BBD, was melted at temperature higher than its melting point. CCO as a liquid lipid phase was added to the melted solid phase, and then approximately 20 mg of RSV and specific amount of (Labrafac™) as cosurfactant were added to the lipid mixture with insistent mixing until homogenous mix was obtained. Up to 10 mL aqueous phase was emulsified with identified amount of surfactant (Tween 80), heated to the same temperature and then gradually added to the lipid phase while stirring till the pre-emulsion formed. The obtained pre-emulsion was homogenized for 5 min at 10,000 rpm using Ultra-Turrax homogenizer (IKA-T25; Germany). Afterward, the formed mixture was exposed for 30 s to sonication using probe sonicator XL-200, Qsnonica (New town, CT; USA). The developed dispersion was set aside at room temperature to cool, which facilitates the formation of NLC.
X1: Lipid phase concentration; X2: Tween 80 concentration; X3: Labrafac™ concentration Y1: particle size (nm) and Y2: EE (%)
Particle Size Analysis
In a disposable cuvette, small aliquot of the prepared RSV-NLCs diluted to 3 mL of distilled water in order to measure the particle sizes and the corresponding polydispersity index (PDI). Using a Malvern zetasizer (Nanoseries, zs; Malvern Instruments, Malvern, UK), such measurement was performed depending on dynamic light scattering technique at 25°C.41
Measuring EE%
The percentage of RSV entrapped into the fabricated NLC formulations determined via centrifugation method utilizing centrifuge (Andreas Hettich GmbH, Co. KG, Germany).42 Concisely, Amicon® ultra- 4 (Ultracel 10K) was used, where sample of the NLC formulation was added in such tube followed by centrifugation for 1 h at 4°C and 6000 rpm. The free drug in the obtained filtrate was diluted and examined spectrophotometrically using spectrophotometer (U.V. Spectrophotometer, JENWAY 6305) at λmax 241 nm. The following equation was used for calculating the percentage of entrapment:
Fourier Transform Infrared Spectroscopy (FTIR)
Compatibility that might be identified between drug and excipient were investigated utilizing FTIR spectrophotometer (FTIR spectrophotometer, SHIMADZU, IRAFFINITY-1S, Japan). The study was performed using KBr pellet method, where KBr disc was prepared by compressing a thin film of the studied sample over KBr layer and allow drying in vacuum. The spectra of pure RSV, placebo NLC and optimized RSV-NLC were recorded at a scale between 4000 and 400 cm−1.43
Morphological Evaluation
Scanning electron microscopy (SEM) (JSM-6390LA, JEOL, Tokyo, Japan) is a microscopic technique that can be used to assess the morphology of the optimized RSV-NLC formulation. Usually, a sample of the formulation was applied to slabs that shield them gold, and then scanned. Next, using various magnifications, the morphology was detected at a 15 kV acceleration voltage under reduced vacuum.44
In vitro Drug Release Study
The in vitro release of RSV from optimized RSV-NLC was evaluated and compared with that released from RSV suspension using dialysis bag method as previously stated.45 Sample of the optimized RSV-NLC was placed into a dialysis bag with a cutoff 10,000 Da. The bag was immersed into a dissolution media of 250 mL PBS pH 6.8 at 37°C and adjusted to rotate at 50 rpm using ERWEKA dissolution system (ERWEKA, GmbH, Heusenstamm, Germany). Samples were withdrawn from media at various time intervals up to 24 h and replaced with equivalent volume of fresh vehicle to insure keeping sink condition. The withdrawn sample were analyzed spectrphotometrically at λmax 241 nm via spectrophotometer (U.V. Spectrophotometer, JENWAY 6305). The study was performed in triplicate.
Kinetic Study
Release of RSV from optimized NLC formula was studied against different kinetic models. Zero order, first order, Higuchi and Korsmeyer–Peppas were the different models to which the release profile was fitted. The drug concentration versus time was plotted to check the most fitted model.46
Stability Testing
It is very essential to determine the stability of the formula when kept at different conditions. Accordingly, the optimized RSV-NLC was kept at closed containers for periods of 1 and 3 months at room temperature (25±2°C/60±5% RH) and at refrigerator (5±2°C). The study was performed according to the International Conference on Harmonization (ICH) guidelines. Certain evaluations were carried out upon storage including particle size, EE and in vitro release.47
Animals
Animal Handling
Male Wistar rats of average weight (170±25 g) were acquired from the Experimental Animal Research Centre at King Saud University, Riyadh, KSA. Rats were kept in ventilated cages under a standard laboratory conditions applying 12 h light/dark cycle at ambient temperature and with free access to standard rodent diet and tap water.
Declaration of Ethical Approval
The protocol of the study was conducted following the approval (IRB23-124) that issued by Institutional Review Board (IRB) of AlMaarefa University. All animal experiments were performed in accordance with the rules and regulations of the Kingdom of Saudi Arabia and the research policies and procedures of IRB of AlMaarefa University, Riyadh.
In vivo Study
Protocol of in vivo Animal Study
Twenty-five rats, divided into five groups were used in the current study. The first group was the control group that was fed a standard commercial rodent diet. The other four groups (20 rats) were fed a high-fat rodent diet (HFD) for 4 weeks that renders the animals obese. HFD would provide the rats with high calories since it contains high concentrations of protein, fat, and carbohydrates. All obese rats were kept on their feeding routine throughout the whole study.48,49 The treated groups were administered the examined formulations orally via oral gavage for two weeks. Accordingly, the animal groups were distributed as follows:
Group I: Control nonobese rats.
Group II: Untreated obese rats.
Group III: Obese rats treated orally with RSV suspension (20 mg/kg).50
Group IV: Obese rats treated orally with placebo NLC.
Group V: Obese rats treated orally with RSV-NLC (20 mg/kg) daily.
Following the specified time of treatment, animals were sacrificed and blood samples were taken, centrifuged at 3.000 rpm for 10 min for serum separation. Serum samples were kept at temperatures of −20°C for further analysis.
Lipid Profile Measurement
Certain blood serum parameters were assessed using protocol of specific kits to measure the level of total cholesterol (T. Chol), triglycerides (TG), low density lipoprotein (LDL), and high density lipoprotein (HDL).
Statistical Analysis
Data were displayed as the mean ± standard deviation (SD) for at least three experiments. Groups considered significantly different from each other when P<0.05. All statistical analysis was confirmed using SPSS statistics software, v. 9 (IBM Corporation, Armonk, NY).
Result and Discussion
Manufacturing Process of RSV-NLC
RSV, as a poorly water-soluble hypolipidemic drug can be administered orally via its incorporation into nanolipid formulation. Consequently, all ingredients that have been chosen for the NLC formulation must fall into the GRAS (generally recognized as safe) category.51 This is to guarantee the biocompatibility and safety of the formulations to be administered orally. Moreover, the broad application of nanolipid formulation is attributed to their composition of generally regarded as safe (GRAS) components, which minimize cytotoxicity.52 For that reason, coconut oil that has been documented to have hypolipidemic activity was utilized since the US Food and Drug Administration (FDA) has classified it as generally recognized as safe (GRAS), which means it is harmless. Furthermore, the RSV-NLC was formulated using Tween 80, which is approved as a GRAS since it is a nonionic, nontoxic, and biocompatible surfactant.53 In addition, a wide variety of literature proved Gelucire 44/14® and Labrafac™ safe to use for oral NLC formulations owing to their ability to improve solubility and oral bioavailability of the medications.54,55
Analyzing the Experimental Design
BBD was used and overall 14 experimental formulations were designed using the assigned independent factors as shown in Table 2 along with their obtained responses. Statistical analysis for the obtained data was performed as listed in Table 3, and it was distinguished that the model F-value was significant for both responses. In addition, P-values for most of the model terms (X1, X2, and X3) were significant as long as their values stayed less than 0.0001. Furthermore, the lack of fit is very important parameter for interpreting the model and predicting the responses. In the current design, lack of fit F-value for both responses was not significant, which is recommended, since it was 5.04 and 1.33 for Y1 and Y2, respectively, therefore, the selected model was appropriate for the study.56
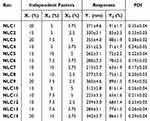 |
Table 2 Experimental Runs and Their Observed Responses as per BBD |
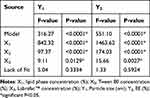 |
Table 3 Statistical Analysis for Both Responses |
X1, lipid phase concentration (%); X2, Tween 80 concentration (%); X3, Labrafac™ concentration (%); Y1, Particle size (nm); Y2, EE (%);*significant P<0.05
It was observed that particle size of all fabricated RSV-NLC was in range of 213±3.7 to 371±4.6 nm while the EE was between 63±1.9 and 91±1.7% as displayed in Table 2. For both responses, it was noteworthy that particle size (Y1) and EE (Y2) increased upon increasing lipid phase concentration (X1). The rationale behind that could be due to aggregation of particles that might happen and resulted in larger particle size upon using higher lipid concentration.57 Consequently, larger particle size could definitely accommodate for larger amount of drug due to space availability, therefore resulted in an increment in the EE of the drug.13 In contrast, while using same lipid concentration, the increment of surfactant (X2) and cosurfactant concentration (X3) would result in decreasing Y1 and Y2. This could be attributed to the role of surfactant and cosurfactant in lowering the interfacial tension that would form particles with smaller size. Moreover, these surfactants would form barriers around the particles, preventing their aggregation, keeping them small.58 Subsequently, since surfactant and cosurfactant keep the particles small, there would be no available space for more drugs to be entrapped to maintain the EE small. The obtained outcomes could be emphasized by polynomial equations generated from the design as follow:
It was obvious that the significant influence of factor X1 on particle size (Y1) and EE (Y2) response was confirmed by the positive sign available in front of X1; however, the negative sign in front of both X2 and X3 denoted the indirect influence of these independent factors on the response of both Y1 and Y2.
Further, a linear correlation was distinguished between the predicted and the actual responses as shown in Table 4 and Figure 1. This confirmed by the values of predicted and adjusted R2 for both responses Y1 and Y2. Whereas, predicted and adjusted R2 for Y1 response was 0.9808 and 0.9864, respectively, however, it was 0.9875 and 0.9940, respectively for Y2 response. The difference between predicted and adjusted R2 in case of all responses was less than 0.2 indicating that there was a reasonable agreement between both values. Likewise, adequate precision is another parameter that could measures the ratio of signal to noise. Generally, ratio greater than 4 is considerable. Y1 and Y2 responses showed adequate precision of 51.4472 and 68.0612, indicating appropriate signal that could certify the model efficiency in operating the design space.
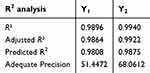 |
Table 4 Summary for Fit Statistics of Observed Responses |
 |
Figure 1 Linear correlation plots for actual versus predicted values for (A) Y1 response and (B) Y2 response. |
For endorsement of the selected factors influence on the obtained responses, more graphs were generated by BBD. Moreover, 3D response surface plot could illustrate the interaction and effects between independent factors and responses as exhibited in Figure 2. In addition, Perturbation plots presented in Figure 3 were created for illustrating the influence of each factor on the investigated response while keeping all other factors constant.59 It seems from both Figure 3A and B that factor X1 possessed the most prominent effect upon response Y1 and Y2, respectively if compared to other factors X2 and X3. This outcome was observed because the curvature of X1 in both responses pointed toward its sensitiveness. As well, the direction of the perturbation plot proved the positive synergistic influence of factor X1 on both; Y1 and Y2 response; however, it exposed an antagonistic effect toward them.
 |
Figure 3 Perturbation plots demonstrating the influence of each independent factor alone on (A) particle size Y1, and (B) EE Y2 response. |
Optimization and Point Prediction
Quality by design approach via BBD was operated to interpret the influence of the selected factors over the obtained responses. The created response surface graphs, numerical optimization in addition to the resulted desirability function were supportive in selecting the optimized formula depending on certain proper constraints. To achieve such appropriate selection, the independent factors were adjusted to be in range, while the responses were directed toward certain desirable properties. In the present study the particle size was adjusted toward minimum value; whereas, the EE oriented to the maximum value. As a result of such orientation, the design suggested certain data that is supposed to be the values of the optimized formulation, which would be selected based on the highest value of desirability function. The higher desirability was 0.508 that directed the concentration to be 13.5% lipid phase, 5% Tween 80 and 5% Labrafac™. The design suggested certain data that is supposed to be the values of the optimized formulation since it showed desirability of 0.508 as appeared in Table 5 and Figure 4. Accordingly, the optimized RSV-NLC was formulated and evaluated for its responses that found to be very close to the predicted values. As per Figure 5, the particle size of the optimized RSV-NLC was assessed and found to be 279.3±5.03 nm with a relative PDI value 0.237±0.22.
 |
Table 5 Predicted and Observed Results of the Optimized RSV-NLC Formulation |
 |
Figure 4 (A) 2D contour desirability plot showing the proposed values of selected factors (A) X1 and X2, (B) X1 and X3, and (C) X2 and X3 that provides the highest desirability value. |
 |
Figure 5 Particle size of the optimized RSV-NLC formulation. |
Fourier Transform Infrared Spectroscopy (FTIR)
In order to determine the prospective drug-excipient interactions, the functional groups of pure RSV, blank NLC and developed RSV-NLC were identified by comparing their peaks using FTIR spectroscopy. As exhibited in Figure 6, the spectrum of the drug displays all the distinctive peaks of RSV, including a broad band stretching at 3380 cm−1 corresponding to O-H and another band stretching at 2900 cm− 1 attributed to C-H group. Other peaks were found at 1550 cm−1 for C=C and at 1515 cm−1 corresponding to N-H bending. These observed peaks were in a great similarity to that of Gholamhosseinzadeh et al.60 Other peaks appeared at 1390 cm−1 and 1300 cm−1 corresponding to C-N stretching and asymmetric vibration of S=O, respectively, which is closely correlated to same finding of previous studies.61,62 RSV spectral bands were visible in the optimized NLCs with a little drop in intensity. This is most likely ascribed to the successful encapsulation of the medication inside the NLC formulation.63 In addition, the lack of any extra peaks or significant shifts in the drug peak wavelength indicates that RSV does not interact with any of the excipients utilized to formulate the NLC.
 |
Figure 6 FTIR spectra of RSV-NLC, pure RSV, and blank NLC. |
Morphological Evaluation
Figure 7 demonstrates the surface morphology and shape of the developed RSV-NLC formulation using a SEM instrument. Generally, NLCs was seen to have distinct, spherical forms and particle sizes that matched the results of dynamic light scattering tests.
 |
Figure 7 Scanning electron microscopy showing the morphology of the optimized RSV-NLC. |
In vitro Drug Release Study
In vitro study was carried out to determine the pattern by which RSV was released from the developed optimized NLC formulation compared to that released from RSV suspension. As depicted in Figure 8, the study was extended for 24 h at 37°C in PBS pH 6.8 indicating a sustained release mechanism. It was obvious that the outline of RSV release from NLC followed a biphasic pattern since it was rapid initially followed by a slow drug release rate. The rationale behind that could be due to some nonentrapped drug present free in the outer shell of the formula thus it would be released faster.64 However, the second phase of the release was slower because the drug was released from the lipid core of the formulation thus it would be released slowly.65 The overall RSV release from NLC reached 93.7±1.47%, while it reached only 34.0± 4.15% when released from suspension after 24 h. The previous smaller release of RSV from suspension could be attributed to the presence of the drug particles in a dispersion state.66 The current investigation was in agreement with Ahmed, who found that the release of RSV from the developed liposome and chitosomes was more than that from drug suspension.66
 |
Figure 8 In vitro release study of RSV from optimized NLC formulations compared with RSV suspension. |
Kinetic Study
The in vitro release of RSV from studied formulations was analyzed for its drug release kinetics using different models. It was apparent that the best fitted kinetic model for RSV release from suspension and NLC was Korsmeyer–Peppas kinetic model when compared to other kinetic models. This is ascribed to the most linear model and the greatest correlation coefficient (R2) obtained by plotting the log of drug release against log time that was 0.9631 and 0.9962 for optimized RSV-NLC and RSV suspension, respectively as shown in Figure 9. Moreover, the “n” value of Korsmeyer–Peppas equation was 0.345 and 0.402 for optimized RSV-NLC and RSV suspension, respectively, which stated that the release pattern from both formulations governed by Fickian diffusion mechanism as long as their n-values (<0.5).67 It is remarkable that Korsmeyer–Peppas kinetic modeling generally refers to a distinctive release from various lipid-based colloidal delivery systems such as liposomes and NLCs.68 Our results were in accordance with previous studies that confirmed the Fickian Korsmeyer–Peppas kinetic release from Indomethacin NLC.69 Likewise, Marathe et al verified that the kinetic release from α-Tocopherol Succinate-Based NLC followed Korsmeyer–Peppas as well.70
 |
Figure 9 Percentage of RSV released from developed suspension and NLC formulation and their kinetics according to (A) zero order (B) first order (C) Higuchi and (D) Korsmeyer–Peppas model. |
Stability Testing
Studying stability of the developed RSV-NLC was performed in terms of the evaluated responses; particle size and EE. The study was conducted at two conditions; room temperature at 25±2°C/60±5% RH and refrigerator at 5±2°C for periods of 1 and 3 months. As per data in Table 6, slight variation was detected in RSV-NLC in relative to the evaluated parameters upon storage at both conditions up to 3 months which is not significant (P<0.05) in terms of particle size and EE. The attained result is a good sign for excellent stability of the formulation and the suitability of the storage conditions.
 |
Table 6 Stability Study of RSV-NLC Formulation Upon Storage for 1 and 3 Months at 25±2°C/60±5% RH and 5±2°C in Relative to Particle Size and EE |
In vivo Animal Study: Lipid Profile Measurement
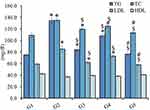
The study was conducted utilizing rats that were fed with a HFD for 4 weeks and treated with different formulations compared with nonobese rats. Figure 10 showed that all rats in all groups fed on HFD exhibited a significant elevation in their evaluated biochemical parameters; TG, TC, and LDL levels (P<0.05) when compared to the nonobese group (G1). However, a nonsignificant decline in HDL levels of these groups was detected when compared to same group (G1) (P<0.05). For obese rats in G3, G4, and G5 treated with RSV suspension, placebo NLC, and RSV-NLC, respectively, a significant reduction in levels of TG, TC, and LDL was distinguished if compared to obese rats in G2 (P<0.05). The reason behind the obtained results could be ascribed to the role of RSV in lowering levels of blood lipid parameters in case of G3.12 Similar results were obtained upon using Rosuvastatin for treating patients with hypertriglyceridemia. It was observed that Rosuvastatin could reduce triglycerides and other lipid parameters such as LDL and TC.71 Moreover, the documented role of CCO incorporated into placebo NLC formulation played a vital role in lowering blood lipid levels in G4.32 In addition, a significant decrease in TG, TC, and LDL levels was prominent in G5 treated with RSV-NLC comparable with G3 and G4 (P<0.05). This could be owed to the dual hypolipidemic effect of both virgin CCO and RSV incorporated into RSV-NLC formulation that provide synergistic action in lowering blood lipid levels. It was evidenced earlier that the lipid lowering effect of CCO was enhanced upon combining with licorice extract.72 Furthermore, it seemed that the pharmacological influence of RSV as a hypolipidemic agent was enhanced following its integration into NLC formulation prepared with CCO. These results came in agreement with Salil and Rajamohan's, investigation who demonstrated the hyperlipidemic and peroxidative effect of coconut in animals fed on high fat cholesterol containing diet.73 Further, another study exhibited the influence of virgin coconut oil in restoring the normal serum lipid profile in diabetic rats.74 It was revealed that the physiologically active polyphenol components found in CCO may be responsible for this characteristic.32 Conversely, it was remarkable that all groups under investigation did not reveal a significant change in their HDL levels compared to the nontreated group (G1) (P<0.5), which could be due to very short period of treatment (14 days).75
Conclusion
The existing study discussed effective manufacturing of nanostructured lipid carrier formulations using coconut oil and loaded with Rosuvastatin. Box–Behnken experimental design was employed to help in selecting the optimized formulation based on selected factors and their observed response such as particle size and entrapment efficiency. The optimized RSV-NLC was evaluated for several studies including compatibility study using FTIR analysis and examining the morphology. In vitro release and kinetic study was performed providing successful release over 24 h following Korsmeyer–Peppas kinetics. Checking the stability of the optimized formulation revealed its suitability to be stored at various conditions. Eventually, treatment with RSV-NLC formulation displayed a significant reduction in biochemical parameters and provided a potential hypolipidemic influence of coconut oil when integrated with Rosuvastatin.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) of AlMaarefa University approval number (IRB23-124).
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this Research work through the project number INST005.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia through the project number INST005. The APC was funded by the same “Grant No, INST005”.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Budreviciute A, Damiati S, Sabir DK, et al. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. 2020;8:574111.
2. Lin H, Li Q, Hu Y, et al. The prevalence of multiple non-communicable diseases among middle-aged and elderly people: the Shanghai Changfeng Study. Eur j epidemiol. 2017;32(2):159–163. doi:10.1007/s10654-016-0219-6
3. Mosca S, Araújo G, Costa V, et al. Dyslipidemia diagnosis and treatment: risk stratification in children and adolescents. J Nutr Metab. 2022;2022:4782344. doi:10.1155/2022/4782344
4. Vodnala D, Rubenfire M, Brook RD. Secondary causes of dyslipidemia. Am j Cardiol. 2012;110(6):823–825. doi:10.1016/j.amjcard.2012.04.062
5. Yanai H, Yoshida H. Secondary dyslipidemia: its treatments and association with atherosclerosis. Glob Health Med. 2021;3(1):15–23. doi:10.35772/ghm.2020.01078
6. Kayani T, Ahmad B, Chang RS, et al. Beyond statins: novel lipid-lowering agents for reducing risk of atherosclerotic cardiovascular disease. Pharmacoepidemiology. 2024;3(1):117–168. doi:10.3390/pharma3010009
7. Hedayatnia M, Asadi Z, Zare-Feyzabadi R, et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020;19(1):42. doi:10.1186/s12944-020-01204-y
8. Rajagopal G, Suresh V, Sachan A. High-density lipoprotein cholesterol: how High. Indian J Endocrinol Metab. 2012;16(8):S236–238. doi:10.4103/2230-8210.104048
9. Dayar E, Pechanova O. Targeted strategy in lipid-lowering therapy. Biomedicines. 2022;10(5):1090. doi:10.3390/biomedicines10051090
10. Rosenson RS. Rosuvastatin: a new inhibitor of HMG-coA reductase for the treatment of dyslipidemia. Expert Rev Cardiovasc Ther. 2003;1(4):495–505. doi:10.1586/14779072.1.4.495
11. Hirpara MR, Manikkath J, Sivakumar K, et al. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: development and in vitro and in vivo evaluations. Int J Biol Macromol. 2018;107:2190–2200. doi:10.1016/j.ijbiomac.2017.10.086
12. Rizwanullah M, Amin S, Ahmad J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J Drug Target. 2017;25(1):58–74. doi:10.1080/1061186x.2016.1191080
13. Elsewedy HS, Shehata TM, Almostafa MM, Soliman WE. Hypolipidemic activity of olive oil-based nanostructured lipid carrier containing atorvastatin. Nanomaterials. 2022;12(13):2160. doi:10.3390/nano12132160
14. Elsayed I, El-Dahmy RM, Elshafeey AH, Abd El Gawad NA, El Gazayerly ON. Tripling the bioavailability of rosuvastatin calcium through development and optimization of an in-situ forming nanovesicular system. Pharmaceutics. 2019;11(6):275. doi:10.3390/pharmaceutics11060275
15. Zdrojewicz Z, Waracki M, Bugaj B, Pypno D, Cabała K. Medical applications of nanotechnology. Postepy Hig Med Dosw. 2015;69:1196–1204. doi:10.5604/17322693.1177169
16. Xie F, Li R, Shu W, Zhao L, Wan J. Self-assembly of Peptide dendrimers and their bio-applications in theranostics. Mater Today Bio. 2022;14:100239. doi:10.1016/j.mtbio.2022.100239
17. Su H, Li S, Yang GZ, Qian K. Janus micro/nanorobots in biomedical applications. Adv Healthcare Mater. 2023;12(16):2202391. doi:10.1002/adhm.202202391
18. Elumalai K, Srinivasan S, Shanmugam A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed Technol. 2024;5:109–122. doi:10.1016/j.bmt.2023.09.001
19. Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innovative Food Sci Emerg Technol. 2013;19:29–43. doi:10.1016/j.ifset.2013.03.002
20. Noore S, Pathania S, Fuciños P, O’Donnell CP, Tiwari BK. Lipid-Based Nanocarriers. In: Noore S, Pathania S, Fuciños P, O’Donnell CP, Tiwari BK, editors. Nanocarriers for Controlled Release and Target Delivery of Bioactive Compounds. Springer Nature Switzerland: Cham; 2024:7–20.
21. Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–313. doi:10.15171/apb.2015.043
22. Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Targeting. 2012;20(10):813–830. doi:10.3109/1061186X.2012.716845
23. Rodrigues da Silva GH, Moura LDD, Carvalho FVD, et al. Antineoplastics Encapsulated in Nanostructured Lipid Carriers. Molecules. 2021;26(22):6929. doi:10.3390/molecules26226929
24. Guilherme VA, Ribeiro LNM, Alcântara ACS, et al. Improved efficacy of naproxen-loaded NLC for temporomandibular joint administration. Sci Rep. 2019;9(1):11160. doi:10.1038/s41598-019-47486-w
25. Cortesi R, Valacchi G, Muresan XM, et al. Nanostructured lipid carriers (NLC) for the delivery of natural molecules with antimicrobial activity: production, characterisation and in vitro studies. J Microencapsul. 2017;34(1):63–72. doi:10.1080/02652048.2017.1284276
26. Fathi HA, Allam A, Elsabahy M, Fetih G, El-Badry M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf B. 2018;162:236–245. doi:10.1016/j.colsurfb.2017.11.064
27. Abdel-Rahman A, Anyangwe N, Carlacci L, et al. The safety and regulation of natural products used as foods and food ingredients. Toxicol Sci. 2011;123(2):333–348. doi:10.1093/toxsci/kfr198
28. DebMandal M, Mandal S. Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pac J Trop Med. 2011;4(3):241–247. doi:10.1016/s1995-7645(11)60078-3
29. Widianingrum DC, Noviandi CT, Salasia SIO. Antibacterial and immunomodulator activities of virgin coconut oil (VCO) against Staphylococcus aureus. Heliyon. 2019;5(10):e02612. doi:10.1016/j.heliyon.2019.e02612
30. Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48(2):151–157. doi:10.3109/13880200903062614
31. Santana LF, Cordeiro KW, Soares FLP, de Cássia Freitas K. < b> Coconut oil increases HDL-c and decreases triglycerides in Wistar rats. Acta Sci Health Sci. 2016;38:185–190.
32. Nevin KG, Rajamohan T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin Biochem. 2004;37(9):830–835. doi:10.1016/j.clinbiochem.2004.04.010
33. Margata L, Silalahi J, Harahap U, Satria D. The effect of hydrolyzed virgin coconut oil on lipid profile and liver enzymes in dyslipidemic rats. Asian J Pharm Clin Res. 2018;11(10):406–409. doi:10.22159/ajpcr.2018.v11i10.27476
34. Setyawati A, Sangkala MS, Malasari S, et al. Virgin Coconut Oil: a Dietary Intervention for Dyslipidaemia in Patients with Diabetes Mellitus. Nutrients. 2023;15(3):564. doi:10.3390/nu15030564
35. Caesario J, Adi S, Qurnianingsih E. The Effect of simvastatin and virgin coconut oil (VCO) combination therapy as triglyceride plasma lowering agent in white dyslipidemic male rat (Rattus norvegicus). metabolism. 2021;4:02.
36. Chinwong S, Chinwong D, Mangklabruks A. Daily consumption of virgin coconut oil increases high-density lipoprotein cholesterol levels in healthy volunteers: a randomized crossover trial. Evid Based Complement Alternat Med. 2017;2017:1–8. doi:10.1155/2017/7251562
37. Sinaga FA, Samosir A, Sinaga R, Ayu E, Manalu N, Ginting A Effect of aerobic exercise and supplementation virgin coconut oil on lipid profile. In
38. Illam SP, Narayanankutty A, Raghavamenon AC. Polyphenols of virgin coconut oil prevent pro-oxidant mediated cell death. Toxicol Mech Methods. 2017;27(6):442–450. doi:10.1080/15376516.2017.1320458
39. Shehata TM, Elnahas HM, Elsewedy HS. Development, Characterization and Optimization of the Anti-Inflammatory Influence of Meloxicam Loaded into a Eucalyptus Oil-Based Nanoemulgel. Gels. 2022;8(5):262. doi:10.3390/gels8050262
40. Agrawal M, Saraf S, Pradhan M, et al. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed Pharmacothe. 2021;141:111919. doi:10.1016/j.biopha.2021.111919
41. Elsewedy HS, Shehata TM, Soliman WE. Tea tree oil nanoemulsion-based hydrogel vehicle for enhancing topical delivery of neomycin. Life. 2022;12(7):1011. doi:10.3390/life12071011
42. Elsewedy HS, Shehata TM, Soliman WE. Shea Butter Potentiates the Anti-Bacterial Activity of Fusidic Acid Incorporated into Solid Lipid Nanoparticle. Polymers. 2022;14(12):2436. doi:10.3390/polym14122436
43. Haroun M, Elsewedy HS, Shehata TM, et al. Significant of injectable brucine PEGylated niosomes in treatment of MDA cancer cells. J Drug Delivery Sci Technol. 2022;71:103322. doi:10.1016/j.jddst.2022.103322
44. Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2–3):85–103. doi:10.1016/j.micron.2011.07.014
45. Li J, Yang M, Xu W. Development of novel rosuvastatin nanostructured lipid carriers for oral delivery in an animal model. Drug Des Devel Ther. 2018;12:2241–2248. doi:10.2147/dddt.s169522
46. Shehata TM, Elsewedy HS. Paclitaxel and Myrrh oil combination therapy for enhancement of cytotoxicity against breast cancer; QbD approach. Processes. 2022;10(5):907. doi:10.3390/pr10050907
47. Elsewedy HS, Shehata TM, Alqahtani NK, Khalil HE, Soliman WE. Date palm extract (phoenix dactylifera) encapsulated into palm oil nanolipid carrier for prospective antibacterial influence. Plants. 2023;12(21):3670. doi:10.3390/plants12213670
48. Shehata TM, Khalil HE, Elsewedy HS, Soliman WE. Myrrh essential oil-based nanolipid formulation for enhancement of the antihyperlipidemic effect of atorvastatin. J Drug Delivery Sci Technol. 2021;61:102277. doi:10.1016/j.jddst.2020.102277
49. Bhatt BA, Dube JJ, Dedousis N, Reider JA, O’Doherty RM. Diet-induced obesity and acute hyperlipidemia reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regulatory Integr Comp Physiol. 2006;290(1):R233–240. doi:10.1152/ajpregu.00097.2005
50. Inam S, Irfan M, Lali NUA, et al. Development and characterization of eudragit(®) EPO-based solid dispersion of rosuvastatin calcium to foresee the impact on solubility, dissolution and antihyperlipidemic activity. Pharmaceuticals. 2022;15(4):492. doi:10.3390/ph15040492
51. Burdock GA, Carabin IG. Generally recognized as safe (GRAS): history and description. Toxicol Lett. 2004;150(1):3–18. doi:10.1016/j.toxlet.2003.07.004
52. Khan S, Sharma A, Jain V. An overview of nanostructured lipid carriers and its application in drug delivery through different routes. Adv Pharm Bull. 2023;13(3):446–460. doi:10.34172/apb.2023.056
53. Nobari Azar FA, Pezeshki A, Ghanbarzadeh B, Hamishehkar H, Mohammadi M. Nanostructured lipid carriers: promising delivery systems for encapsulation of food ingredients. J Agric Food Res. 2020;2:100084. doi:10.1016/j.jafr.2020.100084
54. Ortiz AC, Yañez O, Salas-Huenuleo E, Morales JO. Development of a nanostructured lipid carrier (NLC) by a low-energy method, comparison of release kinetics and molecular dynamics simulation. Pharmaceutics. 2021;13(4):531. doi:10.3390/pharmaceutics13040531
55. Franceschinis E, Roverso M, Gabbia D, et al. Self-Emulsifying Formulations to Increase the Oral Bioavailability of 4,6,4′-Trimethylangelicin as a Possible Treatment for Cystic Fibrosis. Pharmaceutics. 2022;14(9):1806. doi:10.3390/pharmaceutics14091806
56. Zhang H, Choi JP, Moon SK, Ngo TH. A hybrid multi-objective optimization of aerosol jet printing process via response surface methodology. Addit Manuf. 2020;33:101096. doi:10.1016/j.addma.2020.101096
57. Sarheed O, Dibi M, Ramesh KVRNS. Studies on the effect of oil and surfactant on the formation of alginate-based O/W lidocaine nanocarriers using nanoemulsion template. Pharmaceutics. 2020;12(12):1223. doi:10.3390/pharmaceutics12121223
58. Rahman Z, Zidan AS, Khan MA. Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur J Pharm Biopharm. 2010;76(1):127–137. doi:10.1016/j.ejpb.2010.05.003
59. Ghanem HA, Nasr AM, Hassan TH, et al. Comprehensive study of atorvastatin nanostructured lipid carriers through multivariate conceptualization and optimization. Pharmaceutics. 2021;13(2):178. doi:10.3390/pharmaceutics13020178
60. Gholamhosseinzadeh MR, Aghaie H, Zandi MS, Giahi M. Rosuvastatin drug as a green and effective inhibitor for corrosion of mild steel in HCl and H2SO4 solutions. J Mater Res Technol. 2019;8(6):5314–5324. doi:10.1016/j.jmrt.2019.08.052
61. Butt S, Hasan SMF, Hassan MM, Alkharfy KM, Neau SH. Directly compressed rosuvastatin calcium tablets that offer hydrotropic and micellar solubilization for improved dissolution rate and extent of drug release. Saudi Pharm J. 2019;27(5):619–628. doi:10.1016/j.jsps.2019.03.002
62. Mostafa NM, Badawey AM, Lamie NT, Abd El-Aleem -AE-AB. Selective chromatographic methods for the determination of rosuvastatin calcium in the presence of its acid degradation products. J Liquid Chromatogr Related Technol. 2014;37(15):2182–2196. doi:10.1080/10826076.2013.828305
63. Zewail MB, Asaad GF, Swellam SM, et al. Design, characterization and in vivo performance of solid lipid nanoparticles (SLNs)-loaded mucoadhesive buccal tablets for efficient delivery of Lornoxicam in experimental inflammation. Int J Pharm. 2022;624:122006. doi:10.1016/j.ijpharm.2022.122006
64. Elmowafy M, Ibrahim HM, Ahmed MA, Shalaby K, Salama A, Hefesha H. Atorvastatin-loaded nanostructured lipid carriers (NLCs): strategy to overcome oral delivery drawbacks. Drug Delivery. 2017;24(1):932–941. doi:10.1080/10717544.2017.1337823
65. Ravi PR, Aditya N, Kathuria H, Malekar S, Vats R. Lipid nanoparticles for oral delivery of raloxifene: optimization, stability, in vivo evaluation and uptake mechanism. Eur J Pharm Biopharm. 2014;87(1):114–124. doi:10.1016/j.ejpb.2013.12.015
66. Ahmed TA. Development of rosuvastatin flexible lipid-based nanoparticles: promising nanocarriers for improving intestinal cells cytotoxicity. BMC Pharmacol Toxicol. 2020;21(1):14. doi:10.1186/s40360-020-0393-8
67. Chudoba D, Jażdżewska M, Łudzik K, Wołoszczuk S, Juszyńska-Gałązka E, Kościński M. Description of release process of doxorubicin from modified carbon nanotubes. Int J Mol Sci. 2021;22(21):12003. doi:10.3390/ijms222112003
68. Baskararaj S, Panneerselvam T, Govindaraj S, et al. Formulation and characterization of folate receptor-targeted PEGylated liposome encapsulating bioactive compounds from Kappaphycus alvarezii for cancer therapy. 3 Biotech. 2020;10(3):1–18. doi:10.1007/s13205-020-2132-7
69. Choi K-O, Choe J, Suh S, Ko S. Positively charged nanostructured lipid carriers and their effect on the dissolution of poorly soluble drugs. Molecules. 2016;21(5):672. doi:10.3390/molecules21050672
70. Marathe S, Shadambikar G, Mehraj T, Sulochana SP, Dudhipala N, Majumdar S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics. 2022;14(5):1034. doi:10.3390/pharmaceutics14051034
71. Saito Y, Yamada N, Shirai K, et al. Effect of rosuvastatin 5–20mg on triglycerides and other lipid parameters in Japanese patients with hypertriglyceridemia. Atherosclerosis. 2007;194(2):505–511. doi:10.1016/j.atherosclerosis.2006.11.028
72. Lee EJ, Oh H, Kang BG, et al. Lipid-lowering effects of medium-chain triglyceride-enriched coconut oil in combination with licorice extracts in experimental hyperlipidemic mice. J Agric Food Chem. 2018;66(40):10447–10457. doi:10.1021/acs.jafc.8b04080
73. Salil G, Rajamohan T Hypolipidemic and antiperoxidative effect of coconut protein in hypercholesterolemic rats; 2001.
74. Alatawi KA, Alshubaily FA. Coconut products alleviate hyperglycaemic, hyperlipidimic and nephropathy indices in streptozotocin-induced diabetic Wistar rats. Saudi J Biol Sci. 2021;28(8):4224–4231. doi:10.1016/j.sjbs.2021.06.060
75. Shehata TM, Mohafez OM, Hanieh HN. Pharmaceutical formulation and biochemical evaluation of atorvastatin transdermal patches. Indian J Pharm Educ Res. 2018;52(1):54–61. doi:10.5530/ijper.52.1.6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.