Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Xanomeline and Trospium Chloride Versus Placebo for the Treatment of Schizophrenia: A Post Hoc Analysis of Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed
Authors Citrome L , Neugebauer NM, Meli AA, Kando J
Received 29 October 2024
Accepted for publication 12 March 2025
Published 5 April 2025 Volume 2025:21 Pages 761—773
DOI https://doi.org/10.2147/NDT.S503494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Taro Kishi
Leslie Citrome,1 Nichole M Neugebauer,2 Alicia A Meli,2 Judith Kando2
1Department of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla, NY, USA; 2Medical Affairs, Bristol Myers Squibb, Princeton, NJ, USA
Correspondence: Leslie Citrome, Department of Psychiatry and Behavioral Sciences, New York Medical College, 40 Sunshine Cottage Road, Valhalla, NY, 10595, USA, Email [email protected]
Purpose: Describe xanomeline and trospium chloride efficacy and safety/tolerability for the treatment of schizophrenia using number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH).
Methods: Categorical data were extracted from the three 5-week, randomized, double blind, placebo controlled EMERGENT-1, EMERGENT-2, and EMERGENT-3 clinical trials of xanomeline/trospium in adults with schizophrenia experiencing acute psychosis. Efficacy was assessed using the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impression–Severity (CGI-S), and categorical response criteria. Safety and tolerability were assessed using rates of discontinuation and treatment-emergent adverse events (TEAEs). NNT, NNH, and LHH values were calculated for each individual study as well as pooled.
Results: In data from the acute EMERGENT trials, NNT estimates were significant for xanomeline/trospium vs placebo for the pre-specified treatment response threshold of ≥ 30% reduction from baseline in PANSS total score at Week 5 (NNT=5 [95% CI, 4– 8]). NNT estimates for response thresholds of ≥ 20% and ≥ 40% reduction from baseline in PANSS total score and ≥ 1- and ≥ 2-point decrease from baseline in CGI-S score were < 10, indicating a clinically relevant therapeutic benefit of xanomeline/trospium over placebo. Estimates of NNH vs placebo for the most common TEAEs were > 10, with the exception of nausea and vomiting; however, rates of discontinuations due to TEAEs of nausea, dyspepsia, or vomiting were low (NNH=49 [95% CI, 28– 182]). LHH indicated an overall benefit of xanomeline/trospium vs placebo for all assessed outcomes. In indirect comparisons based on published data from trials of available antipsychotics approved for schizophrenia, xanomeline/trospium exhibited comparable or more robust NNT estimates vs placebo and was the least likely agent to be associated with weight gain or somnolence/sedation.
Conclusion: In the 5-week EMERGENT clinical trials, NNT, NNH, and LHH assessments demonstrated a favorable benefit-risk profile for xanomeline/trospium.
Trial Registration: ClinicalTrials.gov identifiers: NCT03697252, NCT04659161, NCT04738123.
Keywords: antipsychotic, xanomeline and trospium chloride, KarXT, schizophrenia, number needed to treat, number needed to harm
Introduction
The limitations of available antipsychotic medications are well known, and new treatments with broader efficacy and improved tolerability are an area of significant unmet need for individuals living with schizophrenia. Xanomeline and trospium chloride, formerly known as KarXT, has been assessed in three clinical trials of adults with acute exacerbations of schizophrenia and is an approved treatment for this population and an investigational therapy for psychosis in Alzheimer’s disease.1–4 A novel mechanism of action distinguishes xanomeline/trospium from previously approved antipsychotics: xanomeline is a dual M1/M4 preferring muscarinic receptor agonist without any binding affinity to the dopamine D2 receptor.5–7 Xanomeline is combined with the peripherally restricted pan-muscarinic receptor antagonist trospium to reduce unwanted cholinergic side effects elicited by xanomeline activity in the periphery.8,9
The clinical development program assessing the efficacy and safety of xanomeline/trospium in schizophrenia included three 5-week, randomized, double-blind, placebo-controlled trials of nearly identical design: the Phase 2 EMERGENT-1 (NCT03697252)1 and Phase 3 EMERGENT-2 (NCT04659161)2 and EMERGENT-3 (NCT04738123)3 trials. The primary endpoint of change from baseline to Week 5 in Positive and Negative Syndrome Scale (PANSS) total score with xanomeline/trospium vs placebo was met in all three trials, demonstrating statistically significant improvement in symptoms of schizophrenia with xanomeline/trospium compared with placebo. Consistent with the activity of xanomeline and trospium at muscarinic receptors, the most common side effects observed with treatment were primarily gastrointestinal, such as constipation and nausea, mild to moderate in intensity, and transient. Notably, xanomeline/trospium was not associated with clinically meaningful adverse events (AEs) commonly observed with currently available antipsychotics, including extrapyramidal movement disorders, hyperprolactinemia, weight gain, metabolic side effects, or somnolence/sedation.10,11 Based on the results of the acute EMERGENT trials, the US Food and Drug Administration (FDA) awarded xanomeline/trospium approval for the treatment of schizophrenia in adults in September 2024.
Evidence-based medicine metrics such as number needed to treat (NNT) and number needed to harm (NNH) can aid physicians in assessing the clinical relevance of results from clinical trials.12 NNT and NNH are measures of effect size and indicate how many patients would need to be treated with one intervention (such as a medication) instead of the comparator (such as another medication or placebo) to encounter one additional outcome of interest. NNT and NNH can also be used to quantify benefit vs risk by calculating the ratio of NNH to NNT (likelihood to be helped or harmed [LHH]). In general, a LHH >1 would mean the likelihood to be helped is greater than the likelihood to be harmed. For a LHH <1, the reverse is true. For a LHH to be meaningful, the efficacy outcome and adverse outcome must be clinically relevant for the person being treated. In this analysis, NNT, NNH, and LHH estimates were calculated for xanomeline/trospium vs placebo and indirectly compared with available oral, first-line, second-generation antipsychotics approved for the treatment of schizophrenia, including aripiprazole, asenapine, brexpiprazole, cariprazine, iloperidone, lumateperone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone, based on previously published data for these agents.
Methods
Trial Design
The pivotal EMERGENT-1, EMERGENT-2, and EMERGENT-3 trials were nearly identical 5-week, randomized, double-blind, placebo-controlled trials of xanomeline/trospium in adults with schizophrenia experiencing acute psychosis.1–3 Briefly, the trials enrolled participants aged 18–60 years (EMERGENT-1) and 18–65 years (EMERGENT-2 and EMERGENT-3) with a confirmed diagnosis of schizophrenia based on criteria in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition,13 a PANSS total score of 80–120 (higher scores reflect greater symptom severity),14 and a recent worsening of psychosis requiring hospitalization. Enrolled participants were randomized 1:1 to receive twice-daily treatment with placebo or xanomeline/trospium starting at a dose of 50-mg xanomeline/20-mg trospium chloride and titrating up to a maximum dose of 125-mg xanomeline/30-mg trospium chloride over a period of 7 days.
All trials were conducted in accordance with the principles of the Declaration of Helsinki, the International Council of Harmonization guidelines for Good Clinical Practice, and regulations of the countries in which the trials took place.1–3 Protocols were approved by centralized institutional review boards in the United States (EMERGENT-1, Copernicus Group, Cary, NC; EMERGENT-2 and EMERGENT-3, WCG IRB, Puyallup, WA) and local ethics committees at trial sites in Ukraine, and all participants submitted informed consent prior to enrollment. Trials followed guidelines for the Consolidated Standards of Reporting Trials (CONSORT).
Outcomes
Efficacy data were extracted from the EMERGENT-1, EMERGENT-2, and EMERGENT-3 trials. In the trials, PANSS assessments were made at Weeks 2, 3 (EMERGENT-2 and EMERGENT-3 only), 4, and 5. Clinical Global Impression–Severity (CGI-S) scores were assessed weekly at Weeks 1 through 5. Data were collected for participants classified as responders at endpoint (Week 5 or early termination [using a last observation carried forward approach]) as assessed using both PANSS total and CGI-S score response criteria thresholds: PANSS total score reductions from baseline to endpoint of ≥20%, ≥30%, ≥40%, and ≥50% and CGI-S score of ≤2 (not at all ill or borderline ill) or ≤3 (not at all ill, borderline ill, or mildly ill) at endpoint.15 Additionally, data were collected for participants who achieved a decrease from baseline in CGI-S score of ≥1, ≥2, or ≥3 points. The denominator for all efficacy outcome measures was the number of participants who had a baseline and ≥1 post-baseline PANSS assessment (the modified intention-to-treat [mITT] population).
Safety and tolerability data were extracted from the three pivotal EMERGENT trials including specific outcomes of clinical interest occurring at any point during the double-blind treatment period. Data were collected for the following safety and tolerability outcomes: discontinuations due to treatment-emergent AEs (TEAEs); dose reductions due to TEAEs; both discontinuations and dose reductions due to a TEAE of nausea, dyspepsia, or vomiting; TEAEs occurring in ≥5% of participants; TEAEs relevant to indirect comparisons with other second-generation antipsychotics, including ≥7% weight increase, akathisia, and somnolence/sedation; participants exhibiting shifts at any time in electrocardiogram QTc interval from <450 msec to ≥450 msec, in total cholesterol from <240 mg/dL to ≥240 mg/dL, in fasting glucose from <126 mg/dL to ≥126 mg/dL, or in fasting triglycerides from <200 mg/dL to ≥200 mg/dL; plasma prolactin values of ≥17 ng/mL (men) and ≥25 ng/mL (women); and plasma prolactin values of ≥34 ng/mL (men) and ≥50 ng/mL (women). Prolactin was assessed in EMERGENT-2 and EMERGENT-3 only. The denominator for AE analyses was the number of randomized participants who received ≥1 dose of trial drug (safety population). The denominator for weight and laboratory variables was the number of participants who had a baseline and ≥1 post-baseline value for a variable while on treatment, and in the case of shifts, met the defined starting threshold.
Data Analysis
NNT estimates, with their respective 95% CIs, were calculated for xanomeline/trospium vs placebo using previously published formulas (Box 1) and pooled when all data elements were available for each trial.16 NNH estimates, with their respective 95% CIs, were also calculated for xanomeline/trospium vs placebo and pooled using all available data.
 |
Box 1 Formulae Used for Calculating NNT, NNH, and LHH |
LHH was calculated for xanomeline/trospium to illustrate potential trade-offs for efficacy and tolerability outcomes, specifically response (≥30% reduction from baseline in the total PANSS score at endpoint [Week 5 or early termination]) vs discontinuation from the trial because of a TEAE; discontinuation from the trial because of an AE of nausea, dyspepsia, or vomiting; dose reduction because of an AE; dose reduction because of an AE of nausea, dyspepsia, or vomiting; weight gain ≥7%; somnolence/sedation AEs; akathisia AEs; and all AEs occurring in ≥5% of participants receiving xanomeline/trospium and at a rate at least twice that observed in the placebo group (nausea, constipation, dyspepsia, vomiting, hypertension, dry mouth, tachycardia). When a NNH estimate is a negative number (ie, when the rate of the AE is higher with placebo than with xanomeline/trospium), the LHH calculation is uninterpretable. In such instances, we took the customary approach to impute a value of 1000 for the NHH to quantify LHH.17,18
If the 95% CI for the NNT or NNH estimate included “infinity” the result was considered not statistically significant at the P<0.05 threshold, and the notation “ns” was used rather than displaying two non-continuous domains of numbers ranging to infinity or negative infinity. The terms “statistically significant” and “non-statistically significant” were used descriptively rather than inferentially. In general, data pooling increases the sample size and narrows the 95% CI, leading to more precise estimates of effect size and these results are included in the body of this report; the results from the individual studies are included in the Supplementary Appendix, including baseline characteristics (Tables S1a, S1b, and S1c), efficacy outcomes (Tables S2a, S2b, and S2c), and safety/tolerability outcomes (Tables S3a, S3b, S3c, S4a, S4b, and S4c). Additional pooled data can also be found in Tables S2d, S3d, and S4d.
Indirect comparisons with other agents used to treat schizophrenia were possible because similar analyses have been performed with most second-generation antipsychotics, including all currently available branded single-agent oral products.19–22
Results
Participants
A total of 683 participants (xanomeline/trospium, n=340; placebo, n=343) were included in the pooled safety population; there were no meaningful differences in demographics and baseline characteristics between treatment groups (Table 1; see Supplementary Appendix for the demographics and baseline characteristics from the individual studies). Efficacy outcomes were examined in the mITT population (xanomeline/trospium, n=314; placebo, n=326).
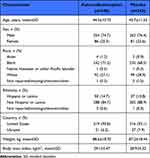 |
Table 1 Demographics and Baseline Characteristics (Safety Population) |
Efficacy
The majority of the NNT estimates for xanomeline/trospium compared with placebo were low (<10). NNT for the outcome measure of ≥30% reduction from baseline to Week 5 in PANSS total score was 5 (95% CI, 4–8); the NNT for outcomes of ≥20% and ≥40% reduction from baseline to Week 5 in PANSS total score were similar, at 5 (95% CI, 4–7) and 8 (95% CI, 6–16), respectively (Table 2; see Supplementary Appendix for results from the individual studies). NNT for xanomeline/trospium compared with placebo for the outcomes of CGI-S score of ≤3 (representing severities of not at all ill, borderline ill, or mildly ill)15 was 8 (95% CI, 5–14) and <10 for CGI-S decreases from baseline of both ≥1 and ≥2 points. Only the highest threshold outcomes, ie, ≥50% reduction from baseline to Week 5 in PANSS total score, ≥3-point decrease from baseline to Week 5 in CGI-S score, and endpoint CGI-S score of ≤2 (not at all ill or borderline ill) yielded NNT estimates >10.
 |
Table 2 NNT Based on PANSS Total and CGI-S Score Criteria (mITT Population) |
Tolerability
Xanomeline/trospium NNH estimates based on TEAEs and laboratory or metabolic endpoints were generally high (>10). The proportions of participants who discontinued treatment due to a TEAE were low and similar between xanomeline/trospium and placebo groups (5.6% vs 4.7%), and the NNH compared with placebo was not significant (Table 3; see Supplementary Appendix for results from the individual studies). The NNH estimates for nausea and vomiting were <10; however, the NNH estimate based on discontinuation due to a TEAE of nausea, dyspepsia, or vomiting was 49, and the NNH estimate based on dose reductions due to the same TEAEs was 170 and non-significant compared with placebo. NNH estimates for all the other most common TEAEs were ≥10. NNH estimates based on metabolic and clinical laboratory outcomes either exceeded 10 or the event rate was higher with placebo than with xanomeline/trospium, resulting in negative NNH values (Table 4).
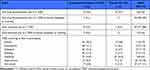 |
Table 3 NNH Based on TEAE (Safety Population) |
 |
Table 4 NNH Based on Shift in QTc Interval, Weight, Metabolic Variables, and Prolactin (Safety Population) |
Likelihood to Be Helped or Harmed
LHH is a ratio of NNT to NNH and reflects the relative benefit vs risk of harm associated with treatment. LHH estimates for the most common TEAEs in the acute EMERGENT trials and those of special interest vs treatment response (defined as ≥30% reduction from baseline in PANSS total score) are presented in Table 5. LHH was >1 for all AEs, indicating that a response is expected to occur more often than any single harm. Regarding discontinuations, the LHH estimate of 21.8 for trial drug discontinuation due to a TEAE suggests that a response is nearly 22× more likely to occur than a discontinuation due to a TEAE. The trend toward greater likelihood of a response vs TEAE remains, albeit to a lesser degree, when considering discontinuation due to a TEAE of nausea, dyspepsia, or vomiting (LHH=9.8). Among the most common TEAEs reported in the acute EMERGENT trials (listed in Table 3) and those of somnolence/sedation, akathisia, and tachycardia, nausea was the most likely to be encountered but was still less common than a response (LHH=1.4); somnolence/sedation was the least likely to occur (LHH=16.6). Weight gain was a highly uncommon event, approximately 200× less likely to occur than a treatment response.
 |
Table 5 Likelihood to Be Helped or Harmed |
Indirect Comparisons
Indirect comparisons of effect size indicate a NNT vs placebo for xanomeline/trospium that is comparable to, or more robust than, other oral, first-line, second-generation agents that are approved by the US Food and Drug Administration for schizophrenia and for which similar data are available (Figure 1). NNT estimates for currently available antipsychotics were calculated using data collected from the pivotal trials of each compound that informed product labeling.20–29 In the published analyses, response was defined as ≥30% reduction from baseline in PANSS total score in the trials of xanomeline/trospium, asenapine, cariprazine, lumateperone, lurasidone, paliperidone, quetiapine extended release, and one of the three pivotal trials of ziprasidone, and as ≥30% reduction from baseline in PANSS total score or a CGI-S score of 1 or 2 for aripiprazole and brexpiprazole.20–29 Although CIs overlap across agents, the NNT estimate of 5 places xanomeline/trospium within the lower range of values (ie, a more robust effect size) calculated for currently available antipsychotics (Figure 1).
 |
Figure 1 Indirect comparisons of efficacy vs placebo. Abbreviations: CGI-S, Clinical Global Impression–Severity; CI, confidence interval; NNT, number needed to treat; PANSS, Positive and Negative Syndrome Scale; XR, extended release. Notes: aResponse defined as ≥30% reduction from baseline in PANSS total score (xanomeline/trospium, asenapine,24,25 cariprazine,21 lumateperone,22 lurasidone,26 paliperidone,29 quetiapine XR,28 and 1 of the 3 pivotal trials of ziprasidone)23 and as ≥30% reduction from baseline in PANSS total score or a CGI-S score of 1 (very much improved) or 2 (much improved; aripiprazole,27 brexpiprazole).20 Data are not available for the pivotal trials of risperidone, olanzapine, quetiapine immediate release, or iloperidone. Figure adapted from Citrome L, Durgam S, Edwards JB, et al. Lumateperone for the treatment of schizophrenia: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(2):22r14631.30 |
A heat map for NNH for the outcomes often observed with antipsychotic use of weight gain ≥7%, somnolence/sedation, and akathisia for xanomeline/trospium and oral, first-line, second-generation antipsychotics vs placebo is shown in Table 6. NNH estimates calculated from placebo-controlled trials in adults with acutely exacerbated schizophrenia symptoms20–29,31–34 were <10 for somnolence/sedation for lumateperone and quetiapine extended release and ≥7% for weight gain for olanzapine and quetiapine immediate release. In this indirect comparison, xanomeline/trospium was one of only three interventions, including aripiprazole and paliperidone, to exhibit a low risk (NNH ≥20) for all three of the outcomes in question. Xanomeline/trospium was the only agent that showed no difference from placebo in terms of weight gain ≥7%, and it displayed relatively high NNH estimates of 83 and 68 for somnolence/sedation and akathisia, respectively.
 |
Table 6 Heat Map of Indirect Comparisons of Acute Tolerability |
Discussion
The inferential statistics used in registrational clinical trials provide a method for distinguishing a probable outcome different from a chance finding but do not necessarily reflect the outcome’s clinical relevance. The clinical relevance of a treatment outcome can be appraised using NNT and NNH effect sizes expressed in clinically intuitive “patient units” that provide measures of a therapy’s relative advantages vs risks. Outside of the clinic, NNT and NNH are also of potential use to regulators assessing benefit: risk ratios during the drug approval process.35 When interpreting these metrics, lower values represent a greater treatment benefit than higher values for NNT estimates for the outcome of a response vs placebo, and estimates of <10 are preferable.12 NNH estimates ≥10 are generally preferred, although lower values may be acceptable for AEs that are mild or moderate, short-lived, do not result in treatment discontinuation, or do not pose a serious health risk.
Here, NNT estimates with xanomeline/trospium for most efficacy outcomes were <10, suggesting the efficacy demonstrated in the acute EMERGENT trials is clinically relevant. NNT was >10 for the efficacy outcomes of ≥50% reduction from baseline to Week 5 in PANSS total score, ≥3-point decrease from baseline to Week 5 in CGI-S score, and endpoint CGI-S score of ≤2, three outcomes that could be considered unusually robust for a 5-week clinical trial. Equally notable is the relative absence of side effects associated with currently available antipsychotics used to treat schizophrenia, such as weight gain/metabolic abnormalities, sedation/somnolence, akathisia, elevation in prolactin, or prolongation of the electrocardiogram QT interval, as quantified by the NNH estimates reported here. The most common AEs with xanomeline/trospium reported in the acute EMERGENT trials were primarily gastrointestinal, mild or moderate in intensity, and transient.1–3 Gastrointestinal symptoms are a major factor in non-adherence with other types of medications and even moderate symptoms could potentially interfere with xanomeline/trospium adherence.36 Here, two TEAEs, nausea and vomiting, had NNH estimates <10 in our analyses; however, the NNH for discontinuation due to a TEAE vs placebo was 109 (not significant) and the NNH for discontinuation due specifically to a TEAE of nausea, dyspepsia, or vomiting vs placebo remained high at 49, suggesting the TEAEs that occurred were manageable or of low enough intensity so as not to interfere with treatment.
LHH is the ratio of NNT to NNH and provides a measure of the relative likelihood of encountering a therapeutic benefit versus an undesirable event or harm. Xanomeline/trospium LHH estimates were >1 for all outcomes measured, meaning that a therapeutic benefit was more likely to be encountered than an adverse outcome. LHH estimates for individual TEAEs range from 1.4 for nausea, one of the most common TEAEs observed in the clinical trials, to 16.6 for somnolence/sedation, a common and bothersome side effect of many available antipsychotics.10,11 The LHH of 200 for clinically significant weight gain (≥7% body weight gain) provides a significant contrast with the relatively high risk of clinically significant weight gain associated with many second-generation antipsychotics.10,11 Taken together, in the present analysis xanomeline/trospium demonstrates the dual attributes of treatment benefit and acceptable tolerability, which to date have been an area of considerable unmet need in schizophrenia. Most available antipsychotics rely on a similar mechanism of antagonism or partial agonism of dopamine D2 receptors and, as a result, exhibit overlapping limitations in efficacy and safety. Xanomeline/trospium, in contrast, is a potent agonist of muscarinic M1/M4 receptors and exhibits no direct dopamine D2 receptor activity.37,38 The novel mechanism may underlie the advantageous benefit to risk profile observed here.
Using indirect comparisons, the xanomeline/trospium NNT estimate of 5 for response over placebo positions this agent among the more efficacious of oral, first-line, second-generation antipsychotics. These findings parallel efficacy analyses based on treatment effect size using standardized mean difference for the continuous measures: in clinical trials, the PANSS total score effect size of 0.65 (in standard deviation units) for xanomeline/trospium compares favorably to the median effect size of 0.42 reported in a meta-analysis of 32 antipsychotics.10,39 Additionally, xanomeline/trospium demonstrated improved tolerability vs the comparators. Tolerability is a major concern in schizophrenia treatment, and side effects contribute to a medication non-adherence rate of approximately 50%.40,41 Among the most common side effects is weight gain, and approximately half of individuals using antipsychotics are considered overweight/obese.42 Weight gain is a source of reduced quality of life and places people treated with antipsychotics at increased risk of cardiovascular morbidity and mortality.43,44 Somnolence/sedation is another side effect observed with nearly all antipsychotics and may impair social and work-related functioning.10,11 Motor side effects are reported by approximately 22% of people treated with second generation antipsychotics,45 may become both serious and chronic, and contribute to poor health outcomes.46–48 In the present analysis, xanomeline/trospium is the only treatment that does not differ from placebo in terms of NNH for ≥7% weight gain and has the highest NNH estimate for somnolence and/or sedation in comparison with available second-generation antipsychotics. While the risk of akathisia with xanomeline/trospium was greater than placebo, the NNH estimate of 68 suggests this risk is low. Overall, the combination of a favorable efficacy profile and low side effect risk distinguishes xanomeline/trospium from comparator agents in these indirect analyses.
This study has several limitations. First, as is necessary in calculating NNT and NNH, the results reported here are restricted to dichotomous outcomes, with the inherent loss of precision that could otherwise be retained with the use of effect size measures such as Cohen’s d for continuous variables. Second, the short duration of the clinical trials prevents examination of NNH for AEs that may manifest after prolonged treatment. Third, the applicability of these results beyond the research environment is uncertain; individuals living with schizophrenia in the general population typically differ from those individuals who meet the strict inclusion and exclusion criteria for participation in clinical trials; also, participants discontinue clinical trials for myriad and complex reasons and as such the NNH estimates of discontinuation due to AEs may not accurately reflect tolerability in a clinical setting. Moreover, this trial enrolled participants from the United States and Ukraine, which may limit the generalizability of the current findings in other countries. However, recent analyses demonstrated persistent efficacy of xanomeline/trospium across a broad range of demographic and baseline subgroups in the EMERGENT trials.39 In addition, NNH and NNT estimates should be interpreted in the context of the underlying percentages because similar estimates may derive from quite different clinical scenarios. For example, a NNT estimate of 10 may result from comparing responder rates of 80% vs 90% or 10% vs 20%. Lastly, indirect comparisons of NNT, NNH, and LHH with other antipsychotics must be regarded with caution due to differences in study design, including trial population characteristics and treatment dosing and duration, as well as when the studies were conducted. Trials of several of the agents included in our analyses employed 6- or 4-week treatment periods as opposed to the 5 weeks used in the EMERGENT clinical program.
Conclusion
Xanomeline/trospium is an efficacious agent for the treatment of schizophrenia with an effect size vs placebo comparable to, or in some instances more robust than, currently available antipsychotics. The favorable safety profile is particularly noteworthy in that xanomeline/trospium exhibits a low risk for motor adverse effects and has the least likelihood to be associated with weight gain or somnolence/sedation among other first-line agents FDA approved for the treatment of schizophrenia.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, LC, upon reasonable request.
Acknowledgments
The authors thank the trial participants and investigators for their roles in this research. Medical writing and editorial support were provided by Sarah Marshall, PhD, and Paula Stuckart of Apollo Medical Communications, part of Helios Global Group, and funded by Karuna Therapeutics, a Bristol Myers Squibb company. Dr Marshall and Ms Stuckart have no conflicts of interest to disclose. Karuna Therapeutics, a Bristol Myers Squibb Company, funded the EMERGENT trials.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Citrome has received payment from Karuna Therapeutics, a Bristol Myers Squibb company, for the design and execution of this analysis and has received other advising, consulting, and/or speaker fees from AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, Biogen, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, Impel, INmune Bio, Intra-Cellular Therapies, Janssen, Karuna Therapeutics, a Bristol Myers Squibb company, Lundbeck, Luye, Lyndra, MapLight, Marvin, MedAvante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neumora, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sumitomo/Sunovion, Supernus, Takeda, Teva, University of Arizona, Vanda, and Wells Fargo, and performed one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research, CME activities organized by medical education companies such as HMP, Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies. He owns stocks from Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer and Reviva (options). Editor in Chief for Current Medical Research and Opinion, Taylor and Francis. Former Editor in Chief for International Journal of Clinical Practice, Wiley. Royalties from UpToDate, Springer Healthcare, and Elsevier. Dr. Neugebauer and Ms. Meli are employees of Bristol Myers Squibb. Dr. Kando is an employee of Bristol Myers Squibb and holds equity in Johnson and Johnson, McKesson, and Takeda Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Brannan SK, Sawchak S, Miller AC, Lieberman JA, Paul SM, Breier A. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717–726. doi:10.1056/NEJMoa2017015
2. Kaul I, Sawchak S, Correll CU, et al. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet. 2024;403(10422):160–170. doi:10.1016/S0140-6736(23)02190-6
3. Kaul I, Sawchak S, Walling DP, et al. Efficacy and safety of xanomeline-trospium chloride in schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2024;81(8):749–756. doi:10.1001/jamapsychiatry.2024.0785
4. NIH. A study to assess efficacy and safety of KarXT for the treatment of psychosis associated with Alzheimer’s disease (ADEPT-1). Available from: https://clinicaltrials.gov/study/NCT05511363.
5. Bodick NC, Offen WW, Levey AI, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54(4):465–473. doi:10.1001/archneur.1997.00550160091022
6. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033–1039. doi:10.1176/appi.ajp.2008.06091591
7. Shannon HE, Rasmussen K, Bymaster FP, et al. Xanomeline, an M(1)/M(4) preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr Res. 2000;42(3):249–259. doi:10.1016/s0920-9964(99)00138-3
8. Breier A, Brannan SK, Paul SM, Miller AC. Evidence of trospium’s ability to mitigate cholinergic adverse events related to xanomeline: Phase 1 study results. Psychopharmacology. 2023;240(5):1191–1198. doi:10.1007/s00213-023-06362-2
9. Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride is undetectable in the older human central nervous system. J Am Geriatr Soc. 2010;58(8):1618–1619. doi:10.1111/j.1532-5415.2010.02988.x
10. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. doi:10.1016/S0140-6736(19)31135-3
11. Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81(5):617–622.
12. Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407–411. doi:10.1111/ijcp.12142
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th Ed.). American Psychiatric Association; 2013.
14. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261
15. Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31(10):2318–2325. doi:10.1038/sj.npp.1301147
16. Citrome L, Juday T, Frech F, Atkins N Jr. Lemborexant for the treatment of insomnia: direct and indirect comparisons with other hypnotics using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2021;82:20m13795. doi:10.4088/JCP.20m13795
17. Citrome L, Sanchez Del Rio M, Dong Y, et al. Benefit-risk assessment of galcanezumab versus placebo for the treatment of episodic and chronic migraine using the metrics of number needed to treat and number needed to harm. Adv Ther. 2021;38(8):4442–4460. doi:10.1007/s12325-021-01848-x
18. Vo P, Wen S, Martel MJ, Mitsikostas D, Reuter U, Klatt J. Benefit-risk assessment of erenumab and current migraine prophylactic treatments using the likelihood of being helped or harmed. Cephalalgia. 2019;39(5):608–616. doi:10.1177/0333102418801579
19. Citrome L. Iloperidone for schizophrenia: a review of the efficacy and safety profile for this newly commercialised second-generation antipsychotic. Int J Clin Pract. 2009;63(8):1237–1248. doi:10.1111/j.1742-1241.2009.02142.x
20. Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978–997. doi:10.1111/ijcp.12714
21. Citrome L. Cariprazine for the treatment of schizophrenia: a review of this dopamine D3-preferring D3/D2 receptor partial agonist. Clin Schizophr Relat Psychoses. 2016;10(2):109–119. doi:10.3371/1935-1232-10.2.109
22. Citrome L, Durgam S, Edwards JB, Davis RE. Lumateperone for the treatment of schizophrenia: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(2). doi:10.4088/JCP.22r14631
23. Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20(5):491–505. doi:10.1016/S0893-133X(98)00090-6
24. Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract. 2009;63(12):1762–1784. doi:10.1111/j.1742-1241.2009.02228.x
25. Citrome L. Role of sublingual asenapine in treatment of schizophrenia. Neuropsychiatr Dis Treat. 2011;7:325–339. doi:10.2147/NDT.S16077
26. Citrome L. Lurasidone for the acute treatment of adults with schizophrenia: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Clin Schizophr Relat Psychoses. 2012;6(2):76–85. doi:10.3371/CSRP.6.2.5
27. Citrome L. The ABC’s of dopamine receptor partial agonists - aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int J Clin Pract. 2015;69(11):1211–1220. doi:10.1111/ijcp.12752
28. Kahn RS, Schulz SC, Palazov VD, et al. Efficacy and tolerability of once-daily extended release quetiapine fumarate in acute schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):832–842. doi:10.4088/jcp.v68n0603
29. Kantrowitz J, Citrome L. Paliperidone: the evidence of its therapeutic value in schizophrenia. Core Evid. 2008;2(4):261–271.
30. Citrome L, Durgam S, Edwards JB, et al. Lumateperone for the treatment of schizophrenia: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2023;84(2).
31. US Food and Drug Administration. Drug Approval Package. Zyprexa (olanzapine), Company: lilly. NDA 20-592. FDA website. Accessed Accessed June 9, 2024, https://pi.lilly.com/us/zyprexa-pi.pdf.
32. RISPERDAL. (risperidone) tablets, for oral use; RISPERDAL (risperidone) oral solution; RISPERDAL M-TAB (risperidone) orally disintegrating tablets. Product label. Janssen Pharmaceutical Companies website. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/RISPERDAL-pi.pdf.
33. US Food and Drug Administration. Drug Approval Package. Fanapt (iloperidone) tablets, Company: vanda Pharmaceuticals. Application No.: 022192. Available from: https://fanaptpro.com/wp-content/uploads/2016/02/Fanapt-Prescribing-Information.pdf.
34. SEROQUEL. (quetiapine) tablets, for oral use. Product label. US Food and Drug Administration website. Revised January 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020639s072lbl.pdf.
35. Mendes D, Alves C, Batel Marques F. Testing the usefulness of the number needed to treat to be harmed (NNTH) in benefit-risk evaluations: case study with medicines withdrawn from the European market due to safety reasons. Expert Opin Drug Saf. 2016;15(10):1301–1312. doi:10.1080/14740338.2016.1217989
36. Baryakova TH, Pogostin BH, Langer R, McHugh KJ. Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems. Nat Rev Drug Discov. 2023;22(5):387–409. doi:10.1038/s41573-023-00670-0
37. Mirza NR, Peters D, Sparks RG. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9(2):159–186. doi:10.1111/j.1527-3458.2003.tb00247.x
38. Thorn CA, Moon J, Bourbonais CA, et al. Striatal, hippocampal, and cortical networks are differentially responsive to the M4-and M1-muscarinic acetylcholine receptor mediated effects of xanomeline. ACS Chem Neurosci. 2019;10(3):1753–1764. doi:10.1021/acschemneuro.8b00625
39. Kaul I, Sawchak S, Claxton A, et al. Efficacy of xanomeline and trospium chloride in schizophrenia: pooled results from three 5-week, randomized, double-blind, placebo-controlled, EMERGENT trials. Schizophrenia. 2024;10(1):102. doi:10.1038/s41537-024-00525-6
40. Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. doi:10.4088/jcp.v63n1007
41. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388(10039):86–97. doi:10.1016/S0140-6736(15)01121-6
42. Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull. 2013;39(2):295–305. doi:10.1093/schbul/sbs082
43. De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Moller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European PSYCHIATRIC ASSociation (EPA), supported by the European association for the study of diabetes (EASD) and the European society of cardiology (ESC). Eur Psychiatry. 2009;24(6):412–424. doi:10.1016/j.eurpsy.2009.01.005
44. Townsend M, Pareja K, Buchanan-Hughes A, et al. Antipsychotic-related stigma and the impact on treatment choices: a systematic review and framework synthesis. Patient Prefer Adherence. 2022;16:373–401. doi:10.2147/PPA.S343211
45. Kadakia A, Brady BL, Dembek C, Williams GR, Kent JM. Burden of EPS in commercial patients with schizophrenia initiating atypical antipsychotics. Am J Manag Care. 2022;28(9):e315–e324. doi:10.37765/ajmc.2022.89163
46. Arya D, Khan T, Margolius AJ, Fernandez HH. Tardive dyskinesia: treatment update. Curr Neurol Neurosci Rep. 2019;19(9):69. doi:10.1007/s11910-019-0976-1
47. Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int. 2014;2014:656370. doi:10.1155/2014/656370
48. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi:10.1056/NEJMoa051688
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Aripiprazole Plasma Concentrations Delivered from Two 2-Month Long-Acting Injectable Formulations: An Indirect Comparison
Harlin M, Chepke C, Larsen F, Bell Lynum KS, Chumki SR, Fitzgerald H, Such P, Madera-McDonough J, Yildirim M, Panni M, Saklad SR
Neuropsychiatric Disease and Treatment 2023, 19:1409-1416
Published Date: 8 June 2023
Investigating the Effect of Adherence to Antipsychotic Therapy on the Length of Stay and Number of Hospitalizations in Patients with Schizophrenia – A Descriptive Analysis
Barliana MI, Ramdini DA, Afifah NN, Alfian SD, Sumiwi SA
Patient Preference and Adherence 2023, 17:2737-2747
Published Date: 1 November 2023

