Back to Journals » Infection and Drug Resistance » Volume 18
Contezolid Harbored Equivalent Efficacy to Linezolid in Tuberculosis Treatment in a Prospective and Randomized Early Bactericidal Activity Study
Authors Jiang G, Liu R, Xue Y, Ge Q, Nie L, Lv Z, Kong Z, Shi J, Chen H, Li H, Wu X, Xie L, Song Y, Huang H , Gao M
Received 21 October 2024
Accepted for publication 1 January 2025
Published 13 January 2025 Volume 2025:18 Pages 261—268
DOI https://doi.org/10.2147/IDR.S499816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guanglu Jiang,1,* Rongmei Liu,2,* Yi Xue,1 Qiping Ge,2 Lihui Nie,2 Zizheng Lv,2 Zhongshun Kong,2 Jin Shi,2 Hongmei Chen,2 Hua Li,2 Xiaoguang Wu,2 Li Xie,2 Yanhua Song,2 Hairong Huang,1 Mengqiu Gao2
1National Clinical Laboratory on Tuberculosis, Beijing Key Laboratory on Drug-Resistant Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mengqiu Gao; Hairong Huang, Email [email protected]; [email protected]
Background: Contezolid (CZD) is an analog of Linezolid (LZD) that has demonstrated potent in vitro and in vivo activity against tuberculosis (TB) while presenting a safer side-effect profile. In this study, we evaluated the early bactericidal activity (EBA) of CZD compared to LZD, with LZD serving as a control.
Methods: Naive, smear-positive pulmonary TB patients were enrolled and randomly assigned to receive either a 14-day monotherapy regimen of 600 mg LZD once daily (QD) or 800 mg CZD twice daily (BID). Sputum samples were collected daily starting one day before treatment initiation and continuing throughout the treatment period. Each sample was processed for the enumeration of acid-fast bacilli (AFB) colonies, and time-to-positivity (TTP) during MGIT960 liquid culture was recorded.
Results: A total of 10 eligible patients were enrolled in each treatment group, although one patient in the CZD group was later excluded from the analysis. The early bactericidal activity (EBA0-14) was 0.08 ± 0.12 log CFU/mL/day (95% CI: − 0.02 to 0.18 CFU/mL/day) in the CZD group, compared to 0.03 ± 0.10 log CFU/mL/day (95% CI: − 0.05 to 0.10 CFU/mL/day) in the LZD group. The increase in time-to-positivity (TTP0-14) was 38.6 ± 43.69 hours (95% CI: − 1.85 to 79 hours) in the CZD group and 27.7 ± 78.21 hours (95% CI: − 28.15 to 83.75 hours) in the LZD group. LZD showed rapid bacterial reduction in sputum during the first two days of treatment, whereas CZD demonstrated superior efficacy after a few days of treatment.
Conclusion: 800 mg BID contezolid exhibited comparable efficacy to 600 mg QD LZD in treating pulmonary TB in this EBA study. While CZD showed slower initial bactericidal action compared to LZD, its efficacy surpassed that of LZD after a few days of treatment. Given its similar efficacy and superior safety profile, contezolid may serve as an alternative to linezolid for the treatment of tuberculosis.
Keywords: contezolid, linezolid, early bactericidal activity, time-to-positivity
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains a significant global health threat. According to the World Health Organization (WHO) Global Tuberculosis Report 2024,1 approximately 1.08 million new TB cases and 1.25 million TB-related deaths were recorded in 2023. Drug-resistant TB, particularly multidrug-resistant TB (MDR-TB) and rifampin-resistant TB (RR-TB), is a growing concern, with an estimated 400,000 cases reported in 2023. MDR-TB, defined by resistance to both isoniazid and rifampin, requires longer treatment durations, results in higher costs, causes more severe side effects, and leads to increased mortality compared to drug-susceptible TB. Despite the use of complex and expensive regimens, the overall success rate of MDR-TB treatment remains below 70%. Thus, the development of novel anti-mycobacterial drugs with improved safety, tolerability, and efficacy is crucial to combat drug-resistant TB.
Linezolid (LZD) is the first oxazolidinone antibiotic developed and exhibits potent activity against drug-resistant Gram-positive bacteria.2 It works by inhibiting bacterial protein synthesis through binding to the 23S rRNA of the bacterial 50S ribosomal subunit, without cross-resistance to other antibiotics. LZD has proven in vitro and in vivo efficacy against Mtb and is included by the World Health Organization (WHO) as a core drug for treating drug-resistant TB.3–5 Recent TB treatment regimens, such as BPaL,6,7 MDR-END8 and TB-PRACTUCAL,9 incorporate LZD, achieving up to a 90% cure rate in MDR-TB and rifampin-resistant TB patients. However, LZD is associated with significant adverse events, including bone marrow suppression, peripheral neuropathy, and optic nerve damage.10 A meta-analysis found that 58.9% of patients on LZD regimens experienced adverse events, with 68.4% of these classified as major, including anemia (38.1%), peripheral neuropathy (47.1%), gastrointestinal issues (16.7%), optic neuritis (13.2%), and thrombocytopenia (11.8%).11 Among anti-TB drugs, LZD has the highest rate of permanent discontinuation due to adverse events (14%). The incidence of these events is dose-dependent, with a higher frequency observed when the daily dosage exceeds 600 mg.12 Although reducing the dosage to 300 mg/day may reduce side effects, this adjustment only minimally mitigates adverse reactions.13 Therefore, there is an urgent need for LZD analogs that maintain strong anti-TB efficacy while offering a better safety profile.
Several oxazolidinones, including tedizolid (TZD), sutezolid (SZD), delpazolid (DZD), and contezolid (CZD), have been developed in recent years.14–17 In 2021, the Chinese FDA approved CZD for treating Gram-positive bacterial infections. While CZD shares a similar core structure with LZD, it has been shown to have reduced mitochondrial protein synthesis inhibition (MPSi), which is linked to myelosuppression, as well as lower monoamine oxidase inhibition (MAOi), which is associated with fewer drug-drug interactions. Studies have demonstrated that CZD exhibits comparable in vitro and in vivo anti-TB activity to LZD.18,19 Notably, preclinical and clinical data suggest that CZD has a lower risk of causing myelosuppression. Despite these promising findings, a direct evaluation of CZD’s efficacy in TB patients has not been conducted, given the complexity of drug combinations used in TB treatment. Thus, in this study, we performed an early bactericidal activity (EBA) evaluation of CZD alongside LZD to assess the in vivo efficacy of both drugs against Mtb.
EBA assays are used to measure the rate at which drugs eliminate acid-fast bacilli (AFB) from sputum during the first two days of chemotherapy. This allows for comparison between new and existing treatments, and helps in determining effective dosing regimens.20 EBA reflects a drug’s ability to target actively metabolizing and rapidly multiplying organisms in cavitary lesions. The concept of EBA has been extended to measure bacterial killing beyond the initial two days, known as extended EBA, which serves as an early indicator of a drug’s sterilizing activity. Extended EBA can provide insights into a drug’s capacity to eradicate slowly replicating, persistent bacilli and predict its potential to shorten TB treatment duration.21 In this study, both EBA and extended EBA were evaluated for LZD and CZD.
Method
Study Procedures
From June 2022 to April 2023, an open-label, active-controlled, randomized clinical trial evaluating EBA of CZD was conducted at Beijing Chest Hospital (Beijing, China). The study protocol received approval from the Ethics Committee of Beijing Chest Hospital, and written informed consent was obtained from all the participants prior to enrollment. This study complied with the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov (ChiCTR2100054786).
Adult patients aged 16–60 years with newly diagnosed smear-positive pulmonary TB, with a smear-positive grade of ≥2+ according to the World Health Organization/International Union Against Tuberculosis and Lung Disease criteria, were enrolled. All the participants had no prior history of anti-TB treatment and were free of serious underlying medical conditions. A simultaneous positive Xpert MTB/RIF assay (Cepheid, USA) with a rifampin-sensitive result was required at recruitment to confirm the presence of Mtb in sputum and to minimize the likelihood of severe drug resistance. Patients who were HIV-positive, pregnant, lactating, or hypersensitive to the tested drug were excluded. Participants were randomly assigned to receive a 14-day single-drug treatment of either 600 mg QD linezolid (LZD; Pfizer Pharmaceuticals Limited, USA) or 800 mg BID contezolid (CZD; Huahai Pharmaceuticals Limited, China). Upon completion of the EBA study, all patients were transitioned to the standard anti-TB chemotherapy. Safety monitoring involved routine observation of vital signs, as well as laboratory assessments of hematology at baseline, on day 3, day 8 during the treatment, and on day 15 which was the first day after the final dose.
Sputum Collection and Microbiological Methodology
Patients were hospitalized throughout the study period. Sputum samples were collected at baseline and daily during the 14-day mono-drug treatment phase. Collections continued over a 16-hour period from 4pm to 8am the next day. Laboratory staff performing the tests were blinded to treatment allocation. Sputum samples were processed as per the instruction of the manufacturer of BACTEC MGIT 960 (Becton Dickinson). Briefly, sputum was digested with a mixture of N-acetyl-L-cysteine and sodium hydroxide (NALC-NaOH), buffered with phosphate-buffered saline (0.067 M, pH 6.8), and then concentrated by centrifugation. The resulting sediments were resuspended in phosphate-buffered saline. Serial ten-fold dilutions ranging from 10−1 to 10–4 were prepared and plated onto Middlebrook 7H10 agar (BD, USA). Plates were incubated at 37°C for up to 42 days, with weekly examinations. Colonies were counted on plates yielding 20–200 visible colonies. Data were reported as log10 CFU (colony-forming units) per milliliter of undiluted sputum. Additionally, each homogenized sediment was processed for MGIT960 liquid culture, and time-to-positivity (TTP) was recorded in hours.
Data Management
Early bactericidal activity (EBA x–y) was calculated as the rate of fall for sputum CFU (expressed in log10 units) during the labeled days of the study drug using the equation: EBA (log10 CFU/mL Sx- log10 CFU/mL Sy)/(y-x), where Sx and Sy indicate colony counts of the given day, respectively. The mean standard deviation (SD) was calculated. The primary efficacy endpoint was the EBA over 14 days measured by the daily rate of change of log10CFU in sputum (EBA CFU0–14). The rate of change was modeled using linear, bilinear, or multiple regression, depending on the best fit for the data. EBA parameters were calculated as weighted averages across study intervals for each patient and then averaged within each treatment group. Secondary endpoints, reflecting CFU reduction, included EBA_CFU days 0–2, EBA_CFU days 0–7, EBA_CFU days 2–14, and EBA_CFU days 7–14. EBA_TTP was assessed similarly, based on the hourly extension of time to positivity (TTP) over the relevant time intervals. The study was not powered for hypothesis testing; exploratory comparisons between experimental groups were conducted using one-way ANOVA, and no statistical comparison was made with the EBA of the control group on standard treatment.
Results
Patient Enrollment
Ten eligible patients were enrolled in each treatment group. One patient from the CZD group was excluded from the efficacy analysis due to failure to cultivate acid-fast bacilli (AFB) colonies. Another patient in the CZD group withdrew on day 8 due to hemoptysis, although their data from the first 7 days were included in the analysis. There were no significant differences in demographic characteristics or clinical symptoms between the two groups (Table 1). Serial monitoring during the 14-day EBA study showed no abnormal hematological changes (Table 2). Although RBC counts were slightly lower in the CZD group on day 3 compared to the LZD group, this was deemed unrelated to the treatment.
 |
Table 1 The Demographic Characteristics of the Enrolled Patients |
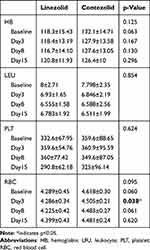 |
Table 2 Outcomes of Serial Hematological Examinations |
EBA Outcomes
The mean baseline AFB concentration, expressed as log10 CFU/mL, was 3.74 ± 0.92 for the LZD group and 3.97 ± 0.98 for the CZD group, with no significant difference between the groups (P > 0.05). The mean EBA0-2, EBA2-7, and EBA0-14 values for the LZD group were 0.05 ± 0.29 log CFU/mL/day (95% CI: −0.16 to 0.25), 0.08 ± 0.19 log CFU/mL/day (95% CI: −0.05 to 0.21), and 0.03 ± 0.10 log CFU/mL/day (95% CI: −0.05 to 0.10), respectively. For the CZD group, the values were −0.05 ± 0.31 CFU/mL/day (95% CI: −0.25 to 0.24), 0.06 ± 0.12 CFU/mL/day (95% CI: −0.06 to 0.16), and 0.08 ± 0.12 log CFU/mL/day (95% CI: −0.02 to 0.18). The magnitude of CFU decrease during different time intervals is shown in Table 3. Over the 14-day mono-drug therapy, the CFU decreased by 1.13 log CFU/mL in the CZD group, compared to 0.43 log CFU/mL in the LZD group. The EBA0-2 and EBA2-7 values were greater in the LZD group, whereas the CZD group showed greater extended EBA during the days 2–14 and 7–14 intervals.
 |
Table 3 Bactericidal Activity of LZD and CZD Determined by CFU Decrease (log10CFU±SD) and TTP Increase (Hour ±SD) |
Bilinear regression analysis identified the best fit for the data, with inflection points occurring at 9 days for LZD and 10.5 days for CZD (Figure 1). During the day 0–9 period, the CFU decrease slope for LZD was −0.1191, shifting to −0.0019 from day 9–14. For CZD, the slope was −0.05465 during days 0–10 and −0.22388 during days 10–14. After day 13, the CFU decrease rate for CZD surpassed that of LZD.
 |
Figure 1 Bilinear regression of the fall in mean log10 CFU from baseline. |
TTP Outcomes
The increase in EBA TTP (0–14 days) was 38.6 ± 43.69 hours (95% CI: −1.85 to 79 hours) for the CZD group and 27.8 ± 78.21 hours (95% CI: −28.15 to 83.75 hours) for the LZD group (Table 3). The LZD group exhibited a more rapid increase in TTP during the initial 2 days (TTP0-2) and days 2–7 (TTP2-7) compared to the CZD group. However, the trend reversed in the longer monitoring period, with CZD showing a greater increase in TTP during the later stages, particularly for EBA TTP0-14, TTP2-14, and TTP7-14 (Table 3).
Bilinear regression analysis was applied to assess changes in TTP (Figure 2). The inflection points occurred at 2 days for LZD and 10 days for CZD. For LZD, the slope of TTP increase from day 0–2 was 13.663, which decreased to 1.391 from day 2–14. In contrast, the CZD group showed a slope of 2.539 from day 0–10, which increased to 5.934 from day 10–14. After day 13, the TTP increase in the CZD group exceeded that of LZD.
 |
Figure 2 Bilinear regression of the mean daily TTP (h) in liquid culture. |
Discussion
Assessing the efficacy of a single anti-TB drug in clinical trials is challenging due to the multi-drug regimens used in TB treatment and the prolonged therapy duration. Typically, new anti-TB drugs are evaluated by incorporating them into multi-drug regimens, often compared with a placebo or a standard drug. However, this approach is costly, labor-intensive, and may not accurately reflect the efficacy of an individual drug. Animal models are another option but are expensive and may not fully replicate human disease conditions. An alternative is the early bactericidal activity (EBA) study, which is a practical and cost-effective method for evaluating the early efficacy of a drug. EBA studies require fewer cases and a shorter duration, making them an ideal tool for assessing both efficacy and short-term toxicity, as well as guiding dose selection for further trials.
Traditional EBA studies for TB have focused on a 2-day period, but this has been extended to 14 days to assess drugs that require a longer duration to reach full efficacy, such as PZA and TMC207.22,23 While 2-day studies are limited in their ability to assess sterilizing activity, 14-day EBA studies, when analyzed using regression models, can predict a drug’s sterilizing efficacy and its potential to shorten treatment duration and prevent relapse.24 As a result, EBA outcomes are now analyzed for both the initial 2 days and subsequent periods.
In this study, the EBA of LZD during the first 2 days (EBA0-2) was 0.05 ± 0.29 log CFU/mL/day, which is lower than the 0.18 ± 0.27 log CFU/mL/day reported by Dietze et al, likely due to their higher baseline bacterial load in sputum.25 However, the EBA from days 2–7 (EBA2-7) was similar between the studies, with values of 0.09 ± 0.17 log CFU/mL/day in Dietze et al’s study compared to 0.08 ± 0.12 log CFU/mL/day in our study. Dietze et al conducted their study over 7 days with two dosing regimens (600 mg QD and 600 mg BID). The 600 mg BID regimen yielded an EBA0-2 of 0.26 ± 0.42 log CFU/mL/day and an EBA2-7 of 0.04 ± 0.11 log CFU/mL/day. A separate study in South Korea with LZD 600 mg BID showed CFU reduction only during the first 2 days, with no significant change in the later intervals (days 2–7, days 2–14, and days 0–14).26 These results align with our findings for LZD 600 mg QD, indicating rapid AFB killing in the early days, followed by a diminished effect and an increase in AFB counts in subsequent days.
In our study, the 800 mg BID CZD group demonstrated superior EBA over 14 days (EBA0-14: 1.13 vs 0.43 for LZD). Our previous research showed that CZD had faster and stronger intracellular bactericidal activity against Mtb compared to LZD, supporting CZD’s potential for superior in vivo efficacy.19 Interestingly, despite being chemical analogs, CZD and LZD displayed differing activity profiles in both early and extended EBA. LZD showed superior EBA during the first 2 days and days 2–7, while CZD outperformed LZD in the later periods (days 2–14 and days 7–14). These findings suggest that LZD may be more effective at rapidly killing active AFB in the early stages, while CZD demonstrates better sterilizing activity over a longer duration, potentially shortening the overall treatment course.
The TTP results mirrored the EBA findings. LZD exhibited a greater increase in TTP from day 0–2, but CZD eventually exceeded LZD in the later stages. The inflection points for both drugs were similar (9 days for LZD vs 10 days for CZD), and the overall trends in CFU reduction and TTP elongation were consistent. CZD proved having a more favorable safety profile compared to LZD, with a lower incidence of myelosuppression and no significant effects on the QT interval at the tested dose (800 mg/day).27–29 In a 28-day trial, neither the 800 mg BID nor 1200 mg BID dosing caused serious adverse events, further supporting the safety of CZD.30,31 Our study manifested that 800 mg BID CZD having non-inferior Mtb killing activity to that of 600 mg QD LZD. Future studies should investigate whether lower doses of CZD can achieve similar EBA efficacy.
This study has several limitations. First, it was an open-label trial, though random assignment was used for treatment allocation, and laboratory staff remained blinded. Second, the CFU enumeration process is labor-intensive and susceptible to variability, which may affect reproducibility. Finally, while EBA studies typically enroll 8–12 patients per group,25,32–34 the small sample size in this study may introduce bias and limit the generalizability of the results.
Conclusion
This study suggests that 800 mg BID CZD demonstrates more sustained AFB-killing activity compared to 600 mg QD LZD in the treatment of pulmonary tuberculosis. With its superior safety profile, CZD holds promise as a viable alternative to LZD for TB treatment. Further investigation is warranted to explore the efficacy of lower doses of CZD, particularly in multidrug-resistant TB.
Data Sharing Statement
All the significant data were presented in this article. There is no other extra meaningful data we could share for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by research funding from the Capital’s Funds for Health Improvement and Research (2022-2-2163), Beijing Public Health Experts Project (G2023-2-002), Tongzhou lianggao talents project (2019B0401715,YHLJ202010) and high-level public health technology talent construction project (Disciplinary backbone −03-49).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report, Geneva, Switzerland, 2024.
2. Spangler SK, Jacobs MR, Appelbaum PC. Activities of RPR 106972 (a new oral streptogramin), cefditoren (a new oral cephalosporin), two new oxazolidinones (U-100592 and U-100766), and other oral and parenteral agents against 203 penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1996;40(2):481–484. doi:10.1128/AAC.40.2.481
3. Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367(16):1508–1518. doi:10.1056/NEJMoa1201964
4. Sotgiu G, Centis R, D’Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J. 2012;40(6):1430–1442. doi:10.1183/09031936.00022912
5. Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2021;57(6):2003300. doi:10.1183/13993003.03300-2020
6. Conradie F, Diacon AH, Ngubane N, et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med. 2020;382(10):893–902. doi:10.1056/NEJMoa1901814
7. Zohaib Ali M, Dutt TS, MacNeill A, et al. A modified BPaL regimen for tuberculosis treatment replaces linezolid with inhaled spectinamides. Elife. 2024;13:RP96190. doi:10.7554/eLife.96190.3
8. Padmapriyadarsini C, Vohra V, Bhatnagar A, et al. Bedaquiline, Delamanid, Linezolid and Clofazimine for Treatment of Pre-extensively Drug-Resistant Tuberculosis. Clin Infect Dis. 2022;76(3):e938–46. doi:10.1093/cid/ciac528
9. Nyang’wa BT, Berry C, Kazounis E, et al. Short oral regimens for pulmonary rifampicin-resistant tuberculosis (TB-PRACTECAL): an open-label, randomised, controlled, phase 2B-3, multi-arm, multicentre, non-inferiority trial. Lancet Respir Med. 2024;12(2):117–128. doi:10.1016/S2213-2600(23)00389-2
10. Hasan T, Medcalf E, Nyang’wa BT, et al. The Safety and Tolerability of Linezolid in Novel Short-Course Regimens Containing Bedaquiline, Pretomanid, and Linezolid to Treat Rifampicin-Resistant Tuberculosis: an Individual Patient Data Meta-analysis. Clin Infect Dis. 2024;78(3):730–741. doi:10.1093/cid/ciad653
11. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment 2017, Lan Z, Ahmad N, Baghaei P, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8(4):383–394. doi:10.1016/S2213-2600(20)30047-3.
12. Park IN, Hong SB, Oh YM, et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother. 2006;58(3):701–704. doi:10.1093/jac/dkl298
13. Padmapriyadarsini C, Oswal VS, Jain CD, et al. Effectiveness and Safety of Varying Doses of Linezolid With Bedaquiline and Pretomanid in Treatment of Drug-Resistant Pulmonary Tuberculosis: open-Label, Randomized Clinical Trial. Clin Infect Dis. 2024;2024:ciae388.
14. Ruiz P, Causse M, Vaquero M, et al. In Vitro Activity of Tedizolid against Mycobacterium tuberculosis.. Antimicrob Agents Chemother. 2019;63(4):e01939–18. doi:10.1128/AAC.01939-18
15. Vera-Cabrera L, Gonzalez E, Rendon A, et al. In vitro activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis. Antimicrobial Agents and Chemotherapy. 2006;50(9):3170–3172. doi:10.1128/AAC.00571-06
16. Barbachyn MR, Hutchinson DK, Brickner SJ, et al. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J. Med Chem. 1996;39(3):680–685. doi:10.1021/jm950956y
17. Cho YS, Lim HS, Lee SH, et al. Pharmacokinetics, Pharmacodynamics, and Tolerability of Single-Dose Oral LCB01-0371, a Novel Oxazolidinone with Broad-Spectrum Activity, in Healthy Volunteers. Antimicrob Agents Chemother. 2018;62(7):e00451–18.
18. Shoen C, DeStefano M, Hafkin B, et al. In Vitro and In Vivo Activities of Contezolid (MRX-I) against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018;62(8):e00493–18. doi:10.1128/AAC.00493-18
19. Wang C, Wang G, Huo F, et al. Novel oxazolidinones harbor potent in vitro activity against the clinical isolates of multidrug-resistant Mycobacterium tuberculosis in China. Front Med. 2022;9:1067516. doi:10.3389/fmed.2022.1067516
20. Jindani A, Aber VR, Edwards EA, et al. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121(6):939–949. doi:10.1164/arrd.1980.121.6.939
21. Sirgel FA, Donald PR, Odhiambo J, et al. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J Antimicrob Chemother. 2000;45(6):859–870. doi:10.1093/jac/45.6.859
22. Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–2405. doi:10.1056/NEJMoa0808427
23. Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52(8):2831–2835. doi:10.1128/AAC.01204-07
24. Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of anti-tuberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167(10):1348–1354. doi:10.1164/rccm.200210-1125OC
25. Dietze R, Hadad DJ, McGee B, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis.Am. J Respir Crit Care Med. 2008;178(11):1180–1185. doi:10.1164/rccm.200806-892OC
26. Kim JS, Kim YH, Lee SH, et al. Early Bactericidal Activity of Delpazolid (LCB01-0371) in Patients with Pulmonary Tuberculosis. Antimicrob Agents Chemother. 2022;66(2):e0168421. doi:10.1128/aac.01684-21
27. Wu X, Li Y, Zhang J, et al. Short-term Safety, Tolerability, and Pharmacokinetics of MRX-I, an Oxazolidinone Antibacterial Agent, in Healthy Chinese Subjects. Clinical Therapeutics. 2018;40(2):322–332.e5. doi:10.1016/j.clinthera.2017.12.017
28. Eckburg PB, Ge Y, Hafkin B. Single- and Multiple-Dose Study To Determine the Safety, Tolerability, Pharmacokinetics, and Food Effect of Oral MRX-I versus Linezolid in Healthy Adult Subjects.. Antimicrobial Agents and Chemotherapy. 2017;61(4):e02181–16. doi:10.1128/AAC.02181-16
29. Wu J, Wu H, Wang Y, et al. Tolerability and Pharmacokinetics of Contezolid at Therapeutic and Supratherapeutic Doses in Healthy Chinese Subjects, and Assessment of Contezolid Dosing Regimens Based on Pharmacokinetic/Pharmacodynamic Analysis. Clin Ther. Clinical Therapeutics. 2019;41(6):1164–1174. doi:10.1016/j.clinthera.2019.04.025
30. Wu J, Wang K, Chen Y, et al. Concentration-response modeling of ECG data from early-phase clinical studies to assess QT prolongation risk of contezolid (CZD) an oxazolidinone antibacterial agent. J Pharmacokinet Pharmacodyn. 2019;46(6):531–541. doi:10.1007/s10928-019-09650-7
31. Wu J, Cao G, Wu H, et al. Evaluation of the Effect of Contezolid (CZD) on the Corrected QT Interval in a Randomized, Double-Blind, Placebo- and Positive-Controlled Crossover Study in Healthy Chinese Volunteers. Antimicrob Agents Chemother. 2020;64(6):e02158–19. doi:10.1128/AAC.02158-19
32. Chambers HF, Kocagoz T, Sipit T, et al. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis. 1998;26(4):874–877. doi:10.1086/513945
33. Gosling RD, Uiso LO, Sam NE, et al. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;168(11):1342–1345. doi:10.1164/rccm.200305-682OC
34. Johnson JL, Hadad DJ, Boom WH, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10(6):605–612.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Clinical Utility of Contezolid-Containing Regimens in 25 Cases of Linezolid-Intolerable Tuberculosis Patients
Wang J, Nie W, Ma L, Li Q, Geng R, Shi W, Chu N
Infection and Drug Resistance 2023, 16:6237-6245
Published Date: 19 September 2023
Sequential Therapy of Linezolid and Contezolid to Treat Hematogenous Lung Abscess Caused by Staphylococcus aureus in a Congenital Cerebral Hypoplasia Patient: A Case Report
Zhou S, Xin C, Liu W
Infection and Drug Resistance 2025, 18:253-260
Published Date: 13 January 2025

