Back to Journals » Journal of Pain Research » Volume 18
A Review of Nonsurgical Neurolytic Procedures for Neuropathic Pain
Authors Gupta M , Abdallah RT, Abd-Elsayed A, Chakravarthy K, Day M, Deer T , Diwan S , Knezevic NN , Mehta ND , Schatman ME , Soin A, Staats P
Received 15 August 2024
Accepted for publication 5 February 2025
Published 25 February 2025 Volume 2025:18 Pages 879—895
DOI https://doi.org/10.2147/JPR.S491330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael A Ueberall
Mayank Gupta,1 Rany T Abdallah,2 Alaa Abd-Elsayed,3 Krishnan Chakravarthy,4 Miles Day,5 Timothy Deer,6 Sudhir Diwan,7 Nebojsa Nick Knezevic,8 Neel D Mehta,9 Michael E Schatman,10 Amol Soin,11 Peter Staats12
1Kansas Pain Management, Overland Park, KS, USA; 2APICO Pain Management, Bear, DE, USA; 3Anesthesiology Department, University Hospital, Madison, WI, USA; 4Solaris Research Institute, San Diego, CA, USA; 5Department of Anesthesiology, Texas Tech University Health Sciences Center, Lubbock, TX, USA; 6The Spine and Nerve Center of the Virginias, Charleston, WV, USA; 7Advanced Spine on Park Avenue; Department of Anesthesiology, Albert Einstein Medical College, Bronx; Pain Attending, Lenox Hill Hospital/Northwell Health, New York, NY, USA; 8Department of Anesthesiology College of Medicine, University of Illinois at Chicago; Advocate Illinois Masonic Medical Center, Chicago, IL, USA; 9Och Spine at Weill Cornell New York Presbyterian Hospital, New York, NY, USA; 10Department of Anesthesiology, Perioperative Care, and Pain Medicine, NYU Grossman School of Medicine; Department of Population Health, Division of Medical Ethics, NYU Grossman School of Medicine, New York, NY, USA; 11Ohio Pain Clinic, Centerville, OH; Department of Surgery, Wright State University, Dayton, OH, USA; 12National Spine & Pain Centers, Premier Pain Centers, Atlantic Beach, FL, USA
Correspondence: Mayank Gupta, Kansas Pain Management, 10995 Quivira Road, Overland Park, KS, USA, Email [email protected]
Introduction: Ideally, a physical or chemical nonsurgical neurolytic procedure provides targeted neurolysis to relieve pain for a suitable length of time without causing complications. This narrative review focuses on five nonsurgical neurolytic procedures that are well-established and well-documented in the literature for the treatment of refractory neuropathic pain and peripheral neuropathies, in particular: two physical nonsurgical neurolytic techniques (cryoablation and radiofrequency ablation) and three chemoneurolytic agents (alcohol injection, phenol injection, and a high-concentration capsaicin 8% topical system).
Methods: Using the definition of nonsurgical physical and chemical neurolytic procedures for neuropathic pain, a focused literature search of the PubMed database for English-language, human studies published through July 2024 included, but was not limited to, the following search terms: “neuropathic pain” AND “cryoablation”, “cryoneurolysis”, “radiofrequency ablation”, “alcohol neurolysis”, “alcohol injection”, “phenol neurolysis”, “phenol injection”, “chemoneurolysis”, “topical capsaicin”, and “TRPV1.” While attempts were made to identify prospective clinical trials for each type of neurolytic procedure, information regarding the conduct and safety and efficacy of some of these nonsurgical neurolytic procedures was primarily limited to case studies and anecdotal evidence.
Results: The risk benefit basis of each technique is discussed, and recommendations for proper use based on the literature are summarized. Most techniques require ultrasound or fluoroscopy guidance. Pain relief typically ranges from 3 to 12 months, with repeat neurolytic procedures often required to maintain suitable levels of pain relief.
Conclusion: The authors provide their insights as to the best utilization of these identified nonsurgical physical and chemoneurolytic procedures for the treatment of refractory neuropathic pain in different patient populations based on neural targets. Together, these five nonsurgical neurolytic techniques provide patients and physicians with a variety of options for the treatment of refractory neuropathic pain.
Keywords: alcohol injection, capsaicin 8%, chemoneurolysis, cryoablation, neuropathic pain, peripheral neuropathy, phenol injection, radiofrequency ablation, topical
Graphical Abstract:

Introduction
Neurolysis, also referred to as ablation, involves the intentional application of physical or chemical neurolytic agents to a nerve to cause nerve fiber degeneration. Neurolytic agents are used to treat both cancer pain and long-term chronic pain of noncancer origin. This narrative review focuses on five nonsurgical neurolytic procedures that are well-established and well-documented in the literature for the treatment of refractory neuropathic pain. Two physical neurolytic procedures utilize cold (cryoneurolysis/ cryoablation) or heat (radiofrequency ablation) to produce lesions, and three neurolytic procedures use chemoneurolytic agents, including alcohol injection, phenol injection, or a high-concentration capsaicin 8% topical system (HCCTS). To the best of our knowledge, this is the first article to review these five nonsurgical neurolytic procedures together. Other potential treatments for neuropathic pain, including low-level laser, high-level laser, LED therapy, and extracorporeal shockwave therapy, were considered to be outside the scope of and, therefore, not included in this review because they are not generally identified in the literature as nonsurgical neurolytic procedures.
Methods
This publication attempts to rationally analyze data for the five identified nonsurgical neurolytic procedures. As this is a narrative review, rather than a systematic or scoping review, PRISMA guidelines were consulted but not strictly followed. Using the definition of nonsurgical neurolytic procedures for neuropathic pain, a focused literature search of the PubMed database for English-language, human studies published through July 2024 included, but was not limited to, the following search terms: “neuropathic pain” AND “cryoablation”, “cryoneurolysis”, “radiofrequency ablation”, “alcohol neurolysis”, “alcohol injection”, “phenol neurolysis”, “phenol injection”, “chemoneurolysis”, “topical capsaicin”, and “TRPV1.” A review of the reference lists of identified sources was also performed to gather additional potentially relevant articles. While attempts were made to identify prospective clinical trials for each type of neurolytic procedure, information regarding the conduct and safety and efficacy of some of these nonsurgical neurolytic procedures was primarily limited to case studies and anecdotal evidence. We acknowledge the potential limitations and inherent bias associated with utilizing a single database (PubMed) and lack of systematic/scoping review methodology. Future work should incorporate broader database searches and systematic methodologies.
Brief Overview of Peripheral Neuropathic Pain
Peripheral neuropathies associated with neuropathic pain include diabetic peripheral neuropathy (DPN), postherpetic neuralgia (PHN), HIV infection, trigeminal neuralgia/neuropathy, painful radiculopathy, amputation (eg, phantom limb pain), peripheral nerve injury (eg, following surgery or trauma), chemotherapy-induced peripheral neuropathy, central post-stroke pain, complex regional pain syndrome, and chronic inflammatory demyelinating polyneuropathy.1,2 It is important to note that not all patients with peripheral neuropathy develop neuropathic pain.2 Neuropathic pain is defined by the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain as pain that occurs as a direct result of damage to or lesions of the somatosensory nervous system.3 Neuropathic pain distribution can be focal or generalized, which is typically symmetrical.2 In the distal extremities, “glove and stocking” distribution can occur due to pain in the feet, calves, hands, and forearms. Patients often describe their spontaneous or evoked pain as burning, shooting, pricking, pins and needles, squeezing, or freezing pain or an electrical-like sensation.4 There may also be abnormal sensations, including dysesthesias (abnormal unpleasant painful, itching, burning, or restrictive sensations) and paresthesias (abnormal sensations such as tingling, numbness, skin crawling, or itching sensations).4 In addition to sensory loss, there may be allodynia (pain due to a stimulus that does not normally provoke pain) and hyperalgesia (unusually severe or more intense pain from a stimulus that normally provokes pain).4
In the largest epidemiologic study of neuropathic pain to date, conducted in the UK, the overall prevalence of neuropathic pain was 9.2%.5 Chronic neuropathic pain occurs more frequently in women (8% vs 5.7% in men) and in individuals over the age of 50 years (8.9% vs 5.6% in those <49 years of age) and most commonly affects the lower back and lower limbs, neck, and upper limbs.6 The prevalence of neuropathic pain is likely to increase due to the aging global population, increased incidence of diabetes mellitus, and increasing rates of cancer and the consequences of chemotherapy, all of which affect sensory nerve fibers.2 The health impact and quality of life burden of neuropathic pain is higher than for non-neuropathic pain, leading to increased drug prescriptions, healthcare visits, sleep disturbances, anxiety, and depression.5,7–9
The Case for Neurolysis
Mechanistically, neuropathic pain is dissimilar to other chronic pain conditions and is, therefore, treated differently.2 As neuropathic pain does not typically respond to analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) or opioids, it can be difficult to successfully treat, with fewer than half of patients experiencing 50% or greater pain relief with currently available pharmacological treatments.10,11 Consequently, symptoms persist and tend to become chronic and refractory. Oral pharmacotherapies for neuropathic pain include anticonvulsants including gabapentinoids, tricyclic/ tetracyclic antidepressants, selective serotonin/ norepinephrine reuptake inhibitors such as duloxetine and venlafaxine, tapentadol, tramadol, and, in some cases, stronger opioids, which carry the potential for dependency. Topical treatments for neuropathic pain include local anesthetics, compounded agents, including ketamine and gabapentin, and capsaicin in multiple formulations. Neurolytic procedures can be used in combination with oral pharmacotherapies without increasing a patient’s pill burden.
If oral pharmacotherapy or topical application of lidocaine or low-dose capsaicin fail to provide sufficient pain relief, then physical and chemical neurolytic techniques, brain neuromodulation/neurostimulation techniques,12,13 or surgical neurolytic techniques may be considered. Surgical neurolytic procedures are permanent and are not ideal for the treatment of neuropathic pain. This review focuses on nonsurgical neurolytic procedures, which interrupt signal transmission and, for some patients, provide lasting pain relief. As illustrated in Table 1, none of the nonsurgical neurolytic (physical and chemical) techniques discussed in this review destroy the nerve; if the dorsal root ganglion is left intact, the nerve is capable of regeneration, and subsequently, pain can theoretically return.
 |
Table 1 Comparison of Representative Nerve Ablation and Modulation Methods |
Pain sensations are transmitted via peripheral nerves to the dorsal root ganglion. The pathology of peripheral disorders that cause neuropathic pain predominantly involves the small, unmyelinated C- fibers and myelinated A-fibers, namely, Aβ- and Aδ-fibers. As opposed to central nervous system damage, peripheral nerves can regenerate following an injury. As briefly reviewed by Stierli et al in 2018,14 the peripheral nerve regenerative process includes guiding axonal regrowth across the site of injury, opening the blood-nerve barrier and controlling the inflammatory response, remodeling the environment surrounding the nerve, and promoting the regrowth of surviving axons. In the case of patients suffering from painful DPN, peripheral nerve fibers neurolysed with HCCTS regenerate in a healthier state with increased nerve fiber density and improved sensory thresholds and axon-reflex vasodilatation.15
Classification of Peripheral Nerve Injury and Appropriate Use of Neurolytic Techniques
Neurolysis involves the deliberate injury of a nerve by freezing or heating or the application of chemicals to cause a temporary degeneration of targeted nerve fibers, causing an interruption along the nerve in the signal nerve transmission. In particular, neurolysis implies the destruction of neurons by placing a needle close to the nerve and either injecting neurodestructive chemical agents or producing damage with a physical method such as cold (ie, cryotherapy) or heat (ir, radiofrequency ablation, RFA). As illustrated in Figure 1, a schematic representation of a peripheral nerve, individual myelinated axons and groups of unmyelinated axons are surrounded by the endoneurium. Collections of axons enclosed by the perineurium are called fascicles. The internal epineurium (epifascicular) lies between fascicles, which make up the peripheral nerve trunk. The external epineurium (epineurial) surrounds the nerve trunk. The prognosis for nerve recovery depends on the extent of peripheral nerve injury (PNI).16
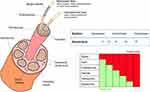 |
Figure 1 Comparison Between Seddon’s and Sunderland’s Classification of Peripheral Nerve Injury. Sunderland subdivided axonotmesis into three types with different degrees of nerve disruption and different capabilities for spontaneous regeneration. Reproduced from Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural ablation and regeneration in pain practice. Korean J Pain. 2016;29(1):3–11. Copyright © The Korean Pain Society, 2016. Creative Commons Attribution Non-Commercial License.16 |
Two grading systems define the degree of PNI. The Seddon classification defines three degrees of PNI: neurapraxia (non-action), axonotmesis, and neurotmesis (cutting).17 Neurapraxia is used to describe a short-lived paralysis followed by recovery without any evidence of true regeneration. Axons are intact but nonfunctional, and there are no structural changes to the myelin. Segmental demyelination results in motor and sensory loss, but no axon disruption or Wallerian degeneration is observed. Wallerian degeneration refers to the active, well-orchestrated process of anterograde degeneration of the distal end of an axon, Schwann cells, and myelin sheaths that occurs after peripheral nerve injury via trauma, toxic, ischemic, or metabolic events. This axon death is distinct from apoptosis and involves breakdown of the blood-nerve barrier, recruitment of circulating macrophages, reorganization of the endoneurial space, changes in the endoneurial extracellular matrix, and increased neurotrophin and cytokine production, as reviewed in 2011 by Dubovy et al.18 With axonotmesis, there is actual damage to the peripheral nerve fibers, which results in complete peripheral degeneration. The axon and its myelin sheath break into fragments, and Wallerian degeneration occurs. In third-degree PNI, neurotmesis, there is complete neural separation, including loss of most of the connective tissue and distortion of the epineurium. Because axonotmesis involves damage to the axon of a nerve fiber but preserves the surrounding connective tissue, while neurotmesis involves complete disruption of both the axon and the connective tissue, axonotmesis is considered to be a less severe nerve injury compared to neurotmesis.
Sunderland19,20 further subcategorized Seddon’s PNI classification system by splitting axonotmesis into three categories based on the severity of nerve injury: second degree, third degree, and fourth degree. In second-degree PNI, there is axonal discontinuity, but the endoneurium, fascicular arrangement, and perineurium are preserved. In third-degree PNI, the myelin, axon, and endoneurium are disrupted, but the fascicular arrangement is preserved. Finally, in fourth-degree PNI, only the epineurium remains intact.
The goal of neurolytic procedures is to produce a reversible injury, which involves third-degree PNI to the myelin, axon, and endoneurium without disrupting the fascicular arrangement, perineurium, and epineurium.16 Ideally, a physical or chemical neurolytic technique provides satisfactory neurolysis for a suitable duration of pain relief without causing complications, which include neuritis (nerve inflammation secondary to injury), anesthesia dolorosa (deafferentation pain after traumatic or surgical injury to the trigeminal nerve), or reflex or motor deficits.16 While the goal is a reversible injury, in the informed consent process, the risk of permanent and irreversible injury should be considered.
Cryoneurolysis (Cryoablation)
Cryoneurolysis/cryoablation uses an “ice ball” formed at the tip of a probe (see Figure 2) that, when applied to a nerve, temporarily interrupts nerve conduction.21,22 After cryoneurolysis, myelin sheaths and axons degenerate via Wallerian degeneration, but nerve structure (perineurium, epineurium, and basal lamina) remain intact, allowing nerve regeneration and physiologic restoration of the neuronal structure.22–24 In cryoneurolysis procedures, neuromas are unlikely to form because inflammatory reactions are minimal.22 To perform cryoneurolysis, the optimal treatment temperature is between –60°C and –100°C. Warmer temperatures lead to insufficient nerve lesions/ pain reduction, and colder temperatures may be associated with permanent nerve damage.25 After cryoneurolysis, damaged axons regenerate at a rate of approximately 1 to 3 mm per day.26
 |
Figure 2 Cryoneurolysis Devices. (A) Needle Tips from Handheld Cryoneurolysis Devices. (B) FDA-Approved Handheld Cryoneurolysis Devices Used in Combination with Ultrasound Guidance. Reproduced from: Biel E, Aroke EN, Maye J, Zhang SJ. The applications of cryoneurolysis for acute and chronic pain management. Pain Practice. 2023;23:204–215. © 2022 The Authors. Pain Practice published by Wiley Periodicals LLC on behalf of World Institute of Pain. Creative Commons CC-BY-NC-ND.27 |
Few randomized studies demonstrating the long-term effects of cryoneurolysis for peripheral mononeuropathies exist, but results have been published for cervicogenic headache, osteoarthritis, and nociceptive knee pain.28–31 Several case reports have suggested heterogeneous responses to cryoneurolysis for the treatment of acute and chronic pain.27 The use of cryoneurolysis to treat chronic lower extremity phantom pain yielded pain relief lasting fewer than 4 months, suggesting success but the need for repeat procedures.32 Recent applications for cryoneurolysis include postoperative pain management following mastectomy,32 total knee arthroplasty,33 or idiopathic trigeminal neuralgia.34
A retrospective cohort study was conducted involving 24 patients with chronic refractory peripheral mononeuropathy who had been treated with cryoneurolysis.22 For inclusion in the study, patients were required to have neuropathic pain attributable to a specific peripheral nerve, be nonresponsive to noninvasive therapy, and experience at least 50% pain relief following two ultrasound-guided prognostic differential blocks (first infiltration with lidocaine 2%; second infiltration with ropivacaine 0.5%). Most of the nerves treated included the intercostal, subcostal, ilioinguinal/ hypogastric, superficial peroneal, and saphenous nerves. More than half of patients received pretreatment with anticonvulsants, opioids, antidepressants, and/or topical lidocaine or capsaicin. The cryoneurolysis procedure was performed by three pain specialists and required a previously placed peripheral venous indwelling catheter, ultrasound guidance, lidocaine 2% before the procedure, and ropivacaine 0.5% after the procedure. Two to three lesions per nerve were created by keeping the probe in place for 2 minutes each at a temperature of minus 78°C. At 1 month, 54.2% reported significant pain reduction of at least 30%. This decreased to 13.8% at 3 months and 9.1% at 6 months, which failed to reach statistical significance. Thus, repeated cryoneurolysis may be a viable treatment option for patients with refractory peripheral mononeuropathy, although additional investigation will be necessary to determine the duration of relief.
In a prospective case study of 22 patients with refractory chronic peripheral neuropathy who underwent cryoneurolysis, pain relief was noted for up to 12 months following the procedure, although half of the patients received repeat cryoneurolysis during this timeframe.35 Cryoneurolysis was performed under sedation and with additional pain medication before, during, and after the intervention. It is important to note that a significantly lower temperature (–135°C to –160°C) was used in this study than that usually applied, and colder temperatures are associated with a higher risk of permanent nerve damage.25,36 In this study, however, there were no complications from the procedures.
Per the 2010 American Society of Anesthesiologists’ task force on chronic pain management and the American Society of Regional Anesthesia and Pain Medicine guidelines (hereafter referred to as the ASA/ASRAPM guidelines) for select patients with lumbar facet joint pain, low back pain (medial branch), post-thoracotomy neuralgia, or peripheral nerve pain, cryoneurolysis can result in pain relief ranging from 1 to 12 months.37
Radiofrequency Ablation: Thermal (Conventional, Continuous) and Pulsed Radiofrequency Ablation
Thermal radiofrequency ablation (RFA) utilizes a constant, high-frequency (500 kHz) alternating current to create a thermal neurodestructive lesion at the active tip of an insulated cannula.16,38 The alternating current induces a coagulative necrosis in the target tissue, and an electrical field causes charged molecules to oscillate and generate heat in the surrounding tissues when the probe temperature reaches between 60°C and 80°C. The magnitude of the tissue destruction is related not only to the distance from the electrode tip but also to tissue temperature, electrode size, and procedure duration.38 As illustrated in the ultrasound-guided images in Figure 3, the lesion that is created with RFA is spherical in shape and well circumscribed, unlike with chemical neurolysis.38 In clinical practice, the cannula should be placed parallel to the target nerve, which can be difficult.39 The entire procedure, from start to finish, usually requires 30 minutes, but time may vary based on anatomical complexity and physician experience.
 |
Figure 3 Pulsed Radiofrequency Ablation in the Thoracic Dorsal Root Ganglion for the Treatment of Postherpetic Neuralgia. (A) Oblique view. The target point is below the pedicle. (B) Anteroposterior view. The needle is advanced to the dorsal root ganglion below the pedicle. (C) Lateral view. The depth of the needle is adjusted under the lateral view. A contrast medium spreads to the left posterior epidural space, and the dorsal root ganglia become apparent. Reproduced from Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural ablation and regeneration in pain practice. Korean J Pain. 2016;29(1):3–11. Copyright © The Korean Pain Society, 2016. Creative Commons Attribution Non-Commercial License.16 |
According to the ASA/ASRAPM guidelines, good evidence exists for the relief of low back pain for periods of 2 to 6 months using conventional (80°C) or thermal (67°C) RFA.37 Pain relief for periods up to 6 months have been reported for patients with neck pain and no radiculopathy.37 Pain relief for periods of up to 3 months have been reported for patients with chronic sacroiliac joint pain.37 Anecdotal reports represent the normative response, but longer durations of effect have been reported in the literature. Given these results, the ASA/ASRAPM guidelines recommend the use of conventional/constant focused RFA of the medial branch nerves to the facet joint for patients with low back (medial branch) pain when previous diagnostic or therapeutic injections of the joint or medial branch nerve have provided temporary relief.37 Additionally, the ASA/ASRAPM guidelines recommend conventional RFA for neck pain and water-cooled RFA for chronic sacroiliac joint pain.37 Finally, the guidelines recommend against the routine use of conventional or thermal RFA of the dorsal root ganglion for the treatment of lumbar radicular pain due to the lack of high-quality evidence and the potential for complications.37
Pulsed RFA also uses a 500 kHz high frequency current, but it is applied at a rate of two bursts per second, with each pulse lasting 20 ms over a 120-second interval.16 During pulsed RFA, each “silent” phase (480 ms) provides time for heat to dissipate, which generally keeps the target tissue below 42°C.38 Here, it is the electromagnetic field rather than heat that is used to treat neuropathic pain.16 Rabbit and rat models have been used to demonstrate that pulsed RFA results in transient endoneurial edema rather than the Wallerian degeneration caused by continuous RFA.38 Studies of the internal ultrastructural components of axons have demonstrated microscopic damage to the mitochondria and mitochondrial membranes and disruption and disorganization of cellular microfilaments and microtubules. Additionally, the microscopic damage appears to be more pronounced in unmyelinated C-fibers compared with thickly myelinated Aδ- and Aβ-fibers.40 Compared with conventional thermal RFA, pulsed RFA should be used for a longer duration and in a repeated manner.16 The advantages of pulsed over continuous RFA are that pulsed RFA has broader applicability to neuropathic pain and is associated with fewer side effects compared to continuous RFA.38 Pulsed RFA is currently used to treat PHN that has scarred the dorsal horn of the spinal cord and the dorsal root ganglion and may be applied to the injured dorsal root ganglion in type 2 complex regional pain syndrome.16,38 However, because peer reviewed studies of pulsed RFA are only now beginning to appear in the medical literature, in the United States, pulsed RFA is seen as a relatively new technique, and many insurance companies do not cover the procedure. As data continue to evolve, this will likely change, particularly as pulsed RFA techniques are frequently performed in Europe with high rates of success.
Chemoneurolysis
Chemoneurolysis is defined as the dose-dependent destruction of a nerve, resulting in necrosis, death, Wallerian degeneration, and a complete conduction block in all fibers contained within the nerve. Neurolytic agents used in chemolysis include injections of either alcohol or phenol or topical application of high-concentration capsaicin (Figures 4–6). Agents such as alcohol and phenol have the potential for off-target tissue damage, which can lead to skin sloughing and damage to other, non-nerve tissue. Concerns with these agents also include post-procedure neuritis. In addition, in some settings, such as the celiac plexus, attention must be paid to the vasculature, since vascular damage and catastrophic injury, although rare, can occur. Chemical neurolysis with alcohol or phenol, thus, is typically a therapy of last resort.41 The ASA/ASRAPM guidelines stipulate that, based on the quality of evidence obtained only from observational studies and case reports, chemoneurolysis should not be used in the routine care of non-cancer patients with chronic pain.37 Unlike alcohol and phenol, HCCTS, although a chemoneurolytic agent, is not toxic to surrounding tissues due to its selectivity for transient receptor potential vanilloid 1 (TRPV1)-expressing nerve fibers.
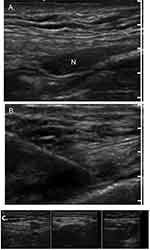 |
Figure 4 Chemoneurolytic Methods for Neuropathic Pain: Alcohol injection. (A) Ultrasound-guided alcohol neurolysis for an inguinal neuroma. (B) Satisfactory needle and injectate placement into the inguinal neuroma. (A) and (B) Reproduced with permission from: Reeves RA, Miller CJ, Wang D, Ng A, Heller JE, Nazarian LN. Use of high-resolution ultrasound to guide alcohol neurolysis for chronic pain. Pain Physician. 2022;25:E1297-E1303. CC-BY-NC.42 (C) Representative images of alcohol neurolysis to the neuroma. Reproduced from Zhang X, et al. Ultrasound-guided alcohol neurolysis and radiofrequency ablation of painful stump neuroma: effective treatments for post-amputation pain. J Pain Res. 2017;10:295–302. © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited under the terms of http://creativecommons.org/licenses/by-nc/3.0/.43 |
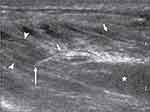 |
Figure 5 Chemoneurolytic Methods for Neuropathic Pain: Phenol injection. 73-year-old man with stump neuroma of right tibial nerve after traumatic amputation 30 years ago. Sonographic image shows phenol instillation into neck (arrowheads) of stump neuroma (asterisk). Tip (long arrow) of needle (short arrows) is positioned intraneurally with fusiform widening of targeted nerve segment caused by injected phenol. Surrounding hypoechoic patchy fluid accumulations are caused by local anesthetic. Reproduced with permission of the American Roentgen Ray Society from: Gruber H, Glodny B, Bodner G, Kopf H, Bendix N, Galiano K, et al. Practical experience with sonographically guided phenol instillation of stump neuroma: predictors of effects, success, and outcome. AJR. 2008;190:1263–1269.44 |
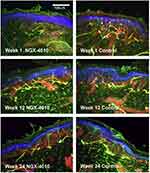 |
Figure 6 Chemoneurolytic Methods for Neuropathic Pain: Capsaicin 8% Topical System. Representative images of epidermal nerve fiber (ENF) density in skin areas exposed to NGX-4010, and in unexposed control areas on the thighs of healthy volunteers. The 6 panels show immunostaining at the time points indicated in the insets. ENF and dermal nerves (DN), immunoreactive for PGP9.5, are stained yellow or green; the basement membrane, immunoreactive for Type IV collagen, at the dermal-epidermal junction (DEJ) and around blood vessels is red, and the epidermis is blue. ENFs are clearly lower in density 1 week following capsaicin patch application as compared with control biopsies. ENFs have increased in density 12 weeks after patch application, but still lower than control biopsies. At 24 weeks, ENF density appears similar to the control biopsy. Scale bar=100 µm. Reproduced from Kennedy WR, Vanhove GF, Lu S-P, Tobias J, Bley KR, Walk D, et al. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain. 2010;11(6):579–58745. Copyright © 2010 American Pain Society. Published by Elsevier Inc. All rights reserved. |
Alcohol Chemoneurolysis via Injection
When injected at a concentration of at least 35% to 60%, ethyl alcohol (ethanol) functions as a nonselective chemoneurolytic agent and can cause a burning sensation on injection.46 Alcohol can spread rapidly from the site of injection; therefore, large-volume injections are often necessary for effective chemoneurolysis.46 For visceral cancer pain or pain recurrence following thermal conventional or continuous RFA, alcohol neurolysis is routinely used to increase the post-procedure duration of pain relief.16 Alcohol neurolysis of peripheral nerves is also used to treat spasticity.47
Once injected, alcohol damages nerves by nonselectively denaturing proteins, extracting cholesterol, phospholipids, and cerebroside from the neural membranes, and precipitating mucoproteins and lipoproteins.48,49 This can lead to retrograde Wallerian degeneration of the nerve fibers.47
Ultrasound is the preferred technique to visualize and localize nerves and is frequently used to guide alcohol neurolysis, as illustrated in Figure 4. Upon intrathecal injection, patients are tilted forward 45 degrees so that the dorsal sensory rootlets but not the ventral motor rootlets are affected.46,50 In some settings, physicians choose fluoroscopy as a mechanism of guidance for alcohol or phenol neurolysis. This method uses landmarks to place the needle and is often preferred by those not proficient in ultrasound and in settings where ultrasound is not available.51
In one retrospective case series of 35 patients, 23 of whom had refractory peripheral neuropathy, ultrasound-guided alcohol neurolysis resulted in a durable average 2- to 3-unit reduction in pain scores at 1 week (P<0.001), 1 month (P<0.001), and 1 year (P=0.002) following alcohol neurolysis; however, one third of patients with aberrant postsurgical anatomy progressed to surgery due to decreased efficacy in this patient subset.42 Overall, 60% of patients reported that alcohol neurolysis resulted in more effective pain relief than oral pain medications. In this study, alcohol neurolysis was performed in an operating room with the patient under moderate sedation and after administration of 1% lidocaine. Approximately 1 to 2 mL of 99% ethanol was injected into the nerve followed, after 2 minutes, by an injection of steroid and local anesthetic to ease postprocedural inflammation. Prior to alcohol neurolysis, patients had undergone a median of two steroid injections, with a median interval between steroid injections of 3.7 months.
Other successful cases of using ultrasound-guided alcohol neurolysis for pain management include neurolysis of the lateral femoral nerve for intractable meralgia paresthetica, a rare sensory entrapment mononeuropathy,52 and in combination with and without RFA for painful stump neuroma.43,53 In a retrospective case series of 6 patients with meralgia paresthetica, 3 mL of 50% alcohol in 0.25% bupivacaine was injected, and resulting pain relief lasted for up to 12 weeks.52 In the initial study of alcohol neurolysis of painful stump neuroma, 1.2 mL of anhydrous alcohol was injected into the nerves proximal to the neuroma, and when pain returned several months later, repeat neurolysis with 0.3 mL of anhydrous alcohol was performed.53 In the second study of painful stump neuroma, 10 mL of local anesthetic was administered around the nerve proximal to the neuroma prior to administering 2 to 5 mL of anhydrous alcohol solution into the neuroma body.43 In this study, 7 of 15 subjects (54%) achieved pain relief following one to three alcohol neurolysis treatments performed 2 weeks apart.
No clear correlation exists between the volume and concentration of alcohol used and associated adverse effects.47 Theoretically, injection of alcohol or phenol (discussed in the next section) could result in seizures, central nervous system depression, and cardiovascular collapse.47 Unlike phenol, a possible complication of alcohol injection is inebriation, potentially limiting its use in children and those with liver impairment.47 Compared to phenol injection, alcohol injection acts more slowly and is more irritating to adjacent tissue.54 However, most studies of alcohol neurolysis have demonstrated limited local site injection pain and sensory dysesthesias that are limited to a few weeks’ duration and that respond well to oral antiepileptic or antidepressant medication.47
Phenol Chemoneurolysis via Injection
Like alcohol, phenol also functions as a chemoneurolytic agent. Phenol is comprised of carbolic acid, phenic acid, phenylic acid, phenyl hydroxide, hydroxybenzene, and oxybenzone.41 When injected near neural structures, phenol causes nonselective nerve destruction by denaturing protein and causing the loss of cellular fatty content, separating the myelin sheath from the axon, and causing axonal edema and Schwann cell dissolution inside basal lamina tubes.46,47,55,56 Ultrasound, as illustrated in Figure 5, is typically used to guide the injection.
Phenol is typically available in an 89% aqueous, glycerin, or lipid solution and must be prepared by the hospital pharmacy.41 When mixed with glycerin, phenol diffuses slowly, resulting in a very limited spread pattern. It is unstable at room temperature and oxidizes in the presence of air and light. The exact concentration of phenol required for neurolysis is not well defined but is thought to range from 3% to 12%.41,46,57
Phenol has local anesthetic properties and often causes a warming sensation upon injection.46 When injected intrathecally, the patient is tilted backward 45 degrees so that the dorsal sensory rootlets are affected but the ventral motor rootlets are not. Phenol has an immediate onset of action and can be titrated to optimize dosing and clinical effect, and injection can be repeated within days rather than weeks or months, if needed.47 According to the ASA/ASRAPM guidelines, an observational study demonstrated that chemoneurolysis using phenol was effective in providing pain relief for patients with neuropathic, facet, or musculoskeletal pain for a period of assessment ranging from 2 to 24 weeks.37
A prospective study of 82 patients involved the use of ultrasound-guided phenol neurolysis for pain management of stump neuroma.44 In this study, up to 0.8 mL of 80% phenol solution was used according to a standardized protocol58 that involved initial instillation of 15 mL of local anesthetic (lidocaine) and repeated phenol neurolysis at 1, 6, and 12 weeks. To prevent spread of the phenol into surrounding soft tissues, the needle was withdrawn during a constant flush with saline solution. Overall, 12 subjects (15%) were pain-free following one to three injections, including 9 subjects who achieved pain relief after the first injection. After 6 months, 38% reported almost unnoticeable pain, and 64% reported pain levels similar to those achieved with other therapies. In 156 phenol installations, there were eight cases (5.1%) of minor complications that were self-limiting with local treatment; these included four cases of unspecific painful local myopathy. In 3 subjects, local skin redness suggested the presence of confined infection, which responded to local therapy. However, major lesions were identified in 1.3% of injections, including one case of infectious erysipeloid, an acute bacterial infection of traumatized skin or other organs, that was treated with systemic antibiotics and one case of toxically induced local soft-tissue necrosis that was treated with surgical resection of the necrotic tissue.
Capsaicin Chemoneurolysis via Topical Application
Capsaicin, a naturally occurring active ingredient found in hot peppers, is a highly selective agonist of the TRPV1 receptor, a calcium-permeable ion channel-receptor complex. TRPV1 receptors are primarily expressed peripherally on small-diameter sensory neurons including C- and Aδ-fibers but can also be found centrally in trigeminal ganglia and the dorsal root.59,60 They are not expressed on neurons in the skin that are involved in cold detection and cold pain or tactile or vibration sensitivity.60,61 When TRPV1 receptors are activated, burning, itching, stinging, and other physiologic responses result.59,60,62
Unlike alcohol and phenol chemoneurolysis, which are delivered via injection, HCCTS is a topically delivered chemoneurolytic agent that is approved by the US Food and Drug Administration for the treatment of neuropathic pain associated with PHN and for neuropathic pain associated with DPN of the feet.63
The effects of capsaicin on pain modulation are complex and driven by multiple mechanisms. First, activation and overstimulation of TRPV1 receptors desensitize the TRPV1-expressing fibers, which suspends pain signals to the brain.64–66 Second, high-concentration capsaicin triggers a chemical cascade that results in temporary, reversible chemoneurolysis of the TRPV1-expressing nerve fibers.15,62,63 High capsaicin concentrations markedly increase intracellular calcium levels, which activate the protease, calpain. Once activated, calpain degrades membranes and cytoplasmic and nuclear substrates, leading to microtubule disorganization and the breakdown of cell architecture, dysfunction of axonal mitochondria, and apoptosis. A mouse model of neuropathic pain demonstrated that calcium/calpain mediates the neurolysis of axonal terminals, which is necessary for long-lasting analgesia. Therefore, high concentrations of capsaicin can lead to long-term “defunctionalization” mediated by the desensitization of capsaicin receptor TRPV1-expressing afferent terminals, temporary loss of membrane potential, inability to transport neurotrophic factors, and reversible retraction or pruning of epidermal nerve fiber terminals.15,62 This deep pruning of nociceptors can prevent the transmission of pain signals and provide significant and sustained analgesia that persists for several months. Other publications have also referenced the chemoneurolytic properties of capsaicin.1,67–71 Finally, unlike any other available pain or neuropathy treatment, in some patients with painful and nonpainful DPN, HCCTS has the potential for disease modification via nerve regeneration and restoration of function of cutaneous nerve fibers.15 Specifically, as demonstrated using skin biopsies, the density of both intraepidermal and subepidermal nerve fibers were significantly increased at 3 months post-HCCTS treatment, and these changes correlated with pain relief and axon-reflex vasodilatation. In fact, subepidermal nerve fiber density, as assessed using a selective marker of regenerating nerve fibers, was not only significantly higher at 3 months than at baseline but also higher than that of control patients. Nerve fiber density after capsaicin application is illustrated in Figure 6.
A matrix topical system with a silicon adhesive has been developed that contains a lipogel with a high concentration of capsaicin (640 µg/cm2) dissolved in glycol ether.64 Using this delivery system not only enables direct, locally confined contact between the skin and the active substance, capsaicin, but it also creates a forced diffusion of high-concentration capsaicin into the skin such that a cutaneous reservoir of bioavailable capsaicin is formed within the epidermis where it activates TRPV1 receptors.64 This reservoir is sufficient to enable pain relief for up to 3 months.63 Each 280 cm2 HCCTS contains a micro-reservoir of 179 mg of capsaicin. Once in the skin, very little systemic absorption of capsaicin occurs; in fact, it is generally so low that it is expected to have little or no pharmacologic or toxicologic relevance.59,62–64,72 Owing to the low systemic exposure, drug-drug interactions between HCCTS and systemic medications are highly unlikely, and dose adjustments are not required for patients with hepatic or renal failure.59,63
HCCTS is applied in a clinical setting by a healthcare professional, and patients can be pretreated with a topical anesthetic or oral medications to proactively address potential application-site pain.63,73 HCCTS remains in place for 30 to 60 minutes, depending on the treatment indication and subsequent body location.63,64 Although HCCTS may be associated with transient application-site reactions, for most patients, these dissipate within 48 hours of application.73
A 12-week, randomized, controlled, Phase 3 trial evaluated the efficacy and safety of a single, 30-minute application of HCCTS relative to placebo for the treatment of 386 patients with painful DPN of the feet. In this trial, HCCTS provided sustained pain relief for up to 12 weeks.74 Compared to placebo, during Weeks 2 to 8, mean daily pain scores improved from baseline to a significantly greater extent with HCCTS (–27.4% vs –20.9%; P=0.025). Analysis of the secondary endpoint, change in average daily pain score from baseline to between Weeks 2 to 12, demonstrated that this reduction in mean daily pain scores was maintained (–28.0% vs –21.0%, P=0.018). Results from a 52-week, open-label, randomized trial75–77 provided evidence of the long-term safety and efficacy of up to seven HCCTS applications for the treatment of painful DPN of the feet. The study also demonstrated that HCCTS does not cause any deterioration in sharp, cold, or warm sensory perception.
Similarly, the efficacy of HCCTS in the treatment of PHN has also been demonstrated in several randomized, controlled trials.78–80 In a multicenter, double-blind, parallel-group trial of 402 patients, those treated with a 60-minute application of HCCTS, compared with those treated with a low-concentration capsaicin control patch, exhibited a significant (P=0.001) 9.7% greater reduction in pain scores 2 to 8 weeks post-application (–29.6% vs –19.9%, respectively) and significant improvements in pain during Weeks 2 to 12 (–29.9% vs –20.4%, P=0.002). Overall, 42% achieved ≥30% reduction in mean pain scores. Irving et al79 also conducted a multicenter, randomized, double-blind study involving 418 patients and found that HCCTS-treated patients had a greater mean reduction from baseline in pain during Weeks 2 to 8 compared with control (–32.0% vs –24.4%, P=0.011). Forty-six percent achieved ≥30% reduction in pain scores compared with 34% of controls (P=0.02). In a third trial,80 HCCTS reduced neuropathic pain in PHN patients—even among those patients who were taking concomitant systemic (oral) neuropathic pain medications.
In clinical trials, most treatment-emergent adverse drug reactions (ADRs) reported with the capsaicin 8% topical system were application-site reactions, including burning, pain, erythema, pruritus, papules, swelling, and dryness, and were generally transient, self-limiting, and typically mild or moderate in intensity.63 No drug-related ADRs led to permanent discontinuation, and no drug-related serious ADRs or deaths occurred.
Conclusion
This narrative review has focused on five nonsurgical neurolytic procedures that may be used to treat patients with refractory neuropathic pain and peripheral neuropathies, in particular. Ideally, a physical or chemical neurolytic technique provides targeted neurolysis to relieve pain for a suitable length of time without causing complications. Two techniques, cryoablation and radiofrequency ablation, are physical techniques that utilize the application of extreme cold and heat, respectively, to temporarily interrupt nerve conduction and relieve pain. Unlike chemoneurolytic lesions, lesions created with cryoablation and radiofrequency ablation tend to be spherical and well circumscribed. There is a potential for permanent nerve damage, although ultrasound or fluoroscopy guidance has made these procedures safer. Effects typically last on the order of 3 to 12 months, although repeat procedures may be required to maintain suitable pain relief. Three additional techniques utilize chemoneurolytic agents. Two injectable chemoneurolytic agents, alcohol and phenol, and one topical chemoneurolytic agent, HCCTS, have been used to relieve neuropathic pain. Both types of injections (alcohol and phenol) require the use of ultrasound or fluoroscopy to guide the injection and may cause posttreatment infection or neuritis. Alcohol and phenol are nonselective agents, which can lead to off-target tissue damage and post-procedure neuritis, whereas HCCTS does not because it is a highly selective agonist of the TRPV1 receptor. HCCTS is associated with temporary (up to 48 hours) application-site reactions, but these are generally transient, self-limiting, and of mild or moderate intensity. With these chemoneurolytic procedures, pain relief, again, is on the order of 3 to 12 months, and, as with the physical neurolytic methods, repeat chemoneurolytic procedures may be required to maintain suitable levels of pain relief. Together, these five nonsurgical neurolytic techniques provide patients and physicians with a variety of options for the treatment of refractory neuropathic pain.
Acknowledgment
Medical writing and editorial assistance was supported by Katherine Hasal, MS, and Averitas Pharma, Inc. Authors thank Content Ed Net for editorial assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Medical writing and editorial assistance was funded by Averitas Pharma.
Disclosure
Dr. Gupta has received financial support from AbbVie, Inc., Abbott Laboratories, Baudax Bio, Inc. Boston Scientific Corporation, GRT US Holding, Inc., Horizon Therapeutics plc, MML US, Inc., Medtronic, Inc., Nalu Medical, Inc., Nevro Corp., PAINTEQ, LLC, Relievant Medsystems, Inc., SPR Therapeutics, Inc., Spinal Simplicity, LLC, Stimwave Technologies, Inc., SurGenTec, and Vertos Medical, Inc.
Dr. Abdallah has received financial support from AbbVie, Inc., Abbott Laboratories, Avanos Medical, Biohaven Pharmaceutical Holding Company, Ltd., Boston Scientific Corporation, Cerapedics, Inc., Kowa Pharmaceuticals America, Inc., Medtronic, Inc., Nalu Medical, Inc., PAINTEQ, LLC, Relievant Medsystems, Inc., SPR Therapeutics, Inc., Saluda Medical Americas, Inc., Scilex Pharmaceuticals, Inc., Stimwave Technologies Incorporated.
Dr. Abd-Elsayed has received financial support from Avanos Medical, Curonix, LLC, and Medtronic, Inc.
Dr. Chakravarthy is a consultant for Medtronic, Mainstay Medical. He has stock options in Neuronoff, Mainstay Medical. He has founders equity in NXTSTIM and Solaris Research Institute.
Dr. Day has received restricted educational grants from Abbott Laboratories, Boston Scientific Corporation, and Medtronic.
Dr. Deer is a consultant for Abbott Laboratories, Boston Scientific Corporation, Comorloc, PAINTEQ, LLC, Saluda Medical, Spina Simplicity, SPR Therapeutics, Inc., and Vertos Medical, Inc.
Dr. Diwan has received financial support from Abbott Laboratories, Aurora Spine, Inc., Boston Scientific Corporation, Foundation Fusion Solutions, LLC, GRT US Holding, Inc., MML US, Inc. Medtronic, Inc., Nevro Corp., SPR Therapeutics, Inc., and Stimwave Technologies Inc.
Dr. Knezevic served on Advisory Boards for Tris Pharma, Inc. and Scielex Pharmaceuticals, Inc.
Dr. Mehta has received financial support from Boston Scientific Corporation, GRT US Holding, Inc., Nevro Corp., and Salix Pharmaceuticals, a division of Bausch Health US, LLC.
Dr. Schatman serves as a research consultant to Modoscript and serves on an AdComm for Syneos Health.
Dr. Soin has an ownership interest in Neuros Medical, Jan One, and Soin Neuroscience, has intellectual property with Synaptix and Neuronoff, and is a consultant at Neuronoff.
Dr. Staats has received financial support from Biotronik, Electrocore, GRT US Holding, Inc., Medtronic, Inc., Nalu Medical, Inc., Nevro Corp., Saluda Medical Americas, Inc., and Vertos Medical, Inc.
The authors report no other conflicts of interest in this work.
References
1. Arora V, Campbell JN, Chung MK. Fight fire with fire: neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol Ther. 2021;220:107743. doi:10.1016/j.pharmthera.2020.107743
2. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi:10.1038/nrdp.2017.2
3. International Association for the Study of Pain. IASP Taxonomy. Pain terms. Neuropathic pain. Available from: www.iasp-pain.org/resources/terminology/#Neuropathicpain.
4. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. doi:10.1152/physrev.00045.2019
5. Baskozos G, Hebert HL, Pascal MMV, et al. Epidemiology of neuropathic pain: an analysis of prevalence and associated factors in UK Biobank. Pain Rep. 2023;8(2):e1066. doi:10.1097/PR9.0000000000001066
6. Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi:10.1016/j.pain.2007.08.013
7. Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152(12):2836–2843. doi:10.1016/j.pain.2011.09.014
8. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
9. Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin: results from a general population survey. J Pain. 2006;7(4):281–289. doi:10.1016/j.jpain.2005.11.008
10. O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27(2):95–112. doi:10.2165/00019053-200927020-00002
11. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(Suppl 10):S22–S32. doi:10.1016/j.amjmed.2009.04.007
12. Attal N, Bouhassira D. Advances in the treatment of neuropathic pain. Curr Opin Neurol. 2021;34(5):631–637. doi:10.1097/WCO.0000000000000980
13. Mekhail NA, Cheng J, Narouze S, Kapural L, Mekhail MN, Deer T. Clinical application of neurostimulation: forty years later. Pain Pract. 2010;10(2):103–112. doi:10.1111/j.1533-2500.2009.00341.x
14. Stierli S, Napoli I, White IJ, et al. The regulation of the homeostasis and regeneration of peripheral nerve is distinct from the CNS and independent of a stem cell population. Development. 2018;145(24):1–12. doi:10.1242/dev.170316
15. Anand P, Privitera R, Donatien P, et al. Reversing painful and non-painful diabetic neuropathy with the capsaicin 8% patch. Clinical evidence for pain relief and restoration of function via nerve fiber regeneration. Front Neurol. 2022;13:998904. doi:10.3389/fneur.2022.998904
16. Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural ablation and regeneration in pain practice. Korean J Pain. 2016;29(1):3–11. doi:10.3344/kjp.2016.29.1.3
17. Seddon HJ. A classification of nerve injuries. BMJ. 1942;2:237–239. doi:10.1136/bmj.2.4260.237
18. Dubovy P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann Anatomy. 2011;193(4):267–275. doi:10.1016/j.aanat.2011.02.011
19. Sunderland S. A classification of nerve injuries producing loss of function. Brain. 1951;74:491–516. doi:10.1093/brain/74.4.491
20. Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi:10.1016/j.clinph.2008.03.018
21. Katz J, Nelson W, Forest R, Bruce D. Cryoanalgesia for post-thoracotomy pain. Lancet. 1980;315(8167):512–513. doi:10.1016/S0140-6736(80)92766-X
22. Nemecek Z, Sturm C, Rauen AC, Reisig F, Streitberger K, Harnik MA. Ultrasound-controlled cryoneurolysis for peripheral mononeuropathies: a retrospective cohort study. Pain Manag. 2023;13(6):363–372. doi:10.2217/pmt-2023-0053
23. Bittman RW, Behbahani K, Gonzalez F, Prologo JD. Interventional cryoneurolysis: what is the same, what is different, what is new? Semin Intervent Radiol. 2019;36(5):374–380. doi:10.1055/s-0039-1696705
24. Moorjani N, Zhao F, Tian Y, Liang C, Kaluba J, Maiwand MO. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur J Cardiothorac Surg. 2001;20(3):502–507. doi:10.1016/S1010-7940(01)00815-6
25. Zhou L, Shao Z, Ou S. Cryoanalgesia: electrophysiology at different temperatures. Cryobiology. 2003;46(1):26–32. doi:10.1016/S0011-2240(02)00160-8
26. Evans PJ. Cryoanalgesia. The application of low temperatures to nerves to produce anaesthesia or analgesia. Anaesthesia. 1981;36(11):1003–1013. doi:10.1111/j.1365-2044.1981.tb08673.x
27. Biel E, Aroke EN, Maye J, Zhang SJ. The applications of cryoneurolysis for acute and chronic pain management. Pain Pract. 2023;23(2):204–215. doi:10.1111/papr.13182
28. Goyal S, Kumar A, Sharma RS, Goyal D, Singh GK. Efficacy of cryoneurolysis in the management of chronic non-cancer pain: a systematic review and meta-analysis. Indian J Anaesth. 2022;66(7):485–497. doi:10.4103/ija.ija_154_22
29. Kvarstein G, Hogstrom H, Allen SM, Rosland JH. Cryoneurolysis for cervicogenic headache—a double blinded randomized controlled study. Scand J Pain. 2019;20(1):39–50. doi:10.1515/sjpain-2019-0086
30. Radnovich R, Scott D, Patel AT, et al. Cryoneurolysis to treat the pain and symptoms of knee osteoarthritis: a multicenter, randomized, double-blind, sham-controlled trial. Osteoarthritis Cartilage. 2017;25(8):1247–1256. doi:10.1016/j.joca.2017.03.006
31. Diep D, Mittal N, Sangha H, Farag J. Cryoneurolysis for non-cancer knee pain: a scoping review. Interv Pain Med. 2023;2(2):100247. doi:10.1016/j.inpm.2023.100247
32. Ilfeld BM, Smith CR, Turan A, et al. Ultrasound-guided percutaneous cryoneurolysis to treat chronic postamputation phantom limb pain: a multicenter randomized controlled trial. Anesthesiology. 2023;138(1):82–97. doi:10.1097/ALN.0000000000004429
33. Swisher MW, Ball ST, Gonzales FB, Cidambi KR, Trescot AM, Ilfeld BM. A randomized controlled pilot study using ultrasound-guided percutaneous cryoneurolysis of the infrapatellar branch of the saphenous nerve for analgesia following total knee arthroplasty. Pain Ther. 2022;11(4):1299–1307. doi:10.1007/s40122-022-00427-4
34. Ashworth BD, Day MR. Treatment of idiopathic trigeminal neuralgia utilizing a novel cryoablation device. Pain Med Case Rep. 2022;6(2):35–38.
35. Yoon JHE, Gretchushkin V, Chaudry A, Bhattarcharji P, Durkin B, Moore W. Cryoneurolysis in patients with refractory chronic peripheral neuropathic pain. J Vasc Interv Radiol. 2016;27(2):239–243. doi:10.1016/j.jvir.2015.11.027
36. Cheng J-G. Cryoanalgesia for refractory neuralgia. J Perioper Sci. 2015;2(2):1–8.
37. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810–833. doi:10.1097/ALN.0b013e3181c43103
38. Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12(1):37–41. doi:10.1007/s11916-008-0008-3
39. Iannuccilli JD, Prince EA, Soares GM. Interventional spine procedures for management of chronic low back pain—a primer. Semin Intervent Radiol. 2013;30(3):307–317. doi:10.1055/s-0033-1353484
40. Liuzzi FJ, Tedeschi B. Peripheral nerve regeneration. Neurosurg Clin N Am. 1991;2(1):31–42. doi:10.1016/S1042-3680(18)30755-1
41. D’Souza RS, Wamer NS. Phenol nerve block. Stat Pearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525978/.
42. Reeves RA, Miller CJ, Wang D, Ng A, Heller JE, Nazarian LN. Use of high-resolution ultrasound to guide alcohol neurolysis for chronic pain. Pain Physician. 2022;25:E1297–E1303.
43. Zhang X, Xu Y, Zhou J, et al. Ultrasound-guided alcohol neurolysis and radiofrequency ablation of painful stump neuroma: effective treatments for post-amputation pain. J Pain Res. 2017;10:295–302. doi:10.2147/JPR.S127157
44. Gruber H, Glodny B, Kopf H, et al. Practical experience with sonographically guided phenol instillation of stump neuroma: predictors of effects, success, and outcomes. AJR Am J Roentgenol. 2008;190(5):1263–1269. doi:10.2214/AJR.07.2050
45. Kennedy W R, Vanhove G F, Lu S, Tobias J, Bley K R, Walk D, Wendelschafer-Crabb G, Simone D A and Selim M M. (2010). A Randomized, Controlled, Open-Label Study of the Long-Term Effects of NGX-4010, a High-Concentration Capsaicin Patch, on Epidermal Nerve Fiber Density and Sensory Function in Healthy Volunteers. The Journal of Pain, 11(6), 579–587. 10.1016/j.jpain.2009.09.019
46. Tariq RA, Mueller M, Green MS. Neuraxial neurolysis. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537157/.
47. Horn LJ, Singh G, Dabrowski ER. Chemoneurolysis with phenol and alcohol: a “dying art” that merits revival. Available from: https://musculoskeletalkey.com/chemoneurolysis-with-phenol-and-alcohol-a-dying-art-that-merits-revival/.
48. Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80(2):290–295. doi:10.1097/00000539-199502000-00015
49. Hsu M. Significance of clinical treatments on peripheral nerve and its effect on nerve regeneration. J Neurol Disord. 2014;2:168. doi:10.4172/2329-6895.1000168
50. Smyth CE, Jarvis V, Poulin P. Brief review: neuraxial analgesia in refractory malignant pain. Can J Anaesth. 2014;61(2):141–153. doi:10.1007/s12630-013-0075-8
51. Ahmed A, Arora D. Fluoroscopy-guided neurolytic splanchnic nerve block for intractable pain from upper abdominal malignancies in patients with distorted celiac axis anatomy: an effective alternative to celiac plexus neurolysis—a retrospective study. Indian J Palliat Care. 2017;23(3):274–281. doi:10.4103/IJPC.IJPC_28_17
52. Ahmed A, Arora D, Kochhar AK. Ultrasound-guided alcohol neurolysis of lateral femoral cutaneous nerve for intractable meralgia paresthetica: a case series. Br J Pain. 2016;10(4):232–237. doi:10.1177/2049463716668811
53. Lim K-B, Kim Y-S, Kim J-A. Sonographically guided alcohol injection in painful stump neuroma. Ann Rehabil Med. 2012;36(3):404–408. doi:10.5535/arm.2012.36.3.404
54. Dockery GL. The treatment of intermetatarsal neuromas with 4% alcohol sclerosing injections. J Foot Ankle Surg. 1999;38(6):403–408. doi:10.1016/S1067-2516(99)80040-4
55. Garland DE, Lucie RS, Waters RL. Current uses of open phenol nerve block for adult acquired spasticity. Clin Orthop Relat Res. 1982;165:217–222. doi:10.1097/00003086-198205000-00033
56. Halpern D. Histologic studies in animals after intramuscular neurolysis with phenol. Arch Phys Med Rehabil. 1977;58(10):438–443.
57. Weksler N, Klein M, Gurevitch B, et al. Phenol neurolysis for severe chronic nonmalignant pain: is the old also obsolete? Pain Med. 2007;8(4):332–337. doi:10.1111/j.1526-4637.2006.00228.x
58. Gruber H, Kovacs P, Peer S, Fischhut B, Bodner G. Sonographically guided phenol injection in painful stump neuroma. AJR. 2004;182:952–954. doi:10.2214/ajr.182.4.1820952
59. Baranidharan G, Das S, Bhaskar A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther Adv Neurol Disord. 2013;6(5):287–297. doi:10.1177/1756285613496862
60. Benitez-Angeles M, Morales-Lazaro SL, Juarez-Gonzalez E, Rosenbaum T. TRPV1: structure, endogenous agonists, and mechanisms. Int J Mol Sci. 2020;21(10):3421. doi:10.3390/ijms21103421
61. Lo Vecchio S, Andersen HH, Arendt-Nielsen L. The time course of brief and prolonged topical 8% capsaicin-induced desensitization in healthy volunteers evaluated by quantitative sensory testing and vasomotor imaging. Exp Brain Res. 2018;236(8):2231–2244. doi:10.1007/s00221-018-5299-y
62. Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anesth. 2011;107(4):490–502. doi:10.1093/bja/aer260
63. QUTENZA® (capsaicin) topical system [prescribing information]. Morristown, NJ: Averitas Pharma, Inc; 2022. Available from: https://www.qutenza.com/pdfs/qutenza_prescribing_information.pdf.
64. Wohlrab J, Neubert RH, Heskamp ML, Michael J. Cutaneous drug delivery of capsaicin after in vitro administration of the 8% capsaicin dermal patch system. Skin Pharmacol Physiol. 2015;28(2):65–74. doi:10.1159/000362740
65. Schreiber AK, Nones CF, Reis R, Chichorro JG, Cunha JM. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 2015;6(3):432–444. doi:10.4239/wjd.v6.i3.432
66. Peppin JF, Pappagallo M. Capsaicinoids in the treatment of neuropathic pain: a review. Ther Adv Neurol Disorder. 2014;7(1):22–32. doi:10.1177/1756285613501576
67. Mishra SK, Hoon MA. Ablation of TRPV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43(1):157–163. doi:10.1016/j.mcn.2009.10.006
68. Yu S-Q, Premkumar LS. Ablation and regeneration of peripheral and central TRPV1 expressing nerve terminals and the consequence of nociception. Open Pain J. 2015;8:1–9. doi:10.2174/1876386301508010001
69. Chung M-K, Campbell JN. Use of capsaicin to treat pain: mechanistic and therapeutic considerations. Pharmaceuticals. 2016;9(4):66. doi:10.3390/ph9040066
70. Wang S, Wang S, Asgar J, et al. Ca2+ and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J Biol Chem. 2017;292(20):8291–8303. doi:10.1074/jbc.M117.778290
71. Wang S, Bian C, Yang J, et al. Ablation of TRPV1+ afferent terminals by capsaicin mediates long-lasting analgesia for trigeminal neuropathic pain. eNeuro.;7(3):ENEURO.0118–20.2020. doi:10.1523/ENEURO.0118-20.2020
72. Babbar S, Marier J-F, Mouksassi M-S, et al. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with a peripheral neuropathic pain. Ther Drug Monit. 2009;31(4):502–510. doi:10.1097/FTD.0b013e3181a8b200
73. Laklouk M, Baranidharan G. Profile of the capsaicin 8% patch for the management of neuropathic pain associated with postherpetic neuralgia: safety, efficacy, and patient acceptability. Patient Prefer Adherence. 2016;10:1913–1918. doi:10.2147/PPA.S76506
74. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18(1):42–53. doi:10.1016/j.jpain.2016.09.008
75. Vinik AI, Perrot S, Vinik EJ, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol. 2016;16(1):251. doi:10.1186/s12883-016-0752-7
76. Vinik AJ, Perrot S, Vinik EJ, et al. Repeat treatment with capsaicin 8% patch (179 mg capsaicin cutaneous patch) effects on pain, quality of life, and patient satisfaction in painful diabetic peripheral neuropathy: an open-label, randomized controlled clinical trial. J Curr Med Res Opin. 2019;2(12):388–401 doi:10.15520/jcmro.v2i12.242.
77. Freynhagen R, Argoff C, Eerdekens M, Engelen S, Perrot S. Progressive response to repeat application of capsaicin 179 mg (8% w/w) cutaneous patch in peripheral neuropathic pain: comprehensive new analysis and clinical implications. Pain Med. 2021;22(10):2324–2336. doi:10.1093/pm/pnab113
78. Backonja M, Wallace MS, Blonsky ER, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7(12):1106–1112. doi:10.1016/S1474-4422(08)70228-X
79. Irving GA, Backonja MM, Dunteman E, et al. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch for the treatment of postherpetic neuralgia. Pain Med. 2011;12:99–109. doi:10.1111/j.1526-4637.2010.01004.x
80. Irving GA, Backonja M, Rauck R, Webster LR, Tobias JK, Vanhove GF. NGX-4010, a capsaicin 8% dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clin J Pain. 2012;28(2):101–107. doi:10.1097/AJP.0b013e318227403d
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Painful Peripheral Neuropathies of the Lower Limbs and/or Lower Extremities Treated with Spinal Cord Stimulation: A Systematic Review with Narrative Synthesis
Burkey AR, Chen J, Argoff CE, Edgar DR, Petersen EA
Journal of Pain Research 2023, 16:1607-1636
Published Date: 18 May 2023

