Back to Journals » Drug Design, Development and Therapy » Volume 19
Visual Research of Global Orphan Drug from a Bibliometric Perspective
Received 12 November 2024
Accepted for publication 4 May 2025
Published 20 May 2025 Volume 2025:19 Pages 4201—4220
DOI https://doi.org/10.2147/DDDT.S506112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Xiaoya Wen,1,* Guangzhi Jin,2,* Chunxing Wu3
1Department of Pharmacy, The Fourth People’s Hospital of GuiYang, Guiyang, Guizhou Province, 550007, People’s Republic of China; 2Department of Hemodialysis Room, The Second People’s Hospital of Guizhou Province, Guiyang, Guizhou Province, 550001, People’s Republic of China; 3Department of Pharmacy, Binzhou Central Hospital, Binzhou, Shandong Province, 251700, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunxing Wu, Department of Pharmacy, Binzhou Central Hospital, Binzhou, Shandong Province, 251700, People’s Republic of China, Email [email protected]
Objective: This study analyzes the current research landscape and trends in orphan drug development, providing insights for future advancements in the field.
Methods: Gathering pertinent material from the China National Knowledge Infrastructure (CNKI) and Web of Science databases. Microsoft Office Excel 2017, VOSview 1.6.20, and CiteSpace 6.3R2 were utilised to summarise the present research state and offer insights into research hotspots in the domain of orphan drugs.
Results: A total of 3598 research papers were included, with Chinese research showing a continuous upward trend, while international research has entered a slow development stage. The field of orphan drug research has formed a sizable research team, and cooperation between international institutions is relatively mature. Meanwhile, the United States and the United Kingdom have strong influence in this research field, while China lacks international cooperation. The research focus in this field mainly involves the development and clinical application of orphan drugs, and domestic and foreign research also has its own emphasis. Maintain consistency in clinical trials and medication support for orphan drugs both domestically and internationally; And foreign research has obvious advantages in the development of orphan drugs. Keyword emergence research indicates that the clinical accessibility and regulatory approval of orphan drugs have become a prominent issue of interest both nationally and globally.
Conclusion: This study systematically summarises and examines the current research status and emerging trends in the global orphan drug sector by analysing relevant literature from 2000 to 2024 through bibliometric methods. It further delineates the similarities and differences in orphan drug research domestically and internationally, offering valuable references for future investigations in this domain.
Keywords: orphan drug, rare disease, bibliometrics, medication guarantee, orphan drug development
Introduction
Rare disease refers to a category of diseases characterised by an exceedingly low incidence rate.1 The China Rare Disease Definition Research Report 2021 indicates that diseases with an incidence rate of fewer than one in 10,000 among newborns, a prevalence rate of less than one in 10,000, or a patient population of fewer than 140,000 should be classified as rare diseases,2 and there are reports that diseases exhibited in up to 6–8% of the world’s patient population should also be included in the category of rare diseases.3 Clarifying the definition of rare diseases has a milestone positive significance, which not only provides a basis for clinical diagnosis and treatment of rare diseases, but also greatly promotes the development and updating of related drugs (orphan drugs) or treatment methods. Orphan drugs are a category of pharmaceuticals utilised for the prevention, treatment, and diagnosis of uncommon diseases, also referred to as rare medications. Although each rare disease affects a small population, there are over 7000 identified rare diseases globally, collectively impacting millions of individuals.4 According to incomplete statistics, the global prevalence of rare diseases is around 4.5%, and there are about 20 million patients with rare diseases in China.4,5 As is well known, rare diseases already lack effective treatment methods, and the current supply of orphan drugs for treating rare diseases is still insufficient,6 which highlights the urgency, necessity, and public welfare of orphan drug research. In 1983, the US Congress passed the Orphan Drug Act, aimed at incentivizing pharmaceutical companies to develop orphan drugs specifically for the treatment of rare diseases.7,8 The act put the United States at the forefront of global orphan drug development and research, and had epoch-making significance for the treatment of rare diseases and the development of orphan drugs. In 2000, the European Union introduced the concept of orphan drug designation and identified medical plausibility, rarity, and medical significant benefit as the three criteria for orphan drug development.9 In 2018, China published the First Catalogue of Rare Diseases,10 resulting in a continuous increase in the number of orphan medications approved and commercialised for the treatment of rare diseases, significantly addressing the medication needs of most people affected by these conditions. Currently, global policy research, drug discovery, accessibility analysis, and clinical application regarding orphan pharmaceuticals have been extensively documented. At present, there are several studies on policy research, medication discovery, accessibility analysis, and clinical applications concerning orphan pharmaceuticals globally. Recent advancements in technology and methodologies, including genome sequencing, gene therapy, and pathway regulation, extensively utilized in orphan drug research and development, may result in several orphan medicines with therapeutic promise being approved for market introduction.11 Bibliometrics enables systematic quantitative analysis of scientific literature. Such studies can map global orphan drug research, identifying key findings, focuses, and trends to guide future advancements in the field. Although bibliometric studies have reported on the current research status and hotspots in the field of orphan drugs, this research only involves the pharmacovigilance of orphan drugs and cannot fully demonstrate the research status and achievements in clinical application, drug development, review and approval of orphan drugs.12 Based on this, this study is intended to use published literature on orphan drugs as a source of data and use bibliometric methods, such as keyword co-occurrence, clustering, and emergence, to compare the results achieved in the field of orphan drugs around the globe and to explore the focus of the research in the field of orphan drugs and its trends, with a view to providing a reference for further research in the field of orphan drugs.
Sources of Data and Methodologies
Literature Retrieval and Inclusion
Web of Science is one of the most comprehensive and widely recognized databases in the world, and due to its timely literature updates and high professionalism and authority, it has become the most commonly used literature retrieval database in bibliometric research.13 This study’s Chinese literature was sourced from the China National Knowledge Infrastructure (CNKI), utilising the keywords “orphan drug” OR “rare drug” OR “rare disease drug” for subject searches on the advanced search page, with the option for “synonym expansion” selected to guarantee the comprehensiveness and precision of the included literature. The search scope was “Medical Science and Technology”, and the search date was 21 June 2024, and the search period was from 2000 to 2024. The results of the search yielded 830 articles, and 263 Chinese articles were included after excluding non-Chinese articles, journal catalogues, short reports, and reports with the theme of rare diseases.
The English literature is derived from Web of Science (WOS), employing the search strategy: Topic = “rare drug(s)” OR “orphan drug(s)” OR “orphan drug design” and other synonyms. The included English literature should meet the following criteria: ① The research topic is related to “orphan drugs”; ② The literature type is “Article” or “Review”; ③ Published from 2000 to 2024. Exclusion criteria: ① Literature in languages other than English; ② Meetings, newspapers, short stories, and non-medical literature. In addition, the search results based on the Web of Science database can classify papers according to the publication time, country (region), language category, publishing house, etc. In this study, papers in English but in Chinese Mainland or Taiwan are classified as Chinese documents. Consequently, 5125 articles were obtained, and 3335 English articles that fulfilled the criteria were eventually included after removing those irrelevant to the issuer.
Data Processing
According to the literature recognition type of Citespace software, download Chinese data in the “Refworks” format for literature; Download English data in the “Plain text file” format and select “Full Record and Cited References”. All literature data will be uniformly named in the “download_XX” format. Use the built-in “CNKI Format Conversion” function in CiteSpace 6.3R2 (Advanced version, the same below) to transpose Chinese literature into “converted” format for analysis; English literature does not require transposition. When conducting keyword co-occurrence analysis, due to the interference of synonyms or near-synonyms on subsequent research results, this study first unified the synonyms or near-synonyms that appeared in the literature before keyword analysis. The terms “rare drug”, “orphan drug”, “orphan drug design”, etc. are all unified as “orphan drug”.
Data Analysis and Observation Metrics
CiteSpace’s built-in “Remove Duplicates” function was used to obtain the number of articles by year, and a scatter plot of the number of articles by year was plotted with the help of Microsoft Office Excel 2017 tables. As two commonly used software in bibliometrics, VOSviewer is a Java-based analysis software, as is Citespace. The critical value of the number of publications by core authors in the field is determined based on Price’s law 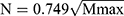 (Mmax is the highest output author’s publication volume).14 In this study, VOSviewer 1.6.20 and Citespace 6.3R2 were used to analyze the authors, institutions, and national collaborations of the Chinese and English literature, and keywords were subjected to co-occurrence, clustering, and emergence analyses in order to clarify the current status and trends of research in the field of orphan drugs. Addition to selecting “Author”, “Institution”, “Keyword” and “Burstness” nodes as needed, the rest of the parameters of the Citespace software were set as follows: according to the time of inclusion of the literature, the Time Slicing of this study was set to 2000–2024, Years per slice was set to every 1 year, and the Cosine (connection strength), With Slices (Connection Range) of the subjects were kept as default and its threshold (top N per slice) = 25. In addition, for keyword co-occurrence, its pruning module selects “Pathfinder” + “Pruning sliced networks”.
(Mmax is the highest output author’s publication volume).14 In this study, VOSviewer 1.6.20 and Citespace 6.3R2 were used to analyze the authors, institutions, and national collaborations of the Chinese and English literature, and keywords were subjected to co-occurrence, clustering, and emergence analyses in order to clarify the current status and trends of research in the field of orphan drugs. Addition to selecting “Author”, “Institution”, “Keyword” and “Burstness” nodes as needed, the rest of the parameters of the Citespace software were set as follows: according to the time of inclusion of the literature, the Time Slicing of this study was set to 2000–2024, Years per slice was set to every 1 year, and the Cosine (connection strength), With Slices (Connection Range) of the subjects were kept as default and its threshold (top N per slice) = 25. In addition, for keyword co-occurrence, its pruning module selects “Pathfinder” + “Pruning sliced networks”.
In addition, the node size in the co-occurrence graph represents the number of publications, and node centrality represents the strength of cooperation. The evaluation indicators for clustering results mainly include clustering module values (modularity, Q) and clustering contour mean values (Silhouette, S), and the evaluation criteria for their credibility are Q >0.3 and S >0.7.15
Results
Analysis of the Volume of Publications
The number of publications is a visual reflection of the current state of research in a particular field, and in this study, we counted the literature related to orphan drug research published in CNKI as well as Web of Science since 2000. The results showed that a total of 3598 articles were retrieved in this field, including 263 Chinese articles, accounting for 7%; There are 3335 English literature, accounting for 93%, as shown in Figure 1. The trend of publications in the field of orphan drugs is shown in Figure 2, indicating a continuous upward trend in Chinese publications, with the highest number of publications in 2023 at 32. The average annual number of publications in English literature is about 136, with the highest number of publications in 2015 at 266. The trend of publication shows that the English literature in the field of orphan drug research has roughly gone through two periods starting from 2015. In the past decade, the publication volume of English literature has sharply declined, and its development has entered a slow period.
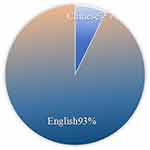 |
Figure 1 Posting percentage. |
 |
Figure 2 Posting trends. |
Analysis of Author Collaboration
Statistics show that a total of 249 scholars have participated in research in the field of orphan drugs in Chinese literature, and 50 scholars have published ≥ 3 articles. According to Price’s law, compared to other biomedical fields, these 50 authors have a high concentration. The co-occurrence network is shown in Figure 3. In terms of English literature, a total of 503 scholars participated in research in the field of orphan drugs, and their co-occurrence network is shown in Figure 4. The graph consists of 503 nodes and 600 lines, with a graph density of only 0.0048. Chinese literature has the highest publication volume, with Bo Zhang (13 articles), Xin Liu (12 articles), Shuyang Zhang (8 articles), Shiwei Gong (8 articles), etc., while English literature is represented by Simoens, Steven (14 articles), Antoniu, Sabina (8 articles), Cassiman, David (7 articles), etc. It can be seen that there is currently no large-scale and influential team of scholars in the field of orphan drug research at home and abroad, but some research teams have taken shape, indicating that there may be great potential for the development of orphan drug research.
 |
Figure 3 Collaboration co-occurrence of Chinese authors. |
 |
Figure 4 Collaboration co-occurrence of English authors. |
Analysis of Institutional Cooperation
Statistics show that Chinese literature involves a total of 176 institutions, with the highest number of publications from institutions such as the International School of Pharmaceutical Business at China Pharmaceutical University (22 articles), the School of Business Administration at Shenyang Pharmaceutical University (16 articles), China Pharmaceutical University (10 articles), and the Department of Pharmacy at Peking Union Medical College Hospital (10 articles). The co-occurrence visualization is shown in Figure 5. In terms of English literature, a total of 354 institutions have participated in research in the field of orphan drugs, and their co-occurrence network is shown in Figure 6. The graph consists of 354 nodes and 562 lines, with a density of 0.0090. The institutions with the highest publication volume in the WOS database include KU Leuven (28 articles), US Food & Drug Administration (FDA) (25 articles), Utrecht University (18 articles), University of London (15 articles), etc. It can be seen that there is relatively little cooperation and exchange between China orphan drug research institutions, while international orphan drug research institutions have formed mature cooperative relationships, and the exchange of experience and complementary cooperation between institutions have been strengthened, which is conducive to the development of research in this field.
 |
Figure 5 Collaboration co-occurrence of Chinese institutions. |
 |
Figure 6 Collaboration co-occurrence of English institutions. |
Analysis of National Cooperation
Analyzing the countries where various researchers are located can help understand the current status and research popularity of orphan drug research worldwide. Statistics show that scholars in the field of orphan drug research come from 63 countries (regions) around the world. The co-occurrence network of cooperation among countries is shown in Figure 7, and the countries with a large number of publications and their centrality are shown in Table 1. It can be seen that research in the field of orphan drugs is international, and countries have contributed to a certain degree of enthusiasm in this field, with higher publication volume and centrality in the United States and England; Although People’s R China has a higher number of publications, its centrality is 0. This suggests that Chinese scholars should further strengthen international cooperation based on previous research and promote the high-quality development of orphan drug research in China.
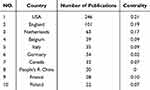 |
Table 1 Countries with More Posts and Centrality |
 |
Figure 7 Visualization of national cooperation. |
Analysis of Highly Cited and Emergence
The citation count of literature can reflect the overall research status of a certain research field, and highly cited literature is the knowledge foundation of research in this field; And citation emergence analysis can also identify literature that has received much attention during a specific time period.16 The highly cited Chinese and English literature in the field of orphan drug research are shown in Tables 2 and 3; It can be seen that the content of highly cited literature in China mainly focuses on the current development status of orphan drugs and a review of comparative studies at home and abroad; As Zhang S and others have found,17 rare disease patients in China have been plagued by the issue of accessibility to orphan drugs. Statistics show that about 11% of rare disease treatment drugs in China are not included in the scope of medical insurance payment. The research content of highly cited English literature mainly tends to focus on the accessibility of orphan drugs (access OR availability) and the development of orphan drugs (reposition OR discovery). Meanwhile, the emergence of citations indicates that there has been an increase in the emergence of five articles, including Access to Orphan Drugs: A Comprehensive Review of Legislations, Regulations and Policies in 35 Countries (Gammie T, 2015, PLOS ONE, V10, P0, DOI), in recent years. These articles with high emergence intensity mainly explore the medication guarantee of orphan drugs, involving the accessibility, acquisition, and reimbursement of orphan drugs, as shown in Figure 8. The “strength” value in the figure represents the intensity of attention given to the literature, and the red interval represents the duration of attention given to the literature. Chan A Y L4 and others explored the implementation policies and reimbursement systems of orphan drugs in many Eurasian countries. The results showed that since 2013, the number of countries that have established orphan drug policy guarantees has been rapidly increasing. Countries have also strengthened investment in regulating orphan drug prices, incentivizing market supply, and encouraging research and development to improve the accessibility of orphan drugs.
 |
Table 2 High Cited Chinese Articles |
 |
Table 3 High Cited English Articles |
 |
Figure 8 English literature emergence. |
Keyword Co-Occurrence Analysis
As a refined expression and high-level summary of academic research, keyword co-occurrence is a commonly used method in bibliometrics to reveal the research focus of a field, and high-frequency keywords with high centrality are the most important.18 After keyword synonymous merging, the Chinese literature involved 287 keywords and appeared a total of 785 times. The English literature involves 608 keywords, appearing a total of 2976 times. Obtain the co-occurrence network of Chinese and English keywords in the field of orphan drug research using the “Keyword” operation node of CiteSpace software, as shown in Figures 9 and 10. The size of nodes in the graph is related to the frequency of keyword occurrence. The Chinese keyword co-occurrence network consists of 287 nodes and 536 lines, with a graph density of 0.0131. The co-occurrence network of English keywords consists of 608 nodes and 1531 connections, with a graph density of 0.0083. The frequency and centrality of keywords are key indicators for measuring their importance, with higher frequency indicating higher research attention; Centrality>0.1 indicates that it is in a central position in the co-occurrence network.19 In this study, the frequency and centrality of keywords such as orphan drugs, rare diseases, drug development, accessibility, health technology assessment, clinical trials, and drug development were relatively high, forming a co-occurrence network centered around them. These keywords mainly involve the research and clinical application of orphan drugs, as shown in Table 4.
 |
Table 4 Frequency and Centrality of Keywords |
 |
Figure 9 Co-occurrence network of Chinese keywords. |
 |
Figure 10 Co-occurrence network of English keywords. |
Keyword Emergence Analysis
Emergent words refer to the phenomenon of a sharp increase in keywords during a specific period of time, which reflects the degree of attention given to keywords during this period and also reveals the research evolution and hotspots in this field. Using the “Burstness” function of CiteSpace software to obtain prominent keywords in the field of orphan drugs, as shown in Figures 11 and 12. “Strength” in the figures represents mutation intensity, and the red area indicates that the keywords emerged during this period, making it a research hotspot. In terms of Chinese literature, keywords with high mutation intensity such as medication guarantee (2022–2024, 2.53), management system (2002–2013, 2.08), incentive policies (2015–2019, 1.81), drug policies (2015–2019, 1.81), clinical trials (2018–2024, 1.75), affordability (2021–2024, 1.72), accessibility (2020–2024, 1.48), and research and development status (2012–2015, 1.38) suggest that the research and development of orphan drugs in China has undergone a transformation from policy research and data management to clinical trials and medication guarantee. In terms of English literature, prominent keywords such as clinical trials (2009–2011, 2.95), pregnancy x receptor (2001–2010, 2.7), drug discovery (2003–2008, 1.93), and molecular cloning (2004–2012, 1.51) from 2000 to 2015 suggest that during this period, international research in this field mainly focused on the discovery of orphan drugs. In recent years, research in the field of orphan drugs has focused on ensuring the use of orphan drugs, with relevant emerging terms including reimbursement (2015–2020, 4.41), availability (2017–2020, 2.83), coverage (2020–2022, 2.74), cost (2020–2024, 2.67), access (2022–2024, 2.51), etc.
 |
Figure 11 Emergence keywords of Chinese articles. |
 |
Figure 12 Emergence keywords of English articles. |
Keyword Clustering Analysis
Cluster analysis is based on keyword co-occurrence to classify keywords that are closely related. Perform K-means clustering analysis on keywords using CiteSpace’s log - likelihood rate (LLR) method, and present the clustering results of Chinese and English literature, as shown in Figures 13 and 14. The results showed that Chinese literature obtained 10 (#0 - #9) clusters, with cluster Q = 0.6270 and S = 0.7579. The English literature obtained 9 clusters (#0 - #8), with cluster Q = 0.7681 and S = 0.9158. This indicates that the clustering results obtained are highly homogeneous and closely related, and the clustering is reasonable. Using “Landscape” to display the temporal continuity of the research directions represented by each clustering result, as shown in Figures 15 and 16. The LLR label words are explanations for the clustering results, it can be seen that in terms of Chinese literature, the research directions represented by clusters such as treatment (# 0), orphan drugs (# 1), and accessibility (# 3) are guaranteed in terms of temporal continuity, making them relatively stable research directions in the field of orphan drugs; In terms of English literature, research directions represented by clusters such as rare diseases (# 0), drug discovery (# 1), orphan drug designation (# 2), and clinical trials (# 4) are relatively stable. The clustering results and their label definitions are shown in Tables 5 and 6. Combining the clustering label words based on LLR algorithm, it can be seen that there is consistency in the research of Chinese and English literature, such as both focusing on clinical trials and medication guarantee of orphan drugs. Chinese literature related clustering includes treatment (# 0), clinical trials (# 2), accessibility (# 3), Gaucher’s disease (# 6), and medication guarantee (# 8); English literature related clustering includes rare diseases (# 0), clinical trials (# 4), act (# 5), etc; The Chinese literature clustering labels related to it involve budget impact analysis, supply guarantee, coverage, medical insurance, special approval, registration characteristics, accessibility, priority drugs, and heavyweight drugs; The clustering labels for English literature involve reimbursement, availability, priority review vouchers, drug approval, clinical trials, etc. At the same time, research on both Chinese and English literature has its own focus. For example, Chinese literature is particularly prominent in orphan drug research policies, and related clusters include policy (# 4), excitation mechanism (# 5), Japan (# 7), and Britain (# 9); Related tag words include international comparison, strategy research, optimal strategy, incentive, incentive measures, research and development incentives, etc. English literature has demonstrated advantages in the development of orphan drugs, involving clustering drug discovery (# 1), pregnane x receptor (# 3), and marin compound (# 7), among others; Cluster label words include pharmaceutical industry, agonists, protein-coupled receptors, drug development, investment, pregnane x receptor, etc.
 |
Table 5 Clustering Results of Chinese Articles |
 |
Table 6 Clustering Results of English Articles |
 |
Figure 13 Clustering results of Chinese keywords. |
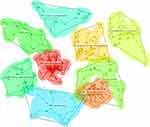 |
Figure 14 Clustering results of English keywords. |
 |
Figure 15 The overview of Chinese clustering. |
 |
Figure 16 The overview of English clustering. |
Discussion
This study employs bibliometric analysis of pertinent research literature on orphan pharmaceuticals from 2000 to 2024, examining and summarising publishing trends, author institutions, national collaboration, and the co-occurrence and emergence of keywords. In recent years, the number of orphan drug research in China has been gradually increasing, but despite this, this research field is still the most reported by foreign research. The research in this domain encompasses various facets, including accessibility, clinical application, orphan drug development, and the assurance of orphan drug medication. Domestic and international studies exhibit distinct research orientations, with their respective findings offering substantive references for orphan drug development.
Data indicate that 3598 eligible papers were incorporated in the domain of orphan drug research, with English literature comprising 93% of the total. The research accomplishments in the domain of orphan pharmaceuticals are notably prolific, with associated academic studies continually emerging. As of October 2022, research by Shijun Luo et al revealed that China had 194 orphan medications available, encompassing 219 dosage forms, of which 165 were included in the national basic medical insurance catalogue.20 Recent statistics21 reveal that the FDA requires 244 days to evaluate marketing applications for new orphan pharmaceuticals, but the clinical development duration for these treatments is roughly 7.2 years. Currently, the trend in published articles indicates a general increase in research on orphan pharmaceuticals in China; the average annual number of publications in the international orphan drug domain is approximately 136. Compared with other biomedical fields (such as tumors and cardiovascular diseases), the number of clinical trials and published papers related to orphan drugs shows a low publication rate.22 Although academic achievements in the field of orphan drugs are relatively limited within the scope of biomedicine, research related to orphan drugs has certain academic value and clinical significance. The current decline in academic reports in the field of orphan drugs abroad may be the result of multiple factors such as policies, markets, and technology. Due to difficulties in recruiting rare disease patients and strong heterogeneity, this will to some extent lead to difficulties in standardizing clinical trial data related to orphan drug development and insufficient sample size.23 Meanwhile, technologies related to orphan drug development, such as gene therapy and viral vector delivery, also face challenges such as immune rejection and off target effects.24 In addition, with the strengthening of patent protection and data confidentiality awareness, relevant research institutions or enterprises are more inclined to disclose their results through patent protection rather than academic papers. Future policy makers need to balance incentive measures and payment pressures, while the academic community needs to adapt to the dissemination mode of new research results to promote knowledge sharing and innovative breakthroughs in the field of orphan drugs.
The findings reveal that the research focus of highly cited Chinese literature predominantly pertains to the development status of orphan pharmaceuticals and comparative studies both domestically and internationally, with notable scholars including Bo Zhang, Xin Liu, Shuyang Zhang, and Shiwei Gong. Xin Liu et al25 examined the global application status of orphan pharmaceuticals and discovered that 51.85% of orphan drugs available in China were solely dependent on imports. It can be seen that there is still a certain gap between China and developed countries around the world (such as the United States, the European Union, and Japan) in the research and development of orphan drugs. The main reasons include difficulties in diagnosing rare diseases, limited clinical research, lack of resources for single disease patients, and difficulties in conducting clinical trials for children.26 The research on extensively referenced English literature centres on the accessibility and advancement of orphan pharmaceuticals, with notable scholars including Simoens Steven, Antoniu Sabina, Cassiman David, and others, who have several publications. The research and development of orphan drugs is more specialised and challenging than that of other pharmaceuticals due to the exceedingly low incidence rate of rare diseases, the substantial expense of clinical trials, the limited target market, the complexities of implementing randomised controlled studies, the geographical distribution of patients, and other contributing factors.5 Analyzing the collaboration between authors and institutions in this field can roughly describe the overall status of global orphan drug research, which can help relevant researchers adjust their research strategies in a timely manner and allocate resources reasonably, such as mature author collaboration relationships, complete institutional cooperation and communication, etc. This can further promote the development of orphan drug research to deeper and broader levels. On the other hand, scholars in the field of orphan drug research currently come from 63 countries and regions around the world, indicating that there is a trend towards international cooperation in the research of orphan drugs. It is worth noting that although China’s achievements in the field of orphan drugs are commendable, its international cooperation still needs to be strengthened.
The reasons for the lack of international cooperation in China are multifaceted. Firstly, there are significant differences in the definition of rare diseases and orphan drug policies among countries around the world, including China. These differences will require Chinese pharmaceutical companies to adapt to different regulatory frameworks in cross-border cooperation, and increase coordination costs and complexity.22 At the same time, the development of orphan drugs relies heavily on cutting-edge technologies such as gene editing and monoclonal antibodies, and there is still a significant gap between China and developed countries such as Europe and America in these areas; In addition, the inherent characteristics of small market size and high research and development costs of orphan drugs have also reduced the enthusiasm of Chinese drug research and development enterprises or institutions for orphan drug cooperation or research.27 These factors have made the development and use of orphan drugs in China difficult. Liu J et al28 pointed out that in sharp contrast to the approval of orphan drugs in the United States, the average approval delay for orphan drugs in China is 5.9 ± 6.07 years, and the clinical supply rate is also less than 30%. Therefore, in future orphan drug research, China should focus on aligning with international standards, simplifying the approval process for cross-border orphan drug clinical trials, strengthening technical cooperation, and seeking diversified funding support and cooperation models to promote the in-depth development of domestic research in this field.
The co-occurrence results show that keywords with high frequency and centrality mainly involve the development and clinical application of orphan drugs, and related keywords include orphan drugs, rare diseases, drug development, accessibility, health technology assessment, clinical trials, drug development, etc. As drugs for diagnosing, treating, or preventing rare diseases, orphan drugs have characteristics that distinguish them from other common drugs, such as high research and development costs, long cycles, and low market demand. This leads to challenges in the treatment of rare diseases, as investigations29 have found that although the Japanese government has already introduced relevant measures to encourage the development of drugs for rare disease treatment, the supply of orphan drugs for difficult to treat or rare diseases in Japan is still plagued by the phenomenon of “drug lag”. Cluster Q and S values indicate that the clustering results based on keywords are reliable, and the research directions represented by each cluster suggest that there is global consistency in orphan drug clinical trials and medication assurance. Chinese literature related clusters include treatment (# 0), clinical trials (# 2), accessibility (# 3), Gaucher’s disease (# 6), and medication guarantee (# 8); English literature related clustering includes rare diseases (# 0), clinical trials (# 4), drug repurposing (# 5), etc. The enactment of the Orphan Drug Act in the United States injected momentum into the treatment of rare diseases and the research of orphan drugs, and also provided guarantees for the medication of related patients.30 At present, clinical trials of rare disease drugs still have shortcomings such as small scale and low quality of evidence.31 However, in 2018, the FDA approved 34 new orphan drugs, which was the first time in history that orphan drugs accounted for more than half of new drug approvals since the enactment of the Orphan Drug Act.32 At the same time, relevant clustering suggests that Chinese research is more prominent in orphan drug policies; International research has shown strong advantages in the development of orphan drugs. The development of orphan drugs is full of uncertainty and risk,33,34 and incentive measures from regulatory agencies have removed obstacles to the development of orphan drugs; In the meantime, some new research and development strategies, such as protein replacement therapy, monoclonal antibodies, antisense oligonucleotides, etc,35 will greatly accelerate the development process of related orphan drugs. It can be seen that the research scope in this field is relatively broad and comprehensive, covering various dimensions of orphan drugs from research and development to clinical practice.
Research has found that current global research hotspots in this field are focused on ensuring the use of orphan drugs; At the same time, research in the global orphan drug field also has its own focus while maintaining consistency. In the early stages of research, international studies in this field mainly focused on the discovery of orphan drugs, while China mainly focused on policy research and accessibility of orphan drugs, with less involvement in the development and discovery of orphan drugs; It can be seen that the research and development of orphan drugs in China has seriously lagged behind developed countries such as Europe and America. Fang Zhongjian and others36 found that the use of orphan drugs in China mainly relies on imports and generic drugs, and the research and development of orphan drugs is still in its early stages. With the increasing awareness of the urgency and necessity of orphan drug research and development, China has issued measures such as the Technical Guidelines for Clinical Research and Development of Rare Disease Drugs and the Statistical Guidelines for Clinical Research of Rare Disease Drugs (Trial), and introduced corresponding incentive policies and increased investment in technology funds to encourage the research and development of innovative drugs for children, innovative drugs for the treatment of rare diseases (orphan drugs), and innovative drugs included in breakthrough treatment drug programs. The approval time for innovative drugs, including orphan drugs, has been shortened to accelerate their listing speed. China has established a supportive environment for orphan drug research and development. It is believed that with the support of national policies and increased investment, the research and development of orphan drugs in China will enter a new stage.
In addition, there are also some shortcomings in this study, such as the fact that some orphan drugs only appear in the title of the article under the trade name or generic name during literature search, and the search terms specified in the search equation such as “orphan drug” or “orphan drug” do not appear. This may result in the omission of relevant literature during the inclusion of literature search in this study, which may lead to some bias in the analysis results.
Conclusion
This study examines the present research landscape in the global orphan drug sector through bibliometric analysis, highlighting both consistencies and disparities in worldwide orphan drug research. At present, the focus of global research is on ensuring the accessibility of orphan drugs, while the development of orphan drugs in China is still underdeveloped. We should address this issue by clarifying clinical diagnosis, improving information networks, developing medical technology innovation, encouraging drug research and development, and balancing drug prices, in order to accelerate orphan drug innovation and benefit rare disease patients worldwide.
Data Sharing Statement
The dataset generated and analyzed in the current research can be obtained from Web of Science databases (https://www.webofscience.com/wos/author/author-search) and China National Knowledge Infrastructure databases (https://www.cnki.net).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferreira CR. The burden of rare diseases. Am J Med Genet A. 2019;179(6):885–892. doi:10.1002/ajmg.a.61124
2. Lu Y, Han J. The definition of rare disease in China and its prospects. Intractable Rare Dis Res. 2022;11(1):29–30. doi:10.5582/irdr.2022.01034
3. Sharma A, Jacob A, Kumar D, et al. Orphan drug: development trends and strategies. J Pharm Bioallied Sci. 2010;2(4):290–299. doi:10.4103/0975-7406.72128
4. Chan AYL, Chan VKY, Olsson S, et al. Access and unmet needs of orphan drugs in 194 countries and 6 areas: a global policy review with content analysis. Value Health. 2020;23(12):1580–1591. doi:10.1016/j.jval.2020.06.020
5. Feng S, Liu S, Zhang S, et al. National rare diseases registry system of china and related cohort studies: vision and roadmap. Hum Gene Ther. 2018;29(2):128–135. doi:10.1089/hum.2017.215
6. Liu M, Lu Y, Liu Y, et al. Orphan drug policy analysis in China. Front Pharmacol. 2024;15:1278710. doi:10.3389/fphar.2024.1278710
7. Roberts AD, Wadhwa R. Orphan drug approval laws. In: StatPearls. Treasure Island (FL): StatPearls; 2021.
8. Huang YJ, Chao WY, Chang LC, et al. Orphan drug development: the impact of regulatory and reimbursement frameworks. Drug Discov Today. 2022;27(6):1724–1732. doi:10.1016/j.drudis.2022.03.002
9. Micallef J, Blin O. Orphan drug designation in Europe: a booster for the research and development of drugs in rare diseases. Therapie. 2020;75(2):133–139. doi:10.1016/j.therap.2020.02.003
10. National Health Commission of the People’s Republic of China, Ministry of Science and Technology of the People’s Republic of China, Ministry of Industry and Information Technology of the People’s Republic of China, et al. Notice on the publication of the first batch of rare disease catalogue. Bulletin Nat Health Commission People’s Republic of China. 2018. 05: 15–19.
11. Chirmule N, Feng H, Choudhury MC, et al. Orphan drug development: challenges, regulation, and success stories. J Biosci. 2024;49(1):30. doi:10.1007/s12038-024-00425-y
12. Tian M, Wang S, Lu Y, et al. A bibliometric study of rare diseases in English and Chinese databases from 1985 to 2024 based on CiteSpace. Intractable Rare Dis Res. 2025;14(1):14–28. doi:10.5582/irdr.2024.01062
13. Cheng K, Zhang H, Guo Q, et al. Emerging trends and research foci of oncolytic virotherapy for central nervous system tumors: a bibliometric study. Front Immunol. 2022;13:975695. doi:10.3389/fimmu.2022.975695
14. Veiga-Del-Baño JM, Cámara MÁ, Motas M, et al. Mapping of emerging contaminants in coastal waters research: a bibliometric analysis of research output during 1986-2022. Mar Pollut Bull. 2023;194(Pt A):115366. doi:10.1016/j.marpolbul.2023.115366
15. Wei T, Jin Q. Research trends and hotspots in exercise interventions for liver cirrhosis: a bibliometric analysis via CiteSpace. Medicine. 2024;103(28):e38831. doi:10.1097/MD.0000000000038831
16. Lu C, Liu M, Shang W, et al. Knowledge mapping of Angelica sinensis (Oliv.) Diels (Danggui) research: a scientometric study. Front Pharmacol. 2020;11:294. doi:10.3389/fphar.2020.00294
17. Zhang S, Chen L, Zhao Y, et al. Orphan drug development in China: progress and challenges. Lancet. 2019;394(10204):1127–1128. doi:10.1016/S0140-6736(19)32179-8
18. Wang J, Dong P, Zheng S, et al. Advances in gut microbiome in metabonomics perspective: based on bibliometrics methods and visualization analysis. Front Cell Infect Microbiol. 2023;13:1196967. doi:10.3389/fcimb.2023.1196967
19. Chunxing W, Xue Z, Bolong W. The research hot spots and frontiers of Acori Tatarinowii Rhizoma based on bibliometrics. J Foshan University. 2023;41(05):42–52. doi:10.13797/j.cnki.jfosu.1008-0171.2023.0045
20. Demirci E, Knicley J, Fiorentino L. Clinical development and marketing application review times for novel orphan-designated drugs. Front Med Lausanne. 2024;11:1404922. doi:10.3389/fmed.2024.1404922
21. Shijun L, Yulin Z, Shiwei G, et al. Analysis of treatment drugs for 121 rare diseases in China and their medical coverage status. Chin J Hosp Pharm. 2024;44(10):1192–1198+1220. doi:10.13286/j.1001-5213.2024.10.13
22. Tambuyzer E, Vandendriessche B, Austin CP, et al. Therapies for rare diseases: therapeutic modalities, progress and challenges ahead. Nat Rev Drug Discov. 2020;19(2):93–111. doi:10.1038/s41573-019-0049-9
23. Mishra S, Venkatesh MP. Rare disease clinical trials in the European Union: navigating regulatory and clinical challenges. Orphanet J Rare Dis. 2024;19(1):285. doi:10.1186/s13023-024-03146-5
24. Dunbar CE, High KA, Sadelain M, et al. Gene therapy comes of age. Science. 2018;359(6372):eaan4672. doi:10.1126/science.aan4672
25. Shuobing C, Feng Y. Suggestions on the development of real world study on the rare diseases and orphan drugs. J Int Pharm Res. 2019;46(09):699–704. doi:10.13220/j.cnki.jipr.2019.09.009
26. Huang R, Wei Y, Hu J, et al. The progress of, challenges faced by, and future of rare disease patient organizations in China. Intractable Rare Dis Res. 2019;8(2):158–160. doi:10.5582/irdr.2019.01069
27. Blin O, Lefebvre MN, Micallef J, et al. Orphan drug clinical development. Therapie. 2020;75(2):141–147. doi:10.1016/j.therap.2020.02.004
28. Liu J, Yu Y, Shao R, et al. Long way to go: progress of orphan drug accessibility in China from 2017 to 2022. Front Pharmacol. 2023;14:1138996. doi:10.3389/fphar.2023.1138996
29. Song P, Gao J, Tang W, et al. New opportunity for orphan drug development in Japan: early exploratory clinical trial bases promote drug translation from basic studies to clinical application. Intractable Rare Dis Res. 2012;1(2):95–97. doi:10.5582/irdr.2012.v1.2.95
30. Mikami K. Orphans in the market: the history of orphan drug policy. Soc Hist Med. 2019;32(3):609–630. doi:10.1093/shm/hkx098
31. Kubota Y, Narukawa M. Randomized controlled trial data for new drug application for rare diseases in Japan. Ther Innov Regul Sci. 2022;56(4):659–666. doi:10.1007/s43441-022-00404-1
32. Darrow JJ, Avorn J, Kesselheim AS. FDA approval and regulation of pharmaceuticals, 1983-2018. JAMA. 2020;323(2):164–176. doi:10.1001/jama.2019.20288
33. Ko JM. Advancing orphan drug development for rare diseases. Clin Exp Pediatr. 2024;67(7):356–357. doi:10.3345/cep.2023.01109
34. Hunter NL, Rao GR, Sherman RE. Flexibility in the FDA approach to orphan drug development. Nat Rev Drug Discov. 2017;16(11):737–738. doi:10.1038/nrd.2017.151
35. Yoo HW. Development of orphan drugs for rare diseases. Clin Exp Pediatr. 2024;67(7):315–327. doi:10.3345/cep.2023.00535
36. Zhongjian F, Leting L, Yulu F, et al. Exploration and inspiration of orphan drug registration systems at home and abroad. Chin J New Drugs. 2024;33(10):978–983. doi:10.3969/j.issn.1003-3734.2024.10.003
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

