Back to Journals » Cancer Management and Research » Volume 17
Advances in Multimodal Imaging Techniques for Evaluating and Predicting the Efficacy of Immunotherapy for NSCLC
Authors Liu J , Xie M, Shen J, Yao J, Lin X, Bao X, Zhang X, Liang Y, Yang Y, Jiang G, Diao X, Han W, Du H, Xue X, Wu J
Received 11 February 2025
Accepted for publication 17 May 2025
Published 7 June 2025 Volume 2025:17 Pages 1073—1086
DOI https://doi.org/10.2147/CMAR.S522136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Jingyi Liu,1 Mei Xie,2 Jing Shen,1 Jie Yao,2 Xuwen Lin,2 Xinyu Bao,3 Xin Zhang,3 Yiran Liang,2 Yun Yang,1 Gege Jiang,1 Ximeng Diao,1 Wenya Han,4 Hai Du,5 Xinying Xue,3,6 Jianlin Wu1
1Department of Radiology, Affiliated Zhongshan Hospital of Dalian University, Dalian, 116001, People’s Republic of China; 2Department of Respiratory and Critical Care, Emergency and Critical Care Medical Center, Beijing Shijitan Hospital, Capital Medical University, Beijing, 100038, People’s Republic of China; 3Department of Respiratory and Critical Care, Affiliated Hospital of Shandong Second Medical University, Weifang, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Taihe Hospital, Hubei University of Medicine, Shiyan, 442000, People’s Republic of China; 5Ordos Central Hospital, Ordos City, 017000, People’s Republic of China; 6Department of Respiratory and Critical Care, Xuanwu Hospital, Capital Medical University, Beijing, 100038, People’s Republic of China
Correspondence: Jianlin Wu, Department of Radiology, Affiliated Zhongshan Hospital of Dalian University, Dalian, 116001, People’s Republic of China, Email [email protected] Xinying Xue, Department of Respiratory and Critical Care, Xuanwu Hospital, Capital Medical University, Beijing, 100038, People’s Republic of China, Email [email protected]
Abstract: Immunotherapy has emerged as a transformative treatment for non-small cell lung cancer (NSCLC), yet its clinical benefits remain variable among patients. Early and accurate evaluation of treatment response is critical to guide therapeutic adjustments and improve outcomes. This review synthesizes recent advancements in multimodal imaging techniques—computed tomography (CT), positron emission tomography (PET)/CT, magnetic resonance imaging (MRI), and radiomics—for evaluating and predicting immunotherapy efficacy in NSCLC. We analyze the strengths and limitations of conventional morphological criteria (eg, RECIST, iRECIST) and highlight emerging quantitative biomarkers, including CT texture analysis, metabolic parameters (MTV, TLG), and diffusion-weighted MRI metrics. Notably, radiomics demonstrates promise in decoding tumor heterogeneity, PD-L1 expression, and immune microenvironment features, while immuno-PET probes targeting immune checkpoints offer novel insights into immune activity in vivo. Challenges such as pseudo-progression, nodal immune flare, and discrepancies between imaging responses and pathological responses are critically discussed. By integrating morphological, metabolic, and microenvironmental data, multimodal imaging enhances precision in patient stratification and therapeutic monitoring. Future research should prioritize multicenter, AI-driven radiomics validation and targeted tracer development to optimize NSCLC immunotherapy management. This review provides clinicians and researchers with new directions for utilizing multimodal imaging techniques in developing personalized treatment strategies.
Keywords: non-small cell lung cancer, immunotherapy, efficacy prediction, CT, PET, MRI
Introduction
Lung cancer is one of the most common malignancies in the world. According to the American Cancer Society, approximately 234,580 new lung cancer cases (116,310 in men and 118,270 in women) and 125,070 lung cancer-related deaths (65,790 in men and 59,280 in women) were reported in 2024.1 Lung cancer is broadly classified into two major histological subtypes: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). SCLC, accounting for approximately 10–15% of cases, is characterized by rapid progression, early metastasis, and a strong association with smoking; in contrast, NSCLC constitutes 80–85% of all lung cancer diagnoses and encompasses distinct histological subtypes, including adenocarcinoma (the most prevalent subtype, arising from peripheral lung glands), squamous cell carcinoma (originating in central bronchial epithelial cells), and large cell carcinoma (a less common, poorly differentiated variant).2,3 The clinical and molecular heterogeneity of NSCLC highlights the critical need for advanced multimodal imaging techniques to evaluate and predict immunotherapy efficacy, address its biological diversity, and improve patient stratification. Surgery remains the primary and most effective treatment for primary lung tumors, while chemotherapy, radiation therapy, immunotherapy, targeted therapy, or their combination are often used for advanced-stage cancer.4,5 Over recent years, the treatment of lung cancer has evolved profoundly owing to the advent of immune checkpoint inhibitors, including antibodies targeting programmed cell death protein 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4).6–11 For instance, in PD-L1-positive (tumor proportion score (TPS)≥50%) advanced NSCLC, pembrolizumab monotherapy achieved a 31.9% 5-year overall survival (OS) rate, compared to 5% with chemotherapy; similarly, nivolumab demonstrated a 14% 4-year OS rate in previously treated NSCLC patients.12,13 Immunotherapy can block or inhibit ligand-receptor interactions,14,15 thereby overcoming T-cell exhaustion, enhancing immune activity, and exerting anti-tumor effects.16 However, the efficacy of immunotherapy varies from patient to patient.17 For example, a US study has discovered that the difference between people who do and do not respond to immunotherapy may have to do with an immune cell known as CD5+ dendritic cells (DCs).18 CD5+ DCs, a subset of conventional DCs, play a critical role in enhancing T-cell priming and effector function. Specifically, CD5+ DCs promote the activation of CD5loCD4+ helper T cells and neoantigen-specific CD8+ cytotoxic T cells, which are essential for anti-tumor immunity. Their density in tumor-draining lymph nodes correlates with improved survival outcomes in NSCLC patients. In addition to CD5+ DCs, multiple factors contribute to the variability in immunotherapy response in NSCLC patients, including tumor microenvironment components such as PD-L1 expression (high PD-L1 expression is associated with improved response to PD-1/PD-L1 inhibitors),19 tumor-infiltrating lymphocytes (high densities of CD8+ T cells correlate with better outcomes),20 and tumor mutational burden (TMB) (higher TMB predicts improved response to immunotherapy);21 host factors such as gut microbiome (specific gut bacteria modulate systemic immunity via the gut-lung axis)22 and inflammatory status (elevated systemic inflammation is linked to poor response and increased toxicity).23 These factors collectively underscore the complexity of immunotherapy response and emphasize the need for multimodal tools to predict patient outcomes. Therefore, early evaluation and prediction of immunotherapy efficacy in NSCLC patients is crucial.
Imaging examinations remain an important method for diagnosis, efficacy evaluation, and prediction of lung cancer.24 Traditional imaging is based on morphological structures for diagnosing and evaluating diseases. However, with the advent of the era of precision medicine and individualized therapy, functional imaging has been developing rapidly; it offers the possibility to detect microscopic alterations earlier than morphological changes of tumors,25 thereby facilitating early identification of treatment response, enabling timely therapeutic strategy adjustments, and contributing to the optimization of clinical decision-making in NSCLC immunotherapy. Such clinical detection through imaging techniques enhances the accuracy of tumor biological assessment and supports personalized interventions that may influence patient outcomes by addressing therapeutic efficacy at a critical stage prior to overt morphological changes.26 In this study, we discuss multimodal imaging techniques (CT, PET/CT, MRI) and radiomics (a method that extracts a large number of features from medical images using data-characterization algorithms) in evaluating and predicting the efficacy of immunotherapy in NSCLC patients. The advantages and shortcomings of various techniques and indicators mentioned above are also noted. We also discuss new directions for future research, which may provide new ideas for the selection of imaging methods for clinical evaluation and prediction of the efficacy of immunotherapy in NSCLC patients. The multimodal imaging techniques and indicators mentioned in this study are summarized in Figure 1.
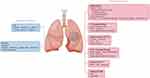 |
Figure 1 Multimodal imaging technology approach for evaluation and prediction of NSCLC immunotherapy efficacy. Source: Original illustrations created using Figdraw.com (License ID. YIISIefef2). |
Imaging Techniques to Evaluate the Efficacy of Immunotherapy in NSCLC Patients
Computed Tomography (CT)
CT is one of the most commonly used methods for examining lung tumors. A CT scan of the chest is the cornerstone of lung cancer imaging, based on which further management is decided. It possesses high density and spatial resolution and has a good observation effect on the morphology and size of lesions. NSCLC can be centrally located masses, invading the peripherally situated lesions or mediastinal structures that invade the chest wall.27
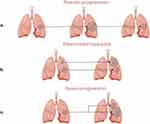
Conventional NSCLC treatments, such as radiotherapy and chemotherapy, mainly rely on cytotoxic drugs used to induce apoptosis or directly kill tumor cells, and their efficacy is mainly reflected in the tumor size change.27 The Response Evaluation Criteria in Solid Tumors (RECIST)28 and RECIST 1.1 published in the European Journal of Cancer29 use the change in tumor size before and after treatment as the tumor burden to evaluate the efficacy of treatment. The schematic diagram of non-contrast CT based on the RECIST 1.1 standard for immunotherapy efficacy evaluation is shown in Figure 2. Measurable lesions are defined as lesions not on the non-measurable list below and having the longest diameter of ≥10 mm on spiral CT with 5 mm reconstructed section thickness in the axial plane (not the sagittal or coronal planes in CT imaging).28 The detailed classification and definition of the assessment criteria are summarized in Table 1. However, imaging findings of tumors treated with immunotherapy are distinct from those treated with conventional systemic therapy, and various rule sets have been published. Eg, some immunotherapy patients present with specific response patterns, such as pseudo-progression, dissociated response and hyper-progression of the disease, pseudo-progression is defined as a temporary increase in tumor size followed by shrinkage, mostly due to immune cell infiltration or therapy-related necrosis and edema; dissociated response is defined as concurrent growth and regression of different lesions; hyper-progression is defined as the acceleration growth of tumor after immunotherapy.26,30 The schematic diagram of special response patterns after immunotherapy for NSCLC is shown in Figure 3. In the CheckMate-159 trial (NCT02259621), a pilot study evaluating neoadjuvant nivolumab in 21 patients with resectable stage I, II, or IIIA NSCLC, 20 patients underwent complete tumor resection. Among these, 9 patients (45%) achieved major pathological response (MPR), defined as ≤10% residual viable tumor cells in the resected specimen. Notably, while the objective response rate assessed by RECIST criteria was only 10%, this discrepancy highlights that MPR—a histopathological endpoint indicative of profound tumor regression—may capture the effects of immunotherapy not fully detectable by imaging alone.31 Although the small sample size (n=21) limits the generalizability of these findings, they underscore the potential value of integrating pathological assessments with imaging-based criteria to comprehensively evaluate treatment responses in early-stage NSCLC. More evidence indicates that some patients with MPR cannot achieve objective remission by RECIST criteria alone, as pathological regression and radiographic response may exhibit substantial discrepancies due to tumor heterogeneity and immune-related inflammatory changes.32 Moreover, Luo et al pointed out that progression-free survival (PFS) varies greatly within the stable disease (SD) range, showing heterogeneity among SD subgroups; the RECIST criteria define “SD” as more responsive to the rate of tumor proliferation than to the tumor response to immunotherapy and patients with PFS > 6 months and no tumor growth as “SD responders”.33 This conclusion further supports the inconsistencies in the evaluation of imaging efficacy and prognosis of immunotherapy patients. The possible reasons are necrosis, inflammatory infiltration, mesenchymal formation, and fibrosis, which appear after traditional NSCLC treatment is applied.34 After immunotherapy, some NSCLC lesions also show special pathological signs such as cholesterol cleft, a large number of lymphocytes aggregation and tertiary lymphoid structure based on the above pathological changes,35 which makes diagnosis challenging. Thus, in early 2017, the Immune Response Evaluation Criteria in Solid Tumors (iRECIST) has been proposed.36 This criteria introduced two new concepts—unconfirmed progressive disease (iUPD) and confirmed progressive disease (iCPD)—which provisionally assesses the RECIST 1.1 criteria for progressive disease (PD) as iUPD and require re-evaluation after 4–6 weeks to confirm iCPD. If the tumor evaluated as iUPD does not change during subsequent treatment, it is redefined as iUPD at the next evaluation. Although this criterion defines the delayed effect of tumor immunotherapy, it evaluates the efficacy by measuring the diameter of the tumor lesion before and after treatment; however, it cannot predict the degree of tumor response to immunotherapy. The detailed classification and definition of the assessment criteria are summarized in Table 1.
 |
Table 1 Morphology and Metabolic Response Evaluation Criteria |
Currently, most of the efficacy evaluation criteria use a single long diameter of the largest cross-section to measure the change in tumor size, often ignoring the asymmetry of some tumor changes. The 2017 Fleischner Society guidelines recommend measuring pulmonary nodule size using the average of the long and short axes of the largest cross-section, as outlined in their management criteria for incidentally detected solid and subsolid nodules.37 In addition, gross tumor volume (GTV) is more representative of changes in tumor size on a three-dimensional level, but its measurement method is more complex than tumor diameter measurement. In the future, it may be possible to provide a more accurate description of lesion size based on mean tumor diameter or GTV and thus evaluate the efficacy of the treatment.
Positron Emission Tomography (PET/CT)
PET/CT plays a dual pivotal role in the management of NSCLC. First, as a standard imaging modality for clinical staging (recommended by guidelines such as National Comprehensive Cancer Network), PET/CT integrates 18F-FDG metabolic activity with anatomical CT features to accurately delineate primary tumors, lymph node metastases, and distant metastases, thereby critically guiding therapeutic strategies.38,39 Second, its quantitative metabolic parameters (eg, maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG)) reflect tumor microenvironment heterogeneity and serve as key biomarkers for predicting immunotherapy responses.40 Thus, PET/CT not only serves as a cornerstone for staging and response assessment but also provides a potential tool for tailoring individualized immunotherapy regimens. In 1999, the European Organization for Research and Treatment of Cancer (EORTC) introduced the first criteria for tumor assessment based on 18F-FDG PET/CT.41 A few years later, the EORTC criteria were replaced by the Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) criteria42 for evaluating the efficacy of solid tumors using 18F-FDG tracer (as shown in Table 1). In the following years, a number of PET/CT evaluation criteria for evaluating tumor response to immunotherapy have been proposed, including PECRIT criteria, PERCIMT criteria, imPERCISTS criteria, and iPERCIST criteria;43–46 however, a majority of studies have focused on evaluating patients with melanoma, and there is limited research data on the application of these criteria in patients with NSCLC. Also, Cascone’s analysis of the NEOSTAR trial identified a “nodal immune flare” (NIF) phenomenon,47 where 16% (7/44) of NSCLC patients treated with neoadjuvant immunotherapy demonstrate radiologically abnormal nodes post-therapy that upon pathological evaluation are devoid of cancer and demonstrate de novo non-caseating granulomas, this phenomenon was not observed in patients after neoadjuvant chemotherapy (0% (0/28)); NIF is characterized by abnormally enlarged lymph nodes on imaging and high 18F-FDG uptake on PET/CT. However, no significant changes in tumor size (p=0.089) and SUVmax (p=0.142) values were found between NIF and No-NIF patients after neoadjuvant immunotherapy, making it impossible to differentiate NIF from actual disease progression on imaging. Therefore, this phenomenon may be misinterpreted as tumor progression, and further examination should be performed if abnormal lymph nodes suspected of disease progression are detected after treatment. NIF should be differentiated from tumor infiltration in conjunction with pathology.
Imaging Techniques to Predict the Efficacy of Immunotherapy in NSCLC Patients
Multimodal CT Examination
Routine CT Scan
Conventional CT scans are used to predict the prognosis of immunotherapy by measuring the diameter of tumor lesions based on RECIST and its derived criteria. Tazdait et al48 compared the predictive value of the RECIST 1.1, irRECIST,49 and iRECIST criteria for evaluating the efficacy of immunotherapy in patients with NSCLC. Based on survival analyses, the authors concluded that RECIST 1.1 criteria underestimated the benefit of PD-1 or PD-L1 inhibitors in approximately 11% of the evaluated population, suggesting that the irRECIST and iRECIST criteria are more accurate compared to RECIST 1.1 criteria.48
Prior studies have demonstrated that CT texture analysis (CTTA) can differentiate genetic mutations and tumor grades in certain tumors, including lung cancer.50 CTTA can quantify the pixel values inside the lesion, obtain the distribution characteristics and patterns of the pixel values, and provide microscopic information that is not easily accessible to the naked eye, thus evaluating the intra-tumor heterogeneity. A retrospective study of 54 NSCLC patients demonstrated CTTA as a new independent predictor of survival.51 Cox analysis showed coarse-texture CTTA (filter=2.5) was the strongest survival predictor (OR=56.4, 95% CI=4.79–665, P=0.001), surpassing PET stage (OR=3.85, P=0.002) and SUVmax (P=0.076). Patients with low CTTA uniformity (≤0.624) had 100% 30-month mortality versus 53% survival in high-uniformity group (P<0.0014), highlighting CTTA’s potential for enhancing NSCLC risk stratification. Shen et al52 extracted texture features of pre-immunotherapy enhanced CT images of NSCLC patients, including histogram, absolute gradient, tour matrix, grayscale covariance matrix, autoregressive model, and wavelet transform, and analyzed each texture feature. The results showed that the pre-immunotherapy enhanced CTTA could predict the efficacy of immunotherapy for NSCLC with a sensitivity and specificity of 88.2% and 76.3% (AUC = 0.812; 95% CI: 0.706–0.919).52 In addition, a Zerunian study proposed that the mean value of positive pixels, a CTTA parameter, was significantly associated with lower OS and PFS (P < 0.0035), which could be used as a predictive imaging biomarker for OS and PFS in patients with NSCLC treated with first line immunotherapy.53 CT histogram analysis (CTHA) consists of algorithms for extracting numerical parameters from diagnostic images and analyzing the distribution of CT values within regions of interest. In a multicenter retrospective study of 104 platinum-refractory NSCLC patients treated with nivolumab (median age: 67 years; 66.4% male; 72.1% non-squamous histology), Ravanelli et al demonstrated that CTHA using spatial scale filters (SSF) could stratify survival outcomes. Coarse-texture kurtosis at SSF=4 mm inversely correlated with OS (adjusted HR=0.476, 95% CI: 0.29–0.77; P=0.0028), while kurtosis at SSF=6 mm predicted shorter PFS (HR=0.556, 95% CI: 0.36–0.86; P=0.0088). These findings underscore the prognostic value of baseline CTHA in identifying high-risk subgroups among immunotherapy-treated NSCLC patients.54
Energy Spectrum CT
The energy spectrum CT technique includes single-energy imaging, material separation, and effective atomic number, which can acquire a wide range of parameters that provide value in lesion efficacy assessment. In a prospective single-arm Phase II trial (NCT04326153) involving 20 patients with potentially resectable stage IIIA/IIIB NSCLC (90% squamous carcinoma), Sun et al conducted a comparative analysis of single-source dual-energy CT (ssDECT) and postoperative pathology. Among patients achieving pathological complete response (pCR) after neoadjuvant chemoimmunotherapy (8/20, 40%), ssDECT demonstrated reductions in average iodine values (ΔAP=−3.17%; ΔAP=−6.61%) and normalized iodine concentration (ΔAP=−6.09%; ΔAP=−20.25%) compared to baseline. Notably, ssDECT accurately differentiated residual necrotic/reparative tissue from viable tumor in pCR patients, outperforming conventional CT, which misclassified 62.5% (5/8) of pCR cases as stable disease. This highlights ssDECT’ s potential for resolving discrepancies between imaging and pathological responses in immunotherapy-treated NSCLC.55
Possible Future Directions
The CT value can quantitatively measure the absorption rate of X-rays by different tissues. Necrosis, fibrosis, and special pathological signs, which often appear in tumor lesions after treatment, lead to corresponding changes in the CT values of the lesions.56 CT three-dimensional volumetric imaging can identify the tumor lesion and the surrounding adjacent tissues, thus providing a more intuitive description of the morphology and structure of the tumor lesion. Also, three-dimensional volume measurement is more accurate than the RECIST standard measurement.57,58 Moreover, CT perfusion imaging provides information about the blood supply inside the tumor and has some predictive value for the outcome of NSCLC patients after treatment.58 Dual-source CT demonstrates high sensitivity in detecting intratumoral hemorrhage, as evidenced by its ability to identify hemorrhagic response in 14% (4/29) of solid and lymph node target lesions in NSCLC patients undergoing anti-angiogenic therapy. By utilizing virtual non-enhanced images and iodine-enhanced material decomposition, this modality accurately differentiates post-treatment lesion enlargement caused by hemorrhage (eg, attenuation >50 hU on virtual nonenhanced images) from true tumor progression, thereby providing reliable net tumor enhancement measurements without additional radiation exposure.59
In conclusion, all of the above multiple CT imaging tests have different values in predicting the efficacy of NSCLC immunotherapy. However, these methods are still in the exploratory stage, and detailed research data and in-depth research are required.
CT Radiomics
Radiomics is a rapidly evolving field of research concerned with extracting quantitative metrics, ie, advanced quantitative imaging features with high throughput from CT, PET or MRI images.60 Its key advantage is that it can capture microscopic tumor features typically unobservable to the naked eye. Radiomics data can be used to construct models that correlate image features with clinical and biological endpoints, thereby characterizing or predicting the onset, progression, and clinical outcome of disease from the three-dimensional level of the lesion.61
CT Radiomics Based on Lesion Characterization
Trebeschi et al performed radiomics-based characterization of each lesion on preprocessed contrast-enhanced CT imaging data and developed and validated a non-invasive machine-learning capable of distinguishing between immunotherapy response and non-response biomarkers.62 Their data suggest that radiomics characterization of lesions can serve as a noninvasive biomarker of response to immunotherapy, which was significant in predicting the efficacy of immunotherapy for NSCLC (AUC=0.83, p<0.001), and may show utility in improving patient stratification in neoadjuvant and palliative settings.
CT Radiomics Based on PD-L1 Expression
Tumor cell expression of PD-L1 is approved as a diagnostic biomarker for immunotherapy in NSCLC patients. Tian et al developed a CT-based deep learning model to predict PD-L1 expression (cutoff ≥50%) in NSCLC patients, stratifying patients into high PD-L1 expression signature (PD-L1ES) score and low PD-L1ES score groups. The low PD-L1ES score group demonstrated significantly improved PFS (median PFS: 363 days, 95% CI: 363~) compared to the high PD-L1ES score group (median PFS: 183 days, 95% CI: 122~257), with a hazard ratio (HR) of 2.57 (95% CI: 1.22~5.44; P = 0.010).63 Furthermore, integrating this model with clinical variables (age, sex, smoking history, and family history) enhanced prognostic stratification capabilities (combined model HR: 3.53, 95% CI: 1.86~6.72; P < 0.001).
CT Radiomics Based on Tumor Immune Micro Environment Phenotypes
Immune cells in the tumor immune microenvironment (TIME) have an important role in tumor resistance.64 Studies have shown that CD8+ T cell infiltration within tumors correlates with the efficacy and survival of immunotherapy.65 TIME can be classified into three immune phenotypes: immune-inflamed, immune-excluded, and immune-desert.66 The immunoinflammatory phenotype has a considerable CD8 cell infiltration and often responds to immunotherapy,67 which is opposite to the characteristics seen in the immune-desert tumor (less CD8 cell infiltration); in the latter case, transforming growth factor-β, activation of myeloid-derived suppressor cells, and angiogenesis prevent T-cell infiltration within the tumor.68 Thus, the TIME phenotype may influence the efficacy of immunotherapy. Sun et al developed and validated a biomarker for tumor-infiltrating CD8 cells based on enhanced CT radiomics. This new radiomics profile was able to classify tumors with high and low CD8 infiltration abundance (AUC=0.74), predict gene expression of CD8 cells (AUC=0.67), and classify the tumor immunophenotype (AUC=0.76); the median survival of patients with a high radiomics score (24.3 months) was significantly greater than that of patients with a null predictor (11.5 months) at 6 months post-immunotherapy (P=0.0081, HR=0.58).69
Multi-Parametric PET/CT
Routine PET/CT Parameters
PET/CT parameters include SUVmax, mean standardized uptake value (SUVmean), MTV, and TLG. MTV and TLG are more often used to predict efficacy.70 Monaco et al71 analyzed 92 patients with NSCLC treated with nivolumab, pembrolizumab, or atezolizumab and showed that the median MTV was significantly lower in those who achieved disease control (complete response (CR), partial response (PR), SD) than in those who did not achieve disease control (PD)(77 vs.160.2, P=0.039). Also, patients with MTV and TLG values below median values had longer OS (P=0.03 and 0.05).71 Moreover, Bauckneht et al analyzed 45 patients with imaging evaluations of PD during treatment with nivolumab. They found that MTV was an independent predictor of OS (HR=1.001; 95% CI: 1.001–1.002) and could identify patients responding well to immunotherapy.72 In addition, a retrospective study analyzing 57 patients with NSCLC undergoing immunotherapy found that MTV (P=0.028) and TLG (P=0.035) were significantly associated with disease progression, whereas SUVmax was not significantly correlated with disease progression and prognosis.73 In addition, Ling et al systematically evaluated 13 related studies and found that baseline MTV may be highly predictive of survival while baseline TLG only predicts OS in a small proportion of patients; they also concluded that baseline SUVmax and SUVmean are unsuitable for use as prognostic indicators for patients with advanced or metastatic NSCLC who receive immunotherapy.74 This phenomenon may be related to the activation of the TIME. Immunotherapy induces an increase in the in-flow and activity of immune cells, such as T-lymphocytes, in the TIME, which, in turn, increases the uptake of 18F-FDG by the lesion; this condition masks the actual therapeutic response and may even lead to the generation of false-positive results.75–77 Therefore, although SUVmax and SUVmean are commonly used semi-quantitative parameters for PET/CT, whether they can be used as predictors of prognosis in patients undergoing immunotherapy remains controversial.
Special PET/CT Parameters
In addition to the above commonly used PET/CT parameters, Popinat et al analyzed lean body mass, fat body mass, muscle body mass, visceral fat mass and sub-cutaneous fat (SCFM) parameters and discovered that patients with low SCFM (<5.69 kg/m2) had a poorer prognosis (P=0.04). Also, SCFM resulted as an important prognostic factor in stage IV NSCLC treated with nivolumab.78
PET/CT Radiomics
PET/CT Radiomics Based on Lesion Characterization
PET/CT imaging is still in a rapid development stage. Polverari et al evaluated the value of imaging radiomics based on baseline 18F-FDG PET/CT images of primary foci in predicting the efficacy of immunotherapy, where patients with high tumor volume (P=0.035), high TLG values (P=0.037) and heterogeneity expressed by “skewness” (P=0.032) and “kurtosis” (P=0.046) had a higher probability of poor immunotherapy outcome. Volume and heterogeneity were considered to be associated with disease progression.73
PET/CT Radiomics Based on PD-L1 Expression
PET/CT imaging histologic features have some value in predicting PD-L1 expression status.79 Valentinuzzi et al analyzed immunotherapy [18F] FDG PET radiomics signature (iRADIOMICS) and compared them with the PD-L1 TPS and iRECIST criteria in predicting prognosis, finding that iRADIOMICS (AUC=0.90; 95% CI: 0.78–1.00) had better predictive power than the PD-L1 TPS (AUC=0.60; 95% CI: 0.37–0.83) and iRECIST criteria (month 4) (AUC=0.86; 95% CI: 0.72–1.00).80
PET/CT Radiomics Based on Tumor Immune Micro Environmental Profiles
Tong et al used a machine learning model based on PET/CT radiomics and clinical features to predict the TIME profiles of NSCLC and found that CD8 cell expression could represent the temporal profile of NSCLC. Also, combined PET/CT imaging histology-clinical model (AUC = 0.932) was superior to the PET/CT imaging histology model (AUC = 0.907, P = 0.0326) and the clinical model (AUC = 0.868, P = 0.0036) in predicting higher immune scores and more immune-activated pathways in the high CD8 cell group than in the low CD8 cell group (P = 0.0421).81 Thus, the authors suggested that a combined 18F-FDG PET/CT radiomics-clinical model could be a clinical noninvasive method for detecting the immune status of NSCLC tumors and predicting the efficacy of immunotherapy.
Immuno-PET Imaging
In recent years, immuno-PET/CT imaging has developed rapidly. Today, novel radiopharmaceuticals can identify specific immune system targets and are under investigation in pre-clinical and clinical settings. For example, several radiolabeled 18F, 89Zr, and 64Cu have been tested against the expression of tumor-infiltrating lymphocytes at the PD-1/PD-L1 checkpoints and the aggregation of CD8+ T cells.82–84 Niemeijer et al used the 18F-BMS-986192 and 89Zr-nivolumab tracers for the first time in humans and demonstrated the feasibility of non-invasive quantitative analysis of PD-L1 and PD-1 with both tracers.85 Moreover, Smit et al used 89Zr-durvalumab to detect PD-L1 in 13 patients. The tracer detected a higher number of tumor foci; also, tumor uptake of the tracer was higher in patients with treatment response or stable disease than in patients with disease progression, but the difference was not statistically significant (median SUVpeak, 4.9 vs 2.4; P=0.06)).86 The reason for this may be the small sample size, which needs to be expanded for more in-depth studies in the future.
Liu et al evaluated the feasibility of the PET tracer [68Ga] Ga-NOTA-Nb 109 for PD-L1 immuno-PET imaging in patient-derived xenografts (PDX) (nude mice) with lung adenocarcinoma and squamous cell carcinoma, showing that [68Ga] Ga-NOTA-Nb 109 could accurately and sensitively assess PD-L1 expression in NSCLC PDX models.87 New molecular probe binding sites, such as tracers targeting binding to granzyme B and tumor-associated macrophages have been demonstrated to have a good ability to assess the early response to immunotherapy in mouse cancer models.88–90 This type of study is important for screening patients who may respond to immunotherapy and guiding the development of appropriate therapeutic strategies for such patients. Further in-depth clinical studies based on large sample sizes of NSCLC patients are awaited in the future.
Multimodal MRI
Diffusion-weighted imaging (DWI) MRI is widely used to evaluate and predict the efficacy of NSCLC immunotherapy.91 This imaging approach is based on detecting the Brownian motion of water molecules in the tissues, which enables the assessment of the tumor cell and tissue microstructure. Its parameters, including real diffusion coefficient (D) value, macroscopic diffusion kurtosis (Kapp), and apparent diffusion coefficient (ADC) values, can be used to predict the efficacy of treatment. Bao et al predicted the efficacy of neoadjuvant chemo-immunotherapy (NCIT) in resectable NSCLC patients by predictive value of intravoxel incoherent motion (IVIM) DWI and diffusion kurtosis imaging (DKI) and found that the post-NCIT ADC, ΔADC, and ΔD value changes were significantly higher in the pCR group (mean value=0.982, 0.162, and 0.129) than in the non-pCR group (mean value=0.834, 0.014, and −0.110), whereas the pre-NCIT D, post-NCIT Kapp, and ΔKapp in the pCR group (mean value=0.721, 0.846, and −0.005) were significantly lower than those in the non-pCR group (mean value=0.845, 1.028, and 0.168). Also, the multivariate logistic regression analysis showed that the pre-NCIT D and post-NCIT Kapp values were the independent predictors for response and that the combined predictive model consisting of IVIM-DWI and DKI had the best predictive efficacy (AUC=0.889).92 Furthermore, Karayama et al assessed the efficacy of immunotherapy using IVIM-MRI, showing that an increase in ADC at 8 weeks, decreased in ADCkurt (kurtosis of ADC) and ΔADCkurt at 4 weeks after immunotherapy are associated with longer PFS, a decreased ΔADCskew (skewness of ADC) at 4 weeks was associated with longer PFS and OS. This study suggests that changes in ADC histograms can help predict long-term efficacy after immunotherapy.93 In addition, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), which can show information on intra-tissue perfusion and tumor heterogeneity, has been used to identify benign and malignant nodules in the lungs; however, this method has been less frequently used in NSCLC immunotherapy studies.94
Summary and Prospect
Within the landscape of NSCLC immunotherapy, multimodal imaging techniques have emerged as pivotal tools for evaluating treatment response and predicting clinical outcomes, fulfilling the critical clinical need for early identification of non-responders to facilitate timely therapeutic modifications. CT remains the cornerstone for morphological assessment, with quantitative analyses (eg, volumetric measurements, texture/radiomics features) providing deeper insights into tumor heterogeneity that surpass traditional size-based criteria such as RECIST, which frequently misclassifies immune-related responses including pseudo-progression. PET/CT integrates metabolic information, with parameters such as MTV and TLG demonstrating prognostic value, although challenges remain in differentiating immune-induced inflammation (eg, nodal immune flare) from true progression. Immuno-PET/CT demonstrates potential for non-invasive detection of immune checkpoint expression (eg, PD-L1/PD-1); however, clinical translation remains constrained by limited cohort sizes and challenges in tracer specificity. MRI, particularly DWI, delivers microstructural insights into tumor response but remains constrained by motion artifacts and restricted clinical availability when compared with CT and PET.
This review elucidates how multimodal imaging—through capturing morphological, metabolic, and microenvironmental features—augments histopathological evaluation, allowing refined stratification of NSCLC patients undergoing continued immunotherapy. However, persisting challenges encompass heterogeneity in radiomics feature extraction across studies and the difficulty in differentiating immune-related phenomena (eg, inflammatory changes versus true progression) with single-modality assessments. Future investigations should emphasize multicenter validation of multimodal imaging-pathology integration, development of AI-driven algorithms to decipher complex radiomics signatures, and exploration of targeted immuno-PET tracers to optimize patient stratification and therapeutic monitoring. By addressing these limitations, multimodal imaging could advance precision immunotherapy through enabling earlier identification of treatment-responsive cohorts and refining clinical decision-making to ameliorate NSCLC prognosis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
National Natural Science Foundation of China (82071911, 62176166, 62076254), Dalian Science and Technology Innovation Fund (2021JJ12SN38), Inner Mongolia Autonomous Region Natural Science Foundation (2023MS08031), and Capital Medical University Outstanding Young Talent Program (A2310).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
2. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409–436. doi:10.3322/caac.21731
3. Amin H, Ibrahim IM, Hassanein EHM. Weaponizing chitosan and its derivatives in the battle against lung cancer. Int J Biol Macromol. 2024;272(Pt 2):132888. doi:10.1016/j.ijbiomac.2024.132888
4. Chiu LC, Lin SM, Lo YL, Kuo SC, Yang CT, Hsu PC. Immunotherapy and vaccination in surgically resectable non-small cell lung cancer (NSCLC). Vaccines. 2021;9(7):689. doi:10.3390/vaccines9070689
5. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi:10.1038/nature25183
6. Paulsen EE, Kilvaer TK, Khanehkenari MR, et al. Assessing PDL-1 and PD-1 in non-small cell lung cancer: a novel immunoscore approach. Clin Lung Cancer. 2017;18(2):220–233.e8. doi:10.1016/j.cllc.2016.09.009
7. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236(1):219–242. doi:10.1111/j.1600-065X.2010.00923.x
8. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi:10.1038/nri3862
9. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–1218. doi:10.1038/ni.2762
10. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi:10.1097/coc.0000000000000239
11. Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi:10.1126/science.1202947
12. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39(21):2339–2349. doi:10.1200/jco.21.00174
13. Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408. doi:10.1016/s1470-2045(19)30407-3
14. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi:10.1158/2159-8290.Cd-18-0367
15. Lee HT, Lee JY, Lim H, et al. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep. 2017;7(1):5532. doi:10.1038/s41598-017-06002-8
16. Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936–3943. doi:10.1158/1078-0432.Ccr-13-0895
17. Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75. doi:10.1007/978-3-319-40389-2_3
18. He M, Roussak K, Ma F, et al. CD5 expression by dendritic cells directs T cell immunity and sustains immunotherapy responses. Science. 2023;379(6633):eabg2752. doi:10.1126/science.abg2752
19. Jassem J, de Marinis F, Giaccone G, et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. 2021;16(11):1872–1882. doi:10.1016/j.jtho.2021.06.019
20. Yan Q, Li S, He L, Chen N. Prognostic implications of tumor-infiltrating lymphocytes in non-small cell lung cancer: a systematic review and meta-analysis. Front Immunol. 2024;15:1476365. doi:10.3389/fimmu.2024.1476365
21. Alessi JV, Ricciuti B, Wang X, et al. Impact of TMB/PD-L1 expression and pneumonitis on chemoradiation and durvalumab response in stage III NSCLC. Nat Commun. 2023;14(1):4238. doi:10.1038/s41467-023-39874-8
22. Zhang H, Xu Z. Gut-lung axis: role of the gut microbiota in non-small cell lung cancer immunotherapy. Front Oncol. 2023;13:1257515. doi:10.3389/fonc.2023.1257515
23. Burke M, Rashdan S. Management of immune-related adverse events in patients with non-small cell lung cancer. Front Oncol. 2021;11:720759. doi:10.3389/fonc.2021.720759
24. Tárnoki ÁD, Tárnoki DL, Dąbrowska M, et al. New developments in the imaging of lung cancer. Breathe. 2024;20(1):230176. doi:10.1183/20734735.0176-2023
25. Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological advancements in cancer diagnostics: improvements and limitations. Cancer Rep. 2023;6(2):e1764. doi:10.1002/cnr2.1764
26. Eze C, Schmidt-Hegemann NS, Sawicki LM, et al. PET/CT imaging for evaluation of multimodal treatment efficacy and toxicity in advanced NSCLC-current state and future directions. Eur J Nucl Med Mol Imaging. 2021;48(12):3975–3989. doi:10.1007/s00259-021-05211-8
27. Purandare NC, Rangarajan V. Imaging of lung cancer: implications on staging and management. Indian J Radiol Imaging. 2015;25(2):109–120. doi:10.4103/0971-3026.155831
28. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi:10.1093/jnci/92.3.205
29. Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho. 2009;36(13):2495–2501.
30. Wang GX, Kurra V, Gainor JF, et al. Immune checkpoint inhibitor cancer therapy: spectrum of imaging findings. Radiographics. 2017;37(7):2132–2144. doi:10.1148/rg.2017170085
31. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi:10.1056/NEJMoa1716078
32. Chen X, Ma K. Neoadjuvant therapy in lung cancer: what is most important: objective response rate or major pathological response? Curr Oncol. 2021;28(5):4129–4138. doi:10.3390/curroncol28050350
33. Luo J, Wu S, Rizvi H, et al. Deciphering radiological stable disease to immune checkpoint inhibitors. Ann Oncol. 2022;33(8):824–835. doi:10.1016/j.annonc.2022.04.450
34. Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–740. doi:10.1016/j.jtho.2020.01.005
35. Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018;29(8):1853–1860. doi:10.1093/annonc/mdy218
36. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi:10.1016/s1470-2045(17)30074-8
37. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner society 2017. Radiology. 2017;284(1):228–243. doi:10.1148/radiol.2017161659
38. Volpi S, Ali JM, Tasker A, Peryt A, Aresu G, Coonar AS. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann Transl Med. 2018;6(5):95. doi:10.21037/atm.2018.01.25
39. Li Y, Deng J, Ma X, Li W, Wang Z. Diagnostic accuracy of CT and PET/CT radiomics in predicting lymph node metastasis in non-small cell lung cancer. Eur Radiol. 2025;35(4):1966–1979. doi:10.1007/s00330-024-11036-4
40. Qiao T, Cheng Z, Duan Y. Innovative applications and future trends of multiparametric PET in the assessment of immunotherapy efficacy. Front Oncol. 2024;14:1530507. doi:10.3389/fonc.2024.1530507
41. Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European organization for research and treatment of cancer (EORTC) PET study group. Eur J Cancer. 1999;35(13):1773–1782. doi:10.1016/s0959-8049(99)00229-4
42. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1(Suppl 1):122s–50s. doi:10.2967/jnumed.108.057307
43. Cho SY, Lipson EJ, Im HJ, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58(9):1421–1428. doi:10.2967/jnumed.116.188839
44. Sachpekidis C, Anwar H, Winkler J, et al. The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45(8):1289–1296. doi:10.1007/s00259-018-3972-9
45. Ito K, Teng R, Schöder H, et al. (18)F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J Nucl Med. 2019;60(3):335–341. doi:10.2967/jnumed.118.213652
46. Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019;9(1):8. doi:10.1186/s13550-019-0473-1
47. Cascone T, Weissferdt A, Godoy MCB, et al. Nodal immune flare mimics nodal disease progression following neoadjuvant immune checkpoint inhibitors in non-small cell lung cancer. Nat Commun. 2021;12(1):5045. doi:10.1038/s41467-021-25188-0
48. Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi:10.1016/j.ejca.2017.10.017
49. Bohnsack O, Hoos A, Ludajic K. Adaptation and modification of the immune related response criteria (IRRC): irRECIST. J Clin Oncol. 2014;32(15_suppl):e22121–e22121. doi:10.1200/jco.2014.32.15_suppl.e22121
50. Digumarthy SR, Padole AM, Lo Gullo R, Singh R, Shepard JO, Kalra MK. CT texture analysis of histologically proven benign and malignant lung lesions. Medicine. 2018;97(26):e11172. doi:10.1097/md.0000000000011172
51. Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol. 2012;22(4):796–802. doi:10.1007/s00330-011-2319-8
52. Shen L, Fu H, Tao G, Liu X, Yuan Z, Ye X. Pre-immunotherapy contrast-enhanced CT texture-based classification: a useful approach to non-small cell lung cancer immunotherapy efficacy prediction. Front Oncol. 2021;11:591106. doi:10.3389/fonc.2021.591106
53. Zerunian M, Caruso D, Zucchelli A, et al. CT based radiomic approach on first line pembrolizumab in lung cancer. Sci Rep. 2021;11(1):6633. doi:10.1038/s41598-021-86113-5
54. Ravanelli M, Agazzi GM, Milanese G, et al. Prognostic and predictive value of histogram analysis in patients with non-small cell lung cancer refractory to platinum treated by nivolumab: a multicentre retrospective study. Eur J Radiol. 2019;118:251–256. doi:10.1016/j.ejrad.2019.07.019
55. Sun C, Ma X, Meng F, et al. Tumor microenvironment(TME) and single-source dual-energy CT(ssDECT) on assessment of inconformity between RECIST1.1 and pathological remission in neoadjuvant immunotherapy of NSCLC. Neoplasia. 2024;50:100977. doi:10.1016/j.neo.2024.100977
56. Zhang BW, Zhang Y, Ye JD, Qiang JW. Use of relative CT values to evaluate the invasiveness of pulmonary subsolid nodules in patients with emphysema. Quant Imaging Med Surg. 2021;11(1):204–214. doi:10.21037/qims-19-998
57. Mozley PD, Bendtsen C, Zhao B, et al. Measurement of tumor volumes improves RECIST-based response assessments in advanced lung cancer. Transl Oncol. 2012;5(1):19–25. doi:10.1593/tlo.11232
58. Fraioli F, Anzidei M, Zaccagna F, et al. Whole-tumor perfusion CT in patients with advanced lung adenocarcinoma treated with conventional and antiangiogenetic chemotherapy: initial experience. Radiology. 2011;259(2):574–582. doi:10.1148/radiol.11100600
59. Kim YN, Lee HY, Lee KS, et al. Dual-energy CT in patients treated with anti-angiogenic agents for non-small cell lung cancer: new method of monitoring tumor response? Korean J Radiol. 2012;13(6):702–710. doi:10.3348/kjr.2012.13.6.702
60. Kumar V, Gu Y, Basu S, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234–1248. doi:10.1016/j.mri.2012.06.010
61. Rogers W, Thulasi Seetha S, Refaee TAG, et al. Radiomics: from qualitative to quantitative imaging. Br J Radiol. 2020;93(1108):20190948. doi:10.1259/bjr.20190948
62. Trebeschi S, Drago SG, Birkbak NJ, et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann Oncol. 2019;30(6):998–1004. doi:10.1093/annonc/mdz108
63. Tian P, He B, Mu W, et al. Assessing PD-L1 expression in non-small cell lung cancer and predicting responses to immune checkpoint inhibitors using deep learning on computed tomography images. Theranostics. 2021;11(5):2098–2107. doi:10.7150/thno.48027
64. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi:10.1038/nature21349
65. Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv Exp Med Biol. 2017;1036:19–31. doi:10.1007/978-3-319-67577-0_2
66. Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865–1874. doi:10.1158/1078-0432.Ccr-15-1507
67. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi:10.1038/nature14011
68. Mojsilovic S, Mojsilovic SS, Bjelica S, Santibanez JF. Transforming growth factor-beta1 and myeloid-derived suppressor cells: a cancerous partnership. Dev Dyn. 2022;251(1):105–124. doi:10.1002/dvdy.339
69. Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–1191. doi:10.1016/s1470-2045(18)30413-3
70. Kudura K, Ritz N, Templeton AJ, et al. Predictive value of total metabolic tumor burden prior to treatment in NSCLC patients treated with immune checkpoint inhibition. J Clin Med. 2023;12(11):3725. doi:10.3390/jcm12113725
71. Monaco L, Gemelli M, Gotuzzo I, et al. Metabolic parameters as biomarkers of response to immunotherapy and prognosis in non-small cell lung cancer (NSCLC): a real world experience. Cancers. 2021;13(7):1634. doi:10.3390/cancers13071634
72. Bauckneht M, Genova C, Rossi G, et al. The role of the immune metabolic prognostic index in patients with non-small cell lung cancer (NSCLC) in radiological progression during treatment with nivolumab. Cancers. 2021;13(13):3117. doi:10.3390/cancers13133117
73. Polverari G, Ceci F, Bertaglia V, et al. (18)F-FDG pet parameters and radiomics features analysis in advanced Nsclc treated with immunotherapy as predictors of therapy response and survival. Cancers. 2020;12(5):1163. doi:10.3390/cancers12051163
74. Ling T, Zhang L, Peng R, Yue C, Huang L. Prognostic value of (18)F-FDG PET/CT in patients with advanced or metastatic non-small-cell lung cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. 2022;13:1014063. doi:10.3389/fimmu.2022.1014063
75. Lang D, Wahl G, Poier N, et al. Impact of PET/CT for assessing response to immunotherapy-A clinical perspective. J Clin Med. 2020;9(11):3483. doi:10.3390/jcm9113483
76. Anwar H, Sachpekidis C, Winkler J, et al. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45(3):376–383. doi:10.1007/s00259-017-3870-6
77. Somarouthu B, Lee SI, Urban T, Sadow CA, Harris GJ, Kambadakone A. Immune-related tumour response assessment criteria: a comprehensive review. Br J Radiol. 2018;91(1084):20170457. doi:10.1259/bjr.20170457
78. Popinat G, Cousse S, Goldfarb L, et al. Sub-cutaneous Fat Mass measured on multislice computed tomography of pretreatment PET/CT is a prognostic factor of stage IV non-small cell lung cancer treated by nivolumab. Oncoimmunology. 2019;8(5):e1580128. doi:10.1080/2162402x.2019.1580128
79. Zhao X, Zhao Y, Zhang J, Zhang Z, Liu L, Zhao X. Predicting PD-L1 expression status in patients with non-small cell lung cancer using [(18)F]FDG PET/CT radiomics. EJNMMI Res. 2023;13(1):4. doi:10.1186/s13550-023-00956-9
80. Valentinuzzi D, Vrankar M, Boc N, et al. [18F]FDG PET immunotherapy radiomics signature (iRADIOMICS) predicts response of non-small-cell lung cancer patients treated with pembrolizumab. Radiol Oncol. 2020;54(3):285–294. doi:10.2478/raon-2020-0042
81. Tong H, Sun J, Fang J, et al. A machine learning model based on PET/CT radiomics and clinical characteristics predicts tumor immune profiles in non-small cell lung cancer: a retrospective multicohort study. Front Immunol. 2022;13:859323. doi:10.3389/fimmu.2022.859323
82. Pandit-Taskar N, Postow MA, Hellmann MD, et al. First-in-humans imaging with (89)Zr-Df-IAB22M2C Anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med. 2020;61(4):512–519. doi:10.2967/jnumed.119.229781
83. Natarajan A, Mayer AT, Reeves RE, Nagamine CM, Gambhir SS. Development of novel ImmunoPET tracers to image human PD-1 checkpoint expression on tumor-infiltrating lymphocytes in a humanized mouse model. Mol Imaging Biol. 2017;19(6):903–914. doi:10.1007/s11307-017-1060-3
84. Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel radiotracer for ImmunoPET imaging of PD-1 checkpoint expression on tumor infiltrating lymphocytes. Bioconjug Chem. 2015;26(10):2062–2069. doi:10.1021/acs.bioconjchem.5b00318
85. Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9(1):4664. doi:10.1038/s41467-018-07131-y
86. Smit J, Borm FJ, Niemeijer AN, et al. PD-L1 PET/CT imaging with radiolabeled durvalumab in patients with advanced-stage non-small cell lung cancer. J Nucl Med. 2022;63(5):686–693. doi:10.2967/jnumed.121.262473
87. Liu Q, Wang X, Yang Y, et al. Immuno-PET imaging of PD-L1 expression in patient-derived lung cancer xenografts with [(68)Ga]Ga-NOTA-Nb109. Quant Imaging Med Surg. 2022;12(6):3300–3313. doi:10.21037/qims-21-991
88. Zhou H, Wang Y, Xu H, et al. Noninvasive interrogation of CD8+ T cell effector function for monitoring early tumor responses to immunotherapy. J Clin Invest. 2022;132(16). doi:10.1172/jci161065
89. Parker CC, Bin Salam A, Song PN, et al. Evaluation of a CD206-targeted peptide for PET imaging of macrophages in syngeneic mouse models of cancer. Mol Pharm. 2023;20(5):2415–2425. doi:10.1021/acs.molpharmaceut.2c00977
90. Wang J, Lu Y, Zhang R, et al. Modulating and imaging macrophage reprogramming for cancer immunotherapy. Phenomics. 2024;4(4):401–414. doi:10.1007/s43657-023-00154-6
91. Yuan Z, Niu XM, Liu XM, et al. Use of diffusion-weighted magnetic resonance imaging (DW-MRI) to predict early response to anti-tumor therapy in advanced non-small cell lung cancer (NSCLC): a comparison of intravoxel incoherent motion-derived parameters and apparent diffusion coefficient. Transl Lung Cancer Res. 2021;10(8):3671–3681. doi:10.21037/tlcr-21-610
92. Bao X, Bian D, Yang X, et al. Multiparametric MRI for evaluation of pathological response to the neoadjuvant chemo-immunotherapy in resectable non-small-cell lung cancer. Eur Radiol. 2023;33(12):9182–9193. doi:10.1007/s00330-023-09813-8
93. Karayama M, Yoshizawa N, Sugiyama M, et al. Intravoxel incoherent motion magnetic resonance imaging for predicting the long-term efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer. Lung Cancer. 2020;143:47–54. doi:10.1016/j.lungcan.2020.03.013
94. Wu W, Zhou S, Hippe DS, et al. Whole-lesion DCE-MRI intensity histogram analysis for diagnosis in patients with suspected lung cancer. Acad Radiol. 2021;28(2):e27–e34. doi:10.1016/j.acra.2020.01.025
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Path to Personalized Treatment in KRAS-Mutant Non-Small Cell Lung Cancer: A Review of Targeted Therapies and Immunotherapy
Shu CL, Liu YL
Cancer Management and Research 2022, 14:3485-3492
Published Date: 16 December 2022
NOTCH1 Mutations Predict Superior Outcomes of Immune Checkpoint Blockade in Non-Small Cell Lung Cancer
Huang Q, Cao H, Yao Q, Zhou X, Li H, Bai Q, Hu H
ImmunoTargets and Therapy 2023, 12:165-173
Published Date: 5 December 2023
Progressive Disease with Mixed Response After Immunotherapy in Non-Small Cell Lung Cancer
Lv J, Yan W, Zhang R, Chen X, Ren Z, Chen D, Yu J
Journal of Inflammation Research 2024, 17:6317-6327
Published Date: 11 September 2024
Clinical Effect of Treatment with Metformin for Type 2 Diabetes on Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: A Retrospective Study
Wang Y, Sun Y, Hu J, Ma H
International Journal of General Medicine 2024, 17:6595-6604
Published Date: 31 December 2024
Theranostic Role of Advanced Nanotechnological Tools in Early Brain Metastases in Lung Cancer: An Updated Review
Wu Y, Sun C, Jin K, Dong C
International Journal of Nanomedicine 2025, 20:7215-7232
Published Date: 6 June 2025