Back to Journals » Drug Design, Development and Therapy » Volume 19
Effect of Esketamine Compared with Sufentanil Combined with Propofol in Patients Undergoing First Trimester Surgical Abortion: A Randomized, Double-Blinded Clinical Trial
Authors Guan Y , Wang H, Cong X , Zhang B , Lin Y , Wang X
Received 30 December 2024
Accepted for publication 9 April 2025
Published 13 April 2025 Volume 2025:19 Pages 2873—2883
DOI https://doi.org/10.2147/DDDT.S515006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Tuo Deng
Yingchao Guan,1 Haochen Wang,1,2 Xiaojing Cong,1,2 Beibei Zhang,1 Yusong Lin,1,2 Xiaodong Wang1,2
1Department of Anesthesiology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai City, Shandong Province, People’s Republic of China; 2The Second School of Clinical Medicine of Binzhou Medical University, Yantai City, Shandong Province, People’s Republic of China
Correspondence: Xiaodong Wang, Department of Anesthesiology, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, and The Second School of Clinical Medicine of Binzhou Medical University, No. 70 heping Road, Huancui District, Weihai City, Shandong, People’s Republic of China, Tel +86-13508914062, Email [email protected]
Background: We explored whether esketamine anesthesia during first-trimester surgical abortion can reduce intraoperative hemodynamic fluctuations and improve patients’ respiratory function.
Methods: A total of 197 patients who underwent a first-trimester surgical abortion were included in the analysis. Patients were randomly assigned to either the esketamine anesthesia group (group E, n=98) or sufentanil anesthesia group (group S, n=99). The primary outcomes were systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR), respiratory rate (RR) and end-tidal carbon dioxide partial pressure (PetCO2) during the surgery. Secondary outcomes included body movement, apnea, hypoxemia, postoperative nausea and vomiting (PONV), dizziness, anesthesia recovery time, Richmond Agitation and Sedation Scale (RASS) score, and postoperative pain.
Results: Patients in Group E had a more stable intraoperative SBP (p=0.001), DBP (p=0.014), MBP (p=0.003), and HR (p=0.001). There was no significant difference in intraoperative RR between the two groups (p=0.108); however, PetCO2 in group E remained at preoperative levels, whereas it increased in group S during surgery (p< 0.001). The risk of apnea and hypoxemia in group E was lower (RR 0.32, 95% CI [0.13, 0.76], p=0.006; RR 0.13, 95% CI [0.03, 0.54], p=0.001). The incidence of intraoperative body movement (50% vs 27%, p=0.003), postoperative dizziness (45% vs 30%, p=0.024), and nausea (7% vs 0%, p=0.007) was higher in group E. There were no differences in anesthesia recovery time, postoperative RASS score, pain, or vomiting.
Conclusion: Compared with sufentanil, esketamine anesthesia during the first trimester surgical abortion can maintain stable intraoperative hemodynamics and respiratory function during surgery and reduce apnea and hypoxemia. Esketamine may increase the risk of dizziness and PONV after surgical abortion.
Plain Language Summary: Induced abortion is a simple and common health care procedure. The combination of sedative drugs and anesthesia is currently the main anesthetic regimen for abortion. However, the choice of analgesic drug remains controversial. Esketamine is a promising alternative that has been widely used in painless treatment. However, reports on the use of esketamine in abortion surgery are insufficient, and its effectiveness and adverse reactions require further in-depth and comprehensive exploration. Therefore, we designed this prospective randomized controlled trial. We found that compared to sufentanil, esketamine anesthesia during first-trimester surgical abortion could maintain stable intraoperative hemodynamics and respiratory function during surgery and reduce apnea and hypoxemia, but might increase the risk of dizziness and PONV.
Keywords: esketamine, hemodynamics, respiration, abortion, randomized clinical trial
Introduction
Induced abortion is a simple and common health care procedure. According to World Health Organization (WHO), 6/10 of unintended and 3/10 of all pregnancies end in induced abortion,1 and most of them are first trimester abortions2 which are commonly in outpatient setting using aspiration.3 However, 14.4% of total abortions carried out every year were estimated to be least safe,4 and patients usually have adverse experience such as pain and anxiety.5 For clinicians, safe and comfortable abortion surgery requires enough health workers of different types who are trained to provide safe and respectful care, and moderate sedation or general anesthesia helps eliminate patients’ negative emotions and improve comfort during abortion procedure.6
The combination of sedative drugs and anesthesia is currently the main anesthetic regimen for abortion. Propofol has become a widely used sedative drug because of its rapid and thorough metabolism, ideal anesthetic effect, and low incidence of adverse reactions.7 However, the choice of analgesic drugs remains controversial. Opioid μ receptor agonists, such as fentanyl and sufentanil, are currently the main analgesics used clinically,8,9 but its effects such as dizziness, nausea, vomiting, and respiratory depression may still lead to adverse outcomes in patients. As an N-methyl-D-aspartate (NMDA) receptor antagonist, esketamine is the s-enantiomer of ketamine and is a promising alternative that has been widely used in multiple painless treatments. Our previous study showed that using esketamine instead of sufentanil in hysteroscopic surgery anesthesia resulted in stable hemodynamics, improved anesthesia recovery quality, and did not increase the incidence of adverse reactions such as nausea, vomiting, and dizziness.10 Other studies have also revealed the benefits of using esketamine, including decreasing propofol dosage and reducing the incidence of respiratory depression.11–13 However, there are still insufficient reports on the use of esketamine in abortion surgery, and its effectiveness and adverse reactions require further in-depth and comprehensive exploration.
Therefore, we designed a prospective randomized controlled trial to investigate whether esketamine intravenous anesthesia during first-trimester aspiration abortion surgery helps maintain stable intraoperative vital signs, reduce perioperative adverse outcomes, and promote postoperative recovery.
Materials and Methods
This single-center, prospective, double-blind, randomized clinical trial was conducted at Weihai Municipal Hospital following the guidelines for the applicable Consolidated Standards of Reporting Trials (CONSORT)14 for conducting and reporting clinical trials as well as Declaration of Helsinki,15 and was approved by the Ethics Committee of Weihai Municipal Hospital (approval no.2023071). The trial was registered before patient enrollment in the Chinese Clinical Trial Registry (registry no. ChiCTR2300079120; last updated on December 26, 2023). Data from patients undergoing first-trimester surgical abortion in our hospital between December 30, 2023, and November 30, 2024, were prospectively collected.
Patients Enrollment and Visits
One day before surgery, the researchers preliminarily screened patients who met the research protocol based on the Hospital Information System (HIS) and completed preoperative visits. Patients and their relatives will be fully aware of the potential benefits and risks of participating in the study and will be informed that they can request withdrawal from the study at any stage without affecting their clinical treatment. Written consent was obtained in a private setting according to the institutional guidelines.
The inclusion criteria were as follows: patients over 18 years old who would undergo first-trimester surgical abortion; no serious comorbidities such as unstable angina, poor control of severe hypertension, etc.; able to cooperate in communication; and complete follow-up indicators. Exclusion criteria: previous history of major surgeries, such as cardiac surgery, etc.; contraindications to the use of esketamine, such as severe hypertension and poorly controlled asthma; allergy to research medication; patients with mental illness cannot cooperate with follow-up. Patient participation will be suspended if they withdraw their informed consent form, major perioperative adverse events occur, do not follow the established anesthesia plan for anesthesia, change the surgical method, or perform other surgeries simultaneously.
Sample Size Estimation and Random Grouping
Patients were randomized in a 1:1 ratio into two groups using a block randomization method: esketamine (Jiangsu Hengrui Medicine Co., LTD, Jiangsu Province, China) anesthesia group (group E) and sufentanil (Yichang Humanwell Pharmaceutical Co., LTD, Hubei Province, China) anesthesia group (group S). Patients in group E received anesthesia induction with esketamine and propofol, whereas those in group S received sufentanil and propofol. We set the block length to alternate between 4 and 6, and the patients entered the blocks sequentially based on their enrollment time. Within each block, four or six random numbers are sequentially generated based on the length of the block. Two or three participants with smaller random numbers were divided into Group E, whereas the remaining participants were divided into Group S. Randomized grouping was performed by a dedicated researcher and sealed in an envelope until the patient entered the operating room. This was a double-blind study, and participants were unaware of the grouping. To prevent researchers from becoming aware of this grouping, we set up a dedicated dispensing researcher for drug preparation. After the patient entered the operating room, this researcher opened the envelope with randomized information, prepared the corresponding dose of esketamine or sufentanil according to their grouping and weight, diluted it to 10 milliliters with saline, and labeled it as “Analgesics”, so as to ensured that the anesthesiologists, surgical abortion operators, and data-collecting researchers were not aware of the grouping information.
According to our previous study, heart rate after anesthesia induction with esketamine and propofol is 79.79±1.25 beats per minute (bpm), while it is 69.82±1.33 bpm with sufentanil and propofol.10 The sample size was calculated using PASS (version 21.0.3, NCSS Corporation, USA). We considered the average heart rates of Groups E and S to be 75 and 70 bpm, respectively, with a standard deviation of 10 bpm, an alpha level of 0.05, and a statistical power level of 0.90. Using two-sided tests, the sample size was calculated for the 86 patients in each group. Considering a loss to follow-up of 10%, 95 patients were required for each group.
Perioperative Management
All patients underwent cervical preconditioning with laminaria (KL-30; Ken Medical Co., Ltd., Hyogo, Japan) at least 30 min before surgery. Successful cervical pretreatment meant that the laminaria expanded sufficiently, with a diameter of over 7 mm, and the 6 mm diameter cervical dilation stick could smoothly pass through the cervix. The patients fasted for 8 hours with food and 2 hours with water, and no preoperative medication was administered before anesthesia. After entering the operating room (OR), the patients underwent routine monitoring while O2 was delivered at a flow rate of 2 L/min. A nasal cannula was placed to measure the respiratory rate (RR) and end-tidal carbon dioxide partial pressure (PetCO2) using an anesthetic machine (A5 type, Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China). After surgical positioning was completed, the anesthesiologist designated by the research group (with over three years of anesthesia experience) administered anesthesia. For patients in group E, anesthesia induction was conducted using propofol 2.0–2.5 mg/kg+esketamine 0.1 mg/kg, and patients in group S received propofol 2.0–2.5 mg/kg+sufentanil 0.1 ug/kg. We required a Modified Observer’s Assessment of Alertness/Sedation Scale (MOAA/S) score of ≤1 before the surgery started,16 which meant that patients responded only after painful trapezius squeeze or no response after painful trapezius squeeze. A single dose of 30–50 mL of propofol was used when the MOAA/S score was ≥2 or when intraoperative body movement affected the surgical progress.
All surgeries were performed by gynecologists with >5 years of clinical experience. After anesthesia, the cervix was gradually dilated using a metal stick within 3 min until a 10-millimeter-diameter stick was smoothly inserted. Physicians completed the aspiration procedure via electric vacuum aspiration in the standard manner. After the surgery, no additional painkillers or antiemetics were administered. All the patients were resuscitated by monitoring. After recovery of consciousness, further observation was conducted until the patient’s vital signs were stable, no significant discomfort was reported, and the patient could leave the hospital accompanied by family members.
The physician performing anesthesia could decide whether to use vasoactive medications (atropine, ephedrine, dopamine, etc). Throughout the perioperative period, and the researcher recorded when and what kind of vasoactive drugs the physician used. SpO2 threshold for hypoxia was defined as 94%.17 When SpO2≥94%, no intervention was performed; when 90%<SpO2≤94%, we firstly tried jaw-lift maneuver; when SpO2≤90% or it did not elevate after jaw-lift maneuver, we performed ventilator-assisted breathing. When patients had apnea but SpO2 can be maintained above 94%, we did not intervene but closely observed the patient’s breathing and SpO2 changes. If SpO2 further decreased, ventilator-assisted breathing should be adopted until spontaneous breathing was recovered. When observing whether the breathing has recovered, we would briefly pause the ventilation and immediately resume after observation.
Outcomes Measurement
All data were collected by the designated researcher of the research group who would not participate in randomization, blinding, study drug configuration, or data statistical analysis. The primary outcomes included systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR), RR, and PetCO2 at six time points: entry into the operating room (T1), completion of anesthesia induction (T2), completion of cervical dilation (T3), beginning of uterine cavity aspiration (T4), end of surgery (T5), and anesthesia recovery (T6). Secondary outcomes included apnea after anesthesia induction, defined as the inability to observe the PetCO2 waveform and the patient’s respiratory movement even after fully opening the airway; intraoperative hypoxemia, defined as a patient with SpO2 <94% or progressive decline, requiring artificial ventilation assistance; body movement when the uterine cavity aspiration began and an extra dose of propofol was needed; anesthesia recovery time, which was defined as the time required from the end of the surgery to when patients could follow the doctor’s order of body movement; dizziness, pain intensity scored on a numeric pain rating scale (NRS; 0–10), postoperative nausea and vomiting (PONV)18 and Richmond Agitation and Sedation Scale (RASS)19 when patients achieved anesthesia recovery.
Statistical Analysis
Statistical analyses were conducted by a designated researcher who was not involved in any part of the study implementation process. Missing data were handled using pairwise deletion and data analysis followed the Per-Protocol Set. Continuous data are presented as means and standard deviations, and were analyzed using a t-test for normally distributed variables or the Mann–Whitney U-test for nonparametric variables. Categorical data are presented as numbers and were analyzed using the χ2 or Fisher’s exact test, as appropriate. Relative Risk (RR) was calculated, where zeros caused problems, 0.5 was added to all cells for that result.20 Repeated measurement data were analyzed using repeated-measures analysis of variance (ANOVA), and comparisons of each measurement time were performed using multivariate analysis of variance. Statistical significance was set at p values <0.05. The results were analyzed using IBM SPSS Statistics for Windows (version 27; IBM Corporation, USA).
Results
In total, 202 patients were assessed for eligibility. Two patients declined to participate and one patient was excluded for surgery cancellation. The remaining 199 patients were randomly assigned to different groups. Two patients in group E were excluded from further analysis because of laminaria allergy (n=1) and surgical method changes (n=1), while no patients were excluded from group S. Finally, 197 patients were analyzed, with 98 in group E and 99 in group S (Figure 1).
 |
Figure 1 Consort Flow Diagram. Notes: Group E Esketamine anesthesia group; Group S Sufentanil anesthesia group. |
The demographic characteristics of the patients, including age, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, Mallampati classification, surgical history, and laboratory and electrocardiogram (ECG) findings, were not significantly different between the two groups (Table 1).
 |
Table 1 Characteristics of Participants Before Surgery |
According to our results, there was a significant difference in the HR (p<0.001). Patients in both groups experienced a transient increase in HR after anesthesia induction, which gradually decreased with the progress of surgery. However, the HR of patients in group E was slightly higher than in group S, and the difference at the T2-T4 time point was significant (83.020 ± 10.027 vs 79.222 ± 9.951 bpm, p=0.008; 78.235 ± 8.324 vs 73.364 ± 10.207 bpm, p<0.001; 75.459 ± 10.270 vs 71.040 ± 11.136 bpm, p=0.004)(Figure 2A). The results of blood pressure also showed similar differences, with smaller changes in SBP, DBP, and MBP in group E compared to the preoperative levels (p=0.001, p=0.014, p=0.0.03)(Figure 2B–D).
The RR of group S decreased compared to preoperative levels after anesthesia until T3, and gradually returned to preoperative levels, while the RR in group E did not show a significant decrease after anesthesia, and even remained slightly faster than preoperative levels during surgery. There was a significant difference at T2-T6 (p=0.009 at T2, p<0.001 at T3-6)(Figure 2E). There were also differences in the PetCO2. After anesthesia, the PetCO2 levels in group S gradually increased during the surgical process and returned to preoperative levels after awakening, whereas patients in group E maintained slightly lower PetCO2 levels than the preoperative levels (p<0.001)(Figure 2F). There were significant differences in PetCO2 levels at T3 (27.674 ± 4.249 vs 32.505 ± 7.956 mmHg, p<0.001), T4 (27.265 ± 4.098 vs 33.879 ± 2.576 mmHg, p<0.001), T5 (26.510 ± 3.599 vs 34.081 ± 3.948 mmHg, p<0.001), and T6 (27.245 ± 4.498 vs 31.556 ± 3.396 mmHg, p<0.001)(Figure 2F). Meanwhile, patients in group E had a lower incidence of apnea (6 (6.12%) vs 19 (19.19%), RR 0.32, 95% CI [0.13–0.76], p=0.006) and a lower risk of intraoperative hypoxemia (2 (2.04%) vs 16 (16.16%), RR 0.13, 95% CI [0.03–054], p=0.001) (Table 2). All apnea events occurred at T2 in both groups. Patients in group E gradually recovered spontaneous breathing after a brief pause, but 4% of the patients in group S did not recover spontaneous breathing until T3 (Table 3).
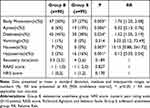 |
Table 2 Secondary Outcomes |
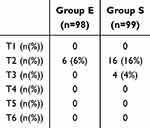 |
Table 3 Apnea at Different Time Points |
The incidence of body movement in group E was higher (47 (50.0%) vs 27 (27.27%), RR 1.76, 95% CI [1.20–2.58], p=0.003), and all patients reporting PONV occurred in group E (nausea, 7 (7.14%); RR 15.15; 95% CI [0.88, 261.72]; p=0.007); vomiting 1 (1.02%); RR 3.03; 95% CI [0.12, 73.49]; p=0.314). There were no statistically significant differences in the RASS and pain scores.
Discussion
Considering the surgical duration is usually within 10 minutes,21 we designed a relatively small dosage of esketamine to avoid unnecessary delayed anesthesia recovery and adverse drug reactions, such as dissociation, anxiety, nausea, increased blood pressure, and headache.22 In fact, according to Chen et al23 esketamine 0.3 mg/kg for intravenous anesthesia in patients undergoing surgery abortion can lead to over 10% of potential psychiatric symptoms. Our study suggests that a single low dose of esketamine combined with propofol is safe and can provide ideal anesthesia for surgical abortion. Although our study found that the blood pressure of patients in group E was more stable, considering that the blood pressure of both groups at various time points was within the normal range, it is difficult to conclude that this difference is clinically significant. Similar results have also been reported in other studies. Lin et al24 found that in lung tumor percutaneous radiofrequency ablation, the intraoperative MAP in the esketamine plus dexmedetomidine sedation group was above 100 mmHg, whereas in the sufentanil plus dexmedetomidine sedation group, the MAP was between 85 and 95 mmHg. However, in clinical practice, we cannot say that an MAP above 100 mmHg is more beneficial for patients than an MAP between 85 and 95 mmHg. Nevertheless, we consider that the esketamine-based anesthesia protocol has advantages in preventing intraoperative hypotension and bradycardia, which can be attempted in patients with potential risks, such as those with preoperative sinus bradycardia and fatigue. Further clinical research is needed to evaluate the benefits of using esketamine for anesthesia in such patients.
We conducted a comprehensive evaluation of patients’ intraoperative respiratory conditions. We found that esketamine helped maintain a comparable RR throughout the entire procedure as in the preoperative stage; however, patients in group S experienced a certain period of slowed RR after anesthesia and then gradually recovered to preoperative levels until surgery began, and the risk of hypoxemia was more than seven times higher than that in group E. According to our results, all apnea events occurred at T2, indicating that anesthesia induction is a high-risk time point for apnea. Clinicians should closely monitor patients breathing and oxygenation to reduce adverse events. These results are consistent with previous studies.25–27 Although measuring PetCO2 through a nasal cannula may not accurately reflect its actual value, its changes have clinical reference value. Patients in Group S experienced an increase in PetCO2 during surgery, indicating a decrease in minute volume (MV). However, at T4, the patients recovered to a comparable preoperative RR, suggesting a possible decrease in tidal volume (TV). Conversely, patients in group E showed a slight decrease in PetCO2 during surgery. Considering that the patient’s RR was slightly faster during surgery, this might suggest that the patient’s TV should not change so that a slightly higher MV can be maintained. Many researchers have explored the mechanisms underlying the effects of esketamine on respiration. Jonkman et al28 involved health and young volunteers and found that anesthetic drugs might inhibit respiration by suppressing ventilation CO2 chemosensitivity, and esketamine could increase the sensitivity to avoid hypopnea. Animal experiments have shown that ketamine abolishes the coupling between loss of conscientiousness and upper airway dilator muscle dysfunction, and increases the flow rate, respiratory rate, and effective inspiratory time.29 In addition, ketamine can be metabolized into hydroxynorketamine (HNK), which may act on glutamatergic modulation of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR), leading to exciting respiratory activity.30,31
Anesthesia recovery time, RAAS, and pain score showed no differences between the two groups, although the patients in group E seemed to have faster anesthesia recovery. However, the other outcomes were somewhat controversial with those of previous studies. We found that patients in Group E had a higher proportion of intraoperative body movements, PONV, and dizziness. Kan et, al.26 conducted a meta-analysis that revealed that esketamine could reduce body movement (RR 0.60, 95% CI [0.41, 0.88]) while increasing the risk of dizziness (RR 1.99, 95% CI[1.17, 3.37]). Xu et al showed that esketamine did not increase the risk of PONV after gastroscopy,32 and similar results were observed among patients undergoing esketamine sedation during cervical conization.33 Moreover, esketamine-based opioid-free anesthesia (OFA) or postoperative patient-controlled intravenous analgesia (PCIA) can reduce the risk of PONV.34,35 Our results might be related to the esketamine dosage. However, we cannot determine whether an increase in esketamine can decrease the risk of PONV and body movement, and potential side effects of esketamine might occur. Monitoring of pain-analgesia balance might reveal the reasons for this difference, such as quantitative oxidative index (qNOX), nociception level (NOL), and cardiovascular dysplasia (CARDIAN).36 Therefore, caution should be exercised when choosing esketamine for anesthesia in high-risk patients with PONV. When administering esketamine anesthesia, clinicians should ensure that propofol is within reach and can be added at any time, and protective control should be implemented to prevent the patient from affecting the surgical procedure or causing injury.
This study has several limitations. First, as a single-center study with a limited number of involved patients, we designed 0.1 mg/kg of esketamine based on our previous experience and analysis, without comparing the effects of different doses. Therefore, all conclusions of this study are based on this anesthesia induction dose, and we cannot prove whether this dose is optimal. In fact, Shen et al found in their study on painless abortion patients that, anxious patients require higher doses of esketamine to achieve effective analgesia and alleviate propofol injection pain.37 Second, we mainly included East Asian populations; whether this conclusion applies to other populations requires further exploration. Third, esketamine has potential psychiatric symptoms, and we did not assess the preoperative psychological state of the patients (including anxiety, depression, and preoperative sleep status), which may lead to increased perioperative psychological risks for patients. Indeed, Jiang et al reported that esketamine could treated postoperative depressive symptoms at 7 days and 42 days after abortion surgery,38 which suggested that esketamine might have potential medium-to-long-term psychological effects, although it cannot be determined as positive or negative yet. Fourth, we did not evaluate the safety and effectiveness of esketamine induction in cases of long-term opioid drug exposure, and further research is needed for this specific population. Finally, our evaluation indicators for intraoperative respiratory status are not sufficient (such as tidal volume). Using advanced equipment, such as Electrical Impedance Tomography (EIT), and incorporating more indicators (atelectasis, hyperventilation, tidal volume, etc)., we could evaluate the impact of different anesthesia induction regimens on the patient’s respiratory function more comprehensively. Therefore, further research can be conducted by designing large-sample, multi-center clinical studies that include patients from different regions, cultural backgrounds, and basic preoperative conditions, using different gradients of anesthesia-inducing drugs, and incorporating more comprehensive outcome indicators, to provide more reference and basis for the use of esketamine in painless diagnosis and treatment.
Conclusion
The use of esketamine for first-trimester surgical abortion anesthesia is beneficial for maintaining intraoperative hemodynamic stability, improving intraoperative respiratory function, and reducing the risk of apnea and hypoxemia; however, it may increase the risk of intraoperative body movement, postoperative dizziness, and PONV. Further exploration of the esketamine anesthesia regimen for first-trimester surgical abortion can help reduce the occurrence of adverse effects and benefit patients.
Data Sharing Statement
We would like to share individual deidentified participant data, and researchers can apply to correspondence author to view raw data for scientific research purposes.
Consent for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors a worldwide license to the Publishers and its licensees in perpetuity, in all forms, formats, and media (whether known now or created in the future), to: i) publish, reproduce, distribute, display, and store the Contribution; ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts, and/or abstracts of the Contribution; iii) create any other derivative work(s) based on the Contribution; iv) to exploit all subsidiary rights in the Contribution; v) the inclusion of electronic links from the Contribution to third-party material where-ever it may be located; and vi) license any third party to do any or all of the above.
Acknowledgments
We would like to thank Shihong Zhang, chief physician of the Gynecology Department, Weihai Municipal Hospital, for careful assessment of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by Youth General Project Category of Natural Science Foundation of Shandong Province, China (Grant No. ZR2024QH295).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Abortion. Available at: https://www.who.int/news-room/fact-sheets/detail/abortion.
2. Guttmacher Institute. Abortion in the United States, Available from: https://www.guttmacher.org/fact-sheet/induced-abortion-united-states.
3. O’Connell K, Jones HE, Simon M, Saporta V, Paul M, Lichtenberg ES. National abortion federation members. first-trimester surgical abortion practices: a survey of national abortion federation members. Contraception. 2009;79(5):385–392.PMID: 19341852. doi:10.1016/j.contraception.2008.11.005
4. Ganatra B, Gerdts C, Rossier C, et al. Global, regional, and subregional classification of abortions by safety, 2010-14: estimates from a Bayesian hierarchical model. Lancet. 2017;390(10110):2372–2381.PMID: 28964589; PMCID: PMC5711001. doi:10.1016/S0140-6736(17)31794-4
5. Free LL, Sheeder J, Cohen RH. Effects of aromatherapy on patient satisfaction with procedural abortion at less than 10 weeks’ gestation: a randomized controlled trial. Contraception. 2024;130:110311. PMID: 37858617. doi:10.1016/j.contraception.2023.110311
6. Renner RM, Jensen JT, Nichols MD, Edelman AB. Pain control in first-trimester surgical abortion: a systematic review of randomized controlled trials. Contraception. 2010;81(5):372–388.PMID: 20399943. doi:10.1016/j.contraception.2009.12.008
7. Chen L, Zhou Y, Cai Y, Bao N, Xu X, Shi B. The ED95 of nalbuphine in outpatient-induced abortion compared to equivalent sufentanil. Basic Clin Pharmacol Toxicol. 2018;123(2):202–206.PMID: 29626849. doi:10.1111/bcpt.13022
8. Gao W, Sha B, Zhao Y, Fan Z, Liu L, Shen X. Comparison of simultaneous and sequential administration of fentanyl-propofol for surgical abortion: a randomized single-blinded controlled trial. Artif Cells Nanomed Biotechnol. 2017;45(5):1045–1050.PMID: 27707001. doi:10.1080/21691401.2016.1239106
9. Gokhale P, Lappen JR, Waters JH, Perriera LK. Intravenous sedation without intubation and the risk of anesthesia complications for obese and non-obese women undergoing surgical abortion: a retrospective cohort study. Anesth Analg. 2016;122(6):1957–1962.PMID: 27177015. doi:10.1213/ANE.0000000000001335
10. Guan Y, Pan H, Cong X, et al. Effect of esketamine on haemodynamic fluctuations in patients undergoing hysteroscopic surgery: a prospective, double-blind randomized clinical trial. Br J Clin Pharmacol. 2024;90(11):2754–2762.PMID: 38958172. doi:10.1111/bcp.16165
11. Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401.PMID: 31860599. doi:10.1097/EJA.0000000000001134
12. Chen Y, Chen J, Wang Q, et al. Safety and tolerability of esketamine in propofol based sedation for endoscopic variceal ligation with or without injection sclerotherapy: randomized controlled trial. Dig Endosc. 2023;35(7):845–854.PMID: 36808150. doi:10.1111/den.14539
13. Zhan Y, Liang S, Yang Z, et al. Efficacy and safety of subanesthetic doses of esketamine combined with propofol in painless gastrointestinal endoscopy: a prospective, double-blind, randomized controlled trial. BMC Gastroenterol. 2022;22(1):391.PMID: 35987996; PMCID: PMC9392938. doi:10.1186/s12876-022-02467-8
14. Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT. Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi:10.1186/1741-7015-8-18
15. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects, JAMA. 2013;310(20):2191–2194. PMID: 24141714. doi:10.1001/jama.2013.281053
16. Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the observer’s assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–251. PMID: 2286697.
17. O’Driscoll BR, Howard LS, Earis J, Mak V; British Thoracic Society Emergency Oxygen Guideline Group; BTS Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1–ii90. PMID: 28507176. doi:10.1136/thoraxjnl-2016-209729
18. Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–448.PMID: 32467512. doi:10.1213/ANE.0000000000004833
19. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344.PMID: 12421743. doi:10.1164/rccm.2107138
20. Deeks JJ, Higgins JPT. Statistical algorithms in review manager 5. 2010. Available from: https://training.cochrane.org/.
21. Moayedi G, Tschann M. Pain management for first-trimester uterine aspiration. Obstet Gynecol Surv. 2018;73(3):174–181.PMID: 29595873. doi:10.1097/OGX.0000000000000544
22. Ceban F, Rosenblat JD, Kratiuk K, et al. Prevention and management of common adverse effects of ketamine and esketamine in patients with mood disorders. CNS Drugs. 2021;35(9):925–934.PMID: 34363603. doi:10.1007/s40263-021-00846-5
23. Chen J, Zou X, Hu B, et al. Effect of different doses of esketamine compared with fentanyl combined with propofol on hypotension in patients undergoing painless abortion surgery: a prospective, randomized, double-blind controlled clinical trial. BMC Anesthesiol. 2022;22(1):305.PMID: 36171562; PMCID: PMC9516803. doi:10.1186/s12871-022-01848-6
24. Lin Z, Li S, Zhou Y, et al. A comparative study of esketamine-dexmedetomidine and sufentanil-dexmedetomidine for sedation and analgesia in lung tumor percutaneous radiofrequency ablation (PRFA): a randomized double-blind clinical trial. BMC Anesthesiol. 2023;23(1):304.PMID: 37684574; PMCID: PMC10486108. doi:10.1186/s12871-023-02266-y
25. Lu C, Ren J, Guo X, Qian J. Effects of remimazolam combined with esketamine anesthesia on circulatory and respiratory function during painless gastroenteroscopy. Contrast Media mol Imaging. 2022:1079099. PMID: 36304777; PMCID: PMC9581615. doi:10.1155/2022/1079099
26. Kan Z, Min W, Dai Y, Zhang P. Intravenous esketamine as an adjuvant for sedation/analgesia outside the operating room: a systematic review and meta-analysis. Front Pharmacol. 2024;15:1287761. PMID: 39021840; PMCID: PMC11252540. doi:10.3389/fphar.2024.1287761
27. Chen H, Ding X, Xiang G, et al. Analysis of the efficacy of subclinical doses of esketamine in combination with propofol in non-intubated general anesthesia procedures - a systematic review and meta-analysis. BMC Anesthesiol. 2023;23(1):245.PMID: 37479982; PMCID: PMC10360232. doi:10.1186/s12871-023-02135-8
28. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–1127.PMID: 29661389. doi:10.1016/j.bja.2018.02.021
29. Eikermann M, Grosse-Sundrup M, Zaremba S, et al. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116(1):35–46.PMID: 22108392; PMCID: PMC3246073. doi:10.1097/ALN.0b013e31823d010a
30. Pace RW, Mackay DD, Feldman JL, Del negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582(Pt 1):113–125.PMID: 17446214; PMCID: PMC2075310. doi:10.1113/jphysiol.2007.133660
31. Kamp J, Van Velzen M, Olofsen E, Boon M, Dahan A, Niesters M. Pharmacokinetic and pharmacodynamic considerations for NMDA-receptor antagonist ketamine in the treatment of chronic neuropathic pain: an update of the most recent literature. Expert Opin Drug Metab Toxicol. 2019;15(12):1033–1041.PMID: 31693437. doi:10.1080/17425255.2019.1689958
32. Xu Y, Zheng Y, Tang T, Chen L, Zhang Y, Zhang Z. The effectiveness of esketamine and propofol versus dezocine and propofol sedation during gastroscopy: a randomized controlled study. J Clin Pharm Ther. 2022;47(9):1402–1408.PMID: 35488787. doi:10.1111/jcpt.13678
33. Si J, Li X, Wang Y, Feng N, Cui M. Effects of adding low-dose esketamine to sufentanil and propofol sedation during cervical conization: a single-centre, randomized controlled trial. BMC Anesthesiol. 2024;24(1):15.PMID: 38178016; PMCID: PMC10765731. doi:10.1186/s12871-023-02389-2
34. Li X, He X, Li M, et al. The effect of esketamine combined with sufentanil based patient-controlled intravenous analgesia for postoperative pain in patients undergoing third molar surgery and maxillofacial trauma: a randomized clinical trial. BMC Oral Health. 2024;24(1):1460.PMID: 39623402; PMCID: PMC11610279. doi:10.1186/s12903-024-05273-8
35. Yan H, Chen W, Chen Y, et al. Opioid-free versus opioid-based anesthesia on postoperative pain after thoracoscopic surgery: the use of intravenous and epidural esketamine. Anesth Analg. 2023;137(2):399–408.PMID: 37267129. doi:10.1213/ANE.0000000000006547
36. Martinez-Vazquez P, Jensen EW. Different perspectives for monitoring nociception during general anesthesia. Korean J Anesthesiol. 2022;75(2):112–123.PMID: 35172074; PMCID: PMC8980281. doi:10.4097/kja.22002
37. Shen Y, Yin L, Hu B, Xia Y, Zhang L. Preoperative anxiety’s impact on the median effective dose of esketamine for alleviating propofol injection pain in patients undergoing painless abortion: a randomized, double-blind, controlled trial. Drug Des Devel Ther. 2024;18:5863–5872. PMID: 39670280; PMCID: PMC11635161. doi:10.2147/DDDT.S482019
38. Jiang M, Li Q, Mao M, et al. Evaluation of clinical effects of Esketamine on depression in patients with missed miscarriage: a randomized, controlled, double-blind trial. J Affect Disord. 2023;329:525–530. PMID: 36863473. doi:10.1016/j.jad.2023.02.127
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effects of Remimazolam Tosilate Combined with Esketamine on Anesthetic Efficacy and Psychiatric Symptoms in Patients Undergoing Ambulatory Surgery: A Randomized Controlled Study
Yu X, Xu X, Wang J, Wang Z, Zhang Y
Drug Design, Development and Therapy 2025, 19:4527-4535
Published Date: 30 May 2025


