Back to Journals » International Journal of Nanomedicine » Volume 20
Empagliflozin-Pretreated MSC-Derived Exosomes Enhance Angiogenesis and Wound Healing via PTEN/AKT/VEGF Pathway
Authors Wang H , Bai Z, Qiu Y, Kou J, Zhu Y, Tan Q, Chen C, Mo R
Received 30 December 2024
Accepted for publication 8 April 2025
Published 22 April 2025 Volume 2025:20 Pages 5119—5136
DOI https://doi.org/10.2147/IJN.S512074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lijie Grace Zhang
Hao Wang,1,2,* Zihao Bai,3,* Yan Qiu,1,4,* Jiaxi Kou,1,4,* Yanqing Zhu,1,4 Qian Tan,1,4 Chen Chen,5 Ran Mo1,4
1Department of Burns and Plastic Surgery, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Cardiothoracic Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 3Nanjing Children’s Hospital, Clinical Teaching Hospital of Medical School, Nanjing, Jiangsu, People’s Republic of China; 4Department of Burns and Plastic Surgery, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 5Department of Nutrition, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ran Mo, Email [email protected] Chen Chen, Email [email protected]
Background: Diabetic wounds are a common and challenging complication of diabetes, characterized by delayed healing and increased risk of infection. Current treatment methods are limited and often ineffective in promoting wound repair. Mesenchymal stem cell (MSC)-derived exosomes have shown promise in regenerative medicine, but enhancing their therapeutic potential remains a key area of research.
Methods: In this study, MSCs were pretreated with empagliflozin (EMPA), and exosomes were isolated using ultracentrifugation. The morphology, size, and protein markers of EMPA-Exos were characterized. Their effects on human umbilical vein endothelial cells (HUVECs) were assessed using EdU assays, CCK-8 assays, scratch assays, Transwell assays, and Matrigel tube formation assays. The PTEN/AKT/VEGF signaling pathway was analyzed through Western blotting. In vivo, diabetic mouse wound models were used to evaluate the healing efficacy of EMPA-Exos.
Results: EMPA pretreatment enhanced the functional properties of MSC-derived exosomes, significantly improving HUVECs’ proliferation, migration, invasion, and angiogenesis compared to non-pretreated exosomes (P < 0.05). Transcriptomic analysis and pathway activation studies revealed that EMPA-Exos promoted angiogenesis through the PTEN/AKT/VEGF signaling pathway. In vivo experiments demonstrated accelerated wound healing and increased vascularization in diabetic mice treated with EMPA-Exos (P < 0.05).
Conclusion: EMPA-pretreated MSC-derived exosomes effectively enhance angiogenesis and accelerate diabetic wound healing by activating the PTEN/AKT/VEGF signaling pathway. This strategy offers a promising approach for improving diabetic wound repair and provides a potential new therapeutic avenue in regenerative medicine.
Keywords: exosomes, adipose-derived mesenchymal stem cells, Ad-MSCs, empagliflozin, diabetic wound healing, angiogenesis
Introduction
Diabetes wound is a prevalent chronic metabolic disease. In recent years, the incidence of diabetes has been rising annually due to increased aging, higher obesity rates, and the impact of unhealthy lifestyles. The prevalence of diabetes has escalated from an estimated 4% in 1995 to 6.1% in 2021,1 with an annual increase rate of 3.73 per 100,000.2 The International Diabetes Federation (IDF) projects that by 2045, approximately 783 million people worldwide will have diabetes. With the growing number of diabetic patients, diabetes-related complications, particularly diabetic wounds, are increasingly becoming a global public health issue.3
Current conventional treatments for diabetic wounds include debridement, infection control, dietary management, and skin grafting. However, traditional treatment methods often have low cure rates, high recurrence rates, and are less effective in preventing amputations and restoring skin integrity.4,5 Studies indicate that more than 20% of diabetic patients suffer from chronic, non-healing diabetic wounds throughout their lives, and the mortality associated with diabetic wounds is significantly higher compared to those without diabetic wounds.6,7 This not only severely impacts patients’ physical and mental health but also places a considerable burden on families and society.8 Therefore, further research into diabetic wound treatment is necessary to explore new mechanisms and therapies. Successful diabetic wound repair is closely related to local angiogenesis, which effectively promotes nutrient and oxygen supply to the wound area, thereby facilitating key processes such as fibroblast proliferation, collagen synthesis, and re-epithelialization.9–11 Consequently, novel angiogenesis-targeting treatments hold significant promise for diabetic wound care and have broad clinical application potential.
Adipose-derived mesenchymal stem cells (Ad-MSCs) are pluripotent stem cells obtained from adipose tissue, known for their self-renewal and multi-directional differentiation capabilities. They are widely used in tissue engineering and regenerative medicine.12,13 Previous studies have shown that Ad-MSCs can promote the repair of various tissues and organs by secreting growth factors and other substances. Recent research suggests that Ad-MSCs primarily exert their biological effects through the secretion of exosomes via paracrine signaling.14,15 Exosomes, extracellular vesicles with diameters of 30–150 nm, are synthesized within cells and transported to the extracellular environment through the endoplasmic reticulum and Golgi apparatus. They contain various bioactive molecules such as proteins, nucleic acids, and lipids. Ad-MSCs can regulate target cell physiological processes by transferring these exosomes to target cells.16 Exosomes play crucial roles in intercellular communication, immune regulation, and tissue repair. Compared to Ad-MSCs, exosomes offer advantages such as better stability, fewer immune rejection responses, convenient administration, and ease of internalization into recipient cells.17 Research shows that exosomes have a wide range of applications in various fields, including disease diagnosis, treatment, and prognosis.18–20
However, there are still several challenges in the research and clinical translation of Ad-MSCs, such as issues with immune compatibility, stability, heterogeneity, differentiation, and migration capacity, which limit their widespread application.21 To overcome these research bottlenecks, studies have found that regulating Ad-MSCs with small molecules, hypoxia, or structural stimuli can enhance their functionality, survival, and therapeutic effects.22–24 To better replicate the microenvironment in which MSCs exist in the human body, hypoxia is commonly used as an intervention for MSCs. Studies have shown that exosomes secreted by hypoxia-treated Ad-MSCs contain a higher concentration of protective microRNAs (eg, miR-125b, miR-612, miR-126), which play a significant role in the repair of various conditions, including myocardial infarction, bone healing, and angiogenesis. The identification of these microRNAs provides new insights into the clinical application of MSCs in regenerative medicine.25,26
Empagliflozin (EMPA), a sodium-glucose co-transporter 2 (SGLT2) inhibitor, reduces glucose reabsorption and lowers blood glucose levels, making it a crucial therapeutic agent for diabetes management. Beyond its hypoglycemic effects, EMPA has been shown to offer multiple additional benefits, particularly in cardiovascular health. Research indicates that EMPA can mitigate mitochondrial oxidative stress, protect the endothelial barrier function of cardiac microvascular endothelial cells, prevent cellular senescence, promote endothelial cell migration, and enhance angiogenesis.27–29 Given EMPA’s potential in improving diabetes-related complications and mitigating endothelial cell damage, this study further explores its application in diabetic wound healing.
This study aims to investigate the effects of EMPA pre-treatment on the exosomes secreted by Ad-MSCs on the biological functions of human umbilical vein endothelial cells (HUVECs) under hyperglycemic conditions. It examines how these exosomes enhance HUVECs proliferation, migration, invasion, and tube formation, thereby promoting angiogenesis in diabetic wounds and accelerating wound healing. Additionally, the study explores the impact of empagliflozin-treated exosomes (EMPA-Exos) on the PTEN/AKT/VEGF signaling pathway in diabetic wounds. The findings of this research are expected to improve the quality of life for diabetic patients and provide new insights and methods for wound healing research.
Methods
Cell Culture and EMPA Pretreatment
All experiments were approved by the Ethics Committee of Nanjing University Affiliated Drum Tower Hospital. Ad-MSCs were obtained from discarded adipose tissue during surgery. Briefly, type I collagenase solution (Beyotime, China) was added to chopped adipose tissue and digested on a shaker at 37°C for 20 minutes until no visible particles remained. The digestion solution was then centrifuged at 1000 rpm for 5 minutes, and the pellet was resuspended in 5% FBS ECM medium (Sciencell, USA). After 24 hours, the medium was replaced to remove non-adherent cells. HUVECs were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China) and cultured in complete medium containing 5% fetal bovine serum (Gibco, USA). The high glucose (HG) group had a glucose concentration of 35 mmol/L, while the low glucose control group (LG/Con) had a glucose concentration of 5.6 mmol/L.
Exosome Isolation and Characterization
Ad-MSCs were pre-treated with 500 nM EMPA in 10% exosome-free FBS complete medium for 48 hours. Subsequently, cell supernatants were collected and filtered through a 0.22 μm membrane. Exosomes were isolated from the supernatant by ultracentrifugation: 300 g for 10 minutes, 2000 g for 10 minutes, 10000 g for 30 minutes, and 100000 g for 70 minutes. The obtained exosomes were dissolved in PBS and stored at −80° for subsequent use. Exosome morphology was observed using transmission electron microscopy (TEM), exosome markers (CD9 and Alix) were detected by Western blot, and nanoparticle tracking analysis (NTA) was used to measure the size distribution of the exosomes. The bicinchoninic acid (BCA) assay was used to quantify exosomal protein content, thereby reflecting the concentration of exosomes.
Exosome Uptake
Exosomes were labeled with the red fluorescent dye PKH26 (Sigma-Aldrich, USA). PKH26-labeled exosomes were co-cultured with HUVECs for 10 hours. After co-culture, HUVECs were washed twice with PBS and fixed with 4% paraformaldehyde for 15 minutes. The cytoplasm was stained with Actin-Tracker (Beyotime, China) for 30 minutes, and the nucleus was stained with DAPI. After washing with PBS, fluorescence was observed using a laser scanning confocal microscope (Nikon A1, Japan).
Transwell Assay
The Transwell assay was used to evaluate cell invasion. Cell suspensions without serum were placed in the upper chamber, while complete medium was placed in the lower chamber. After incubation at 37°C for 48 hours, cells in the upper chamber were removed with a cotton swab. The remaining cells were fixed with formaldehyde and stained with crystal violet. Cells were then observed under an optical microscope, and the number of cells was quantified using ImageJ.
CCK-8 Assay
Cell viability of HUVECs was assessed using the CCK-8 assay. HUVECs were seeded into 96-well plates and treated with LG, HG, HG + Exos (100 μg/mL), and HG + EMPA-Exos (100 μg/mL). After 24 hours, 100 μL of CCK-8 solution was added to each well. The plate was incubated at 37°C for 1 hour, and absorbance at 450 nm was measured using a spectrophotometer (BioTek, USA).
EDU Assay
The EDU assay was used to assess HUVEC proliferation. HUVECs were seeded into 24-well plates and treated with LG, HG, HG + Exos (100 μg/mL), and HG + EMPA-Exos (100 μg/mL). Cells were co-incubated with EDU reagent for 12 hours, then fixed and stained with formaldehyde. Fluorescence was observed using a laser scanning confocal microscope (Nikon A1, Japan).
Tube Formation Assay
Tube formation was assessed using Matrigel (Corning, USA). Matrigel was added to each well of a pre-chilled 96-well plate and allowed to solidify on ice. HUVECs were seeded into the Matrigel-coated wells and treated with LG, HG, HG + Exos (100 μg/mL), and HG + EMPA-Exos (100 μg/mL). After incubation at 37°C for 6 hours, tube formation was observed under an optical microscope (Olympus, Japan) and the number of capillaries was quantified using ImageJ.
Wound Healing Assay
The wound healing assay was used to assess HUVEC migration. HUVECs were seeded into 96-well plates and treated with LG, HG, HG + Exos (100 μg/mL), and HG + EMPA-Exos (100 μg/mL). When cells reached 90% confluence, wounds were created using a 1 mL pipette tip. After washing with PBS to remove suspended cells, images were taken at 0 hours and 24 hours. Wound healing was quantified using ImageJ.
Protein Extraction
Cells were seeded into 6-well plates and treated with LG, HG, HG + Exos (100 μg/mL), and HG + EMPA-Exos (100 μg/mL). Cells were washed twice with PBS, and 1 mL of RIPA buffer was added to each well. Protein extracts were centrifuged at 12000 rpm at 4°C for 15 minutes. The supernatant (80 μL) was mixed with 20 μL of 5× SDS loading buffer and heated at 100°C for 10 minutes.
Western Blot Analysis
Western blotting was performed to detect exosome expression and mechanisms. Protein extracts were separated by gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% BSA solution, incubated overnight at 4°C with primary antibodies, and then washed. Secondary antibodies were incubated at room temperature for 1 hour. Bands were visualized using chemiluminescent detection and quantified with ImageJ software. As a loading control, GAPDH and β-actin were used to ensure equal protein loading across all samples. Bands were visualized using chemiluminescent detection and quantified using ImageJ software. The intensity of the bands was measured and normalized to GAPDH and β-actin levels to ensure consistency and reproducibility across different experimental runs. Catalog Numbers for Antibodies Used: PTEN (Rabbit origin, CAT#ET1606-43, HUABIO); VEGF (Rabbit origin, CAT#ER30607, HUABIO); AKT (Rabbit origin, CAT#9272, Cell Signaling Technology); P-AKT (Rabbit origin, CAT#4060T, Cell Signaling Technology); GAPDH (Mouse origin, CAT#AC002, ABclonal); β-Actin (Rabbit origin, CAT#AC026, ABclonal); CD9 (Rabbit origin, CAT#ab236630, Abcam); Alix (Rabbit origin, CAT#ab275377, Abcam); PageRuler™ Marker (CAT#26616, Thermo Fisher Scientific); p-PI3K (Rabbit origin, CAT#HA721672, HUABIO); PI3K (Rabbit origin, CAT#4292, Cell Signaling Technology); HIF-1α (Rabbit origin, CAT#10006421, Cayman); Calnexin (Rabbit origin, CAT#ER1803-42, HUABIO).
Animal Model
Wound healing studies were conducted using 6–8 week old C57BL/6 and db/db mice. Animal experiments were permitted by the Medical School for Animal Use and Care Committee of Nanjing Drum Tower (Ethics Approval Number: 2024-749-01) and were performed according to the guidelines of NIH (USA). Hair was shaved from the dorsal area of the mice using a razor, followed by depilatory cream and cleaning with water. The next day, mice were anesthetized with isoflurane, and a 1 cm diameter wound was created using a puncher, followed by disinfection with iodine. db/db mice were injected with 100 µL PBS, 100 µL exosomes (100 µg exosomes in 100 µL PBS), or 100 µL EMPA-Exos (100 µg EMPA-Exos in 100 µL PBS). Each group consisted of 5 mice. Mice were housed in SPF conditions, and images were taken at days 0, 3, 7, 10, and 14 using a smartphone. Wound healing was analyzed using ImageJ. On day 14, mice were euthanized, and tissues were collected, fixed in formaldehyde, embedded in paraffin, and sectioned for further analysis.
Histology, Immunohistochemistry, and Immunofluorescence Analysis
Paraffin blocks were sectioned into 5 μm thick slices. Hematoxylin and eosin (H&E) staining was used to observe wound healing length, while Masson’s trichrome staining was used to assess collagen synthesis and deposition.
Paraffin sections were deparaffinized in water, subjected to antigen retrieval to restore antigen activity, and incubated overnight at 4°C with VEGF antibody. Sections were then incubated with secondary antibodies and ABC complex to enhance signals. Brown precipitates were visualized using DAB substrate, and sections were observed under a microscope.
For immunofluorescence, paraffin sections were deparaffinized in water and blocked with 1.5% goat serum in the darkroom to prevent non-specific binding. Sections were incubated with CD31 and α-smooth muscle actin (α-SMA) antibodies, followed by treatment with Alexa Fluor 488 and Cy3-conjugated secondary antibodies for visualization. Fluorescence images were observed using a fluorescence microscope in the darkroom to determine the location and expression levels of target proteins.
Transcriptomic Analysis
HUVEC were allocated into three groups—Control, HG, and HG+EMPA-Exos—with three biological replicates per group. After culturing under defined conditions, total RNA was extracted using Trizol, followed by DNase I treatment and magnetic bead purification. Only samples with an RNA concentration >200 ng/μL and an RNA Integrity Number (RIN) ≥8.0 were processed further. mRNA was enriched using Oligo (dT) magnetic beads and subsequently fragmented prior to cDNA library construction. Library quality was assessed with an Agilent 2100 bioanalyzer before sequencing on an Illumina platform at BGI. Raw sequencing data were subjected to quality control with FastQC to remove low-quality reads, and gene expression was quantified in transcripts per million (TPM) using RSEM. Differentially expressed genes (DEGs) were identified using edgeR (p < 0.05) and further analyzed via GO and KEGG enrichment analyses.
Statistical Analysis
Statistical analyses were conducted using data from at least three independent experiments. For each dataset, means and standard deviations were reported. Data were analyzed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). Differences between two groups were assessed using Student’s t-test. For comparisons involving more than two groups, one-way ANOVA was performed followed by Tukey’s multiple comparisons test. A p-value < 0.05 was considered statistically significant.
Results
Characterization of Ad-MSCs-Derived Exosomes
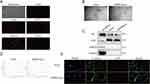
To investigate the role of EMPA-Exos in promoting diabetic wound healing (Figure 1), we first isolated Ad-MSCs. Ad-MSCs exhibited fibroblast-like morphology in culture. Immunofluorescence analysis confirmed the presence of surface markers CD29, CD44, CD75, and CD105, while CD34 was absent (Figure 2A). To determine the optimal concentration of EMPA for treating mesenchymal stem cells, we assessed MSC viability using the CCK-8 assay after 24 and 48 hours of treatment. The results indicated that MSCs showed the best viability at 500 nM EMPA after 48 hours compared to other concentrations and controls (Supplementary Figure 1). Consequently, we used 500 nM EMPA for 48 hours as the standard condition for subsequent in vitro experiments, consistent with previous studies.30
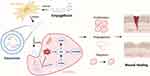 |
Figure 1 Schematic diagram of EMPA-Exos accelerating diabetic wound repair through enhancing angiogenesis by activation of the PTEN/AKT/VEGF pathway. Created with BioRender.com. |
To investigate the effect of EMPA-Exosomes on diabetic wound healing, exosomes were isolated from both untreated and EMPA-treated Ad-MSCs via ultracentrifugation. TEM revealed that both exosomes and EMPA-Exosomes had a characteristic cup-shaped, double-layered membrane structure (Figure 2B). Western blotting confirmed the presence of exosome positive markers CD9 and Alix, as well as the negative markers Calnexin and GAPDH in both Ad-MSC-derived exosomes and EMPA-Exosomes (Figure 2C). NTA showed that the size distribution of exosomes and EMPA-Exosomes was predominantly between 50 and 150 nm (Figure 2D). These findings indicate that Ad-MSC-derived exosomes and EMPA-Exosomes are similar in morphology, surface markers, and particle size, with EMPA pretreatment not altering these characteristics. To assess if HUVECs could internalize exosomes, PKH26-labeled exosomes and EMPA-Exosomes were co-cultured with HUVECs for 10 hours. Both exosomes and EMPA-Exosomes were found to be internalized by HUVECs (Figure 2E).
EMPA-Exosomes Enhance HUVECs Proliferation, Migration, Invasion, and Angiogenesis Induced by High Glucose
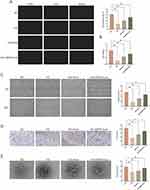
To explore whether EMPA-Exosomes promote diabetic wound healing by enhancing HUVEC functions, we first evaluated their effect on HUVEC proliferation using the EdU assay. Compared to the HG group, the number of EdU-positive cells was higher in both exosome and EMPA-Exosome groups, with EMPA-Exosomes showing the highest number of positive cells (Figure 3A). Additionally, CCK-8 assays demonstrated that HG conditions inhibited HUVEC survival, while both exosomes and EMPA-Exosomes provided protective effects, with EMPA-Exosomes showing the most significant protection (Figure 3B). Scratch assays assessed the effect of EMPA-Exosomes on HUVEC migration, revealing that both exosomes and EMPA-Exosomes improved migration under high glucose conditions, with EMPA-Exosomes exhibiting a stronger effect (Figure 3C and D). Transwell assays further showed that EMPA-Exosomes enhanced HUVECs invasion under high glucose conditions. Tube formation assays on Matrigel evaluated the ability of HUVECs to form capillary networks. Results indicated that while exosomes improved tube formation under high glucose conditions, EMPA-Exosomes showed the most pronounced improvement (Figure 3E). These results collectively demonstrate that EMPA-Exosomes effectively restore proliferation, migration, and angiogenic capacity of HUVECs impaired by high glucose.
EMPA-Exosomes Enhance HUVECs Angiogenesis Through the PTEN/AKT/VEGF Pathway
To elucidate the mechanism by which EMPA-Exosomes promote angiogenesis in HUVECs, transcriptomic analysis was performed on HUVECs, high glucose-treated HUVECs, and EMPA-Exosome-treated HUVECs. The results are shown in Figure 4A. Intersection analysis of differentially expressed genes between the control and high glucose groups, and between the high glucose and EMPA-Exosome-treated groups, followed by GO and KEGG analysis, revealed that differential genes primarily activated lipid metabolism, angiogenesis, and inflammatory responses in HUVECs (Figure 4B). KEGG analysis suggested that these genes might act through pathways such as TNF, NF-κB, and AKT (Figure 4C). To verify if EMPA-Exosomes promote angiogenesis through the AKT signaling pathway, we analyzed the expression of PTEN/AKT/VEGF pathway-related proteins using Western blotting. The results showed that HG reduced the expression of phosphorylated AKT (p-AKT), PI3K (p-PI3K), HIF-1α and VEGF, whereas both exosomes and EMPA-Exosomes increased their expression, with EMPA-Exosomes demonstrating the most substantial effect. Additionally, high glucose increased PTEN expression, which was significantly reduced by both exosomes and EMPA-Exosomes, with EMPA-Exosomes showing the strongest inhibitory effect (Figure 4D and E). These results indicate that EMPA-Exosomes enhance HUVECs angiogenesis by activating the PTEN/AKT/VEGF pathway.
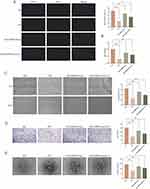
LY294002 Inhibits HUVECs Angiogenesis by Suppressing the PTEN/AKT/VEGF Pathway
To further investigate the role of the PTEN/AKT/VEGF pathway in EMPA-Exosome-induced HUVEC angiogenesis, we used the PI3K inhibitor LY294002. The EdU and CCK-8 assays showed that LY294002 inhibited the protective effect of EMPA-Exosomes on HUVEC proliferation under high glucose conditions (Figure 5A and B). Scratch assays and Transwell migration experiments indicated that LY294002 partially suppressed the migration and invasion enhancement induced by EMPA-Exosomes (Figure 5C and D). Additionally, the angiogenesis assays demonstrated that LY294002 inhibited the improvement in vascular formation promoted by EMPA-Exosomes (Figure 5E). Western blotting results showed that LY294002 significantly reduced the expression of phosphorylated AKT, PI3K, HIF-1α and VEGF proteins, while increasing PTEN expression (Figure 6A and B). Overall, these results suggest that LY294002 inhibits EMPA-Exosome-induced HUVEC angiogenesis, highlighting the critical role of the PTEN/AKT/VEGF pathway in the process.
EMPA-Exosomes Promote in vivo Wound Healing in Diabetic Mice
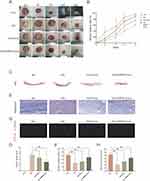
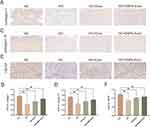
To further evaluate the therapeutic effect of EMPA-Exosomes on diabetic wound healing, full-thickness skin defects were created on the backs of C57BL/6 and db/db diabetic mice. Subcutaneous injections revealed that the exosome-treated group accelerated wound closure compared to the model group, with the most significant effects observed on days 7, 10, and 14 post-surgery (Figure 7A and B). Histological analysis with HE staining showed improved epithelial regeneration and wound healing in the EMPA-Exosome-treated group compared to both the exosome-only and model groups (Figure 7C and D). Masson’s trichrome staining indicated more extensive collagen deposition in the EMPA-Exosome group, suggesting superior matrix remodeling (Figure 7E and F). Immunofluorescence staining for CD31 (endothelial cells) and α-SMA (smooth muscle cells) showed more mature blood vessels in the EMPA-Exosome-treated diabetic wounds (Figure 7G and H). Additionally, immunohistochemistry results indicated that EMPA-Exosomes significantly enhanced collagen I and III synthesis (Figure 8A–D) and increased VEGF expression (Figure 8E and F). Collectively, these results demonstrate that EMPA-Exosomes accelerate diabetic wound healing by enhancing angiogenesis.
Discussion
Diabetic wound are a common complication in diabetic patients, characterized by slow healing, susceptibility to infection, and frequent recurrence. These issues not only cause significant patient suffering but also increase the healthcare burden.31,32 Current treatments, including wound debridement, antibiotic therapy, and topical applications, often fail to effectively promote wound healing and are associated with side effects and limitations.33 Thus, exploring new strategies to enhance diabetic wound healing is of considerable practical and clinical significance.
One major limiting factor in diabetic wound healing is impaired angiogenesis. Increasing evidence indicates that endothelial cell dysfunction caused by hyperglycemia is a primary cause of vascular problems, leading to diabetic complications.11,34 High glucose environments activate inflammatory signaling pathways, leading to endothelial cell apoptosis and dysfunction, which in turn contributes to diabetic vascular complications.35–38 In this study, we found that high glucose conditions inhibited HUVEC vitality and proliferation, leading to cellular dysfunction and limiting their migration, angiogenesis, and wound healing capabilities.
Recent advancements in regenerative medicine have provided new hope for treating various refractory clinical diseases. Ad-MSCs have become a focus of regenerative medicine research due to their unique abilities for self-renewal, differentiation, and immune modulation.39–41 MSCs, sourced from adipose tissue, bone marrow, umbilical cord, and other tissues, can differentiate into various cell types including osteoblasts, chondrocytes, and adipocytes. Besides direct tissue repair, MSCs can also promote tissue regeneration through the secretion of various bioactive molecules.42–45 This paracrine effect offers a promising new approach for treating chronic wounds such as diabetic ulcers.
Exosomes, nanometer-sized membrane vesicles secreted by cells, contain a variety of bioactive molecules including proteins, RNAs, and lipids. Studies have shown that MSC-derived exosomes can transfer information between cells and regulate the physiological functions of recipient cells. Utilizing MSC-derived exosomes in diabetic wounds offers significant biological functionality while avoiding the immune responses and rejection issues associated with direct MSC application.46,47 However, research on exosomes has encountered some challenges. Recent studies have found that small molecule compounds, hypoxia, structural stimuli, or engineering modifications can enhance their functionality. For example, Muyu et al demonstrated that atorvastatin-pretreated MSC-derived exosomes (ATV-Exos) significantly promoted diabetic wound healing and angiogenesis through AKT/eNOS pathway activation and upregulation of miR-221-3p.22 Hypoxia-preconditioned MSC-derived extracellular vesicles, by transferring miR-612, inhibit TP53 and activate HIF-1α-VEGF signaling pathways, significantly promoting angiogenesis and showing potential for treating ischemic diseases.23 Liu et al used engineering methods to incorporate superparamagnetic nanoparticles into exosomes to enhance their cardiac targeting efficiency, thus improving the therapeutic effects of exosomes.24
EMPA is a SGLT2 inhibitor that primarily lowers blood glucose levels by inhibiting renal glucose reabsorption.48–50 It is widely used for treating type 2 diabetes and has been found to have cardiovascular and renal protective effects beyond glucose lowering, possibly through mechanisms such as improving endothelial function, reducing inflammation, and oxidative stress.51–53 Recent studies have explored combining drug pre-treatment with stem cell therapy to enhance the therapeutic effects of exosomes.54–56 Pre-treating MSCs with EMPA may enhance their exosome secretion and biological activity by modulating intracellular signaling pathways, potentially providing a new strategy for diabetic wound treatment.
In this study, we pretreated MSCs with EMPA. After exposing MSCs to an appropriate concentration of EMPA for 2 days, we isolated and extracted exosomes using ultracentrifugation. To ensure the purity and quality of the exosomes, we characterized their morphology and size using transmission electron microscopy and nanoparticle tracking analysis, confirming no significant changes before and after EMPA treatment. Tracing experiments showed that EMPA-pretreated exosomes, like untreated exosomes, could be internalized by cells and exert their effects. In vitro experiments demonstrated that EMPA-Exosomes significantly enhanced HUVEC proliferation, invasion, migration, and angiogenesis. Transcriptomic analysis revealed significant enrichment of several signaling pathways in the EMPA-treated group, particularly those related to angiogenesis. Activation of the PTEN/AKT/VEGF pathway is crucial for stimulating angiogenesis, including HUVEC vitality and tube formation. PTEN indirectly inhibits VEGF-mediated angiogenesis by suppressing AKT phosphorylation, while AKT phosphorylation directly promotes VEGF-mediated angiogenesis. Regulation of this pathway is significant for angiogenesis and treatment of related diseases.57–59
To further validate the key role of the PTEN/AKT/VEGF signaling pathway in the enhanced functionality of EMPA-pretreated exosomes, we used the AKT pathway inhibitor LY294002. Adding LY294002 to HUVECs revealed its impact on EMPA-pretreated exosome-induced angiogenesis. LY294002 significantly inhibited the proliferation, migration, and angiogenesis induced by EMPA-pretreated exosomes, confirming the critical role of the PTEN/AKT/VEGF pathway in the enhanced functionality of these exosomes. VEGF promotes angiogenesis by specifically binding to cell surface receptors with tyrosine kinase activity.59–61 In vivo experiments using a diabetic mouse wound model demonstrated that local injection of EMPA-pretreated exosomes significantly accelerated wound healing compared to the control group. Histological analyses, including HE staining, Masson’s trichrome staining, CD31 and α-SMA immunofluorescence staining, and collagen I and III, as well as VEGF immunohistochemistry, supported these findings. The results showed increased mature blood vessels, richer granulation tissue, and more uniform and orderly collagen deposition in the treatment group. Immunohistochemistry analysis indicated a significant upregulation of VEGF expression in the treatment group, further supporting that EMPA-pretreated exosomes promote angiogenesis through the PTEN/AKT/VEGF signaling pathway.
When comparing EMPA-preconditioned exosomes to other exosome preconditioning methods (such as hypoxia, small molecules, or metal particle stimulation), EMPA demonstrates significant advantages. Firstly, EMPA, as an SGLT2 inhibitor widely used in clinical practice, exhibits low cellular stress and high safety, avoiding the cellular damage and long-term stability issues often associated with hypoxia and other methods. Secondly, EMPA not only promotes angiogenesis but also provides a more comprehensive biological effect by improving endothelial function, reducing inflammation, and mitigating oxidative stress. In contrast to other methods, EMPA preconditioning is more clinically translatable, with simpler, more standardized protocols, making it better suited to support the widespread application of exosome-based therapies. Therefore, EMPA preconditioning offers a unique advantage in enhancing exosome-mediated angiogenesis and wound healing potential.
Exosomes, as a promising drug delivery system, offer advantages such as low immunogenicity and efficient crossing of biological barriers, making them widely applicable in clinical treatments. The key to large-scale production of exosomes lies in optimizing the production process. While traditional ultracentrifugation is commonly used for exosome isolation, it has limitations in terms of efficiency and yield. Therefore, the use of chromatography and filtration technologies has emerged as an alternative, significantly improving production efficiency while minimizing damage to exosome functionality.62 In addition, optimizing cell sources and culture conditions during production is crucial. By adjusting cell culture media, increasing cell density, and utilizing optimized transfection techniques, the yield and quality of exosomes can be further enhanced. To ensure the stability of exosomes, it is recommended to store them at −80°C for long-term storage, effectively preserving their structure and biological activity. For short-term storage, 4°C is feasible, but long-term storage may lead to changes in exosome size and reduced bioactivity. To minimize damage during freezing and thawing, it is advised to reduce the number of freeze-thaw cycles and use stabilizers (such as trehalose or DMSO) for protection.63,64 Furthermore, storing exosomes in natural biological fluids (such as serum) has been shown to maintain better stability compared to purified buffer solutions. However, despite exosomes’ naturally low immunogenicity, engineered exosomes may still induce immune responses during clinical applications, and further research is needed to ensure their immunological safety.65 Therefore, optimizing exosome production, storage, and immunogenicity remains a critical focus for future research.
While the study yielded positive results, several limitations should be noted. First, the in vivo wound healing experiments were conducted over 14 days, which were sufficient for evaluating healing rates and histological changes. However, a longer follow-up (eg, 28 days) would provide better insight into long-term processes like collagen remodeling and scar formation. Additionally, evaluating pro-inflammatory and anti-inflammatory markers in the wound environment could offer a more comprehensive understanding of healing. The relatively small sample size (n=5 per group) is another limitation, and future studies could benefit from power calculations to optimize sample size for detecting subtle biological effects. Furthermore, while we focused on the PTEN/AKT/VEGF pathway, other signaling pathways (eg, IL-17, TNF, NF-κB) identified in transcriptomic analysis were not fully explored. Future studies should incorporate genetic knockdown approaches to assess these pathways’ roles in EMPA-Exos’ effects. Although we confirmed that HUVECs internalize EMPA-Exos via PKH26 labeling, the exact mechanism of internalization (eg, receptor-mediated endocytosis or direct fusion) was not investigated. This should be explored in future research to better understand their therapeutic potential.
Conclusion
This study demonstrates that empagliflozin-pretreated MSC-derived exosomes significantly enhance angiogenesis and accelerate diabetic wound healing by activating the PTEN/AKT/VEGF signaling pathway. This approach shows promise as a novel and effective strategy for improving wound repair in diabetic patients, offering potential benefits over current treatments and advancing regenerative medicine. Given the clinical approval of empagliflozin, this strategy holds significant translational potential, with clinical trials potentially starting within 2–3 years following further comprehensive studies. Future research should focus on optimizing exosome dosage and exploring additional downstream targets and mechanistic.
Ethics Approval and Consent to Participate
All experiments were approved by the Ethics Committee of Nanjing University Affiliated Drum Tower Hospital (ID: 2024-749-01). Ad-MSCs were obtained from discarded adipose tissue during surgery, with informed consent provided by the patients. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi:10.2337/diacare.21.9.1414
2. Balooch Hasankhani M, Mirzaei H, Karamoozian A. Global trend analysis of diabetes mellitus incidence, mortality, and mortality-to-incidence ratio from 1990 to 2019. Sci Rep. 2023;13(1):21908. doi:10.1038/s41598-023-49249-0
3. Chen AC, Lu Y, Hsieh CY, et al. Advanced biomaterials and topical medications for treating diabetic foot ulcers: a systematic review and network meta-analysis. Adv Wound Care. 2024;13(2):97–113. doi:10.1089/wound.2023.0024
4. Oprita EI, Iosageanu A, Craciunescu O. Natural polymeric hydrogels encapsulating small molecules for diabetic wound healing. Gels. 2023;9(11). doi:10.3390/gels9110867
5. Chen P, Vilorio NC, Dhatariya K, et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. 2024;40(3):e3644. doi:10.1002/dmrr.3644
6. Xie P, Deng B, Zhang X, et al. Time in range in relation to amputation and all-cause mortality in hospitalised patients with diabetic foot ulcers. Diabetes Metab Res Rev. 2022;38(2):e3498. doi:10.1002/dmrr.3498
7. Armstrong DG, Tan TW, Boulton AJM, et al. Diabetic foot ulcers: a review. JAMA. 2023;330(1):62–75. doi:10.1001/jama.2023.10578
8. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
9. Krishnan ST, Quattrini C, Jeziorska M, et al. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care. 2007;30(12):3058–3062. doi:10.2337/dc07-1421
10. Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Acc Chem Res. 2021;54(5):1080–1093. doi:10.1021/acs.accounts.0c00864
11. Veith AP, Henderson K, Spencer A, et al. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. doi:10.1016/j.addr.2018.09.010
12. Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19. doi:10.1186/1756-8722-5-19
13. Lin R, Zhang T, Gao J. Apoptotic vesicles of MSCs: the natural therapeutic agents and bio-vehicles for targeting drug delivery. Small. 2023;19(47):e2301671. doi:10.1002/smll.202301671
14. Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther. 2016;16(4):489–506. doi:10.1517/14712598.2016.1131976
15. Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10(13):5979–5997. doi:10.7150/thno.40122
16. Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. doi:10.1186/s40364-019-0159-x
17. Lamichhane TN, Sokic S, Schardt JS, et al. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21(1):45–54. doi:10.1089/ten.TEB.2014.0300
18. Yu D, Li Y, Wang M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56. doi:10.1186/s12943-022-01509-9
19. Chang WT, Lee WC, Lin YW, et al. Transpulmonary expression of exosomal microRNAs in idiopathic and congenital heart disease-related pulmonary arterial hypertension. J Am Heart Assoc. 2023;12(23):e031435. doi:10.1161/JAHA.123.031435
20. Siregar FM, Haryana SM, Anggrahini DW, Dinarti LK, Hartopo AB. The potential of circular RNAs as biomarkers in pulmonary arterial hypertension related to congenital heart disease. Congenit Heart Dis. 2024;19(4):375–388.
21. Zhou T, Yuan Z, Weng J, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14(1):24. doi:10.1186/s13045-021-01037-x
22. Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350. doi:10.1186/s13287-020-01824-2
23. Liu W, Li L, Rong Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi:10.1016/j.actbio.2019.12.020
24. Liu S, Chen X, Bao L, et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng. 2020;4(11):1063–1075. doi:10.1038/s41551-020-00637-1
25. Ge L, Xun C, Li W, et al. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnology. 2021;19(1):380. doi:10.1186/s12951-021-01126-6
26. Xiao C, Wang K, Xu Y, et al. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ Res. 2018;123(5):564–578. doi:10.1161/CIRCRESAHA.118.312758
27. Zhou H, Wang S, Zhu P, et al. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi:10.1016/j.redox.2017.12.019
28. Han JX, Luo LL, Wang YC, et al. SGLT2 inhibitor empagliflozin promotes revascularization in diabetic mouse hindlimb ischemia by inhibiting ferroptosis. Acta Pharmacol Sin. 2023;44(6):1161–1174. doi:10.1038/s41401-022-01031-0
29. Marshall VWHM, Wright LK. Sodium-glucose cotransporter 2 inhibitors in adult and pediatric congenital heart disease: review of emerging data and future directions. Congenit Heart Dis. 2024;19(4):419–433.
30. Chi B, Zou A, Mao L, et al. Empagliflozin-pretreated mesenchymal stem cell-derived small extracellular vesicles attenuated heart injury. Oxid Med Cell Longev. 2023;2023:7747727. doi:10.1155/2023/7747727
31. Dixon D, Edmonds M. Managing diabetic foot ulcers: pharmacotherapy for wound healing. Drugs. 2021;81(1):29–56. doi:10.1007/s40265-020-01415-8
32. Cho H, Blatchley MR, Duh EJ, et al. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267–288. doi:10.1016/j.addr.2018.07.019
33. Shi X, Li H, Guo F, et al. Novel ray of hope for diabetic wound healing: hydrogen sulfide and its releasing agents. J Adv Res. 2024;58:105–115. doi:10.1016/j.jare.2023.05.009
34. Wang X, Li R, Zhao H. Enhancing angiogenesis: innovative drug delivery systems to facilitate diabetic wound healing. Biomed Pharmacother. 2024;170:116035. doi:10.1016/j.biopha.2023.116035
35. Zhou C, She X, Gu C, et al. FTO fuels diabetes-induced vascular endothelial dysfunction associated with inflammation by erasing m6A methylation of TNIP1. J Clin Invest. 2023;133(19). doi:10.1172/JCI160517
36. Huang G, Cheng Z, Hildebrand A, et al. Diabetes impairs cardioprotective function of endothelial progenitor cell-derived extracellular vesicles via H3K9Ac inhibition. Theranostics. 2022;12(9):4415–4430. doi:10.7150/thno.70821
37. Otto M, Bucher C, Liu W, et al. 12(S)-HETE mediates diabetes-induced endothelial dysfunction by activating intracellular endothelial cell TRPV1. J Clin Invest. 2020;130(9):4999–5010. doi:10.1172/JCI136621
38. Lu X, Qin L, Guo M, et al. A novel alginate from Sargassum seaweed promotes diabetic wound healing by regulating oxidative stress and angiogenesis. Carbohydr Polym. 2022;289:119437. doi:10.1016/j.carbpol.2022.119437
39. Kadri N, Amu S, Iacobaeus E, et al. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol Immunol. 2023;20(6):613–625. doi:10.1038/s41423-023-01022-z
40. Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14(1):195. doi:10.1186/s13045-021-01208-w
41. Wechsler ME, Rao VV, Borelli AN, et al. Engineering the MSC secretome: a hydrogel focused approach. Adv Healthc Mater. 2021;10(7):e2001948. doi:10.1002/adhm.202001948
42. Boulestreau J, Maumus M, Jorgensen C, et al. Extracellular vesicles from mesenchymal stromal cells: therapeutic perspectives for targeting senescence in osteoarthritis. Adv Drug Deliv Rev. 2021;175:113836. doi:10.1016/j.addr.2021.113836
43. Palanisamy CP, Pei J, Alugoju P, et al. New strategies of neurodegenerative disease treatment with extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs). Theranostics. 2023;13(12):4138–4165. doi:10.7150/thno.83066
44. Psaraki A, Ntari L, Karakostas C, et al. Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology. 2022;75(6):1590–1603. doi:10.1002/hep.32129
45. Bertolino GM, Maumus M, Jorgensen C, et al. Therapeutic potential in rheumatic diseases of extracellular vesicles derived from mesenchymal stromal cells. Nat Rev Rheumatol. 2023;19(11):682–694. doi:10.1038/s41584-023-01010-7
46. Li Y, Zhu Z, Li S, et al. Exosomes: compositions, biogenesis, and mechanisms in diabetic wound healing. J Nanobiotechnology. 2024;22(1):398. doi:10.1186/s12951-024-02684-1
47. Zhao H, Li Z, Wang Y, et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front Cell Dev Biol. 2023;11:1029671. doi:10.3389/fcell.2023.1029671
48. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi:10.1056/NEJMoa1515920
49. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018;41(12):2560–2569. doi:10.2337/dc18-1749
50. Kim ES, Deeks ED. Empagliflozin/Linagliptin: a review in type 2 diabetes. Drugs. 2015;75(13):1547–1557. doi:10.1007/s40265-015-0457-z
51. Shanmuganathan M, Goswami RM. Empagliflozin in heart failure. N Engl J Med. 2021;384(4):386. doi:10.1056/NEJMc2033669
52. Paneni F. Empagliflozin across the stages of diabetic heart disease. Eur Heart J. 2018;39(5):371–373. doi:10.1093/eurheartj/ehx519
53. Tuttle KR, Hauske SJ, Canziani ME, et al. Efficacy and safety of aldosterone synthase inhibition with and without empagliflozin for chronic kidney disease: a randomised, controlled, Phase 2 trial. Lancet. 2024;403(10424):379–390. doi:10.1016/S0140-6736(23)02408-X
54. Ning Y, Huang P, Chen G, et al. Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway. BMC Med. 2023;21(1):96. doi:10.1186/s12916-023-02778-x
55. Zhao Q, Yang J, Liu B, et al. Exosomes derived from mangiferin-stimulated perivascular adipose tissue ameliorate endothelial dysfunction. Mol Med Rep. 2019;19(6):4797–4805. doi:10.3892/mmr.2019.10127
56. Yoon YM, Lee JH, Song KH, et al. Melatonin-stimulated exosomes enhance the regenerative potential of chronic kidney disease-derived mesenchymal stem/stromal cells via cellular prion proteins. J Pineal Res. 2020;68(3):e12632. doi:10.1111/jpi.12632
57. Zhou C, Kuang Y, Li Q, et al. Endothelial S1pr2 regulates post-ischemic angiogenesis via AKT/eNOS signaling pathway. Theranostics. 2022;12(11):5172–5188. doi:10.7150/thno.71585
58. Davies EM, Gurung R, Le KQ, et al. PI(4,5)P(2)-dependent regulation of endothelial tip cell specification contributes to angiogenesis. Sci Adv. 2023;9(13):eadd6911. doi:10.1126/sciadv.add6911
59. Song S, Zhang G, Chen X, et al. HIF-1alpha increases the osteogenic capacity of ADSCs by coupling angiogenesis and osteogenesis via the HIF-1alpha/VEGF/AKT/mTOR signaling pathway. J Nanobiotechnology. 2023;21(1):257. doi:10.1186/s12951-023-02020-z
60. Nagy JA, Dvorak AM. Dvorak HF.VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi:10.1146/annurev.pathol.2.010506.134925
61. Ge Y, Wang Q, Yao Y, et al. Framework nucleic acids-based VEGF signaling activating system for angiogenesis: a dual stimulation strategy. Adv Sci. 2024;11(21):e2308701. doi:10.1002/advs.202308701
62. Qu Q, Fu B, Long Y, et al. Current strategies for promoting the large-scale production of exosomes. Curr Neuropharmacol. 2023;21(9):1964–1979. doi:10.2174/1570159X21666230216095938
63. Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017;20(1):1. doi:10.1208/s12248-017-0160-y
64. Ahmadian S, Jafari N, Tamadon A, et al. Different storage and freezing protocols for extracellular vesicles: a systematic review. Stem Cell Res Ther. 2024;15(1):453. doi:10.1186/s13287-024-04005-7
65. Cully M. Exosome-based candidates move into the clinic. Nat Rev Drug Discov. 2021;20(1):6–7. doi:10.1038/d41573-020-00220-y
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Mesenchymal Stem Cell-Derived Exosomes Hold Promise in the Treatment of Diabetic Foot Ulcers

Wang H, Wu S, Bai X, Pan D, Ning Y, Wang C, Guo L, Guo J, Gu Y
International Journal of Nanomedicine 2025, 20:5837-5857
Published Date: 6 May 2025