Back to Journals » International Journal of Nanomedicine » Volume 20
Advancing CNS Therapeutics: Enhancing Neurological Disorders with Nanoparticle-Based Gene and Enzyme Replacement Therapies
Authors Liu S, Li H, Xi S, Zhang Y, Sun T
Received 31 December 2023
Accepted for publication 12 December 2024
Published 4 February 2025 Volume 2025:20 Pages 1443—1490
DOI https://doi.org/10.2147/IJN.S457393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. RDK Misra
Shuhan Liu,1– 3 Haisong Li,4 Shiwen Xi,1,2 Yuning Zhang,1,2,* Tianmeng Sun1,2,5,6,*
1Key Laboratory of Organ Regeneration and Transplantation of Ministry of Education, Institute of Immunology, The First Hospital, Jilin University, Changchun, Jilin, People’s Republic of China; 2National-Local Joint Engineering Laboratory of Animal Models for Human Diseases, Changchun, People’s Republic of China; 3Cancer Center, The First Hospital, Jilin University, Changchun, Jilin, People’s Republic of China; 4Department of Neurosurgery, The First Hospital, Jilin University, Changchun, Jilin, People’s Republic of China; 5International Center of Future Science, Jilin University, Changchun, People’s Republic of China; 6State Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianmeng Sun; Yuning Zhang, The First Hospital of Jilin University, 71 Xinmin St, Changchun, Jilin, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Given the complexity of the central nervous system (CNS) and the diversity of neurological conditions, the increasing prevalence of neurological disorders poses a significant challenge to modern medicine. These disorders, ranging from neurodegenerative diseases to psychiatric conditions, not only impact individuals but also place a substantial burden on healthcare systems and society. A major obstacle in treating these conditions is the blood-brain barrier (BBB), which restricts the passage of therapeutic agents to the brain. Nanotechnology, particularly the use of nanoparticles (NPs), offers a promising solution to this challenge. NPs possess unique properties such as small size, large surface area, and modifiable surface characteristics, enabling them to cross the BBB and deliver drugs directly to the affected brain regions. This review focuses on the application of NPs in gene therapy and enzyme replacement therapy (ERT) for neurological disorders. Gene therapy involves altering or manipulating gene expression and can be enhanced by NPs designed to carry various genetic materials. Similarly, NPs can improve the efficacy of ERT for lysosomal storage disorders (LSDs) by facilitating enzyme delivery to the brain, overcoming issues like immunogenicity and instability. Taken together, this review explores the potential of NPs in revolutionizing treatment options for neurological disorders, highlighting their advantages and the future directions in this rapidly evolving field.
Keywords: central nervous system, nanoparticle, gene therapy, enzyme replacement therapy, lysosomal storage disorders, neurological disorders
Introduction
With the steady growth of the aging population and the continuous progress of medical technology, the prevalence of neurological disorders has gradually increased, becoming the most common, debilitating, and underserved disease.1 There is a wide variety of central nervous system (CNS) diseases, including neurodegenerative diseases, autoimmune diseases, vascular diseases, tumors, metabolic diseases, psychiatric disorders, and others. The treatment of neurological disorders is a formidable challenge for contemporary medicine, largely because of the complexity of the brain and the diversity of neurological disorders that limit therapeutic efficacy.2 This complexity is exacerbated by unique physiologic barrier, the blood-brain barrier (BBB), which is a dynamic interface between blood and brain parenchyma that acts as a selective impediment to the passage of substances.
The BBB comprises continuous cerebrovascular endothelial cells and their intercellular tight junctions, intact basement membranes, pericytes, and membranes surrounded by astrocyte foot plates.3 This barrier not only protects the brain from harmful substances in the circulation but also restricts the passage of most therapeutic drugs, thereby limiting and complicating treatment options for many brain disorders.4 What’s more, the BBB is actively involved in the process of efflux transport, which is mediated by various efflux transporters such as multidrug resistance protein 1 (MDR1)/P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance-associated proteins (MRPs). These transporters play a crucial role in protecting the CNS by actively pumping out potentially harmful substances or xenobiotics from the brain back into the bloodstream, therefore preventing their accumulation within brain tissue.5 Therefore, there is a need to explore more effective treatments based on the enormous challenges facing neurological disorders.
Nanotechnology has ushered in a new era in the treatment of neurological disorders, providing innovative approaches to overcome long-standing challenges in drug delivery and treatment efficacy.6 Nanoparticles (NPs) have unique physicochemical properties such as small size and large surface area, which help them to cross the BBB and enable drug delivery to the lesion sites.7 In addition, surface modification of NPs, such as coating with polyethylene glycol (PEG) to prolong their circulatory half-life, or modification of targeting molecules on their surfaces to promote selective binding of NPs to receptors or transporters within the BBB for targeted delivery therapies, can minimize systemic side effects and improve therapeutic efficacy. Furthermore, surface modifications enhance the permeability of NPs to cross the BBB and biocompatibility in the body, thereby improving the efficiency of drug delivery.8 Hence, NPs have been extensively studied in a variety of neurological disorders and have become a promising tool in this field owing to their advantages. As research progresses, these nanoscale tools are expected to revolutionize neurotherapeutics.
Gene therapy aims to treat or prevent disease by altering or manipulating gene expression, and it focuses primarily on correcting defective genes that contribute to the development of disease. NPs can be designed to carry various forms of genetic material such as DNA, miRNA, siRNA, and mRNA, depending on the target disease and the desired therapeutic effect.9 This versatility makes them suitable for different types of gene therapy. Enzyme Replacement Therapy (ERT) is a therapeutic approach used primarily for lysosomal storage disorders (LSDs) that are genetic disorders often lacking specific enzymes, leading to the accumulation of toxic substances in the cells and causing severe neurological damage.10 However, unfavorable properties of the enzyme, including immunogenicity, lack of targeting, and instability, will diminish the clinical significance of ERT.11 NP-based enzyme delivery systems have multiple advantages, which protect the stability of drugs in vivo, promote drug release and enrichment across the BBB at CNS lesion sites, reduce drug immunogenicity, and achieve controlled release. Thus, NPs have emerged as a promising vehicle for delivering genes and enzymes to the CNS.12,13 The combination of nanotechnology with gene therapy and ERT marks a major leap forward in the field of CNS disease treatment. This review delves into the application of NPs in neurological disorders with a special focus on gene therapy and ERT, providing insights into future directions and potential breakthroughs in this emerging field.
Classification and Treatment of Neurological Disorders
Neurological disorders represent a diverse and complex group of diseases affecting the CNS in various ways. These disorders range from neurodegenerative conditions, autoimmune diseases, vascular disorders, and tumors to traumatic injuries, metabolic diseases, and psychiatric conditions.14 Each type of disease has its own unique pathogenesis and pathologic features, resulting in different symptoms and therefore requiring specific treatment approaches.15
Neurodegenerative Diseases
Neurodegenerative diseases are a group of disorders characterized by the progressive degeneration and death of neurons. These diseases typically involve chronic inflammation, oxidative stress, mitochondrial dysfunction, impaired protein clearance, and gradual loss of neuron structure and function, leading to cognitive, motor, and sensory impairments depending on the area of the nervous system that is affected.16 Common neurodegenerative diseases include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). For example, AD is associated with the buildup of β-amyloid (Aβ) plaques and tau protein tangles, leading to synaptic dysfunction and neuronal death. PD is characterized by the accumulation of α-synuclein (α-syn) proteins, which form Lewy bodies and lead to the death of dopamine-producing neurons in the substantia nigra. HD results from a genetic mutation leading to the accumulation of the huntingtin protein, causing neuronal death primarily in the basal ganglia. ALS is marked by the progressive degeneration of motor neurons, often linked to mutations in the SOD1 gene, among others.17
Treatment options for neurodegenerative diseases are limited and mainly focus on symptom management. Drugs like cholinesterase inhibitors (for AD) or dopamine agonists (for PD) can help alleviate symptoms but do not halt disease progression. Physical therapy, cognitive therapy, and dietary adjustments may help improve quality of life.18,19 Recent advances in gene therapy have opened up promising avenues for the treatment of neurodegenerative diseases. For instance, adeno-associated virus (AAV) vectors were utilized to deliver complementary deoxyribonucleic acid (cDNA) coding for human apolipoprotein E2 (ApoE2) as a potential treatment for AD in ApoE4 homozygotes (NCT03634007). Similarly, PD is another area where gene therapy is making strides. AAV2-mediated delivery of the GDNF gene (NCT04167540) and the AXO-Lenti-PD vector for dopamine synthesis (NCT03720418) are being evaluated for their potential to restore dopaminergic neuron function and alleviate motor symptoms. In the case of HD, a Phase I/II trial (NCT04120493) is testing the safety and efficacy of AAV5-miRNA, designed to silence the mutant huntingtin gene and slow disease progression. In the treatment of spinal muscular atrophy (SMA), FDA-approved Zolgensma (AVXS-101) has provided success for SMA Type 1 (NCT03381729), which replaces the defective SMN1 gene. ASOs targeting SMN2 gene to alter SMN2 splicing for increasing the fraction of transcripts containing exon 7 have also shown efficacy (NCT02386553). These clinical trials offer hope for more effective treatments in the future, though these are not yet widely available.
Autoimmune Diseases
Autoimmune diseases occur when the immune system mistakenly attacks healthy cells and tissues in the CNS, resulting in inflammation and damage. These conditions can affect the brain and nerves, leading to a variety of neurological symptoms. Common autoimmune neurological diseases include multiple sclerosis (MS), autoimmune encephalitis, and neuromyelitis optica.20 The underlying cause of autoimmune neurological diseases is often the loss of immune tolerance, where the body’s immune system fails to distinguish between foreign pathogens and its own cells. This leads to the production of antibodies and immune cells that target the nervous system.21 For instance, MS is characterized by the immune system attacking the myelin sheath, causing disrupted electrical signals between the brain and body. This leads to the formation of scar tissue (sclerosis) at sites of myelin damage, neuronal loss and brain atrophy.22
Common treatment strategies for autoimmune brain diseases primarily focus on modulating the immune response to prevent further neurological damage. Immunosuppressive therapies, including corticosteroids and drugs such as rituximab or azathioprine, are widely used to reduce immune-mediated attacks on the CNS. Monoclonal antibodies target specific antigens to block pathological immune responses. Anti-inflammatory agents, such as corticosteroids, help control acute inflammation during disease flare-ups. Plasmapheresis is employed to remove harmful autoantibodies from the bloodstream, while intravenous immunoglobulin (IVIG) is used to modulate the immune system and provide passive immunity. These treatments aim to preserve neurological function and improve patient outcomes.23 In clinical trials for MS, monoclonal antibodies targeting B cells and T cells, such as the anti-CD52 monoclonal antibody alemtuzumab and the humanized anti-CD20 monoclonal antibody ocrelizumab, have demonstrated promising efficacy, offering hope for MS treatment.24
Vascular Diseases
Vascular diseases refer to disorders that affect the blood vessels (arteries, veins, and capillaries) that supply blood to the nervous system. These diseases can lead to serious neurological complications due to impaired blood flow, resulting in insufficient oxygen and nutrient supply to the brain. Stroke (ischemic and hemorrhagic) is the most common type of vascular disease, resulting from the interruption of blood flow to the brain, leading to brain tissue necrosis, inflammation, and a cascade of cell death in affected areas. This may be due to thrombosis, embolism, or hemorrhage.25
The treatment of vascular diseases differs due to different pathogenesis. For ischemic stroke, thrombolytic agents like tissue plasminogen activator are used to dissolve clots. Antiplatelet agents and anticoagulants are common in prevention.26 The treatment of hemorrhagic stroke focuses on controlling the bleeding and reducing intracranial pressure. Surgical interventions may include clipping or coiling of aneurysms, and decompressive craniectomy may be used to relieve pressure.27 Some stem cell therapies have been explored in clinical trials for enhancing functional recovery by modulating immune responses, providing neuroprotection, and restoring damaged neural circuits in the brain.28
Tumors
Tumors of the nervous systems originate from the brain or metastasize from other areas. Tumorigenesis involves uncontrolled cell growth, often due to genetic mutations affecting growth-regulating pathways. Tumors are highly invasive, disrupting normal brain function. They can compress brain structures, increase intracranial pressure, and invade surrounding tissue. The most common primary brain tumors include gliomas (eg, glioblastoma multiforme (GBM), astrocytomas, oligodendrogliomas), meningiomas, medulloblastomas, and ependymomas.29 Metastatic tumors are more common, arising from cancers like lung, breast, or melanoma that spread to the brain.30
Treatment strategies for CNS tumors depend on the tumor type, location, size, and patient’s condition. Surgical resection, radiotherapy, and chemotherapy (eg, temozolomide for GBM) are standard treatment modalities.31 Novel therapies, including targeted therapy and immunotherapy, are under investigation.32 Clinical trials are ongoing to explore innovative therapies for CNS tumors, such as DC vaccination and bevacizumab combined with conventional therapy in GBM, may provide help to overcome the limitations of current therapies.33,34
Traumatic Injuries
Traumatic injuries to the CNS are caused by an external force, which may lead to significant neurological deficits, including motor, sensory, cognitive, and autonomic dysfunctions. The severity of traumatic injuries can range from mild to severe, with long-lasting consequences. Common pathological features of traumatic brain injury include brain edema, increased intracranial pressure, diffuse axonal injury, and BBB disruption. These injuries can lead to a range of cognitive, motor, and emotional impairments.35 Spinal cord injury is caused by trauma to the spinal cord, resulting in partial or complete loss of motor and sensory functions below the injury site. The damage can be the result of direct trauma, or it can occur due to secondary processes such as ischemia, inflammation, and free radical formation.36
The acute management of traumatic injuries focuses on reducing intracranial pressure, mitigating inflammation, preventing further neural damage, and stabilizing the patient. Surgical interventions are frequently required to decompress affected nerve tissues. Long-term rehabilitation is essential to restore neurological function. Additionally, neuroprotective and regenerative therapies are designed to minimize secondary injury and promote neural regeneration and repair.37
Metabolic Diseases
Metabolic diseases of the nervous system refer to a group of inherited or acquired disorders that affect the brain and nervous system due to abnormalities in the metabolism of essential molecules such as proteins, lipids, and carbohydrates. These diseases often result from enzyme deficiencies or genetic mutations that impair the breakdown, storage, or production of crucial substances. Common examples include LSDs, mitochondrial disorders, and leukodystrophies. In LSDs, defective enzymes lead to the accumulation of toxic substances in the lysosomes, causing cell damage and degeneration of neural tissue.38 Mitochondrial disorders such as Leigh syndrome involve impaired energy production due to dysfunctional mitochondria, which disrupts the energy supply needed by neurons and results in neurodegeneration.39 Leukodystrophies involve abnormalities in the myelin sheath that insulates nerve fibers, leading to progressive loss of motor function and cognition.40
The treatment of metabolic diseases affecting the nervous system is challenging, as these disorders often involve widespread neurological deterioration. Current therapies include ERT to compensate for the missing or defective enzyme, and substrate reduction therapy to reduce the accumulation of toxic substances by limiting their production.41 Recent advancements of clinical trials in ERT have shown promise in addressing the neurological manifestations of various LSDs. For example, trials investigating intrathecal administration of enzymes such as idursulfase for MPS II (NCT00920647, NCT02055118) and recombinant human heparan N-sulfatase for MPS IIIA (NCT01155778) aim to bypass the BBB and directly deliver therapeutic enzymes to the CNS, thereby potentially mitigating cognitive and neurological decline. Similarly, in Neuronal Ceroid Lipofuscinoses (CLN2 disease), studies on BMN 190 (cerliponase alfa) (NCT01907087, NCT02678689) have focused on restoring enzyme function within the CNS to slow the progression of neurodegeneration. Additionally, ongoing research in other conditions like Gaucher Disease Type 3 (NCT00001211) and metachromatic leukodystrophy (NCT01510028) further underscores the potential of ERT in alleviating neurological symptoms. These trials collectively highlight the growing focus on refining ERT approaches to effectively target the CNS, offering hope for improved management of these debilitating disorders.42
Psychiatric Diseases
Psychiatric diseases encompass a wide range of mental health disorders that affect cognitive function, mood, behavior, and emotional regulation. These diseases, such as depression, schizophrenia, bipolar disorder, and anxiety disorders, are influenced by both genetic and environmental factors, and involve complex changes in brain structure, function, and neurotransmitter systems. Psychiatric disorders often involve dysregulation in neurotransmitter systems, such as dopamine, serotonin, and glutamate pathways, which are critical for mood regulation, cognition, and emotional responses.43
The treatment of psychiatric diseases is complex and multifactorial, and treatment strategies are often multimodal, including pharmacotherapy, psychotherapy, and sometimes brain stimulation techniques.44 Psychotropic medications, including antidepressants, antipsychotics, and mood stabilizers, are the mainstay of treatment. Psychotherapy and cognitive-behavioral therapy are also essential components of management.45 Several clinical trials have been conducted for the treatment of psychiatric diseases. For schizophrenia, in addition to traditional targets such as 5HT and dopamine receptor D2R, new targets like D-amino acid oxidase and GlyT1, which are related to NMDA receptor activation, have emerged. These novel targets aim to enhance NMDA receptor activity by targeting mechanisms associated with its activation.46 In the treatment of bipolar disorder type I, endoxifen targets estrogen receptors and protein kinase C. For depression, zuranolone has been approved by the US FDA for postpartum depression, lumateperone has been approved for schizophrenia and bipolar disorder, and pimavanserin has been approved for the treatment of psychosis in PD.47,48
To summarize, the treatment of neurological disorders presents significant challenges due to the complexity of these diseases. Many neurological conditions, such as neurodegenerative and metabolic disorders, are associated with intricate pathologies involving multiple molecular pathways. This makes it difficult for single therapies to fully alleviate symptoms or slow disease progression. Current treatment strategies for these disorders vary widely, often focusing on symptom management rather than addressing root causes, particularly for progressive conditions like neurodegenerative diseases. Although clinical trials can offer new and promising therapeutic approaches for diseases with limited treatment options, current therapies still face significant limitations, particularly when it comes to crossing the BBB and effectively targeting the CNS. Future research must focus on enhancing the efficiency of drug delivery systems that bypass the BBB.
Mechanisms by Which Nanoparticles Penetrate the Blood-Brain Barrier
The BBB consists of endothelial cells with tight junctions, pericytes, astrocytes and basement membranes. This complex structure ensures the formation of a highly selective barrier that controls the entry of substances from the blood into the brain parenchyma.49 The tight junctions between endothelial cells limit paracellular transport, allowing only passive diffusion of lipophilic small molecules to pass through.50 The selectivity of this barrier poses a great challenge for drug delivery. Most blood-borne substances including more than 98% of small molecule drugs and all macromolecular therapeutics are prevented from entering the brain.51
Nanotechnology provides a library of advanced materials can serve as a powerful toolkit for targeted drug delivery. Intensive research in BBB anatomy and physiology has further facilitated the development of brain-targeting strategies to enhance BBB penetration.51 The transport pathways of drug molecules through the BBB include paracellular and transcellular diffusion, receptor-mediated transcytosis, cell-mediated transcytosis, carrier-mediated transport, and adsorption-mediated transcytosis (Figure 1).52 A detailed understanding of these mechanisms is critical to understanding how NPs can be effectively used in the treatment of neurological disorders.
 |
Figure 1 Transport mechanism of the solute or substance BBB shuttle. Reproduced from Wang J, Yu Y, Zhang C, Song J, Zheng Z, Yan W. New advances in diagnosis and treatment of nano drug delivery systems across the blood-brain barrier. Nanocomposites. 2023/12/31 2023;9(1):116–127. Creative Commons CC BY license (http://creativecommons.org/licenses/by/4.0/).52 © 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group. |
Passive Diffusion
Passive diffusion is the simplest form of substance transport across the BBB without expending energy.53 This mechanism is driven by the concentration gradient and is highly dependent on the molecular size and lipophilicity of the substance, with small, nonpolar, and lipophilic molecules being able to diffuse through the endothelial cell membrane of the BBB.54 The BBB permits the diffusion of certain gases (eg, oxygen and carbon dioxide); small molecules (eg, ethanol and nicotine); and some lipophilic substance (eg, anesthetic agents) to diffuse into brain tissue.55
Although passive diffusion is a natural transport mechanism, it has very limited applicability in drug delivery. The mechanism of passive diffusion is utilized to engineer enhanced drug permeation through the BBB, which can increase the lipophilicity and decrease the molecular weight of drug molecules.56 A study developed amphiphilic yellow-emissive carbon dots (Y-CDs) measuring 3 nm in diameter. The contents of primary and carboxyl groups on CDs were 6.12×10−5 and 8.13×10−3 mmol/mg, respectively, showing the potential for loading small molecule drugs via bioconjugation. Y-CDs were localized in the central canal of the spinal cord of zebrafish, displaying the ability to cross the BBB, which might be because of passive diffusion resulting from the amphiphilic nature of Y-CDs. In addition, Y-CDs can inhibit the overexpression of human amyloid precursor protein and Aβ after entering the cells, indicating potential for preventing Aβ-related AD.57
Carrier-Mediated Transport
Carrier-mediated transport (CMT) is an important mechanism for the delivery of essential nutrients and therapeutic drugs to the brain. This process requires specific carrier proteins that bind to their respective substrates, transport the substrate, and cross the BBB. The main substances include glucose, amino acids, nucleosides, and others.58 Glucose transporters (especially glucose transporter protein 1, GLUT1) facilitate glucose transport across the BBB.59 Essential amino acids are transported to the brain via specific amino acid transporters.60 Nucleosides such as adenosine and thymidine are transported by nucleoside transporters.61 These substances play important roles in brain function and metabolism, and the specificity of CMT allows for the controlled and efficient transport of these molecules into the brain, thereby maintaining the delicate balance of the CNS environment.
Recent research has explored the potential of NPs to enhance drug delivery via interactions with CMT-associated transporters. However, it is important to clarify that NPs, due to their larger size, cannot directly traverse the BBB through the narrow pores of these transporters as small molecules do. Instead, when NPs are engineered to target specific transporters, they can bind to these transporters and form clusters, triggering a process known as receptor-mediated endocytosis rather than true CMT. In this process, transporters act more like receptors, facilitating the internalization of the NPs into the endothelial cells via endocytosis, after which the NPs are released into the brain via exocytosis.7 The most commonly studied CMT transporter is GLUT1, which is expressed at significantly higher levels in cerebrovascular endothelial cells than many other transporters and receptors. A study reported glucose-based NPs to traverse the BBB in the context of glycemic control.62 This nanocarrier was constructed by self-assembly of negatively charged PEG-poly(α,β-aspartic acid) and positively charged PEG-poly([5-aminopentyl]-α,β-aspartamide) block copolymers. The surface of the polymeric NPs was decorated with multiple glucose molecules with a controlled density ranging from 0, 10, and 25 to 50%. By injecting the Gluc(6)/m formulations intravenously, the 25% Gluc(6)/m formulation showed the highest brain uptake in glycemic-controlled mice compared to the free-feeding group. These results suggest that glycosylated nanocarriers can cross the BBB via the GLUT1 transporter.
Receptor-Mediated Transcytosis
Receptor-mediated transcytosis (RMT) is a highly selective mechanism wherein a molecule (such as a protein, peptide, or antibody) binds to certain receptors on the surface of cerebrovascular endothelial cells, allowing it to pass through the BBB. Key substances that pass through the BBB via RMT include transferrin (Tf), insulin, and low-density lipoprotein receptors (LDLRs).63 Iron in the bloodstream binds to Tf, a transport protein, and the Tf-iron complex binds to the Tf receptors (TfRs) on cerebrovascular endothelial cells. The binding triggers endocytosis, a process by which the cell membrane wraps around the Tf-iron complex to form a vesicle that enters the cell.64 Insulin or insulin-like growth factors (IGFs) in the blood bind to insulin receptors on BBB endothelial cells, triggering internalization after receptor-mediated endocytosis.65 Low-density lipoprotein (LDL), which carries cholesterol in the blood, binds to LDLRs on BBB endothelial cells, then the LDL-LDLR complex is internalized and the LDL is translocated into the cell.66
RMT mechanism is frequently employed to allow for the targeted distribution of NPs to pass through the BBB. A study reported that a short peptide derived from Aβ1-42 modified the surface of liposomes to develop bio-inspired liposomes (SP-sLip) that interact with the lipid-binding domain of exchangeable apolipoproteins to form a protein corona. In vivo, SP-sLip could absorb plasma apolipoproteins A1, E, J (ApoA1, E, and J), thereby exposing the receptor-binding domains of apolipoproteins on the liposome surface for multiple receptor recognition (LRP1/ApoE, LRP2/ApoJ, and SR-B1/ApoA1) and then crossed the BBB enabling brain-targeted delivery.67
Adsorptive-Mediated Transcytosis
Adsorptive-mediated transcytosis (AMT) relies on electrostatic interactions between cationic molecules and anionic sites on the surface of the endothelial cells of the BBB.68,69 Once the cationic molecules contact with the endothelial cell surface, they adhere to it by adsorption.70 This process involves the invagination of the surrounding cell membrane, culminating in the formation of vesicles (endosomes) within the cytoplasm. The drug-containing vesicles then move across the cerebrovascular endothelium from the luminal side (facing the blood) to the tubular side (facing the brain tissue), which in turn fuses with the cell membrane and releases its contents into the brain tissue, achieving the passage of the substance across the BBB.71
NPs can be manipulated to increase the positive charge to enhance interactions with the negatively charged surface of the cerebrovascular endothelial cell membranes, which in turn promotes their uptake and transcytosis via AMT. The main strategies include surface-modified peptides and the application of positively charged nanomaterials.72,73 For example, cell-penetrating peptides (CPPs) such as penetratin and the Syn-B vectors are used to promote NP passage through the BBB.74 Additionally, using positively charged materials as components of NPs, such as liposomes or cationic polymers (eg, polyethyleneimine [PEI] or chitosan) can significantly increase the positive charge.75,76 A study reported dual functionalized liposomes (Tf-CPP liposomes) for RMT and AMT to cross the BBB by modifying their surfaces with Tf and CPPs, respectively. The penta peptide QLPVM enhanced transport across the BBB into GBM tissue in the brain in vivo and enhanced cellular penetration.73
Cell-Mediated Transcytosis
Cell-mediated transcytosis involves the cells to carry therapeutic drugs across the BBB by utilizing the cells’ natural transporter mechanisms.77 For example, some immune cells such as macrophages and monocytes, take advantage of their natural migration into the brain to act as carriers of drugs, especially in inflammation or infection situations where the BBB is compromised.52 Mesenchymal stem cells (MSCs) have shown promise to cross the BBB. Their inherent ability to localize the site of injury and potential for genetic modification make them a versatile tool for drug delivery.78 Neural stem cells (NSCs) have a natural affinity for brain tissue and can migrate across the BBB.79 In addition, modified erythrocytes have been explored as potential carriers for drug delivery to the brain for their biocompatibility and long circulation time in the bloodstream.80
Cell-based carriers offer unique opportunities to improve the targeted delivery of NPs, extend their circulation time and facilitate the transport of NPs across challenging physiological barriers.81 It has been reported that activated effector/memory CD4+ helper T cells (CD4+ TEM cells) can serve as carriers for the delivery of polymer NPs across the BBB. This study demonstrated that 200-nm polystyrene NPs functionalized with maleimide groups were covalently conjugated to CD4+ TEM cells via thiol groups on the cell surface. Using an in vitro BBB model, NP-modified CD4+ TEM cells effectively transported NPs across the BBB under both static and physiological flow conditions. Systemic administration in mice confirmed their ability to enter the brain parenchyma, highlighting the potential of this T-cell subset for targeted brain delivery.77
In all, the mechanisms by which NPs penetrate the BBB have been explored for NP-based therapies. While these mechanisms offer promising strategies to overcome the challenges posed by the BBB, each method has its limitations. Passive diffusion is highly restricted due to the selective nature of the BBB, allowing only small and lipophilic molecules to pass through. CMT and RMT, though more specific, may suffer from limited cargo capacity and inefficiency when delivering larger therapeutic molecules. Additionally, AMT can be non-selective, raising concerns about off-target effects and potential toxicity. Therefore, future research needs to focus on improving the selectivity and efficiency of these delivery mechanisms to improve the treatment outcomes for CNS disorders.
Nanoparticle Approaches for Central Nervous System Drug Delivery
NPs, given their small size and tunable physicochemical properties, offer a new approach to bypass the BBB and enable targeted and controlled drug delivery to the brain. Various types of NPs are being explored for their potential application in diagnostic, therapeutic, and targeted therapies of CNS disorders (Figure 2).
 |
Figure 2 Types of NPs for drug delivery and their application in various CNS disorders. Created with BioRender.com. |
Types of Nanoparticles for Central Nervous System Drug Delivery
Organic Nanoparticles
Organic NPs that can penetrate the BBB and deliver drugs to the CNS are currently being investigated, mainly based on lipids, polymers, and biomolecules. These organic NPs can cross the BBB and increase the drug concentration in the brain parenchyma by surface modification to enhance surface charge, lipophilicity, biocompatibility, and brain-targeting ability.
Liposome NPs, spherical vesicles made of phospholipid layers, offer advantages for targeted drug delivery in neurological disorders.82 Their natural phospholipid composition reduces toxicity, making them suitable for repeated use.82 Liposomes can encapsulate various drugs, including small molecules, proteins, and nucleic acids, enabling synergistic therapies.83 Additionally, positively charged liposomes enhance stability and therapeutic efficacy by interacting with negatively charged biomolecules.84
Solid lipid NPs (SLN) are solid at room and body temperatures, including fatty acids, triglycerides, waxes, or other lipidic substances.85 SLNs can be designed to release the drug payload in a controlled manner.86 Moreover, the solid core is less susceptible to degradation and fusion than other lipid carriers, giving them better stability of the encapsulated drug.87
Polymeric NPs are made from biodegradable polymers such as chitosan, alginate, gelatin, as well as poly(lactic acid) (PLA), polyglycolic acid (PGA), and poly(lactic-co-glycolic acid) (PLGA).88–90 Polymeric NPs can encapsulate a wide range of therapeutic agents, including small molecules, proteins, and nucleic acids. Their biocompatible breakdown products reduce long-term toxicity risks.91 These NPs can encapsulate both hydrophilic and hydrophobic drugs, enhancing combination therapy,91 and offer controlled drug release.92
Dendrimers are highly regular and symmetrically branched tree-like macromolecules with controlled size and shape.93 The interior of dendrimers can encapsulate drugs, and the branched structure provides a high surface area to volume ratio, allowing for greater functionalization and interaction with the surrounding environment.94 In addition, dendrimers have high catalytic efficiency and their high surface area to volume ratio provides a large number of active sites for catalysis.95
Micelles are molecular structures that form spontaneously when amphiphilic molecules reach a certain concentration in a liquid.96 Typical micelles are spherical in structure, with the hydrophobic tails pointing inward, and the hydrophilic heads pointing outward. As a result, the interior of the micelle can encapsulate hydrophobic drugs, and its hydrophilic portion can connect water-soluble drugs.97
Protein- and peptide-based NPs are constructed using naturally occurring biomolecules such as proteins (eg, albumin, gelatin, or ferritin) or peptides (short-chain amino acids) as the primary building blocks, which are typically well tolerated and can be broken down into non-toxic by-products.98 These NPs can encapsulate a wide range of therapeutic agents, and the immunogenicity of the NPs can be minimized by using endogenous proteins or peptides.99
Inorganic Nanoparticles
Inorganic NPs have properties such as high surface area to volume ratio, optical properties, and surface modification functions and can be conjugated with a wide range of therapeutic drugs, targeting ligands, and imaging probes to deliver drugs to specific regions or cell types.100 These NPs include metal NPs, quantum dots, and silica NPs, among others, each with their own advantages in drug delivery to the brain.
Metal NPs are composed of metallic elements, typically ranging in size from 1 to 100 nm. Their small size results in a high surface area to volume ratio, which enhances their reactivity with other substances and provides more active sites for reactions.101 Commonly used metallic NPs include gold (Au), silver, and iron oxide NPs. AuNPs are known for their biocompatibility and ease of functionalization for targeted drug delivery and photothermal therapy.102 Silver NPs have antimicrobial properties and therefore have the potential to treat brain infections or brain tumors.103,104 Iron NPs are commonly used in magnetic applications, and magnetic iron oxide NPs can be used under magnetic guidance, such as magnetic resonance imaging (MRI) contrast agents and brain tumor thermotherapy.105,106
Quantum dots are NPs typically made from semiconductor materials such as silicon, cadmium selenide, cadmium sulphide, or indium arsenide, and typically have diameters of 2–10 nm.107 Quantum dots have unique optical properties that vary by size, making them valuable for brain imaging and tracking therapeutic drugs. Their ability to label proteins or structures enables detailed real-time bioimaging.108
Silica NPs (SiNPs) are made primarily from silicon dioxide (SiO2), and can be synthesized in a variety of shapes. Some SiNPs are designed with porous-like structures to enable controlled drug release and enhanced drug loading.109 Given the chemical inertness of silica, SiNPs maintain the stability of the delivered drug and biocompatible to minimize immunogenicity and toxicity.110
Strategies to Manipulate Nanoparticles for Blood-Brain Barrier Crossing
Surface Modification
Surface modification of NPs is a key strategy to enhance their ability to cross the BBB for effective treatment of neurological disorders. This approach involves altering the surface physicochemical properties of NPs or extending the half-life of NPs to improve their interaction with cerebrovascular endothelial cells, thereby facilitating their entry into the brain.
Specific ligands attached to the surface of NPs is a widely studied approach, and these ligands can bind to the corresponding receptors on the BBB and mediate transcytosis.111 For example, attachment of Tf or insulin to NPs can be utilized to enhance the ability to cross the BBB via their respective receptors on the BBB.51 NPs can also be designed to mimic or incorporate substances that naturally cross the BBB, such as glucose, to improve their transport across the BBB.112
PEG is often used to modify the surface of NPs to reduce the opsonization and clearance by the mononuclear phagocyte system. This modification prolongs the circulation time of NPs in the bloodstream, thus increasing the chances of NPs crossing the BBB.113 In addition, the length of the PEG chains also affects the ability of NPs to cross the BBB, and this needs to be optimized to strike a balance between prolonged circulation time and effective BBB penetration.114
The surface charge of NPs significantly affects their interaction with the BBB. Positively charged NPs have been shown to enhance interaction with negatively charged cell membranes, but they also pose a higher risk of toxicity. Optimizing the surface charge of NPs to enhance BBB permeability, while minimizing adverse effects is the focus of current research.52
Size and Shape Optimization
The size and shape of NPs significantly affect their biodistribution, cellular uptake, and interaction with biological barriers.115,116 The size of NPs greatly affects their ability to cross the BBB. Studies have shown that NPs with diameters of approximately 10–100 nm are more likely to cross the BBB.117 This size range helps to evade rapid clearance mechanisms while allowing effective interaction with the BBB.
The shape of NPs also plays an important role in their BBB crossing. Earlier, it was thought that spherical NPs showed better penetration than rod- or irregular-shaped NPs.118 However, recent studies have shown that non-spherical shapes, such as rod or disk NPs, may enhance BBB traversal owing to their unique interactions with biological membranes.117 Alternatively, studies have also explored the role of NP aspect ratio (ratio of width to height) on BBB permeation, and higher aspect ratios may contribute to more efficient crossing of the BBB.72 The surface area of NPs is another aspect that is affected by size and shape. NPs with a larger surface area (relative to their volume) can carry more therapeutic drugs and have more interaction sites with the BBB, thus potentially enhancing their ability to cross the BBB.119
Magnetic Guidance
Magnetic NPs, usually consisting of an iron oxide core, are biocompatible and can be directed to specific brain regions using an external magnetic field. This approach can be targeted to minimize systemic side effects and improve therapeutic efficacy.120 It has been shown that the successful use of magnetic fields to guide NPs containing drugs or genetic materials across the BBB has shown significant therapeutic effects on neurological disorders in animal models.121
Moreover, magnetic guidance offers the advantage of real-time control over NP movement, allowing for dynamic adjustments during treatment to optimize therapeutic outcomes. The ability to fine-tune the localization of NPs with an external magnetic field could lead to highly precise treatment strategies, especially in conditions where targeting specific brain regions is critical, such as in localized brain tumors or focal areas of neurodegeneration.122 Recent studies have demonstrated the effectiveness of magnetic fields in guiding NPs across the BBB to deliver drugs, genes, or other therapeutic materials, resulting in significant therapeutic effects in various neurological disorder models.123,124
Endosomal Escape of Nanoparticles
After being internalized by cells, NPs are typically encapsulated within endosomes or lysosomes. One of the critical challenges in NP-mediated delivery of therapeutic agents is ensuring their release from endosomes or lysosomes into the cytosol, a process known as endosomal escape.125 Several strategies have been developed to facilitate endosomal escape. For instance, some cationic polymer NPs, such as PEI, absorb protons in the acidic environment of endosome, leading to an influx of chloride ions and water. This causes the endosome to swell and eventually rupture, releasing the cargo into the cytosol through the proton sponge effect.126 Additionally, NPs can be designed using materials that are stable at physiological pH but become destabilized in the acidic environment of the endosome. These materials undergo conformational changes that disrupt the endosomal membrane and release the therapeutic cargo.127 Some NPs also incorporate CPPs or fusogenic peptides on their surface, which interact with the endosomal membrane and promote its destabilization, facilitating the release of the encapsulated cargo into the cytosol.128 Moreover, certain NPs are engineered with redox-sensitive materials containing disulfide bonds that are cleaved in the cytosol environment.129 Briefly, these escape mechanisms are crucial for the successful application of NP-based therapies in CNS diseases. By leveraging these mechanisms, the intracellular delivery and activity of therapeutic agents can be enhanced, paving the way for more effective treatments of neurological disorders.
In conclusion, both organic and inorganic NPs have shown potential for effectively crossing the BBB. Each class offers unique advantages: organic NPs are often biocompatible and biodegradable, making them ideal for safe and sustained drug release, while inorganic NPs, due to their stability and magnetic properties, provide opportunities for real-time imaging and targeted delivery. Future research must focus on overcoming these challenges by developing multifunctional nanoparticles that can combine the biocompatibility of organic materials with the precision of inorganic nanostructures. Optimizing strategies to manipulate NPs for BBB crossing such as surface modification, size and shape optimization, and the use of magnetic or endosomal escape materials can further enhance BBB penetration for CNS disorders.
Nanoparticle-Based Gene Therapy for Neurological Disorders
Gene therapy is a revolutionary medical technique that involves the introduction, modification, or manipulation of genes within cells to treat or prevent diseases.130 This can be accomplished in a variety of ways, including replacing disease-causing genes with healthy copies, inactivating malfunctioning genes, or introducing new or modified genes to help treat a disease.131 In general, gene therapy can be categorized into gene augmentation and gene silencing approaches based on the nature of the effect on genetic activity.132 Gene augmentation therapy involves gene replacement or gene addition to enhance the expression of a specific missing/dysfunctional gene by administering the appropriate pDNA, mRNA, or CRISPR/Cas9. Gene silencing, on the other hand, utilizes substances such as siRNA, miRNA molecules, or antisense oligonucleotides (ASOs) to silence genes through RNA interference, thereby suppressing the expression of undesirable genes.133 Therefore, gene enhancement and gene silencing therapies have a promising future in a variety of neurological gene-related disorders.
However, the brain poses unique challenges for gene therapy, and the development of effective delivery carriers is a significant focus of current research.134 Nowadays, NP-based delivery system are being developed as a new tool to improve brain drug delivery for gene therapy.135 NPs are capable of carrying various types of genetic materials, including DNA plasmids, RNA, CRISPR/Cas9, and other gene-editing tools. This versatility in drug delivery has led to a wide range of applications. Here we shall summarize successful examples of NP-mediated delivery of nucleic acids for gene therapy applications, mainly focusing on DNA, siRNA, mRNA, miRNA, ASOs, and CRISPR/Cas9 gene-editing tools (Table 1), highlighting the technical diversity in different neurological disorders.
 |
Table 1 Applications of NP-Based Gene Therapy for Neurological Disorders |
DNA Delivery
As an essential component of cells, DNA plays a role in the growth, development, function, and reproduction in nearly all known organisms. When DNA is mutated, it can contribute to the development of certain diseases.206 As a result, gene therapy targeting DNA is widely studied. Plasmid DNA is a small, circular, double-stranded DNA molecule that is most commonly found in bacteria, but is also found in archaea and eukaryotes. It is capable of replicating independently of chromosomal DNA. Plasmid DNA is widely used as a genetic engineering tool in biotechnology and molecular biology, and is also used in various types of gene therapy.207 There are a number of studies that utilize NPs to deliver plasmid DNA into the body for neurological disorders.
Plasmid DNA delivered by NPs was widely studied in neurodegenerative diseases such as AD. Vgf is a neurotrophin-stimulating protein that plays a role in learning, synaptic activity, and neurogenesis, which is down-regulated in the brains of AD patients. A study developed liposomal NPs encapsulating pVGF, decorated with GLUT-1 targeting ligands and brain-targeting CPPs. The liposomal NPs showed higher vgf transfection efficiency in brain cells and BBB models.136 NPs targeted at silencing or knocking down the BACE1 gene, such as RVG29 modified dendrimers137 and dopamine modified poly-lysine NPs,138 have also been investigated to reduce Aβ production and plaque formation in the brain (Figure 3A and B). For PD treatment, Aly et al developed NPs for the intranasal delivery of the plasmid DNA targeting human glial cell line-derived neurotrophic factor (hGDNF) gene. The expressed hGDNF had neuroprotective properties and supported the survival of dopaminergic neurons, therefore slowing or halting PD progression.139 Huang et al designed nano-MgO composites functionalized with nerve growth factor (NGF) and loaded with plasmid DNA designed to silence the SNCA gene to reduceα-syn accumulation and improve synaptic plasticity in the hippocampus.140
 |
Figure 3 DNA delivery by NPs of gene therapy for neurological disorders. (A) Two modes of drug loading using brain-targeted DGLs vector. The therapeutic peptide was covalently linked to DGLs, while therapeutic plasmid was encapsulated by DGLs through electrostatic interactions. Reproduced with permission from Liu Y, An S, Li J et al. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials. Feb 2016;80:33–45.137 Copyright © 2015 Elsevier Ltd. All rights reserved. (B) Preparation of CRISPR/Cas9 plasmids-loaded nano-biohybrid complexes. Reproduced with permission from Shen J, Lu Z, Wang J et al. Traceable Nano-Biohybrid Complexes by One-Step Synthesis as CRISPR-Chem Vectors for Neurodegenerative Diseases Synergistic Treatment. Advanced materials (Deerfield Beach, Fla). Jul 2021;33(27):e2101993.138 Copyright 2021, Wiley-VCH. (C) Schematic illustration for the synthesis of polymer and the formation of polymer/DNA NPs. Reproduced with permission from Gao S, Tian H, Xing Z et al. A non-viral suicide gene delivery system traversing the blood brain barrier for non-invasive glioma targeting treatment. Journal of Controlled Release. 2016/12/10/ 2016;243:357–369.144 Copyright 2016, Elsevier. |
NPs were also investigated in cerebral infarction and traumatic brain injuries (TBI) treatment. Shen et al developed OX26 antibody-conjugated polyglutamic acid (PGA)-based NPs to deliver Meg3 short hairpin RNA (shRNA) plasmid, which promoted angiogenesis by upregulating the expression of vascular endothelial growth factor A (Vegfa) and its receptor Vegfr2, thereby enhancing blood vessel formation and improving recovery after stroke.141 A nano-plumber was designed to regulate and restore the glymphatic-lymphatic system after TBI. The NPs co-encapsulated pro-DHA and a VEGF-C plasmid, targeting dysregulated microglia and promoting lymphangiogenesis, thereby sustaining brain homeostasis.142
For brain tumor treatment, the herpes simplex virus thymidine kinase (HSVtk) was used as a therapeutic gene, known as the suicide gene therapy. HSVtk could convert ganciclovir into a toxic form, inducing tumor cell apoptosis. There were some studies focused on pHSVtk delivery into brain tumor cells, such as GBM cell membrane-coated PEI25k/pHSVtk complexes143 and angiopep-2-modified poly(L-lysine)-grafted PEI copolymer (Figure 3C).144 Angiopep-2 was used to bind to the lipoprotein receptor-associated protein 1 (LRP-1) receptor on cerebrovascular endothelial cells, which in turn crossed the BBB and delivered plasmid targeting to GBM tissues.
In summary, plasmid DNA, as a tool for gene therapy, is widely investigated in the treatment of neurological diseases. NPs, through various surface modifications or administration routes, can achieve brain delivery, showing promising potential for application.
RNA Delivery
siRNA
siRNA, or small interfering RNA, is a double-stranded RNA molecule, usually about 20–25 nucleotides long, that plays a key role in the RNA interference pathway. siRNA base pairs with the complementary sequence of the RNA-induced silencing complex (RISC) to direct the RISC to a specific target mRNA. Once bound, the RISC cleaves the mRNA, causing its degradation and preventing it from being used as a template for protein synthesis, thus preventing the expression of the protein encoded by the gene.208 Because of their specific targeting and gene silencing properties, siRNAs have potential therapeutic applications in a variety of diseases, especially those caused by gene overexpression or mutations.209 The application of NPs for their in vivo delivery for the treatment of neurological disorders has also been widely investigated.
In treating neurodegenerative diseases such as AD, some studies developed NPs to deliver BACE1 siRNA to reduce Aβ production and slowed disease progression, such as angiopep-2 peptides modified exosome-liposome hybrid nanovesicles loaded with BACE1 siRNA and TREM2 plasmid,145 phosphatidic acid-functionalized high-density lipoprotein (pHDL) nanoscavengers loaded with curcumin and BACE1 siRNA (Figure 4A),146 and dendrigraft poly-l-lysine (DGL)-based NPs co-loaded with BACE1 siRNA and rapamycin.147 In addition, Zhang et al designed magnesium (Mg²⁺) and cyclophilin D (CypD) siRNA-loaded lipid nanocarriers to halt mitochondrial dysfunction in AD. Mg²⁺ helped to counteract calcium overload in mitochondria. As CypD regulated the mitochondrial permeability transition pore (mPTP), the siRNA downregulated CypD, preventing the opening of mPTP and thereby protecting mitochondria from damage.148
 |
Figure 4 siRNA delivery by NPs of gene therapy for neurological disorders. (A) Schematic illustration of pHDL/Cur-siBACE1 preparation. Reproduced with permission from Zhang H, Jiang W, Zhao Y et al. Lipoprotein-Inspired Nanoscavenger for the Three-Pronged Modulation of Microglia-Derived Neuroinflammation in Alzheimer’s Disease Therapy. Nano letters. Mar 23 2022;22(6):2450–2460.146 Copyright 2022, American Chemical Society. (B) Schematic for the preparation of siRNA-loaded PLGA NPs by a modified nanoprecipitation method. DSPE-PEG was used to impart stealth character. In addition, polysorbate 80 (PS 80), poloxamer 188 (F-68), DSPE-PEG-GSH, or DSPE-PEG-transferrin (Tf) was used to augment BBB penetration. Reproduced with permission from Li W, Qiu J, Li XL et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Science advances. Jan 2021;7(1).152 Copyright 2021, The Authors, CC BY-NC 4.0. (C) Illustration of formulating bioreducible BAMPA-O16B/siRNA lipoplex. Reproduced with permission from Liu S, Liu J, Li H et al. An optimized ionizable cationic lipid for brain tumor-targeted siRNA delivery and glioblastoma immunotherapy. Biomaterials. Aug 2022;287:121645. doi:10.1016/j.biomaterials.2022.121645.153 Copyright 2022, Elsevier. (D) Schematic of nanoconstruct synthesis. Reproduced with permission from Ngamcherdtrakul W, Bejan DS, Cruz-Muñoz W et al. Targeted Nanoparticle for Co-delivery of HER2 siRNA and a Taxane to Mirror the Standard Treatment of HER2+ Breast Cancer: Efficacy in Breast Tumor and Brain Metastasis. Small (Weinheim an der Bergstrasse, Germany). Mar 2022;18(11):e2107550.165 Copyright 2022, John Wiley and Sons. |
For ischemic stroke treatment, Lin et al designed superparamagnetic iron oxide NPs (SPIO)-loaded micelles based on the polymer PAsp(DMA)-Lys-(CA)2 (PALC). The NPs were loaded with siRNA and ASOs targeting the Pnky lncRNA to promote the differentiation of NSCs into neurons, allowing for the replacement of lost or damaged neurons in the brain.149 PLGA-PAMAM NPs loaded with CircOGDH siRNA were developed to target the ischemic penumbra in stroke. The silence the CircOGDH gene reduced neuronal apoptosis and improved neurological outcomes in a mouse model of focal brain ischemia.150 Solid lipid NPs loaded with TGF-β1 siRNA and curcumin were developed for treating cerebral injury after intracerebral hemorrhage to reduce inflammation and provide additional neuroprotective effects.151 Additionally, PLGA-based NPs to deliver siRNA targeting the tau gene were engineered to treat TBI. The NPs reduced tau protein levels and mitigated the neurodegenerative processes associated with TBI (Figure 4B).152
For tumor treatment, there are studies focus on silencing the expression of immune checkpoints, programmed cell death-ligand 1 (PD-L1). The delivery of siRNA to tumor tissue can induce a reduction in PD-L1 expression by tumor cells, which in turn triggers a T cell-dependent anti-tumor immune response. This strategy has been developed with some NPs, such as ionizable cationic lipid NPs co-delivering CD47 siRNA (Figure 4C)153 and 2-deoxy-D-glucose modified lipid polymer NPs co-delivering temozolomide (TMZ).154 STAT3 (signal transducer and activator of transcription 3) gene is related to tumor cell proliferation and angiogenesis. A cation-free siRNA micelle platform was developed to deliver STAT3 siRNA to reduce drug resistance and increase sensitivity to TMZ.155 What’s more, the Polo-like kinase 1 (PLK1) gene is associated with cell proliferation and survival in GBM cells. NPs have been investigated to silence this gene via siRNA to cause tumor cell apoptosis, such as a virus-mimicking NP with envelope protein of Japanese encephalitis virus embedded into the lipid membranes156 and hyaluronan-enveloped nanomicelles for enhanced nose-to-brain delivery.157 In addition, Epidermal growth factor receptor (EGFR), associated with tumor growth, is targeted for treatment using EGFR siRNA delivered via endoplasmic reticulum (ER) membrane-decorated hybrid nanoplexes.158
Various target genes have also been investigated for CNS tumor treatment. Tang et al developed selenium-engineered mesoporous silica nanocapsules for cofilin-1 siRNA delivery, enhancing radiation-induced apoptosis in radiotherapy-resistant GBM.159 Wang et al designed iron oxide NPs coated with a chitosan-PEG-PEI co-polymer and conjugated with chlorotoxin to deliver O6-methylguanine-DNA methyltransferase (MGMT)-targeting siRNA, increasing TMZ sensitivity by impairing DNA repair.160 A cholesterol-modified T7 peptide-based nanomicelle system delivered Slit2 siRNA intranasally, inhibiting glioma growth and remodeling the tumor microenvironment.161 Cationic lipid-polymer hybrid NPs, combined with microbubble-enhanced focused ultrasound, delivered siRNA targeting the smoothened (SMO) gene, inducing tumor cell apoptosis.162 Core-shell lipoplexes modified with 89WP peptide effectively delivered c-Myc siRNA for GBM treatment.163 Hyperosmotic nanochains loaded with SHMT1 siRNA displayed BBB crossing ability via an osmotic pressure-driven mechanism, activating nuclear factor of activated T cells-5 (NFAT5) and inhibiting DNA synthesis in tumor cells.164 Furthermore, in brain metastasis from breast cancer, mesoporous silica NPs co-delivering human epidermal growth factor receptor-2 (HER2) siRNA and docetaxel showed therapeutic efficacy in HER2-positive breast cancer treatment (Figure 4D).165
In brief, NPs enable the efficient delivery of siRNA across the BBB, facilitating targeted gene silencing within the brain. siRNA-loaded NP therapies show significant potential in reducing pathogenic gene expression, slowing disease progression, and improving outcomes in conditions such as neurodegenerative diseases, ischemic stroke, TBI, and tumors.
mRNA
mRNA, or messenger RNA, is a type of RNA that is synthesized during the process of transcription, in which a piece of DNA is used as a template to create a complementary RNA strand. mRNAs play a crucial role in translating genetic information from DNA to proteins and have recently received much attention in the medical field,84 particularly in the development of mRNA vaccines, such as those against COVID-19. The use of mRNAs as genetic material for the treatment of neurological disorders has also been studied. However, systemic delivery of naked mRNA is difficult to realize, as it cannot penetrate cell membranes given its large size and negative charge, and is easily degraded in blood circulation.
The application of NPs for mRNA delivery shows promises in treating neurodegenerative and functional diseases. Polyplex nanomicelles loaded with neprilysin mRNA were shown to degrade Aβ peptides, potentially slowing AD progression and mitigating its symptoms.166 This research group also utilized this nanocarrier to deliver brain-derived neurotrophic factor (BDNF) mRNA to treat olfactory dysfunction. BDNF protein aided in the recovery of the olfactory epithelium and supported the repair and regeneration of damaged neurons, leading to enhanced neurological recovery from sensory nerve disorders.167
For stroke treatment, lipid NPs targeting M2 microglia and loaded with interleukin 10 (IL-10) mRNA promoted anti-inflammatory responses and restored BBB integrity. The mannose moiety was incorporated to target the CD206 receptor on M2 microglia (Figure 5A).168 Similarly, DA-PEI2k (deoxycholic acid-conjugated polyethylenimine) polymeric NPs loaded with HO1 mRNA demonstrated anti-inflammatory and antioxidant effects by degrading heme into biliverdin, iron, and carbon monoxide.169 Vascular cell adhesion molecule-1 (VCAM-1)-targeted lipid NPs were also designed to deliver thrombomodulin mRNA to inflamed cerebral vasculature, therefore reducing brain edema and stabilizing the BBB.170
 |
Figure 5 mRNA delivery by NPs of gene therapy for neurological disorders. (A) Schematic of targeted mIL-10-encapsulated LNPs. Reproduced with permission from Gao M, Li Y, Ho W et al. Targeted mRNA Nanoparticles Ameliorate Blood–Brain Barrier Disruption Postischemic Stroke by Modulating Microglia Polarization. ACS nano. 2024/01/30 2024;18(4):3260–3275.168 Copyright 2024, American Chemical Society. (B) Synthesis of the ABNPs@mRNA biomimetic NPs. Polyethylenimine (PEI) complex mRNA forms the inner core, which was then coated with citraconic anhydride-grafted poly-L-lysine, functionalized with ApoE peptide-decorated red blood cell membrane to obtain the ABNPs@mRNA biomimetic NPs. Reproduced with permission from Liu Y, Zhang D, An Y et al. Non-invasive PTEN mRNA brain delivery effectively mitigates growth of orthotopic glioblastoma. Nano Today. 2023/04/01/ 2023;49:101,790.172 Copyright 2023 Elsevier. (C) Illustration for the preparation process of IL-12 mRNA@cRGD-CM-CaCO3 NPs. Reproduced with permission from Zhao P, Tian Y, Lu Y et al. Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis. Journal of nanobiotechnology. Dec 10 2022;20(1):525.173 Copyright 2022, The Authors. Creative Commons Attribution 4.0 International License. |
For tumor treatment, mRNA encoding tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) using TransIT-mRNA delivered via intracranial injection induced apoptosis in tumor cells by activating death receptors and the caspase cascade, thereby reducing tumor size.171 ApoE functionalized red blood cell membrane-camouflaged biomimetic NPs were designed to deliver PTEN mRNA to GBM cells, restoring its tumor-suppressing functions by inhibiting the PI3K-AKT pathway and increasing apoptosis (Figure 5B).172 Additionally, cRGD-modified cancer cell membrane-coated calcium carbonate NPs were used to deliver interleukin-12 (IL-12) mRNA, releasing CO2 in acidic environments to induce necroptosis and trigger an anti-tumor immune response (Figure 5C).173
Based upon the studies, although mRNA plays a crucial role in protein synthesis, its direct application is limited by its large size, negative charge, and biodegradability. NPs have been used to deliver mRNA for the treatment of related neurological diseases, such as degrading Aβ peptides in AD, promoting neuroprotective and anti-inflammatory responses in stroke, and inducing tumor cell apoptosis in GBM. By effectively expressing genes in target cells, NPs have become a promising mRNA delivery solution.
miRNA
miRNAs, or microRNAs, are small non-coding RNA molecules that direct the RISC to bind to a target mRNA by binding to a complementary sequence on the target mRNA molecule. This binding can lead to degradation of the target mRNA or inhibit its translation into proteins.210 miRNAs are involved in various cellular processes, including apoptosis (programmed cell death), proliferation, and metabolism, ensuring that genes are expressed at the right level and the right time. In addition, miRNAs are key regulators of developmental timing and cell differentiation. They can turn off silenced genes that are required for cells to move from one developmental stage to another or to maintain the characteristics of a particular cell type. Thus, by regulating miRNAs, it is possible to modulate gene expression at the post-transcriptional level and thus treat related diseases.211
Applying NP delivery of miRNAs to treat neurological disorders is an effective approach in preclinical studies. miR-124 plays a variety of roles including regulation of neurogenesis, maintenance of neuronal identity, and neuroprotection, and its therapeutic effects have been explored in a variety of diseases. For neurodegenerative diseases like PD, RVG29-linked PLGA NPs were developed to deliver miR-124, downregulating the MEKK3/NF-κB pathway and reducing pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α), IL-6, and iNOS, enhancing neuroprotection in a PD model.174 For AD treatment, DNA nanoflowers co-delivering miR-124 and rutin resulted in downregulation of BACE1 and amyloid precursor protein (App), reduction of Aβ generation, combined with the anti-oxidative and anti-inflammatory effects.175 Enveloped RNA-cell permeating peptide nanocomplexes delivering miR-132 mimics ameliorated neurodegenerative processes by regulating multiple mRNA targets and signaling pathways related to inflammation, neuronal health, and apoptosis, as well as reducing Aβ accumulation.176
For treating ischemic brain injury, miR-124 was targeted using calcium-based metal-organic framework NPs to promote the differentiation of NSCs into mature neurons (Figure 6A).177 Polymeric nanocapsules delivering miR-21 enhanced AKT, hypoxia inducible factor-1α (HIF-1α), and VEGF expression, reducing infarct size and improving outcomes in a rat ischemia model.178 For multiple sclerosis (MS) therapy, a polymeric NP was synthesized using conjugated chitosan, tragacanthic acid, and glutathione (GSH) to ameliorate the pathological progress of chronic demyelination by delivering miR-219. The NPs led to miR-219 overexpression, downregulation of ApoE, upregulation of crystallin αB, reduction of inflammation in the brain, and enhanced neuronal regeneration.179
 |
Figure 6 miRNA delivery by NPs of gene therapy for neurological disorders. (A) Diagram of the effective loading of miR-124 onto the surface of Ca-MOF by hydrogen bonds between the −NH2 group and −OH group and the rapid cleavage of the hydrogen bonds upon exposure to an acidic pH. Reproduced with permission from Yang H, Han M, Li J et al. Delivery of miRNAs through Metal-Organic Framework Nanoparticles for Assisting Neural Stem Cell Therapy for Ischemic Stroke. ACS nano. Sep 27 2022;16(9):14,503–14,516.177 Copyright 2022, American Chemical Society. (B) Scheme of polymeric miRNA nanomedicine construction. Reproduced with permission from Liu Y, Zheng M, Jiao M et al. Polymeric nanoparticle mediated inhibition of miR-21 with enhanced miR-124 expression for combinatorial glioblastoma therapy. Biomaterials. Sep 2021;276:121036. doi:10.1016/j.biomaterials.2021.121036.180 Copyright 2021 Elsevier. (C) Schematic of the synthesis of B1L@SpAcDex-ATMO-21 NPs. Reproduced with permission from Zheng T, Wang W, Mohammadniaei M et al. Anti-MicroRNA-21 Oligonucleotide Loaded Spermine-Modified Acetalated Dextran Nanoparticles for B1 Receptor-Targeted Gene Therapy and Antiangiogenesis Therapy. Advanced science (Weinheim, Baden-Wurttemberg, Germany). Feb 2022;9(5):e2103812.181 © 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH. Creative Commons CC BY license. |
For managing brain tumors, miR-21, an oncogene overexpressed in GBM related to tumor growth and angiogenesis, has been targeted using NPs to inhibit its expression. Studies have explored delivering anti-miR-21 via angiopep-2 peptide-modified polymeric NPs co-delivering miR-124 (Figure 6B),180 spermine-modified acetalated dextran NPs (Figure 6C),181 as well as angiopep-2 and TAT peptides-modified lipid polymer micelles,182 all showing therapeutic efficacy in GBM. Other miRNAs have also been explored as targets for CNS tumor gene therapy. Gold-liposome NPs loaded with miR-92b inhibitors reduced tumor cell proliferation and promoted apoptosis.183 Lipid self-assembling NPs delivering miR-603 inhibited MGMT expression, reducing the tumor’s resistance to temozolomide (TMZ) and enhancing chemotherapeutic efficacy.184
In brief, miRNAs modulate gene expression at the post-transcriptional level, making them valuable targets for disease intervention. NPs for miRNA delivery have shown promise in preclinical studies, particularly for neurodegenerative diseases, ischemic brain injury, MS, and tumors. These strategies demonstrate the potential of miRNA-loaded NPs to enhance therapeutic outcomes by targeting specific genes involved in disease progression and tumor growth.
Antisense Oligonucleotides Delivery
ASOs are synthetic short strands of DNA or RNA designed to bind specifically to mRNA produced by a particular gene, which then recruit the enzyme RNase H to degrade the RNA strand in the RNA-DNA double strand. This leads to a reduction in the production of genetically encoded proteins.212 Therefore, ASO can serve as a gene therapy strategy; however, due to its negative charge and susceptibility to degradation by nucleases in vivo, the development of efficient delivery systems is necessary to enhance its therapeutic efficacy.
There are current studies utilizing NPs to improve ASO delivery. For AD treatment, Li et al reported tetrahedral DNA framework-based NPs with triphenylphosphine for mitochondrial targeting, cholesterol for CNS penetration, and ASOs to silence miRNA-34a, restoring mitochondrial function and reducing apoptosis (Figure 7A).185 Ma et al designed neurotransmitter-derived lipid NPs loaded with ASOs targeting tau mRNA to reduce the accumulation of tau protein and mitigate neurodegeneration (Figure 7B).186 Israel et al developed D-peptide functionalized polymalic acid-based NPs delivering ASOs targeting BACE1 gene, reducing Aβ production, by decreasing BACE1 expression.187 For PD treatment, Sun et al reported ZAAM, a NSC membrane-coated and Apt 19S-conjugated penetrable NP, loaded with ASOs that transiently suppressed the RNA-binding protein PTBP1, thereby reducing neuronal apoptosis and promoting the conversion of astrocytes to neurons (Figure 7C).188 A kind of cyclodextrin-based NP was developed to deliver ASOs targeting the mutant huntingtin (mHTT) gene associated with HD. The RVG-targeted NPs reduced mHTT expression in neuronal cells and HD patient-derived fibroblasts, offering a strategy for treating HD.189 Another research team also reported apolipoprotein A-I nanodisks to deliver ASOs targeting mHTT.190
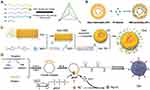 |
Figure 7 ASO delivery by NPs of gene therapy for neurological disorders. (A) Schematic Legend of the composition process of tetrahedral DNA framework-based NPs. Reproduced with permission from Li W, Peng X, Mei X, Dong M, Li Y, Dong H. Multifunctional DNA Tetrahedron for Alzheimer’s Disease Mitochondria-Targeted Therapy by MicroRNA Regulation. ACS applied materials and interfaces. May 17 2023;15(19):22,977–22,984.185 Copyright 2023, American Chemical Society (B) Schematic illustration of formulating NT-lipidoid–doped LNPs. Reproduced with permission from Ma F, Yang L, Sun Z et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Science advances. Jul 2020;6(30):eabb4429.186 Copyright 2020 The Authors. Creative Commons Attribution-NonCommercial license. (C) Design of the ASO-loaded, aptamer Apt 19S-conjugated, NSC membrane-coated NPs. Reproduced with permission from Sun Y, Kong J, Ge X, Mao M, Yu H, Wang Y. An Antisense Oligonucleotide-Loaded Blood-Brain Barrier Penetrable Nanoparticle Mediating Recruitment of Endogenous Neural Stem Cells for the Treatment of Parkinson’s Disease. ACS nano. Mar 14 2023;17(5):4414–4432.188 Copyright 2023, American Chemical Society (D) Synthesis scheme of spherical nucleic acid nanostructure based self-assembly. Reproduced with permission from Yu W, Xuan C, Liu B et al. Carrier-free programmed spherical nucleic acid for effective ischemic stroke therapy via self-delivery antisense oligonucleotide. Nano Research. 2023/01/01 2023;16(1):735–745.191 Copyright 2022, Tsinghua University Press. |
ASOs have been explored for treating ischemic stroke and SMA. A spherical nucleic acid nanostructure co-encoded with caspase-3 ASOs and TfR aptamers was developed to reduce neuronal apoptosis, providing neuroprotection and mitigating the damage caused by ischemic stroke (Figure 7D).191 SMA is a genetic disorder characterized by the progressive loss of motor neurons in the spinal cord, leading to muscle weakness and atrophy. This condition is primarily caused by mutations in the survival motor neuron (SMN) gene. CPP-conjugated ASOs targeting SMN2 corrected splicing, increasing full-length SMN protein production for treating this disease.192
ASOs have also been explored for tumor treatment, particularly to silence miR-21 in GBM. Cholesterol-conjugated ASO co-micelles193 and spermine-modified acetalated dextran NPs181 were used to load miR-21 ASOs, promoting apoptosis by upregulating expression of pro-apoptotic genes such as PTEN and PDCD4, thus reducing tumor growth. Protein corona-assisted DNA cubes delivered PLK1-targeting ASOs to inhibit GBM growth. The DNA cubes were synthesized through self-assembly of oligonucleotides, and protein corona formed on their surface in biological environment and facilitated their transport across the BBB to silence PLK1 expression.194
In neuropsychiatric disorders such as depression and schizophrenia, border-associated macrophages (BAMs) contribute to the pathophysiology by promoting neuroinflammation. Calero et al designed lipid NPs functionalized with mannosylated lipids to specifically target the mannose receptors on BAMs. By loading with ASOs target the L-PGDS, an enzyme involved in the production of prostaglandin D2, the NPs silenced the L-PGDS gene, reducing the production of pro-inflammatory mediators and thus modulating neuroinflammation.195
In summary, NP-based delivery systems have shown promising advancements in enhancing the targeting and efficacy of ASOs, particularly in the treatment of neurodegenerative diseases, ischemic stroke, SMA, as well as GBM and neuropsychiatric disorders. These studies suggest that ASO-loaded NPs have the potential to cross the BBB and modulate gene expression to achieve therapeutic effects.
CRISPR/Cas9 Gene-Editing Tools Delivery
CRISPR/Cas9 is a revolutionary technology that combines Cas9 nuclease with single-guide RNA (sgRNA) and cuts the target DNA for gene editing. It presents significant advantages over conventional therapies, including ease of use, straightforward design, and the ability to perform multiplexed gene editing, enabling simultaneous modification of multiple genes. Consequently, it has emerged as a highly suitable tool for a wide range of genome editing applications, particularly in gene therapy research.213 However, two components, namely Cas9 endonuclease and sgRNA, need to be introduced into the target cell.214 Co-delivering two RNAs with significantly different sizes such as Cas9 mRNA (4.5 kb) and sgRNA (0.1 kb) into the brain remains challenging. Therefore, noninvasive delivery of NP-encapsulated CRISPR/Cas9 complexes is urgently needed to promote gene therapy for neurological disorders.
For neurodegenerative diseases like AD and PD treatment, Wang et al designed GSH-responsive silica nanocapsules to deliver Cas9 mRNA and sgRNA targeting App and tyrosine hydroxylase (Th) genes, achieving 6.1% and 3.9% editing rates in wild-type mice, respectively.196 Yang et al designed nanozyme-boosted metal-organic framework NPs synthesized by encapsulating cerium dioxide nanozymes within MIL-100 (Fe) and modified with PEI and KLVFFAED peptide. The NPs were loaded with a CRISPR activation system plasmid designed to activate the Nrf2 gene, therefore promoting the expression of antioxidant proteins and restoring redox homeostasis (Figure 8A).197 Shen et al used superparamagnetic iron oxide NPs functionalized with fluvastatin and RVG peptides to deliver CRISPR-Cas9 plasmids, knocking out BACE1 gene in neurons, reducing Aβ production.138 Park et al also targeted BACE1 with CRISPR-Cas9 ribonucleoproteins (RNPs) using amphiphilic peptide (R7L10), lowering Aβ plaque formation and alleviating cognitive deficits in AD mouse models.198 They also developed nanocomplexes by mixing the R7L10 amphiphilic peptides with Cas9 activator targeting Adam10 gene, which was involved in non-amyloidogenic processing of APP, thus reducing Aβ plaques. The Cas9 activator nanocomplexes upregulated Adam10, enhancing α-secretase activity, which led to reduced Aβ plaque formation and improved cognitive function in AD models.199
 |
Figure 8 CRISPR/Cas9 delivery by NPs of gene therapy for neurological disorders. (A) Process of preparation of the nanozyme-boosted MOF-CRISPR platform. Reproduced with permission from Yang J, Qin G, Liu Z et al. A Nanozyme-Boosted MOF-CRISPR Platform for Treatment of Alzheimer’s Disease. Nano letters. Aug 14 2024;24(32):9906–9915.197 Copyright 2024, American Chemical Society. (B) Schematic of CRISPR–Gold synthesis. DNA oligonucleotide-conjugated GNPs bind to Cas9 or Cpf1 RNPs, and subsequent PAsp(DET) polymer encapsulation generates CRISPR–Gold. Reproduced with permission from Lee B, Lee K, Panda S et al. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nature biomedical engineering. Jul 2018;2(7):497–507.200 Copyright 2018, The Authors. (C) In situ free-radical polymerization was used to synthesize disulfide–cross-linked nanocapsules containing Cas9/sgRNA and functionalized with angiopep-2 targeting ligand. Reproduced with permission from Zou Y, Sun X, Yang Q et al. Blood-brain barrier-penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Science advances. Apr 22 2022;8(16):eabm8011.202 Copyright 2022 The Authors, CC BY-NC 4.0. |
Fragile X syndrome (FXS) is a genetic disorder and caused by a mutation in the FMR1 gene on the X chromosome, which leads to a deficiency or absence of the FMRP protein, important for normal neural development and synaptic function. Lee et al used CRISPR-Gold nanovehicles to deliver Cas9 and Cpf1 RNPs for mGluR5 gene editing in neurons, astrocytes, and microglia, rescuing FXS-related behaviors in mouse models (Figure 8B).200 Mucopolysaccharidosis type I (MPS-I) is a lysosomal storage disorder caused by mutations in the α-L-iduronidase (IDUA) gene, leading to a deficiency of the enzyme IDUA, which results in the accumulation of glycosaminoglycans (GAGs), causing severe multi-system damage, particularly affecting the nervous system. A study reported ionizable lipid NPs loaded with mRNA encoding adenine base editors and sgRNA targeting IDUA mutations, corrected the W392X mutation in the IDUA gene, reducing GAG accumulation and alleviating disease symptoms.201
For tumor treatment, CRISPR-Cas9 can also be employed by targeting oncogenic viruses and promoting the expression of tumor suppressor genes. One research group designed angiopep-2-modified disulfide–cross-linked polymeric shell (Figure 8C)202 and angiopep-2-decorated guanidinium and fluorine functionalized polymeric NP203 to deliver Cas9/gRNA RNPs, knocking out PLK1 gene in GBM cells. They also developed Cas12a RNP nanocapsules for dual gene editing of EGFR and PLK1, inhibiting tumor growth and improving survival in mouse models.204 Rui et al highlighted carboxylated branched PBAE NPs for CRISPR-Cas9 editing to knockout the CXCR4 gene, disrupting tumor growth, metastasis, and resistance to therapy.205
Overall, CRISPR/Cas9 is a powerful tool capable of multiplex gene editing, and the applications include editing genes implicated in neurodegenerative diseases, as well as genetic disorders like fragile X syndrome and MPS-IH. In tumor therapy, CRISPR/Cas9 has shown potential in knocking out oncogenes and enhancing tumor suppressor genes, offering a promising strategy for cancer gene therapy.
In short, gene therapy involves the use of various gene-editing and regulatory tools, including DNA, siRNA, mRNA, miRNA, ASOs, and CRISPR/Cas9, all of which hold significant promise for treating CNS diseases. However, despite the potential of these approaches, there are significant barriers to their clinical application. NPs provide a valuable solution to these challenges, as they can protect genetic material from degradation and facilitate its delivery across the BBB. The main challenges include the efficient delivery of large genetic materials across the BBB, the potential for off-target effects, and the need for long-term safety data. Future directions will likely focus on optimizing the balance between therapeutic efficacy and safety, particularly for long-term or chronic treatments. Overcoming these barriers is essential to fully realize the potential of NP-based gene therapy in treating complex neurological disorders.
Nanoparticle-Based Enzyme Replacement Therapy for Neurological Disorders
Enzyme deficiency disorders are a diverse group of diseases caused by deficiency or malfunction of specific enzymes, which may lead to the accumulation of toxic substances or disruption of key metabolic pathways, thus ultimately resulting in systemic damage and dysfunction.215 Enzyme deficiency disorders can be categorized based on the type of enzyme affected and the metabolic pathway involved.216 Among them, LSDs are the most widely studied enzyme deficiency disorders and can be broadly categorized as lipid storage disorders, mucopolysaccharidoses, glycoprotein storage disorders, and mucolipidoses, based on the nature of the main storage substance involved.217 Certain LSDs affect the CNS, disrupting normal metabolic pathways and leading to the accumulation of toxic substances in the brain, which can damage nerve cells and impair brain function. Table 2 summarizes the main types of LSDs that invade the CNS according to the accumulated general substrate.
 |
Table 2 Classification of LSDs Affect the CNS |
ERT has emerged as a promising therapy for these LSDs, which aims to correct these deficiencies through the administration of recombinant enzymes.218 To date, a dozen enzymes have been approved by the FDA/EMA to treat LSDs and metabolic diseases. However, as the BBB prevents macromolecules including enzymes from entering the brain, how to deliver therapeutic enzymes across the BBB remains a major challenge.219 NP-based delivery systems provide a novel and effective method to facilitate enzyme delivery to the brain and overcome the limitations of conventional ERT. The design of NPs for enzyme delivery is critical. The NPs typically need to be small enough to pass through the BBB, yet large enough to encapsulate the desired enzyme.220 Surface modifications such as the decoration of targeted ligands or the use of biocompatible coatings can further enhance their ability to reach target cells in the brain.8 Here we summarize examples of NP-mediated delivery of enzymes for ERT applications by focusing on lipid storage disorders (gangliosidoses, sphingolipidoses, leukodystrophies, neuronal ceroid lipofuscinoses (NCLs)), MPSs, and glycoprotein storage disorders (Table 3), highlighting the potential of NP-based systems to overcome challenges associated with ERT.
 |
Table 3 Applications of NP-Based ERT for LSDs Affected Nervous System |
Lipid Storage Disorders
Gangliosidoses
Gangliosidoses are a group of inherited metabolic disorders characterized by the accumulation of gangliosides in cells of the nervous system.231 The two most common types of gangliosidoses are GM1 gangliosidosis and GM2 gangliosidosis.232 GM1 gangliosidosis is an autosomal recessive disorder due to β-galactosidase (β-gal) deficiency, which causes GM1 gangliosides to accumulate in lysosomes, particularly in the brain. One of the pathologic features of GM1 gangliosidosis is impaired autophagy, ie, decreased fusion of autophagosomes and lysosomes to degrade cellular waste products.233 In addition, GM2 gangliosidosis is characterized by the accumulation of GM2 gangliosides in neurons owing to the lack of specific enzymes needed to break down these complex molecules, which will lead to progressive cell damage and death. GM2 gangliosidosis includes several genetically distinct disorders, the best known of which are Tay-Sachs disease and Sandhoff disease.234 Tay-Sachs disease is caused by a deficiency in the enzyme hexosaminidase A; it is most common in certain populations such as Ashkenazi Jews, and typically presents in infancy with symptoms such as muscle weakness, deterioration of motor skills, and severe nerve damage.235 Sandhoff disease is similar to Tay-Sachs disease, but results from the deficiency of both β-hexosaminidase A and B enzymes. It affects a broader range of body tissues and exhibits symptoms similar to those of Tay-Sachs disease, including developmental delays, motor weakness, and neurologic deterioration.236
ERT can be effective in treating systemic deficiencies, but it is limited by immunogenicity and the shortened half-life of intravenous enzymes. NPs have been explored as efficient carriers for systemic enzyme delivery. Paruchuri et al reported a hyaluronic acid-b-polylactic acid (HA-PLA) polymer delivery system that enabled enzyme-responsive and sustained delivery of β-gal to promote the self-repair process of cellular autophagy.221 In the presence of model cognate enzymes, HA-PLA polymer vesicles had a higher release rate owing to enzymatic degradation (Figure 9A). Autophagy was reduced in the GM1 gangliosidosis (GM1SV3) cell model, but enhanced in GM1SV3 cells treated with polymeric NPs loaded with β-gal compared to healthy cells. After 24 h of treatment with NPs, the fusion of lysosomes and autophagosomes in GM1SV3 cells was restored to the normal level of healthy cells (Figure 9B). Another research group utilized polyethylene glycol-b-poly(lactic acid) (PEGPLA) polymersomes as vehicles for β-gal delivery to restore enzymatic activity and reduce the accumulation of GM1 gangliosides. Limited release of PEGPLA polymersomes was observed at physiological pH (7.4), while the release was significantly increased in lysosomes at acidic pH (4.8) (Figure 9C).222 The research indicated NPs were promising delivery system for enzymatic treatments to the brain in gangliosidoses. Therefore, NPs show promise as delivery systems for ERT in treating gangliosidoses, restoring autophagic function and reducing ganglioside accumulation in the brain.
 |
Figure 9 NP-based ERT for gangliosidoses. (A) Release of encapsulated fluorescein isothiocyanate-dextran from hyaluronic acid-polylactic acid (HA-PLA) polymersomes when incubated with hyaluronidase (●), citrate phosphate buffer (pH 4.8) (♦), and β-galactosidase (β-gal) (▲) (n = 4, * = p < 0.05, ** = p < 0.01). (B) Co-localization (%) in untreated healthy (NSV3) and diseased (GM1SV3) cells, GM1SV3 cells treated with free β-gal (6 h treatment), β-gal-loaded HA-PLA polymersomes for 6, 12, and 24 h. Star (*) indicate a significant difference compared to NSV3 cells and diamond (◆) indicates a significant difference in colocalization compared to GM1SV3 cells (n = 3, * = p < 0.05, ** = p < 0.025). Reproduced from Paruchuri BC, Smith S, Larsen J. Enzyme-responsive polymersomes ameliorate autophagic failure in a cellular model of GM1 gangliosidosis. Original Research. Frontiers in Chemical Engineering. 2022;4. Creative Commons Attribution License (CC BY).221 Copyright 2022, Paruchuri et al, published by Frontiers Media. (C) Release curves demonstrating in vitro release of AF488 β-gal from poly(ethylene glycol)-b-poly(lactic acid) polymersomes under pH 4.8 and pH 7.4 conditions (n = 3). (* = p < 0.05, ** = p < 0.025). Reproduced with permission from Kelly JM, Gross AL, Martin DR, Byrne ME. Polyethylene glycol-b-poly(lactic acid) polymersomes as vehicles for enzyme replacement therapy. Nanomedicine (London, England). Dec 2017;12(23):2591–2606.222 Copyright 2017 Taylor & Francis. |
Sphingolipidoses
Sphingolipidoses refer to a group of inherited metabolic disorders caused by genetic mutations that result in the deficiency or malfunction of specific enzymes required for sphingolipid metabolism, characterized by the accumulation of sphingolipids in a variety of tissues, including the CNS.237 Sphingolipids play a key role in cell membrane structure and cell signaling. The accumulation of sphingolipids leads to cellular dysfunction and ultimately to a variety of clinical conditions.237 Sphingolipid disorders include several subtypes, such as Gaucher disease and Niemann-Pick disease. Gaucher disease is caused by a deficiency of the enzyme glucocerebrosidase (GCase), leading to the accumulation of intracellular glucocerebroside.238 Niemann-Pick disease is a group of inherited metabolic disorders in which mutations in specific genes lead to the deficiency of acid sphingomyelinase (ASM). This will cause the accumulation of sphingolipids and cholesterol in various cellular lysosomes in the body, resulting in a range of symptoms that severely affect the health and function of patients.239
Combining nanotechnology delivery systems with ERT therapy is a promising treatment modality. LNPs delivering recombinant GCase (rGCase) were developed for Gaucher disease, bypassing the BBB via intranasal administration and restoring enzymatic activity in microglia, reducing substrate accumulation.223 Furthermore, Solomon et al investigated how transcytosis pathways at the BBB were altered in Niemann-Pick disease, and how these alterations affected the effectiveness of nanocarrier-based therapeutics in crossing the BBB. They synthesized polystyrene NPs targeting TfR, ganglioside GM1, or intercellular adhesion molecule-1 (ICAM-1), revealing disease-specific pathway modifications that impact brain delivery (Figure 10A).224 They also utilized these polystyrene NPs functionalized with antibodies targeting CAMs like ICAM-1, PECAM-1, and VCAM-1, with triple-targeted NPs showing the best brain targeting.225 Muntimadugu et al described ICAM-1-modified PLGA NPs encapsulating ASM enzymes to restore the deficient enzyme activity, thereby reducing the accumulation of sphingomyelin in Niemann-Pick disease. They found NPs were transported to lysosomes and released active enzymes under lysosomal conditions, and targeted peripheral organs and the brain in vivo. Compared with the free enzyme, the encapsulated PLGA NPs enhanced the delivery of the enzyme to the organs, especially the brain tissue (Figure 10B-D), and were superior to the coated PLGA NPs, providing guidance for future applications.226 Based on the studies, NPs are investigated to treat sphingolipidoses by effectively crossing the BBB and restoring enzymatic function in the neurological disorders.
 |
Figure 10 NP-based ERT for sphingolipidoses. (A) Nanocarriers (NCs) targeted to the transferrin receptor (TfR), ganglioside GM1 or ICAM-1, associated to the clathrin, caveolar or cell adhesion molecule (CAM) routes, respectively. Reproduced with permission from Solomon M, Loeck M, Silva-Abreu M et al. Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases. Journal of controlled release: official journal of the Controlled Release Society. Sep 2022;349:1031–1044.224 Copyright 2022 Elsevier. (B-D) In vivo biodistribution of ASM encapsulated vs coated anti-ICAM PLGA NP formulations. (B) Enzyme presence in blood was analyzed at the indicated times using a gamma counter and expressed as the percentage of the injected dose (% ID). (C) Enzyme biodistribution in the indicated organs was assessed 30 min after injection and expressed as the tissue-to-blood localization ratio. (D) The specificity index was calculated as the localization ratio of respective NP formulation over the localization ratio of the free enzyme. Data are the mean ± SEM and statistics were assessed by Student’s t-test (p < 0.05). * Compares each NP formulation vs free 125I-ASM at respective times and locations; # Compares NP encapsulated vs coated enzyme for respective times and locations. Reproduced with permission from Muntimadugu E, Silva-Abreu M, Vives G et al. Comparison between Nanoparticle Encapsulation and Surface Loading for Lysosomal Enzyme Replacement Therapy. International journal of molecular sciences. Apr 6 2022;23(7).226 Copyright 2022 The authors. Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/. |
Leukodystrophies
Leukodystrophies are a group of rare genetic disorders that primarily affect the white matter of the CNS.240 White matter consists of nerve fibers covered by myelin, which insulates and protects the nerve fibers and facilitates the rapid transmission of nerve signals. In leukodystrophies, there is a defect in the production or maintenance of myelin, leading to progressive deterioration of myelin.241 The symptoms depend on the specific type of leukodystrophy and the extent of myelin damage, such as problems with movement, balance, speech, vision, hearing, and cognitive development. In severe cases, there may be grave physical and intellectual disabilities.240 There are several types of leukodystrophies such as metachromatic leukodystrophy, Krabbe disease, adrenoleukodystrophy, and Alexander disease. Each has its own specific genetic cause and clinical manifestations.242 Krabbe disease is a fatal pediatric neurodegenerative LSD caused by insufficient activity of the enzyme galactosylceramidase (GALC), which degrades galactosylceramide (a major component of myelin sheaths) and other terminal β-galactose–containing sphingolipids. GALC dysfunction results in elevated levels of cytotoxic D-galactosylsphingosine in neural tissues, which leads to extensive degeneration of oligodendrocytes and Schwann cells, followed by destructive demyelination.243
An enzyme delivery system was reported based on the encapsulation of cross-linked enzyme aggregates (CLEAs) into PLGA NPs with brain-targeting peptide functionality, which was capable of encapsulating GALCs and preserving the enzyme activity to treat Krabbe disease.227 These NPs were functionalized using targeted peptides angiopep-2, g7, or Tf-binding peptide to allow the NPs to pass through the BBB (Figure 11A). The result demonstrated restoration of enzyme activity in different organs of the CNS and peripheral nervous system as well as in typical accumulation zones after intraperitoneal injection of GALC CLEA NPs in Twitcher mice, which showed a return of enzyme activity in the brain to the level seen in unaffected mice (Figure 11B-G). This approach offers a promising therapeutic strategy for treating CNS involvement in LSDs like Krabbe disease.
 |
Figure 11 NP-based ERT for leukodystrophies. (A) Graphical summary of the experiment. Peptide-modified PLGA was produced by covalent linking of each peptide to a previously activated form of PLGA. GALC CLEAs were obtained by precipitation of GALC in acetone in the presence of glutaraldehyde, resulting in Schiff’s base formation between enzyme molecules. Last, targeted GALC CLEA NPs were obtained by nanoprecipitation. (B) Legend. Untreated: WT (WT-UT), heterozygous (HET-UT), and TWI (TWI-UT). Targeted GALC CLEA ATTO 633 NPs (TWI Targ-NPs): TWI-GALC Ang2 NPs (lot a and lot b), TWI-GALC g7 NPs (lot a and lot b), and TWI-GALC Tf2 NPs (lot a and lot b). Control treatments (TWI Controls): GALC CLEA ATTO 633 NPs (TWI-GALC NPs lot a and lot b) and free rm-GALC (GALC-lot a and lot b). (C-G) GALC activity of the organs. (C) Brain GALC activity. ***P < 0.001 hET versus WT; ****P < 0.0001 TWI, TWI Targ-NPs, and TWI Controls versus WT; +P < 0.05 TWI versus HET; #P < 0.05 TWI Controls versus TWI; ####P < 0.0001 TWI Targ-NPs versus TWI one-way ANOVA (Tukey’s test). Means± SEM (n= 3 to 12 per group). (D) Liver GALC activity. **P < 0.01 TWI versus WT and #P < 0.05 TWI Controls versus TWI, one-way ANOVA (Tukey’s test). ^P < 0.05 TWI Targ-NPs versus WT and ^^P< 0.01 TWI Targ-NPs versus TWI, Student’s t test. Means ± SEM (n = 3 to 6 per group). (E) Kidney GALC activity. ***P < 0.001 TWI and TWI Controls versus WT and ****P < 0.0001 TWI Targ-NPs versus WT, one-way ANOVA (Tukey’s test). Means ± SEM (n = 3 to 6 per group). (F) Sciatic nerve GALC activity. ****P < 0.0001 all groups versus WT, +P < 0.05 TWI versus HET, ++P < 0.01 WT, TWI Targ-NPs and TWI Controls versus HET, one-way ANOVA (Tukey’s test). Means ± SEM (n = 3 to 8 per group). (G) Spinal cord GALC activity. ****P < 0.0001 hET, TWI Targ-NPs and TWI Controls versus WT, +P < 0.05 TWI TargNPs versus HET, and ++P < 0.01 TWI and TWI Controls versus HET, one-way ANOVA (Tukey’s test). Means ± SEM (n = 3 to 7 per group). Reproduced from Del Grosso A, Galliani M, Angella L et al. Brain-targeted enzyme-loaded nanoparticles: A breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease. Science advances. Nov 2019;5(11):eaax7462.227 Copyright 2019 The Authors. Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC). |
Neuronal Ceroid Lipofuscinoses
NCLs are a group of inherited, progressive neurodegenerative disorders primarily affecting children and characterized by the accumulation of lipofuscin, a pigment composed of fats and proteins, in body tissues, particularly in neurons, leading to neurodegeneration and related symptoms. NCLs are caused by gene mutations and inherited in an autosomal recessive pattern.244 Each type of NCL is associated with a different gene mutation that affects specific enzymes or proteins involved in cellular function. There are several types of NCLs, each of which can be categorized according to age of onset as infantile NCL (INCL or CLN1 disease), late infantile NCL (LINCL or CLN2 disease), juvenile NCL (JNCL or CLN3 disease), and adult NCL (Kufs disease or CLN4 disease).245 In all types of NCL, the CNS is significantly affected, resulting in varying degrees of neurodegeneration and associated symptoms. The progression and severity of symptoms may vary depending on specific disease and individual factors.246
INCL, also known as CLN1 disease or Santavuori-Haltia disease, is caused by a mutation in the CLN1 gene that results in a deficiency of the lysosomal enzyme palmitoyl-protein thioesterase-1 (PPT1), which leads to an abnormal accumulation of lipofuscin in the lysosomes of cells, including the brain.247 There is currently no cure for INCL, and treatment is primarily supportive, focusing on controlling symptoms and improving the quality of life. ERT is one of the most promising treatments. One study introduces peptide-based stealth liposomes that inhibited serum protein adsorption and used Tf-driven internalization on the BBB to deliver PPT1 (Figure 12A).228 These enzyme-loaded NPs were able to restore stable levels of PPT1 activity, comparable to free enzyme, in the fibroblasts from CLN1 patients. Nanocarrier encapsulation did not alter uptake or intracellular translocation of enzymes. In addition, the NPs were able to halve the level of palmitoylated proteins, restoring them to a state similar to that in normal cells (Figure 12B,C). As a result, NP-based enzyme delivery systems demonstrate potential in treating INCL by effectively restoring PPT1 activity and reducing lipofuscin accumulation in affected cells.
 |
Figure 12 NP-based ERT for NCLs. (A) Liposome NPs exploit Tf-driven internalization to inhibit serum protein adsorption, deliver PPT1 enzyme to the TfR-mediated pathway, and restore stable levels of enzyme activity in the fibroblasts of patients with CLN1. (B) Dose-dependent internalization of vesicles (L-TF2-0 and L-TF2-2.5) or free PPT1 in fibroblasts derived from CLN1 patient. Data are reported as mean ± standard error. Two-way ANOVA Dunnett’s test vs L-TF2-0, *p < 0.0125. (C) Quantification of the palmitoylated protein after treatment in patient-derived fibroblasts. Data are reported as the average ± standard error. Unpaired t-test vs untreated (T0), ****p < 0.0001. Reproduced with permission from Santi M, Finamore F, Cecchettini A et al. Protein Delivery by Peptide-Based Stealth Liposomes: A Biomolecular Insight into Enzyme Replacement Therapy. Molecular pharmaceutics. Dec 7 2020;17(12):4510–4521.228 Copyright 2020 American Chemical Society. |
Mucopolysaccharidoses
MPSs are caused by deficiency in the enzymes required to metabolize glycosaminoglycans (GAGs), a group of extracellular heteropolysaccharides that play multiple roles in human physiology. As a result, the absence or dysfunction of the enzyme that breaks down GAGs results in the accumulation of GAGs in cells, blood, and connective tissues, and affected patients typically develop progressive multisystemic symptoms in early childhood.248 There are several types of MPS, each of which is classified according to a specific enzyme deficiency and the resulting symptoms.249 Some types of MPS can affect the CNS.10 MPS I (Hurler, Hurler-Scheie, Scheie syndrome) is caused by a deficiency of the enzyme α-L-iduronidase (IDUA). All forms of MPS I involve the CNS, but the severity varies.250 MPS II (Hunter syndrome) is caused by a deficiency of the enzyme iduronate-2-sulfatase (IDS). CNS involvement is common in MPS II, especially in severely ill patients.251 MPS III (Sanfilippo syndrome) has four subtypes, and is caused by the deficiency of an enzyme needed to break down heparan sulfate. MPS III particularly affects the CNS. It primarily affects the brain and is characterized by severe neurological symptoms, including developmental delays, severe behavioral problems, sleep disturbances, and progressive cognitive decline.252 MPS VII (Sly syndrome) is caused by a deficiency of β-glucuronidase. It also affects the CNS, with symptoms including developmental delays and cognitive impairment.253 In brief, the severity and specific symptoms of MPS vary from person to person, and treatment options are limited, usually focusing on controlling symptoms and improving the quality of life.254
Some progress has been made in applying therapies such as ERT or hematopoietic stem cell transplantation. The main treatment is weekly infusions of a functional enzyme, but this enzyme cannot cross the BBB to reach the CNS.255 Laronidase-functionalized multiple-wall lipid-core nanocapsules replaced the deficient IDUA enzyme and enhanced enzyme activity in different organs within 4 h and 24 h in MPS I.229 Another study used a delivery method based on g7-functionalized PLGA NPs (g7-NPs) loaded with the therapeutic enzyme IDS for treating MPS II.230 These NPs were administered to fibroblasts from patients with MPS II for 7 days in vitro, and it was found that the measured induced IDS activity was identical to that detected in healthy cells (Figure 13A), while the GAG content was reduced to non-pathological levels (Figure 13B). In vivo experiments in MPS II mice administered g7-NPs-IDS weekly revealed a significant reduction in gel deposition in the brain (Figure 13C) and liver (Figure 13D) tissues, as well as a reduction in LAMP2 (Figure 13E), CD68 (Figure 13F), and GFAP (Figure 13G) markers. These NPs reduced GAG accumulation in the brain and addressed the neurological symptoms of the disease, suggesting a generalized improvement in inflammatory response and pathology in the brain. Muntimadugu et al reported PLGA NPs coated with ICAM-1 for BBB crossing to deliver hyaluronidase (HAse) enzyme, thereby restoring the deficient enzyme activity and reducing the accumulation of glycosaminoglycans in MPS IX treatment.226 Hence, drawing from these studies, NPs offer potential in treating MPS by crossing the BBB, restoring enzyme activity, and reducing GAG accumulation, particularly in the CNS.
 |
Figure 13 NP-based ERT for MPSs. (A) Induced IDS activity expressed in nmoles of 4MU (4-Methylumbelliferyl) released in 4 h per mg of protein (nmol/4 h/mg protein) (B) GAG content (μg GAG/mg protein) after 7 days treatment in fibroblasts from MPSII patients. (C-D) Histochemical and biochemical analysis of GAG in the (C) brain and (D) liver of Ids-ko mice treated with 0.9% NaCl (untreated, UT), g7-NPs, free IDS, g7-NPs-IDS, and in wt mice, after 6 weeks of treatment. (E-G) Quantification of positive cells to (E) LAMP2, (F) CD68, and (G) GFAP in the cerebral cortex and hippocampus. Data are mean ± SD. For each time point, asterisks indicate a statistically significant difference from UT cells (two-tailed Student’s t-test for A and B, Mann–Whitney U-test for C-G * p < 0.05, ** p < 0.01, *** p < 0.001), while hash marks indicate a statistically significant difference between IDS and u-NPs-IDS treated cells (two-tailed Student’s t-test for A and B, Mann–Whitney U-test for C-D, # p < 0.05, ## p < 0.01, ### p < 0.001. Reproduced from Rigon L, Salvalaio M, Pederzoli F et al. Targeting Brain Disease in MPSII: Preclinical Evaluation of IDS-Loaded PLGA Nanoparticles. International journal of molecular sciences. Apr 24 2019;20(8).230 © 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). |
Glycoprotein Storage Disorders
Glycoprotein storage disorders, also called glycoproteinoses, are a group of autosomal recessive metabolic disorders in which a deficiency of the enzyme responsible for the breakdown of glycoproteins causes them to accumulate in lysosomes and is characterized by an abnormal accumulation of glycoproteins in various tissues of the body leading to cell and tissue dysfunction.256 There are several types of glycoproteinoses, each associated with a deficiency of a specific enzyme. Examples include aspartylglucosaminuria (aspartylglucosaminidase deficiency), fucosidosis (α-L-fucosidase deficiency), mannosidosis (α-mannosidase deficiency), sialidosis (neuraminidase deficiency), and Schindler disease (α-N-acetylgalactosaminidase).257 Symptoms can vary greatly depending on the specific disorder and severity of the enzyme deficiency. Common symptoms include developmental delays, mental retardation, skeletal abnormalities, coarse facial features, and organ hypertrophy. Some patients also experience neurological symptoms such as ataxia, seizures, and vision problems. Treatment of glycoproteinoses usually focus on controlling symptoms and improving the patient’s quality of life.258 ERT has limited efficacy in glycoproteinoses because of the BBB. Therefore, nanomedicine delivery systems may have a promising application in improving the treatment of glycoproteinoses because of their ability to cross the BBB.
To sum up, for the CNS dysfunction caused by a variety of hereditary LSDs, effective delivery of active enzymes to the lesion sites in the brain is the key to treatment. In the context of ERT, NPs help deliver vital enzymes to the brain, thereby addressing the underlying causes of various metabolic diseases of the CNS. Although not much research has been done, this approach has shown promising applications in these LSD disorders. However, while NPs have been optimized for enhanced CNS targeting, achieving sufficient therapeutic enzyme concentrations in affected brain regions remains a challenge. Future research should focus on refining NP formulations to improve BBB crossing efficiency, enhancing targeting specificity, and prolonging enzyme activity within the CNS.
Challenges and Future Perspectives for Nanoparticle-Based Therapies
The field of NP-based gene therapy and ERTs for neurological disorders is rapidly advancing, demonstrating significant potential to overcome the challenges posed by the BBB. However, despite promising progress, several critical challenges and future directions need to be addressed to fully realize the clinical potential of these therapies.
First, targeted delivery and specificity are key areas of focus for future research. The complexity of the CNS, coupled with the challenges of crossing the BBB, requires a multidisciplinary approach involving nanotechnology, molecular biology, pharmacology, and clinical medicine. While current NPs can enhance drug accumulation in the brain through physicochemical optimization and surface modification, there is still a need to improve their brain-targeting capabilities and specificity. This will ensure that therapeutic agents are delivered precisely to affected areas without off-target effects.259 Future research should prioritize the development of brain-specific target and multifunctional NPs capable of targeting multiple markers to enhance both specificity and therapeutic efficacy.
Second, the scalability and manufacturing of NP delivery systems are also areas of concern. The transition from laboratory research to clinical application requires scalable and cost-effective manufacturing processes. The complexity of NP synthesis and functionalization presents significant hurdles.260 Developing standardized synthesis protocols and scalable manufacturing techniques is essential for the clinical application of NP-based therapies.
Third, long-term safety and biocompatibility have been focal points in the study of NP delivery systems. The long-term safety of NPs remains a critical concern, particularly regarding their potential accumulation in the liver and other organs, as well as unforeseen side effects, immune responses, and the ethical implications of gene editing.261 Future research should prioritize long-term biocompatibility and toxicity assessments in animal models, followed by careful monitoring in clinical trials to ensure that NPs do not cause adverse reactions over extended periods.
Finally, personalized medicine represents a future direction for the use of NPs in treating neurological disorders. The heterogeneity of neurological diseases suggests that a one-size-fits-all approach may be ineffective. Developing NP-based personalized therapies that take into account the unique genetic, epigenetic, and environmental factors of each patient could lead to more effective and tailored treatments.262
Overall, although NP-based delivery systems show remarkable promise in bypassing the BBB and providing targeted drug delivery to the CNS, several challenges limit their efficacy. Key limitations include potential off-target effects, immunogenic responses, and the need for precise control over NP biodegradability and clearance. Furthermore, achieving both high treatment efficacy and safety remains challenging, especially with concerns about long-term NP accumulation in CNS tissues and peripheral organs. Future research therefore prioritizes improving NP design to enhance targeting accuracy, BBB permeability, and controlled release mechanisms, fostering more effective and safer treatments for complex neurological diseases.
Conclusion
The burgeoning field of NP applications in neurological disorders, particularly in the realms of gene therapy and ERT, represents a significant stride in the pursuit of more effective treatments for neurological conditions. This review has described the mechanisms by which NPs cross the BBB, a key obstacle in neurotherapeutic delivery, and highlighted the multifaceted advantages of NPs as drug delivery systems for the treatment of neurological disorders. Owing to their multifunctionality and surface modifiability, NPs can be engineered to specific sizes, shapes, and surface characteristics that not only protect the loaded genetic material and enzymes from degradation in the in vivo environment but also facilitate the crossing of the BBB, which ensures that the therapeutic agents reach the intended site of action within the brain. NPs thus provide a unique platform for CNS drug delivery.
The current research landscape is rife with advancements in nanotechnology, and organic and inorganic NPs all have been widely explored for therapeutic applications in the treatment of neurological disorders, each with their own unique properties and applications. For example, liposomal NPs are of interest for their biocompatibility and ability to encapsulate both hydrophilic and hydrophobic drugs, while polymeric NPs offer enhanced stability and controlled release of therapeutic drugs. Inorganic NPs, such as quantum dots and silica NPs, have been studied for their robustness and potential in diagnostic imaging and therapeutic delivery. Despite these advances, the clinical translation of NP-based therapies still faces challenges. Issues such as potential toxicity, long-term stability, and the body’s immune response to NPs need to be further addressed. In addition, quantitative production and batch-to-batch consistency are important for clinical applications. Therefore, future research should focus on optimizing the design and functionalization of NPs to improve their efficiency and safety. Furthermore, the field is moving towards personalized medicine, in which treatment plans are tailored to the individual patient. NPs offer the possibility of personalized therapies, but this requires a deeper understanding of disease mechanisms and patient-specific responses to treatment.
In conclusion, NPs have great potential for application in the treatment of neurological disorders, especially for gene therapy and ERT. Their ability to cross the BBB and deliver therapeutic agents directly to lesions in the brain paves way for more effective and minimally invasive treatments, but the road to clinical application is rife with challenges. Continued research, innovation, and interdisciplinary collaboration will be necessary to overcome these obstacles and realize the full potential of these therapies in improving patient prognosis. As research in this area progresses, it is expected that NP-based therapies will become a cornerstone in the treatment of neurological disorders.
Abbreviations
CNS, central nervous system; NP, nanoparticle; BBB, blood-brain barrier; ERT, enzyme replacement therapy; LSD, lysosomal storage disorder; AD, Alzheimer’s disease; PEG, polyethylene glycol; Aβ, β-amyloid; CMT, carrier-mediated transport; GLUT1, glucose transporter protein 1; RMT, receptor-mediated transcytosis; Tf, transferrin; TfR, transferrin receptor; IGF, insulin-like growth factor; LDL, low-density lipoprotein; AMT, adsorptive-mediated transcytosis; CPP, cell-penetrating peptide; GBM, glioblastoma; MSC, mesenchymal stem cell; NSCs, neural stem cells; SLN, solid lipid nanoparticle; PLA, poly(lactic acid); PGA, polyglycolic acid; PLGA, poly(lactic-co-glycolic acid); SPR, surface plasmon resonance; Au, gold; MRI, magnetic resonance imaging; SiO2, silicon dioxide; EPR, enhanced permeability and retention; AAV, adeno-associated virus; BDNF, brain-derived neurotrophic factor; CM, cell membrane; RISC, RNA-induced silencing complex; LNP, lipid nanoparticle; SDT, sonodynamic therapy; FUS, focused ultrasound; GSH, glutathione; ASO, antisense oligonucleotide; PD, Parkinson’s diseases; sgRNA, single-guide RNA; VGF, vascular growth factor; hGDNF, human glial cell line-derived neurotrophic factor; ShRNA, short hairpin RNA; NGF, nerve growth factor; HSVtk, herpes simplex virus thymidine kinase; GBM, glioblastoma multiforme; GCV, ganciclovir; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; LRP, low-density lipoprotein receptor-related protein; MMP9, matrix metalloproteinase 9; SPIO, superparamagnetic iron oxide; mPTP, mitochondrial permeability transition pore; α-syn, α-synuclein; PD-L1, programmed cell death-ligand 1; TMZ, temozolomide; EGFR, epidermal growth factor receptor; NRP-1, neuropilin-1; PLK1, Polo-like kinase 1; HD, Huntington’s disease; VEGF, vascular endothelial growth factor; ER, endoplasmic reticulum; MGMT, O6-methylguanine-DNA methyltransferase; SMO, smoothened; MB-FUS, microbubble-enhanced focused ultrasound; HIF-1α, hypoxia inducible factor-1α; HER2, human epidermal growth factor receptor-2; Tf, transferrin; TBI, traumatic brain injuries; TNF-α, tumor necrosis factor-α; IL-10, interleukin 10, VCAM-1, vascular cell adhesion molecule-1; EPR, enhanced permeability and retention; ASO, antisense oligonucleotides; mHTT, mutant huntingtin; apoA-1, apolipoprotein A-I; SMA, spinal muscular atrophy; ALS, amyotrophic lateral sclerosis; MS, multiple sclerosis; App, amyloid precursor protein; Th, tyrosine hydroxylase; sgRNA, single guide RNA; RNPs, ribonucleoproteins; FXS, Fragile X syndrome; IDUA, α-L-iduronidase; CXCR4, chemokine (C-X-C motif) receptor 4; β-gal, β-galactosidase; HA, hyaluronic acid; ASM, acid sphingomyelinase; CAM, cell adhesion molecule; GALC, galactosylceramidase; CLEA, cross-linked enzyme aggregate; NCL, neuronal ceroid lipofuscinose; PPT1, palmitoyl-protein thioesterase-1; MPS, mucopolysaccharidose; GAG, glycosaminoglycan;ApoE, apolipoprotein E; GCase, glucocerebrosidase; rGCase, recombinant glucocerebrosidase; ICAM-1, intercellular cell adhesion molecule-1; PECAM-1, platelet-endothelial cell adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; IDS, iduronate 2-sulfatase; HAse, hyaluronidase.
Acknowledgments
This work was supported by grants from National Key Research and Development Program of China (2021YFA1100700), NSFC (82325029, 32171379, U22A20156, 91642208), Department of Human Resource and Social Security of Jilin Province (2022DJ02), the Bethune Medical Department of Jilin University (2022JBGS01), Health Science and Technology Capacity Enhancement Program of Jilin Province (2022JC056), Science and Technology Development Program Project of Jilin Province (YDZJ202301ZYTS063), and the Fundamental Research Funds for the Central Universities, JLU.
Disclosure
The authors declare no conflict of interest.
References
1. Teleanu RI, Preda MD, Niculescu AG. et al. Current Strategies to Enhance Delivery of Drugs across the Blood-Brain Barrier. Pharmaceutics. 2022;14(5). doi:10.3390/pharmaceutics14050987
2. Amin ND, Paşca SP. Building Models of Brain Disorders with Three-Dimensional Organoids. Neuron. 2018;100(2):389–405. doi:10.1016/j.neuron.2018.10.007
3. Andjelkovic AV, Stamatovic SM, Phillips CM, Martinez-Revollar G, Keep RF. Modeling blood-brain barrier pathology in cerebrovascular disease in vitro: current and future paradigms. Fluids Barriers CNS. 2020;17(1):44. doi:10.1186/s12987-020-00202-7
4. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: from Physiology to Disease and Back. Physiol Rev. 2019;99(1):21–78. doi:10.1152/physrev.00050.2017
5. Lingineni K, Belekar V, Tangadpalliwar SR, Garg P. The role of multidrug resistance protein (MRP-1) as an active efflux transporter on blood–brain barrier (BBB) permeability. Mol Diver. 2017;21(2):355–365. doi:10.1007/s11030-016-9715-6
6. Naqvi S, Panghal A, Flora SJS. Nanotechnology: a Promising Approach for Delivery of Neuroprotective Drugs. Front Neurosci. 2020;14:494. doi:10.3389/fnins.2020.00494
7. Hersh AM, Alomari S, Tyler BM. Crossing the Blood-Brain Barrier: advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int J Mol Sci. 2022;23(8). doi:10.3390/ijms23084153
8. Teixeira MI, Lopes CM, Amaral MH, Costa PC. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): a current overview of active targeting in brain diseases. Colloids Surf B. 2023;221:112999. doi:10.1016/j.colsurfb.2022.112999
9. Cullis PR, Hope MJ. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol Ther. 2017;25(7):1467–1475. doi:10.1016/j.ymthe.2017.03.013
10. Sato Y, Okuyama T. Novel Enzyme Replacement Therapies for Neuropathic Mucopolysaccharidoses. Int J Mol Sci. 2020;21(2):400. doi:10.3390/ijms21020400
11. Kim DH, Lee HS, Kwon T-W, et al. Single enzyme nanoparticle, an effective tool for enzyme replacement therapy. Arch Pharmacal Res. 2020;43(1):1–21. doi:10.1007/s12272-020-01216-3
12. Khan AR, Yang X, Fu M, Zhai G. Recent progress of drug nanoformulations targeting to brain. J Controll Release. 2018;291:37–64. doi:10.1016/j.jconrel.2018.10.004
13. Zeb A, Gul M, Nguyen -T-T-L, Maeng H-J. Controlled release and targeted drug delivery with poly(lactic-co-glycolic acid) nanoparticles: reviewing two decades of research. J Pharm Invest. 2022;52(6):683–724. doi:10.1007/s40005-022-00584-w
14. Jin GZ, Chakraborty A, Lee JH, Knowles JC, Kim HW. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J Tissue Eng. 2020;11:2041731419897460. doi:10.1177/2041731419897460
15. Rodrigues-Neves AC, Ambrósio AF, Gomes CA. Microglia sequelae: brain signature of innate immunity in schizophrenia. Transl Psychiatry. 2022;12(1):493. doi:10.1038/s41398-022-02197-1
16. Rehman MU, Sehar N, Dar NJ, et al. Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: an update on current advances and impediments. Neurosci Biobehav Rev. 2023;144:104961. doi:10.1016/j.neubiorev.2022.104961
17. Ciurea AV, Mohan AG, Covache-Busuioc RA, et al. Unraveling Molecular and Genetic Insights into Neurodegenerative Diseases: advances in Understanding Alzheimer’s, Parkinson’s, and Huntington’s Diseases and Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2023;24(13):10809. doi:10.3390/ijms241310809
18. Krellman JW, Mercuri G. Cognitive Interventions for Neurodegenerative Disease. Curr Neurol Neurosci Rep. 2023;23(9):461–468. doi:10.1007/s11910-023-01283-1
19. Martemucci G, Khalil M, Di Luca A, Abdallah H, D’Alessandro AG. Comprehensive Strategies for Metabolic Syndrome: how Nutrition, Dietary Polyphenols, Physical Activity, and Lifestyle Modifications Address Diabesity, Cardiovascular Diseases, and Neurodegenerative Conditions. Metabolites. 2024;14(6). doi:10.3390/metabo14060327
20. Ortiz GG, Torres-Mendoza BMG, Ramírez-Jirano J, et al. Genetic Basis of Inflammatory Demyelinating Diseases of the Central Nervous System: multiple Sclerosis and Neuromyelitis Optica Spectrum. Genes. 2023;14(7):1319. doi:10.3390/genes14071319
21. Zhang F, Gao X, Liu J, Zhang C. Biomarkers in autoimmune diseases of the central nervous system. Front Immunol. 2023;14:1111719. doi:10.3389/fimmu.2023.1111719
22. Coutinho Costa VG, Araújo SE, Alves-Leon SV, Gomes FCA. Central nervous system demyelinating diseases: glial cells at the hub of pathology. Front Immunol. 2023;14:1135540. doi:10.3389/fimmu.2023.1135540
23. Magdalena C, Clarissa A, Sutandi N. Comparative Analysis of Treatment Outcomes in Patients with Neuromyelitis Optica Spectrum Disorder Treated with Rituximab, Azathioprine, and Mycophenolate Mofetil: a Systematic Review and Meta-analysis. Innov Clin Neurosci. 2022;19(4–6):51–64.
24. Gabelić T, Barun B, Adamec I, Krbot Skorić M, Habek M. Product review on MAbs (alemtuzumab and ocrelizumab) for the treatment of multiple sclerosis. Hum Vaccines Immunother. 2021;17(11):4345–4362. doi:10.1080/21645515.2021.1969850
25. Musmar B, Adeeb N, Ansari J, Sharma P, Cuellar HH. Endovascular Management of hemorrhagic Stroke. Biomedicines. 2022;10(1). doi:10.3390/biomedicines10010100
26. Ceulemans A, Spronk HMH, ten Cate H, van Zwam WH, van Oostenbrugge RJ, Nagy M. Current and potentially novel antithrombotic treatment in acute ischemic stroke. Thromb Res. 2024;236:74–84. doi:10.1016/j.thromres.2024.02.009
27. Zhao W, Wu C, Stone C, Ding Y, Ji X. Treatment of intracerebral hemorrhage: current approaches and future directions. J Neurol Sci. 2020;416:117020. doi:10.1016/j.jns.2020.117020
28. Kawabori M, Shichinohe H, Kuroda S, Houkin K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int J Mol Sci. 2020;21(19):7380. doi:10.3390/ijms21197380
29. Ostrom QT, Francis SS, Barnholtz-Sloan JS. Epidemiology of Brain and Other CNS Tumors. Curr Neurol Neurosci Rep. 2021;21(12):68. doi:10.1007/s11910-021-01152-9
30. Wanleenuwat P, Iwanowski P. Metastases to the central nervous system: molecular basis and clinical considerations. J Neurol Sci. 2020;412:116755. doi:10.1016/j.jns.2020.116755
31. Rampling R, James A, Papanastassiou V. The present and future management of malignant brain tumours: surgery, radiotherapy, chemotherapy. J Neurol Neurosurg. 2004;75 Suppl 2(Suppl 2):ii24–30. doi:10.1136/jnnp.2004.040535
32. Li S, Wang C, Chen J, et al. Signaling pathways in brain tumors and therapeutic interventions. Sig Transd Target Ther. 2023;8(1):8. doi:10.1038/s41392-022-01260-z
33. Hotchkiss KM, Batich KA, Mohan A, Rahman R, Piantadosi S, Khasraw M. Dendritic cell vaccine trials in gliomas: untangling the lines. Neuro Oncol. 2023;25(10):1752–1762. doi:10.1093/neuonc/noad088
34. Fu M, Zhou Z, Huang X, et al. Use of Bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer. 2023;23(1):544. doi:10.1186/s12885-023-11043-6
35. Freire MAM, Rocha GS, Bittencourt LO, Falcao D, Lima RR, Cavalcanti J. Cellular and Molecular Pathophysiology of Traumatic Brain Injury: what Have We Learned So Far? Biology. 2023;12(8). doi:10.3390/biology12081139
36. Hu X, Xu W, Ren Y, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Sig Transd Target Ther. 2023;8(1):245. doi:10.1038/s41392-023-01477-6
37. Tsai MH, Wu CY, Wu CH, Chen CY. The Current Update of Conventional and Innovative Treatment Strategies for Central Nervous System Injury. Biomedicines. 2024;12(8):1894. doi:10.3390/biomedicines12081894
38. Ellison S, Parker H, Bigger B. Advances in therapies for neurological lysosomal storage disorders. J Inherit Metabol Disease. 2023;46(5):874–905. doi:10.1002/jimd.12615
39. Ardissone A, Bruno C, Diodato D, et al. Clinical, imaging, biochemical and molecular features in Leigh syndrome: a study from the Italian network of mitochondrial diseases. Orphanet J Rare Diseases. 2021;16(1):413. doi:10.1186/s13023-021-02029-3
40. Wolf NI, ffrench-Constant C, van der Knaap MS. Hypomyelinating leukodystrophies — unravelling myelin biology. Nat Rev Neurol. 2021;17(2):88–103. doi:10.1038/s41582-020-00432-1
41. Critchley BJ, Gaspar HB, Benedetti S. Targeting the central nervous system in lysosomal storage diseases: strategies to deliver therapeutics across the blood-brain barrier. Mol Ther. 2023;31(3):657–675. doi:10.1016/j.ymthe.2022.11.015
42. Rajan DS, Escolar ML. Evolving therapies in neuronopathic LSDs: opportunities and challenges. Metab Brain Dis. 2022;37(7):2245–2256. doi:10.1007/s11011-022-00939-0
43. Morozova A, Zorkina Y, Abramova O, et al. Neurobiological Highlights of Cognitive Impairment in Psychiatric Disorders. Int J Mol Sci. 2022;23(3):1217. doi:10.3390/ijms23031217
44. Correll CU, Cortese S, Croatto G, et al. Efficacy and acceptability of pharmacological, psychosocial, and brain stimulation interventions in children and adolescents with mental disorders: an umbrella review. World Psychiatry. 2021;20(2):244–275. doi:10.1002/wps.20881
45. Yuan S, Wu H, Wu Y, et al. Neural Effects of Cognitive Behavioral Therapy in Psychiatric Disorders: a Systematic Review and Activation Likelihood Estimation Meta-Analysis. front psychol. 2022;13:853804. doi:10.3389/fpsyg.2022.853804
46. Lin CH, Chen YM, Lane HY. Novel Treatment for the Most Resistant Schizophrenia: dual Activation of NMDA Receptor and Antioxidant. Curr Drug Targets. 2020;21(6):610–615. doi:10.2174/1389450120666191011163539
47. Goes FS. Diagnosis and management of bipolar disorders. BMJ. 2023;381:e073591. doi:10.1136/bmj-2022-073591
48. Mansuri Z, Reddy A, Vadukapuram R, Trivedi C, Amara A. Pimavanserin in the Treatment of Parkinson’s Disease Psychosis: meta-analysis and Meta-regression of Randomized Clinical Trials. Innov Clin Neurosc. 2022;19(1–3):46–51.
49. Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front Physiol. 2020;11:914. doi:10.3389/fphys.2020.00914
50. Dong X. Current Strategies for Brain Drug Delivery. Theranostics. 2018;8(6):1481–1493. doi:10.7150/thno.21254
51. Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood-brain barrier: structure, regulation, and drug delivery. Sig Transd Target Ther. 2023;8(1):217. doi:10.1038/s41392-023-01481-w
52. Wang J, Yu Y, Zhang C, Song J, Zheng Z, Yan W. New advances in diagnosis and treatment of nano drug delivery systems across the blood-brain barrier. Nanocomposites. 2023;9(1):116–127. doi:10.1080/20550324.2023.2256466
53. Sharma A, Fernandes DC, Reis RL, et al. Cutting-edge advances in modeling the blood-brain barrier and tools for its reversible permeabilization for enhanced drug delivery into the brain. Cell Biosci. 2023;13(1):137. doi:10.1186/s13578-023-01079-3
54. Correia AC, Monteiro AR, Silva R, Moreira JN, Sousa Lobo JM, Silva AC. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv Drug Deliv Rev. 2022;189:114485. doi:10.1016/j.addr.2022.114485
55. Xiong B, Wang Y, Chen Y, et al. Strategies for Structural Modification of Small Molecules to Improve Blood-Brain Barrier Penetration: a Recent Perspective. J Med Chem. 2021;64(18):13152–13173. doi:10.1021/acs.jmedchem.1c00910
56. Velásquez-Jiménez D, Corella-Salazar DA, Zuñiga-Martínez BS, et al. Phenolic compounds that cross the blood-brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021;12(21):10356–10369. doi:10.1039/d1fo02017j
57. Zhou Y, Liyanage PY, Devadoss D, et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood-brain barrier and inhibitors of β-amyloid. Nanoscale. 2019;11(46):22387–22397. doi:10.1039/c9nr08194a
58. Markowicz-Piasecka M, Markiewicz A, Darłak P, et al. Current Chemical, Biological, and Physiological Views in the Development of Successful Brain-Targeted Pharmaceutics. Neurotherapeutics. 2022;19(3):942–976. doi:10.1007/s13311-022-01228-5
59. Koepsell H. Glucose transporters in brain in health and disease. Pflugers Archiv. 2020;472(9):1299–1343. doi:10.1007/s00424-020-02441-x
60. Zaragozá R. Transport of Amino Acids Across the Blood-Brain Barrier. Front Physiol. 2020;11:973. doi:10.3389/fphys.2020.00973
61. Chiang HL, Wu KC, Chen YY, et al. The Critical Role of Equilibrative Nucleoside Transporter-2 in Modulating Cerebral Damage and Vascular Dysfunction in Mice with Brain Ischemia-Reperfusion. Pharm Res. 2023;40(11):2541–2554. doi:10.1007/s11095-023-03565-2
62. Anraku Y, Kuwahara H, Fukusato Y, et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat Commun. 2017;8(1):1001. doi:10.1038/s41467-017-00952-3
63. Zhang S, Gan L, Cao F, et al. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res Bull. 2022;190:69–83. doi:10.1016/j.brainresbull.2022.09.017
64. Khoo TC, Tubbesing K, Rudkouskaya A, et al. Quantitative label-free imaging of iron-bound transferrin in breast cancer cells and tumors. Redox Biol. 2020;36:101617. doi:10.1016/j.redox.2020.101617
65. Zhang W, Liu QY, Haqqani AS, et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS. 2020;17(1):47. doi:10.1186/s12987-020-00209-0
66. Siddiqui H, Yevstigneyev N, Madani G, McCormick S. Approaches to Visualising Endocytosis of LDL-Related Lipoproteins. Biomolecules. 2022;12(2):158. doi:10.3390/biom12020158
67. Zhang Z, Guan J, Jiang Z, et al. Brain-targeted drug delivery by manipulating protein Corona functions. Nat Commun. 2019;10(1):3561. doi:10.1038/s41467-019-11593-z
68. Liu HJ, Xu P. Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor. Adv Drug Deliv Rev. 2022;191:114619. doi:10.1016/j.addr.2022.114619
69. Song J, Lu C, Leszek J, Zhang J. Design and Development of Nanomaterial-Based Drug Carriers to Overcome the Blood-Brain Barrier by Using Different Transport Mechanisms. Int J Mol Sci. 2021;22(18). doi:10.3390/ijms221810118
70. Wong KH, Riaz MK, Xie Y, et al. Review of Current Strategies for Delivering Alzheimer’s Disease Drugs across the Blood-Brain Barrier. Int J Mol Sci. 2019;20(2):381. doi:10.3390/ijms20020381
71. René CA, Parks RJ. Delivery of Therapeutic Agents to the Central Nervous System and the Promise of Extracellular Vesicles. Pharmaceutics. 2021;13(4):492. doi:10.3390/pharmaceutics13040492
72. Lombardo SM, Schneider M, Türeli AE, Günday Türeli N. Key for crossing the BBB with nanoparticles: the rational design. Beilstein J Nanotechnol. 2020;11:866–883. doi:10.3762/bjnano.11.72
73. Lakkadwala S, Dos Santos Rodrigues B, Sun C, Singh J. Biodistribution of TAT or QLPVM coupled to receptor targeted liposomes for delivery of anticancer therapeutics to brain in vitro and in vivo. Nanomedicin. 2020;23:102112. doi:10.1016/j.nano.2019.102112
74. D’Souza A, Dave KM, Stetler RA, S Manickam D. Targeting the blood-brain barrier for the delivery of stroke therapies. Adv Drug Deliv Rev. 2021;171:332–351. doi:10.1016/j.addr.2021.01.015
75. Georgieva JV, Kalicharan D, Couraud PO, et al. Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro. Mol Ther. 2011;19(2):318–325. doi:10.1038/mt.2010.236
76. Cortés H, Alcalá-Alcalá S, Caballero-Florán IH, et al. A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes. 2020;10(9):212. doi:10.3390/membranes10090212
77. Ayer M, Schuster M, Gruber I, et al. T Cell-Mediated Transport of Polymer Nanoparticles across the Blood-Brain Barrier. Adv Healthcare Mater. 2021;10(2):e2001375. doi:10.1002/adhm.202001375
78. Huang X, Zhang F, Wang H, et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013;34(7):1772–1780. doi:10.1016/j.biomaterials.2012.11.032
79. Morgenroth A, Baazaoui F, Hosseinnejad A, et al. Neural Stem Cells as Carriers of Nucleoside-Conjugated Nanogels: a New Approach toward Cell-Mediated Delivery. ACS Appl Mater Interfaces. 2023;15(18):21792–21803. doi:10.1021/acsami.2c23283
80. Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta pharmaceutica Sinica B. 2019;9(4):675–689. doi:10.1016/j.apsb.2019.01.011
81. Ayer M, Klok HA. Cell-mediated delivery of synthetic nano- and microparticles. J Controll Release. 2017;259:92–104. doi:10.1016/j.jconrel.2017.01.048
82. Vashist A, Manickam P, Raymond AD, et al. Recent Advances in Nanotherapeutics for Neurological Disorders. ACS Appl Bio Mater. 2023;6(7):2614–2621. doi:10.1021/acsabm.3c00254
83. Pandian SRK, Vijayakumar KK, Murugesan S, Kunjiappan S. Liposomes: an emerging carrier for targeting Alzheimer’s and Parkinson’s diseases. Heliyon. 2022;8(6):e09575. doi:10.1016/j.heliyon.2022.e09575
84. Liu J, Chang J, Jiang Y, et al. Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles. Adv Mater. 2019;31(33):e1902575. doi:10.1002/adma.201902575
85. Rehman M, Ihsan A, Madni A, et al. Solid lipid nanoparticles for thermoresponsive targeting: evidence from spectrophotometry, electrochemical, and cytotoxicity studies. Int j Nanomed. 2017;12:8325–8336. doi:10.2147/ijn.S147506
86. Arduino I, Liu Z, Rahikkala A, et al. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater. 2021;121:566–578. doi:10.1016/j.actbio.2020.12.024
87. Madkhali OA. Perspectives and Prospective on Solid Lipid Nanoparticles as Drug Delivery Systems. Molecules. 2022;27(5):1543. doi:10.3390/molecules27051543
88. Nunes D, Andrade S, Ramalho MJ, Loureiro JA, Pereira MC. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: a Systematic Review on In Vivo Findings. Polymers. 2022;14(5):1010. doi:10.3390/polym14051010
89. Marante T, Viegas C, Duarte I, Macedo AS, Fonte P. An Overview on Spray-Drying of Protein-Loaded Polymeric Nanoparticles for Dry Powder Inhalation. Pharmaceutics. 2020;12(11):1032. doi:10.3390/pharmaceutics12111032
90. Yan C, Gu J, Yin S, et al. Design and Preparation Naringenin Loaded Functional Biomimetic Nano-Drug Delivery System for Alzheimer’s Disease. J Drug Targeting. 2023;2023:1–44. doi:10.1080/1061186x.2023.2290453
91. Sánchez A, Mejía SP, Orozco J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules. 2020;25(16):3760. doi:10.3390/molecules25163760
92. Castro K, Costa JM, Campos MGN. Drug-loaded polymeric nanoparticles: a review. Int J Polym Mater Polym Biomater. 2022;71(1):1–13. doi:10.1080/00914037.2020.1798436
93. Patel P, Patel V, Patel PM. Synthetic strategy of dendrimers: a review. J Indian Chem Soc. 2022;99(7):100514. doi:10.1016/j.jics.2022.100514
94. Karimi S, Namazi H. A photoluminescent folic acid-derived carbon dot functionalized magnetic dendrimer as a pH-responsive carrier for targeted doxorubicin delivery. New J Chem. 2021;45(14):6397–6405. doi:10.1039/D0NJ06261H
95. Shiri P, Amani AM. A brief overview of catalytic applications of dendrimers containing 1,4-disubstituted-1,2,3-triazoles. Monatshefte Für Chemie - Chemical Monthly. 2021;152(4):367–385. doi:10.1007/s00706-021-02753-3
96. Shi C, Sun Y, Wu H, et al. Exploring the effect of hydrophilic and hydrophobic structure of grafted polymeric micelles on drug loading. Int J Pharm. 2016;512(1):282–291. doi:10.1016/j.ijpharm.2016.08.054
97. Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine: Nanotechnology, Biology and Medicine. 2010;6(6):714–729. doi:10.1016/j.nano.2010.05.005
98. Varanko A, Saha S, Chilkoti A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv Drug Deliv Rev. 2020;156:133–187. doi:10.1016/j.addr.2020.08.008
99. Yin L, Yuvienco C, Montclare JK. Protein based therapeutic delivery agents: contemporary developments and challenges. Biomaterials. 2017;134:91–116. doi:10.1016/j.biomaterials.2017.04.036
100. Luther DC, Huang R, Jeon T, et al. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv Drug Deliv Rev. 2020;156:188–213. doi:10.1016/j.addr.2020.06.020
101. Ndolomingo MJ, Bingwa N, Meijboom R. Review of supported metal nanoparticles: synthesis methodologies, advantages and application as catalysts. J Mater Sci. 2020;55(15):6195–6241. doi:10.1007/s10853-020-04415-x
102. He Y, Gao Q, Lv C, Liu L. Improved photothermal therapy of brain cancer cells and photogeneration of reactive oxygen species by biotin conjugated gold photoactive nanoparticles. Journal of Photochemistry and Photobiology B: Biology. 2021;215:112102. doi:10.1016/j.jphotobiol.2020.112102
103. Vanin Dos Santos Lima M, Beloni de Melo G, Gracher Teixeira L, et al. Green synthesis of silver nanoparticles using Ilex paraguariensis extracts: antimicrobial activity and acetilcolinesterase modulation in rat brain tissue. Green Chem Lett Rev. 2022;15(1):128–138. doi:10.1080/17518253.2021.2024896
104. Ejidike IP, Clayton HS. Green synthesis of silver nanoparticles mediated by Daucus carota L.: antiradical, antimicrobial potentials, in vitro cytotoxicity against brain glioblastoma cells. Green Chem Lett Rev. 2022;15(2):298–311. doi:10.1080/17518253.2022.2054290
105. Israel LL, Galstyan A, Holler E, Ljubimova JY. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J Controll Release. 2020;320:45–62. doi:10.1016/j.jconrel.2020.01.009
106. Sheervalilou R, Shirvaliloo M, Sargazi S, Ghaznavi H. Recent advances in iron oxide nanoparticles for brain cancer theranostics: from in vitro to clinical applications. Expert Opin Drug Delivery. 2021;18(7):949–977. doi:10.1080/17425247.2021.1888926
107. Mohamed WAA, Abd El-Gawad H, Mekkey S, et al. Quantum dots synthetization and future prospect applications. Nanotechnol Rev. 2021;10(1):1926–1940. doi:10.1515/ntrev-2021-0118
108. Li D, Ushakova EV, Rogach AL, Qu S. Optical Properties of Carbon Dots in the Deep-Red to Near-Infrared Region Are Attractive for Biomedical Applications. Small. 2021;17(43):e2102325. doi:10.1002/smll.202102325
109. Tikhani F, Jouyandeh M, Jafari SH, et al. Cure Index demonstrates curing of epoxy composites containing silica nanoparticles of variable morphology and porosity. Prog Org Coat. 2019;135:176–184. doi:10.1016/j.porgcoat.2019.05.017
110. Karthik R, Raymon A. Effect of silicone oxide nano particles on dielectric characteristics of natural ester. 2016 IEEE international conference on high voltage engineering and application (ICHVE). 2016;2016;1–3.
111. Anthony DP, Hegde M, Shetty SS, Rafic T, Mutalik S, Rao BSS. Targeting receptor-ligand chemistry for drug delivery across blood-brain barrier in brain diseases. Life Sci. 2021;274:119326. doi:10.1016/j.lfs.2021.119326
112. Min HS, Kim HJ, Naito M, et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angewandte Chemie. 2020;59(21):8173–8180. doi:10.1002/anie.201914751
113. Vanbilloen WJF, Rechberger JS, Anderson JB, Nonnenbroich LF, Zhang L, Daniels DJ. Nanoparticle Strategies to Improve the Delivery of Anticancer Drugs across the Blood-Brain Barrier to Treat Brain Tumors. Pharmaceutics. 2023;15(7). doi:10.3390/pharmaceutics15071804
114. Zhang W, Mehta A, Tong Z, Esser L, Voelcker NH. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv Sci. 2021;8(10):2003937. doi:10.1002/advs.202003937
115. Xia Q, Huang J, Feng Q, et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int j Nanomed. 2019;14:6957–6970. doi:10.2147/ijn.S214008
116. Hadji H, Bouchemal K. Effect of micro- and nanoparticle shape on biological processes. J Controll Release. 2022;342:93–110. doi:10.1016/j.jconrel.2021.12.032
117. Nowak M, Brown TD, Graham A, Helgeson ME, Mitragotri S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng Transl Med. 2020;5(2):e10153. doi:10.1002/btm2.10153
118. Tan Q, Zhao S, Xu T, et al. Getting drugs to the brain: advances and prospects of organic nanoparticle delivery systems for assisting drugs to cross the blood-brain barrier. J Mat Chem B. 2022;10(45):9314–9333. doi:10.1039/d2tb01440h
119. Betzer O, Shilo M, Opochinsky R, et al. The effect of nanoparticle size on the ability to cross the blood-brain barrier: an in vivo study. Nanomedicine. 2017;12(13):1533–1546. doi:10.2217/nnm-2017-0022
120. Busquets MA, Espargaró A, Sabaté R, Estelrich J. Magnetic Nanoparticles Cross the Blood-Brain Barrier: when Physics Rises to a Challenge. Nanomaterials. 2015;5(4):2231–2248. doi:10.3390/nano5042231
121. Gupta R, Chauhan A, Kaur T, Kuanr BK, Sharma D. Transmigration of magnetite nanoparticles across the blood-brain barrier in a rodent model: influence of external and alternating magnetic fields. Nanoscale. 2022;14(47):17589–17606. doi:10.1039/d2nr02210a
122. Thomas RG, Kim S, Tran TA, et al. Magnet-Guided Temozolomide and Ferucarbotran Loaded Nanoparticles to Enhance Therapeutic Efficacy in Glioma Model. Nanomaterials. 2024;14(11). doi:10.3390/nano14110939
123. Tencomnao T, Klangthong K, Pimpha N, et al. Acceleration of gene transfection efficiency in neuroblastoma cells through polyethyleneimine/poly(methyl methacrylate) core-shell magnetic nanoparticles. Int j Nanomed. 2012;7:2783–2792. doi:10.2147/ijn.S32311
124. Stephen ZR, Kievit FM, Veiseh O, et al. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O6-benzylguanine to brain tumors. ACS nano. 2014;8(10):10383–10395. doi:10.1021/nn503735w
125. Wei PS, Thota N, John G, et al. Enhancing RNA-lipid nanoparticle delivery: organ- and cell-specificity and barcoding strategies. J Controll Release. 2024;375:366–388. doi:10.1016/j.jconrel.2024.08.030
126. Li Y, Li C, Yan J, et al. Polymeric micellar nanoparticles for effective CRISPR/Cas9 genome editing in cancer. Biomaterials. 2024;309:122573. doi:10.1016/j.biomaterials.2024.122573
127. Liu S, Cheng Q, Wei T, et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nature Mater. 2021;20(5):701–710. doi:10.1038/s41563-020-00886-0
128. Oude Egberink R, van Schie DM, Joosten B, et al. Unraveling mRNA delivery bottlenecks of ineffective delivery vectors by co-transfection with effective carriers. Eur j Pharm Biopharm. 2024;202:114414. doi:10.1016/j.ejpb.2024.114414
129. Kanjilal P, Dutta K, Thayumanavan S. Thiol-Disulfide Exchange as a Route for Endosomal Escape of Polymeric Nanoparticles. Angewandte Chemie. 2022;61(37):e202209227. doi:10.1002/anie.202209227
130. Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372). doi:10.1126/science.aan4672
131. Sayed N, Allawadhi P, Khurana A, et al. Gene therapy: comprehensive overview and therapeutic applications. Life Sci. 2022;294:120375. doi:10.1016/j.lfs.2022.120375
132. Afonso-Reis R, Afonso IT, Nóbrega C. Current Status of Gene Therapy Research in Polyglutamine Spinocerebellar Ataxias. Int J Mol Sci. 2021;22(8):4249. doi:10.3390/ijms22084249
133. Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107(2):222–239. doi:10.1016/j.pharmthera.2005.03.004
134. Ingusci S, Verlengia G, Soukupova M, Zucchini S, Simonato M. Gene Therapy Tools for Brain Diseases. Front Pharmacol. 2019;10:724. doi:10.3389/fphar.2019.00724
135. Luo M, Lee LKC, Peng B, Choi CHJ, Tong WY, Voelcker NH. Delivering the Promise of Gene Therapy with Nanomedicines in Treating Central Nervous System Diseases. Adv Sci. 2022;9(26):e2201740. doi:10.1002/advs.202201740
136. Arora S, Singh J. In vitro and in vivo optimization of liposomal nanoparticles based brain targeted vgf gene therapy. Int J Pharm. 2021;608:121095. doi:10.1016/j.ijpharm.2021.121095
137. Liu Y, An S, Li J, et al. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials. 2016;80:33–45. doi:10.1016/j.biomaterials.2015.11.060
138. Shen J, Lu Z, Wang J, et al. Traceable Nano-Biohybrid Complexes by One-Step Synthesis as CRISPR-Chem Vectors for Neurodegenerative Diseases Synergistic Treatment. Adv Mater. 2021;33(27):e2101993. doi:10.1002/adma.202101993
139. Aly AE, Harmon B, Padegimas L, et al. Intranasal delivery of hGDNF plasmid DNA nanoparticles results in long-term and widespread transfection of perivascular cells in rat brain. Nanomedicin. 2019;16:20–33. doi:10.1016/j.nano.2018.11.006
140. Huang SY, Dong ZS, Chen ZH, et al. Nano-MgO composites containing plasmid DNA to silence SNCA gene displays neuroprotective effects in Parkinson’s rats induced by 6-hydroxydopamine. Eur J Pharmacol. 2022;922:174904. doi:10.1016/j.ejphar.2022.174904
141. Shen J, Zhao Z, Shang W, et al. Fabrication of a nano polymer wrapping Meg3 ShRNA plasmid for the treatment of cerebral infarction. Artif Cells Nanomed Biotechnol. 2018:46(2):894–903. doi:10.1080/21691401.2018.1471483
142. Tong S, Xie L, Xie X, et al. Nano-Plumber Reshapes Glymphatic-Lymphatic System to Sustain Microenvironment Homeostasis and Improve Long-Term Prognosis after Traumatic Brain Injury. Adv Sci. 2023;10(34):2304284. doi:doi:10.1002/advs.202304284
143. Han S, Lee Y, Lee M. Biomimetic cell membrane-coated DNA nanoparticles for gene delivery to glioblastoma. J Controll Release. 2021;338:22–32. doi:10.1016/j.jconrel.2021.08.021
144. Gao S, Tian H, Xing Z, et al. A non-viral suicide gene delivery system traversing the blood brain barrier for non-invasive glioma targeting treatment. J Control Release. 2016;243:357–369. doi:10.1016/j.jconrel.2016.10.027
145. Jiang S, Cai G, Yang Z, et al. Biomimetic Nanovesicles as a Dual Gene Delivery System for the Synergistic Gene Therapy of Alzheimer’s Disease. ACS nano. 2024;18(18):11753–11768. doi:10.1021/acsnano.3c13150
146. Zhang H, Jiang W, Zhao Y, et al. Lipoprotein-Inspired Nanoscavenger for the Three-Pronged Modulation of Microglia-Derived Neuroinflammation in Alzheimer’s Disease Therapy. Nano Lett. 2022;22(6):2450–2460. doi:10.1021/acs.nanolett.2c00191
147. Yang X, Yang W, Xia X, et al. Intranasal Delivery of BACE1 siRNA and Rapamycin by Dual Targets Modified Nanoparticles for Alzheimer’s Disease Therapy. Small. 2022;18(30):2203182. doi:doi:10.1002/smll.202203182
148. Zhang Q, Song Q, Yu R, et al. Nano-Brake Halts Mitochondrial Dysfunction Cascade to Alleviate Neuropathology and Rescue Alzheimer’s Cognitive Deficits. Adv Sci. 2023;10(7):e2204596. doi:10.1002/advs.202204596
149. Lin B, Lu L, Wang Y, et al. Nanomedicine Directs Neuronal Differentiation of Neural Stem Cells via Silencing Long Noncoding RNA for Stroke Therapy. Nano Lett. 2021;21(1):806–815. doi:10.1021/acs.nanolett.0c04560
150. Liu Y, Zhang T, Zou X, et al. Penumbra-targeted CircOGDH siRNA-loaded nanoparticles alleviate neuronal apoptosis in focal brain ischaemia. Strok vasc neurol. 2024;9(2):134–144. doi:10.1136/svn-2022-002009
151. Abudurexiti M, Xue J, Li X, et al. Curcumin/TGF-β1 siRNA loaded solid lipid nanoparticles alleviate cerebral injury after intracerebral hemorrhage by transnasal brain targeting. Colloids Surf. B. 2024;237:113857. doi:10.1016/j.colsurfb.2024.113857
152. Li W, Qiu J, Li XL, et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Sci Adv. 2021;7(1):6889. doi:10.1126/sciadv.abd6889
153. Liu S, Liu J, Li H, et al. An optimized ionizable cationic lipid for brain tumor-targeted siRNA delivery and glioblastoma immunotherapy. Biomaterials. 2022;287:121645. doi:10.1016/j.biomaterials.2022.121645
154. Liu D, Cheng Y, Qiao S, et al. Nano-Codelivery of Temozolomide and siPD-L1 to Reprogram the Drug-Resistant and Immunosuppressive Microenvironment in Orthotopic Glioblastoma. ACS nano. 2022;16(5):7409–7427. doi:10.1021/acsnano.1c09794
155. Jiang T, Qiao Y, Ruan W, et al. Cation-Free siRNA Micelles as Effective Drug Delivery Platform and Potent RNAi Nanomedicines for Glioblastoma Therapy. Adv Mater. 2021;33(45):e2104779. doi:10.1002/adma.202104779
156. Zhu H, Wang YF, Wang ZG, Pang DW, Liu SL. Regulation of Protein Conformation Enables Cell-Selective Targeting of Virus-Mimicking Nanoparticles for siRNA Therapy of Glioblastoma. Adv Mater. 2024;36(29):e2401640. doi:10.1002/adma.202401640
157. Yang Y, Zhang X, Wu S, et al. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy. J Controll Release. 2022;342:66–80. doi:10.1016/j.jconrel.2021.12.034
158. Qiu C, Liang ST, Tu QC, et al. Enhanced tumor penetration across the blood-brain barrier: endoplasmic reticulum membrane hybrid siRNA nanoplexes. Mat Today Nano. 2024;25:100442. doi:10.1016/j.mtnano.2023.100442
159. Tang X, Wang Z, Xie Y, et al. Radiation-Triggered Selenium-Engineered Mesoporous Silica Nanocapsules for RNAi Therapy in Radiotherapy-Resistant Glioblastoma. ACS nano. 2023;17(4):4062–4076. doi:10.1021/acsnano.3c00269
160. Wang K, Kievit FM, Chiarelli PA, et al. siRNA nanoparticle suppresses drug-resistant gene and prolongs survival in an orthotopic glioblastoma xenograft mouse model. Adv Funct Mater. 2021;31(6):2007166. doi:10.1002/adfm.202007166
161. Tang L, Zhang R, Wang Y, et al. A simple self-assembly nanomicelle based on brain tumor-targeting peptide-mediated siRNA delivery for glioma immunotherapy via intranasal administration. Acta Biomater. 2023;155:521–537. doi:10.1016/j.actbio.2022.11.013
162. Guo Y, Lee H, Fang Z, et al. Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci Adv. 2021;7(18). doi:10.1126/sciadv.abf7390
163. Hu Y, Jiang K, Wang D, et al. Core-shell lipoplexes inducing active macropinocytosis promote intranasal delivery of c-Myc siRNA for treatment of glioblastoma. Acta Biomater. 2022;138:478–490. doi:10.1016/j.actbio.2021.10.042
164. Pandey S, Lee MC, Lim J, et al. SHMT1 siRNA-Loaded hyperosmotic nanochains for blood-brain/tumor barrier post-transmigration therapy. Biomaterials. 2022;281:121359. doi:10.1016/j.biomaterials.2021.121359
165. Ngamcherdtrakul W, Bejan DS, Cruz-Muñoz W, et al. Targeted Nanoparticle for Co-delivery of HER2 siRNA and a Taxane to Mirror the Standard Treatment of HER2+ Breast Cancer: efficacy in Breast Tumor and Brain Metastasis. Small. 2022;18(11):e2107550. doi:10.1002/smll.202107550
166. Lin CY, Perche F, Ikegami M, Uchida S, Kataoka K, Itaka K. Messenger RNA-based therapeutics for brain diseases: an animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J Controll Release. 2016;235:268–275. doi:10.1016/j.jconrel.2016.06.001
167. Baba M, Itaka K, Kondo K, Yamasoba T, Kataoka K. Treatment of neurological disorders by introducing mRNA in vivo using polyplex nanomicelles. J Controll Release. 2015;201:41–48. doi:10.1016/j.jconrel.2015.01.017
168. Gao M, Li Y, Ho W, et al. Targeted mRNA Nanoparticles Ameliorate Blood–Brain Barrier Disruption Postischemic Stroke by Modulating Microglia Polarization. ACS nano. 2024;18(4):3260–3275. doi:10.1021/acsnano.3c09817
169. Oh J, Kim SM, Lee EH, et al. Messenger RNA/polymeric carrier nanoparticles for delivery of heme oxygenase-1 gene in the post-ischemic brain. Biomater Sci. 2020;8(11):3063–3071. doi:10.1039/d0bm00076k
170. Marcos-Contreras OA, Greineder CF, Kiseleva RY, et al. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood-brain barrier. Proc Natl Acad Sci USA. 2020;117(7):3405–3414. doi:10.1073/pnas.1912012117
171. Peng H, Guo X, He J, et al. Intracranial delivery of synthetic mRNA to suppress glioblastoma. Mol ther Oncol. 2022;24:160–170. doi:10.1016/j.omto.2021.12.010
172. Liu Y, Zhang D, An Y, et al. Non-invasive PTEN mRNA brain delivery effectively mitigates growth of orthotopic glioblastoma. Nano Today. 2023;49:101790. doi:10.1016/j.nantod.2023.101790
173. Zhao P, Tian Y, Lu Y, et al. Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis. J Nanobiotechnol. 2022;20(1):525. doi:10.1186/s12951-022-01731-z
174. Gan L, Li Z, Lv Q, Huang W. Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int J Pharm. 2019;567:118449. doi:10.1016/j.ijpharm.2019.118449
175. Ouyang Q, Liu K, Zhu Q, et al. Brain-Penetration and Neuron-Targeting DNA Nanoflowers Co-Delivering miR-124 and Rutin for Synergistic Therapy of Alzheimer’s Disease. Small. 2022;18(14):e2107534. doi:10.1002/smll.202107534
176. Samaridou E, Walgrave H, Salta E, et al. Nose-to-brain delivery of enveloped RNA - cell permeating peptide nanocomplexes for the treatment of neurodegenerative diseases. Biomaterials. 2020;230:119657. doi:10.1016/j.biomaterials.2019.119657
177. Yang H, Han M, Li J, et al. Delivery of miRNAs through Metal-Organic Framework Nanoparticles for Assisting Neural Stem Cell Therapy for Ischemic Stroke. ACS nano. 2022;16(9):14503–14516. doi:10.1021/acsnano.2c04886
178. Liu C, Wen J, Li D, et al. Systemic delivery of microRNA for treatment of brain ischemia. Nano Res. 2021;14(9):3319–3328. doi:10.1007/s12274-021-3413-8
179. Shamaeizadeh N, Varshosaz J, Mirian M, Aliomrani M. Glutathione targeted tragacanthic acid-chitosan as a non-viral vector for brain delivery of miRNA-219a-5P: an in vitro/in vivo study. Int J Biol Macromol. 2022;200:543–556. doi:10.1016/j.ijbiomac.2022.01.100
180. Liu Y, Zheng M, Jiao M, et al. focused on mediated inhibition of miR-21 with enhanced miR-124 expression for combinatorial glioblastoma therapy. Biomaterials. 2021;276:121036. doi:10.1016/j.biomaterials.2021.121036
181. Zheng T, Wang W, Mohammadniaei M, et al. Anti-MicroRNA-21 Oligonucleotide Loaded Spermine-Modified Acetalated Dextran Nanoparticles for B1 Receptor-Targeted Gene Therapy and Antiangiogenesis Therapy. Adv Sci. 2022;9(5):e2103812. doi:10.1002/advs.202103812
182. Zhang C, Zhao X, Zhang J, et al. Dual-modified brain-targeting lipid polymer micelle delivery of anti-miRNA-21 for achieving oral treatment of glioma. J Drug Delivery Sci Technol. 2024;96:105721. doi:10.1016/j.jddst.2024.105721
183. Grafals-Ruiz N, Rios-Vicil CI, Lozada-Delgado EL, et al. Brain Targeted Gold Liposomes Improve RNAi Delivery for Glioblastoma. Int j Nanomed. 2020;15:2809–2828. doi:10.2147/ijn.S241055
184. Campani V, Zappavigna S, Scotti L, et al. Hybrid lipid self-assembling nanoparticles for brain delivery of microRNA. Int J Pharm. 2020;588:119693. doi:10.1016/j.ijpharm.2020.119693
185. Li W, Peng X, Mei X, Dong M, Li Y, Dong H. Multifunctional DNA Tetrahedron for Alzheimer’s Disease Mitochondria-Targeted Therapy by MicroRNA Regulation. ACS Appl Mater Interfaces. 2023;15(19):22977–22984. doi:10.1021/acsami.3c03181
186. Ma F, Yang L, Sun Z, et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci Adv. 2020;6(30):eabb4429. doi:10.1126/sciadv.abb4429
187. Israel LL, Sun T, Braubach O, et al. β-Amyloid targeting nanodrug for neuron-specific delivery of nucleic acids in Alzheimer’s disease mouse models. J Controll Release. 2023;361:636–658. doi:10.1016/j.jconrel.2023.08.001
188. Sun Y, Kong J, Ge X, Mao M, Yu H, Wang Y. An Antisense Oligonucleotide-Loaded Blood-Brain Barrier Penetrable Nanoparticle Mediating Recruitment of Endogenous Neural Stem Cells for the Treatment of Parkinson’s Disease. ACS nano. 2023;17(5):4414–4432. doi:10.1021/acsnano.2c09752
189. Mendonça MCP, Sun Y, Cronin MF, Lindsay AJ, Cryan JF, O’Driscoll CM. Cyclodextrin-Based Nanoparticles for Delivery of Antisense Oligonucleotides Targeting Huntingtin. Pharmaceutics. 2023;15(2). doi:10.3390/pharmaceutics15020520
190. Caron NS, Aly AE, Findlay Black H, et al. Systemic delivery of mutant huntingtin lowering antisense oligonucleotides to the brain using apolipoprotein A-I nanodisks for Huntington disease. J Controll Release. 2024;367:27–44. doi:10.1016/j.jconrel.2024.01.011
191. Yu W, Xuan C, Liu B, et al. Carrier-free programmed spherical nucleic acid for effective ischemic stroke therapy via self-delivery antisense oligonucleotide. Nano Res. 2023;16(1):735–745. doi:10.1007/s12274-022-4402-7
192. Dastpeyman M, Sharifi R, Amin A, et al. Endosomal escape cell-penetrating peptides significantly enhance pharmacological effectiveness and CNS activity of systemically administered antisense oligonucleotides. Int J Pharm. 2021;599:120398. doi:10.1016/j.ijpharm.2021.120398
193. Lee Y, Ha J, Kim M, Kang S, Kang M, Lee M. Antisense-oligonucleotide co-micelles with tumor targeting peptides elicit therapeutic effects by inhibiting microRNA-21 in the glioblastoma animal models. J Adv Res. 2023;53:249–260. doi:10.1016/j.jare.2023.01.005
194. Kim KR, Kang JH, Thai HBD, et al. Systemic Brain Delivery of Oligonucleotide Therapeutics Enhanced by Protein Corona-Assisted DNA Cubes. Small Methods. 2024:e2400902. doi:10.1002/smtd.202400902
195. Calero M, Moleiro LH, Sayd A, et al. Lipid nanoparticles for antisense oligonucleotide gene interference into brain border-associated macrophages. Front Mol Biosci. 2022;9:887678. doi:10.3389/fmolb.2022.887678
196. Wang Y, Wang X, Xie R, Burger JC, Tong Y, Gong S. Overcoming the Blood-Brain Barrier for Gene Therapy via Systemic Administration of GSH-Responsive Silica Nanocapsules. Adv Mater. 2023;35(6):e2208018. doi:10.1002/adma.202208018
197. Yang J, Qin G, Liu Z, et al. A Nanozyme-Boosted MOF-CRISPR Platform for Treatment of Alzheimer’s Disease. Nano Lett. 2024;24(32):9906–9915. doi:10.1021/acs.nanolett.4c02272
198. Park H, Oh J, Shim G, et al. In vivo neuronal gene editing via CRISPR-Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer’s disease. Nat Neurosci. 2019;22(4):524–528. doi:10.1038/s41593-019-0352-0
199. Park H, Hwang Y, Kim J. Transcriptional activation with Cas9 activator nanocomplexes rescues Alzheimer’s disease pathology. Biomaterials. 2021;279:121229. doi:10.1016/j.biomaterials.2021.121229
200. Lee B, Lee K, Panda S, et al. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat Biomed Eng. 2018;2(7):497–507. doi:10.1038/s41551-018-0252-8
201. Palanki R, Bose SK, Dave A, et al. Ionizable Lipid Nanoparticles for Therapeutic Base Editing of Congenital Brain Disease. ACS nano. 2023;17(14):13594–13610. doi:10.1021/acsnano.3c02268
202. Zou Y, Sun X, Yang Q, et al. Blood-brain barrier-penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci Adv. 2022;8(16):eabm8011. doi:10.1126/sciadv.abm8011
203. Ruan W, Jiao M, Xu S, et al. Brain-targeted CRISPR/Cas9 nanomedicine for effective glioblastoma therapy. J Controll Release. 2022;351:739–751. doi:10.1016/j.jconrel.2022.09.046
204. Ruan W, Xu S, An Y, et al. Brain-Targeted Cas12a Ribonucleoprotein Nanocapsules Enable Synergetic Gene Co-Editing Leading to Potent Inhibition of Orthotopic Glioblastoma. Adv Sci. 2024;11(33):e2402178. doi:10.1002/advs.202402178
205. Rui Y, Wilson DR, Choi J, et al. Carboxylated branched poly(β-amino ester) nanoparticles enable robust cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci Adv. 2019;5(12):eaay3255. doi:10.1126/sciadv.aay3255
206. Kantor A, McClements ME, MacLaren RE. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int J Mol Sci. 2020;21(17). doi:10.3390/ijms21176240
207. Martínez-Puente DH, Pérez-Trujillo JJ, Zavala-Flores LM, et al. Plasmid DNA for Therapeutic Applications in Cancer. Pharmaceutics. 2022;14(9):1861. doi:10.3390/pharmaceutics14091861
208. Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nature Biotechnol. 2004;22(3):326–330. doi:10.1038/nbt936
209. Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nature Mater. 2013;12(11):967–977. doi:10.1038/nmat3765
210. Cui Y, Qi Y, Ding L, et al. miRNA dosage control in development and human disease. Trends Cell Biol. 2023. doi:10.1016/j.tcb.2023.05.009
211. Asl ER, Amini M, Najafi S, et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: a crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021;278:119499. doi:10.1016/j.lfs.2021.119499
212. Masaki Y, Tabira A, Hattori S, Wakatsuki S, Seio K. Insertion of a methylene group into the backbone of an antisense oligonucleotide reveals the importance of deoxyribose recognition by RNase H. Org Biomol Chem. 2022;20(45):8917–8924. doi:10.1039/d2ob01667b
213. Xiao-Jie L, Hui-Ying X, Zun-Ping K, Jin-Lian C, Li-Juan J. CRISPR-Cas9: a new and promising player in gene therapy. J Med Genet. 2015;52(5):289–296. doi:10.1136/jmedgenet-2014-102968
214. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi:10.1126/science.1225829
215. Sun A. Lysosomal storage disease overview. Ann translat Med. 2018;6(24):476. doi:10.21037/atm.2018.11.39
216. González-Davis O, Villagrana-Escareño MV, Trujillo MA, Gama P, Chauhan K, Vazquez-Duhalt R. Virus-like nanoparticles as enzyme carriers for Enzyme Replacement Therapy (ERT). Virology. 2023;580:73–87. doi:10.1016/j.virol.2023.01.017
217. Leal AF, Nieto WG, Candelo E, Pachajoa H, Alméciga-Díaz CJ. Hematological Findings in Lysosomal Storage Disorders: a Perspective from the Medical Laboratory. Ejifcc. 2022;33(1):28–42.
218. Marchetti M, Faggiano S, Mozzarelli A. Enzyme Replacement Therapy for Genetic Disorders Associated with Enzyme Deficiency. Curr. Med. Chem. 2022;29(3):489–525. doi:10.2174/0929867328666210526144654
219. Desnick RJ, Schuchman EH. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Ann Rev Genomics Hum Genet. 2012;13:307–335. doi:10.1146/annurev-genom-090711-163739
220. Kelly JM, Bradbury A, Martin DR, Byrne ME. Emerging therapies for neuropathic lysosomal storage disorders. Prog neurobiol. 2017;152:166–180. doi:10.1016/j.pneurobio.2016.10.002
221. Paruchuri BC, Smith S, Larsen J. Enzyme-responsive polymersomes ameliorate autophagic failure in a cellular model of GM1 gangliosidosis. Front Chem Eng. 2022;4. doi:10.3389/fceng.2022.997607
222. Kelly JM, Gross AL, Martin DR, Byrne ME. Polyethylene glycol-b-poly(lactic acid) polymersomes as vehicles for enzyme replacement therapy. Nanomedicine. 2017;12(23):2591–2606. doi:10.2217/nnm-2017-0221
223. Goldsmith M, Abramovitz L, Braunstein H, Horowitz M, Peer D. Quantitative analysis of recombinant glucocerebrosidase brain delivery via lipid nanoparticles. Nano Futures. 2018;2(4):045003. doi:10.1088/2399-1984/aadd34
224. Solomon M, Loeck M, Silva-Abreu M, et al. Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases. J Controll Release. 2022;349:1031–1044. doi:10.1016/j.jconrel.2022.07.022
225. Papademetriou I, Tsinas Z, Hsu J, Muro S. Combination-targeting to multiple endothelial cell adhesion molecules modulates binding, endocytosis, and in vivo biodistribution of drug nanocarriers and their therapeutic cargoes. J Controll Release. 2014;188:87–98. doi:10.1016/j.jconrel.2014.06.008
226. Muntimadugu E, Silva-Abreu M, Vives G, et al. Comparison between Nanoparticle Encapsulation and Surface Loading for Lysosomal Enzyme Replacement Therapy. Int J Mol Sci. 2022;23(7):4034. doi:10.3390/ijms23074034
227. Del Grosso A, Galliani M, Angella L, et al. Brain-targeted enzyme-loaded nanoparticles: a breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease. Sci Adv. 2019;5(11):eaax7462. doi:10.1126/sciadv.aax7462
228. Santi M, Finamore F, Cecchettini A, et al. Protein Delivery by Peptide-Based Stealth Liposomes: a Biomolecular Insight into Enzyme Replacement Therapy. Mol Pharmaceut. 2020;17(12):4510–4521. doi:10.1021/acs.molpharmaceut.0c00615
229. Mayer FQ, Adorne MD, Bender EA, et al. Laronidase-functionalized multiple-wall lipid-core nanocapsules: promising formulation for a more effective treatment of mucopolysaccharidosis type I. Pharm Res. 2015;32(3):941–954. doi:10.1007/s11095-014-1508-y
230. Rigon L, Salvalaio M, Pederzoli F, et al. Targeting Brain Disease in MPSII: preclinical Evaluation of IDS-Loaded PLGA Nanoparticles. Int J Mol Sci. 2019;20(8):2014. doi:10.3390/ijms20082014
231. Sipione S, Monyror J, Galleguillos D, Steinberg N, Kadam V. Gangliosides in the Brain: physiology, Pathophysiology and Therapeutic Applications. Front Neurosci. 2020;14:572965. doi:10.3389/fnins.2020.572965
232. Lecarie E. Gangliosidoses. In: Volkmar FR, editor. Encyclopedia of Autism Spectrum Disorders. Springer International Publishing; 2021:2155–2157.
233. Rha AK, Maguire AS, Martin DR. GM1 Gangliosidosis: mechanisms and Management. Appli clin genet. 2021;14:209–233. doi:10.2147/tacg.S206076
234. Leal AF, Benincore-Flórez E, Solano-Galarza D, et al. GM2 Gangliosidoses: clinical Features, Pathophysiological Aspects, and Current Therapies. Int J Mol Sci. 2020;21(17). doi:10.3390/ijms21176213
235. Flotte TR, Cataltepe O, Puri A, et al. AAV gene therapy for Tay-Sachs disease. Nature Med. 2022;28(2):251–259. doi:10.1038/s41591-021-01664-4
236. Prabu SL, Sara Josen T, Umamaheswari A, Puratchikody A. Chapter 24 - Sandhoff disease: pathology and advanced treatment strategies. In: Dureja H, Murthy SN, Wich PR, Dua K, editors. Drug Delivery Systems for Metabolic Disorders. Academic Press; 2022:351–358.
237. Abed Rabbo M, Khodour Y, Kaguni LS, Stiban J. Sphingolipid lysosomal storage diseases: from bench to bedside. Lipids Health Dis. 2021;20(1):44. doi:10.1186/s12944-021-01466-0
238. Chauhan K, Olivares-Medina CN, Villagrana-Escareño MV, et al. Targeted Enzymatic VLP-Nanoreactors with β-Glucocerebrosidase Activity as Potential Enzyme Replacement Therapy for Gaucher’s Disease. ChemMedChem. 2022;17(19):e202200384. doi:10.1002/cmdc.202200384
239. Geberhiwot T, Wasserstein M, Wanninayake S, et al. Consensus clinical management guidelines for acid sphingomyelinase deficiency (Niemann-Pick disease types A, B and A/B). Orphanet J Rare Diseases. 2023;18(1):85. doi:10.1186/s13023-023-02686-6
240. van der Knaap MS, Schiffmann R, Mochel F, Wolf NI. Diagnosis, prognosis, and treatment of leukodystrophies. Lancet Neurol. 2019;18(10):962–972. doi:10.1016/s1474-4422(19)30143-7
241. Maegawa GHB. Lysosomal Leukodystrophies Lysosomal Storage Diseases Associated With White Matter Abnormalities. J Child Neurol. 2019;34(6):339–358. doi:10.1177/0883073819828587
242. Modesti NB, Evans SH, Jaffe N, Vanderver A, Gavazzi F. Early recognition of patients with leukodystrophies. Curr Probl Pediatr Adolesc Health Care. 2022;52(12):101311. doi:10.1016/j.cppeds.2022.101311
243. Weinstock NI, Kreher C, Favret J, et al. Brainstem development requires galactosylceramidase and is critical for pathogenesis in a model of Krabbe disease. Nat Commun. 2020;11(1):5356. doi:10.1038/s41467-020-19179-w
244. Mole SE, Anderson G, Band HA, et al. Clinical challenges and future therapeutic approaches for neuronal ceroid lipofuscinosis. Lancet Neurol. 2019;18(1):107–116. doi:10.1016/s1474-4422(18)30368-5
245. Kose M, Kose E, Ünalp A, et al. Neuronal ceroid lipofuscinosis: genetic and phenotypic spectrum of 14 patients from Turkey. Neurol Sci. 2021;42(3):1103–1111. doi:10.1007/s10072-021-05067-8
246. Specchio N, Ferretti A, Trivisano M, et al. Neuronal Ceroid Lipofuscinosis: potential for Targeted Therapy. Drugs. 2021;81(1):101–123. doi:10.1007/s40265-020-01440-7
247. Kamate M, Reddy N, Detroja M, Hattiholi V. Neuronal Ceroid Lipofuscinoses in Children. Ann Indian Acad Neurol. 2021;24(2):192–197. doi:10.4103/aian.AIAN_61_20
248. Penon-Portmann M, Blair DR, Harmatz P. Current and new therapies for mucopolysaccharidoses. Pediatr Neonatol. 2023;64 Suppl 1:S10–s17. doi:10.1016/j.pedneo.2022.10.001
249. Saville JT, McDermott BK, Fletcher JM, Fuller M. Disease and subtype specific signatures enable precise diagnosis of the mucopolysaccharidoses. Genet med. 2019;21(3):753–757. doi:10.1038/s41436-018-0136-z
250. Kubaski F, de Oliveira Poswar F, Michelin-Tirelli K, et al. Mucopolysaccharidosis Type I. Diagnostics. 2020;10(3):161. doi:10.3390/diagnostics10030161
251. Okuyama T, Eto Y, Sakai N, et al. Iduronate-2-Sulfatase with Anti-human Transferrin Receptor Antibody for Neuropathic Mucopolysaccharidosis II: a Phase 1/2 Trial. Mol Ther. 2019;27(2):456–464. doi:10.1016/j.ymthe.2018.12.005
252. Gustavsson S, Ohlin Sjöström E, Tjernberg A, et al. Intravenous delivery of a chemically modified sulfamidase efficiently reduces heparan sulfate storage and brain pathology in mucopolysaccharidosis IIIA mice. Mol Gene Metabol Rep. 2019;21:100510. doi:10.1016/j.ymgmr.2019.100510
253. Gónzalez-Meneses A, Pineda M, Bandeira A, et al. Description of the molecular and clinical characteristics of the mucopolysaccharidosis type VII Iberian cohort. Orphanet J Rare Diseases. 2021;16(1):445. doi:10.1186/s13023-021-02063-1
254. McBride KL, Flanigan KM. Update in the Mucopolysaccharidoses. Seminar Pediatr Neurol. 2021;37:100874. doi:10.1016/j.spen.2021.100874
255. Muenzer J, Wraith JE, Beck M, et al. A Phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet med. 2006;8(8):465–473. doi:10.1097/01.gim.0000232477.37660.fb
256. Suzuki T. Catabolism of N-glycoproteins in mammalian cells: molecular mechanisms and genetic disorders related to the processes. Mol Aspect Med. 2016;51:89–103. doi:10.1016/j.mam.2016.05.004
257. Malm D, Stensland HMFR, Nilssen Ø. Glycoproteinoses. Lysosomal Storage Disorder. 2022;2022:203–210.
258. Michalski JC, Klein A. Glycoprotein lysosomal storage disorders: alpha- and beta-mannosidosis, fucosidosis and alpha-N-acetylgalactosaminidase deficiency. BBA. 1999;1455(2–3):69–84. doi:10.1016/s0925-4439(99)00077-0
259. Gao J, Xia Z, Gunasekar S, Jiang C, Karp JM, Joshi N. Precision drug delivery to the central nervous system using engineered nanoparticles. Nature Rev Mater. 2024;9(8):567–588. doi:10.1038/s41578-024-00695-w
260. Liu X, Meng H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. VIEW. 2021;2(5):20200190. doi:10.1002/VIW.20200190
261. Nikzamir M, Akbarzadeh A, Panahi Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J Drug Delivery Sci Technol. 2021;61:102316. doi:10.1016/j.jddst.2020.102316
262. Li H, Guan M, Zhang NN, et al. Harnessing nanomedicine for modulating microglial states in the central nervous system disorders: challenges and opportunities. Biomed Pharmacothe. 2024;177:117011. doi:10.1016/j.biopha.2024.117011
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

