Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Development and Validation of an Interpretable Machine Learning Model for Prediction of the Risk of Clinically Ineffective Reperfusion in Patients Following Thrombectomy for Ischemic Stroke
Authors Hu X , Qi D, Li S, Ye S, Chen Y, Cao W, Du M, Zheng T, Li P, Fang Y
Received 11 February 2025
Accepted for publication 14 April 2025
Published 3 May 2025 Volume 2025:21 Pages 621—631
DOI https://doi.org/10.2147/TCRM.S520362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Xiaolong Hu,1,2,* Dayong Qi,2,* Suya Li,2 Shifei Ye,2 Yue Chen,2 Wei Cao,2 Meng Du,2 Tianheng Zheng,2 Peng Li,2 Yibin Fang1– 3
1Tongji University School of Medicine, Tongji University Affiliated Shanghai 4th People’s Hospital, Shanghai, People’s Republic of China; 2Department of Neurovascular Disease, Tongji University Affiliated Shanghai 4th People’s Hospital, Shanghai, People’s Republic of China; 3Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yibin Fang, Tongji University Affiliated Shanghai 4th People’s Hospital, Sanmen Road 1279, Hongkou District, Shanghai, People’s Republic of China, Email [email protected]
Background: Despite successful recanalization after thrombectomy in patients with acute ischemic stroke, poor prognosis often persists. This study aimed to investigate the factors contributing to clinically ineffective reperfusion (CIR), develop and validate a machine-learning model to predict CIR, and provide guidance for future clinical treatments.
Methods: We collected data from patients undergoing thrombectomy at Shanghai Fourth People’s Hospital between December 2021 and June 2024. The clinical variables were compared between the clinically ineffective and effective recanalization groups using univariate analysis. Four machine learning models were developed: random forest (RF), support vector machine (SVM), decision tree (DT), and k-nearest neighbor (KNN). Model performance was evaluated using receiver operating characteristic (ROC) curves and heatmap visualization. The SHAP method rank the feature importance and provided interpretability for the final model.
Results: Among the four machine learning models, the RF model showed the best performance, with an area under the curve (AUC) of 0.96 (95% CI: 0.91– 1.0), accuracy of 0.93, and specificity of 0.97 on the test dataset. The SHAP algorithm identified the number of endovascular thrombectomy (EVT) attempts as the key factor influencing CIR. Based on the RF model, a web-based calculator for CIR prediction is available at https://ineffectivereperfusion.shinyapps.io/calculate/. The final model included ten parameters: EVT attempts, diabetes mellitus, previous ischemic stroke, National Institutes of Health Stroke Scale (NIHSS score), preoperative infarction in the basal ganglia, baseline diastolic blood pressure, clot burden score (CBS)/basilar artery on computed tomography angiography (BATMAN) score, stroke cause, collateral grade, and MLS.
Conclusion: We developed and validated the first interpretable machine learning model for CIR prediction after EVT, surpassing traditional methods. Our CIR risk prediction platform enables early intervention and personalized treatment. The number of EVT attempts has emerged as a key determinant, underscoring the need for optimized procedural timing to improve outcomes.
Keywords: machine learning, clinically ineffective reperfusion, predictive model, acute ischemic stroke, online predictive platform
Introduction
Acute ischemic stroke is caused by the obstruction of cerebral blood vessels, leading to interrupted blood supply, hypoxia, and necrosis of brain tissue.1–3 It manifests as acute focal neurological deficits and is associated with high rates of disability and mortality.4 Reperfusion therapy is considered the first-line treatment for acute ischemic stroke caused by large-vessel occlusion.5 Timely restoration of blood flow to the brain is associated with better clinical outcomes.6,7 However, several studies8,9 have shown that patients with acute stroke undergoing thrombectomy often achieve recanalization (modified treatment in cerebral infarction (mTICI) grade 2b-3) but fail to attain functional independence (modified Rankin scale (mRS) score 0–2). Clinically ineffective reperfusion (CIR) is regarded as a treatment failure despite successful vascular recanalization.10 Therefore, there is an urgent need to enhance understanding of CIR-associated risk factors and develop effective predictive methods to facilitate personalized diagnostic, treatment, and follow-up strategies. Prognostic models based on machine learning methods often outperform traditional statistical models in terms of predictive accuracy.11 In this study, we retrospectively analyzed the risk factors for CIR in patients undergoing thrombectomy at our cerebrovascular center. We developed the first machine learning model for CIR prediction, which demonstrated high accuracy. The SHAP algorithm was used to interpret the model and a web-based calculator was developed to facilitate its clinical application.
Methods
Study Design and Population
We collected data from patients with acute ischemic stroke who underwent thrombectomy at Shanghai Fourth People’s Hospital between December 2021 and June 2024. The inclusion criteria were: (1) underwent thrombectomy; (2) had complete imaging and clinical data; and (3) had complete clinical follow-up information. The exclusion criteria were: (1) pre-stroke mRS > 2 due to physical disability, prior history of cancer, or surgery; (2) lack of complete imaging data; and (3) loss to follow-up after surgery. The study was conducted with Institutional Review Board approval from the Shanghai Fourth Hospital affiliated with Tongji University in China (No: 2024218–001). As this study was retrospective, informed consent was waived. The guidelines outlined in the Declaration of Helsinki were followed in this study.
Assessment of Study Outcomes
CIR was defined as a postoperative mTICI grade ≥ 2b and an mRS score > 2 at three months post-discharge. Imaging results were independently assessed by two senior neurosurgeons, both blinded to the predictor information. In cases of disagreement, a third blinded neurosurgeon was consulted, and the final decision was reached by consensus.
Variables Selection
Initially, univariate analysis was conducted to identify statistically significant variables. 22 statistically significant parameters were identified. Using the Lasso algorithm, we further reduced these to 16 parameters, followed by mRMR selection to obtain the top 10 statistically significant variables, which were subsequently included in the machine learning models.
Model Development, Validation and Performance Evaluation
We optimized the hyperparameters of random forest (RF), support vector machine (SVM), k-nearest neighbor (KNN), and decision tree (DT) machine learning models using a grid search with five-fold cross-validation. The hyperparameters were selected based on the highest area under the curve (AUC) score obtained on the validation set. The ranges of hyperparameters tested for each model are as follows: RF: n_estimators (50, 100, 200), max_depth (5, 10, 20), min_samples_split (2, 5, 10), min_samples_leaf (1, 2, 4), max_features (“sqrt”, “log2”); SVM: C (0.1, 1, 10, 100), kernel (“linear”, “rbf”, “poly”, “sigmoid”), gamma (“scale”, “auto”, 0.01, 0.1, 1), degree (for “poly”, 2, 3, 4); KNN: n_neighbors (3, 5, 7, 10), weights (“uniform”, “distance”), metric (“euclidean”, “manhattan”, “minkowski”); DT: criterion (“gini”, “entropy”), max_depth (5, 10, 20), min_samples_split (2, 5, 10), min_samples_leaf (1, 2, 4). Th four machine learning models—RF, SVM, DT, and KNN—were applied to predict CIR risk in patients with ischemic stroke undergoing thrombectomy, and all data were randomly split into training (70%) and testing (30%) sets. The training set was used to construct the models and the test set was used to evaluate them. Model performance was assessed using multiple metrics, including the receiver operating characteristic (ROC) curve, negative predictive value (NPV), positive predictive value (PPV), sensitivity, specificity, accuracy, and F1 score, and the best predictive model was selected based on its performance in the testing dataset. A heat map was used to analyze the performance of the optimal model.
Model Explanation
The SHAP algorithm addresses interpretability challenges posed by the black-box nature of machine learning models. It ranks the importance of input parameters and explains the model’s final outputs. By quantifying the contribution of each parameter across individual samples, the SHAP algorithm enhances model interpretability and transparency.
Developing an Online CIR Risk-Prediction Platform
To facilitate clinical applications, we integrated the optimal model into a Shiny-based web platform to develop an online calculator. The calculator provides the predicted probability of CIR by inputting the relevant parameters of the patients undergoing thrombectomy.
Results
Baseline Clinical Information
A total of 177 patients who underwent thrombectomy were included in this study, 60 of whom were classified as having CIR 3 months postoperatively. The clinical characteristics of the patients are summarized in Table 1. Significant differences were observed in several clinical features between the clinically effective reperfusion and clinically ineffective reperfusion groups. Patients in the CIR group, compared to the non-CIR group, tended to be older (78 vs 71 y) and were more likely to have a history of previous ischemic stroke, diabetes mellitus, atrial fibrillation, and hyperlipidemia. They also had higher NIHSS scores (15 vs 10), systolic blood pressure (145 vs 135 mm Hg), diastolic blood pressure (DBP: 87 vs 78 mm Hg), serum glucose levels (7.95 vs 6.4 mmol/L), endovascular thrombectomy (EVT) attempts (5 vs 1), infarct core volumes (27.5 vs 11 mL), hypoperfusion intensity ratios (0.5 vs 0.4), door-to-magnetic resonance imaging (MRI) times (48 vs 42 min), and door-to-puncture times (48.5 vs 36 min). Additionally, collateral grades and clot burden scores (CBS) were significantly lower in the CIR group than in the clinically effective reperfusion group, and patients with cardioembolic stroke or those not receiving postoperative anticoagulant or antiplatelet therapy were more likely to experience CIR (p < 0.05).
 |
Table 1 Baseline Characteristics of Patients with Effective and Ineffective Reperfusion |
Selection of Predictor Variables
We employed the mRMR and LASSO algorithms for feature selection (Figure 1). Based on the univariate analysis, 22 statistically significant parameters were identified. Using the LASSO algorithm, we further reduced these to 16 parameters, followed by mRMR selection to obtain the top 10 statistically significant variables, which were subsequently included in the machine learning models (RF, DT, SVM, and KNN). The importance score for each variable was calculated and visualized using a bar plot.
 |
Figure 1 Feature selection. (a) CV,10-fold cross-validation. (b) Lasso, Least absolute shrinkage and selection operator. (c) mRMR, max-relevance and min-redundancy. |
Model Construction and Performance Evaluation
We utilized 10-fold internal cross-validation to construct four machine learning models. The RF model demonstrated the best performance, achieving an AUC of 0.96 (95% CI: 0.91–1.0), accuracy of 0.93, and specificity of 0.97 in the test dataset (Figure 2 and Table 2).
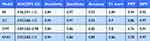 |
Table 2 AUC, Sensitivity, Specificity, Accuracy, F1 Score, PPV and NPV of mL Model |
Heatmap Analysis of RF Model Variables
A heatmap was generated to illustrate the performance of the RF model in predicting CIR risk after thrombectomy (Figure 3). Different colors represented the actual values of clinical characteristics across patients. The heatmap analysis showed that the RF model accurately distinguished between the CIR and clinically effective reperfusion groups, demonstrating its excellent classification ability and potential utility in managing high-risk thrombectomy patients.
Model Explanation
The SHAP algorithm was used to evaluate the importance of each variable in the RF model and its contribution to the final prediction (Figure 4). The bar plot emphasized that EVT attempts, with the highest SHAP value (Figure 4b), were the most significant risk factors for CIR. Collateral grade (Figure 4b) was identified as the most critical imaging parameter for predicting CIR.
An Online CIR Risk-Prediction Platform
As shown in Figure 5, an online CIR risk-prediction platform was developed based on the optimal model to predict the probability of CIR three months after discharge in patients following thrombectomy. By inputting the ten required parameters, the web calculator (at the following link: https://ineffectivereperfusion.shinyapps.io/calculate/.automatically) outputs the predicted probability of CIR.
 |
Figure 5 An online CIR risk-prediction platform was developed to predict the probability of CIR three months after discharge in patients following thrombectomy. |
Discussion
Timely thrombectomy and complete vascular recanalization are often associated with improved prognosis in patients with acute ischemic stroke; however, complete vascular recanalization does not always guarantee favorable clinical outcomes, as numerous factors influence prognosis.12–16 This study retrospectively analyzed the clinical parameters of patients with acute ischemic stroke treated at our cerebrovascular center, identified the risk factors for clinical CIR, and developed the first machine learning model for CIR prediction, aiding in personalized treatment planning for future patients.
This study identified 22 risk factors for CIR, with the number of EVT attempts being the most critical. Following acute ischemic stroke, a higher number of thrombectomy attempts during standard procedures increases the likelihood of CIR, a finding corroborated by other studies.17 Procedure durations exceeding 60 min and more than three thrombectomy attempts are significant contributors to CIR, emphasizing the importance of achieving first-pass recanalization.18,19 Our study demonstrated that CIR is associated with infarction location, with preoperative infarction involving the basal ganglia identified as a significant risk factor. Patients with ischemic stroke who did not have preoperative infarction in the basal ganglia area were more likely to achieve favorable postoperative outcomes. Previous studies20,21 have shown that core infarct volume is associated with prognosis, a finding supported by our results. Larger core infarcts were associated with worse prognosis; however, the presence of a large infarct alone was not predictive of outcomes, possibly due to the study’s threshold of 80 mL for defining large infarcts. Our findings do not support the conclusion that larger core infarcts are unrelated to prognosis. This study incorporated four temporal parameters, of which three (door-to-MRI time, door-to-puncture time, and puncture-to-recanalization time) demonstrated statistically significant associations with patient outcomes. Our findings indicate that shorter time to diagnostic imaging, earlier surgical intervention, and more rapid vascular recanalization are consistently associated with a more favorable prognosis. Indeed, when patients experience acute cerebral infarction, neurons immediately begin to undergo cell death due to ischemia and hypoxia.22 However, timely vascular recanalization effectively salvages endangered brain tissue within the ischemic penumbra and significantly mitigates the degree of neurological functional impairment, thereby improving long-term patient outcomes.23
Previous studies have reported that even with successful recanalization, a subset of patients with acute ischemic stroke caused by distal medium vessel occlusion (DMVO) may experience poor outcomes or mortality.24,25 In this study, among 106 patients with middle cerebral artery (MCA) occlusion, 32 (30.2%) exhibited clinically ineffective reperfusion (CIR), a proportion higher than that reported in the literature.
Consistent with prior findings,26,27 our results demonstrated that endovascular thrombectomy yielded better outcomes in patients with low baseline NIHSS scores compared to those with higher scores. Notably, the CIR group had significantly higher NIHSS scores (mean ± SD: XX ± XX) than the clinically effective reperfusion group (XX ± XX, P < 0.05), suggesting that the severity of initial neurological deficits may influence reperfusion efficacy.
Previous studies have successfully developed and validated early mortality prediction models for patients with acute ischemic stroke undergoing EVT, demonstrating the significance of machine learning algorithms in predicting outcomes.28 These findings align with our hypothesis that precise predictive tools can effectively optimize stroke treatment strategies and significantly improve patient survival rates.
Based on 10 CIR-related parameters, we developed four machine learning models, with the RF model achieving the highest AUC, accuracy, and specificity. Random forest29,30 is an ensemble learning algorithm that constructs multiple decision trees and aggregates their votes to complete classification tasks. Each tree is trained on different subsets of data (via bootstrap sampling) and random feature subsets, reducing overfitting and improving generalization. The RF algorithm captures nonlinear relationships within data, making it well-suited for handling complex datasets. The DT model31 also performed well (AUC: 0.93, accuracy: 93%), but its lower specificity (91%) suggests a slightly higher false positive rate, likely due to its tendency to overfit. The SVM model32 had the lowest AUC (0.89) and accuracy (85%), potentially owing to its sensitivity to hyperparameter tuning and small sample size. The KNN model33 achieved moderate performance (AUC: 0.92), but its lower specificity (86%) suggests that it may be more affected by high-dimensional data and noise. RF emerged as the most suitable model for CIR prediction, balancing sensitivity (0.84) and specificity (0.97) while minimizing false positives and false negatives. The ensemble nature of RF makes it more robust than DT, whereas its automatic feature selection enhances generalization, addressing the limitations observed in SVM and KNN.
To enhance the model interpretability, we employed the SHAP algorithm.34,35 SHAP, based on game theory, calculates the marginal contributions of features to prediction outcomes, ensuring consistent and locally accurate explanations. It quantifies feature importance and interactions, revealing the decision-making logic of the predictive model. Finally, we developed an online CIR risk prediction platform using the RF model. By entering ten key parameters, clinicians can obtain the predicted probability of the 3-month CIR for patients undergoing EVT. This can assist in risk stratification, prognosis estimation, and treatment discussions with patients and their families. We recognize that there may be potential barriers to implementation, including clinical workflow integration, for which the calculator needs to be seamlessly embedded into electronic health records for real-time access, and data privacy and security, to ensure compliance with healthcare data protection regulations.
Our study yielded valuable findings, but it has several limitations. First, our study may be subject to bias from unmeasured confounders because not all relevant clinical variables were included in the analysis. Factors such as comorbidities, prior stroke history, and detailed neuroimaging biomarkers might have influenced the predictive model, but were not fully accounted for. Future studies should incorporate a more comprehensive set of variables to enhance the model accuracy. Second, owing to the relatively small sample size, our model may be prone to overfitting, meaning it might perform well on the training data but generalize poorly to independent datasets. Cross-validation techniques have been applied to mitigate this problem. Finally, the limited sample size may have affected the statistical power, making some subgroup comparisons less reliable. Future studies with larger multicenter datasets are required to validate the model’s performance across different patient populations.
Conclusion
This study identified the risk factors associated with CIR, highlighting the number of EVT attempts as the most critical determinant. We developed the first machine-learning model to accurately predict CIR. Furthermore, a CIR risk-prediction platform was built based on the RF model, enhancing the clinical utility and value.
Abbreviations
AUC, area under the curve; BATMAN, basilar artery on computed tomography angiography; CBS, clot burden score; CIR, clinically ineffective reperfusion; DT, decision tree; EVT, endovascular thrombectomy; KNN, k-nearest neighbor; LASSO, least absolute shrinkage and selection operator; mRMR, minimum redundancy maximum relevance; mRS, modified Rankin scale; mTICI, modified treatment in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale; NPV, negative predictive value; PPV, positive predictive value; RF, random forest; ROC, receiver operating characteristic; SVM, support machine vector.
Acknowledgments
The data was anonymized or maintained with confidentiality.
Xiaolong Hu and Dayong Qi contributed equally to this paper and should be considered as co-first authors.
Funding
This work was supported by The study was supported by National Natural Science Foundation of China (No. 82071279), Medical research subject of Hongkou District Health Committee (No. 2022-28), Scientific funding of Shanghai fourth people’s hospital (No. sykyqd06701).
Disclosure
There are no competing interests.
References
1. Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48(11):1654–1663. doi:10.1097/CCM.0000000000004597
2. Huang ZX, Alexandre AM, Pedicelli A, et al. AI prediction model for endovascular treatment of vertebrobasilar occlusion with atrial fibrillation. NPJ Digit Med. 2025;8(1):78. doi:10.1038/s41746-025-01478-5
3. Xu Y, Alexandre AM, Pedicelli A, et al. Predicting symptomatic intracranial hemorrhage after endovascular treatment of vertebrobasilar artery occlusion: PEACE score. J Neurointerv Surg;2025. jnis–2024–022802. 10.1136/jnis-2024-022802
4. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480 687. Circulation. 2017;135(8):759–771. doi:10.1161/CIRCULATIONAHA.116.025250
5. Widimsky P, Snyder K, Sulzenko J, et al. Acute ischaemic stroke: recent advances in reperfusion treatment. Eur Heart J. 2023;44(14):1205–1215. doi:10.1093/eurheartj/ehac684
6. Happi Ngankou E, Gory B, Marnat G, et al. Thrombectomy complications in large vessel occlusions: incidence, predictors, and clinical impact in the ETIS registry. Stroke. 2021;52(12):e764–e768. doi:10.1161/STROKEAHA.121.034865
7. Jia B, Ren Z, Mokin M, et al. Current status of endovascular treatment for acute large vessel occlusion in china: a real-world nationwide registry. Stroke. 2021;52(4):1203–1212. doi:10.1161/STROKEAHA.120.031869
8. Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (Aurora): a systematic review and individual patient data meta-analysis. Lancet. 2022;399(10321):249–258. doi:10.1016/S0140-6736(21)01341-6
9. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
10. Hussein HM, Georgiadis AL, Vazquez G, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR. Am J Neuroradiol. 2010;31(3):454–458. doi:10.3174/ajnr.A2006
11. Hu X, Deng P, Ma M, et al. A machine learning model based on results of a comprehensive radiological evaluation can predict the prognosis of basal ganglia cerebral hemorrhage treated with neuroendoscop. Front Neurol. 2024;15:1406271. doi:10.3389/fneur.2024.1406271
12. Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J. Neurointerv Surg. 2019;11(5):433–438. doi:10.1136/neurintsurg-2018-014127
13. Rubiera M, Garcia-Tornel A, Olivé-Gadea M, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke. 2020;51(6):1736–1742. doi:10.1161/STROKEAHA.120.029212
14. Pasarikovski CR, Keith J, da Costa L, et al. Optical coherence tomography imaging after endovascular thrombectomy: a novel method for evaluating vascular injury in a swine model. J Neurosurg. 2020;134(3):870–877. doi:10.3171/2019.12.JNS192881
15. Caiazzo G, Musci RL, Frediani L, et al. State of the art: no-reflow phenomenon. Cardiol Clin. 2020;38(4):563–573. doi:10.1016/j.ccl.2020.07.001
16. Brinjikji W, Pasternak J, Murad MH, et al. Anesthesia-related outcomes for endovascular stroke revascularization: a systematic review and meta-analysis. Stroke. 2017;48(10):2784–2791. doi:10.1161/STROKEAHA.117.017786
17. Jahan R, Saver JL, Schwamm LH, et al. Association Between Time To Treatment With Endovascular Reperfusion Therapy And Outcomes In Patients With Acute Ischemic Stroke Treated In Clinical Practice. JAMA. 2019;322(3):252–263. doi:10.1001/jama.2019.8286
18. Li G, Sun Y, Liu T, et al. Predictive factors of poor outcome and mortality among anterior ischaemic stroke patients despite successful recanalisation in China: a secondary analysis of the CAPTURE trial. BMJ open. 2023;13(12):e078917. doi:10.1136/bmjopen-2023-078917
19. Sun D, Huo X, Raynald R, et al. First pass effect of mechanical thrombectomy for acute vertebrobasilar artery occlusion: data from the ANGEL-ACT registry. J. Neurointerv Surg. 2023;15(12):1201–1206. doi:10.1136/jnis-2023-020065
20. Ng FC, Yassi N, Sharma G, et al. Cerebral edema in patients with large hemispheric infarct undergoing reperfusion treatment: a HERMES meta-analysis. Stroke. 2021;52(11):3450–3458. doi:10.1161/STROKEAHA.120.033246
21. Nie X, Leng X, Miao Z, et al. Clinically ineffective reperfusion after endovascular therapy in acute ischemic stroke. Stroke. 2023;54(3):873–881. doi:10.1161/STROKEAHA.122.038466
22. Mao R, Zong N, Hu Y, et al. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull. 2022;38(10):1229–1247. doi:10.1007/s12264-022-00859-0
23. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774.
24. Salim H, Musmar B, Adeeb N, et al. Outcomes of mechanical thrombectomy in anticoagulated patients with acute distal and medium vessel stroke. Eur. Stroke J. 2024;9(4):896–906.
25. Salim HA, Yedavalli V, Musmar B, et al. Mechanical thrombectomy versus intravenous thrombolysis in distal medium vessel acute ischemic stroke: a multinational multicenter propensity score-matched Study. J Stroke. 2024;26(3):434–445. doi:10.5853/jos.2024.01389
26. Kühn AL, Puri AS, Salim HA, et al. Multicenter evaluation of mechanical thrombectomy for distal medium vessel occlusions with national institute of health stroke scale scores ≥ 6 and ≤ 6. J. Neurol. 2024;271(9):5853–5863.
27. Dmytriw AA, Ghozy S, Salim HA, et al. Assessment of thrombectomy versus combined thrombolysis and thrombectomy in patients with acute ischemic stroke and medium vessel occlusion. Radiology. 2024;312(2):e233041. doi:10.1148/radiol.233041
28. Chen Y, Zhou S, Yang S, et al. Developing and predicting of early mortality after endovascular thrombectomy in patients with acute ischemic stroke. Front Neurosci. 2022;16:1034472. doi:10.3389/fnins.2022.1034472
29. Hu J, Szymczak S. A review on longitudinal data analysis with random forest. Brief Bioinform. 2023;24(2):bbad002. doi:10.1093/bib/bbad002
30. Wallace ML, Mentch L, Wheeler BJ, et al. Use and misuse of random forest variable importance metrics in medicine: demonstrations through incident stroke prediction. BMC Med Res Methodol. 2023;23(1):144. doi:10.1186/s12874-023-01965-x
31. Hammann F, Drewe J. Decision tree models for data mining in hit discovery. Expert Opin Drug Discov. 2012;7(4):341–352. doi:10.1517/17460441.2012.668182
32. Valkenborg D, Rousseau AJ, Geubbelmans M, et al. Support vector machines. Am. J Orthod Dentofacial Orthop. 2023;164(5):754–757. doi:10.1016/j.ajodo.2023.08.003
33. Chen Q, Li D, Tang CK. KNN Matting. IEEE Trans Pattern Anal Mach Intell. 2013;35(9):2175–2188. doi:10.1109/TPAMI.2013.18
34. Fryer D, Strümke I, Nguyen H. Shapley values for feature selection: the good, the bad, and the axioms. IEEE Access. 2021;9:144352–144360. doi:10.1109/ACCESS.2021.3119110
35. Wang K, Tian J, Zheng C, et al. Interpretable prediction of 3-year all-cause mortality in patients with heart failure caused by coronary heart disease based on machine learning and SHAP. Comput Biol Med. 2021;137:104813.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




