Back to Journals » Cancer Management and Research » Volume 17
Combination Therapy of Transarterial Chemoembolization, Lenvatinib, and PD-1 Inhibitors Achieves Significant Tumor Response in Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Case Report
Authors Fu Y, Wu J, Wu J , Li Y , Zeng Z, Liu D, Li H, Ou X, Lin Z, Wei S, Song H, Yan M
Received 10 December 2024
Accepted for publication 4 April 2025
Published 13 April 2025 Volume 2025:17 Pages 793—799
DOI https://doi.org/10.2147/CMAR.S511319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Yangkai Fu,1 Junyi Wu,1,2 Jiayi Wu,1,2 Yinan Li,1 Zhenxin Zeng,1 Deyi Liu,1 Han Li,1 Xiangye Ou,1 Zhongtai Lin,1 Shaoming Wei,1 Huachun Song,1 Maolin Yan1,2
1Shengli Clinical Medical College of Fujian Medical University, Fuzhou, Fujian Province, People’s Republic of China; 2Department of Hepatobiliary Pancreatic Surgery, Fujian Provincial Hospital, Fuzhou University Affiliated Provincial Hospital, Fuzhou, Fujian Province, People’s Republic of China
Correspondence: Maolin Yan, The Shengli Clinical Medical College of Fujian Medical University, Department of Hepatobiliary Pancreatic Surgery, Fujian Provincial Hospital, Dongjie Road 134, Fuzhou, Fujian Province, 350001, People’s Republic of China, Email [email protected]
Abstract: Combination therapy plays a critical role in optimizing surgical outcomes for patients with locally advanced hepatocellular carcinoma (HCC) complicated by bile duct tumor thrombus (BDTT). Current neoadjuvant strategies integrate local and systemic modalities to reduce tumor burden and recurrence rate. However, the combination of transarterial chemoembolization (TACE), lenvatinib, and PD-1 inhibitors (triple therapy) as a neoadjuvant regimen for HCC with BDTT has not been previously reported. Here, we present the case of a 61-year-old man with HBV-associated HCC and BDTT, initially deemed high-risk for direct resection due to tumor size (7 cm) and biliary involvement. The patient underwent one session of TACE followed by two months of lenvatinib (12 mg/day) and sintilimab (200 mg every 3 weeks). Post-treatment contrast-enhanced MRI revealed complete resolution of BDTT and partial response of the primary tumor. Subsequent right hemihepatectomy confirmed extensive tumor necrosis (> 90%) with negative margins. At 15-month follow-up, surveillance imaging showed no recurrence. The patient experienced only grade 1 hypertension, managed without treatment interruption. This case highlights the potential of triple therapy as a neoadjuvant approach to downstage advanced HCC with BDTT, enabling curative resection while maintaining a manageable safety profile. Further studies are warranted to validate its efficacy in larger cohorts and define optimal treatment protocols.
Keywords: hepatocellular carcinoma, bile duct tumor thrombus, combination therapy, lenvatinib, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the leading cause of cancer-related deaths globally, with approximate 45% of new HCC cases worldwide originating from China.1,2 With advancing insights into the molecular mechanisms of HCC, future research will prioritize precision-based therapeutic strategies. For instance, leveraging tumor hypoxia scores, CTNNB1 mutation status,3 and radiomics-based prediction of tumor immune microenvironment status may enable more accurate prediction of therapeutic responses and optimization of personalized treatment regimens.4 Current research priorities are shifting toward developing integrated therapeutic strategies, exploring multimodal combination regimens that incorporate targeted therapies, immunotherapies, locoregional interventions, and systemic treatments.
In the context of the increasing development of precision medicine, there is a need for an in-depth study of HCC with different clinical characteristics. The reported incidence of BDTT is relatively uncommon, ranging from 0.4%-12.9%.5–7 HCC with bile duct tumor thrombus (BDTT) occurs when HCC invades the biliary tree,7 often accompanied by jaundice and decreased liver function. Patients with HCC and BDTT who undergo conservative management have a median survival ranging from 1–3 months.8 The overall survival (OS) of patients with HCC and BDTT at 1 and 3 years (62% and 30%, respectively) is poor compared with those free of BDTT (89% and 73%).9
Surgical resection is considered the primary treatment for HCC combined with BDTT. Numerous studies have reported that surgical resection leads to better outcomes and improves long-term survival compared to other palliative treatments.10,11 Additionally, studies have shown that patients with HCC and BDTT can achieve similar survival outcomes similar to those without BDTT after radical surgery.11–13 However, many patients with HCC and BDTT cannot benefit from surgery during their initial hospital visit because of factors such as underlying hepatic dysfunction, sustained cholestasis, poor general conditions, uncontrollable comorbidities and high risk of recurrence.14,15 The promising outcomes of recent studies combining locoregional and systemic therapies in advanced HCC have prompted a paradigm shift, with researchers re-evaluating the repositioning of these regimens as neoadjuvant strategies.16,17 In alignment with existing evidence, the Chinese Expert Consensus on HCC Neoadjuvant Therapy recommends TACE combined with targeted agents and immunotherapies as a feasible neoadjuvant option.18
Considering the challenging clinical scenario and limited evidences in the literature, we report the case of a patient with HCC and BDTT treated with transarterial chemoembolization (TACE), lenvatinib and programmed death 1 (PD-1) inhibitors (triple therapy) and achieves significant tumor response. This study aimed to investigate the efficacy and safety of triple therapy in patients with HCC and BDTT.
Case Presentation
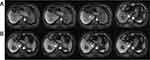
A 61-year-old male with a 20-year history of chronic hepatitis B virus (HBV) infection presented with an incidentally discovered hepatic mass during routine physical examination. The patient denied symptoms of jaundice, abdominal pain, or weight loss. Initial laboratory evaluation revealed preserved liver function: total bilirubin 13.4 μmol/L (normal range: 3.4–20.5 μmol/L), direct bilirubin 3.7 μmol/L (normal: <5.1 μmol/L), and Child-Pugh class A (score 5). Tumor marker analysis demonstrated normal alpha-fetoprotein (AFP: 4.19 ng/mL; normal: <7 ng/mL) but markedly elevated protein induced by vitamin K absence or antagonist-II (PIVKA-II: 3662 mAU/mL; normal: <40 mAU/mL). Contrast-enhanced magnetic resonance imaging (MRI) identified a 7-cm hypervascular mass in the right hepatic lobe, exhibiting arterial phase enhancement and delayed washout, consistent with HCC. Notably, a BDTT extending from the right posterior hepatic duct to the right hepatic duct was observed, accompanied by dilatation of the right anterior bile duct (Figure 1A). Based on HBV history, characteristic imaging findings, and elevated PIVKA-II, the patient was diagnosed with HCC with BDTT, staged as Barcelona Clinic Liver Cancer (BCLC) stage A.
TACE was administered once at the initial stage of treatment, on 12 July 2023 (Figure 2). Iodized oil (15 mL) and pirarubicin were mixed and injected into the super-selective tumor artery via a microcatheter. The feeding arteries were selectively embolized using gelatin sponge particles until complete arterial flow stasis was observed. Lenvatinib (Lenvatinib Mesilate Capsules, 12mg orally once daily, Eisai Co.) was started on 12 July 2023 after informed consent was obtained from the patient. Sintilimab (Sintilimab Injection, 200 mg intravenously once every 3 weeks, Cinda Biopharmaceutical (Suzhou) Co.) was injected on 14 July 2023 and 5 August 2023, respectively. One-month post-lenvatinib initiation, the patient developed grade 1 hypertension (systolic blood pressure: 140–159 mmHg) per Common Terminology Criteria for Adverse Events (CTCAE v5.0), without associated symptoms (eg, headache, dizziness). Blood pressure was stabilized within 3 days using valsartan (80 mg daily), with no interruption of lenvatinib therapy.
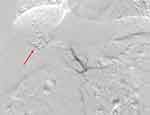
After two months of combined therapy, contrast-enhanced MRI demonstrated complete resolution of the BDTT and partial response (according to modified Response Evaluation Criteria in Solid Tumors criteria) of the primary tumor (Figure 1B). The patient underwent right hemihepatectomy on September 12, 2023, following a two-week lenvatinib washout period. Intraoperative blood loss was 200 mL. Histopathological examination confirmed poorly differentiated HCC with extensive tumor necrosis (>90% necrosis rate; Figure 3A and B). Postoperatively, antiviral therapy for HBV was maintained, and surveillance MRI at 3-month intervals revealed no recurrence at the latest follow-up (15 months post-surgery; Figure 3C and D).
Discussion
In contrast to digestive system malignancies, which often infiltrate the lymph nodes, HCC tends to invade adjacent vascular structures within the liver parenchyma.19 HCC with BDTT represents a rare yet unique subtype of HCC. Currently, this entity remains understudied, with significant gaps in evidence-based guidelines for its management at the international level. Notably, major oncology guidelines including the BCLC staging system and the American Joint Committee on Cancer (AJCC) TNM classification have not incorporated specific criteria or treatment algorithms for BDTT. Researchers in Southeast Asia, including China, have proposed surgery, TACE, radiotherapy, and comprehensive treatment combining multiple therapeutic approaches.20 Generally, the primary treatment objective for HCC with BDTT is to select an optimal method that effectively eliminates or controls the primary lesion, while prioritizing the extension of patient survival and enhancement of the overall quality of life through integrated multidisciplinary approaches.
TACE is considered a viable treatment and has shown favorable long-term survival outcomes compared to the best conservative management.21,22 Several retrospective studies have reported median survival times of 6–11 months following TACE for BDTT.23–25 Shen et al retrospectively compared patients undergoing neoadjuvant TACE with elective curative liver resection and those undergoing curative liver resection alone. They found that neoadjuvant TACE significantly reduced the operative risk of curative liver resection and significantly prolonged the median survival in patients with HCC and BDTT.24 Another study indicated that TACE combined with 125I seed stent in the treating patients with HCC and BDTT, improved the median survival time compared to patients who received TACE alone. Combining TACE with other treatments has been suggested to improve efficacy.26 Few reports exist on surgical treatment post-TACE. Sakata et al reported a case of a 78-year-old female patient diagnosed as HCC with BDTT, where the tumour was primarily located in the S1 segment, and the embolus extended to the common hepatic duct. The patient was treated with TACE followed by surgery after endoscopic biliary drainage, tumor-free survival for 9 months after hepatectomy.22
In recent years, targeted therapy has achieved significant curative effects in HCC.3,18,27 However, clinical studies investigating the prognostic implications of BDTT are sparse, in contrast to the extensive research conducted on HCC with portal vein or hepatic vein tumor thromboses. Sorafenib’s efficacy and safety profile in unresectable HCC with BDTT was first systematically investigated by Tanaka et al.28 This retrospective cohort study enrolled 175 patients with advanced HCC, including 165 cases without BDTT and 10 with BDTT. No significant differences in objective response rate (ORR) or disease control rate were observed between the two groups (ORR: 13.9% vs 20.0%, P=0.42; DCR: 68.5% vs 80.0%, P=0.21). Median OS and time to progression also did not differ significantly between the groups (12.6 months vs 10.3 months, P=0.35; 6.8 months vs 5.1 months, P=0.47). Notably, 50% of BDTT patients (n=5) developed biliary complications, including obstructive jaundice and biliary hemorrhage. However, these adverse events were managed effectively through endoscopic interventions, allowing uninterrupted sorafenib administration without dose reduction. The study preliminarily suggested that sorafenib may maintain comparable antitumor activity in BDTT-positive HCC patients, with manageable safety profile.
Several literatures have revealed that TACE combined with lenvatinib and PD-1 inhibitors achieved better efficacy in the treatment of advanced HCC, with progression-free survival ranging from 6.3 to 22.5 months, and OS ranging from 15.7 to 29 months.29–31 A randomized controlled trial evaluated the efficacy and safety of preoperative TACE in patients with stage A/B HCC classified under the BCLC system and exceeding the Milan criteria. The findings indicated that preoperative TACE significantly improved survival outcomes in this patient group.32 In a retrospective analysis of a triple-regimen neoadjuvant therapy for resectable HCC patients with high-risk recurrence factors, investigators reported an ORR of 83.33% (according to modified response evaluation criteria in solid tumors criteria), a pathological complete response rate of 26.1%, and a MPR rate of 84.3%. Compared to patients undergoing direct surgery, those receiving neoadjuvant therapy showed a marked reduction in microvascular invasion and a higher rate of R0 resections. Importantly, these benefits were achieved without increased intraoperative bleeding or prolonged operative times.33 The possible mechanism lies: TACE-induced hypoxia may upregulate PD-L1 expression in residual tumor cells, thereby sensitizing the microenvironment to PD-1 inhibitors. Concurrent lenvatinib suppresses compensatory angiogenesis, creating a durable antitumor immune response. This mechanistic synergy aligns with recent studies on multimodal HCC therapy.30,31 Additionally, in the neoadjuvant setting, immune checkpoint inhibitors more effectively enhance the de novo induction of T cell-mediated immunity, expansion of pre-existing antitumor T cells, and diversification of the tumor-specific T cell repertoire compared to their use in the adjuvant setting post-tumor resection.27
The safety of combining TACE, lenvatinib, and PD-1 inhibitors requires careful consideration due to overlapping toxicities and potential synergistic risks. In our case, the patient experienced only grade 1 hypertension, which demonstrating the manageable toxicity of this regimen when administered with proactive monitoring. A multicenter, real-world study involving 1244 patients with advanced HCC receiving first-line targeted therapy combined with PD-1 inhibitors, with or without transarterial chemoembolization (TACE), demonstrated that the TACE-based combination therapy significantly improved median OS, median PFS, and ORR compared to targeted-ICI therapy alone, while exhibiting an acceptable safety profile.34
For our patient, characterized by a large tumor invading the bile ducts, the high recurrence risk following direct surgery prompted the adoption of a triple neoadjuvant therapy approach. To our knowledge, triple therapy for treating HCC with BDTT has not yet been reported. This patient treated with triple therapy achieved partial response on preoperative imaging and the BDTT regressed significantly compared to the pre-treatment period. Additionally, postoperative pathology showed a major pathologic response with negative right bile duct incision margin. However, its long-term efficacy needs to be further confirmed in a large cohort of cases. The timing of surgery and options for postoperative adjuvant therapy also warrant further investigation.
In conclusion, TACE, combined with lenvatinib and PD-1 inhibitors may be a safe and effective treatment option for patients with HCC with BDTT.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and received full ethical approval from the Ethics Review Committee of The Fujian Provincial Hospital, including approval to publish the case details.
Patient Consent for Publication
Written informed consent was obtained from the patient for the publication of anonymized data and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 2024;46(3):221–231. doi:10.3760/cma.j.cn112152-20240119-00035
3. Zheng J, Wang S, Xia L, et al. Hepatocellular carcinoma: signaling pathways and therapeutic advances. Sig Transduct Target Ther. 2025;10:35.
4. Wu J, Liu W, Qiu X, et al. A noninvasive approach to evaluate tumor immune microenvironment and predict outcomes in hepatocellular carcinoma. Phenomics. 2023;3(6):549–564. doi:10.1007/s43657-023-00136-8
5. Wu JY, Sun JX, Wu JY, et al. Impact of bile duct tumor thrombus on the long‑term surgical outcomes of hepatocellular carcinoma patients: a propensity score matching analysis. Ann Surg Oncol. 2022;29:949–958. doi:10.1245/s10434-021-10799-0
6. Kim GM, Kim HC, Hur S, et al. Sloughing of biliary tumour ingrowth of hepatocellular carcinoma after chemoembolization. Eur Radiol. 2016;26(6):1760–1765. doi:10.1007/s00330-015-3974-y
7. Ikenaga N, Chijiwa K, Otani K, et al. Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion. J Gastrointest Surg. 2009;13:492–497. doi:10.1007/s11605-008-0751-0
8. Choi J, Shim JH, Park DH, et al. Clinical usefulness of endoscopic palliation in patients with biliary obstruction caused by hepatocellular carcinoma. Digestion. 2013;88(2):87–94. doi:10.1159/000353200
9. Noda T, Nagano H, Tomimaru Y, et al. Prognosis of hepatocellular carcinoma with biliary tumor thrombi after liver surgery. Surgery. 2011;149:371–377. doi:10.1016/j.surg.2010.08.006
10. An J, Lee KS, Kim KM, et al. Clinical features and outcomes of patients with hepatocellular carcinoma complicated with bile duct invasion. Clin mol Hepatol. 2017;23(2):160–169. doi:10.3350/cmh.2016.0088
11. Oba A, Takahashi S, Kato Y, et al. Usefulness of resection for hepatocellular carcinoma with macroscopic bile duct tumor thrombus. Anticancer Res. 2014;34(8):4367–4372.
12. Chang H, Xu J, Mu Q, Qin C, Zhang Z, Wu T. Occult hepatocellular carcinoma: a case report of a special icteric-type hepatoma and literature review. Eur J Cancer Care. 2010;19(5):690–693. doi:10.1111/j.1365-2354.2008.01035.x
13. Wong TC, Cheung TT, Chok KS, et al. Outcomes of hepatectomy for hepatocellular carcinoma with bile duct tumour thrombus. HPB. 2015;17(5):401–408. doi:10.1111/hpb.12368
14. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
15. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
16. Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8(1):58. doi:10.1038/s41392-022-01235-0
17. Guo C, Zhang J, Huang X, et al. Preoperative sintilimab plus transarterial chemoembolization for hepatocellular carcinoma exceeding the Milan criteria: a phase II trial. Hepatol Commun. 2023;7(3):e0054. doi:10.1097/HC9.0000000000000054
18. Alliance of Chinese Expert Consensus on Neoadjuvant Therapy for Hepatocellular Carcinoma, Committee of Digestive Surgery of Chinese Research Hospital Association, Committee of Liver Cancer, Chinese Anti-Cancer Association. Chinese expert consensus on neoadjuvant therapy for hepatocellular carcinoma (2023 edition). Zhonghua Wai Ke Za Zhi. 2023;61(12):1035–1045. doi:10.3760/cma.j.cn112139-20230914-00121
19. Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9(3):385–391. doi:10.3748/wjg.v9.i3.385
20. Xiang X, Lau WY, Wu ZY, et al. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: a multicenter study. Eur J Surg Oncol. 2019;45(8):1460–1467. doi:10.1016/j.ejso.2019.03.042
21. Dai QS, Gu HL, Ye S, et al. Transarterial chemoembolization vs. conservative treatment for unresectable infiltrating hepatocellular carcinoma: a retrospective comparative study. mol Clin Oncol. 2014;2(6):1047–1054. doi:10.3892/mco.2014.391
22. Sakata J, Kobayashi T, Takizawa K, et al. Surgical resection after transarterial chemoembolization for hepatocellular carcinoma with bile duct tumor thrombus-report of a long-term survivor. Gan to Kagaku Ryoho. 2019;46(2):297–299.
23. Yang B, Jie L, Yang T, et al. TACE plus lenvatinib versus TACE plus sorafenib for unresectable hepatocellular carcinoma with portal vein tumor thrombus: a prospective cohort study. Front Oncol. 2021;11:821599. doi:10.3389/fonc.2021.821599
24. Shen Y, Li P, Cui K, et al. Neoadjuvant transcatheter arterial chemoembolization for biliary tumor thrombosis: a retrospective study. Int J Technol Assess Health Care. 2016;32(4):212–217. doi:10.1017/S0266462316000374
25. Ebara C, Yamazaki S, Moriguchi M, et al. Complete remission by transarterial infusion with cisplatin for recurrent bile duct tumor thrombus of hepatocellular carcinoma: report of a case. World J Surg Oncol. 2013;11:78. doi:10.1186/1477-7819-11-78
26. Li S, He X, Dang L, et al. Efficacy of 125I Versus Non-125I Combined with Transcatheter Arterial Chemoembolization for the Treatment of Unresectable Hepatocellular Carcinoma with Obstructive Jaundice. Dig Dis Sci. 2018;63(2):321–328. doi:10.1007/s10620-017-4899-x
27. Lucas MW, Versluis JM, Rozeman EA, et al. Personalizing neoadjuvant immune-checkpoint inhibition in patients with melanoma. Nat Rev Clin Oncol. 2023;20:408–422. doi:10.1038/s41571-023-00760-3
28. Tanaka T, Kuzuya T, Ishigami M, et al. Efficacy and safety of sorafenib in unresectable hepatocellular carcinoma with bile duct invasion. Oncology. 2020;98(9):621–629. doi:10.1159/000507051
29. Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-pd-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi:10.2147/JHC.S332420
30. Li X, Chen J, Wang X, et al. Outcomes and prognostic factors in initially unresectable hepatocellular carcinoma treated using conversion therapy with lenvatinib and TACE plus PD-1 inhibitors. Front Oncol. 2023;13:1110689. doi:10.3389/fonc.2023.1110689
31. Liu J, Li Z, Zhang W, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;12:709060. doi:10.3389/fphar.2021.709060
32. Fang C, Luo R, Zhang Y, et al. Hepatectomy versus transcatheter arterial chemoembolization for resectable BCLC stage A/B hepatocellular carcinoma beyond Milan criteria: a randomized clinical trial. Front Oncol. 2023;13:1101162. doi:10.3389/fonc.2023.1101162
33. Wu JY, Wu JY, Li YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for neoadjuvant treatment of resectable hepatocellular carcinoma with high risk of recurrence: a multicenter retrospective study. Front Oncol. 2021;12:985380. doi:10.3389/fonc.2022.985380
34. Jin ZC, Chen JJ, Zhu XL, et al. Immune checkpoint inhibitors and anti-vascular endothelial growth factor antibody/tyrosine kinase inhibitors with or without transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma (CHANCE2201): a target trial emulation study. E Clin Med. 2024;72:102622.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Trial Designs for Integrating Novel Therapeutics into the Management of Intermediate-Stage Hepatocellular Carcinoma
Su YY, Liu YS, Hsiao CF, Hsu C, Chen LT
Journal of Hepatocellular Carcinoma 2022, 9:517-536
Published Date: 2 June 2022
Macrotrabecular-Massive Hepatocellular Carcinoma: Light and Shadow in Current Knowledge
Sessa A, Mulé S, Brustia R, Regnault H, Galletto Pregliasco A, Rhaiem R, Leroy V, Sommacale D, Luciani A, Calderaro J, Amaddeo G
Journal of Hepatocellular Carcinoma 2022, 9:661-670
Published Date: 27 July 2022
Comparison of the Efficacy and Safety of Transarterial Chemoembolization with or without Lenvatinib for Unresectable Hepatocellular Carcinoma: A Retrospective Propensity Score–Matched Analysis
Chen YX, Zhang JX, Zhou CG, Liu J, Liu S, Shi HB, Zu QQ
Journal of Hepatocellular Carcinoma 2022, 9:685-694
Published Date: 1 August 2022
Application and Resistance Mechanisms of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma
Tao M, Han J, Shi J, Liao H, Wen K, Wang W, Mui S, Li H, Yan Y, Xiao Z
Journal of Hepatocellular Carcinoma 2023, 10:1069-1083
Published Date: 10 July 2023
Efficacy and Safety of Lenvatinib Plus Programmed Death-1 Inhibitors with or Without Transarterial Chemoembolization in the Treatment of Unresectable Hepatocellular Carcinoma
Jin M, Jiang ZQ, Qin JH, Qin HX, Jiang KW, Ya HX, Gu J, Gui MR, Li YH, Xu LK, Fu HX, Xiao XH, Li SQ
Journal of Hepatocellular Carcinoma 2024, 11:2309-2320
Published Date: 25 November 2024