Back to Journals » Infection and Drug Resistance » Volume 18
Biomarkers for Early Predicting In-Hospital Mortality in Severe Fever with Thrombocytopenia Syndrome and Differentiating It from Hemorrhagic Fever with Renal Syndrome
Authors Chen C, Zheng Y, Li X, Shen B , Bi X
Received 24 August 2024
Accepted for publication 1 March 2025
Published 12 March 2025 Volume 2025:18 Pages 1355—1366
DOI https://doi.org/10.2147/IDR.S492942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Chaochao Chen,1,* Yuwei Zheng,1,* Xuefen Li,2 Bo Shen,1 Xiaojie Bi1
1Department of Laboratory Medicine, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai, Taizhou, 317000, People’s Republic of China; 2Department of Laboratory Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaojie Bi; Bo Shen, Department of Laboratory Medicine, Taizhou Hospital of Zhejiang Province affiliated to Wenzhou Medical University, No. 150, Ximen Street, Linhai, Taizhou, 317000, People’s Republic of China, Tel +86 13757693182, Email [email protected]; [email protected]
Purpose: Severe fever with thrombocytopenia syndrome (SFTS) has a high mortality rate and is easily misdiagnosed as hemorrhagic fever with renal syndrome (HFRS), particularly in resource-limited rural areas where early diagnosis remains challenging. This study used routine laboratory parameters, epidemiology and clinical manifestations to develop a model for the early diagnosis of SFTS and identify fatal risk factors, ultimately reducing mortality of SFTS.
Patients and Methods: This retrospective cohort study included 141 SFTS and 141 HFRS patients. Of these, 94 patients with SFTS were allocated to the model cohort for mortality risk identification by using multivariable Cox regression analysis. Sensitivity, specificity, and predictive values were calculated from validation cohort to assess the clinical values. Then, we analyzed 62 SFTS and 113 HFRS using multivariable logistic regression to identify SFTS. Receiver operating characteristic (ROC) curve analysis was used to evaluate their diagnostic value.
Results: Multivariate Cox regression analysis showed that blood urea nitrogen (BUN) ≥ 10.22mmol/L activated partial thromboplastin time (APTT) ≥ 58.05s and D-dimer ≥ 4.68mg/L were the risk factors for death in SFTS. This combined indicators had an area under the curve (AUC) of 0.91 (95% CI: 0.847– 0.973), with a sensitivity and specificity of 86%, respectively. Any indicator was achieved the cutoff, and sensitivity and specificity in the validation group were 93% and 54%. Multivariable logistic regression showed that age (OR: 1.10) and initial laboratory indicators including WBC (OR: 0.48), Cr (OR: 0.86), CK (OR: 1.01), and APTT (OR: 1.09) can be used to identify SFTS from HFRS. This model achieved an AUC value of 0.97 (95% CI: 0.977– 0.999) and 0.98 (95% CI: 0.958– 1.000) in validation cohort.
Conclusion: In resource-limited rural hospitals, the integration of routine laboratory parameters with epidemiology and clinical manifestations demonstrates enhanced sensitivity for early SFTS identification and mortality risk stratification to reduce mortality rate.
Keywords: differential diagnosis, dynamic change, hemorrhagic fever with renal syndrome, risk factors, severe fever with thrombocytopenia syndrome
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an acute viral hemorrhagic fever caused by the SFTS virus (SFTSV),1 with a mortality rate ranging from 5% to 30%.2,3 It was first reported in the Ta-pieh Mountains of central China in 2009.4 In 2011, Taizhou Hospital of Zhejiang Province in China diagnosed and successfully treated the first patient with SFTS in the province.5 Taizhou is a high-incidence region for SFTS within Zhejiang Province, with a total of 140 cases of SFTS reported up to 2018, an average annual incidence of 0.29/100,000 population, and a case fatality rate of 14.29%.6 Given its location within an endemic area and wealth of experience in diagnosis and treatment, Taizhou is well suited to conducting research on SFTS.
Sun et al7 revealed that the SFTS progresses rapidly, a mere 3-day delay in diagnosis can lead to a two-fold increase in the SFTS mortality rate, highlighting the critical importance of early diagnosis and treatment. SFTS diagnosis relies on polymerase chain reaction (PCR) testing for viral RNA, which is predominantly conducted at prefecture-level or higher Centers for Disease Control and Prevention (CDCs). Consequently, diagnosis of SFTS remains challenging in rural areas with limited healthcare infrastructure.
Notably, the epidemiological profiles (predominance in rural areas) and clinical manifestations (fever with hemorrhagic tendencies) of SFTS overlap significantly with those of hemorrhagic fever with renal syndrome (HFRS). Both conditions are caused by viruses belonging to the Bunyaviridae family.8 The SFTS mortality rate is substantially higher than that of HFRS,9,10 and unlike HFRS, SFTS can be transmitted from person to person. In resource-limited rural settings with limited diagnostic capacity, patients with SFTS may be misdiagnosed with HFRS, potentially increasing their risk of death. Thus, early identification of SFTS is critical, requiring integration of epidemiological history, clinical evaluation, and routine laboratory parameters to distinguish it from HFRS.
Most previous studies11,12 have separately investigated the epidemiological histories and clinical characteristics of SFTS and HFRS. In contrast, this study analyzes the epidemiological profiles, clinical data, and laboratory parameters of both SFTS and HFRS patients, aiming to preliminarily differentiate SFTS from HFRS and further explore risk factors for SFTS mortality. These findings provide a source of reference for clinicians working primary healthcare facilities for early detection of SFTS and identifying patients with SFTS who are at highest risk of death.
Materials and Methods
Sample Collection
From January 1, 2016, to April 15, 2024, 143 patients with SFTS patients were enrolled in the study. Two patients without laboratory indicators were excluded, leaving 141 patients with SFTS patients in the study. We also collected data on 141 patients with HFRS admitted from January 1, 2016, to September 2, 2022.
The exclusion criteria were as follows: (1) patients with underlying liver and kidney diseases and (2) incomplete clinical data. Data collection included demographic characteristics, epidemiological exposure history, clinical symptoms, laboratory test results, treatment plan and outcome.
Cohort for Prediction of SFTS Mortality
Following the 7:3 principle, we designated the period from January 1, 2016, to June 10, 2022, as the model cohort (n = 94), and the period from June 11, 2022, to April 15, 2024, as the validation cohort p(n = 47) (Figure 1). A priori G*Power analysis demonstrated that the sample sizes of the two groups were sufficient to achieve statistical powers of 0.93 in the model cohort and 0.71 in the validation cohort.
 |
Figure 1 Study flowchart. |
Cohort for the Differentiation Between SFTS and HFRS
The model cohort comprised 62 SFTS and 113 HFRS patients admitted to our hospital between January 1, 2016, and April 1, 2020, while the validation cohort included 31 SFTS patients and 28 HFRS patients between April 2, 2020, to September 2, 2022. G*Power analysis demonstrated statistical powers of 0.99 in the model cohort and 0.89 in the validation cohort.
Definition Indicators
The indicators were defined as follows: (1) Definition of the date of onset: The date of onset was defined as the date on which the symptoms started, based on patient’s chief complaint. (2) Definition of fever: Fever was defined as a body temperature >37.3°C during the patient’s illness. (3) Dynamic graph data selection: If there were more than one result on the first date in a time period, the mean of the values was calculated.
The dynamic laboratory data were then classified into four groups: fever stage (1–6 days), deterioration/organ failure (7–12 days), improvement/death (13–15 days) and convalescence (≥16 days) based on previous studies.13,14
Hematological Tests
Blood counts were measured using a Sysmex 2100D routine hematology analyzer (Sysmex, Kobe, Japan) and a Mindray BC series automatic blood cell analyzer (Mindray, Shenzhen, China). Routine blood coagulation tests were performed using an automatic coagulation analyzer (Stago, Cedex, France) and supporting reagents. Biochemical indicators were detected using an AU5800 Beckman Library automatic biochemical analyzer (Beckman Coulter, Brea, CA, USA) and supporting reagents.
Serological Tests
Specific immunoglobulin M anti-Epstein-Barr virus hemagglutinin Factor (IgM anti-EHF) antibodies were detected using the corresponding enzyme-linked immunosorbent assay (ELISA) kit (Shandong Kanghua Biological, China), and SFTSV RNA was measured using an ABI 7500 quantitative PCR (Applied Biosystems, Waltham, MA, USA) and supporting reagents (Daan, Guangzhou, China).
Statistical Analysis
We conducted a power analysis to ensure that the sample size was adequate for the study’s objectives using G*Power 3.1 (Universität Duisburg-Essen, Germany), with a power value set to exceed 0.70. SPSS (version 26.0; IBM Corp., Armonk, NY, USA) and GraphPad Prism (version 9.0; GraphPad Software, San Diego, CA, USA) were used for the statistical analysis and mapping. Continuous variables were expressed as the median and interquartile range (IQR), and the Mann–Whitney U-test was used for comparisons between two groups. Categorical variables were expressed as frequencies and percentages and the chi-squared test was used for comparisons between groups. Cutoff points were identified following Youden’s index of receiver operator characteristic (ROC) curve. The area under the curve (AUC) was used to evaluate the diagnostic values. Cox regression analysis screened for risk factors of death in patients with SFTS. The results were reported as hazard ratios (HR) along with their 95% confidence intervals (CI). Sensitivity, specificity, and predictive values were calculated from validation cohort to assess the clinical value. Multivariate logistic regression was used to establish the models. P values <0.05 were considered statistically significant.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Taizhou Hospital of Zhejiang Province (KL20240640, date of approval 27 June, 2024). Informed consent was waived by our institutional review board due to the retrospective nature of our study, and data were anonymized and kept confidential.
Results
Epidemiologic and Clinical Characteristics of the Cohorts
A mean of 18 patients with confirmed SFTS were admitted per year, and the mortality rate was 24%. The incidence was highest between April and September. In 2023, the 35 patients were admitted with confirmed SFTS, with ten deaths (Figure 2).
Compared to survivors, non-survivors were older (73.5[66.0–78.0] vs 66.0[57.0–73.0], p<0.01) and more likely to have neurological changes (97.2% vs 32.38%, p<0.001). The rates of blood transfusion and secondary infections were higher in non-survivors than in survivors (61.11% vs 26.67%, p < 0.001; 38.89% vs 7.62%, p < 0.001) (Table 1 and Figure S1).
 |
Table 1 Clinical Characteristics in All SFTS Patients |
Compared with patients with HFRS, patients with SFTS were older (66[59.0–72.3] vs 49[38.0–59.5], p < 0.01) and more likely to have basic diseases (48.4% vs 18.6%) and had significantly shorter length of hospital stay (7.5[3.0–11.3] days vs 12.0[9.0–16.0] days, p < 0.01) (Table S1).
Laboratory Data of the Cohorts in All SFTS Patients
Among the patients with SFTS, the initial peripheral laboratory test results in survivors and non-survivors are compared in Tables 2 and S2. The neutrophil counts and levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), lactate dehydrogenase (LDH), creatine kinase (CK), blood urea nitrogen (BUN), creatinine (Cr), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrin/fibrinogen degradation products (FIB), thrombin time (TT), and D-dimer in non-survival were higher than those in survivors, and the decline in platelet count was more pronounced in the non-survival group (42[26–61] vs 56[39–74]10×9/L, p < 0.05).
 |
Table 2 Laboratory Data of the Cohorts in All SFTS Patients |
Dynamic Analysis of Laboratory Indicators of Patients With Fatal SFTS
On days 4–6 following disease onset, the APTT, BUN, Cr, and D-dimer levels were higher in non-survivors than in survivors, and these markers continued to rise until days 12–15. Between days 7 and 9 after disease onset, the levels of ALT, AST, LDH, and TT showed a greater increase in non-survivors than in survivors. These levels continued to rise until days 12 to 15 (Figure 3).
Multivariable Cox Regression to Identify Predictors of Mortality in SFTS
Multivariable Cox regression showed that initial laboratory indicators, including BUN (OR: 3.15, 95% CI: 1.45–8.06, p < 0.01), APTT (OR: 4.60, 95% CI: 1.24–17.00, p < 0.05), and D-dimer (OR: 3.95, 95% CI: 1.52–10.24, p < 0.01), could be used as potential indicators of the risk of death in patients with SFTS (Table 3). The predictive model for mortality was: P=−4.04+2.26×BUN (0, no; 1, yes)+2.18×APTT (0, no; 1, yes)+2.25×D-dimer (0, no; 1, yes). This combined model had an AUC of 0.91 (95% CI: 0.847–0.973). The sensitivity and specificity were both 86% (Figure 4A). In the validation group of 47 SFTS, the sensitivity and specificity were 93% and 54%, for any of the following conditions (BUN ≥10.22 or APTT ≥58.05 or D-dimer≥4.68) (Figure 4B). In addition, the sensitivity and specificity were 77% and 79% according to the predictive model (Figure 4C).
 |
Table 3 Univariate/Multivariate Cox Analysis for Prediction the Mortality Risk in SFTS |
Multivariable Logistic Regression to Identify Factors Distinguishing SFTS from HFRS
Variables including age (OR: 1.10), CK (OR: 1.01), Cr (OR: 0.957), WBC count (OR: 0.48), APTT (OR: 1.09) differed significantly between the SFTS and HFRS groups, and were included in the multivariable logistic regression model (Tables 4 and S3). The results showed that the AUC of the combined Age-CK-Cr-WBC-APTT was 0.97 (95% CI: 0.977–0.999) with a sensitivity of 0.95 and specificity of 0.96 (Figure 5A). To further evaluate the performance of this model, we used a validation dataset consisting of 31 SFTS and 28 HFRS patients. The AUC of the validation group was 0.98 (95% CI: 0.958–1.000) (Figure 5B).
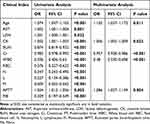 |
Table 4 Univariate/Multivariate Logistic Regression Analysis to Distinguish SFTS and HFRS |
Discussion
SFTS can spread at a rapid rate, has a high mortality rate and can be easily confused with HFRS.15 Early diagnosis of SFTS is difficult in rural areas with limited medical resources; therefore, using routine laboratory test results is crucial for diagnosis of SFTS and identifying risk factors for death. Our study revealed that BUN ≥10.22mmol/L, APTT ≥58.05s, and D-dimer ≥4.68mg/L serve as independent predictors of SFTS-related mortality. Additionally, patients are more likely to be SFTS with Age ≥60.5y, WBC ≤4.25×109/L, Cr ≤103.5μmol/L, CK ≥323U/L, APTT ≥51.05s.
Consistent with other studies,15,16 our data showed that SFTS was prevalent from April to September, with a high mortality rate in Taizhou. In 2023, the number of patients with SFTS increased sharply, with the highest mortality rate even in the off-season. Primary care physicians should consider the possibility of SFTS in patients with fever of unknown origin and a history of outdoor work.
Zuo et al17 reported that in patients with SFTS, the risk of pulmonary infection was associated with the time from the onset to admission. In our study, less than 80% of the non-survivors with SFTS were admitted within 7 days after onset, whereas more than 90% of the survivors were admitted within 7 days. Therefore, the early diagnosis and identification of patients at high risk of death are important, especially as no specific antiviral drugs are available to treat SFTSV.
In this study, BUN, APTT, and D-dimer levels were identified as risk factors for death in patients with SFTS. Wang et al18 and Cao et al19 reported that BUN is a promising early warning biomarker for adverse outcomes in patients with SFTS. In our study, the BUN levels of non-survivors continued to increase, notably, two patients experienced an increase of up to 7-fold. The APTT and D-dimer levels were markedly increased in the non-survivors. Tang et al20 found that APTT and D-dimer were risk factors for death in SFTS patients, suggesting that these patients had coagulation dysfunction. High D-dimer level was associated with 28-day mortality in patients with infection or sepsis identified in the emergency department.21 We emphasized that clinicians should closely monitor the dynamic changes in BUN, APTT, and D-dimer levels to minimize the occurrence of fatal events.
Notably, in the validation group of 47 SFTS, the sensitivity and specificity were 93% and 54%, for any of the following conditions (BUN ≥ 10.22 or APTT ≥ 58.05 or D-dimer ≥ 4.68). We hypothesized that the primary hospital could improve the sensitivity of predicting disease-related mortality by considering individual biomarker and clinical manifestations. However, we divided the validation group by time cutoff, which may lead to variations in clinical symptom severity and individual immune system heterogeneity between the two groups. Our study correctly classified 31 SFTS patients in the validation cohort. Notably, 15 surviving patients were misclassified as being at high risk for mortality, and 73.3% (11/15) patients had no basic disease, which sharply contrasts with the model cohort, while 68% of patients had hypertension or diabetes. Cases 32 and 43 were referred to our hospital after receiving empirical antiviral therapy (eg, ribavirin) at primary care hospitals. Cases 22 and 39 had a strong immune response ability (lymphocyte counts: 1.95 × 109/L and 0.9 × 109/L, respectively). Studies22,23 have demonstrated that a weaker immune response during the early disease stages is correlated with significantly elevated mortality rates. Meanwhile, we also combined three indicators to predict the model’s sensitivity at 77% and specificity at 79%.
SFTS and HFRS have similar clinical manifestations in the early stage.10 Therefore, focusing solely on clinical manifestations is inadequate for the diagnosis of the SFTS in rural hospitals in Taizhou. Our data showed that the majority of patients with SFTS were mainly in older adults, whereas patients with HFRS were considerably younger. This finding is consistent with those of previous studies:24,25 Older adults are more likely to be exposed to SFTS through agricultural activities.25 We found that the early damage to the heart and liver was more obvious in SFTS patients than HFRS, whereas renal injury was more severe in HFRS patients, consistent with the results of studies.26,27 A validation group was developed containing of 31 SFTS patients, which led to correct assignment of 27 patients. Four of 31 patients have a history of hypertension or heart disease and long-term medication, and antibiotic therapy before admission may influenced the efficiency of our model. Our newly established model based on age, WBC count, APTT, Cr, and CK levels could provide a basis for early recognition of SFTS and HFRS for primary care physicians.
There are limitations that should be considered. First, a small sample size increases the risk of over-fitting during mortality risk factor screening, potentially introducing selection bias. Second, there is a lack of uniform intervals between onset to admission, and interventions may confound laboratory/clinical baseline data at admission. We should comprehensively collect more external SFTS cases and laboratory indicators to identify additional biomarkers in further study that will assist in recognizing SFTS and its risk factors for mortality.
Conclusions
Patients with SFTS present mainly with leukopenia, thrombocytopenia, abnormal liver and renal indicators, and abnormal coagulation, which can easily be confused with HFRS in resource-limited rural hospitals. Age, WBC count, APTT, Cr, and CK levels were useful for distinguishing patients with SFTS from those with HFRS. Patients with SFTS have a relatively high mortality rate when BUN ≥10.22mmol/L, APTT ≥58.05s and D-dimer ≥4.68mg/L. We emphasize that in rural hospitals, the combination of routine laboratory parameters with epidemiological exposure history, and clinical manifestations shows improved sensitivity for early identification of SFTS and mortality risk stratification, which could help to reduce mortality rates.
Abbreviations
WBC, white blood cell; N, neutrophils; L, lymphocyte; M, monocyte; RBC, red blood cell; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; LDH, lactate dehydrogenase; CK, creatine kinase; BUN, blood urea nitrogen; Cr, creatinine; PT, prothrombin time; APTT, activated partial thromboplastin time; Fib, fibrin; TT, thrombin time; ROC, receiver operating characteristic; AUC, area under the curve; CDCs: Centers for Disease Control and Prevention.
Acknowledgments
We would like to thank the nurses in Taizhou Hospital of Zhejiang Province for sampling specimens and thank the patients for being enrolled in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Medical Science and Technology Project of Zhejiang Province 2024KY522.
Disclosure
The authors declare no competing interests in this work.
References
1. Abudurexiti A, Adkins S, Alioto D, et al. Taxonomy of the order bunyavirales: update 2019. Arch Virol. 2019;164(7):1949–1965. doi:10.1007/s00705-019-04253-6
2. Chen QL, Zhu MT, Chen N, et al. Epidemiological characteristics of severe fever with thtrombocytopenia syndrome in China, 2011-2021. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43(6):852–859. doi:10.3760/cma.j.cn112338-20220325-00228
3. Heo D, Kang YM, Song K, et al. Clinical score system to differentiate severe fever with thrombocytopenia syndrome patients from patients with scrub typhus or hemorrhagic fever with renal syndrome in Korea. J Korean Med Sci. 2020;35(11). doi:10.3346/jkms.2020.35.e77
4. Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. New Engl J Med. 2011;364(16):1523–1532. doi:10.1056/NEJMoa1010095
5. Z CH, T WW, R XJ, et al. Diagnosis and treatment of severe fever with thrombocytopenia associated with a novel bunyavirus in Zhejiang Province: the first case report. Chin J Clin Infect Dis. 2011;04(4):195–196. doi:10.3760/cma.j.issn.1674-2397.2011.04.002
6. Li JL, Hu YF, Weng J, et al. Analysis on the epidemic characteristics of fever with thrombocytopenia syndrome in Taizhou. Prev Med. 2019;31(12):1267–1268,1272. doi:10.19485/j.cnki.issn2096-5087.2019.12.019
7. Sun J, Gong Z, Ling F, et al. Factors associated with severe fever with thrombocytopenia syndrome infection and fatal outcome. Sci Rep-UK. 2016;6:33175. doi:10.1038/srep33175
8. Wang X, Ren X, Ge Z, et al. Clinical manifestations of death with severe fever and thrombocytopenia syndrome: a meta-analysis and systematic review. J Med Virol. 2021;93(6):3960–3968. doi:10.1002/jmv.26518
9. Wang W, Liu Y, Zhang R, Sun J, Jiang J, Wang H. Comparison of epidemiological characteristics between hemorrhagic fever with renal syndrome patients and severe fever with thrombocytopenia syndrome patients. J Med Virol. 2024;96(8):e29845. doi:10.1002/jmv.29845
10. Qi R, Qin XR, Wang L, et al. Severe fever with thrombocytopenia syndrome can masquerade as hemorrhagic fever with renal syndrome. PLoS Neglect Trop D. 2019;13(3):e0007308. doi:10.1371/journal.pntd.0007308
11. Wang N, Yin JX. Epidemic process and influencing factors of hemorrhagic fever with renal syndrome: a review. Zhongguo Xue XI Chong Bing Fang Zhi Za Zhi. 2021;34(2):200–203. doi:10.16250/j.32.1374.2021163
12. He F, Zheng X, Zhang Z. Clinical features of severe fever with thrombocytopenia syndrome and analysis of risk factors for mortality. Bmc Infect Dis. 2021;21(1):1253. doi:10.1186/s12879-021-06946-3
13. Wang G, Chang H, Jia B, et al. Nucleocapsid protein-specific IgM antibody responses in the disease progression of severe fever with thrombocytopenia syndrome. Ticks Tick-Borne Dis. 2019;10(3):639–646. doi:10.1016/j.ttbdis.2019.02.003
14. Chen C, Li P, Li KF, et al. Animals as amplification hosts in the spread of severe fever with thrombocytopenia syndrome virus: a systematic review and meta-analysis. Int J Infect Dis. 2019;79:77–84. doi:10.1016/j.ijid.2018.11.017
15. Lu QB, Li H, Jiang FC, et al. The differential characteristics between severe fever with thrombocytopenia syndrome and hemorrhagic fever with renal syndrome in the endemic regions. Open Forum Infect Di. 2019;6(12):ofz477. doi:10.1093/ofid/ofz477
16. Liang S, Li Z, Zhang N, et al. Epidemiological and spatiotemporal analysis of severe fever with thrombocytopenia syndrome in Eastern China, 2011-2021. Bmc Public Health. 2023;23(1):508. doi:10.1186/s12889-023-15379-3
17. Zuo Y, Wang H, Huang J, et al. Pulmonary infection in patients with severe fever with thrombocytopenia syndrome: a multicentre observational study. J Med Virol. 2023;95(4):e28712. doi:10.1002/jmv.28712
18. Wang Y, Qin LH, Zhang K, et al. Blood urea nitrogen to albumin ratio is a novel predictor of fatal outcome for patients with severe fever with thrombocytopenia syndrome. J Med Virol. 2024;96(6):e29731. doi:10.1002/jmv.29731
19. Cao K, Ma A, Zhang K, et al. Blood urea nitrogen-to-serum albumin ratio predicts fatal outcomes in severe fever with thrombocytopenia syndrome patients. Am J Trop Med Hyg. 2024;111(1):113–120. doi:10.4269/ajtmh.23-0811
20. Tang N, Yuan P, Luo M, Li D. Prolonged coagulation times in severe fever with thrombocytopenia syndrome virus infection, the indicators of heparin-like effect and increased haemorrhagic risk. Brit J Haematol. 2024;204(5):1999–2006. doi:10.1111/bjh.19364
21. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
22. Zhao J, Lu QB, Li H, et al. Sex differences in case fatality rate of patients with severe fever with thrombocytopenia syndrome. Front Microbiol. 2021;12:738808. doi:10.3389/fmicb.2021.738808
23. Lu QB, Cui N, Hu JG, et al. Characterization of immunological responses in patients with severe fever with thrombocytopenia syndrome: a cohort study in China. Vaccine. 2015;33(10):1250–1255. doi:10.1016/j.vaccine.2015.01.051
24. Wang F, Wu Y, Jiao J, Wang J, Ge Z. Risk Factors and Clinical Characteristics of Severe Fever with Thrombocytopenia Syndrome. Int J Gen Med. 2020;13:1661–1667. doi:10.2147/IJGM.S292735
25. Seo JW, Kim D, Yun N, Kim DM. Clinical update of severe fever with thrombocytopenia syndrome. Viruses. 2021;13(7). doi:10.3390/v13071213
26. Yang Z, Hu Q, Feng Z, Sun Y. Development and validation of a nomogram for predicting severity in patients with hemorrhagic fever with renal syndrome: a retrospective study. Open Med-Warsaw. 2021;16(1):944–954. doi:10.1515/med-2021-0307
27. Kim MC, Chong YP, Lee SO, et al. Differentiation of severe fever with thrombocytopenia syndrome from scrub typhus. Clin Infect Dis. 2018;66(10):1621–1624. doi:10.1093/cid/cix1119
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Comprehensive Analysis Exploring the Vital Role of the Systemic Immune-Inflammatory Index Upon Admission in Severe Hemorrhagic Fever with Renal Syndrome
Yao L, Wang X, Wang Z, Wang X
International Journal of General Medicine 2024, 17:4857-4866
Published Date: 23 October 2024
Predictors of Severity in Hemorrhagic Fever with Renal Syndrome
Huang L, Wu J, Luo J, Gu W
International Journal of General Medicine 2025, 18:2033-2045
Published Date: 9 April 2025





