Back to Journals » Nature and Science of Sleep » Volume 16
Washed Microbiota Transplantation Improves the Sleep Quality in Patients with Inflammatory Bowel Disease
Authors Li Q, Liu Y, Zhang Z, Zhang S , Ding X, Zhang F
Received 27 February 2024
Accepted for publication 18 July 2024
Published 2 August 2024 Volume 2024:16 Pages 1141—1152
DOI https://doi.org/10.2147/NSS.S460882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Qianqian Li,1,* Yujie Liu,2,* Zulun Zhang,1 Sheng Zhang,1 Xiao Ding,2 Faming Zhang1,3
1Department of Microbiota Medicine & Medical Center for Digestive Diseases, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, 210011, People’s Republic of China; 2Department of Medicine & Therapeutics, the Chinese University of Hong Kong, Hong Kong, People’s Republic of China; 3National Clinical Research Center for Digestive Diseases, Xi’an, 710032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Faming Zhang, Medical Center for Digestive Diseases, Second Affiliated Hospital of Nanjing Medical University, 121 Jiangjiayuan, Nanjing, 210011, People’s Republic of China, Email [email protected]
Purpose: There is scarce evidence to support the effectiveness of faecal microbiota transplantation (FMT) in improving sleep among individuals with inflammatory bowel disease (IBD). Our study aimed to evaluate the effect of washed microbiota transplantation (WMT) (the new method of FMT) on the sleep of patients with IBD in short term.
Patients and Methods: This prospective study was conducted as part of two interventional clinical trials (starting on February 2013 and expected to end on December 2025) and placed significant emphasis on evaluating sleep quality in patients with IBD. To measure subjective sleep, we used the Pittsburgh sleep quality index (PSQI). The primary endpoint was the PSQI score one month after WMT.
Results: This stage study included 52 eligible patients evaluated by PSQI questionnaire who underwent WMT from January 2020 to March 2021 and 47 patients were enrolled for analysis. The age of the patients ranged from 13 to 60 years, with a mean of 33.4 years, and 57.4% (25/47) of the patients were male. The PSQI scores for all 47 patients one month after undergoing WMT were significantly lower (Cohen d = 0.59, p < 0.001) compared to the baseline. Moreover, baseline PSQI score was correlated with the difference value of the PSQI score before and after WMT (post-PSQI minus pre-PSQI) (r = 0.61, p < 0.05).
Conclusion: The study suggests that WMT might be a helpful intervention for improving the sleep quality of patients with IBD, encouraging clinicians to consider its use in clinical practice for addressing poor sleep in IBD patients.
Clinical Trial Registration: ClinicalTrials.gov; ID: NCT01793831, NCT01790061.
Keywords: faecal microbiota transplantation, transendoscopic enteral tubing, sleep disorder, Crohn’s disease, ulcerative colitis, inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a group of idiopathic inflammatory disorders that includes Crohn’s disease (CD) and ulcerative colitis (UC). It is characterised by a chronic relapsing and remitting courses and along with a heterogeneous clinical presentation of chronic diarrhoea, rectal bleeding, abdominal pain, and weight loss. Although the precise cause of IBD remains unclear, it is believed to result from a complex interplay of genetic susceptibility, environmental factors, gut microbiota, and abnormal innate and adaptive immune responses.1 Patients with IBD frequently suffer from a significant reduction in quality of life, severe fatigue, work impairment, depression, anxiety, and poor sleep.2–5
Several studies have highlighted a high prevalence of sleep disturbances in IBD patients, regardless of disease activity.4,6 Increasing human and animal studies have demonstrated that sleep could potentially be improved by probiotics and prebiotics aiming at modulating the gut microbiota.7,8 Faecal microbiota transplantation (FMT), an approach for reconstructing the composition and function of the gut microbiota, has shown promising therapeutic value in IBD by inducing and maintaining clinical improvement or remission.9 In our team, the methodology of FMT was coined as washed microbiota transplantation (WMT), which is the new method of FMT. Compared to crude FMT, WMT is safer, offers a more precise and feasible dosage of enriched microbiota, and ensures quality control more effectively.10–12 The methodology of WMT was released by a consensus panel of the FMT-standardization study group in 2019.13
A previous clinical study with a small sample size and our recent publication with 73 patients both demonstrated that FMT or WMT could improve sleep quality in individuals with irritable bowel syndrome.14,15 Another clinical study found that WMT significantly improved symptoms of autism spectrum disorder, including gastrointestinal symptoms and sleep disorders, in children.16 In addition, our previous animal study by Wang et al reported that WMT ameliorated reduced total sleep time induced by staying up late near light in the stress tree shrew.17 However, there have been no prior studies focusing on evaluating the effects of FMT on sleep in IBD patients, and poor sleep is a serious concern as it contributes to more frequent relapses,18 worse subjective clinical symptoms,19 and mood and psychological disorders.20–22 Of note, several patients with IBD provided feedback that their sleep quality improved after undergoing WMT in clinical practice, which motivated us to make dedicated efforts to investigate the effects of WMT on sleep in IBD patients. Improved sleep quality may be as a result of WMT mediating the microbiota-gut-brain axis through activation of the vagus nerve and improving immune responses, blood–brain barrier, intestinal permeability, levels of bacterial metabolite, reactive oxygen species and the serotonergic system, an alleviation of gastrointestinal symptoms that can disrupt sleep, or both.23
The main purpose of this study was to investigate the potential effects of WMT on the subjective assessment of short-term sleep quality in patients with IBD, utilizing the Pittsburgh sleep quality index (PSQI). A multivariate regression model was employed to identify independent factors that could predict a more favourable effect of WMT on sleep quality in IBD. To better assess sleep disturbances and daytime dysfunction in patients with poor sleep, a self-evaluation questionnaire on sleep was specifically designed. Overall, this study aimed to contribute further evidence on the effects of WMT for improving sleep quality in patients with IBD.
Materials and Methods
Participants
This prospective study, as part of two interventional clinical trials (NCT01793831 and NCT01790061) (starting on February 2013 and expected to end on December 2025), evaluated patients with IBD undergoing WMT in our centre placing a particular emphasis on assessing sleep quality. All eligible patients were provided with written informed consent forms prior to participation. Inclusion criteria were patients who had been diagnosed with IBD through a combination of typical clinical symptoms, endoscopy, and histological criteria for at least three months, patients with inactive and active CD or active UC (Mayo score 3–12), and patients who failed to achieve satisfactory efficacy for IBD from the previous therapies. Patients were excluded if accompanied by other severe diseases, including other intestinal diseases (eg, Clostridioides difficile infection), malignant neoplasm, cardiopulmonary failure, and serious liver and kidney disease, refused to complete the questionnaire online and attend follow-up, and underwent FMT or WMT before.
The assessment of clinical efficacy and sleep quality was performed by clinical research coordinators and clinicians at baseline and one month after WMT. The study specifically focused on the evaluation of sleep quality using the PSQI one month after WMT, which served as the second outcome measure in the aforementioned clinical trials. Furthermore, the study aimed to identify independent factors that may contribute to a more favourable effect of WMT on sleep quality in patients with IBD one month after treatment. Additionally, the study described any changes observed in sleep condition at night and the adverse effects during the day among patients with poor sleep at baseline after WMT through sleep self-evaluation questionnaire. Before WMT, baseline demographic characteristics and sleep condition were recorded: age, gender, age of IBD onset, duration of disease, current smoker, PSQI score, time for falling sleep, time of sleep, duration of poor sleep. In addition, baseline IBD-related characteristics were assessed, including the Harvey–Bradshaw index (HBI), partial Mayo score (PMS), disease activity, previous therapy and previous surgery related to IBD. Additionally, delivery route and frequency of WMT (single or multiple WMTs) were recorded.
Donor Screening, WMT Procedure and Clinical Outcome Assessment
Donors from the China Microbiota Transplantation System (fmtBank) were selected following the strict screening criteria. Healthy adults, adolescents, and children (aged 6–24 years old) are considered as potential donors in the clinical practice. Candidates were informed of the potential risks and benefits of WMT for recipients and should provide written informed consent. As shown in Supplementary Figure 1, questionnaire screening, face-to-face screening and laboratory screening are taken step-by-step to exclude candidates based on the criteria including age, physiology, pathology, psychology, integrity, time, environment, and recipient status.12,24
The new methodology of FMT was coined as WMT (Supplementary Figure 1), which is dependent on the automatic washing process (GenFMTer, FMT Medical, Nanjing) and related delivering consideration. The preparation of washed microbiota was performed in hospital and WMT was provided as legal medical technology approved by the institute ethical committee. The washed microbiota comes in two forms, including fresh and frozen. The time from faeces defecation of a donor, laboratory preparation for enriching microbiota to the time of microbiota delivering or storing was limited within one hour, which is regarded as the “one-hour protocol”.11 Additionally, in clinical practice, we use 10% glycerol as a protective agent to freeze and store the freshly prepared washed microbiota suspension for future use. Before 2015, most patients underwent single WMT via gastroscopy. Although several clinical studies at our centre consistently indicated that the frequency of WMT in the short term did not correlate with clinical efficacy,9,11 the step-up WMT strategy increasingly utilized more frequent sessions in serious conditions, which showed enhanced long-term benefits.25,26 Therefore, since the development of colonic and mid-gut transendoscopic enteral tubing (TET) (FMT Medical, Nanjing, China) in 2015, the recommended frequency of WMT has been three times via TET.27,28
Clinical efficacy was evaluated in all patients via HBI and PMS one month after WMT. Clinical remission was defined as HBI ≤ 429 or PMS ≤ 1.9 The Common Terminology Criteria for Adverse Events (AEs) (version 5.0) was applied to describe the intensity and relativity of AE with WMT. Only WMT-attributed AEs were reported in the study. In our present clinical studies, the use of antibiotics before WMT treatment in patients was not a prerequisite. The use of probiotics was prohibited after WMT. The use of antibiotics and pharmacological or nonpharmacological treatment for sleep disorders was not recommended without first communicating with the clinicians during the study period.
Sleep Measure
The PSQI, a validated questionnaire for assessing sleep quality, was used to subjectively determine sleep quality at baseline and one month after WMT. The PSQI is a reliable, well-validated, self-reported, and standardised measure of sleep quality over the month prior to completion.30 The 19 self-rated questions (each weighted equally on a 0 to 3 scale), grouped into seven component scores, assess a variety of factors related to sleep quality, including estimates of sleep duration, sleep onset latency, and the frequency and severity of specific sleep-related problems. Total scores range from 0 to 21, and a total score >5 indicates clinically significant poor sleep.31 In addition, we provided a self-evaluation questionnaire on sleep condition at night and the adverse effects during the day for IBD patients with poor sleep at baseline and one month after WMT (Supplementary Figure 2).
Statistical Analysis
Graphing and analysis of the data were performed using IBM SPSS Statistics 20, GraphPad 8, Origin 2021, and Microsoft Excel 365. Continuous data are expressed as mean and range. The paired t-test was used to analyse differences between two paired samples (the measure of effect size is Cohen d).32 The Mann–Whitney U-test was employed to analyse differences between two independent samples. Spearman’s rank correlation analysis (the measure of effect size is r)33 was used to assess the correlations between the difference value of PSQI score (D-value) (post-WMT-PSQI minus pre-WMT-PSQI) and the baseline characteristics or the seven components of baseline PSQI.
We used univariate analysis assessing whether demographic and IBD-related variables (including age, gender, age of onset, duration of IBD, current smoking status, history of previous surgery, type of IBD, baseline HBI/PMS, and baseline disease activity), details of baseline PSQI score (baseline PSQI score, categorization of sleep quality as good or poor, the seven components of the PSQI, and duration of poor sleep), and WMT methods (delivery route and frequency) were related to the better effect of WMT on sleep in IBD. Variables with p < 0.15 in the univariate analysis were included in the multivariable logistic regression analysis (the measure of effect size is Odds ratio (OR)34) (sleep latency, sleep duration, sleep disturbances, use of sleep medicine, daytime dysfunction and baseline HBI/PMS) to identify the independent predictors of the better effect of WMT on sleep, however factors contributing to potential imprecision and those susceptible to clinician influence were excluded. A p value < 0.05 was considered statistically significant.
Results
Study Population
This stage and single-centre study included 52 eligible patients evaluated by PSQI questionnaire who underwent WMT between January 2020 and March 2021. Five patients were excluded due to withdrawal from the study (n = 2) and absence at follow-up (n = 3). Of the 47 patients included in the analysis. Data from 47 patients were collected at baseline, during the WMT procedure, and one month after WMT, including demographic and IBD-related characteristics, PSQI score, WMT delivery route and frequency, and the safety of WMT. Sleep self-evaluation about sleep disturbances and daytime dysfunction of patients was employed for the 25 patients with poor sleep. The clinical efficacy of WMT against IBD was assessed using HBI/PMS for the 36 patients in the active stage of the disease.
Patient Characteristics and Clinical Outcomes of WMT
The demographic and IBD-related characteristics and the WMT procedure for all 47 patients were shown in Table 1. The age of the patients ranged from 13 to 60 years, with a mean of 33.4 years, and 57.4% (25/47) of the patients were male. The mean duration of IBD for all patients was 5.1 years. The mean baseline PSQI score was 7 (PSQI score > 5 indicates poor sleep), and 53.2% (25/47) of the patients were with poor sleep. In total, 68.1% (32/47) of the patients experienced mild or moderate IBD. Regarding the procedure, 42.6% (20/47) of the patients underwent WMT through mid-gut TET, 95.5% (21/22) of the UC patients underwent WMT through colonic TET, and 76.6% (36/47) of all patients and 95.5% (21/22) of UC patients underwent multiple WMTs.
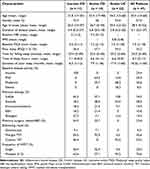 |
Table 1 Baseline Characteristics of 47 Patients with IBD |
Among the 36 patients with active IBD, the clinical remission rates were 50% (7/14) for CD and 55% (12/22) for UC one month after WMT (Supplementary Figure 3A). Furthermore, HBI and PMS significantly decreased one month after WMT, regardless of the status of the disease (inactive CD, active CD, and active UC were p < 0.01, p < 0.01, and p < 0.001, respectively) (Supplementary Figure 3B). No serious AEs associated with WMT were observed during or after WMT. During the follow-up period, five episodes of AEs were observed in five patients, with an AE rate of 10.6% (5/47). All AEs occurred within 24 hours of WMT and included fever, diarrhoea, and dizziness; recovery occurred without medication in the short term.
WMT Improving the Sleep Quality of Patients in IBD
The distribution of patients with different disease statuses and sleep conditions at baseline is depicted in Figure 1A. Following one month of WMT, a significant decrease in the PSQI score was observed in all 47 patients (Cohen d = 0.59, [95% CI = 0.28–0.9], p < 0.001), with a mean post-PSQI score of 4.68 compared to a mean pre-PSQI score of 6.98 (Figure 1B). Subgroup analysis revealed a significant reduction in the PSQI score one-month post-WMT, irrespective of disease status (inactive CD [p = 0.005], active CD [p = 0.039], and active UC [p = 0.044], Figure 1C). Correlation analyses indicated that improvements in sleep quality following WMT were not solely attributed to ameliorated clinical symptoms (changes of HBI/PMS) (r = 0.24, p = 0.1). Moreover, baseline PSQI score and the D-value of PSQI score before and after WMT were correlated (r = 0.61, p < 0.05), suggesting that patients with poorer sleep at baseline experienced greater improvements following WMT (Figure 1D). Further analysis of the correlation between the seven components of baseline PSQI and D-value revealed that patients experiencing more severe daytime dysfunction (r = 0.59, p < 0.05) and longer sleep latency (indicating greater difficulty in falling asleep) (r = 0.47, p < 0.05) exhibited greater improvements in sleep post-treatment (Figure 1E).
We further compared the seven components of the PSQI for all 47 patients before and after WMT (Supplementary Table 1). The results revealed that the total sleep quality (Cohen d = 0.6, p < 0.001), sleep onset latency (Cohen d = 0.3, p = 0.046), and sleep duration (Cohen d = 0.31, p = 0.041) were effectively improved. Additionally, sleep disturbances (Cohen d = 0.5, p = 0.001) and daytime dysfunction (Cohen d = 0.6, p < 0.001) were alleviated one month after WMT.
WMT Making Favourable Effects on the Patients with Poor Sleep at Baseline in IBD
This study also analysed the sleep improvement from WMT in IBD patients with poor sleep at baseline. Twenty-five patients with poor sleep achieved obviously decreased PSQI scores (pre-WMT: mean ± SD = 9.92 ± 3.38 vs post-WMT: mean ± SD = 5.8 ± 3.03) undergoing WMT (Cohen d = 1.04, [95% CI = 0.54–1.5], p < 0.001) (Figure 2A). The PSQI scores of patients with poor sleep decreased obviously one month after WMT, regardless of the status of the disease (inactive CD [p = 0.141], active CD [p = 0.014], and active UC [p = 0.005], (Figure 2B)). According to self-reports by the patients with poor sleep regarding sleep conditions at night and adverse effects during the day, more than 50% reported that the symptoms of difficulty falling sleep (50%, 7/14), difficulty maintaining sleep (60%, 6/10), emotional instability (58.3%, 7/12), early awakening (57.1%, 4/7), lack of energy and enthusiasm (83.3%, 5/6), and loss of memory and concentration (50%, 2/4) disappeared after WMT (Figure 2C).
Predictors of a Better Outcome from WMT on Sleep in IBD
To identify the factors that were significantly correlated with a more favourable effect of WMT on sleep one month after treatment, univariable and multivariable logistic regression analysis were used to isolate independent predictors for sleep quality of patients with IBD one month after WMT (Table 2). We choose the median of the PSQI D-value (PSQI of pre-WMT minus PSQI of 1mo-post-WMT) in all 47 patients as cut-off and divided them into two groups for univariate and multivariate analysis. One group (PSQI D-value ≥ 2, n = 24) represented better effect from WMT for sleep, and the other group (PSQI D-value < 2, n = 23) had relatively poor effect. Based on univariate analysis by Mann–Whitney U-test, patients with a higher baseline PSQI score (p = 0.002), poor sleep (p = 0.014), higher sleep latency score (p = 0.007), sleep disturbances (p = 0.011), and daytime dysfunction (p = 0.01) achieved remarkably better outcomes (the PSQI score decreased ≥2) in sleep quality one month after WMT.
 |
Table 2 Predictors at Baseline for Better Sleep Quality One Month After WMT in IBD |
Further multivariate regression analysis revealed that sleep latency score was an independent predictor of sleep quality one month after WMT (effect size OR = 2.56, 95% CI = 1.083–6.057; p = 0.032) (Table 2). Patients with a higher sleep latency score (ie, longer time required to fall asleep and more difficulty falling sleep) at baseline were more likely to achieve better sleep quality outcomes one month after WMT.
Discussion
Poor sleep is common in IBD patients and causes decreased quality of life, work impairments, emotional disorders, more frequent relapses, and worsening of clinical symptoms.2,35 According to our findings, IBD patients undergoing WMT will likely experience significant effect, such as clinical remission and improved clinical symptoms, with a low incidence of AEs, and may achieve better sleep quality after WMT. The current study evaluated the PSQI score one month after WMT for IBD patients and identified the factors that could predict short-term therapeutic success or failure regarding sleep.
Our study reported that the PSQI scores for all 47 patients one month after WMT was significantly decreased compared with the baseline (a 33% reduction of PSQI scores, pre-mean was 6.98, post-mean was 4.68). Most patients with poor sleep at baseline (52%, 13/25) achieved satisfactory sleep quality with a PSQI score < 5 (normal sleep quality) from WMT. WMT effectively shortened the time for falling sleep, extended the total sleep time, and alleviated sleep disturbances as well as daytime dysfunction. However, 13 patients with poor sleep still experienced continued sleep difficulties after WMT. Hashash et al35 carried out a study about the effects of brief behavioural therapy for sleep in IBD and found that sleep quality improved with a 44% reduction of PSQI scores (PSQI scores: pre-mean was 10.61, post-mean was 5.92). Most participants (54%, 28/52) achieved a PSQI score < 5. In addition, clinical guidelines recommended the use of behavioural sleep treatments as a first line treatment for insomnia especially amongst individuals with chronic diseases.36 Considering the effects of WMT on improving sleep are close to that of behavioural therapy, more evidence should be provided to clarify the values of WMT for sleep.
The precise mechanisms of therapies targeting gut microbiota promoting sleep remain elusive. Liu et al reported that WMT moderated gut microbiota to improve the behavioural and sleeping disorders symptoms of patients with autism spectrum disorder via reducing toxic metabolic production and improving detoxification.37 There is scarce direct evidence from relevant studies proving the mechanisms through which WMT improves sleep. Two animal studies reported that using antibiotics to clear gut microbiota could diminish serotonin production and alter the permeability of the blood-brain barrier, thus contributing to a decrease in sleep quality.38,39 We hold the view that WMT may enhance serotonin levels and improve blood-brain barrier permeability by rebuilding gut microbiota, thereby improving sleep. Additionally, WMT may also regulate sleep by altering gut microbiota composition and levels of inflammatory factors, melatonin, and bile acids.40–42 Further studies are needed to fully understand the potential mechanisms of WMT for improving sleep.
Our study shows that WMT not only improved sleep quality in patients with IBD, but also benefits the clinical symptoms of IBD, including abdominal pain, haematochezia and diarrhoea. Additionally, a clinical study reported that undergoing FMT from healthy donors improved insomnia symptoms among patients with IBS. Meanwhile, the above study revealed that the FMT also normalized stool forms and alleviated gastrointestinal symptoms.14 An unsolved question is whether the interindividual variability in gut microbiota composition and metabolism among donors with healthy sleep plays a role in the efficacy of WMT to improve sleep among patients with sleep disorders. However, which bacteria play a key role and the maintenance time of WMT’s sleep-improving effects is currently scarce, so it is difficult to draw conclusions. Previous literature reported that poor sleep was associated with an increased risk of disease flares in IBD, and improving sleep disturbances in patients with IBD might contribute to better clinical outcomes.2,43–45 In addition to our findings, more evidence is needed to clarify whether WMT could contribute to more stable and sustained clinical outcomes by improving the sleep of IBD patients.
Furthermore, 36.4% (4/11) of IBD patients with an inactive disease status experienced poor sleep, and 75% (3/4) of above patients had better sleep after undergoing WMT. In line with our results, a previous study reported that up to 33.3% of CD patients in remission persistently reported poor sleep.46 In addition, a study reported that patients with inactive IBD and poorer sleep quality had a higher risk of disease relapse at six-month follow-up than patients without sleep disturbances.43 Therefore, we believe that it is necessary to investigate sleep with available medical interventions, regardless of IBD activity status.47 The present findings indicate that IBD patients in remission may benefit from WMT and improved sleep. Although our study found no significant correlation between the improvement in IBD symptoms and sleep quality of patients using Spearman correlation analysis (r = 0.24, p = 0.1), it’s important to consider our sample size and the limitation that correlation analysis alone cannot conclusively determine whether sleep improvements were not attributable to reduced IBD symptoms after WMT.
Several pharmacological and nonpharmacological interventions have been suggested and are included in international guidelines to manage sleep disturbances.48 Pharmacological therapies, such as hypnotics and benzodiazepine-like agents, are frequently prescribed to patients with IBD to treat depressive and anxiety symptoms and sleep disturbances, although these can cause daytime sleepiness, impaired cognitive function, and are associated with a risk of dependency.49 Cognitive behavioural therapy for sleep disturbances, which includes sleep restriction, stimulus control, and cognitive restructuring,50 is a non-pharmacological intervention that has been demonstrated to be effective in improving sleep (assessed by the PSQI).51 In addition, a two-phase open trial exploring interventions for sleep reported that behavioural therapy improved sleep and fatigue and produced additional benefits for psychiatric symptoms in IBD.35 As the relationship between sleep and the gut microbiota continues to be verified, several strategies for improving sleep by manipulating the gut microbiota have been suggested, including probiotic and prebiotic interventions and FMT.23 The current study provided evidence that targeting gut microbiota through WMT might improve sleep in patients with sleep disorders. Further studies are needed to fully understand the therapeutic potential of WMT for sleep disturbances and to clarify the mechanism.
The present study had several limitations. Firstly, it lacked objective measurements of sleep, such as polysomnography or actigraphy. Some clinical outcomes relied on a self-evaluation questionnaire. Secondly, to enhance the validity of the conclusions, future studies with larger sample sizes and a randomized controlled design should establish a scientific predictive model. Thirdly, the study did not assess the correlation between changes in gut-microbiota composition and function and sleep quality. Fourthly, colonic TET, as a breakthrough of microbiota delivery in microbiota medicine,52 would not affect the patients’ sleep, but the delivery of WMT via nasal-jejunal (mid-gut) tube might reasonably affect sleep in some patients. So, the inclusion of patients using nasojejunal tubes might introduce bias. Additionally, the lack of questionnaires for depression, anxiety, or fatigue at baseline or during follow-up prevents us from excluding the possibility that improvements in sleep quality were secondary outcomes.
Conclusions
The results of this study suggest that WMT could be a promising intervention for enhancing the sleep quality of patients with IBD, especially those with initially poorer sleep quality who may experience particularly significant improvements. These findings provide valuable insights for clinicians and highlight the potential of WMT as a clinical intervention for addressing poor sleep in IBD patients.
Abbreviations
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; FMT, faecal microbiota transplantation; WMT, washed microbiota transplantation; PSQI, Pittsburgh sleep quality index; HBI, Harvey–Bradshaw index; PMS, partial Mayo score; TET, transendoscopic enteral tubing; AEs, adverse events.
Data Sharing Statement
Data of this study will be available upon request from the corresponding author.
Ethics Approval
The present study was strictly conducted in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards. Approval of the clinical study was granted by the institutional ethics committee of the Second Affiliated Hospital of Nanjing Medical University.
Acknowledgments
The authors are grateful to all participants of the study and appreciate the kindly help from Jie Zhang for providing data from China Microbiota Transplantation System (www.fmtbank.org).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Natural Science Foundation of China (81873548, 81600417); the National Clinical Research Center for Digestive Diseases, Xi’an, China (2015BAI13B07); and Nanjing Medical University Fan Daiming Research Funds for Holistic Integrative Medicine.
Disclosure
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–317. doi:10.1038/nature10209
2. Ballesio A, Zagaria A, Baccini F, Micheli F, Di Nardo G, Lombardo C. A meta-analysis on sleep quality in inflammatory bowel disease. Sleep Med Rev. 2021;60:101518. doi:10.1016/j.smrv.2021.101518
3. D’Silva A, Fox DE, Nasser Y, et al. Prevalence and risk factors for fatigue in adults with inflammatory Bowel Disease: a systematic review with meta-analysis. Clin Gastroenterol Hepatol. 2022;20(5):995–1009e7. doi:10.1016/j.cgh.2021.06.034
4. Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22(11):1748–1753. doi:10.1111/j.1440-1746.2006.04820.x
5. Zimmerman J. Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: nature, severity, and relationship to gastrointestinal symptoms. Dig Dis Sci. 2003;48(4):743–749. doi:10.1023/a:1022840910283
6. Benhayon D, Youk A, McCarthy FN, et al. Characterization of relations among sleep, inflammation, and psychiatric dysfunction in depressed youth with Crohn disease. J Pediatr Gastroenterol Nutr. 2013;57(3):335–342. doi:10.1097/MPG.0b013e31829641df
7. Aslan CNN, Acik M, TertemIz OF, et al. Effect of prebiotic and probiotic supplementation on reduced pain in patients with fibromyalgia syndrome: a double-blind, placebo-controlled randomized clinical trial. Psychol Health Med. 2023:1–14. doi:10.1080/13548506.2023.2216464
8. Freijy TM, Cribb L, Oliver G, et al. Effects of a high-prebiotic diet versus probiotic supplements versus synbiotics on adult mental health: the “Gut Feelings” randomised controlled trial. Front Neurosci. 2022;16:1097278. doi:10.3389/fnins.2022.1097278
9. Li Q, Ding X, Liu K, et al. Fecal microbiota transplantation for ulcerative colitis: the optimum timing and gut microbiota as predictors for long-term clinical outcomes. Clin Transl Gastroenterol. 2020;11(8):e00224. doi:10.14309/ctg.0000000000000224
10. Zhang T, Lu G, Zhao Z, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020. doi:10.1007/s13238-019-00684-8
11. Ding X, Li Q, Li P, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019;42(7):869–880. doi:10.1007/s40264-019-00809-2
12. Lu G, Wang W, Li P, Wen Q, Cui B, Zhang F. Washed preparation of faecal microbiota changes the transplantation related safety, quantitative method and delivery. Microb Biotechnol. 2022;15(9):2439–2449. doi:10.1111/1751-7915.14074
13. Fecal Microbiota Transplantation-standardization Study Group. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J. 2020;133(19):2330–2332. doi:10.1097/CM9.0000000000000954
14. Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506–512. doi:10.1016/j.jad.2018.04.038
15. Zhang Z, Li Q, Zhang S, et al. Washed microbiota transplantation targeting both gastrointestinal and extraintestinal symptoms in patients with irritable bowel syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2023;127:110839. doi:10.1016/j.pnpbp.2023.110839
16. Pan ZY, Zhong HJ, Huang DN, Wu LH, He XX. Beneficial effects of repeated washed microbiota transplantation in children with autism. Front Pediatr. 2022;10:928785. doi:10.3389/fped.2022.928785
17. Wang J, Li Q, Huang Q, et al. Washed microbiota transplantation accelerates the recovery of abnormal changes by light-induced stress in tree shrews. Front Cell Infect Microbiol. 2021;11:685019. doi:10.3389/fcimb.2021.685019
18. Uemura R, Fujiwara Y, Iwakura N, et al. Sleep disturbances in Japanese patients with inflammatory bowel disease and their impact on disease flare. Springerplus. 2016;5(1):1792. doi:10.1186/s40064-016-3408-6
19. Swanson GR, Burgess HJ, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev Clin Immunol. 2011;7(1):29–36. doi:10.1586/eci.10.83
20. Navabi S, Gorrepati VS, Yadav S, et al. Influences and impact of anxiety and depression in the setting of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24(11):2303–2308. doi:10.1093/ibd/izy143
21. Szigethy E, Murphy SM, Ehrlich OG, et al. Mental health costs of Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2021;27(1):40–48. doi:10.1093/ibd/izaa030
22. Hertenstein E, Feige B, Gmeiner T, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105. doi:10.1016/j.smrv.2018.10.006
23. Sen P, Molinero-Perez A, O’Riordan KJ, McCafferty CP, O’Halloran KD, Cryan JF. Microbiota and sleep: awakening the gut feeling. Trends Mol Med. 2021;27(10):935–945. doi:10.1016/j.molmed.2021.07.004
24. Li Q, Zhang T, Ding X, et al. Enhancing patient adherence to fecal microbiota transplantation maintains the long-term clinical effects in ulcerative colitis. Eur J Gastroenterol Hepatol. 2020. doi:10.1097/MEG.0000000000001725
25. Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015;13:298. doi:10.1186/s12967-015-0646-2
26. Liu Y, Ji X, Huang Y, et al. Older patients benefit more from sequential courses of washed microbiota transplantation than younger population with ulcerative colitis. Scand J Gastroenterol. 2023;58(8):890–899. doi:10.1080/00365521.2023.2185476
27. Long C, Yu Y, Cui B, et al. A novel quick transendoscopic enteral tubing in mid-gut: technique and training with video. BMC Gastroenterol. 2018;18(1):37. doi:10.1186/s12876-018-0766-2
28. Peng Z, Xiang J, He Z, et al. Colonic transendoscopic enteral tubing: a novel way of transplanting fecal microbiota. Endosc Int Open. 2016;4(6):E610–E613. doi:10.1055/s-0042-105205
29. Xiang L, Ding X, Li Q, et al. Efficacy of faecal microbiota transplantation in Crohn’s disease: a new target treatment? Microb Biotechnol. 2020;13(3):760–769. doi:10.1111/1751-7915.13536
30. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
31. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
32. Jeppesen P, Wolf RT, Nielsen SM, et al. Effectiveness of transdiagnostic cognitive-behavioral psychotherapy compared with management as usual for youth with common mental health problems: a randomized clinical trial. JAMA Psychiatry. 2021;78(3):250–260. doi:10.1001/jamapsychiatry.2020.4045
33. Brydges CR. Effect size guidelines, sample size calculations, and statistical power in gerontology. Innov Aging. 2019;3(4):igz036. doi:10.1093/geroni/igz036
34. Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015;76(7):e857–e861. doi:10.4088/JCP.15f10150
35. Hashash JG, Knisely MR, Germain A, et al. Brief behavioral therapy and bupropion for sleep and fatigue in young adults with Crohn’s disease: an exploratory open trial study. Clin Gastroenterol Hepatol. 2022;20(1):96–104. doi:10.1016/j.cgh.2020.09.047
36. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504.
37. Liu NH, Liu HQ, Zheng JY, et al. Fresh washed microbiota transplantation alters gut microbiota metabolites to ameliorate sleeping disorder symptom of autistic children. J Microbiol. 2023. doi:10.1007/s12275-023-00069-x
38. Sun N, Hu H, Wang F, et al. Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav Immun. 2021;92:102–114. doi:10.1016/j.bbi.2020.11.032
39. Ogawa Y, Miyoshi C, Obana N, et al. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci Rep. 2020;10(1):19554. doi:10.1038/s41598-020-76562-9
40. Wang X, Wang Z, Cao J, Dong Y, Chen Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 2023;11(1):17. doi:10.1186/s40168-022-01452-3
41. Lee HJ, Hong JK, Kim JK, et al. Effects of probiotic NVP-1704 on mental health and sleep in healthy adults: an 8-week randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13(8). doi:10.3390/nu13082660
42. Thompson RS, Gaffney M, Hopkins S, et al. Ruminiclostridium 5, Parabacteroides distasonis, and bile acid profile are modulated by prebiotic diet and associate with facilitated sleep/clock realignment after chronic disruption of rhythms. Brain Behav Immun. 2021;97:150–166. doi:10.1016/j.bbi.2021.07.006
43. Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11(8):965–971. doi:10.1016/j.cgh.2013.01.021
44. Hao G, Zhu B, Li Y, Wang P, Li L, Hou L. Sleep quality and disease activity in patients with inflammatory bowel disease: a systematic review and meta-analysis. Sleep Med. 2020;75:301–308. doi:10.1016/j.sleep.2020.08.032
45. Ali T, Madhoun MF, Orr WC, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19(11):2440–2443. doi:10.1097/MIB.0b013e3182a0ea54
46. Iskandar HN, Linan EE, Patel A, et al. Self-reported sleep disturbance in Crohn’s disease is not confirmed by objective sleep measures. Sci Rep. 2020;10(1):1980. doi:10.1038/s41598-020-58807-9
47. Barnes A, Mountifield R, Baker J, Spizzo P, Bampton P, Mukherjee S. Systematic review and meta-analysis of sleep quality in inactive inflammatory bowel disease. JGH Open. 2022;6(11):738–744. doi:10.1002/jgh3.12817
48. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi:10.1111/jsr.12594
49. Doghramji K, Jangro WC. Adverse effects of psychotropic medications on sleep. Psychiatr Clin North Am. 2016;39(3):487–502. doi:10.1016/j.psc.2016.04.009
50. Reynolds AC, Sweetman A, Crowther ME, et al. Is cognitive behavioral therapy for insomnia (CBTi) efficacious for treating insomnia symptoms in shift workers? A systematic review and meta-analysis. Sleep Med Rev. 2022;67:101716. doi:10.1016/j.smrv.2022.101716
51. Cheung JMY, Jarrin DC, Ballot O, Bharwani AA, Morin CM. A systematic review of cognitive behavioral therapy for insomnia implemented in primary care and community settings. Sleep Med Rev. 2019;44:23–36. doi:10.1016/j.smrv.2018.11.001
52. Zhang F, Lu G, Wang X, Wu L, Li R, Nie Y. Concept, breakthrough, and future of colonic transendoscopic enteral tubing. Chin Med J. 2024;137(6):633–635. doi:10.1097/CM9.0000000000003020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



