Back to Journals » Drug Design, Development and Therapy » Volume 19
Effects of Remimazolam Tosilate Combined with Esketamine on Anesthetic Efficacy and Psychiatric Symptoms in Patients Undergoing Ambulatory Surgery: A Randomized Controlled Study
Authors Yu X, Xu X, Wang J, Wang Z, Zhang Y
Received 27 January 2025
Accepted for publication 21 May 2025
Published 30 May 2025 Volume 2025:19 Pages 4527—4535
DOI https://doi.org/10.2147/DDDT.S519732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Lymperopoulos
Xuan Yu, Xinpeng Xu, Jing Wang, Zhao Wang, Yi Zhang
Department of Anesthesiology, The Second Affiliated Hospital of Zunyi Medical University, Zunyi, People’s Republic of China
Correspondence: Yi Zhang, Department of Anesthesiology, The Second Affiliated Hospital of Zunyi Medical University, The Intersection of Xinglong Avenue and Xinpu Avenue, Zunyi, 563000, People’s Republic of China, Email [email protected]
Background: The choice of anesthetic drugs is crucial in ambulatory surgery. Esketamine has anesthetic, analgesic, and sedative effects, but it is associated with dose-dependent psychiatric symptoms. Benzodiazepines can alleviate these symptoms, but traditional drugs like midazolam may prolong the recovery time. Remimazolam tosilate as a novel benzodiazepine, has not been fully explored in terms of its effects when combined with esketamine and its impact on psychiatric symptoms.
Methods: A total of 249 patients undergoing elective laparoscopic surgery were enrolled. Randomly divided into the esketamine group (Group E), the esketamine plus midazolam group (Group EM), and the esketamine plus remimazolam tosilate group (Group ER). The primary outcome was the incidence of adverse reactions. The secondary outcomes included hemodynamics at different time points: at rest in the room (T0), immediately post-intubation (T1), immediately post-extubation (T2), 30 minutes following extubation (T3), and immediately after leaving the PACU (T4). Moreover, we also documented the Riker Sedation-Agitation Scale (SAS) scores at T2-4, as well as extubation time and PACU stay duration.
Results: Patients in Group ER had a significantly lower incidence of postoperative diplopia and blurry vision compared to Groups E and EM (p< 0.05). Postoperative psychiatric symptoms were significantly lower in Groups EM and ER than in Group E (p< 0.05). At the T1 time point, Groups EM and ER displayed significantly lower MAP and HR, compared to Group E (p< 0.05). Groups E and ER displayed a shorter extubation time than Group EM (p< 0.05); the PACU stay of Group ER was shorter than those of Groups E and EM (p< 0.05). The SAS scores decreased from T2 to T4 in Groups EM and ER than in Group E (p< 0.05).
Conclusion: The combination of remimazolam tosilate and esketamine effectively reduces postoperative psychiatric symptoms, enhances hemodynamic stability, and improves recovery quality, making it a viable anesthetic strategy for ambulatory surgery.
Keywords: remimazolam tosilate, esketamine, ambulatory surgery, psychiatric symptoms
Introduction
Ambulatory surgery is a surgical approach, in which patients undergo admission, surgery, and discharge within a single working day. It is beneficial for reducing hospital stays, accelerating bed turnover, and enhancing the efficacy of medical resource utilization.1 The rational selection of anesthetic drugs is crucial for link in ensuring the anesthesia effect of ambulatory surgery and the rapid recovery of patients. Abdominal surgeries (such as laparoscopic cholecystectomy and hernia repair) are common types of ambulatory surgeries. However, due to situations like pneumoperitoneum irritation and visceral traction reflex, they place greater demands on the selection of anesthetic drugs and also pose greater challenges.
Traditional induction drugs for ambulatory surgery, such as propofol, often cause hypotension. Esketamine, with its advantages of providing anesthesia, analgesia, and sedation without suppressing cardiopulmonary function,2 has become one of the ideal choices for anesthesia management in ambulatory surgery. However, the dose-dependent psychiatric symptoms induced by esketamine, such as nightmares and dissociative phenomena,3 have attracted the attention of anesthesiologists.
Studies have shown that benzodiazepines can reduce adverse psychiatric reactions to esketamine through the non-specific central inhibitory effects mediated by the gamma-aminobutyric acid type A (GABAA) receptor.4 Midazolam is the most widely used benzodiazepine sedative in clinical practice. Research indicates that its sedative-hypnotic effects help reduce the use of general anesthetics during surgery, and its unique anterograde amnesia can prevent patients from experiencing intraoperative awareness.5 However, its metabolites remain highly active and continue to exert sedative-hypnotic effects within the body, thereby significantly prolonging the patient’s recovery time.6
Remimazolam tosilate is a novel ultrashort-acting benzodiazepine. Compared with midazolam, it has higher clearance efficiency, a smaller volume of distribution, and a shorter half-life.7,8 In the fast-paced environment of ambulatory surgery, which demands rapid patient turnover, these characteristics give it an advantage in reducing postoperative recovery time and improving the efficiency of anesthesia recovery.
Although previous studies have focused on the interaction between benzodiazepines and esketamine, the effects of the combination of remimazolam tosylate and esketamine in ambulatory surgery, as well as its potential impact on the adverse reaction to esketamine, remain underexplored. This study aimed to investigate the specific effects of the combination of remimazolam tosylate and esketamine in reducing dose-dependent psychiatric symptoms associated with esketamine and the impact of this coadministration regimen on patient’ recovery quality, thereby providing scientific evidence for optimizing anesthesia protocols in ambulatory surgery and promoting rapid patient recovery.
Methods
Study Design
The present unicentric, double-blind, randomized, controlled trial was carried out between July 2023 to February 2024 in Second Affiliated Hospital of Zunyi Medical University. This study was performed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) criteria and adhering to the Declaration of Helsinki. This study has been approved by the Ethics Committee of the Second Affiliated Hospital, Zunyi Medical University, China (number: KYLL-2022-026). It has been registered with the Chinese Clinical Trial Registration Center (https://www.chictr.org.cn, ChiCTR2300073319, registration date: July 6th, 2023). After understanding the study’s objectives, procedures, and potential risks, all enrolled patients or their family members provided signed informed consent.
Patient Eligibility
The inclusion criteria were: Patients scheduled for day-case abdominal surgery under general anesthesia; aged 18–60 years, participants without sex discrimination, and those categorized as grade I–II, according to American Society of Anesthesiologists (ASA) guidelines. The exclusion criteria were: patients with preoperative dysfunctions of major organs like heart, lungs, liver, and kidneys; those contradicted for esketamine and benzodiazepines; patients who used sedative-analgesic drugs recently; those with cognitive impairment or mental disorders; those who underwent laparotomy; patients with surgery duration >1 hour, and participants with missing postoperative follow-up data.
Randomization and Blinding
Before anesthesia, we randomized all patients into Group E, Group EM or Group ER at the 1:1:1 ratio. Random numbers were generated using SPSS 26.0 software, and then random envelopes were created. During the grouping process, an anesthesiologist who did not participate in the research opened the envelopes. An anesthesiologist who was not involved in this study was responsible for the drug preparation. It is worth emphasizing that throughout the entire study period, neither the surgeons, nurses, patients, anesthesiologists, nor the outcome observers were aware of the specific grouping of the patients.
Anesthetic Procedure
All patients were instructed to abstain from drinking and eating for six and eight hours before surgery, respectively. In the operating room, we established an intravenous line and carried out fluid resuscitation as needed. Routine monitoring of patients’ basic vital signs included mean arterial pressure (MAP) and heart rate (HR). The anesthetic induction protocol was the same for all patients. All the patients were administered intravenous sufentanil (0.3ug/kg), propofol (0.2mg/kg), and rocuronium (1mg/kg) consequently. Groups E, EM, and ER were administered esketamine (0.3mg/kg, Jiangsu Hengrui Pharmaceutical Co., Ltd., China),9 esketamine (0.3 mg/kg) plus midazolam (0.08 mg/kg, Jiangsu Enhua Pharmaceutical, Co., Ltd., China),10 and esketamine (0.3 mg/kg) and remimazolam tosilate (0.3 mg/kg, Jiangsu Hengrui Pharmaceutical Co., Ltd., China).11 After, anesthesia was induced, the three groups underwent tracheal intubation and pressure control mode ventilation. The ventilator parameters were adjusted to maintain the PETCO2 at 35–45 mmHg. Anesthesia was sustained with propofol (4–12 mg/kg/h), remifentanil 0.2–0.5 (μg/kg/h), and sevoflurane (1–2 MAC); the muscle relaxants were used whenever needed. The target blood pressure was maintained at ±20% of the baseline value, and the target heart rate was 60–100 beats/min. A decrease in MAP of 20% from baseline or a d rop to below 60 mmHg for at least 1 minute is considered hypotension, which will be treated with ephedrine until the MAP returns to the normal range. A heart rate that drops below 50 beats per minute is considered bradycardia, and will be managed with 0.3 mg of atropine, which can be repeated based on the patient’s heart rate. All patients received an anesthetic loading dose of tropisetron (5mg) and flurbiprofen axetil (50mg), ten minutes before the operation’s completion. However, propofol, remifentanil, and sevoflurane were immediately discontinued postoperatively, and the patients were transferred to the Post-Anesthesia Care Unit (PACU). If a patient’s postoperative VAS score was >3 points, a rescue analgesic plan comprising intravenous ketorolac tromethamine (30 mg) injection was implemented. In case of postoperative psychiatric adverse reactions, propofol sedation was administered as a corrective measure.
Observational Indicators
Our primary outcome was the incidence of adverse reactions in patients (during the induction and postoperative recovery periods). To assess specific psychiatric symptoms such as hallucinations and nightmares, observers conduct interviews with patients upon awakening and make preliminary judgments based on the patients’ self-reports. For the evaluation of symptoms like delirious speech, a comprehensive consideration of the patients’ behavior, mental status, and clinical observations is required. For example, observers examine the patients’ eye contact, emotional stability, and awareness of the surrounding environment.
The secondary outcomes included recording patients’ demographic data, MAP, and HR at rest upon admission (T0), immediately after intubation (T1), immediately following extubation (T2), 30 minutes post-extubation (T3), and immediately before leaving the PACU (T4). Additionally, the quality of patients’ postoperative awakening was assessed through several measures, including the Riker Sedation-Agitation Scale (SAS) scores at time points T2 to T4. The Ricker Sedation-Agitation Scale Criteria are as follows: 1 point, unarousable, with no or only minimal response to noxious stimuli, and inability to communicate or follow commands; 2 points, very sedated, responding only to physical stimuli, unable to communicate or follow commands, with spontaneous movements; 3 points, sedated and drowsy, responsive to verbal stimuli or gentle shaking, able to follow simple commands but quickly returning to sleep; 4 points, calm and cooperative, easily arousable, and able to follow commands. 5 points, agitated and anxious or physically restless, but calmed with verbal reassurance; 6 points, very agitated, requiring protective restraints and repeated verbal reassurance, possibly biting on the endotracheal tube; 7 points, dangerously agitated, characterized by pulling at the endotracheal tube, attempting to remove various catheters, climbing over window bars, attacking medical staff, or struggling in bed. Additional parameters included postoperative extubation time, defined as the interval from the cessation of anesthetic drug administration to removal of the endotracheal tube, and the duration of stay in the post-anesthesia care unit (PACU), defined as the time from PACU admission until the patient meets discharge criteria and is released.
Sample Size Calculation and Statistical Analysis
Based on the results of the preliminary trial, this study used the incidence of postoperative adverse reactions in patients as the main observation index. The proportion in Group E was 62%, that in Group EM was 43%, and that in Group ER was 33%. With a significance level of α = 0.05 and a power of 1 - β = 90%, the PASS 15.0 software was used to calculate the sample size, and the result was n = 73 patients per group. Considering a dropout rate of 20%, it was calculated that 91 patients were planned to be included in each group of this study.
The PASS 15.0 software was used to calculate the sample size. Postoperative adverse reactions were the primary observational indicators. We enrolled 94 patients in each group, based on the results of a preliminary pre-trial, with a significance level of α = 0.05, a power of 1-β = 90%, and a dropout rate of 20%.
Data analysis was conducted using SPSS 26.0 software. The normally distributed data were represented as mean ± standard deviation (±s). Inter-group comparisons were made using one-way ANOVA. However, non-normal data were represented as a median and interquartile range [M(IQR)], followed by the Kruskal–Wallis test. Categorical data were expressed as the number of cases (%), and inter-group comparisons were made using the chi-squared (χ2) test. A p-value <0.05 indicated statistical significance.
Results
Initially, we enrolled 282 patients. However, 21, 7, and 5 patients were excluded due to surgery duration >1 hour, missing data, and implementation of open surgery, respectively. Finally, 249 patients were included and 82, 84, 83 patients were categorized into Groups E, ER, and EM, respectively. Figure 1 shows the flowchart depicting the study’s procedure.
 |
Figure 1 Flowchart depicting the study’s procedure Baseline characteristics. |
No statistically significant differences were observed in gender, age, BMI, ASA classification, type of surgery, surgery duration, or vasoactive drug perioperative use among the three groups (p>0.05, Table 1).
 |
Table 1 Comparison of the General Characteristics of the Three Groups |
Primary Outcomes
Group ER experienced a significant reduction in postoperative diplopia/blurry vision compared to the groups E and EM (p<0.05). Groups EM and ER showed a significant reduction in postoperative psychological reactions (p<0.05) than Group E. There was no statistically significant difference in the incidence of adverse reactions like postoperative nausea, vomiting, and increased secretions during the induction and postoperative phases among the three groups (p>0.05, Table 2).
 |
Table 2 Incidence of Adverse Reactions |
Secondary Outcomes
Groups EM and ER had significantly lower MAP and HR at the T1 time point than Group E (p<0.05). And there were no statistically significant differences in vital signs at any other time points (p>0.05, Table 3).
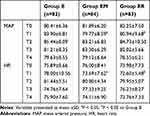 |
Table 3 Comparison of MAP and HR at Different Time Points |
Groups E and ER had quicker extubation times than Group EM (p<0.05); Group ER stayed less in PACU, compared to Groups E and EM (p<0.05). Groups EM and ER showed significantly reduced SAS scores at T2-4 (p<0.05) than Group E; however, no statistical differences were observed at other time points (p>0.05, Table 4).
 |
Table 4 Comparison of the Quality of Postoperative Awakening Among Three Groups |
Discussion
Ambulatory surgery emphasizes rapid recovery and safety. Ideal drugs should take effect quickly, allow for a rapid recovery of consciousness, and have few side effects. The common anesthesia regimen for day surgery, which combines propofol, midazolam, and opioids, aims to achieve rapid awakening and adequate analgesia. However, it can cause hemodynamic changes in patients, affecting perioperative physiological stability and postoperative recovery.12
Esketamine, a new type of anesthetic, sedative, and analgesic drug, is a non-competitive N-methyl-D-aspartic acid (NMDA) receptor antagonist. It dose not inhibit respiration, mildly stimulates circulation, and result in a short awakening time.13 Multiple domestic and international studies have shown that esketamine, a novel anesthetic sedative and analgesic drug,13 has no impact on postoperative recovery, has minimal side effects, and exhibits favorable safety characteristics when used at a dose of 0.3 mg/kg for anesthesia induction.14–16 Therefore, this study selected 0.3mg/kg of esketamine as the induction dose. However, this result is inconsistent with our study findings. The results of this study show that compared with the two groups using midazolam or remimazolam tosylate in combination, the incidence of postoperative diplopia/blurred vision and psychiatric symptoms such as speech confusion, nightmares, and hallucinations was higher in Group E, which used esketamine alone, with statistically significant differences.
Gastaldon17 found that the FAERS database contains 962 cases of esketamine-related adverse reactions, among which 18 were esketamine-related neurological adverse reactions. Xu18 indicated that intravenous injection of 0.25mg/kg esketamine before skin incision in cesarean section could produce sedative and analgesic effects, but it significantly increased the incidence of intraoperative neurological and psychiatric symptoms. We speculate that the esketamine’s psychoactive effects might be related to its function as an NMDA receptor antagonist. As an NMDA receptor antagonist, esketamine interacts with a receptor involved in several perceptual, emotional, behavioral, and cognitive alterations.19 It is worth noting that the elimination half-life of esketamine is 2–3 hours.20 And our selected surgeries were ambulatory surgery, this might be related to the drug’s metabolism. In addition, the differences in drug metabolism among individuals are a factor that cannot be ignored. Due to reasons such as genetic polymorphism, the activities of enzymes involved in drug metabolism vary among different patients.
The research indicates that medications capable of GABA receptor activation can be utilized to mitigate the esketamine-induced psychiatric side effects.21 Our study revealed that the concomitant usage of various benzodiazepines can effectively reduce postoperative diplopia/blurry vision and psychiatric symptoms. Midazolam and remimazolam tosilate were GABA receptor agonists,22 may exert its effects on the brainstem reticular formation and the limbic system through GABA receptors. This inhibits the excitability of the central nervous system and reduces esketamine’s excitatory actions.23 Furthermore, might mitigate esketamine’s psychoactive adverse effects by inducing calcium (Ca2+) influx and activating glutamate NMDA receptors as well as counteracting the antagonistic effects of esketamine on these receptors.24
However, when different benzodiazepines are used in combination with esketamine, there are differences in their effects on patients’ awakening time and recovery quality. Studies have shown that although midazolam can prevent the psychiatric symptoms caused by esketamine, it may prolong the patient’s awakening time and even affect postoperative cognitive function.25,26 The results of this study show that, the group EM significantly reduced the incidence of postoperative diplopia/blurred vision and psychiatric symptoms. However, the time to extubation and the length of stay in the PACU were significantly longer in the group EM than in the group ER. This may be due to the fact that the metabolites of midazolam still retain some pharmacological activity.27 In contrast, remimazolam tosilate, with its high clearance rate and short duration of action, has attracted much attention. Its main metabolites exhibit almost no pharmacological activity and can be rapidly hydrolyzed by nonspecific esterases in the blood,28 accelerates the patient’s awakening process. Consequently, remimazolam tosilate displays a more rapid recovery than midazolam. In addition, the extubation time in Group E was also significantly shorter than that in Group EM, which may be closely related to the pharmacological properties of esketamine. Compared to the traditional racemic ketamine, esketamine exhibits potent pharmacological properties and has a higher body clearance rate. This contributes to faster postoperative recovery in patients.29 Additionally, when esketamine was co-administered with different benzodiazepines, the SAS scores during the recovery period were significantly lower than those in the control group. This might be potentially related to the increased incidence of postoperative agitation due to esketamine’s sympathomimetic effects. Moreover, benzodiazepines might significantly prevent postoperative agitation by reducing sympathetic tone and inhibiting catecholamine release.30
In terms of hemodynamics, esketamine maintains stable vital signs during the anesthesia induction period. However, there is a slight increase in MAP and HR immediately after intubation, which may be due to its sympathomimetic effect and the stimulation of intubation. After the combined use of benzodiazepines, MAP and HR at time T1 are lower compared to when esketamine is used alone, and the hemodynamics are more stable This benefits from the synergistic sedative effect between benzodiazepines and esketamine, as well as the balancing effect of benzodiazepines on the sympathomimetic stimulation of esketamine.26
Limitations
Our study had several limitations. First, our sample size was a single-center trial. Thus, increasing the sample size can bolster the results’ statistical power and external validity. Second, the optimal induction dose of esketamine remains unclear. Future studies can further explore this to reduce adverse reactions and optimize the quality of recovery from anesthesia. Furthermore, the postoperative follow-up period in this study was relatively short, making it difficult to evaluate the long - term recovery of patients and potential delayed adverse reactions. Therefore, it is highly necessary to extend the long-term postoperative follow-up. In addition, this study has certain limitations in the assessment of subjective endpoints such as psychiatric symptoms. In this study, psychiatric symptoms were mainly judged through patients’ self-reports and observations by medical staff. There is a lack of more objective and quantitative assessment indicators. Future studies can adopt more standardized and objective assessment tools.
Conclusions
In summary, the combination of remimazolam tosilate and esketamine effectively reduces postoperative psychiatric symptoms, enhances hemodynamic stability, and improves recovery quality, making it a viable anesthetic strategy for ambulatory surgery.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.
Acknowledgments
Our thanks should go to the Department of Anesthesiology of the Second Affiliated Hospital of Zunyi Medical University for their incredible supports. Thanks to professor Zhao Wang for guidance on this study, to professor Yi Zhang for writing assistance and proof reading the article, to Ms. Xinpeng Xu and Jing Wang for providing language help and data acquisition.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by Beijing Hongyi Medical Development Foundation “Assisting Medical Take-off” Clinical Research Fund in Sedation and Analgesia [HY20220037-A-23], Zunyi City Science and Technology Plan Project [Zunyi Ke He HZ No (2022) 408], Guizhou Province Health and Health Commission Science and Technology Fund Project [gzwkj2023-400].
Disclosure
The authors have no competing interests in this work.
References
1. Rajan N, Rosero EB, Joshi GP. Patient selection for adult ambulatory surgery: a narrative review. Anesth Analg. 2021;133(6):1415–1430. doi:10.1213/ANE.0000000000005605
2. Kohtala S. Ketamine-50 years in use: from anesthesia to rapid antidepressant effects and neurobiological mechanisms. Pharmacol Rep. 2021;73(2):323–345. doi:10.1007/s43440-021-00232-4
3. Mion G, Himmelseher S. Esketamine: less drowsiness, more analgesia. Anesth Analg. 2024;139(1):78–91. doi:10.1213/ANE.0000000000006851
4. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. doi:10.1124/pr.117.015198
5. Kim J, Choi SM, Park YS, et al. Dexmedetomidine versus midazolam for sedation during endobronchial ultrasound-guided transbronchial needle aspiration: a randomised controlled trial. Eur J Anaesthesiol. 2021;38(5):534–540. doi:10.1097/EJA.0000000000001370
6. Li WX, Luo RY, Chen C, et al. Effects of propofol, dexmedetomidine, and midazolam on postoperative cognitive dysfunction in elderly patients: a randomized controlled preliminary trial. Chin Med J. 2019;132(4):437–445. doi:10.1097/CM9.0000000000000098
7. Keam SJ. Remimazolam: first Approval. Drugs. 2020;80(6):625–633. doi:10.1007/s40265-020-01299-8
8. Hirano T, Kimoto Y, Kuratani N, et al. Remimazolam for pediatric procedural sedation: results of an institutional pilot program. J Clin Med. 2023;12(18):5937. doi:10.3390/jcm12185937
9. Weng M, Wang D, Zhong J, et al. Comparison between esketamine and alfentanil for hysteroscopy: a prospective, double-blind, randomized controlled trial. Drug Des Devel Ther. 2024;18:3629–3641. doi:10.2147/DDDT.S472651
10. Hong W, Short TG, Hui TW. Hypnotic and anesthetic interactions between ketamine and midazolam in female patients. Anesthesiology. 1993;79(6):1227–1232. doi:10.1097/00000542-199312000-00013
11. Zhang L, Wang Z, Liu Y, et al. Comparison of remimazolam tosilate and propofol sedation on the early postoperative quality of recovery in patients undergoing day surgery: a prospective randomized controlled trial. Drug Des Devel Ther. 2024;18:1743–1754. doi:10.2147/DDDT.S456675
12. Jor O, Maca J, Koutna J, et al. Hypotension after induction of general anesthesia: occurrence, risk factors, and therapy. A prospective multicentre observational study. J Anesth. 2018;32(5):673–680. doi:10.1007/s00540-018-2532-6
13. Correia-Melo FS, Leal GC, Carvalho MS, et al. Comparative study of esketamine and racemic ketamine in treatment-resistant depression: protocol for a non-inferiority clinical trial. Medicine. 2018;97(38):e12414. doi:10.1097/MD.0000000000012414
14. Xu Z, Lang Y, Xu X, et al. The ED50 and ED95 of esketamine for preventing early postoperative pain in patients undergoing laparoscopic cholecystectomy: a prospective, double-blinded trial. BMC Anesthesiol. 2023;23(1):385. doi:10.1186/s12871-023-02357-w
15. Liu J, Yin J, Yin J, et al. Effect of esketamine-based opioid-sparing anesthesia strategy on postoperative pain and recovery quality in patients undergoing total laparoscopic hysterectomy: a randomized controlled trail. Heliyon. 2024;10(3):e24941. doi:10.1016/j.heliyon.2024.e24941
16. Lin YJ, Chen SL, Zheng XL, et al. Dose-response study of propofol combined with two different doses of esketamine for laryngeal mask airway insertion in women undergoing hysteroscopy. Heliyon. 2024;10(9):e30511. doi:10.1016/j.heliyon.2024.e30511
17. Gastaldon C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Psychother Psychosom. 2021;90(1):41–48. doi:10.1159/000510703
18. Xu LL, Wang C, Deng CM, et al. Efficacy and safety of esketamine for supplemental analgesia during elective cesarean delivery: a randomized clinical trial. JAMA Network Open. 2023;6(4):e239321. doi:10.1001/jamanetworkopen.2023.9321
19. Moore TJ, Alami A, Alexander GC, et al. Safety and effectiveness of NMDA receptor antagonists for depression: a multidisciplinary review. Pharmacotherapy. 2022;42(7):567–579. doi:10.1002/phar.2707
20. Orsolini L, Salvi V, Volpe U. Craving and addictive potential of esketamine as side effects. Expert Opin Drug Saf. 2022;21(6):803–812. doi:10.1080/14740338.2022.2071422
21. Chu Q, Mao M, Bai Y, et al. Midazolam attenuates esketamine-induced overactive behaviors in mice before the sedation, but not during the recovery. Front Vet Sci. 2022;9:829747. doi:10.3389/fvets.2022.829747
22. Lebedeva YA, Zakharova AV, Sitdikova GF, et al. Ketamine-Midazolam anesthesia induces total inhibition of cortical activity in the brain of newborn rats. Bull Exp Biol Med. 2016;161(1):15–19. doi:10.1007/s10517-016-3334-1
23. Yi X, Xu W, Li A. The clinical application of remimazolam benzenesulfonate combined with esketamine intravenous anesthesia in endoscopic retrograde cholangiopancreatography. Biomed Res Int. 2022;2022:5628687. doi:10.1155/2022/5628687
24. Zhou XH, Zhang CC, Wang L, et al. Remimazolam induced cognitive dysfunction in mice via glutamate excitotoxicity. Transl Neurosci. 2022;13(1):104–115. doi:10.1515/tnsci-2022-0220
25. Chen H, Ding X, Xiang G, et al. Correction: analysis of the efficacy of subclinical doses of esketamine in combination with propofol in non-intubated general anesthesia procedures - a systematic review and meta-analysis. BMC Anesthesiol. 2023;23(1):355. doi:10.1186/s12871-023-02326-3
26. Trimmel H, Helbok R, Staudinger T, et al. S(+)-ketamine: current trends in emergency and intensive care medicine. Wien Klin Wochenschr. 2018;130(9–10):356–366. doi:10.1007/s00508-017-1299-3
27. Zaporowska-Stachowiak I, Szymański K, Oduah MT, et al. Midazolam: safety of use in palliative care: a systematic critical review. Biomed Pharmacother. 2019;114:108838. doi:10.1016/j.biopha.2019.108838
28. Schüttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651.
29. Yang M, Liu X, Yang D, et al. Effect of remimazolam besylate compared with propofol on the incidence of delirium after cardiac surgery: study protocol for a randomized trial. Trials. 2021;22(1):717. doi:10.1186/s13063-021-05691-x
30. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265–282.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Comparison of Remimazolam Tosilate and Etomidate on Hemodynamics in Cardiac Surgery: A Randomised Controlled Trial
Hu B, Zhang M, Wu Z, Zhang X, Zou X, Tan L, Song T, Li X
Drug Design, Development and Therapy 2023, 17:381-388
Published Date: 8 February 2023
Effect of Esketamine Compared with Sufentanil Combined with Propofol in Patients Undergoing First Trimester Surgical Abortion: A Randomized, Double-Blinded Clinical Trial
Guan Y, Wang H, Cong X, Zhang B, Lin Y, Wang X
Drug Design, Development and Therapy 2025, 19:2873-2883
Published Date: 13 April 2025

