Back to Journals » Journal of Pain Research » Volume 18
Absorbable Antibacterial Envelope Reduces Surgical Site Infections for Intrathecal Pain Pump Implants: A Retrospective Analysis
Authors Robinson CL , Chiang MC , Patel AS, Chan KS, Ori A, Yong RJ, Ang SP
Received 25 January 2025
Accepted for publication 13 May 2025
Published 15 May 2025 Volume 2025:18 Pages 2479—2482
DOI https://doi.org/10.2147/JPR.S519430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Christopher L Robinson,1 Michael C Chiang,2 Akash S Patel,1 Kheng Sze Chan,1 Arti Ori,1 R Jason Yong,1,* Samuel P Ang1,*
1Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, MA, USA; 2Department of Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Cambridge, MA, USA
*These authors contributed equally to this work
Correspondence: Samuel P Ang, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, 75 Francis St, Boston, MA, 02115, USA, Email [email protected]
Introduction
Surgical site infections (SSIs) remain a significant contributor to healthcare expenditures, amounting to ~$3 billion annually in the USA with each hospital-acquired SSI adding an average total cost of $28,219 to the overall healthcare system.1 Currently, limited, yet growing evidence exists for the utility of prophylactic approaches.1 An absorbable antibacterial envelope (AAE), TYRX (Medtronic, Minneapolis, USA), has been previously used to reduce device implant SSI rates among cardiac and certain neuromodulation devices.2,3 However, its utility in reducing infection rates for intrathecal pain pumps (ITPP) has only been briefly examined.4 Thus, the aim of this retrospective study was to evaluate the reduction in associated SSI rates with the use of AAE for ITPP implants.
Methods
A retrospective analysis with Institutional Review Board approval (#2024P000789) at a single institution (Brigham and Women’s Hospital) was performed on patients ≥ 18 years of age who underwent ITPP implant by any of nine pain management providers between January 1, 2016 to June 30, 2023 (Table 1). ITPP was defined as any intrathecal drug delivery system being implanted that contained either opioid or local anesthetic as the primary medication being delivered. Patients either had the AAE (off-label use for antibacterial prophylaxis) or no AAE implanted along with their ITPP. Exclusion criteria included prior intrathecal pump-related infections or lack of follow-up data within 12 months following implant of device. Data collected included age, gender, indications for device implant, co-morbidities, supplementary infection prevention prophylaxis, in addition to any SSI within 12 months after device implant, as previously described (Tables 1 and 2).2 Categorical data were analyzed using Fisher’s exact test and continuous data were analyzed using unpaired t-test. Statistical analysis was performed using GraphPad Prism Version 10.4.1. The requirement for informed consent was waived due to the retrospective nature of the study and minimal risk to participants. All data were de-identified prior to analysis to ensure patient confidentiality. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Results
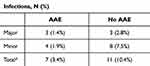
A total of 313 patients who had ITPP implants met inclusion criteria with 207 in the AAE implanted group and 106 in the no AAE implanted group (Table 1). The average ages in the AAE and no AAE group were 59.7 ± 11.8 yrs and 58.3 ± 12.8 yrs (p = 0.10), and women were predominant in both groups, 53.1% (110/207) and 66.0% (70/106) (p = 0.03), respectively. The top indication for ITPP implant in the AAE group was post-laminectomy syndrome (45.4%, 94/207), and for the no AAE group, it was cancer pain (42.5%, 45/106). The leading co-morbidity for both groups was obesity, with 38.2% (79/207) and 28.3% (30/106), respectively. In the AAE and no AAE group, 3.4% (7/207) and 10.4% (11/106) developed SSIs (p = 0.02), respectively. Patients in the AAE group had a lower likelihood of developing an infection (OR = 0.33, 95% CI [0.13, 0.87]).
Discussion
The AAE releases minocycline and rifampicin over the course of a minimum of 7 days and is fully absorbed by 9 weeks.5 First used in a large-scale study to prevent infections during placement of cardiac implantable electronic devices (CIED), the AAE demonstrated a reduction of 40% in infection rate with benefits extending beyond the 12-month observation period, and its use has now been extended to specific neuromodulation devices.2,6
Unfortunately, this same benefit did not appear to extend to neuromodulation devices, as seen in a single-center retrospective study evaluating the infection rates among spinal cord stimulation (SCS) device implants.7 No statistically significant difference in infection rates (p = 0.6) was observed between the group with and without TYRX (one specific absorbable antibacterial envelope), but the authors did note that all the infections in the TYRX group could be managed with oral antibiotics and six out of the seven infections in the no TYRX group required intravenous antibiotics.7 One criticism of this study was that the four cases of SSI in the TYRX group were not confirmed objectively with culture data, but were counted as SSI based on the action of intravenous antibiotics being administered for suspected infection.7 On the contrary, six of the seven infections in the no TYRX group were confirmed with cultures, and if the calculation were to include only confirmed cases, there would have been a difference of zero to six infections (p = 0.014).7
The present study does examine the use of AAEs for ITTP implants. This retrospective analysis of 313 total patients who met inclusion criteria demonstrated a statistically significant lower rate of overall surgical site infections between the AAE and no AAE groups, 3.4% and 10.4%, respectively (p = 0.02). There was also a lower rate of both major and minor surgical site infections in the AAE group (Table 2). Major infections were defined as SSIs requiring revision surgery, such as washout, or device explant. Minor infections encompassed all other SSIs. Unlike the prior study, all infections in the given study were culture-confirmed, and our cohort had approximately 50% more participants.7
While the use of AAEs for ITTP implants was used off-label for antimicrobial prophylaxis, AAEs have been used for a wide range of device implants for the neurosurgical domain including deep brain stimulators and spinal cord stimulators.4 Intrathecal drug-delivery system implantation infection rates traditionally range from approximately 2.5–9%.1 Potential SSI with intrathecal drug-delivery system implants can result in life-threatening complications such as meningitis.1 The results of our retrospective analysis suggest that AAEs may be a feasible way to reduce SSIs but further prospective studies are needed.
Limitations
Our study was limited by its retrospective nature, which can be prone to selection and observatory bias. Propensity score matching to adjust for any biases was not employed given the low number of events in both groups, especially in the AAE group. Furthermore, it could potentially limit statistical power and ability to assess any meaningful associations. If the events in the AAE group were reduced, it could inflate the association.
Conclusions
Our retrospective study of AAEs in the interventional pain space demonstrated a statistically significant decrease in association with ITPP-implant SSIs. To further evaluate the efficacy of the AAE for ITPP implants, multi-center, randomized controlled trials are needed.
Disclosure
Dr R Yong is a consultant for Medtronic outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Provenzano DA, Hanes M, Hunt C, et al. ASRA pain medicine consensus practice infection control guidelines for regional anesthesia and pain medicine. Reg Anesth Pain Med. 2025:rapm–2024–105651. doi:10.1136/RAPM-2024-105651
2. Tarakji KG, Mittal S, Kennergren C, et al. Antibacterial envelope to prevent cardiac implantable device infection. New England Journal of Medicine. 2019;380(20):1895–1905. doi:10.1056/NEJMOA1901111/SUPPL_FILE/NEJMOA1901111_DATA-SHARING.PDF
3. de Oliveira HM, Barbosa LM, Zamora FV, et al. Use of antibacterial envelopes in neuromodulation surgeries with implantable device insertion: a systematic review and meta-analysis. Neurosurgery. 2024. doi:10.1227/NEU.0000000000003242
4. Ahmed SU, Persad AR, Mercure-Cyr R, et al. Use of antibacterial envelopes for prevention of infection in intrathecal pump implantation. Neuromodulation. 2022;25(6):S18. doi:10.1016/j.neurom.2022.04.031
5. DEMONSTRATED CIED STABILIZATION, REDUCED INFECTION TYRXTM absorbable antibacterial envelope. Available from: https://www.medtronic.com/content/dam/medtronic-com/products/cardiac-rhythm/infection-control/documents/tyrx-sell-sheet.pdf.
6. Mittal S, Wilkoff BL, Kennergren C, et al. The World-wide Randomized Antibiotic Envelope Infection Prevention (WRAP-IT) trial: long-term follow-up. Heart Rhythm. 2020;17(7):1115–1122. doi:10.1016/J.HRTHM.2020.02.011
7. Kristensen MKS, Filtenborg JT, Miscov R, Gulisano HA, Bjarkam CR. Use of an antibacterial envelope in spinal cord stimulation reduces the rate and severity of iatrogenic infections. World Neurosurg. 2024;185:e820–e826. doi:10.1016/J.WNEU.2024.02.134
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.