Back to Journals » International Journal of General Medicine » Volume 17
A Comprehensive Analysis Exploring the Vital Role of the Systemic Immune-Inflammatory Index Upon Admission in Severe Hemorrhagic Fever with Renal Syndrome
Authors Yao L , Wang X, Wang Z , Wang X
Received 26 July 2024
Accepted for publication 19 October 2024
Published 23 October 2024 Volume 2024:17 Pages 4857—4866
DOI https://doi.org/10.2147/IJGM.S480204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Lihua Yao,1,2 Xinlu Wang,1 Zihao Wang,1 Xiaozhong Wang1
1Department of Clinical Laboratory, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, 330000, People’s Republic of China; 2Department of Clinical Laboratory, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, 637000, People’s Republic of China
Correspondence: Xiaozhong Wang, Department of Clinical Laboratory, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Mingde Road No. 1, Nanchang, Jiangxi, 330000, People’s Republic of China, Email [email protected]
Objective: To explore the value of the systemic immune-inflammatory index (SII) and the systemic inflammatory response index (SIRI) in patients with severe hemorrhagic fever with renal syndrome (HFRS) upon admission.
Methods: This study included a total of 165 patients with HFRS, who were divided into mild and severe groups based on the severity of the disease. By reviewing medical records, we collected the white blood cell (WBC), SII, and SIRI values of patients upon admission. Univariate and multivariate logistics regression analyses were performed to identify risk factors for severe HFRS. The receiver operating characteristic (ROC) curve was applied to calculate the area under the ROC curve (AUC) to analyze the predictive value of SII and SIRI for severe HFRS, and the results were compared with WBC and SIRI.
Results: Compared with the mild HFRS group, patients in the severe HFRS group had a longer duration of illness (P < 0.05), higher levels of WBC, neutrophil (NEUT), lymphocyte (LYMP), monocyte (MONO), procalcitonin (PCT), SIRI, alanine transaminase (ALT), and creatinine (Scr) (P < 0.05), while lower levels of ALB, platelet (PLT), platelet-to-lymphocyte rate (PLR), and SII, with statistically significant differences (P < 0.05). Binary logistics regression analysis indicated that WBC (OR: 1.190, 95% CI: 1.032– 1.371), SII (OR: 0.967, 95% CI: 0.951– 0.984), and SIRI (OR: 4.743, 95% CI: 2.077– 10.830) were risk factors for severe HFRS. The AUCs of WBC, SII, and SIRI for predicting severe HFRS were 0.765, 0.803, and 0.785, respectively.
Conclusion: Low levels of SII and high levels of WBC and SIRI upon admission are risk factors for severe HFRS and have certain value in predicting the progression of HFRS to severe cases, among which SII exhibits the best predictive value.
Keywords: hemorrhagic fever with renal syndrome, systemic immune inflammatory index, systemic immune-response index, risk factors, predictive score
Introduction
HFRS refers to a zoonotic disease caused by Hantavirus infection.1 Rodents are the main source of infection, and the body becomes infected by inhaling aerosols of virus-carrying rodent urine, saliva, feces, and other excretions.2 Despite the widespread promotion of vaccines, the incidence of HFRS in China is still on an upward trend.3 The main pathophysiology of HFRS is damage to the endothelial cells of systemic small blood vessels and capillaries, leading to vascular leakage, acute kidney injury, and coagulation disorders, clinically characterized by fever, bleeding, hypotension, and kidney damage. However, there is no specific medication available, and treatment is mainly symptomatic, with a mortality rate of 12%.4 Many cytokines (such as interleukin-34, interleukin-10, interferon inducible protein10, transforming growth factor-β3)5,6 are associated with the severity of HFRS, but due to their high detection costs, they are not widely used in clinical practice. Currently, there is a lack of inexpensive and practical indicators for predicting severe HFRS.
The complete blood count ratio is a novel non-specific inflammatory marker, including the neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), PLR, SII, and SIRI. These indicators can well reflect the overall immune status and inflammatory response process, and they are easily accessible and inexpensive. Currently, they have been widely used in assessing inflammatory diseases, such as the diagnosis and prognosis of COVID-19 and hepatitis B-related liver diseases.7,8 Previous studies9,10 have found that PLR, NLR, and SII have certain predictive value for the prognosis of Crimean-Congo hemorrhagic fever patients. However, there have been no reports on the severity of HFRS regarding SII and SIRI. Therefore, this study aims to explore the value of SII and SIRI in severe HFRS patients upon admission, providing a reference for the diagnosis and treatment of severe HFRS patients.
Materials and Methods
Study Design and Setting
This study retrospectively analyzed 165 patients diagnosed with HFRS at the Second Affiliated Hospital of Nanchang University from January 1, 2015, to December 30, 2023. The diagnostic and classification criteria for HFRS were based on the “Diagnostic Criteria for Epidemic Hemorrhagic Fever” (WS278-2008),11 which include suspected, clinical, and confirmed cases. Cases that met the clinical and laboratory diagnostic criteria were included in this study.
Suspected HFRS cases refer to patients with the following characteristics: (1) Epidemiological history: having a history of traveling to HFRS epidemic areas within two months before onset, with contact history with rodents or their secretions and excretions during that period; (2) Clinical symptoms and signs: a. Fever, excluding other febrile diseases; b. Having typical manifestations such as “three reds”, “three pains”, and “three swellings”; c. Gastrointestinal symptoms such as diarrhea, nausea, and vomiting.
Clinically diagnosed HFRS cases refer to suspected cases with one of the following manifestations: (1) Blood routine: increased white blood cell count and decreased platelet count, with the possibility of atypical lymphocytes; (2) Renal injury manifestations: proteinuria, hematuria, oliguria, polyuria, flocculent substances in urine, increased serum creatinine, and urea nitrogen; (3) Hypotensive shock; (4) Going through a five-stage disease course.
Laboratory-confirmed HFRS cases refer to cases that meet one of the following criteria on the basis of suspected or clinical diagnosis: (1) Positive HFRS serum-specific IgM antibody; (2) The titer of serum-specific IgG antibody in the recovery period is ≥ 4 times higher than that in the acute phase; (3) Detection of Hantavirus nucleic acid from patient specimens; (4) Isolation of Hantavirus from patient specimens.
Additionally, according to the clinical classification criteria in the 2021 version of the “Expert Consensus on Prevention and Treatment of Hemorrhagic Fever with Renal Syndrome”,12 atypical, mild, and moderate cases were classified as the mild group (n=115), while severe and critical cases were classified as the severe group (n=50). All included subjects were excluded for having scrub typhus, dengue fever, malignant tumors, hematological diseases, kidney diseases, autoimmune diseases, and pregnancy.
Data Collection
Within 24 hours of admission, we utilized the Hospital Information System (HIS) to meticulously review and gather clinical medical records pertaining to patients from two distinct patient groups. The general information [age, gender, hypertension, diabetes], clinical manifestations [life signs, disease course, length of hospital stay], and laboratory data at admission [WBC, NENT, LYMPH, MONO, red blood cell (RBC), hemoglobin (HGB), PLT, direct bilirubin (D-IBL), ALT, aspartate aminotransferase (AST), albumin (ALB), urea, serum creatinine (Scr), uric acid (UA), prothrombin time(PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB)] were recorded for both groups. All test results were conducted by the laboratory department of the Second Affiliated Hospital of Nanchang University. NLR was calculated as neutrophil count divided by lymphocyte count, MLR as monocyte count divided by lymphocyte count, PLR as platelet count divided by lymphocyte count, SII as (neutrophil count multiplied by platelet count) divided by lymphocyte count, and SIRI as (neutrophil count multiplied by monocyte count) divided by lymphocyte count.
Statistical Methods
Statistical analysis was performed using SPSS 26.0 software. Count data were expressed as cases (%), and comparisons between groups were conducted using chi-square tests or Fisher’s exact test. Measurement data that conformed to normal distribution were expressed as mean ± standard deviation, and comparisons between two groups were performed using independent sample t-tests. Measurement data that did not conform to normal distribution were expressed as M (Q1, Q3), and comparisons between two groups were conducted using the Mann–Whitney U-test. Binary logistic regression analysis was used to analyze the risk factors for severe HFRS. The ROC curve was employed to assess the effectiveness of SII, SIRI, and various indicators in diagnosing severe HFRS.
Results
Demographic Characteristics of HFRS Patients
During the study period, a total of 188 patients were diagnosed with HFRS. Among them, patients who died within 48 hours of admission (n=1), pregnant women (n=2), patients with co-existing scrub typhus and/or dengue fever (n=4), patients with malignant tumors, hematological diseases, kidney diseases, and autoimmune diseases (n=6), and patients with incomplete data (n=10) were excluded. Ultimately, a total of 165 hFRS patients were included in this study. Patients with atypical, mild, and moderate cases were classified into the mild group (n=115), while severe and critical cases were classified into the severe group (n=50) (Figure 1).
 |
Figure 1 Flow chart of patients selected hemorrhagic fever with renal syndrome. |
Baseline Characteristics of Patients with Mild and Severe HFRS
Compared with patients in the mild HFRS group, there were no differences in age, gender, smoking, alcohol consumption, hypertension, diabetes, and hospital stay for patients in the severe HFRS group (P > 0.05). However, the duration of illness was significantly shorter in patients with severe HFRS, and the difference was statistically significant (P < 0.05) (Table 1).
 |
Table 1 Clinical Characteristics of Patients with Hemorrhagic Fever with Renal Syndrome in Mild and Severe Groups |
Univariate Analysis of Clinical and Laboratory Data Between Patients with Mild and Severe HFRS
A univariate analysis was conducted on the clinical features and laboratory data of patients with mild and severe HFRS. Compared with patients in the mild HFRS group, patients in the severe HFRS group showed significantly higher levels of WBC, NENT, Lymph, Mono, PCT, ALT, SII, and SIRI, with statistically significant differences (P < 0.05). Additionally, PT and APTT were significantly prolonged (P < 0.05), while PLT, PLR, ALB, and SII decreased significantly (P < 0.05) (Table 2).
 |
Table 2 The Comparison of Laboratory Data Between Patients with Mild and Severe Hemorrhagic Fever with Renal Syndrome |
Binary Logistic Regression Analysis of Severe HFRS
After excluding the influence of collinearity and incorporating the statistically significant indicators from the univariate analysis of patients with mild and severe HFRS, the binary logistic regression analysis results indicated that high levels of WBC (OR: 1.190, 95% CI: 1.032–1.371), SIRI (OR: 4.743, 95% CI: 2.077–10.830), and low levels of SII (OR: 0.967, 95% CI: 0.951–0.984) were risk factors for severe HFRS (Table 3).
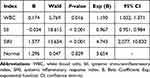 |
Table 3 Multivariate Logistic Regression Analysis |
The Value of WBC, SIRI, and SII in Predicting Severe HFRS Patients at Admission
The predictive effect of risk factors such as WBC, SIRI, and SII on severe HFRS patients was evaluated by plotting ROC curves. The AUC values under the ROC curves for WBC, SIRI, and SII in predicting severe HFRS were 0.773 (95% CI: 0.683–0.846, P < 0.05), 0.785 (95% CI: 0.711–0.858, P < 0.05), and 0.803 (95% CI: 0.729–0.876, P < 0.05) respectively (Table 4 and Figure 2). The predictive value of SII was superior to WBC and SIRI, with a sensitivity and specificity of 0.78 and 0.76 for SII. The cutoff value was 130.88 (Figure 2).
 |
Table 4 The Value of WBC, SII and SIRI in Predicting Patients with Severe HFRS |
Discussion
HFRS is a natural epidemic disease that can affect various organs and systems throughout the patient’s body, and severe cases can be life-threatening, becoming a global public health issue.2 In this study, we found that WBC, SII, and SIRI at admission were potential predictors for early identification of patients with HFRS progressing to severe cases. We provide three pieces of evidence to support this finding: (1) The WBC, NEUT, LYMPH, MONO, PCT, ALT, Scr, and SIRI were significantly higher in patients with severe HFRS compared to those with mild cases at admission; (2) The SII level was significantly lower in patients with severe HFRS at admission compared to those with mild cases; (3) Low SII and high WBC, SIRI levels at admission are risk factors for severe HFRS. WBC, SII, and SIRI have certain value in predicting severe HFRS, among which SII shows the best performance.
Currently, the main criteria for diagnosing severe HFRS rely on clinical classification standards, including body temperature, symptoms of poisoning, bleeding conditions, the degree of kidney function impairment, the presence of shock, and the presence of multiple organ failure. Additionally, immune-related serum proteins also aid in the early assessment of the severity and progression of HFRS patients.6 However, the clinical classification standards involve intricate calculations of multiple variables, and the detection of immune-related serum proteins is costly, limiting their use in clinical practice. Therefore, it is of great significance to find simple and inexpensive indicators for early assessment of disease progression in HFRS patients.
This study found through univariate analysis that WBC, NEUT, LYMPH, MONO, PCT, ALT, and Scr were significantly higher in severe HFRS patients compared to mild cases at admission (Table 2). When the Hantavirus invades the body, it triggers an inflammatory cytokine storm,13 leading to excessive activation of immune cells and a significant increase in WBC, consistent with previous findings.14–17 However, some studies have suggested that a decrease in WBC in HFRS indicates poor prognosis.18 In the initial stage of HFRS, under the influence of chemokines, neutrophils are the first to be recruited to the infection site to kill pathogens, leading to a significant increase in neutrophils. Meanwhile, as the Hantavirus invades capillary endothelial cells, it also attacks bone marrow cells and immune cells, stimulating the lymphocytes to proliferate excessively and revert to a more primitive state, showing blastoid morphology, resulting in an increase in abnormal lymphocytes in the peripheral blood. Multiple previous studies have found that lymphocyte infiltration increases significantly during viral infections.19–21 The increase in monocytes may be related to the activation of the monocyte-macrophage system. Systemic inflammatory responses triggered by cytokines and inflammatory mediators are closely related to an increase in PCT.
The liver damage caused by HFRS is considered to be due to the direct damage of liver cells by Hantavirus and the ischemia and hypoxia of the liver caused by extensive small vessel inflammation. Elisaf et al22 found that liver involvement is closely related to renal insufficiency and thrombocytopenia. Some studies have also found that neutrophils activated by Hantavirus play an important role in renal injury in HFRS.23 In addition, it has been found that pathogenic Hantavirus enters vascular endothelial cells through the membrane surface receptor αvβ3 integrin and inhibits the regulatory effect of β3 on the vascular permeability changes mediated by vascular endothelial cell growth factors, leading to impaired endothelial barrier function, plasma and protein extravasation, and impaired intestinal edema protein absorption. Furthermore, the reduced protein synthesis in the liver leads to a significant decrease in protein levels in HFRS patients. This study found that patients in the severe HFRS group showed a sharp decline in ALB in the early stage (Table 2). Previous studies have found that serum albumin levels are correlated with the severity of HFRS.24
As the condition progresses, HFRS patients experience hyperfunction of humoral immune response, leading to rapid formation of circulating immune complexes that adhere to the surface of platelets, causing PLT aggregation and phagocytosis by monocyte-macrophage systems. Additionally, the most abundant receptor on the platelet surface, αvβ3 integrin, similar to αvβ3, can interact with Hantavirus through its β3 subunit, affecting PLT function and antibody-mediated destruction by monocyte-macrophage systems. Furthermore, widespread damage to small vascular endothelial cells results in the activation of vWF and Factor III, inducing the activation of the extrinsic coagulation pathway and significant consumption of platelets. Therefore, PLT levels in severe cases are significantly lower than those in mild cases, consistent with previous research results,25,26 suggesting that PLT is correlated with the severity of Hantavirus infection. Moreover, numerous studies have shown that complete blood count and its ratios are widely used to evaluate the progression and prognosis of inflammatory diseases and viral infections.7,27–29 The exact mechanisms of how SII and SIRI assess the severity of HFRS remain unclear, but they can be attributed to the role of inflammatory cytokine storms in the pathophysiology of disease progression.30,31 Consequently, monitoring inflammatory markers in the early stages of the disease is crucial for assessing the severity of patients’ conditions. As a new inflammatory response index, SII and SIRI reflect the changing trends of neutrophils, lymphocytes, monocytes, and platelets, offering economic, comprehensive, easily accessible metrics that can comprehensively reflect patients’ inflammatory and immune status compared to traditional inflammatory markers. Through binary logistic regression analysis, this study found that WBC (OR: 1.190, 95% CI: 1.032–1.371), SII (OR: 0.967, 95% CI: 0.951–0.984), and SIRI (OR: 4.743, 95% CI: 2.077–10.830) are risk factors for severe HFRS. Further ROC curve analysis assessed the predictive efficacy of WBC, SII, and SIRI for severe HFRS, suggesting that SII has the best value in predicting severe HFRS, with an AUC of 0.803, sensitivity of 78%, and specificity of 76%. SIRI and WBC followed closely, with AUCs of 0.785 and 0.773, respectively. Some studies32,33 have found a positive correlation between SII and the severity of stable coronary heart disease and acute pancreatitis.
Limitations
The limitations of this study are as follows: (1) This study is a retrospective single-center study, and multi-center and prospective studies are needed to further verify the accuracy and universality of the results; (2) Only indicators within 24 hours of admission were collected, which cannot reflect the entire course of the disease. Subsequent studies will further assess the correlation between changes in indicators such as the 3rd, 5th, and 7th days after admission and the disease; (3) All confirmed HFRS patients were given antiviral treatment upon admission, and further research is needed to explore whether antiviral drugs have an impact on SII and SIRI; (4) The study did not classify HFRS according to clinical stages (mild-type, moderate-type, severe-type, critical-type) to investigate whether SII and SIRI have the same value for different stages.
Conclusion
The results of this study indicate that WBC, SII, and SIRI are risk factors for severe HFRS, with SII having a higher value in early prediction of severe HFRS compared to WBC and SIRI. SII can serve as a more convenient and accessible reference indicator for assessing severe HFRS, but further studies are still needed to verify its effectiveness.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study conformed to the guidelines of the Helsinki Declaration. Ethics approval was obtained by the Research Ethics Committee of the Second Affiliated Hospital of Nanchang University. And the Ethics Committee waived the requirement for informed consent due to the retrospective and observational nature of the investigation, as well as the anonymity of the data.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
The authors would like to thank all the patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by National Natural Science Foundation of China (NO.81860034).
Disclosure
The authors report no conflicts of interest in this work.
References
1. SEHGAL A, MEHTA S, SAHAY K, et al. Hemorrhagic fever with renal syndrome in Asia: history, pathogenesis, diagnosis, treatment, and prevention. Viruses. 2023;15(2):561. doi:10.3390/v15020561
2. TARIQ M, KIM DM. Hemorrhagic fever with renal syndrome: literature review, epidemiology, clinical picture and pathogenesis. Infect Chemother. 2022;54(1):1–19. doi:10.3947/ic.2021.0148
3. GUO Q, XU J, SHI Q, et al. Acute pancreatitis associated with hemorrhagic fever with renal syndrome: a cohort study of 346 patients. BMC Infect Dis. 2021;21(1):267. doi:10.1186/s12879-021-05964-5
4. HEYMAN P, VAHERI A, LUNDKVIST A, et al. Hantavirus infections in Europe: from virus carriers to a major public-health problem. Expert Rev Anti Infect Ther. 2009;7(2):205–217. doi:10.1586/14787210.7.2.205
5. TANG K, ZHANG C, ZHANG Y, et al. Elevated plasma interleukin 34 levels correlate with disease severity-reflecting parameters of patients with haemorrhagic fever with renal syndrome. Infect Dis. 2019;51(11–12):847–853. doi:10.1080/23744235.2019.1672887
6. LEE GY, KIM WK, NO JS, et al. Clinical and immunological predictors of hemorrhagic fever with renal syndrome outcome during the early phase. Viruses. 2022;14(3):595. doi:10.3390/v14030595
7. FOIS AG, PALIOGIANNIS P, SCANO V, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. 2020;25(23):5725. doi:10.3390/molecules25235725
8. MAO W, WU J. Haematologic indices in hepatitis B virus-related liver disease. Clin Chim Acta. 2020;500:135–142. doi:10.1016/j.cca.2019.10.007
9. EREN SH, ZENGIN S, BUYUKTUNA SA, et al. Clinical severity in forecasting platelet to lymphocyte ratio in Crimean-Congo hemorrhagic fever patients. J Med Microbiol. 2016;65(10):1100–1104. doi:10.1099/jmm.0.000330
10. GUNDOGDU O, AVCI O. Relationship between systemic immune-inflammation index and mortality in intensive care patients diagnosed with Crimean-Congo Hemorrhagic Fever. J Coll Physicians Surg Pak. 2022;32(12):1538–1543.
11. Department of Health of the People’s Republic of China. Diagnostic criteria for epidemic hemorrhagic fever: WS 278-2008. Beijing:China Standards Press;2008:1–11
12. JIANG H, HUANG CX, BAI XF, et al. Expert consensus on the prevention and treatment of hemorrhagic fever with renal syndrome. Infect Dis Immun. 2022;2(4):224–232. doi:10.1097/ID9.0000000000000054
13. KHAIBOULLINA SF, MARTYNOVA EV, KHAMIDULLINA ZL, et al. Upregulation of IFN-gamma and IL-12 is associated with a milder form of hantavirus hemorrhagic fever with renal syndrome. Eur J Clin Microbiol Infect Dis. 2014;33(12):2149–2156. doi:10.1007/s10096-014-2176-x
14. DUCHIN JS, KOSTER FT, PETERS CJ, et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The hantavirus study group. N Engl J Med. 1994;330(14):949–955. doi:10.1056/NEJM199404073301401
15. LAHDEVIRTA J. Nephropathia epidemica in Finland. A clinical histological and epidemiological study. Ann Clin Res. 1971;3:1–54.
16. LEE JS. Clinical features of hemorrhagic fever with renal syndrome in Korea. Kidney Int Suppl. 1991;35:S88–93.
17. ZAKI SR, GREER PW, COFFIELD LM, et al. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease [J]. Am J Pathol. 1995;146(3):552–579.
18. KLINGSTROM J, SMED-SORENSEN A, MALEKI KT, et al. Innate and adaptive immune responses against human Puumala virus infection: immunopathogenesis and suggestions for novel treatment strategies for severe hantavirus-associated syndromes. J Intern Med. 2019;285(5):510–523. doi:10.1111/joim.12876
19. HUANG C, JIN B, WANG M, et al. Hemorrhagic fever with renal syndrome: relationship between pathogenesis and cellular immunity. J Infect Dis. 1994;169(4):868–870. doi:10.1093/infdis/169.4.868
20. LEWIS RM, LEE HW, SEE AF, et al. Changes in populations of immune effector cells during the course of haemorrhagic fever with renal syndrome. Trans R Soc Trop Med Hyg. 1991;85(2):282–286. doi:10.1016/0035-9203(91)90058-7
21. CHEN LB, YANG WS. Abnormalities of T cell immunoregulation in hemorrhagic fever with renal syndrome. J Infect Dis. 1990;161(5):1016–1019. doi:10.1093/infdis/161.5.1016
22. ELISAF M, STEFANAKI S, REPANTI M, et al. Liver involvement in hemorrhagic fever with renal syndrome. J Clin Gastroenterol. 1993;17(1):33–37. doi:10.1097/00004836-199307000-00010
23. STRANDIN T, MAKELA S, MUSTONEN J, et al. Neutrophil activation in acute hemorrhagic fever with renal syndrome is mediated by Hantavirus-infected microvascular endothelial cells. Front Immunol. 2018;9:2098. doi:10.3389/fimmu.2018.02098
24. KIM YO, YOON SA, KU YM, et al. Serum albumin level correlates with disease severity in patients with hemorrhagic fever with renal syndrome. J Korean Med Sci. 2003;18(5):696–700. doi:10.3346/jkms.2003.18.5.696
25. DU H, LI J, JIANG W, et al. Clinical study of critical patients with hemorrhagic fever with renal syndrome complicated by acute respiratory distress syndrome [J]. PLoS One. 2014;9(2):e89740. doi:10.1371/journal.pone.0089740
26. WANG M, WANG J, WANG T, et al. Thrombocytopenia as a predictor of severe acute kidney injury in patients with Hantaan virus infections. PLoS One. 2013;8(1):e53236. doi:10.1371/journal.pone.0053236
27. WANG RH, WEN WX, JIANG ZP, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage [J]. Front Immunol. 2023;14:1115031. doi:10.3389/fimmu.2023.1115031
28. HANBERG JS, FREIBERG MS, GOETZ MB, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic inflammatory biomarkers in human immunodeficiency virus (HIV), hepatitis C virus (HCV), and HIV/HCV coinfection. Open Forum Infect Dis. 2019;6(10):ofz347. doi:10.1093/ofid/ofz347
29. KOSIDLO JW, WOLSZCZAK-BIEDRZYCKA B, MATOWICKA-KARNA J, et al. Clinical significance and diagnostic utility of NLR, LMR, PLR and SII in the course of COVID-19: a literature review. J Inflamm Res. 2023;16:539–562. doi:10.2147/JIR.S395331
30. GARANINA E, MARTYNOVA E, DAVIDYUK Y, et al. Cytokine storm combined with humoral immune response defect in fatal hemorrhagic fever with renal syndrome case, Tatarstan, Russia. Viruses. 2019;11(7):601. doi:10.3390/v11070601
31. KHAIBOULLINA SF, LEVIS S, MORZUNOV SP, et al. Serum cytokine profiles differentiating hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Front Immunol. 2017;8:567. doi:10.3389/fimmu.2017.00567
32. CANDEMIR M, KIZILTUNC E, NURKOC S, et al. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72(6):575–581. doi:10.1177/0003319720987743
33. LIU X, GUAN G, CUI X, et al. Systemic immune-inflammation index (SII) can be an early indicator for predicting the severity of acute pancreatitis: a retrospective study. Int J Gen Med. 2021;14:9483–9489. doi:10.2147/IJGM.S343110
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Biomarkers for Early Predicting In-Hospital Mortality in Severe Fever with Thrombocytopenia Syndrome and Differentiating It from Hemorrhagic Fever with Renal Syndrome
Chen C, Zheng Y, Li X, Shen B, Bi X
Infection and Drug Resistance 2025, 18:1355-1366
Published Date: 12 March 2025
Predictors of Severity in Hemorrhagic Fever with Renal Syndrome
Huang L, Wu J, Luo J, Gu W
International Journal of General Medicine 2025, 18:2033-2045
Published Date: 9 April 2025


