Back to Journals » Drug Design, Development and Therapy » Volume 19
Comparison Between Low-Dose Esketamine and Dexmedetomidine on Postoperative Recovery Quality Among Patients Undergoing Humeral Trauma Surgery in Interscalene Brachial Plexus Block: A Randomized, Double-Blind, Controlled Trial
Authors Chen J, Qi Y , Zhang J, Sun B, Zhang M, Meng X, Zhou M, Wang L
Received 28 November 2024
Accepted for publication 29 April 2025
Published 5 May 2025 Volume 2025:19 Pages 3645—3655
DOI https://doi.org/10.2147/DDDT.S507427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Jiao Chen,1,2,* Yu Qi,1,2,* Jun Zhang,1,3,* Bin Sun,1,2 Meng Zhang,2 Xiangdi Meng,2 Meiyan Zhou,1,2 Liwei Wang1,2
1The Xuzhou Clinical College of Xuzhou Medical University, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, Xuzhou Central Hospital, Jiangsu, People’s Republic of China; 3Department of Bone and Joint Surgery, Xuzhou Central Hospital, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meiyan Zhou, Department of Anesthesiology, Xuzhou Central Hospital, Xuzhou, People’s Republic of China, Tel +1 771 298 8959, Email [email protected]; Liwei Wang, Department of Anesthesiology, Xuzhou Central Hospital, Xuzhou, People’s Republic of China, Tel +1 895 217 0255, Email [email protected]
Purpose: Patients with humeral fracture often suffer from post-traumatic neuropsychiatric sequelae, which can cause immense anxiety or fear and worsen recovery. In this report, we examined the effect of low-dose esketamine versus dexmedetomidine on postsurgical recovery among patients who underwent humerus surgery with interscalene brachial plexus block.
Patients and Methods: In this prospective, randomized, controlled study, 141 patients aged 18 to 65 years who underwent humerus reduction and internal fixation were recruited. Patients were randomly assigned to two groups: esketamine (Group E: received 0.2 mg/kg (i.v.) esketamine administration, with subsequent continuous 0.15mg/kg/h infusion); dexmedetomidine (Group D: received 10-min 0.8μg/kg dexmedetomidine infusion, with 0.4ug/kg/h maintenance infusion). All infusions were terminated at closure of surgical incisions. Our major endpoint was the Quality of Recovery-40 (QoR-40) score on postoperative day 1 (POD-1). The secondary outcomes were QoR-40 POD-3, the intraoperative modified observer’s assessment of alert/Sedation (MOAA/S) scores at 5 min (T1) and 10 min (T2) post i.v. administration, at operation initiation (T3), at 10 min interval (T4), 30 min interval (T5) post operation, and at the end of operation (T6), Numeric Rating Scale (NRS) at POD-1, additional postoperative analgesic usage and hospital stays. In addition, we analyzed safety indices, such as hemodynamic profile, postoperative nausea and vomiting, adverse events (AEs) involving the central nervous system.
Results: The QoR-40 scores on POD-1 for Group E were substantially elevated relative to Group D. The T4 and T5 MOAA/S scores of Group D were lower relative to Group E. In comparison to Group E, Group D exhibited reduced T1 and T2 Mean arterial pressure (MAP) and T1-T6 Heart rate (HR). Lastly, we observed no marked alteration in other postsurgical AEs between the two patient cohorts.
Conclusion: Continuous low-dose esketamine infusion seems safely and tolerably, it significantly improves the postoperative recovery quality among patients with ASA I or II receiving elective humeral trauma surgery.
Keywords: esketamine, dexmedetomidine, quality of postoperative recovery, QoR-40, Humeral trauma surgery
Introduction
Traumatic injury is of serious global concern. Emerging evidences revealed that traumatic injury is often accompanied with post-traumatic neuropsychiatric sequelae, namely, anxiety, depression, hyperarousal, sleep disruption, nightmares and pain, which severely impact patient postoperative quality of life.1,2 According to the Global Burden of Disease report completed in 2019, orthopaedic trauma incidences have risen by 70% since 1990s. Following lower limb fractures, upper limb fractures are the second leading form of new fractures,3 among which humeral fractures are relatively high and are linked to significant postoperative pain requiring multimodal analgesia.4,5 Orthopedic management is typically done with interscalene brachial plexus block owing to its high efficacy, reduced hospital duration, diminished hospital costs and lack of general anesthesia associated complications.6 Unfortunately, patients who only received brachial plexus nerve block often experience anxiety and fear.
Emerging evidence revealed that moderate sedation/analgesia usage can elevate patient tolerance during unpleasant or lengthy interventions by alleviating anxiety, discomfort, and pain. According to 2018 American Society of Anesthesiologists (ASA) moderate procedural sedation and analgesia recommendations, dexmedetomidine and esketamine are both effective as intraoperative sedation and analgesia.7 Dexmedetomidine specifically targets α2-adrenergic receptor and induces sedation and analgesia.8 One meta-analysis reported that i.v. dexmedetomidine can significantly enhance postoperative quality of life among adult patients.9 Compared with esketamine, dexmedetomidine has augmented sedation failure, high rates of hypotension and bradycardia.10,11 In addition, dexmedetomidine has a slower onset (10–15min), while esketamine acts within as little as 30 seconds.12 More recently, increasing clinical investigations report considerable efficacy and safety of low-dose esketamine. A recent study showed that subanesthetic dose of esketamine (0.15–0.3 mg/kg/h) can improve the sedative and analgesic effects during liposuction surgery.13 Clinical report showed that intraoperative intravenous esketamine can improve the QoR-40 scores in breast surgery patients.14 Till date, there are no reports on the potential superiority of esketamine over dexmedetomidine among trauma patients receiving humeral fracture surgery. Herein, we investigated the effects of low-dose esketamine on the early recovery of patients undergoing elective humeral fracture surgery.
Materials and Methods
Research Design and Subjects
This prospective, double-blind, RCT was approved by Xuzhou Central Hospital (XZXY-LK-20231029-0168) and registered with the Chinese Clinical Trial Registry prior to patient recruitment (ChiCTR2300077248). The study adhered to the Declaration of Helsinki and its latter amendments. We also acquired written informed consent from all individuals prior to the initiation of the study. Lastly, this study strictly abided by the Consolidated Standards of Reporting Trials (CONSORT) reporting criteria for RCT.
This investigation was conducted between November 25, 2023, and August 31, 2024, at the Xuzhou Central Hospital, concluding with the final follow-up of the last patient. In total, we examined 141 patients who received elective humeral trauma surgery, between 18 and 65 years of age, with the ASA physical status stratification between I and II (I being healthy patient and II being patient with mild systemic disease). The following patients were excluded from analysis: allergy to any medication used in this study; esketamine contraindications, namely, glaucoma, large vessel aneurysm, and so on; severe cardiopulmonary, hepatic, and renal impairment; cognitive decline or history of psychiatric or neurological disorders; preoperative atrioventricular block or bradycardia; and lastly, refusal to participate in study or fail to complete the questionnaire.
Randomization and Blinding
Eligible participants were randomly assigned in a 1:1 ratio to either the esketamine group (Group E) or the dexmedetomidine group (Group D) using computer generated randomization sequence (Figure 1). To confirm blinding, group allocations were sealed in numbered envelopes, before delivery to an anesthesia nurse who was not linked to the investigation. The anesthesia nurse prepared the study drugs according to group assignment and provided esketamine or dexmedetomidine.
 |
Figure 1 CONSORT illustration depicting the patient selection process. Group E esketamine-treated patients, Group D dexmedetomidine-treated patients. |
Patients, responsible anesthesiologists and the investigator who was responsible for patient recruitment, data collection, and follow-up assessments were blinded to group assignment. In the event of an emergent situation, such as an unanticipated and precipitous decline in a subject’s clinical condition, responsible anesthesiologists are authorized to alter or cease the administration of the investigational agent. The protocol of blinding may be breached solely in instances where it is demonstrably necessary for clinical management purposes.
Monitoring
Prior to surgery, all patients underwent an 8-h fast from solids and a 2-h fast from clear fluids. Peripheral venous access was established after they entered the operating room. Subsequently, we closely monitored the vital signs via pulse oximetry (SpO2), electrocardiogram (ECG), heart rate (HR), and noninvasive blood pressure (NBP) measurement. Patients received 5 L/min oxygen via face mask.
Anesthesia Protocol
To ensure the consistency of medication administration, both groups received the same administration route. Group E received intravenous injection 0.2mg/kg esketamine diluted to 10 mL, followed by 20 mL normal saline over 10 minutes, and then an infusion of 0.15mg/kg/h esketamine until incision closure. Group D received an injection of 10 mL normal saline, followed by an infusion of 0.8µg/kg dexmedetomidine diluted to 20 mL over 10 minutes, and then an infusion of 0.4ug/kg/h dexmedetomidine until skin closure. All pumps are covered with opaque paper to ensure blindness for other personnel.
An ultrasound-guided interscalene brachial plexus block was conducted following the initial 10-min of i.v. administration. All ultrasound-guided blocks were performed by a team of senior anesthesiologists with extensive experience in regional anesthesia. Patients were laid supine, with their heads turned to the opposite side. After standard sterile preparation, a 3.5 MHz linear array transducer (EDGE® ultrasound machine, Sonosite Inc USA) was used to identify the brachial plexus between the anterior and middle scalene muscles. Under real-time ultrasound guidance, a short-bevel needle (25-gauge, 5 cm) was inserted toward the brachial plexus using in-plane technique with a lateral-to-medial direction. A slight withdrawal confirmed the absence of blood or air, after which 15 mL of 0.5% ropivacaine was administered. The sensory blockade efficacy was assessed using pinprick tests along the musculocutaneous, radial, median, and ulnar distribution at 5 min intervals until an effective blockade, i.e, complete absence of pinprick sensation, was established. In case the patient experienced pain in the surgical location, additional analgesics were introduced or we switched to a different anesthetic method, and the patient was excluded from the study altogether.
Postoperative Management
Hemodynamic parameters, including NBP, HR, and SpO2 were monitored and documented till surgery termination. Adverse events (AEs) were defined as bradycardia (HR < 50 bpm), hypertension (systolic blood pressure (SBP) >140 mmHg or >20% rise in baseline value), hypotension (SBP < 90 mmHg or a > 20% decline from baseline value) and respiratory depression (pulse oxygen saturation <93% or respiratory rate <8 beats/min), and were provided with i.v. atropine 0.5 mg, urapidil 10–25mg, and ephedrine 5–10 mg, respectively. Patients were given an i.v. administration of 50 mg flurbiprofen during incision closure, and they received i.v. 30 mg ketorolac administration every 8 h for one day postoperation. If the NRS scores ≥4, rescue dose of 5mg oxycodone hydrochloride was given.
Outcome Measurements
The major endpoint was the postoperative day 1 (POD-1) QoR-40 score. The score was based off a questionnaire that evaluated 5 aspects of patient recovery: physical comfort (12 queries), emotional status (9 queries), physical independence (5 queries), psychological support (7 queries), and pain (10 queries). Individual items presented a 5-point rating (1 = none of the time, 2 = some of the time, 3 = usually, 4 = most of the time, 5 = all of the time), and the summation score was between 40 and 200 points. All participants received a detailed description of all questions the day before surgery.
Our secondary endpoint included the POD-3 QoR-40 score, intraoperative hemodynamic alterations, such as, MAP and HR at baseline (T0), 5 min (T1), 10 min (T2) post administration, at operation initiation (T3), 10 min (T4), 30 min (T5) post-operation, and at the end of operation (T6); the intraoperative MOAA/S (A scoring scale used to evaluate a patient’s behavioral response to stimulation). The MOAA/S score ranges from 5(fully alert) to 0(completely sedated) Table S1 and NRS scores (A tool used to assess the level of pain). Pain intensity is measured on a scale from 0 to 10, where 0 represents no pain and 10 represents the most intense pain. Figure S1 at POD1; additional postoperative analgesic usage; hospitalization duration; intra- and postoperative AEs, namely, decreased oxygen saturation, respiratory depression, hemodynamic instability, postoperative nausea and vomiting (PONV, Table S2) postoperative shivering, nightmares, hallucination, dizziness and agitation.
Sample Size Calculation and Statistical Analyses
The appropriate sample size was determined in PASS version 15.0 via analysis of the results of our pilot study. The average QoR-40 scores of Groups E and D were 179.1 and 173.0 and the standard deviations (SDs) were 8.37 and 9.76, with an α of 0.05, β of 0.1, and 48 patients were required per cohort. Considering a dropout rate of 20%, we included 120 individuals in this study.
All statistical analyses were conducted using SPSS software version 26.0 and GraphPad Prism version 10.0. For continuous data, the Shapiro–Wilk test and histograms were used to assess the distribution of data. Data with normal distribution were examined via two independent sample t-test, and provided as mean ± SD. Variables with non-normal distribution were assessed via the Mann–Whitney U-test and are expressed as median (interquartile range). Lastly, Categorical data were examined via the chi-squared (χ2) and Fisher’s exact tests and are presented as absolute numbers (%). Repeated normally distributed variables (MAP, HR) were examined using analysis of covariance (ANCOVA), baseline MAP and HR used as covariates to more accurately assess the impact of groups on the results. The sphericity was evaluated through Mauchly’s test, if violated, the Greenhouse–Geisser correction was employed for degrees of freedom adjustment. Lastly, a generalized estimating equation (GEE) was employed for the analysis of repeated abnormally distributed variables (QoR-40, MOAA/S). Two-tailed p-value <0.05 were considered as statistically significance.
Results
Patients Demographics and Clinical Profiles
The study details are summarized in Figure 1. In total, 115 patients who chose to receive elective humeral trauma surgery were recruited in this study. Among them, 98 patients (48 from Group E and 50 from Group D) completed the study, and 17 patients were eliminated from analysis due to the following reasons: 5 were lost to follow-up, 3 requested patient-controlled analgesia (PCA), 6 failed to complete the questionnaire, and 3 received incomplete motor and sensory block.
Preoperative use of analgesic or sedative medications was documented. Patients routinely received 0.3g oral acetaminophen every 8h. If the NRS scores ≥4, they received 50mg tramadol hydrochloride tablet every 6 h. Patients who requested management of poor sleep quality received 0.4mg oral alprazolam. No other differences were present in the baseline characteristics or intraoperative data between the two cohorts (p>0.05) (Table 1).
 |
Table 1 Clinical Characteristics and Demographics of Patients |
Recovery Quality (QoR-40) Score Alterations
Alterations in the QoR-40 score over time are summarized in Table 2. We conducted a GEE comparing both patient cohorts in overall QoR-40 on PODs 1 and 3 after correcting the effect of baseline QoR-40 (Table 2). There was an interaction between time and group (p<0.001). The QoR-40 on POD-1 was substantially high among Group E versus D (p<0.001), which indicates a significant improvement in postoperative recovery. The estimated QoR-40 differences on POD-1 between both cohorts was 6.91 (95% CI 4.48,9.35), and no marked difference was evident in QoR-40 on POD 3 (p=0.299). In case of the five dimensions, Group E exhibited marked enhancement in physical comfort and emotional status on POD1 relative to Group D (p<0.001, p=0.014). No substantial difference was found in the remaining dimensional QoR-40 scores (p > 0.05) (Table S3).
 |
Table 2 Comparison of QoR-40 Scores Between the Two Patient Cohorts at Varying Time Points |
Perioperative Hemodynamic Alterations
MAP alterations over the study period are presented in Figure 2a. According to Mauchly’s test, we revealed violation of the sphericity assumption (W = 0.084, p<0.001). Thus, we corrected the degrees of freedom using the Greenhouse–Geisser estimates of sphericity (e). The main effect of the group and the interaction effect between time and patient cohort reached significance (F = 11.39, p = 0.001, and F = 38.86, p<0.001, respectively). MAP was elevated at T1 and T2 compared to Group D (p<0.05).
HR alterations over time are presented in Figure 2b. On the basis of Mauchly’s test, we revealed violation of the sphericity assumption (W = 0.095, p<0.001). Thus, we corrected the degrees of freedom using the Greenhouse–Geisser estimates of sphericity (e). We uncovered marked significant between time and patient grouping (F = 13.99, p<0.001, and F = 74.26, p<0.001, respectively). Relative to Group E values, HR was diminished at T1-T6 (p<0.05).
Intraoperative MOAA/S Score Alterations
Table 3 reveals intraoperative MOAA/S score alterations. We conducted generalized estimated equation (GEE) to evaluate the T1-T6 MOAA/S scores of both patient cohorts. The estimated T4 and T5 MOAA/S differences were 0.46 (95% CI 0.21, 0.71, p<0.01) and 0.91 (95% CI 0.77,1.05, p<0.01), respectively. No obvious difference was observed in the MOAA/S score at other time points (p>0.05).
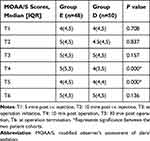 |
Table 3 Intraoperative MOAA/S Scores |
Postoperative Data Assessment
Table 4 list the postoperative data of all participants. In Group E, three subjects experienced nightmares, while one reported dizziness. Their symptoms were temporary and resolved with psychological counseling by the nurse without the use of medication. There were no reports of postoperative shivering, hallucinations or agitation in either group. We also found no marked differences in the PONV and NRS incidences at POD-1, as well as oxycodone hydrochloride usage and hospitalization duration between the two cohorts (p>0.05). Furthermore, no significant respiratory AEs were reported in either group post-surgery (Table S4).
 |
Table 4 Postoperative Clinical Information |
Discussion
Based on our observation, low-dose esketamine (0.2 mg/kg esketamine, with subsequent continuous 0.15mg/kg/h infusion) significantly improved the POD-1 recovery among patients with ASA I or II undergoing elective humeral trauma surgery.
Patients suffering from humeral fracture frequently experience anxiety and pain related to anesthesia and surgery. Procedural sedation and analgesia (PSA), which is aligned with the theory of enhanced Recovery After Surgery (ERAS), is a gold standard practice for alleviating anxiety, discomfort and pain during invasive diagnostic and therapeutic interventions.15 Being a frequently indicated drug in PSA, the safety and efficacy of esketamine have been reported in multiple investigations.16,17 Zhu et al16 demonstrated that 4ug/kg/h esketamine administration partially augmented POD-1 recovery quality among patients undergoing modified radical mastectomy, which is similar to our findings. Lee et al18 also reported that intraoperative dexmedetomidine enhanced POD-1 QoR among patients undergoing video-guided thoracoscopic surgery. This study found that esketamine is superior to dexmedetomidine in enhancing POD-1 recovery. The estimated QoR-40 difference on POD-1 between the two groups was 6.91 (95% CI 4.48, 9.35), which not only reached statistical significance (p < 0.05) but also surpassed the minimal clinically important difference (MCID) threshold of 6.3 points, as reported by Myles et al.19 Moreover, patients who received esketamine demonstrated significantly better scores in physical comfort and emotional well-being when comparing the five QoR-40 dimensions on POD-1 (Tables 2 and S3).
Physical comfort, which encompasses 12 items, is a critical indicator of postoperative well-being. An elevated physical comfort score on POD-1 reflects effective pain management and suggests a minimal occurrence of adverse effects such as nausea, vomiting, or dizziness. Moreover, it indicates a substantial recovery in appetite and gastrointestinal functions, contributing to overall physical ease.20 The emotional state score, comprising 9 items, is equally important. A high score in this domain indicates an absence of pronounced anxiety, depression, or emotional lability, highlighting the patients’ ability to cope with the stressors of postoperative recovery.20 The combination of high scores in both physical comfort and emotional state suggests that patients in the esketamine group experienced a high quality of life during their postoperative recovery.21 This is typically associated with superior clinical outcomes, such as faster discharge, fewer postoperative complications and greater patient satisfaction. The major difference observed in the study may be the specific antidepressant influence of esketamine.22,23 The underlying mechanism may involve NMDA receptor antagonism, alpha κ and μ opioid receptor (KOR and MOR) antagonism, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) activation, and brain-derived neurotrophic factor (BDNF) upregulation.23,24
We also reviewed several pertinent studies examining the impact of varying dosages on postoperative recovery quality. Notably, Xu et al25 demonstrated that intraoperative intravenous low-dose esketamine (0.25 mg/kg bolus followed by a continuous infusion of 0.12 mg/kg/h) enhanced early postoperative recovery in patients undergoing laparoscopic radical resection of colorectal cancer. Additionally, Zhang et al26 reported that 0.5 mg/kg/h esketamine infusion improved the quality of postoperative recovery and reduced pain on POD-1. Moreover, the combination of 0.2 mg/kg esketamine with dexmedetomidine was found to be safe for lung tumor percutaneous radiofrequency ablation, offering fewer hemodynamic disturbances, milder respiratory depression, shorter recovery times, and higher radiologist satisfaction due to better sedation depth control.27 These studies provide valuable insights and reinforce our findings that intravenously administered low-dose esketamine significantly enhances early quality of recovery (QoR) in patients undergoing elective humeral trauma surgery. According to the 9th edition of Miller’s Anesthesia,28 for general anesthesia with esketamine, the minimum required blood concentration had ranged from 0.3 to 1.0 mg/L; however, a blood concentration of ≥0.05 mg/L was sufficient to elevate the pain threshold. The pharmacokinetic characteristics of esketamine in the Chinese population indicated an average half-life of roughly 4 hours, a distribution volume between 5 and 10 L/kg, and a clearance rate (Cl) of approximately 1.08 L/kg/h.29 In our study, we administered an initial dose of 0.2 mg/kg of esketamine, followed by a continuous infusion at 0.15 mg/kg/h. Given the average weight of patients in the esketamine group was 69.8 kilograms, the steady-state plasma concentration (Css) was calculated using the formula Css = R/Cl, with Cl estimated at around 1.08 L/kg/h and R being the infusion rate, resulting in a Css of 0.139 mg/L in our study. Our pharmacokinetic analysis confirmed that the achieved C₀ (0.027 mg/L) was below the typical therapeutic range of 0.3–1.0 mg/L for general anesthesia, indicating a potential underdosing during induction. We observed enhanced QoR-40 scores on postoperative day 1, even at this subanesthetic concentration, suggesting potential clinical benefits at lower doses.
Herein, we revealed that HR and MAP were strongly different between the two patient cohorts (Figure 2). Earlier reports suggest that esketamine stimulates the cardiovascular system in a concentration-reliant fashion via its sympathomimetic pathway.30,31 A study by Zhou et al32 demonstrated that esketamine upregulated MAP values relative to placebo before and after skin incision. Likewise, we demonstrated that Group E produced elevated T1 and T2 MAP, which remained within 20% of the basal blood pressure relative to Group D. Dexmedetomidine is a robust anesthetic, particularly when combined with a regional nerve block. Its many advantages are preserving airway reflexes, expanding tracheal smooth muscles, and inhibiting the cough response without inducing respiratory depression.33 Unfortunately, the sympathetic inhibition of dexmedetomidine greatly increases the chances of bradycardia and hypotension.34,35 Similar to earlier reports,36 we demonstrated that dexmedetomidine-treated patients had reduced T1-T6 HR relative to esketamine-treated patients. Based on this evidence, low-dose esketamine infusion is potentially effective in stabilizing the intraoperative hemodynamic status.
Recent reports reveal that dexmedetomidine-mediated sedation is very potent among older patients.12 Herein, we demonstrated that dexmedetomidine significantly reduced the MOAA/S scores in T4 and T5 relative to esketamine, indicating that dexmedetomidine also produces deeper sedation among younger patients. Our data corroborated the data from the Hansol Kim et al study.37 Notably, although we referred to prior investigations18,38 and employed a reduced dexmedetomidine dose, we still achieved deeper sedation in Group D, which raises the necessity of additional future discussions on the appropriate and optimal dexmedetomidine dose for clinical use.
Lastly, we observed no obvious alterations in PONV, CNS AEs, or hospitalization duration between the two patient cohorts. Earlier reports suggested that ketamine use is often restricted due to associated AEs of the CNS.39 In this report, we found no difference in neurological side effects between the two cohorts. One reason is that, esketamine is reported to induce lesser psychotomimetic influences, relative to the racemic mixture and R-isomer.40 The other is that participants of the current study were administered low-dose esketamine, which has been safely employed in many clinical trials.41
This study has several limitations. First, we did not monitor circulating esketamine content. Second, we focused on elective surgical patients with no comorbidities (ASA I–II) and included only a 3-day follow-up, which limits the generalizability of our results and the ability to assess long-term effects. Further research is needed to evaluate esketamine use in elderly patients to confirm the applicability of our findings. Future studies will aim to conduct larger, more diverse trials that build on the current work. Third, the optimal dosage and medication route of esketamine requires further clarification. Although our proposed dosage showed efficacy, it is potentially sub-optimal. Lastly, our sample population was relatively small, and study was conducted at a single-center. Hence, we warrant further multi-center and large population-based studies to validate our findings.
Conclusion
Intravenously administered low-dose esketamine is safe and well tolerated in humeral surgery, it can significantly enhance early QoR among patients with ASA I or II undergoing elective humeral trauma surgery.
Date Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. Any information we share will be deidentified.
Ethic Approval and Informed Consent
This study strictly abided by the CONSORT reporting criteria for RCT. The study was ethically endorsed by the Xuzhou Central Hospital (XZXY-LK-20231029-0168) and registered at the Chinese Clinical Trial Registry (ChiCTR2300077248). All subjects provided written informed consent prior to the initiation of the study.
Acknowledgments
The authors would like to acknowledge the support of patients who have generously given their time to complete the questionnaires for the research protocol.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Jiangsu Province’s Key Project on Elderly Health Research (NO. LKZ2023016), Jiangsu Province’s Key Discipline/Laboratory of Medicine (No. JSDW202231), Xuzhou Medical Key Talent Training Project (No. XWRCHT20210033), Xuzhou Medical Young Reserve Talent Program (No. XWRCHT20220017).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Straus LD, An X, Ji Y, et al. Utility of wrist-wearable data for assessing pain, sleep, and anxiety outcomes after traumatic stress exposure. JAMA Psychiatry. 2023;80(3):220–229. doi:10.1001/jamapsychiatry.2022.4533
2. Robles TF, Rünger D, Sumner JA, Elashoff D, Shetty V. Salivary inflammatory biomarkers as a predictor of post-traumatic stress disorder and depressive symptom severity in trauma patients: a prospective study. Brain Behav Immun. 2024;119:792–800. doi:10.1016/j.bbi.2024.05.011
3. Wu A-M, Bisignano C, James SL. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021;2(9):e580–e592. doi:10.1016/S2666-7568(21)00172-0
4. Borys M, Zyzak K, Hanych A, et al. Survey of postoperative pain control in different types of hospitals: a multicenter observational study. BMC Anesthesiol. 2018;18(1):83. doi:10.1186/s12871-018-0551-3
5. Ootes D, Lambers KT, Ring DC. The epidemiology of upper extremity injuries presenting to the emergency department in the United States. Hand. 2012;7(1):18–22. doi:10.1007/s11552-011-9383-z
6. Cozowicz C, Poeran J, Memtsoudis SG. Epidemiology, trends, and disparities in regional anesthesia for orthopedic surgery. Br J Anaesth. 2015;115(Suppl 2):ii57–ii67. doi:10.1093/bja/aev381
7. Ractice P. Practice guidelines for moderate procedural sedation and analgesia 2018: a report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology. 2018;128(3):437–479. doi:10.1097/ALN.0000000000002043
8. Bilotta F, Pugliese F. The evolving clinical use of dexmedetomidine. Lancet. 2020;396(10245):145–147. doi:10.1016/S0140-6736(20)30902-8
9. Miao M, Xu Y, Li B, et al. Intravenous administration of dexmedetomidine and quality of recovery after elective surgery in adult patients: a meta-analysis of randomized controlled trials. J Clin Anesth. 2020;65:109849. doi:10.1016/j.jclinane.2020.109849
10. Weerink MAS, Struys M, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi:10.1007/s40262-017-0507-7
11. Inagaki Y, Morita K, Ozaki M, et al. The efficacy and safety of dexmedetomidine for procedural sedation in patients receiving local anesthesia outside the intensive care unit: a prospective, double-blind, randomized clinical phase III trial in Japan. Yonago Acta Med. 2022;65(1):26–43. doi:10.33160/yam.2022.02.005
12. Mion G, Himmelseher S. Esketamine: less drowsiness, more analgesia. Anesth Analg. 2024;139(1):78–91. doi:10.1213/ANE.0000000000006851
13. Chen H, Zhi J, Wang L, et al. Subanesthetic dose of esketamine improves the sedative and analgesic effects of dexmedetomidine and remifentanil in liposuction anesthesia: a prospective, double-blinded, randomized controlled trial. Drug Des Devel Ther. 2024;20(18):3645–3658. doi:10.2147/DDDT.S470891
14. Huang Z, Liu N, Hu S, et al. Effect of dexmedetomidine and two different doses of esketamine combined infusion on the quality of recovery in patients undergoing modified radical mastectomy for breast cancer - a randomised controlled study. Drug Des Devel Ther. 2023;28(17):2613–2621. doi:10.2147/DDDT.S422896
15. Hinkelbein J, Lamperti M, Akeson J, et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol. 2018;35(1):6–24. doi:10.1097/EJA.0000000000000683
16. Zhu M, Xu S, Ju X, et al. Effects of the different doses of esketamine on postoperative quality of recovery in patients undergoing modified radical mastectomy: a randomized, double-blind, controlled trial. Drug Des Devel Ther. 2022;16:4291–4299. doi:10.2147/DDDT.S392784
17. Brinck ECV, Maisniemi K, Kankare J, et al. Analgesic effect of intraoperative intravenous S-ketamine in opioid-naïve patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth Analg. 2021;132(1):69–79. doi:10.1213/ANE.0000000000004729
18. Lee SH, Lee CY, Lee JG, et al. Intraoperative dexmedetomidine improves the quality of recovery and postoperative pulmonary function in patients undergoing video-assisted thoracoscopic surgery: a CONSORT-prospective, randomized, controlled trial. Medicine. 2016;95(7):e2854. doi:10.1097/MD.0000000000002854
19. Myles PS, Myles DB, Galagher W, et al. Minimal clinically important difference for three quality of recovery scales. Anesthesiology. 2016;125(1):39–45. doi:10.1097/ALN.0000000000001158
20. Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
21. Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth. 2013;111(2):161–169. doi:10.1093/bja/aet014
22. Liu P, Li P, Li Q, et al. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J Invest Surg. 2021;34(8):883–888. doi:10.1080/08941939.2019.1710626
23. Lewis V, Rurak G, Salmaso N, et al. An integrative view on the cell-type-specific mechanisms of ketamine’s antidepressant actions. Trends Neurosci. 2024;47(3):195–208. doi:10.1016/j.tins.2023.12.004
24. Wei Y, Chang L, Hashimoto K. A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol Biochem Behav. 2020;190:172870. doi:10.1016/j.pbb.2020.172870
25. Xu Y, He L, Liu S, et al. Intraoperative intravenous low-dose esketamine improves quality of early recovery after laparoscopic radical resection of colorectal cancer: a prospective, randomized controlled trial. PLoS One. 2023;18(6):e0286590. doi:10.1371/journal.pone.0286590
26. Zhang J, Wang F, Dang J, et al. Effect of intraoperative infusion of esketamine on quality of postoperative recovery in patients undergoing laparoscopic bariatric surgery: a randomized controlled trial. Pain Ther. 2023;12(4):979–992. doi:10.1007/s40122-023-00519-9
27. Lin Z, Li S, Zhou Y, et al. A comparative study of esketamine-dexmedetomidine and sufentanil-dexmedetomidine for sedation and analgesia in lung tumor percutaneous radiofrequency ablation (PRFA): a randomized double-blind clinical trial. BMC Anesthesiol. 2023;23(1):304. doi:10.1186/s12871-023-02266-y
28. Miller RD, Pardo MC, eds. Miller’s Anesthesia.
29. Wang J, Huang J, Yang S, et al. Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019;13:4135–4144. doi:10.2147/DDDT.S224553
30. Sigtermans M, Dahan A, Mooren R, et al. S(+)-ketamine effect on experimental pain and cardiac output: a population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111(4):892–903. doi:10.1097/ALN.0b013e3181b437b1
31. Trimmel H, Helbok R, Staudinger T, et al. S(+)-ketamine: current trends in emergency and intensive care medicine. Wien Klin Wochenschr. 2018;130(9–10):356–366. doi:10.1007/s00508-017-1299-3
32. Zhou N, Liang X, Gong J, et al. S-ketamine used during anesthesia induction increases the perfusion index and mean arterial pressure after induction: a randomized, double-blind, placebo-controlled trial. Eur J Pharm Sci. 2022;179:106312. doi:10.1016/j.ejps.2022.106312
33. Chen X, Xin D, Xu G, et al. The efficacy and safety of remimazolam tosilate versus dexmedetomidine in outpatients undergoing flexible bronchoscopy: a prospective, randomized, blind, non-inferiority trial. Front Pharmacol. 2022;13:902065. doi:10.3389/fphar.2022.902065
34. Sharp DB, Wang X, Mendelowitz D. Dexmedetomidine decreases inhibitory but not excitatory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2014;1574:1–5. doi:10.1016/j.brainres.2014.06.010
35. De Cassai A, Boscolo A, Geraldini F, et al. Effect of dexmedetomidine on hemodynamic responses to tracheal intubation: a meta-analysis with meta-regression and trial sequential analysis. J Clin Anesth. 2021;72:110287. doi:10.1016/j.jclinane.2021.110287
36. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi:10.1001/jama.2009.56
37. Kim H, Kim Y, Bae J, et al. Comparison of remimazolam and dexmedetomidine for intraoperative sedation in patients undergoing lower extremity surgery under spinal anesthesia: a randomized clinical trial. Reg Anesth Pain Med. 2024;49(2):110–116. doi:10.1136/rapm-2023-104415
38. Sherif AA, Elsersy HE. The impact of dexmedetomidine or xylocaine continuous infusion on opioid consumption and recovery after laparoscopic sleeve gastrectomy. Min Anestesiol. 2017;83(12):1274–1282. doi:10.23736/S0375-9393.17.11855-9
39. Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19(9):1033–1046. doi:10.1016/S0196-0644(05)82569-7
40. Muller J, Pentyala S, Dilger J, et al. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6(3):185–192. doi:10.1177/2045125316631267
41. Chen Y, Guo Y, Wu H, et al. Perioperative adjunctive esketamine for postpartum depression among women undergoing elective cesarean delivery: a randomized clinical trial. JAMA Network Open. 2024;7(3):e240953. doi:10.1001/jamanetworkopen.2024.0953
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effect of Dexmedetomidine and Two Different Doses of Esketamine Combined Infusion on the Quality of Recovery in Patients Undergoing Modified Radical Mastectomy for Breast Cancer - A Randomised Controlled Study
Huang Z, Liu N, Hu S, Ju X, Xu S, Wang S
Drug Design, Development and Therapy 2023, 17:2613-2621
Published Date: 28 August 2023
Opioid-Free Anesthesia for Pain Relief After Laparoscopic Cholecystectomy: A Prospective Randomized Controlled Trial
Yu JM, Tao QY, He Y, Liu D, Niu JY, Zhang Y
Journal of Pain Research 2023, 16:3625-3632
Published Date: 30 October 2023
Intranasal Dexmedetomidine-Esketamine Combination Premedication versus Monotherapy for Reducing Emergence Delirium and Postoperative Behavioral Changes in Pediatric Tonsillectomy and/or Adenoidectomy: A Randomized Controlled Trial

Liao Y, Xie S, Zhuo Y, Chen S, Luo Y, Wei Y, Yao Y
Drug Design, Development and Therapy 2024, 18:4693-4703
Published Date: 23 October 2024
Effects of Esketamine Combined with Dexmedetomidine on Early Postoperative Cognitive Function in Elderly Patients Undergoing Lumbar Spinal Surgery: A Double-Blind Randomized Controlled Clinical Trial
Tao QY, Liu D, Wang SJ, Wang X, Ouyang RN, Niu JY, Ning R, Yu JM
Drug Design, Development and Therapy 2024, 18:5461-5472
Published Date: 27 November 2024
The Effects of Opioid-Free Anesthesia with Dexmedetomidine and Esketamine on Postoperative Anesthetic-Related Complications for Hip Surgery in the Elderly
Ye Q, Hu Y, Xing Q, Wu Y, Zhang Y
International Journal of General Medicine 2024, 17:6291-6302
Published Date: 17 December 2024


