Back to Journals » Journal of Inflammation Research » Volume 17
Progressive Disease with Mixed Response After Immunotherapy in Non-Small Cell Lung Cancer
Authors Lv J, Yan W, Zhang R, Chen X, Ren Z, Chen D , Yu J
Received 7 May 2024
Accepted for publication 5 September 2024
Published 11 September 2024 Volume 2024:17 Pages 6317—6327
DOI https://doi.org/10.2147/JIR.S477244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Juncai Lv,1,* Weiwei Yan,1,* Ran Zhang,1 Xi Chen,1,2 Ziyuan Ren,1,2 Dawei Chen,1 Jinming Yu1
1Department of Radiation Oncology and Shandong Provincial Key Laboratory of Precision Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 2Cheeloo College of Medicine, Shandong University Cancer Center, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinming Yu; Dawei Chen, Department of Radiation Oncology and Shandong Provincial Key Laboratory of Precision Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China, Tel +86-531-8798-4777, Fax +86-531-8798-4079, Email [email protected]; [email protected]
Purpose: There exists a dearth of research concerning non-small cell lung cancer (NSCLC) patients experiencing overall progressive disease concomitant with shrinking lesions after immunotherapy. This is a special type of mixed response. We aim to evaluate the clinical characteristics and treatment options of these patients during immunotherapy.
Patients and Methods: We categorized patients into two groups: Progressive Disease with Mixed Responses (PDMR) (n = 31) and Progressive Disease with None Mixed Responses (PDNMR) (n = 144), depending on whether at least one target lesion had shrunk by ≥ 30% at the point of overall progression. Computed tomography scans and magnetic resonance imaging were utilized to evaluate the clinicopathological significance of these patients, and a multivariate analysis was conducted to scrutinize the clinical characteristics and prognosis-influencing factors in these patients.
Results: Patients in the PDMR group had worse staging and a greater proportion of previous radiotherapy. The median overall survival (mOS 22 vs 36.4 months; P = 0.019) and median progression-free survival (mPFS 5.83 vs 9.03 months; P = 0.031) of the PDMR group were shorter than PDNMR group. Longer subsequent OS with continued immunotherapy after PDMR compared with patients who do not continue with immunization after PDMR (mOS 23.9 vs 6.5 months; P = 0.024).
Conclusion: PDMR was primarily observed in stage IV patients and previously irradiated patients. OS and PFS were inferior in patients with PDMR compared to patients with PDNMR. The continuation of immunotherapy in PDMR patients could extend their survival.
Keywords: non-small cell lung cancer, immunotherapy, mixed response, progression
Introduction
Lung cancer remains the leading cause of cancer-related mortality globally. At the time of diagnosis, 57% of lung cancer patients present with metastases, and the five-year survival rate is a mere 5%.1 Non-small cell lung cancer (NSCLC) accounts for the majority (85%) of lung cancer cases.2 The introduction of immune checkpoint inhibitors, particularly the combination of anti-programmed cell death protein 1 (anti-PD-1) and anti-programmed cell death 1 ligand 1 (anti-PD-L1) antibodies with chemotherapy, has significantly improved the prognosis for patients with inoperable NSCLC, enhancing survival outcomes in these patients.3–6
However, immunotherapy differs from conventional cytotoxic agents and targeted therapies due to its unique pharmacological mechanisms, leading to diverse response patterns.7 Evaluating immunological drugs is challenging due to mixed responses (MR), pseudoprogression, and hyperprogression. Furthermore, clinical experience elucidates that some patients diagnosed as “progressive” have local tumor shrinkage. This response pattern represents a distinctive form of mixed responses,8 defined as overall progression according to RECIST 1.1 criteria but concurrent with one or more shrinking lesions. Such patients are often labeled as “progressing” in clinical practice.9 Guidelines recommend switching patients to another therapy line upon progression.4,6 However, disregarding the reduction in specific lesions may result in the loss of potential therapeutic benefits.
The proportion of patients who has progressive disease (PD) with mixed responses (PDMR) is not high, but as the concept of “Customized (N-of-1) combination therapy” continues to be recognized, the prognostic factors related to the survival of patients with PDMR and the subsequent therapeutic regimens need to be urgently explored and adjusted. Although some studies have addressed mixed response, there is a significant gap in the literature regarding MR in the context of PD. To address this gap, we conducted a retrospective investigation at a single center, focusing on patients with inoperable non-small cell lung cancer who underwent immunotherapy (anti-PD-1 or anti-PD-L1) combined with chemotherapy. Our objective is to thoroughly examine their clinicopathological characteristics, identify high-risk subgroups, and evaluate their subsequent management and clinical outcomes.
Patients and Methods
Patients
This study reviewed the medical records of patients diagnosed with inoperable stage III and IV NSCLC who underwent anti-PD-1/anti-PD-L1 therapy, with or without chemotherapy, at the Shandong Cancer Hospital and Research Institute between 2018 and 2022. All patients were pathologically confirmed to have unresectable NSCLC and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Standard follow-up care was administered at our institution, which included computed tomography (CT) scans every 12 weeks and clinical evaluations by the oncology team. The study protocol was approved by the Medical Ethics Committee of the Shandong Cancer Hospital and Research Institute. Clinical data were retrieved from the medical record system, encompassing 175 patients with NSCLC who had complete documentation of disease progression following immunotherapy, thereby permitting further analysis. To mitigate the potential confounding effects of “mixed responses” due to local radiotherapy, patients who exhibited progressive disease within six months post-radiotherapy were excluded from the study.
Clinical Variables
Clinical data collection involves extracting information on patient characteristics, tumor characteristics, treatment modalities, and survival outcomes from medical records. The patients were enrolled and then classified into two groups, namely PDMR and Progressive Disease with None Mixed responses (PDNMR), based on whether there was overall progression with concurrent tumor shrinkage or not. The two groups of patients were classified according to age, gender, smoking habits, tumor location, ECOG, pathological classification of NSCLC, and the presence of brain, liver and bone metastases at the time of pathological diagnosis.
Assessment
Response to treatment was evaluated by a radiologist certified by the board via CT scan and magnetic resonance imaging (MRI), which included the evaluation of metastatic lesions ≥5 mm in the long axis, excluding lymph nodes, and lymph nodes ≥15 mm in the short axis. The sum of all measured lesions determined the tumor load. We have classified the response to treatment into two categories: (1) PDMR - presence of shrinking tumor when overall progression, and (2) PDNMR - no shrinking tumor when overall progression. Subsequent analyses of the PDMR and PDNMR groups involved evaluating overall survival (OS), which is defined as the duration between pathology confirmation and the date of the last follow-up or the date of death. In addition, overall survival 2 (OS2) was analyzed, which is defined as the period from disease progression on immunotherapy until the date of death or the date of the last follow-up. Furthermore, progression-free survival (PFS), which is the time from the beginning of immunotherapy until the assessment for PD, was also examined. It is worth noting that all patients underwent PD evaluation in accordance with RECIST 1.1 criteria: 20% increase in target lesions or the manifestation of new lesions was observed. In order to qualify as a shrinking lesion, a reduction of more than 30% in a single target lesion was required.
Statistical Analysis
Statistical analyses were performed to evaluate the demographic and clinicopathological characteristics of all patients. Depending on the nature of the data and the study objectives, clinical characteristics were compared using the chi-square test, Fisher’s exact test, or Wilcoxon rank-sum test. Differences between the two groups were assessed using the Log rank test, and overall survival was estimated using the Kaplan–Meier method. To identify independent prognostic factors associated with improved survival, Cox proportional hazards modeling was employed. Variables with a two-sided p-value of less than 0.10 in the univariate analysis were subsequently included in the multivariate Cox regression analysis. A two-sided p-value of less than 0.05 was considered indicative of statistical significance across all analyses. All statistical analyses were conducted using SPSS Statistics version 26 (IBM Corporation, NY, USA).
Results
Patient Characteristics
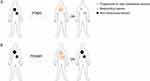
A total of 175 patients diagnosed with inoperable non-small cell lung cancer with metastatic lesions were included in our retrospective study from 2018 to 2022. The program aimed to objectively evaluate the efficacy of immunotherapy for this patient population. Of the patients under study, 105 experienced progression after first-line immunotherapy, while 70 progressed after second-line immunotherapy. The immunotherapy agent of choice was PD-1 inhibitor in 97.71% of patients, while PD-L1 inhibitor was used in 2.29% of patients (Supplementary Table 1). When evaluated as PD, 31 patients exhibited shrinking lesions, and 144 patients did not exhibit any shrinkage. Patients with reducing lesions were designated as being in PDMR. Those without reducing lesions were marked as PDNMR (Table 1). A schematic of the patient categories is displayed (Figure 1). In the PDMR group, 4 patients lacked follow-up information regarding post-progression treatment; 19 continued with immunotherapy, while 8 did not. In contrast, in the PDNMR group, 74 patients continued immunotherapy after progression, whereas 59 did not.
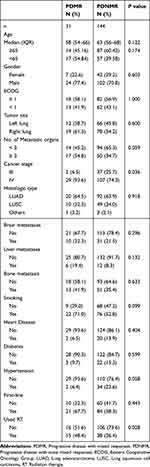 |
Table 1 Demographic and Clinical Characteristics in 175 Non-Small Cell Carcinoma Patients |
OS, PFS and OS2 Comparison Between PDMR and PDNMR
As of April 7, 2023, the final follow-up revealed that 91 patients remained alive. Surprisingly, the overall survival analysis demonstrated that the OS was longer in the PDMR cohort but shorter in PDNMR cohort (median OS 22 vs 36.4 months; P = 0.019). In addition, the PFS was shorter in the PDMR group (mPFS 5.83 vs 9.03 months; P = 0.031), but the difference in OS2 between the two groups was not statistically significant (mOS2 10.3 vs 15.9 months; P = 0.24) (Figure 2). Gender, ECOG status, disease site, tumor histology, and common distant metastatic sites (including liver metastasis, bone metastasis, and brain metastasis), as well as smoking history, cardiac history, diabetes history, and hypertension history exhibited no significant differences between PDMR and PDNMR groups (Table 1). Patients in the PDMR group had a higher proportion of stage IV patients (P = 0.036), as well as a greater proportion of radiotherapy (P = 0.028) applied before progression (excluding those who received radiotherapy within 6 months prior to progression), compared with the PDNMR group.
Univariate analysis revealed that patients with PDMR, liver metastasis, bone metastasis, and male patients had lower OS. Multivariate analysis identified liver metastasis [Hazard Ratio (HR) = 1.87, 95% Confidence intervals (CI): 1.01–3.48; P = 0.046] and PDMR (HR = 1.88, 95% CI: 1.09–3.23; P = 0.023) as independent risk factors for OS (Table 2). All patients were monitored until disease progression following immunotherapy, allowing us to determine the PFS for each patient. Univariate analysis revealed that male gender (HR = 1.59, 95% CI: 1.13–2.24; P = 0.008), liver metastasis (HR = 1.73, 95% CI: 1.06–2.84; P = 0.028), smoking (HR = 1.40, 95% CI: 1.03–1.90; P = 0.032), and PDMR (HR = 1.53, 95% CI: 1.04–2.27; P = 0.033) were associated with shorter PFS. In the multivariate analysis, PDMR (HR = 1.52, 95% CI: 1.03–2.27; P = 0.038), male gender (HR = 1.54, 95% CI: 1.01–2.37; P = 0.024), primary tumor in the left lung (HR = 1.43, 95% CI: 1.05–1.96; P = 0.023), and liver metastasis (HR = 1.82, 95% CI: 1.10–2.99; P = 0.018) were identified as independent risk factors for patients’ PFS (Supplementary Table 2), despite the simultaneous occurrence of PDMR and PFS determination. Following this, we conducted a survival analysis for OS2 and discovered no statistically significant difference between the two groups (mOS2 10.3 vs 15.9 months; P = 0. 24). This implies that patients with shrinking lesions did not have an improved prognosis.
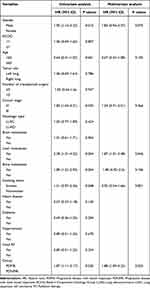 |
Table 2 Univariate and Multivariate Analyses of Clinical Variables on Overall Survival |
Choice of Follow-Up Treatment Program in PDMR and PDNMR
Fifteen patients who could not specify their follow-up regimen after disease progression were excluded from the study, leaving 160 patients for analysis of follow-up treatments. Within the PDMR group, 27 patients were analyzed to determine the relationship between their follow-up regimen and survival time; 4 patients were excluded due to missing follow-up regimen data. Among the 19 individuals who continued to receive immunotherapy, 15 maintained their initial immunotherapy regimen (consisting of PD-1 or PD-L1 inhibitors), while 3 switched to an alternative PD-1 inhibitor, and 1 transitioned from a PD-L1 inhibitor to a PD-1 inhibitor.
In the PDNMR group, 131 patients were continued on maintenance therapy at our institution, with 66 of these patients remaining on immunotherapy. Additionally, 64 patients did not receive further immunologic treatment. Among those who continued immune checkpoint inhibitors (ICIs), 55 patients continued their original immunotherapeutic drug, while 10 switched to a different PD-1 inhibitor, and 1 switched from a PD-1 inhibitor to a PD-L1 inhibitor. Baseline data from the PDMR cohort showed no significant differences in baseline characteristics between patients who continued immunotherapy and those who did not (Supplementary Table 3). The analysis indicates that patients in the PDMR cohort who continued immunotherapy had a higher survival rate (mOS 23.9 vs 6.5 months; P = 0.024). In contrast, continuing immunotherapy in the PDNMR group did not confer a survival benefit (mOS 23.3 vs 15.9 months; P = 0.320) (Figure 3).
Analysis of Hematological Indicators
Furthermore, complete hematological indices were collected from patients within 3 days prior to the commencement of immunotherapy. The analysis revealed that the PDMR group exhibited significantly higher levels of leukocytes, neutrophils, monocytes, and a higher systemic immune-inflammation index (SII) (Table 3). This indicates a potential correlation between the unfavorable prognosis of PDMR and immune cell levels.
 |
Table 3 Hematological Indices During the Three Days Prior to Immunotherapy |
Discussion
This retrospective study encompasses a substantial cohort of patients with inoperable NSCLC who were treated with immunosuppressive agents. It is the first investigation to examine the phenomenon of mixed responses at the point of disease progression, a critical “point of divergence” in clinical practice. At this juncture, some patients may choose to switch to second- or third-line treatment regimens, discontinuing their initial immunotherapy or chemotherapy protocols. Others might opt for an alternative regimen that includes a different immunotherapy drug, while some may decide to incorporate local therapeutic interventions into their primary treatment plan. Our findings challenge the conventional understanding by revealing that patients who experience lesion shrinkage at the time of progression exhibit poorer overall survival rates compared to those who do not experience such shrinkage. This could be associated with the greater proportion of stage IV patients with PDMR in our cohort, or it may be due to higher genetic heterogeneity in patients with mixed responses,10,11 including inconsistent PD-L1 expression across distinct tumor foci in the same patient.12 Furthermore, divergent microenvironmental and tissue type disparities may contribute to the distinct prognoses of both patient categories.13,14 Based on a novel mouse model of synchronous melanoma, some studies have pointed out that genetic variances amid tumors were powerful enough to induce an exclusive tumor immune microenvironment, which consequently led to autonomous regulation of the PD-1/PD-L1 pathway.15 Unfortunately, obtaining histologic specimens from the shrunken lesions proved challenging, as most patients declined biopsy, especially for shrunken lesions. Additionally, invasive procedures were often limited by the size and location of these lesions.
It is important to note that our study takes a clinical perspective. Both the PDMR and PDNMR groups consisted of patients with PD, and the only distinguishing factor between the two groups was the presence or absence of shrinking lesions at the time of progression after immunotherapy. This criterion offers physicians a more convenient means of assessing a patient’s condition. In a study investigating mixed responses in lung cancer patients receiving EGFR-TKI therapy, the occurrence of a mixed remission did not appear to be associated with prognosis.16 This is likely due to the fact that the cohort was evaluated for not only mixed responses of PD but also included MR of partial response (PR), stable disease (SD), with PDMR accounting for a minority of the cases. Additionally, the articles regarding unusual immunotherapy responses indicated that patients with MR had a longer overall survival than those with disease progression.17–22 Similarly, likely due to the absence of distinction between MR, and the fact that only a fraction of MR patients in these studies altered their initial treatment plans, which could have positively impacted MR patient prognoses. Therefore, we can deduce that patients only with MR evaluated as “PD” encounter a decreased OS. The results of the present study do not conflict with the results of the mixed remission-related studies mentioned above; rather, it is an important addition to the mixed responses studies since we focused only on MR of PD.
Given that the PDMR patient group exhibited a shorter PFS compared to the PDNMR group, and no significant difference in OS2 was observed between the two groups, the identification of PDMR does not necessarily indicate a better or worse subsequent survival outcome. The continued use of immune checkpoint inhibitors in the PDMR group may contribute to the observed improvement in subsequent OS2. Therefore, it is crucial to explore optimal treatment strategies for these patients. Tumor lesions often harbor different clones, each of which may respond variably to different therapies. Under therapeutic pressure, drug-sensitive clones may undergo adaptive genetic or environmental changes, potentially leading to resistance and subsequent tumor progression.23–27 Therefore, changing drugs may potentially “waste” the original drug’s response to the sensitive clone in the shrinking lesion in patients experiencing mixed remission. Tazdait M et al conducted a study with five patients with MR, including those with PR, SD, and PD status, who continued immunotherapy after MR, and two of the patients subsequently experienced PR.13 While pseudoprogression did pose a confounding factor in the study, the findings indicate that continuing immunotherapy significantly enhances the prognosis of patients. A retrospective study found that patients in MR had higher survival with continued ICI therapy.14 Our study was more precise in finding that patients in the PDMR group who continued with immunotherapy experienced better subsequent survival times. Conversely, there was no such correlation found in the PDNMR group. As a result, waiver of immunotherapy in patients with PDMR should be carefully considered.
The varying levels of immune cells in both groups may significantly contribute to the differences in prognostic survival rates. Previous studies have shown a strong correlation between elevated SII levels and adverse prognoses in cancer patients undergoing immune checkpoint inhibitor treatment.28 In the context of neoadjuvant therapy, SII is deemed to possess significant predictive value for malignant tumor prognosis, encompassing colorectal, gastric, pancreatic, and breast cancers. High SII values are one of the predictive factors reflecting poorer prognosis.29–32 In a study investigating chemotherapy for inoperable NSCLC, the presence of high levels of neutrophils indicated a poorer overall survival and progression-free survival.33 However, it is important to note that conclusions based on hematologic indices require further examination as the study was constrained by the limited availability of pathology samples. The study is burdened by limitations, including biased tumor staging enrollment, an inadequately large sample size, and a retrospective design confined to a single institution. In addition, since most patients were not tested for PD-L1, we were unable to provide meaningful analyses related to the expression of PD-L1. It is challenging to exclude chemotherapy as a confounding factor, given its common use as a first- or second-line treatment. The efficacy of chemotherapy in these roles could be sustained for a period, potentially influencing the mixed responses observed with immunotherapy. However, since these mixed responses occurred during immunotherapy monotherapy, they are more likely to be strongly associated with the immunotherapy itself. Additionally, MR to chemotherapy has only been reported in isolated cases, and there are currently no reports of PDMR following chemotherapy. The subsequent survival benefit observed in the PDMR group following continued immunotherapy suggests that the shrinking lesions may indeed be attributable to the effects of immunotherapy. Consequently, the occurrence of PDMR and the selection of subsequent treatment regimens still demand careful consideration.
Conclusion
According to our study, PDMR was more likely to occur in stage IV patients and patients who had received radiotherapy. OS and PFS were worse in the PDMR group compared with PDNMR patients, but OS after progression on immunotherapy was not statistically different between the two groups. One reason for this may be that PDMR patients themselves have shorter survival, but continued immunotherapy after progression prolongs survival.
Ethical Approval and Consent to Participate
This study was approved by the Ethics Committee of the Cancer Hospital Affiliated to Shandong First Medical University (SDTHEC2024003177). All organs were donated voluntarily with written informed consent, and these were conducted in accordance with the Declaration of Istanbul. Due to the retrospective nature of this study, the committee waived the requirement for informed consent. We declare that patient information will be kept confidential and that we adhere to the tenets of the Declaration of Helsinki Declaration.
Funding
This work was funded by the National Natural Science Foundation of China (82172676), the Natural Science Foundation of Shandong (ZR2021YQ52, ZR2020LZL016), and the Young Elite Scientist Sponsorship Program by Cast (no. YESS20210137).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA. 2020;70(3):145–164. doi:10.3322/caac.21601
2. Testa U, Castelli G, Pelosi E. Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers. 2018;10(8):248. doi:10.3390/cancers10080248
3. Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32(7):881–895. doi:10.1016/j.annonc.2021.04.008
4. Ettinger DS, Wood DE, Aisner DL, et al. Non–small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J National Compr Cancer Netw. 2022;20(5):497–530. doi:10.6004/jnccn.2022.0025
5. Garassino MC, Gadgeel S, Esteban E, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(3):387–397. doi:10.1016/S1470-2045(19)30801-0
6. Planchard D, Popat S, Kerr K, et al. Correction to: “Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up”. Ann Oncol. 2019;30(5):863–870. doi:10.1093/annonc/mdy474
7. Mushti SL, Mulkey F, Tang S, et al. Immune response evaluation and treatment with immune checkpoint inhibitors beyond clinical progression: response assessments for cancer immunotherapy. Curr Oncol Rep. 2020;22(11):116. doi:10.1007/s11912-020-00974-z
8. Adashek JJ, Subbiah V, Westphalen CB, Naing A, Kato S, Kurzrock R. Cancer: slaying the nine-headed Hydra. Ann Oncol. 2023;34(1):61–69. doi:10.1016/j.annonc.2022.07.010
9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
10. Xu Z, Chen L, Zheng L, et al. Hyperprogressive disease in cervical small cell carcinoma treated by immune checkpoint inhibitor. OTT. 2019;12:8873–8877. doi:10.2147/OTT.S213436
11. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–4250. doi:10.1158/1078-0432.CCR-16-3133
12. Moutafi MK, Tao W, Huang R, et al. Comparison of programmed death-ligand 1 protein expression between primary and metastatic lesions in patients with lung cancer. J Immunother Cancer. 2021;9(4):e002230. doi:10.1136/jitc-2020-002230
13. Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi:10.1016/j.ejca.2017.10.017
14. Zhou H, Sun Y, Xiu W, et al. Overall survival benefit of continuing immune checkpoint inhibitors treatment post dissociated response in patients with advanced lung cancer. J Cancer Res Clin Oncol. 2020;146(11):2979–2988. doi:10.1007/s00432-020-03282-y
15. Qin SS, Han BJ, Williams A, et al. Intertumoral genetic heterogeneity generates distinct tumor microenvironments in a novel murine synchronous melanoma model. Cancers. 2021;13(10):2293. doi:10.3390/cancers13102293
16. Dong ZY, Zhai HR, Hou QY, et al. Mixed responses to systemic therapy revealed potential genetic heterogeneity and poor survival in patients with non-small cell lung cancer. Oncologist. 2017;22(1):61–69. doi:10.1634/theoncologist.2016-0150
17. Sato Y, Morimoto T, Hara S, et al. Dissociated response and clinical benefit in patients treated with nivolumab monotherapy. Invest New Drugs. 2021;39(4):1170–1178. doi:10.1007/s10637-021-01077-7
18. Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. doi:10.1200/JCO.2015.64.0391
19. Tozuka T, Kitazono S, Sakamoto H, et al. Dissociated responses at initial computed tomography evaluation is a good prognostic factor in non-small cell lung cancer patients treated with anti-programmed cell death-1/ligand 1 inhibitors. BMC Cancer. 2020;20(1):207. doi:10.1186/s12885-020-6704-z
20. Wong A, Vellayappan B, Cheng L, et al. Atypical response patterns in renal cell carcinoma treated with immune checkpoint inhibitors—navigating the radiologic potpourri. Cancers. 2021;13(7):1689. doi:10.3390/cancers13071689
21. Humbert O, Cadour N, Paquet M, et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging. 2020;47(5):1158–1167. doi:10.1007/s00259-019-04573-4
22. Vaflard P, Paoletti X, Servois V, et al. Dissociated responses in patients with metastatic solid tumors treated with immunotherapy. Drugs R D. 2021;21(4):399–406. doi:10.1007/s40268-021-00362-3
23. Naing A, Agarwal R, Falchook G, et al. Retreatment after secondary resistance or mixed response: a pilot study. Oncology. 2013;85(6):350–355. doi:10.1159/000355691
24. Naing A, Kurzrock R. Chemotherapy resistance and retreatment: a dogma revisited. Clinical Colorectal Cancer. 2010;9(2):E1–E4. doi:10.3816/CCC.2010.n.026
25. Santini D, Vincenzi B, Addeo R, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol. 2012;23(9):2313–2318. doi:10.1093/annonc/mdr623
26. Cara S, Tannock IF. Retreatment of patients with the same chemotherapy: implications for clinical mechanisms of drug resistance. Ann Oncol. 2001;12(1):23–27. doi:10.1023/a:1008389706725
27. Naing A, Kurzrock R. Dodging a dogma: is treating beyond progression beneficial? Cancer Chemother Pharmacol. 2013;71(5):1385–1386. doi:10.1007/s00280-013-2123-z
28. Tian BW, Yang YF, Yang CC, et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy. 2022;14(18):1481–1496. doi:10.2217/imt-2022-0133
29. Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med. 2020;24(5):2993–3021. doi:10.1111/jcmm.14934
30. Peng X, Wang X, Hua L, Yang R. Prognostic and clinical value of the systemic immune-inflammation index in biliary tract cancer: a meta-analysis. J Immunol Res. 2022;2022:6988489. doi:10.1155/2022/6988489
31. Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273(3):532–541. doi:10.1097/SLA.0000000000003370
32. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
33. Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan multinational trial organisation LC00-03. Eur J Cancer. 2009;45(11):1950–1958. doi:10.1016/j.ejca.2009.01.023
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Path to Personalized Treatment in KRAS-Mutant Non-Small Cell Lung Cancer: A Review of Targeted Therapies and Immunotherapy
Shu CL, Liu YL
Cancer Management and Research 2022, 14:3485-3492
Published Date: 16 December 2022
NOTCH1 Mutations Predict Superior Outcomes of Immune Checkpoint Blockade in Non-Small Cell Lung Cancer
Huang Q, Cao H, Yao Q, Zhou X, Li H, Bai Q, Hu H
ImmunoTargets and Therapy 2023, 12:165-173
Published Date: 5 December 2023
Discover Mutational Differences Between Lung Adenocarcinoma and Lung Squamous Cell Carcinoma and Search for More Effective Biomarkers for Immunotherapy
Nu er lan STE, Yu B, Yang Y, Shen Y, Xu B, Zhan Y, Liu C
Cancer Management and Research 2024, 16:1759-1773
Published Date: 10 December 2024
Clinical Effect of Treatment with Metformin for Type 2 Diabetes on Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: A Retrospective Study
Wang Y, Sun Y, Hu J, Ma H
International Journal of General Medicine 2024, 17:6595-6604
Published Date: 31 December 2024
Advances in Multimodal Imaging Techniques for Evaluating and Predicting the Efficacy of Immunotherapy for NSCLC
Liu J, Xie M, Shen J, Yao J, Lin X, Bao X, Zhang X, Liang Y, Yang Y, Jiang G, Diao X, Han W, Du H, Xue X, Wu J
Cancer Management and Research 2025, 17:1073-1086
Published Date: 7 June 2025