Back to Journals » Drug Design, Development and Therapy » Volume 19
Bioequivalence Study of Single-Pill Capsule Formulation of Amlodipine Plus Benazepril in Healthy Chinese Subjects Under Fasting and Fed Conditions
Authors Song H, Qiu B, Sun X, Guo C, Hu Y, Dong Z, Liu Y , Bai W
Received 24 October 2024
Accepted for publication 3 March 2025
Published 13 March 2025 Volume 2025:19 Pages 1853—1868
DOI https://doi.org/10.2147/DDDT.S498337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Haojing Song,1 Bo Qiu,1 Xue Sun,1 Caihui Guo,1 Yiting Hu,1 Zhanjun Dong,1 Yang Liu,2 Wanjun Bai1
1Department of Pharmacy, Hebei General Hospital, Hebei Key Laboratory of Clinical Pharmacy, Shijiazhuang, 050051, People’s Republic of China; 2Hebei Longhai Pharmaceutical Co., Ltd, Shijiazhuang, 052165, People’s Republic of China
Correspondence: Wanjun Bai; Department of Pharmacy, Hebei General Hospital, Shijiazhuang, 050051, People’s Republic of China, Tel +86-0311-85988326, Email [email protected] Yang Liu, Hebei Longhai Pharmaceutical Co., Ltd, Shijiazhuang, 052165, People’s Republic of China, Tel +86-0311-85158183, Email [email protected]
Background: The aim of the study was to evaluate the pharmacokinetic (PK) properties and safety profiles of test and reference amlodipine/benazepril capsules under both fasting and fed states, determine the bioequivalence between the two formulations, and provide sufficient evidence for new drug application.
Subjects and Methods: The bioequivalence study was conducted utilizing a randomized, open-label design, involving two formulations administered in a single-dose format. Healthy Chinese participants who met the eligibility criteria were administered a single dose of the test or reference amlodipine/benazepril capsule. Blood samples were taken serially for up to 168 hours post-administration during each period, and the plasma levels of amlodipine, benazepril, and benazeprilat were measured using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method. For bioequivalence evaluation, geometric mean ratios comparing the pharmacokinetic parameters of the test drug with those of the reference drug were calculated along with their corresponding 90% confidence intervals. Safety assessments were conducted throughout the duration of the study.
Results: The PK parameters of the test formulation were found to be comparable to those of the reference formulation under both fasting and fed conditions. The 90% confidence intervals (CIs) for the geometric mean ratios comparing the test and reference formulations for the peak concentration (Cmax), the area under the curve from time zero to the last measurable concentration (AUC0-t), and the area under the curve from time zero to observed infinity (AUC0-∞) of amlodipine, benazepril, and benazeprilat fell within the range of 80.00% to 125.00% in both groups. Both formulations were well tolerated by participants, with no serious adverse events reported throughout the trial.
Conclusion: The pharmacokinetic bioequivalence between the test and reference formulation in healthy individuals was confirmed under both fasting and fed states, meeting regulatory standards set for the study. Both drug formulations demonstrated safety and tolerability.
Keywords: Amlodipine, benazepril, benazeprilat, bioequivalence, pharmacokinetics
Introduction
Hypertension is a condition with high blood pressure (SBP ≥140 mmHg or DBP ≥90 mmHg). It’s a major risk factor for heart disease and stroke. Managing hypertension can reduce risk of these conditions and mortality. 1,2 Most patients need two or more medications to reach target blood pressure levels.3,4
The guidelines of the International Society of Hypertension (ISH) in 2020 recommended the preferential utilization of angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers in combination with calcium channel blockers (CCBs) for the treatment of hypertension.5 In the ACCOMPLISH trial,6 a subsequent study compared benazepril with amlodipine or hydrochlorothiazide combination. The findings indicated that the combination of benazepril and amlodipine was more effective than the combination of benazepril and hydrochlorothiazide in preventing cardiovascular events among patients with systolic blood pressure of ≤130 mmHg. Additionally, these benefits persisted in patients with apparent resistant hypertension who were taking four or more medications.7 Furthermore, the combination of ACE inhibitors and CCBs exhibits fewer adverse effects compared to the individual administration of either medication. Clinical studies have demonstrated that the incorporation of ACE inhibitors results in a reduced incidence of peripheral edema induced by dihydropyridine calcium channel blockers.8,9
Currently, an escalating number of national protocols for managing hypertension recommend the utilization of single-pill combinations (SPC) for the treatment of hypertension.10–12 SPC is a composite preparation formulated by integrating two or more medications with distinct mechanisms of action into a single tablet. It can decrease the occurrence of adverse reactions and enhance patient compliance through cooperation with various antihypertensive mechanisms, enabling the utilization of the lowest effective dosage, and thereby achieving the desired antihypertensive effect.
Amlodipine, a calcium channel blocker (CCB), is used to treat hypertension and is mainly metabolized in the liver and excreted through the urine.13 Benazepril, a non-mercapto angiotensin-converting enzyme (ACE) inhibitor, is also used for hypertension and is metabolized to benazeprilat in the liver, which is then excreted by the kidneys.14 Combining benazepril and amlodipine does not significantly alter their pharmacokinetic properties, indicating no pharmacokinetic interaction between the two drugs.15
Amlodipine/benazepril (Lotrel®, Novartis) is a single-pill treatment for hypertension. Clinical trials show its adverse event rate is consistent with placebo, even though higher doses in monotherapy can increase such events. It maintains blood pressure control for 24 hours and is not significantly affected by missed doses.16–18 Additionally, it’s more cost-effective than using the components separately. Therefore, it’s reasonable to consider amlodipine/benazepril for managing hypertension in patients after monotherapy trials.19
The exorbitant cost of genuine products has imparted a financial burden on patients. In comparison to the original products, the reduced costs associated with generic products can alleviate the economic burden on patients and enhance the availability of anti-hypertensive medications. Studies have shown common drugs for chronic diseases that could generate the greatest economic savings upon switching to generic formulations.20 The National Medical Products Administration (NMPA) mandates a comparative bioequivalence study between generic drugs and the original research products as a prerequisite for marketing a new generic drug. If there is no notable disparity in the absorption rate and the extent of absorption between the two products, it is deemed that generic drugs and reference drugs are bioequivalent.
The study aimed to compare the pharmacokinetic parameters and bioequivalence of new amlodipine/benazepril capsules (5/10 mg) with a reference product in healthy Chinese volunteers under both fasting and fed conditions. The goal was to fully understand the new product’s characteristics and provide scientific evidence for its marketing approval in China.
Materials and Methods
Ethics Statement of Human Rights
The study was registered on the Chinese Clinical Trial website (http://www.chinadrugtrials.org.cn/index.html, number: CTR20221788, date: July 18, 2022). The bioequivalence clinical trial was retrospectively registered with the Chinese Clinical Trial Registry, which is recognized by the WHO [https://www.chictr.org.cn/] (number: ChiCTR2400082941, date: April 11, 2024). The Independent Ethics Committee of Hebei General Hospital conducted a review and approved the clinical trial scheme and its subsequent revision On May 25, 2022 (ethical code: 2022–10). In the study, the date on which the first subject provided informed consent was July 26, 2022, whereas the date on which the follow-up for the last subject was completed was December 21, 2022. This study was conducted in strict accordance with the Guidelines for Good Clinical Practice recommended by the National Medical Products Administration (NMPA), the International Conference on Harmonization’s Good Clinical Practice Guidelines, as well as the ethical principles outlined in the Declaration of Helsinki.
Study Population
Prior to the commencement of the study, all participants provided their written informed consent, which encompassed details regarding the research objectives, procedures, and potential risks involved. They underwent a thorough medical evaluation, encompassing routine physical examinations, vital sign assessments, orthostatic hypotension measurements, medical history reviews, laboratory testing, chest radiography, 12-lead electrocardiography, and color Doppler ultrasonography of the renal artery, all of which were conducted to ascertain their health condition and detect any clinically significant diseases.
During the study period, all participants were afforded the option to withdraw at any time. During the screening period, participants of both genders, aged 18 and above, with weights exceeding 50 kg for males and 45 kg for females, and possessing a body mass index (BMI) within the range of 19.0 to 26.0 kg/m2, were eligible for inclusion in the study. Subjects were excluded from the study if they possessed the following medical history or evidence: acute or chronic clinical-related diseases; a history of drug, nicotine, or alcohol abuse; an allergic constitution, particularly an allergy to any component of amlodipine/benazepril capsules; a history of blood donation or acute blood loss exceeding 400 mL within the past three months; or if they had taken any medications or supplements within 30 days prior to the initial administration. Female subjects were excluded if they were pregnant or breastfeeding during the study period, or if they planned to become pregnant one month prior to administration or six months following the conclusion of the study.
Study Drugs
Amlodipine/benazepril capsules (5/10 mg per capsule, batch number: 22030252) manufactured and supplied by Hebei Longhai Pharmaceutical Co., Ltd were used as the test preparations. The reference preparations utilized were amlodipine/benazepril capsules (5/10 mg per capsule, batch number: KV0241), marketed under the brand name Lotrel® and manufactured by Novartis Pharmaceuticals Corporation. During each treatment period, participants were administered a single-pill capsule containing amlodipine/benazepril (5/10 mg) as either the test or reference formulation.
Study Design
This research comprised two distinct clinical trials (the fasting study and the fed study), both of which constituted randomized, open-label, two-formulation, single-dose, two-sequence, two-period crossover bioequivalence studies conducted at the Phase Ι Clinical Research Center of Hebei General Hospital, located in Shijiazhuang, Hebei Province, China. In each trial, participants were allocated randomly to either the T-R or R-T group (where T represented the test product and R represented the reference product) in a 1:1 ratio, based on a random number table produced by SAS statistical software (version 9.4). In the T-R group, the participants were administered the test product during the initial treatment period and subsequently received the reference product in the second treatment period. Conversely, the R-T group underwent the opposite sequence of administration. A washout period of 21 days was implemented to separate the two treatment periods. In the context of fasting, each participant was orally administered a single dose of either the test or reference capsule, accompanied by 240 mL of warm water, following an overnight fast of at least 10 hours. Under fasting conditions, participants in the fed state consumed a standard high-fat breakfast (containing approximately 800–1000 kcal, with approximately 150 kcal from protein, 250 kcal from carbohydrates, and 500–600 kcal from fat) 30 minutes prior to each drug administration, adhering to the same protocol. For one hour prior to and following the administration of the drug, water intake was prohibited. At four hours and ten hours post-drug administration, standardized lunches and dinners were provided, respectively. Throughout the duration of the study, a safety assessment was conducted.
Previous studies indicate that the inter-individual coefficient of variation (CV) for amlodipine and benazepril is less than 30%.21 Consequently, amlodipine/benazepril is classified as a drug with moderate variation. It was assumed that the geometric mean ratio (GMR) of the test (T) to reference (R) was between 0.95 and 1.05, and the CV was 25%. The sample size needed to establish bioequivalence limits of 80.00% to 125.00% between T and R, with 80% power at a significance level of 5%, was determined. Considering a dropout and/or withdrawal rate of approximately 20% and the effects of food on pharmacokinetics, 36 participants in the fed group and 56 in the fasted group were sufficient to meet statistical requirements.
Pharmacokinetic Assessment
Blood samples were collected into coded, pre-cooled K2- EDTA anticoagulation tubes pre-dose (0 h, baseline) and 0.17, 0.33, 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 14, 24, 48, 72, 96, 120, 144 and 168h post-dose followed by centrifugation at 1,700 g at 4°C for 10 min within 1 h of collection. Plasma was collected and stored at −70°C ±10°C within 2 h of collection until their use for analysis. All blood samples were light-proofed throughout collection, processing, and storage.
The PK analysis set comprised all participants randomly assigned to any group who completed periods 1–2 without any major protocol deviations. The primary PK endpoints were Cmax, AUC0-t, and AUC0-∞. The terminal half-life of the analyte in plasma (t1/2), time of maximum plasma concentration (Tmax), AUC_%Extrap, and terminal rate constant (λz) were the secondary endpoints.
Due to data missing caused by subject dropout or exclusion for various reasons, this study did not employ any method for data imputation. For sample concentrations below the lower limit of quantification, a value of “0” was assigned for the time points before Tmax and recorded as missing data after Tmax during PK analysis.
Because of the difference in the half-life of amlodipine, benazepril, and the active metabolite benazeprilat, the AUC0-168h of amlodipine, and the AUC0-48h of benazepril and benazeprilat were used for PK assessment.
Bioequivalent Assessment
To assess the bioequivalence of the test formulations relative to the reference formulations, the 90% CIs for the geometric least-squares mean ratios of the test formulations to the reference formulations were computed. The acceptance criteria for bioequivalence stipulated that 90% CIs for Cmax, AUC0-t, and AUC0-∞ for amlodipine, benazepril, and benazeprilat must fall entirely within the range of 80.00% to 125.00%. For the analysis of Tmax between the two formulations, the Wilcoxon signed-rank test was employed.
Bioanalytical Methods
The plasma concentrations of amlodipine, benazepril, and benazeprilat in healthy individuals were determined using a newly devised and validated HPLC-MS/MS method. An HPLC system from ExionLC AD (SHIMADZU, JAPAN) and a TRIPLE QUAD 6500 Mass Spectrometer with an ESI source (AB SCIEX, USA) were utilized. Briefly, amlodipine-d4, benazepril-d5, and benazeprilat-d5 were incorporated as internal standard, and the precipitation of protein was conducted from the plasma samples. Then 10 μL of the supernatant of amlodipine and 5 μL of the supernatant of benazepril and benazeprilat were injected and separated on an ACE Excel 3 C18-PFP (50 x 2.1mm) column with gradient elution at a flow rate of 0.4 mL/min (mobile phase A, 0.05% formic acid and 2.00 mm NH4Ac in water; mobile phase B, methyl alcohol). The temperature of the column was kept at a constant level of 40°C. The monitored transitions were m/z 409.1/238.1 for amlodipine, m/z 413.2/238.1 for amlodipine-d4, m/z 425.2/190.0 for benazepril, m/z 430.2/190.0 for benazepril-d5, m/z 397.2/351.2 for benazeprilat, and m/z 402.2/356.2 for benazeprilat-d5 in positive electrospray ionization. The optimal instrument settings were configured as follows: The heater temperature was set to 400°C. The ion spray voltage was adjusted to 5500 V. For both amlodipine and amlodipine-d4 (IS), the declustering potential was established at 20 V. The collision energy was set to 18 V for amlodipine and 15 V for amlodipine-d4 (IS). For both benazepril and benazepril-d5 (IS), the declustering potential was set at 100 V, with a collision energy of 44 V for both. The declustering potential for both benazeprilat and benazeprilat-d5 (IS) was configured at 95 V, with collision energies of 29 V and 27 V, respectively. The curtain gas pressure was set to 40 psi, while both gas 1 and gas 2 pressures were adjusted to 50 psi.
Safety Assessment
In this study, all participants were incorporated in the safety analysis. The safety analysis encompassed laboratory examination, combined medications, physical assessments, vital sign monitoring, 12-lead electrocardiography (ECG) evaluations, adverse events (AEs), and serious adverse events (SAEs). Vital signs, including blood pressure, heart rate, and body temperature, were systematically monitored at pre-dose (within 1 h), as well as at 2h, 4h, 6h, 8h, 12h, 24h, 48h, 72h, 96h, 120h, 144h, and 168h following each drug administration during each treatment period. All participants underwent physical examinations, laboratory tests, and 12-lead ECG assessments both at the screening stage and 168 hours following the second administration. Throughout the trial, all AEs were closely monitored by the study doctors or voluntarily reported by the participants. The grading of the severity of AEs was conducted in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, issued by the National Cancer Institute.
Statistical Analysis
Based on the plasma concentration data, the pharmacokinetic parameters for amlodipine, benazepril, and benazeprilat were determined using a non-compartmental analysis model, employing the Phoenix WinNonlin software (Pharsight Corporation, Mountain View, CA, USA; version 8.2). The values of Cmax and Tmax were directly ascertained from the observed plasma concentration-time curve. The AUC0-t and AUC0-∞ were computed employing the trapezoidal method. The elimination rate constant (λz) was ascertained through log-linear regression analysis of the plasma concentration as a function of time during the terminal phase. The t1/2 was subsequently computed as 0.693 divided by λz.
After converting Cmax and AUC0-t to their natural logarithmic values, a linear mixed model was used to analyze the variance (ANOVA) to evaluate the influence of formulation, dosing sequence, trial period, and subjects.
When the AUC%Extrap exceeds 20% for amlodipine, benazepril, or benazeprilat during any period, the AUC0-∞, t1/2, and λz were not statistically analyzed. When the drug plasma concentration before dosing was not below the quantifiable limit (BQL) and was greater than 5% of Cmax, the corresponding cycle data of the subject were not included in the bioequivalence evaluation.
Results
Study Population
During the trial, a total of 281 participants were screened, respectively, and 92 participants were enrolled for the fed and fed groups (n=36 for the fasted group, and n=56 for the fed group). Figure 1 shows the distributions of screening and inclusion criteria for the participants within the two groups. Two participants from the fasted group and four participants from the fed group exited the trial.
 |
Figure 1 Flow Chart of Subjects. Flow chart of the subjects in the fasted group (A). Flow chart of the subjects in the fed group (B). (N) the number of subjects. |
In the fasted group, subject K012 exited the trial due to personal reasons following blood collection within 12 hours of the second period. Additionally, subject K018 was disqualified from the trial because this individual had been in close contact with COVID-19 prior to the second administration period.
In the fed group, subjects C001 and C007 were withdrawn from the trial prior to the first period of administration due to trypanophobia. Subject C038 was withdrawn from the trial prior to the second administration period as a result of a positive urine drug screening. Subject C054 was withdrawn from the trial after blood collection within 96 hours of the second period for personal reasons.
The fundamental characteristics of each subject are outlined in Table 1. All individuals involved in the study fulfilled the established inclusion criteria.
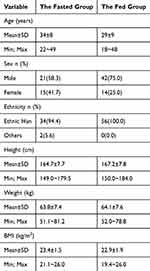 |
Table 1 Demographic Baseline |
PK Results
All pharmacokinetic concentration sets (PKCS) and pharmacokinetic parameter sets (PKPS) were utilized for the analysis of all pharmacokinetic (PK) parameters. In the fasted group, subject K018 from the R-T group withdrew from the trial prior to the second administration period and was therefore excluded from the PKCS and PKPS analysis for amlodipine, benazepril, and benazeprilat. Due to the AUC%Extrap >20% for amlodipine of K012 (the R-T group) during period 2, K021 (the R-T group) across both periods, and K029 (the R-T group) during period 1, the AUC0-∞, t1/2, and λz of were not statistically analyzed. In the fed group, subject C038 in the R-T group withdrawn from the trial prior to the second administration period was excluded from the PKCS and PKPS of amlodipine, benazepril, and benazeprilat. Due to the AUC%Extrap >20% for amlodipine of C023 (the T-R group), C049 (the R-T group), and C054 (the T-R group) in period 2, the AUC0-∞, t1/2 and λz of were not statistically analyzed. Because of the AUC%Extrap >20% of benazepril of C020 (the R-T group) in period 1, the AUC0-∞, t1/2, and λz were not statistically analyzed. The plasma concentration data for benazepril was deemed valid for fewer than 3 points following the Tmax observed in C005 (the R-T group) during Phase 1 and C018 (the T-R group) during Phase 2, consequently, statistical analysis was not conducted for AUC0-∞, t1/2, λz, and AUC%Extrap. The other participants were registered in the PKCS and PKPS within the fasted group and the fed group.
The mean plasma concentration-time curves for amlodipine, benazepril, and benazeprilat in both fasted and fed groups after completing the two cycles are depicted in Figures 2 and 3.
The major PK parameters of amlodipine, benazepril, and benazeprilat in the fasted and fed groups were calculated using a non-compartmental model, and are summarized in Table 2.
 |
Table 2 The PK Parameters of Amlodipine, Benazepril, and Benazeprilat After Oral T and R Formulations in the Fasted and the Fed Groups |
In the bioequivalence trial, the factors of period, sequence, and formula may potentially influence the equivalence between the test preparation and reference preparation. To assess the impact of these extraneous factors on the present study, the linear Mixed Model was employed to conduct a multivariate analysis of variance, following the log transformation of each primary parameter in both the fasting and postprandial states. The results indicated that, as determined by ANOVA, there were statistically significant differences in Cmax, AUC0–t, or AUC0–∞ in the different periods and formulations in the fasting group and the fed group (P < 0.05, the specific values are presented in Table 3). However, the differences did not alter the bioequivalence conclusion.
 |
Table 3 The Results of Variance Analysis of the Main PK Parameters After Logarithmic Transformation |
BE Results
In the fastest group, subject K029 in the R-T group during the two periods was excluded from the Bioequivalence Set (BES) due to the plasma concentration of the drug prior to dosing exceeding 5% of Cmax. For the purpose of pharmacokinetic (PK) bioequivalence evaluation, the remaining subjects in both the fasted and fed groups were included in the bioequivalence analysis set (BES), consistent with the protocols of PKPS.
The 90% CIs and the geometric least-squares mean ratios of amlodipine, benazepril, and benazeprilat for Cmax, AUC0-t, and AUC0-∞ were used to evaluate equivalence, as shown in Table 4. All 90% CIs for Cmax, AUC0-t, and AUC0-∞ were within the established bioequivalence range of 80.00% to 125.00%. The findings demonstrated that the amlodipine/benazepril capsule test product exhibited bioequivalence to the reference product in the fasted group and the fed group.
 |
Table 4 Results of the Equivalence Determination of the T and R Formulations in the Fasted and the Fed Groups |
Bioanalytical Methods Validation
The linear concentration ranges for amlodipine were 0.02 ng/mL to 5.00 ng/mL, for benazepril were 0.30 ng/mL to 300.00 ng/mL, and for benazeprilat were 0.40 ng/mL to 400.00 ng/mL. The lower limits of quantitation (LLOQ) for amlodipine, benazepril, and benazeprilat were 0.02, 0.30, and 0.40 ng/mL, respectively. The intra-batch and inter-batch precision values for amlodipine in plasma samples were less than 7.4% and 9.9%, respectively; for benazepril, they were less than 3.9% and 4.0%, respectively; and for benazeprilat, they were less than 4.7% and 6.2%, respectively. The intra-batch and inter-batch accuracy ranged from −6.2% to 11% and −1.6% to 5.3% for amlodipine, from −5.1% to 6.3% and −1.3% to 3.3% for benazepril, and from −6.0% to 8.3% for benazeprilat, respectively.
Safety Results
The assessment of the safety and tolerability of the amlodipine/benazepril capsule was conducted based on the safety analysis set (SS), which included all subjects who had received at least one dose of the study drug. Both the test and reference formulations demonstrated favorable safety profiles in healthy Chinese participants, irrespective of whether they were in the fasted or fed groups.
The incidence of adverse events (AEs) for both the fasted and fed groups has been summarized in Table 5. In the fasted group, 36 subjects were enrolled in the safety set (SS), with 49 AEs reported for 25 subjects, resulting in an AE incidence rate of 69.44% (25/36). All reported AEs were mild and did not necessitate any treatment. In the fed group, the AE incidence rate was 72.22%, with 84 AEs occurring in 39 subjects. All AEs were classified as mild and did not require further medical intervention. Notably, all AEs either resolved or remained stable upon completion of the study. The results indicated that the incidence and types of AEs were comparable between the two formulations, regardless of whether the subjects were in the fasted or fed state.
 |
Table 5 Summary of AEs of Healthy Subjects in the Fasted and the Fed Groups |
Discussion
In this research, we presented evidence demonstrating the bioequivalence of test and reference amlodipine/benazepril capsules in healthy Chinese participants under both fasting and fed conditions. These conditions met the established acceptance criteria and were deemed to exhibit bioequivalence. Both formulations were well-tolerated, and no serious adverse events (SAEs) were reported.
In accordance with the bioequivalence guidelines issued by the FDA,22 the pharmacokinetic properties of amlodipine, benazepril, and its active metabolite, benazeprilat, were assessed. Benazepril undergoes metabolic conversion in the liver to form its active metabolite, benazeprilat, which binds irreversibly to ACE.23 Benazeprilat is not utilized as an oral medication due to its inadequate absorption from the gastrointestinal tract, exhibiting an oral bioavailability of under 7%.24 Benazeprilat exhibits greater efficacy as an ACE inhibitor compared to benazepril, with an exposure level approximately 10 times higher. Consequently, the study also assessed the bioequivalence of benazeprilat.
In adults, approximately 37% of orally administered benazepril is rapidly absorbed and subsequently metabolized into its active form, benazeprilat, within the liver. Both benazepril and benazeprilat undergo glucuronidation and are subsequently excreted in both urine and bile. A maximum concentration of benazeprilat was observed approximately 1 to 6 hours following administration, with a plasma half-life of 10 to 11 hours. A mere fraction of the administered Lotensin dose is recoverable in the urine in its unchanged form as benazepril, whereas approximately 20% of the dose is excreted as benazeprilat, 4% as benazepril glucuronide, and an additional 8% as benazeprilat glucuronide.25
The pharmacokinetic profiles observed in healthy volunteers in this study were comparable to those previously reported for amlodipine and benazeprilat in references.26–29 Specifically, the Tmax and t1/2 values of amlodipine administered as a single capsule in this study were consistent with those from previous research, suggesting that the rates of absorption and elimination in this study were in line with previous findings.
In our study of amlodipine 5 mg and benazepril 10 mg, the Cmax, Tmax, and AUC0-t of both benazepril and its active metabolite benazeprilat were comparable to those reported in previous research findings,26,27 however, significant differences were observed in the t1/2 of benazeprilat. In previous research findings, the t1/2 was reported to be 17 hours and 14 hours, respectively. However, in our study, the half-life of benazeprilat was found to be approximately 10 hours, which aligns closely with the information provided in the prescription information for Lotensin® (Benazepril hydrochloride). Therefore, it is reasonable to assume that an extended sampling time would result in a more detailed compartmentalization observed in the distribution map of benazeprilat, which is utilized for the calculation of the t1/2. In previous studies,26,27 the average sampling time was 72 hours and 84 hours, whereas in the present study, it was 48 hours. The sampling duration affects the noncompartmental half-life determination, and the sampling scheme spanned over 2 and 4 half-lives was required to have acceptable precision and bias in the noncompartmental t1/2 estimates. The t1/2 of benazeprilat is approximately 10 hours, making a sampling scheme that spans over 48 hours reasonable in the study.
A bioequivalence study of the generic drug was a prerequisite for marketing approval. The 90% CIs of amlodipine, benazepril, and benazeprilat for Cmax, AUC0-t, and AUC0-∞ were within the established bioequivalence range of 80.00% to 125.00%. The findings demonstrated that the amlodipine/benazepril capsule test product exhibited bioequivalence to the reference product in both the fasted and fed groups. The current research offers further substantiation that the single-pill capsule formulation of amlodipine plus benazepril, manufactured by generic companies, demonstrates equivalent clinical efficacy to that of branded products that provide scientific evidence for its marketing approval in China. The active constituents of generic medications are identical to those of their branded counterparts, and the pricing is significantly lower.30 This cost advantage endows generic drugs with the capacity to enhance patient adherence and continuation of therapy.31,32 Upon establishing drug equivalence and bioequivalence with the reference products, the generic versions are authorized for use.
There exist certain research limitations: In this study, the pharmacokinetic parameters of amlodipine/benazepril capsules were assessed in healthy individuals. However, it is possible that the absorption, metabolism, or distribution of amlodipine/benazepril capsules may vary in hypertensive patients. Regarding the impact of food on the pharmacokinetics and bioequivalence of amlodipine/benazepril capsules, our investigation was limited to high-fat diets.
Conclusion
According to the findings of this study, amlodipine/benazepril capsules available in China demonstrate bioequivalence to the reference formulation (Lotrel®) with respect to the rate and extent of absorption, both under fasting and fed conditions. Both formulations exhibited general good tolerability among the healthy Chinese population and may be used interchangeably in clinical practice, thereby alleviating the economic burden on hypertensive patients in China.
Data Sharing Statement
The data set utilized in the present research may be obtained from the corresponding author, Wanjun Bai, upon receipt of a reasonable request.
Acknowledgments
The authors express their gratitude to the participants and the staff involved in this research endeavor.
Informed Consent
All individual participants included in the study provided written informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global status report on noncommunicable diseases. 2014. Available from:. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf.
2. Mills KT, Bundy JD, Kelly TN. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 Countries. Circulation. 2016;134(6):441–450. doi:10.1161/CIRCULATIONAHA.115.018912
3. Salam A, Huffman MD, Kanukula R, et al. Two-drug fixed-dose combinations of blood pressure-lowering drugs as WHO essential medicines: an overview of efficacy, safety, and cost. J Clin Hypertens. 2020;22:1769–1779. doi:10.1111/jch.14009
4. Benjamin IJ, Kreutz R, Olsen MH, et al. Fixed-dose combination antihypertensive medications. Lancet. 2019;394:637–638. doi:10.1016/S0140-6736(19)31629-0
5. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
6. Kjeldsen SE, Jamerson KA, Bakris GL, et al. Avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH). predictors of systolic BP <140 mmHg and systolic BP level by randomly assigned treatment group (benazepril plus amlodipine or hydrochlorothiazide) in the ACCOMPLISH Study. Blood Press. 2012;21(2):82–87. doi:10.3109/08037051.2011.598699
7. Brook RD, Kaciroti N, Bakris G, et al. Cardiovascular benefits of angiotensin-converting enzyme inhibition plus calcium channel blockade in patients achieving tight blood pressure control and with resistant hypertension. Am J Hypertens. 2021;34(5):531–539. doi:10.1093/ajh/hpaa192
8. Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med. 2011;124(2):128–135. doi:10.1016/j.amjmed.2010.08.007
9. Frishman WH, Ram CV, McMahon FG, et al. Comparison of amlodipine and benazepril monotherapy to amlodipine plus benazepril in patients with systemic hypertension: a randomized, double-blind, placebo-controlled, parallel-group study. the benazepril/amlodipine study group. J Clin Pharmacol. 1995;35(11):1060–1066. doi:10.1002/j.1552-4604.1995.tb04027.x
10. Umemura S, Arima H, Arima S, et al. The Japanese Society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertension Res. 2019;42(9):
11. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982–1004. doi:10.1097/HJH.0000000000002453
12. Guideline for the Pharmacological Treatment of Hypertension in Adults [Internet][J]. Geneva: World Health Organization; 2021.
13. Khodadoustan S, Nasri Ashrafi I, Vanaja Satheesh K, Kumar C, Hs S, S C. 2017 evaluation of the effect of time dependent dosing on pharmacokinetic and pharmacodynamics of amlodipine in normotensive and hypertensive human subjects. Clin Exp Hypertens. 2017;39(6):520–526. doi:10.1080/10641963.2017.1281947
14. Balfour JA, Goa KLB. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in hypertension and congestive heart failure. Drugs. 1991;42(3):511–539. doi:10.2165/00003495-199142030-00008
15. Sun JX, Cipriano A, Chan K, John VA. Pharmacokinetic interaction study between benazepril and amlodipine in healthy subjects. Eur J Clin Pharmacol. 1994;47(3):285–289. doi:10.1007/BF02570510
16. Chrysant S. Amlodipine/benazepril combination therapy for hypertensive patients nonresponsive to benazepril monotherapy. Am J Hypertens. 2004;17(7):590–596. doi:10.1016/j.amjhyper.2004.03.679
17. Chrysant SG, Sugimoto DH, Lefkowitz M, et al. The effects of high-dose amlodipine/benazepril combination therapies on blood pressure reduction in patients not adequately controlled with amlodipine monotherapy. Blood Press. 2007;1:10–17. doi:10.1080/08038020701189828
18. Yan P, Fan W. The efficacy and safety of fixed-dose combination of amlodipine/benazepril in Chinese essential hypertensive patients not adequately controlled with benazepril monotherapy: a multicenter, randomized, double-blind, double-dummy, parallel-group clinical trial. Clin Exp Hypertens. 2014;36(4):268–274. doi:10.3109/10641963.2013.810231
19. Faulkner MA, Hilleman DE. Amlodipine/benazepril: fixed dose combination therapy for hypertension. Expert Opin Pharmacother. 2001;2(1):165–178. doi:10.1517/14656566.2.1.165
20. Reichardt Y, Dunkler B, Hronsky D, et al. Comparative effectiveness of branded vs. generic versions of antihypertensive, lipid-lowering and hypoglycemic substances: a population-wide cohort study. Sci Rep. 2020;10(1):5964. doi:10.1038/s41598-020-62318-y
21. LOTREL®((Amlodipine and Benazepril Hydrochloride) Capsules, Tablets. Prescribing Information. Switzerland: Novartis Pharmaceuticals Corporation; 2021.
22. U.S. Food and Drug Administration. Guidance on Benazepril Hydrochloride; 2010.
23. Toutain PL, Lefebvre HP, King JN. Benazeprilat disposition and effect in dogs revisited with a pharmacokinetic/pharmacodynamic modeling approach. J Pharmacol Exp Ther. 2000;292(3):1087–1093. doi:10.1016/S0022-3565(24)35393-5
24. Toutain PL, Lefèbvre HP. Pharmacokinetics and pharmacokinetic/ pharmacodynamic relationships for angiotensin-converting enzyme inhibitors. J Vet Pharmacol Ther. 2004;27(6):515–525. doi:10.1111/j.1365-2885.2004.00601.x
25. Lotensin®(Benazepril Hydrochloride) Tablets. Prescribing Information. Switzerland: Novartis Pharmaceuticals Corporation; 2019.
26. Rezk MR, Badr KA. Development, optimization and validation of a highly sensitive UPLC-ESI-MS/MS method for simultaneous quantification of amlodipine, benazeprile and benazeprilat in human plasma: application to a bioequivalence study. J Pharm Biomed Anal. 2014;98:1–8. doi:10.1016/j.jpba.2014.05.005
27. Chien KL, Chao CL, Su TC. Bioavailability study of fixed-dose tablet versus capsule formulation of amlodipine plus benazepril: a randomized, single-dose, two-sequence, two-period, open-label, crossover study in healthy volunteers. Curr Ther Res Clin Exp. 2005;66(2):69–79. doi:10.1016/j.curtheres.2005.04.005
28. Wang T, Wang Y, Lin S, et al. Evaluation of pharmacokinetics and safety with bioequivalence of amlodipine in healthy Chinese volunteers: bioequivalence study findings. J Clin Lab Anal. 2020;34(6):e23228. doi:10.1002/jcla.23228
29. Chen B, Chen Z, Lv D, Sun Y, Pharmacokinetics CH. Bioequivalence, and safety studies of amlodipine besylate in healthy subjects. Clin Pharmacol Drug Dev. 2022;11(6):717–723. doi:10.1002/cpdd.1064
30. United States Food & Drug Administration. What are generic drugs? Available from: https://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/GenericDrugs/default.htm.
31. Gagne JJ, Choudhry NK, Kesselheim AS, et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med. 2014;161(6):400–407. doi:10.7326/M13-2942
32. Gagne JJ, Kesselheim AS, Choudhry NK, et al. Comparative effectiveness of generic versus brand-name antiepileptic medications. Epilepsy Behav. 2015;52:14–18. doi:10.1016/j.yebeh.2015.08.014
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Evaluation of Pharmacokinetics and Safety with Bioequivalence of Ibuprofen Sustained-Release Capsules of Two Formulations, in Chinese Healthy Volunteers: Bioequivalence Study
Huang C, Yin Z, Yang Y, Mo N, Yang H, Wang Y
Drug Design, Development and Therapy 2023, 17:1881-1888
Published Date: 23 June 2023
Comparative Pharmacokinetics and Bioequivalence Evaluation of Two Formulations of Pramipexole Dihydrochloride Extended-Release Tablets in Healthy Chinese Subjects Under Fasted and Fed States: A Randomized, Open-Label, Single-Dose, Two-Period Crossover Clinical Trial
Yang L, Zhang L, Luo Z
Drug Design, Development and Therapy 2023, 17:2369-2381
Published Date: 15 August 2023
Evaluation of Olaparib Tablet Safety and Pharmacokinetics in Healthy Chinese Male Subjects
Dong R, Chen J, Guo N, Yang Y, Wu J, Wang X, Song Y, Zhang X
Drug Design, Development and Therapy 2024, 18:5529-5539
Published Date: 3 December 2024
Pharmacokinetics and Bioequivalence of Two Fixed-Dose Combination Tablets of Valsartan/Amlodipine (80/5 Mg) in Healthy Chinese Subjects
Tian M, Huang J, Chen Y, Jin Q, Jiang H, Shi C, Mei J, Xu M, Yu X, Yang S
Drug Design, Development and Therapy 2025, 19:11-22
Published Date: 3 January 2025



