Back to Journals » Journal of Hepatocellular Carcinoma » Volume 12
Assessment of Tumor Burden Score as a Feasible and Reliable Tool for Prognosis Prediction for Hepatocellular Carcinoma Undergoing Hepatectomy: A Multicenter, Retrospective Study
Authors Guan R , Zheng Z , Deng M, Mei J , Lin Y
Received 27 July 2024
Accepted for publication 25 January 2025
Published 10 February 2025 Volume 2025:12 Pages 247—260
DOI https://doi.org/10.2147/JHC.S488927
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Renguo Guan,1– 4,* Zehao Zheng,2– 4,* Min Deng,5,* Jie Mei,2– 4 Ye Lin1
1Department of General Surgery, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, People’s Republic of China; 2Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 3State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China; 4Guangdong Provincial Clinical Research Center for Cancer, Guangzhou, People’s Republic of China; 5Department of General Surgery, The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ye Lin, Email [email protected]; Jie Mei, Email [email protected]
Background: Maximum diameter and number are the main parameters of tumor burden in hepatocellular carcinoma (HCC). Tumor burden score (TBS) shows its distinguished ability to stratify patients with HCC undergoing transcatheter arterial chemoembolization (TACE). However, the prognostic accuracy of TBS in HCC undergoing liver resection and its association with the BCLC stage has not been well evaluated.
Methods: A total of 3044 treatment-naïve HCC patients from six independent medical centers undergoing liver resection were retrospectively analyzed. Survival analyses were conducted by plotting Kaplan–Meier curves and the Log rank test. We further investigated whether the tumor burden score was a feasible subclassification criterion across the BCLC stage. Then, we also used TBS to identify HCC patients beyond BCLC criteria who could benefit most from surgical resection. Finally, univariate and multivariate cox analysis was used to determine independent prognostic predictors.
Results: About 44.2% (n=1343) of patients had low TBS, 38.8% (n=1182) had intermediate TBS and 17% (n=519) had high TBS. Overall survival (OS) and recurrence-free survival deteriorated incrementally with increasing TBS (P< 0.0001). Subgroup analysis indicated that there was a significant survival difference among the three TBS groups across the BCLC stage (P< 0.0001). Low TBS group of patients beyond BCLC criteria reported acceptable outcomes compared to intermediate TBS group patients within BCLC criteria, even better than high TBS group (5-year OS: 64.3%, 69.8%, and 56.3%). Finally, low TBS was identified as an independent protective prognostic factor.
Conclusion: Tumor burden score is a feasible and reliable prognostic tool for prognosis prediction and clinical decisions.
Keywords: hepatocellular carcinoma, tumor burden score, BCLC stage, prognosis prediction
Introduction
Hepatocellular carcinoma (HCC) stands out as one of the most aggressive and prevalent malignancies within the gastrointestinal tract, posing significant challenges in both treatment and prognosis due to its complex etiology and often late-stage presentation. It is estimated that 367,700 new cases were diagnosed with HCC and 316,500 cases died from it in China in 2022.1 Surgical interventions, comprising hepatic resection and liver transplantation, continue to be the mainstay of curative treatment for hepatocellular carcinoma (HCC), offering the best chance for long-term survival and disease-free intervals, particularly in patients with early-stage or well-compensated liver function. Unfortunately, a high rate of tumor recurrence leads to a poor 5-year overall survival.2 Thus, it is important to develop a model for predicting prognosis and stratifying optimal candidates for surgical procedures.
Barcelona Clinic Liver Cancer (BCLC) staging system is widely applied for prognosis prediction and treatment recommendation. BCLC staging systems impose strict restrictions on the number and diameter of tumors. This not only limits statistical power but can also lead to an inaccurate causal relationship, thus weakening its ability to predict prognosis.3 Even patients in the same grade may have different prognoses. For example, the 5-year overall survival of a single large HCC (single nodule > 5 cm) fell in between BCLC-A and BCLC-B.4 Wang et al found that the 5-year survival rate of HCC patients with a single tumor smaller than 7 cm is more than twice that of those >7 cm (70% vs 30%).5 Consequently, the current BCLC staging system, while valuable, has limitations in its predictive accuracy for prognosis. It necessitates a refinement or an update to enhance its efficacy in predicting patient outcomes more accurately.
The 2022 BCLC version recommends surgical procedures for HCC patients at an early stage rather than HCC patients at an intermediate and advanced stage, except for the first subgroup within BCLC-B.6 However, there is some heterogeneity in patients with intermediate and advanced stages in real-world clinical practice. Recently, increasing studies have demonstrated that surgical resection may report an acceptable outcome for HCC patients beyond BCLC criteria.7–9 In addition, the China Liver Cancer (CNLC) staging system recommends that some HCC patients with CNLC-II or III stage may still benefit from surgical resection,10 but there is no consensus defining these highly selective HCC patients.11,12 Thus, we need to identify a reliable tool for the selection of patients beyond BCLC criteria for surgical resection.
Tumor burden score (TBS) was first proposed to stratify the tumor burden of colorectal cancer with liver metastasis undergoing liver resection.13 Recently, TBS has shown its distinguished ability to stratify patients with HCC undergoing transcatheter arterial chemoembolization (TACE) and liver transplantation.14,15 However, the prognostic value of TBS in HCC patients who undergo liver resection, as well as its correlation with the BCLC staging system, remains to be thoroughly investigated.
In this study, we aimed to investigate the performance of TBS in predicting the prognosis of HCC undergoing liver resection. Furthermore, our primary research tried to assess whether TBS could be applied as a feasible subclassification criterion for stratifying HCC patients in the BCLC staging system and to identify patients beyond BCLC criteria who are likely to benefit most from resection.
Methods
All procedures followed the ethical standards of the responsible committees on human experimentation (institutional and national) and the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The Institutional Review Board of the Ethics Committee of Guangdong Provincial People’s Hospital approved this study. The study was retrospectively registered at ResearchRegistry.com, and this cohort study complied with the STROCCS statement.16
Patients
This multi-institutional retrospective study collected HCC patients undergoing hepatectomy from 2009 to 2016. HCC patients were from six independent medical centers: Guangdong Provincial People’s Hospital, Sun Yat-Sen University Cancer Center, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Fudan University Shanghai Cancer Center, Affiliated Cancer Hospital and Institute of Guangzhou Medical University, and Dongguan People’s Hospital. Patients were included in this retrospective study meeting the following inclusion criteria: (I) HCC confirmed by pathological diagnosis; (II) A negative surgical margin; (III) Child-Pugh grade A or B; and (IV) Eastern Cooperative Oncology Group performance status less than 2. The following were the exclusion criteria: (I) Patients who were not treatment-naive; (II) Undergoing preoperative adjuvant therapy; (III) Additional malignant cancers; (IV) Undergoing liver transplant after recurrence and (V) Incomplete clinical data. Ultimately, 3044 hCC patients were enrolled in the present analysis.
Treatment
According to the China liver cancer (CNLC) staging system, all patients received normative liver cancer resection. The chief surgeons are all experienced surgeons. Early effective protection liver function and reasonable nutrition support were supplied for all patients. All patients generally underwent scheduled enhanced computerized tomography (CT), Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) assessments, tumor markers and liver function every 2–3 months. All the HBV-HCC patients received anti-viral therapy (like Entecavir or Tenofovir) after surgical resection. Treatment after recurrence depended on the clinical stage, including reoperation, radiofrequency ablation, TACE, and comprehensive treatment.
Variables, Definitions, and Outcomes
Clinical variables retrieved from medical records and imageological examination mainly included age, sexuality, HBV infection, α-fetoprotein (AFP), white blood cell count (WBC), blood platelet count (PLT), alanine aminotransferase (ALT), Child-Pugh grade, tumor number, maximum tumor diameter, portal vein tumor thrombus, differentiation grade and microvascular invasion (MVI). The maximum tumor diameter and tumor number were calculated by preoperative CT and MR. TBS is derived from the following formula:
We utilized X-tile to determine the cut-off values of TBS, and all included patients were sequentially divided into three groups: high TBS (>9.2), intermediate TBS (4.7–9.2), and low TBS (<4.7). The statistical analysis of the cut-off values of TBS was listed in Supplementary Table 1. The foremost outcomes of interest in our retrospective study were overall survival (OS) and (RFS).
Statistical Analysis
Continuous variables with normal distribution were expressed as mean and standard deviations. Variables with abnormal distribution were expressed as median and interquartile ranges (IQR). Categorical variables were analyzed by using Pearson’s chi-square test or Fisher’s exact test. Survival analyses were conducted by plotting Kaplan–Meier curves and the Log rank test. Univariate and multivariate Cox regression analysis were used to identify independent prognostic factors. Then, the predictive ability of TBS grade was evaluated by the time-dependent receiver operating characteristic curve (time-dependent ROC). A calibration curve was plotted to evaluate the consistency between the observed rates and predicted diagnosis. Decision curve analysis (DCA) was also plotted to quantify the net benefits along with the increase in threshold probabilities. All statistical analyses were performed using MedCalc software (version 22), SPSS software (version 26.0) and R software (version 4.2.0). P-value <0.05 was considered statistically significant (two-sided).
Results
Baseline Characteristics
The total population was 56 years old (IQR: 47–65), almost all people were Child-Pugh class A (n=2968, 97.5%), and male (n=2676, 87.9%) and chronically infected with the hepatitis B virus (n=2475, 81.3%). The majority of patients were ALBI grade A (n=2178, 71.6%). About 44.1% (n=1343) of patients had low TBS, 38.8% (n=1182) had intermediate TBS and 17.1% (n=519) had high TBS (Figure 1A). The baseline characteristics of all patients are listed in Table 1.
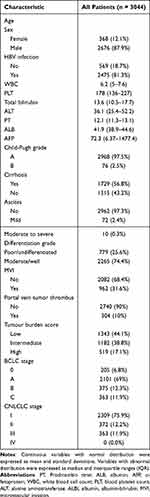 |
Table 1 Baseline Characteristics of Patients with HCC Undergoing Liver Resection |
Distribution of Tumor Burden Score
All of HCC patients with BCLC-0 stage had a low TBS (n=205). Nearly half of patients BCLC-A stage had low TBS (n=1003), 39.0% (n=819) had intermediate TBS and 13.3% (n=279) had high TBS. The percentage of low TBS, intermediate TBS, and high TBS in patients with BCLC-B stage were 20.5, 54.4, and 25.1%, respectively. As for patients with BCLC-C stage, 15.9% had low TBS (n=58), 43.8% (n=159) had intermediate TBS and 40.3% (n=146) had high TBS (Figure 1B). The association between BCLC stages and clinical variables is provided in Supplementary Table 2.
Tumor Burden Score Stratifies Prognosis of HCC Patients Undergoing Resection
The median overall survival was 51.6 months (IQR: 19.6–75.0 months). The 1-, 3-, and 5-year overall survival rates of the entire cohort were 88.9%, 72.9%, and 66.1%, respectively. Further subgroup analysis indicated that the 5-year overall survival rates of the low TBS group, intermediate TBS group, and high TBS group were 79.3%, 60.8%, and 42.3%, respectively, and there was a significant survival difference among different TBS groups (Figure 2A, P<0.0001). As Figure 2B shows, BCLC stage (87.2% vs 72.5% vs 48.1% vs 32.5%, P<0.0001) could also stratify prognosis of HCC patients undergoing resection.
The median recurrence-free survival was 25.9 months (IQR: 8.3–62.7 months). The 1-, 3-, and 5-year recurrence-free survival rates of the entire cohort were 68.9%, 52.1%, and 44.0%, respectively. The 5-year recurrence-free survival rates of different TBS groups were significantly different (Figure 2C, 56.7% vs 38.9% vs 21.6%, log-rank P<0.0001). As Figure 2D shows, similar results of survival analysis were also found based on the BCLC stage (66.2% vs 50.1% vs 23.4% vs 16.0%, P<0.0001).
Considering the inconsistency in the causes of death between the cirrhotic and non-cirrhotic subgroups, we therefore performed subgroup analysis, and as shown in Supplementary Figure 1, TBS also well-delineated stratifies prognosis of HCC patients undergoing resection in the cirrhotic versus non-cirrhotic subgroups. In order to exclude the effect between different hospitals, we divided the patients into internal and external cohorts based on their origin and further sub-grouped them for analysis, as shown in Supplementary Figure 2, where TBS also delineated the prognosis of HCC patients well, both in the internal cohort and in the external cohort subgroups.
Tumor Burden Score as a Feasible Subclassification Criterion Across BCLC Stage
We further investigated whether the tumor burden score was a feasible subclassification criterion across the BCLC stage. Of note, there was a significant overall survival difference between the three TBS score groups across the BCLC stage (P<0.0001, Figure 3A–C). When the survival was stratified by BCLC stage, the 5-year survival rates were 79.6%, 69.2%, and 55.2%, respectively, for low TBS, intermediate TBS, and high TBS (P<0.0001) in patients with BCLC-A stage. High TBS was associated with poor overall survival in comparison with intermediate and low TBS in patients, with BCLC-B stage P<0.0001). Surprisingly, overall survival deteriorated incrementally with increasing TBS in patients with the BCLC-C stage (P<0.001).
There was a significant recurrence-free survival difference between the three TBS score groups across the BCLC stage (P<0.0001). There was a close relationship between increasing TBS and short recurrence-free survival across the BCLC stage. The 5-year recurrence-free survival rates would decrease progressively gradually for low TBS, intermediate TBS, and high TBS in patients with BCLC-A stage (P<0.0001, Figure 3D), BCLC-B stage (Figure 3E), and BCLC-C stage (P<0.0001, Figure 3F). Collectively, TBS could stratify the RFS of HCC patients across different stages of the BCLC stage.
Tumor Burden Score Identifies Patients for Resection Beyond BCLC Criteria
There is no consensus defining the HCC patients beyond BCLC criteria who could benefit most from surgical resection. Thus, we combined TBS within BCLC criteria, which was sequentially divided into six subgroups. The 5-year OS of six subgroups was 81.0%, 69.0%, 55.2%, 62.0%, 41.5%, and 26.2%, respectively (Figure 4A, P<0.0001). The remarkable thing was that low TBS group of patients beyond BCLC criteria reported acceptable outcomes compared to intermediate TBS group patients within BCLC criteria, even better than the high TBS group (6-year OS: 59.7%, 66.3%, and 50.4%).
On the whole, the 5-year RFS of the six subgroups showed a gradual downward trend (Figure 4B, P<0.0001). Consistent with the results of OS, the RFS of high TBS group within BCLC criteria and low TBS group beyond BCLC criteria were evenly matched (6-year RFS: 29.8 vs 30.4%, P=0.19). However, intermediate TBS group within BCLC criteria significantly extended the RFS, compared to low TBS group beyond BCLC criteria (6-year RFS: 42.7 vs 29.8%, P<0.0001). We also performed subgroup analyses based on cirrhosis and different hospital cohorts, and the results similarly corroborated these findings (Supplementary Figures 3 and 4). Collectively, tumor burden score could identify patients for resection beyond BCLC criteria.
Tumor Burden Score as an Independent Prognostic Factor
We further utilized univariate Cox and multivariate Cox analysis to identify independent prognostic factors. The results of the multivariate Cox revealed that intermediate TBS (HR: 1.91, 95% CI: 1.65–2.22, P<0.001), high TBS (HR: 2.90, 95% CI: 2.42–3.46, P<0.001), were independent prognostic factors for OS of HCC patients undergoing resection (Table 2). In a subgroup analysis of stages of the BCLC stage, we also found that both intermediate TBS and high TBS were independently associated with poor OS (P<0.05, Supplementary Table 3).
 |
Table 2 Univariate Cox Analysis and Multivariable Cox Regression Analysis of Predictive Factors for Overall Survival in the Entire Cohort |
As for RFS, the multivariate Cox analysis suggested that intermediate TBS (HR: 1.60, 95% CI: 1.44–1.79, P< 0.001) and high TBS (HR: 2.56, 95% CI: 2.23–2.93, P<0.001) were independent prognostic factors for OS of HCC patients undergoing resection (Table 3). Consistent with the results of OS, subgroup analysis confirmed that both intermediate TBS and high TBS were independent prognostic factors for RFS of HCC patients across BCLC stages (P<0.05, Supplementary Table 4).
 |
Table 3 Univariate Cox Analysis and Multivariable Cox Regression Analysis of Predictive Factors for Recurrence-Free Survival in the Entire Cohort |
Prognostic Accuracy of Tumor Burden Score
First, the AUC of TBS grade for 1, 3, and 5-year OS was 0.701, 0.688, and 0.656, respectively (Figure 5A). As for RFS, the AUC of TBS grade for 1, 3, and 5-year was 0.681, 0.654, and 0.635, respectively (Figure 5B). Next, the decision curve for OS lied above the None and All lines in the 20–40% threshold range (Figure 5C). As for RFS, based on this decision curve, when the threshold of the model was set in the range of 40%–60%, the decision curve was above the None line and the All line (Figure 5D). Ultimately, the calibration curves for 3-year and 5-year OS and RFS probabilities were also plotted and indicated that the nomogram-predicted survival closely corresponded with actual survival outcomes (Figure 6A–F). The above results demonstrated the clinical utility of TBS, which might provide standardized net clinical benefits.
Discussion
In our study, we utilized the data of 3025 hCC patients to confirm that tumor burden score could stratify the prognosis of HCC patients undergoing resection. Further subgroup analysis has shown that tumor burden score could be regarded as a feasible subclassification criterion across the BCLC stage. Moreover, we could use the tumor burden score to identify specific subgroups beyond BCLC criteria which could benefit most from liver resection.
TBS is composed of maximum tumor diameter and tumor number, which are easily obtained from preoperative imaging examination. The TBS score was initially used to predict the prognosis of patients with colorectal cancer with liver metastases.13 A large number of previous studies have demonstrated that the tumor burden score could well distinguish the prognosis of patients treated with TACE.15,17,18 At the same time, Li et al found that the TBS score could also be used to predict the prognosis of patients, with intrahepatic cholangiocarcinoma undergoing liver resection.19 Consistent with the result of a previous study,20 we also found that it could well stratify the prognosis of HCC patients after hepatic resection in our study. There was a negative correlation between TBS and prognosis. The 5-year overall survival rates of the low TBS group, intermediate TBS group, and high TBS group were 79.7%, 61.2%, and 42.7%, respectively. Similar results were also found in the 5-year recurrence-free survival. The highlight of our study was that our study expanded the scope of included patients and further included patients with portal vein tumor thrombus or extrahepatic metastases. Moreover, further multivariate analysis showed that intermediate TBS and high TBS were independent risk factors for HCC patients after hepatic resection. At the same time, previous studies have confirmed that AJCC stage, pathological differentiation grade, metastasis stage, HBV and MVI could predict the prognosis of HCC.21,22 Compared with these factors mentioned above, TBS had better predictive capabilities according to the ROC.
In our study, HCC patients with BCLC-0 grade and BCLC-A were treated with surgical operation rather than radiofrequency ablation. For such patients, radiofrequency ablation was less effective than hepatectomy.23–25 Hepatectomy is also the preferred treatment for patients with liver cancer with a diameter over 3 cm. In contrast to small HCC, large HCCs exhibit a more aggressive phenotype. This heightened malignancy may be attributed to several factors: the irregular morphology of large HCCs, their abundant blood supply, and a propensity for invasion into the surrounding vasculature, leading to the formation of microvascular invasion (MVI) or microsatellite lesions. Minimally invasive procedures such as laparoscopy and robotics have further promoted the widespread application of liver resection. Our previous study has demonstrated that tumor number and tumor size were independent prognostic risk factors for early recurrence.26 Current staging systems, including the BCLC stage, impose strict restrictions on the number and diameter of tumors. Due to heterogeneity, even patients in the same grade may have different prognosis.27,28 In the present study, the subgroup analysis of the BCLC stage confirmed that overall survival deteriorated incrementally with increasing TBS in patients with the BCLC-C stage. Moreover, intermediate TBS and high TBS were independent prognostic factors for OS and RFS of HCC patients across the stage of BCLC stages. The parameters included in TBS are based on continuous variables, so it can well stratify the prognosis of patients with different grades. Thus, the tumor burden score could be regarded as a feasible subclassification criterion across the BCLC stage.
The Japan Society of Hepatology and the Asian Pacific Association for the Study of the Liver HCC Guidelines some HCC patients beyond BCLC criteria may still benefit from surgical resection.29,30 Nevertheless, there is no consensus defining these highly selective HCC patients, and it is an urgent need for a reliable tool for clarifying HCC patients beyond BCLC criteria who are most likely to benefit from hepatic resection.31 In our study, we further evaluated whether tumor burden score could be a reliable tool for identifying patients beyond BCLC criteria for hepatic resection. Surprisingly, the OS of low TBS group of patients beyond BCLC criteria was not inferior to that of intermediate and high TBS group of patients within BCLC criteria. Consistent with the results of OS, the RFS of high TBS group within BCLC criteria and low TBS group beyond BCLC criteria were evenly matched. Thus, for those intermediate-advanced stage HCC patients with low TBS, surgical resection reported an acceptable outcome, compared to early-stage with intermediate and high TBS. In the present investigation, we have deliberately excluded from our analysis patients who had undergone preoperative conversion therapy. Nowadays, multimodal and high-intensity transformational therapies are constantly emerging, which are the main ways for patients with advanced liver cancer to achieve radical resection and long-term survival.32–34 Our team orally presented the results of a prospective, randomized, controlled, Phase III clinical study at the 2021 ASCO meeting that preoperative neoadjuvant FOLFOX-HAIC could reduce preoperative tumor burden and improve the prognosis for patients with resectable BCLC stage A/B hepatocellular carcinoma beyond BCLC criteria. Thus, we have compelling evidence to suggest that HCC patients in the intermediate-advanced stages with low TBS may derive significant therapeutic benefits from hepatic resection.
We acknowledge several potential limitations inherent in our study. First, the primary patient cohort in our research was characterized by a history of HBV infection, which may have influenced the generalizability of our findings. Second, the retrospective nature of our study inevitably introduced selection bias, particularly evident in the process of enrolling patients at an intermediate to advanced stage for surgical intervention. This bias may have affected the objectivity of the results. Consequently, there is a clear necessity for future prospective studies to further validate our conclusions. Moreover, within the TBS formula, the maximum tumor diameter and the number of tumors are assigned equal weight. However, the rationality of this weight distribution has yet to be thoroughly examined and warrants additional investigation in subsequent research endeavors.
In conclusion, TBS stratifies the prognosis of HCC patients undergoing liver resection, and it can be a feasible subclassification criterion across the BCLC stage. Moreover, TBS can identify patients for resection beyond the BCLC criteria. TBS is a feasible and reliable prognostic tool for prognosis prediction and clinical decisions.
Abbreviations
HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; TBS, tumor burden score; OS, overall survival; RFS, recurrence-free survival; CNLC, China Liver Cancer; CT, enhanced computerized tomography; MRI, Gd-EOB-DTPA-enhanced magnetic resonance imaging; AFP, α-fetoprotein; WBC, white blood cell count; PLT, blood platelet count; ALT, alanine aminotransferase; MVI, microvascular invasion; IQR, interquartile ranges.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This multi-institutional retrospective study was approved by the Institutional Review Board of Guangdong Provincial People’s Hospital (KY2024-1113-01). All procedures performed in this study involving human participants were in line with the 1975 Declaration of Helsinki (6th revision, 2008).
Patient Consent
Informed consent was obtained from all individual participants included in the study. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Acknowledgments
Thank Wu-Shen Yu, Yong-Fa Zhang, Chong Zhong, Jia-Hong Wang, and Zhi-Yuan Chen to data acquisition.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Renguo Guan, Zehao Zheng and Min Deng contributed equally to this work.
Funding
This study was supported by the Guangzhou Science and Technology Plan Project (202201010944) and Activation Project of Guangdong Provincial People’s Hospital (8220160353).
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4(1):47–53. doi:10.1016/j.jncc.2024.01.006
2. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev mol Cell Biol. 2020;21(4):225–245. doi:10.1038/s41580-019-0190-7
3. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–141.
4. Jung YK, Jung CH, Seo YS, et al. BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A. J Gastroenterol Hepatol. 2016;31(2):467–474. doi:10.1111/jgh.13152
5. Wang YY, Zhong JH, Xu HF, et al. A modified staging of early and intermediate hepatocellular carcinoma based on single tumour >7 cm and multiple tumours beyond up-to-seven criteria. Aliment Pharmacol Ther. 2019;49(2):202–210. doi:10.1111/apt.15074
6. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
7. Ciria R, Lopez-Cillero P, Gallardo AB, et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41(9):1153–1161. doi:10.1016/j.ejso.2015.05.023
8. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
9. Zhong JH, Torzilli G, Xing H, et al. Controversies and evidence of hepatic resection for hepatocellular carcinoma. BBA Clin. 2016;6:125–130. doi:10.1016/j.bbacli.2016.10.001
10. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
11. Kim H, Ahn SW, Hong SK, et al. Survival benefit of liver resection for Barcelona clinic liver cancer stage B hepatocellular carcinoma. Br J Surg. 2017;104(8):1045–1052. doi:10.1002/bjs.10541
12. Labgaa I, Demartines N, Melloul E. Surgical resection versus transarterial chemoembolization for intermediate stage hepatocellular carcinoma (BCLC-B): an unsolved question. Hepatology. 2019;69(2):923. doi:10.1002/hep.30338
13. Sasaki K, Morioka D, Conci S, et al. The tumor burden score: a new “Metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. 2018;267(1):132–141. doi:10.1097/SLA.0000000000002064
14. Moris D, Shaw BI, McElroy L, Barbas AS. Using hepatocellular carcinoma tumor burden score to stratify prognosis after liver transplantation. Cancers. 2020;12(11):3372. doi:10.3390/cancers12113372
15. Ho SY, Liu PH, Hsu CY, et al. Tumor burden score as a new prognostic marker for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Gastroenterol Hepatol. 2021;36(11):3196–3203. doi:10.1111/jgh.15593
16. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;96:106165. doi:10.1016/j.ijsu.2021.106165
17. Muller L, Hahn F, Auer TA, et al. Tumor burden in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: head-to-head comparison of current scoring systems. Front Oncol. 2022;12:850454. doi:10.3389/fonc.2022.850454
18. Xia D, Wang Q, Bai W, et al. Optimal time point of response assessment for predicting survival is associated with tumor burden in hepatocellular carcinoma receiving repeated transarterial chemoembolization. Eur Radiol. 2022;32(9):5799–5810. doi:10.1007/s00330-022-08716-4
19. Li H, Liu R, Qiu H, et al. Tumor burden score stratifies prognosis of patients with intrahepatic cholangiocarcinoma after hepatic resection: a retrospective, multi-institutional study. Front Oncol. 2022;12:829407. doi:10.3389/fonc.2022.829407
20. Tsilimigras DI, Moris D, Hyer JM, et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br J Surg. 2020;107(7):854–864. doi:10.1002/bjs.11464
21. Wang X, Mao M, He Z, et al. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15(1):221–228. doi:10.7150/ijbs.28720
22. Zhou H, Chen J, Liu K, Xu H. Prognostic factors and predictive nomogram models for early death in elderly patients with hepatocellular carcinoma: a population-based study. Front Mol Biosci. 2023;10:1275791. doi:10.3389/fmolb.2023.1275791
23. Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287(2):461–472. doi:10.1148/radiol.2017162756
24. Yin Z, Jin H, Ma T, Zhou Y, Yu M, Jian Z. A meta-analysis of long-term survival outcomes between surgical resection and radiofrequency ablation in patients with single hepatocellular carcinoma </= 2 cm (BCLC very early stage). Int J Surg. 2018;56:61–67. doi:10.1016/j.ijsu.2018.04.048
25. Chu HH, Kim JH, Kim PN, et al. Surgical resection versus radiofrequency ablation very early-stage HCC (</=2 cm Single HCC): a propensity score analysis. Liver Int. 2019;39(12):2397–2407. doi:10.1111/liv.14258
26. Xia W, Peng T, Guan R, et al. Development of a novel prognostic nomogram for the early recurrence of liver cancer after curative hepatectomy. Ann Transl Med. 2021;9(20):1541. doi:10.21037/atm-21-4837
27. Hwang S, Lee YJ, Kim KH, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015;19(7):1281–1290. doi:10.1007/s11605-015-2849-5
28. Kudo M, Izumi N, Kubo S, et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2020;50(1):15–46. doi:10.1111/hepr.13438
29. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370.
30. Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109–1113. doi:10.1111/hepr.13411
31. Romano F, Chiarelli M, Garancini M, et al. Rethinking the Barcelona clinic liver cancer guidelines: intermediate stage and Child-Pugh B patients are suitable for surgery? World J Gastroenterol. 2021;27(21):2784–2794. doi:10.3748/wjg.v27.i21.2784
32. Zhang Y, Huang G, Wang Y, et al. Is Salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist. 2016;21(12):1442–1449. doi:10.1634/theoncologist.2016-0094
33. Byun HK, Kim HJ, Im YR, Kim DY, Han KH, Seong J. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol. 2019;133:1–8. doi:10.1016/j.radonc.2018.12.025
34. Zhu XD, Huang C, Shen YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. 2021;10(4):320–329. doi:10.1159/000514313
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.








