Back to Journals » Journal of Pain Research » Volume 18
Epigenetics and Herbs: Potential Therapeutic Strategies for Osteoarthritis of the Knee
Authors Zheng Y, Wang J, Wang L, Zheng Y, Qi W
Received 18 February 2025
Accepted for publication 21 May 2025
Published 26 June 2025 Volume 2025:18 Pages 3217—3261
DOI https://doi.org/10.2147/JPR.S517224
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Yihui Zheng, Jiahui Wang, Lei Wang, Yang Zheng,* Wen Qi*
Department of Medicine, Faculty of Chinese Medicine Science Guangxi University of Chinese Medicine, Nanning, Guangxi, 530222, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Zheng; Wen Qi, Department of Medicine, Faculty of Chinese Medicine Science Guangxi University of Chinese Medicine, Nanning, Guangxi, 530222, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Knee osteoarthritis (KOA) is a complex joint disease characterized by progressive cartilage degeneration with programmed cell death of chondrocytes. Programmed cell death (PCD) of chondrocytes plays a central role in the development of knee osteoarthritis, and epigenetics provides new explanations for this complex biological process. By regulating DNA methylation, histone modification and non-coding RNA, epigenetic mechanisms can significantly affect chondrocyte survival and apoptosis without altering the gene sequence. Recent studies have found that TCM can intervene in the epigenetic regulatory network by targeting epigenetic enzymes with active ingredients, non-coding RNA-mediated co-regulation, and epigenetic-metabolic reprogramming. In this paper, we review the latest studies on epigenetics in chondrocyte programmed cell death, focusing on the mechanisms of DNA methylation, histone modification, and non-coding RNAs, such as miRNAs and lncRNAs, and also discuss the interventional roles of TCM in this process, providing therapeutic references to delve into the pathogenesis of KOA.
Keywords: epigenetics, knee osteoarthritis, chondrocytes, programmed death, traditional Chinese medicine
Graphical Abstract:

Knee osteoarthritis (KOA), the most prevalent chronic degenerative joint disorder in elderly populations, is pathologically characterized by progressive cartilage degradation, subchondral bone remodeling, and synovial inflammation. As the sole resident cells in articular cartilage, chondrocytes are pivotal in maintaining extracellular matrix homeostasis through precisely regulated cellular turnover. The disruption of homeostatic equilibrium between chondrocyte survival and programmed cell death (PCD) constitutes a fundamental mechanism driving cartilage degeneration and subsequent joint dysfunction.1 Programmed cell death encompasses distinct genetically regulated pathways of cellular demise, including but not limited to apoptosis, autophagy, pyroptosis, and ferroptosis. These evolutionarily conserved processes are intrinsically programmed to mediate developmental morphogenesis and stress adaptation, yet their dysregulation has been implicated in the pathogenesis of osteoarthritis (OA) through focal chondrocyte depletion.2,3 Emerging evidence highlights the critical role of epigenetic mechanisms-including DNA methylation patterns, histone post-translational modifications, and noncoding RNA networks (eg, miRNAs, lncRNAs) in orchestrating chondrocyte PCD dynamics during KOA progression. Unlike classical genetics that focuses on DNA sequence variations, epigenetic regulation enables context-dependent modulation of gene expression without altering genetic codes, providing a mechanistic link between environmental factors and disease phenotypes.4 Notably, recent investigations have identified aberrant epigenetic signatures associated with imbalanced PCD pathways in KOA-affected chondrocytes. This paradigm shift has spurred interest in therapeutic strategies targeting epigenetic modifiers. Intriguingly, traditional Chinese medicine (TCM) formulations and their bioactive constituents have demonstrated multimodal regulatory effects on chondrocyte PCD pathways in both experimental OA models and clinical observations.5 This review systematically examines emerging mechanisms of epigenetic control in chondrocyte PCD and evaluates evidence supporting TCM-mediated epigenetic modulation, with the aim of informing novel therapeutic approaches for KOA management.
Types of Programmed Death of Chondrocytes in Osteoarthritis of the Knee
Chondrocyte Apoptosis and KOA
Apoptosis is a strictly regulated metabolic pathway crucial for maintaining homeostasis in various adult tissues.6,7 Chondrocyte apoptosis is closely related to articular cartilage destruction and matrix degradation.8 The release of inflammatory mediators and oxidative stress are significant factors mediating chondrocyte apoptosis.9,10 OA, the most prevalent chronic arthritis among the elderly, is characterized by gradual articular cartilage degeneration, synovial inflammation, and pain. Various factors directly or indirectly regulate the anabolic and catabolic pathways of the cartilage matrix to promote cartilage degradation in OA, which is driven by multiple factors that directly or indirectly affect these pathways.11 Chondrocyte apoptosis and the inflammatory response are key factors in OA pathogenesis. OA chondrocytes release inflammatory mediators such as Interleukin (IL)-1β, tumor necrosis factor (TNF) and nitric oxide (NO).12 OA cartilage generates a significant amount of NO, which inhibits cartilage matrix synthesis and boosts MMP activity via a mitochondria-dependent mechanism, and augments the expression of pro-inflammatory cytokines involved in cartilage Extracellular matrix (ECM) breakdown.13 Research has demonstrated that chondrocyte apoptosis mediated by NF-κB signaling is associated with OA.14 Nuclear factor kappa-B (NF-κB) bidirectionally regulates chondrocyte homeostasis. Its crucial subunit RelA can induce anti-apoptotic genes like pik3r1, safeguarding chondrocytes from apoptosis.15 NF-κB can prompt the expression of anti-apoptotic genes to suppress TNF-α-induced cell death, while simultaneously it can also activate TNF-α-induced cell death. Moreover, HIF-2α, a target gene of NF-κB, accelerates Fas-mediated apoptosis of OA chondrocytes, leading to the worsening of OA.16
Chondrocyte Pyroptosis and KOA
Cellular pyroptosis is a phenomenon where cells exhibit morphologically distended deformation and membrane rupture, with the release of cellular contents and activation of inflammatory factors to trigger a robust inflammatory response, along with chromosomal DNA breaks.17,18 The main pathological mechanisms of KOA involve cartilage damage and chondrocyte death.19 In KOA, continuous mechanical stimulation on fragile chondrocytes can generate damage-associated molecular patterns (DAMPs) via mitochondrial damage and other routes. This activates NOD-like receptor heat protein structural domain protein 3 (NLRP3) inflammatory vesicles and secretes matrix metalloproteinase 3 (MMP3) and matrix metalloproteinase 13 (MMP13) that degrade the cartilage matrix. The destruction of chondrocytes then leads to cellular pyrolysis, creating a vicious cycle of tissue damage and disease aggravation. Moreover, oxidized mitochondrial DNA from mitochondrial death and the upregulation of NLRP3 by fusion proteins induces inflammatory death of chondrocytes.20 Interleukin-1β (IL-1β) is a metabolic regulator of degenerative joint pathology.21 It can induce the expression of cartilage matrix degrading enzyme-like genes and stimulate the secretion of various inflammatory mediators by fibroblasts, chondrocytes, and other cells, thereby exacerbating cartilage damage and the inflammatory response of the knee joint.22 The study by Zhao et al’s team23 found that NLRP1 and NLRP3 inflammatory vesicles are highly involved in the pyroptosis of fibroblasts, inducing fibroblast synoviocytes to secrete various pro-inflammatory cytokines like IL-1β, IL-18, and TNF-α, promoting localized synovial inflammation and degradation of the cartilage matrix, and ultimately resulting in the development of KOA.24
Chondrocyte Autophagy and KOA
Autophagy serves as an intracellular degradation system that sustains the energy metabolism steady state in cells. Moderate autophagy can restore damaged chondrocyte function, inhibit chondrocyte ECM degradation and apoptosis. Activating autophagy can effectively alleviate KOA development in animal models.25,26 The key factors of chondrocyte autophagy are Unc-51-like autophagy-activated kinase 1 (ULK1), autophagy-associated target gene (Atg), autophagy effector protein (Beclin-1), and antibody to autophagy microtubule-associated protein light chain 3 (LC3). The upregulation of expressions levels of autophagy positive regulatory proteins like Atg3, Atg5, Atg7, and Beclin-1, and the increase of the LC3 I to LC3 II ratio all indicate the enhancement of the autophagic process.27,28 Phosphatidylinositol 3-kinase (PI3K) forms a complex with Beclin-1 to promote autophagosome formation and induce autophagy.29 Mammalian target of rapamycin (mTOR) is a key regulator of autophagy; its upstream PI3K/protein kinase B (AKT)/mechanistic target of rapamycin complex 1 (mTORC1), which inhibits autophagy, increases apoptosis, and aggravates OA.30 mTORC1 can phosphorylate and inhibit the interaction of ULK1 with AMP-dependent protein kinase (AMPK) and the binding of phosphorylated Atg13 (p-Atg13), both of which suppress ULK1 activity.31,32 This shows that in KOA patients, the autophagy level of chondrocytes is significantly reduced, with the expression of ULK1, Beclin-1, and LC3 being inhibited.33 In other words, promoting the expression of ULK1 and Beclin-1 can stimulate the formation of chondrocyte autophagic vesicles and raise the autophagy level of chondrocytes, which can effectively ameliorate cartilage damage and degeneration and boost the repair of KOA cartilage.34
Chondrocyte Ferroptosis and KOA
Ferroptosis is a novel PCD mode, iron-dependent and distinct from apoptosis, necrosis, and autophagy. When intracellular iron is excessive, hydroxyl radicals are generated under Fenton reaction, promoting lipid peroxidation and inducing ferroptosis.35,36 Excess iron promotes chondrocyte apoptosis and up-regulates matrix-degrading enzyme expression. The Sun et al. Team37 created excess iron conditions with ferric ammonium citrate and found that excess iron accelerated chondrocyte apoptosis, leading to the expression of MMP3 and other matrix-degrading enzymes. Yao et al’s team38 studied the impact of chondrocyte ferroptosis on KOA. They created an inflammatory environment and conditions of iron overload for chondrocytes, where increased levels of lipid reactive oxygen species (ROS) and up-regulated expression of iron-death-related proteins were noted. This led to higher expression of collagen type II (COI II) and lower expression of MMP13. Ferrostatin-1 (Fer-1) could rescue Glutathione Peroxidase 4 (GPX4) and Col II expression and alleviate cartilage erosion. Applying Fer-1 to treated induced cells was found to reduce IL-1β and Fac-induced cytotoxicity, accumulation of ROS and lipid ROS, and expression of ferroptosis-associated proteins, thus slowing KOA’s progression.39
Chondrocyte Necrotic Apoptosis and KOA
Necrotic apoptosis is a highly pro-inflammatory cell death mode mainly initiated by TNF-α. Programmed necrosis transmits cell death signals via the actions of Receptor Interacting Protein Kinase 1 (RIPK1) and Protein Kinase 3 (RIPK3) on Mixed Lineage Kinase Domain-Like (MLKL).40 It promotes the release of intracellular DAMP, like members of the IL-1 family. RIPK1 is involved in activating inflammatory vesicles and cytokines, while ROS drives the release of inflammatory factors, all of which contribute to inflammation development.41,42 Necrostatin-1s (NST-1s), a small-molecule inhibitor of necrotic apoptosis, could reduce the expression of necrotic apoptosis-related factors like RIPK1, RIPK3, and MLKL, thereby slowing OA progression.43,44 The study by the Liang et al. Team45 discovered that Necrostatin-1 (Nec-1) is a potent inhibitor of RIPK1 activity, reducing the expression of PCD-related factors and inhibiting inflammatory factors such as IL-17, IL-1β, IL-6, and TNF-α, and in turn, slowing the damage of articular cartilage and necroinflammation.
Chondrocyte Copper Death and KOA
Copper death is triggered by copper binding to the lipoylated components of the tricarboxylic acid (TCA) cycle, resulting in the aggregation of lipoylated proteins and the subsequent reduction of Fe-S cluster proteins, inducing proteotoxic stress and ultimately cell death.46 Excessive copper interferes with iron-sulfur cofactors and promotes harmful ROS formation by accelerating the Fenton reaction. Ten copper death genes associated with OA are Solute Carrier Family 31 Member 1 (SLC31A1), Pyruvate Dehydrogenase Beta (PDHB), Recombinant Pyruvate dehydrogenase alpha 1 (PDHA1), lipoyltransferase 1 (LIPT1), lipoic acid synthetase (LIAS), dihydrolipoamide dehydrogenase (DLD), Ferredoxin 1 (FDX1), Dihydrolipoamide succinyltransferase (DLST), and Dihydrolipoyl Transacetylase (DLAT).47 The Wang et al. Team48,49 discovered that the copper death gene PDHB could be a risk factor for OA. The two E10 isoforms of the pyruvate dehydrogenase complex, PDHB and PDHA1, are predominantly located in cellular mitochondria and catalyze the conversion of glucose-derived pyruvate to acetyl coenzyme A (CoA); higher CoA accumulation rates result in significant cartilage degeneration and chondrocyte apoptosis. Additionally, a study by the Schamel et al. Team50 indicated that imbalances in copper levels potentially impact bone formation, and imbalances in copper homeostasis and copper proteins affect chondrocyte production, which then influences cartilage development. Meanwhile, metal ions can enhance cellular function and the regenerative capacity of cartilage tissue.
The Role of Epigenetics in Programmed Chondrocyte Death
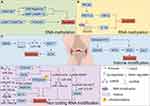
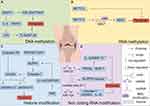
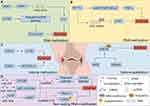
Epigenetics pertains to heritable alterations in gene expression without DNA sequence changes. It’s crucial for the growth and differentiation of all cell types in organisms. The normal epigenetic state of cells alters due to environmental factors or ageing, thus playing a distinctive role in the pathogenesis of some complex multifactorial diseases.51 It belongs to the significant gene-environment interaction process. Epigenetic modifications differ from genomic ones in having broader applications and being reversible. Epigenetic regulation varies by cell type and gene, and phenomena include DNA methylation, RNA methylation, post-translational modifications of histones, RNAs (regulated by non-coding regulatory RNAs), and epigenetic chromatin remodeling (chromatin’s three-dimensional structure).52 See Figure 1. The following molecular mechanisms involved in epigenetic regulation of programmed chondrocyte death are shown in Figures 2–6 and Tables 1–6.
 |
Table 1 DNA Methylation, RNA Methylation, Histone Modifications, and Non-Coding RNA Modifications Regulate Chondrocyte Apoptosis |
 |
Table 2 DNA Methylation, RNA Methylation, Histone Modifications, and Non-Coding RNA Modifications Regulate Chondrocyte Pyroptosis |
 |
Table 3 DNA Methylation, RNA Methylation, Histone Modifications, and Non-Coding RNA Modifications Regulate Chondrocyte Autophagy |
 |
Table 4 DNA Methylation, RNA Methylation, Histone Modifications, and Non-Coding RNA Modifications Regulate Chondrocyte Ferroptosis |
 |
Table 5 RNA Methylation and Non-Coding RNA Modifications Regulate Chondrocyte Necroptosis |
 |
Table 6 RNA Methylation and Non-Coding RNA Modification Regulate Chondrocyte Cuproptosis |
DNA Methylation Regulates Programmed Chondrocyte Death
DNA methylation is a biological process where the cytosine 5 carbon position of Central pattern generators(CpG) in the genome covalently binds a methyl group in the presence of DNA methyltransferases,109 as illustrated in detail in Figure 1A.
Studies have shown that demethylation therapy can inhibit chondrocyte apoptosis by restoring the expression of genes associated with chondrocyte apoptosis such as tumor suppressor genes and apoptotic factors. Epigenomic linkage analysis (EWAS) uncovered KOA-related methylation sites, mainly involving genes related to skeletal development and morphogenesis, by comparing the methylation pattern differences between OA chondrocytes and normal chondrocytes or those differentiated from bone marrow mesenchymal stem cells (BMSCs). These genes fall into four main categories: The first pertains to extracellular matrix homeostasis, encompassing type II collagen fiber α1 gene (COL2A1), type IX collagen α1 (COL9A1), type X collagen α1 chain (COL10A1), aggregated proteoglycans (ACAN), MMP2, MMP3, MMP9, and MMP1, etc.110,111 The second involves inflammation-related molecules like IL-1b, IL-8, IL-32, Transforming growth factor beta-2(TGF-β2), interleukin 1 receptor antagonist (IL1RN), and MMTV integration site family member 11 (WNT11).112,113 The third concerns cartilage maintenance, such as sex-determiningregionY-relatedhighmobilitygroupbox4 (SOX4), sex determining region Y-box 9 (Sox9), Runt-related transcription factor 2 (RUNX2), and Recombinant Superoxide Dismutase 2, Mitochondrial (SOD2).114,115 The fourth relates to growth factors, including bone morphogenetic protein-7 (BMP-7), sclerostin (SOST), and growth differentiation factor 5 (GDF5).116,117 In OA cartilage, numerous metalloproteinase - activating factors display reduced methylation at a single CpG site. This can lead to alterations in the expression of disease - associated genes, such as MMP-9(−36), MMP-13(−110), and ADAMTS-4(−753).118 In articular cartilage chondrocytes from OA patients, the expression of cytokine inhibitory signaling molecules, suppressor of cytokine signaling 2 (SOCS2) and cis-1,2-cyclohexanediamine (CIS-1) is suppressed. Notably, the methylation level of the SOCS2 promoter region does not differ from that in normal subjects.119 Additionally, high methylation of the BMP-7 promoter region correlates with its decreased expression. Adding BMP-7 to chondrocytes can inhibit the expression of inflammatory factors120 and exert an anti-apoptotic effect. DNA methylation regulates leptin expression in OA chondrocytes. RNA interference can downregulate leptin expression, subsequently reducing MMP-13 expression.75 Dou’s team demonstrated53 that overexpression of DNA methyltransferase 3B (DNMT3B) significantly upregulated the expression of Ki67 and Aggrecan, while downregulating the ECM markers MMP3 and MMP13 in chondrocytes and cartilage tissues extracted from DMM induced mice, respectively. Conversely, the addition of RUNX2 reversed the above mentioned regulation by DNMT3B, indicating that RUNX2 positively regulates ECM degradation. In line with this, IL-1β treatment suppressed the cell proliferation marker Ki67 in OA chondrocytes, suggesting inhibited cell proliferation. It was also observed to be involved in the degradation of chondrogenic ECM and the progression of OA. The PI3K/AKT signaling pathway is frequently activated in cancer, and its related genes have been extensively studied, as they are commonly activated in human malignancies. Thioredoxin interacting protein (TXNIP), a tumor suppressor in certain cancers, inhibits cell proliferation and induces apoptosis. Zhang’s team54 discovered that in high - glucose stimulated RSC96 cells, the up - regulation of DNMT1 and DNMT3A was associated with the elevated expression of TXNIP. This, in turn, suppressed the overactivation of the PI3K/AKT pathway, ultimately triggering autophagy and apoptosis. Phosphatase and tensin homolog deleted on chromosome ten (PTEN), a well - known tumor suppressor gene (TSG), down - regulates the PI3K/AKT pathway, thereby inhibiting cancer progression. Activation of the AKT pathway can lead to overexpression of factors that promote cell autophagy and apoptosis. Additionally, DNMT3A influences cell development through the PTEN/PI3K/AKT pathway, as illustrated in detail in Figure 2A.
DNA methyltransferase 1 (DNMT1) mediates chondrocyte pyroptosis by regulating the miR-20a/TXNIP molecular axis through DNA methylation. DNMT1-mediated DNA methylation of the IL-6 and tumor necrosis factor receptor-associated factor 6 (TRAF6) promoters inhibits the expression of IL-6 and TRAF6, subsequently suppressing the LPS induced pyroptotic inflammatory responses. Studies have shown that LPS induces apoptosis and inflammatory responses in cells via activation of the Toll-like receptor 4 (TLR4)/NF-κB pathway. Notably, the down-regulation of apoptosis and inflammatory responses induced by DNMT1 can be reversed by inhibiting PDTC (an NF-κB inhibitor) or TLR4. This indicates that TLR4 and the NF-κB signaling pathway interact to promote LPS induced pyroptotic cell death and inflammation.68 The Sun team69 discovered that after using the DNMT inhibitor 5-aza-2-deoxycytidine (AZA) or knocking down the DNA methyltransferase gene, intracellular overexpression of C-terminal-binding protein (CtBP) occurs. This activates downstream pro inflammatory processes, and the expression of cytokine NLRP3 promotes inflammatory responses in osteoarthritis (OA). Meanwhile, the Zhu et al. Team121 found that high levels of DNMT1 and DNMT3a in mouse and human OA cartilage induce methylation of the peroxisome proliferator-activated receptor γ (PPARγ) promoter, thereby inhibiting PPARγ expression. In DMM mice, inhibiting DNMT1 and DNMT3a with AZA can reverse the methylation of the PPARγ promoter, enhance PPARγ expression, and reduce cartilage destruction, as illustrated in detail in Figure 3A.
DNA methylation can directly modify cellular autophagy-related genes such as Atg1, Atg6, Atg8, and LC3. By regulating the transcription of these genes, it affects the level of cellular autophagy. Additionally, it indirectly impacts autophagy intensity by modifying genes of numerous autophagy-related signaling molecules, including neuron-derived orphanin receptor 1 (NOR1), death-associated protein kinase (DAPK), and SOX1.79 The DNA methylation status of key signaling pathway genes and ATGs (autophagy-related genes) primarily influences chondrocyte autophagy. mTOR, a core regulator of cellular autophagy, modulates autophagy levels in response to various external stimuli. In a favorable metabolic environment, mTOR activates the PI3K/Akt/mTOR signaling pathway and inhibits the autophagy - initiating molecule Atg1/ULK1, thereby regulating autophagy.80 In contrast, the opposite occurs in an unfavorable metabolic environment. AMPK participates in autophagy regulation by acting on mTOR. Metabolic stress - induced cellular autophagy relies on the AMPK signaling pathway, as illustrated in detail in Figure 4A. In this pathway, AMPK, activated by p-LKB1, can activate tuberous sclerosis (TSC), which inhibits mTOR. Alternatively, AMPK directly phosphorylates and inhibits mTOR, inducing autophagy.80 Activation of the type I PI3K pathway inhibits cellular autophagy. It enables the production of p-PtdIns(3,4,5), which binds to Akt/PKB and its PDK1, activating PKB and p-TSC1. This, in turn, affects downstream Rheb and activates mTOR kinases, thus suppressing autophagy onset.73 ATG6 is a component of PI3K complexes I and II and serves as a crucial gene in the autophagy regulatory process.122,123 The mammalian homolog of ATG6, Beclin 1, collaborates with ATG14L to regulate autophagy initiation. Additionally, it binds to multiple proteins, including Vps34 (the catalytic subunit of type III PI3K), mTOR, and BCL-2 proteins, forming complexes that participate in regulating autophagosome maturation and transport.124 Upon receiving a signal, mTOR activity is inhibited. This alleviates its suppression of ULK1 and ATG13, enabling ULK1 activation. Activated ULK1 then phosphorylates ATG13, FIP200, and itself. Concurrently, the activated ULK1 complex translocates from the cytoplasm to the endoplasmic reticulum (ER) as autophagosomal membranes start to form.125 The AMPK signaling pathway also plays a key role. It phosphorylates and activates ULK1 at specific sites, Ser317 and Ser777, thereby inducing autophagy.126
Studies have demonstrated that prolyl hydroxylase 2 (EGLN2) is a potential driver of iron chelation-mediated cell death inhibition, with its hydroxylase activity being iron-dependent.127 Inhibiting stearoyl coenzyme A desaturase-1/fatty acid desaturase-2 directly down-regulates GPX4 expression, reduces the glomerular-stimulating hormone (GSH)/oxidized glutathione ratio, disrupts the redox balance, and triggers iron-mediated lipid peroxidation and mitochondrial dysfunction, ultimately inducing cellular ferroptosis.128 Solute carrier family 7 member 11 (SLC7A11), also known as xCT) has been shown to play a role in maintaining intracellular GSH levels, redox homeostasis, and conferring resistance to ferroptosis.129 The stability of the cystine/glutamate reverse transporter protein xCT is regulated by the mucin-1C-terminal subunit/CD44 variant complex. This complex directly interacts with xCT, enhancing its stability and regulating GSH levels.130 Mucin-1 represses its own gene transcription by increasing histone levels on its promoter and promoting DNA methylation.130 Moreover, elevated DNMT3B levels accelerate DNA methylation of the phosphatase gene - induced kinase 1 (Pink1) promoter, inhibiting Pink1 expression and facilitating ferroptosis - associated brain damage.131 It was shown that glycine-enhanced GPX4 promoter methylation catalyzed by DNMT1, DNMT3A, and DNMT3B induces iron death in rheumatoid arthritis. In addition, DNA dioxygenase 10–11 translocation 2 (TET2), an important demethylating enzyme, inhibits iron death through GPX4 promoter demethylation in airway epithelial cells. A recent study found that inhibition of FSP1 expression through promoter hypermethylation resulted in increased sensitivity of the GSH-GPX4 axis to iron death in an acute lymphoblastic leukemia cell line.91 The DNA methylation inhibitor, 5-azacitidine (5-Aza), inhibited iron death by increasing expression of CDH1.5-Aza decreased methylation levels of procalcitonin β 14 (PCDHB14), a level that induced a decrease in iron death. 5-Aza decreases the methylation level of procalcitonin β 14 (PCDHB14), which induces iron death in hepatocellular carcinoma cells. Tumor Protein 53 (p53)-mediated up-regulation of PCDHB14 promotes ubiquitination and accelerates degradation of p65 mediated by the E3 ubiquitin ligase, RNF182, which inhibits p65-mediated expression of SLC7A11 transcripts.92 Lymphocyte-specific hemolysin (LSH) is a 5-hmC reader. It interacts with WDR structural domain protein 76 (WDR76) to inhibit iron death by promoting the expression of glucose transporter protein 1 (GLUT1), stearoyl coenzyme A desaturase 1 (SCD1), and fatty acid desaturase 2 (FADS2), as illustrated in detail in Figure 5A.
RNA Methylation Regulates Programmed Chondrocyte Death
Methylation modifications have regulatory effects on RNA in eukaryotic cells. N6-methyladenosine (m6A) is the most common methylation modification. m6A is the sixth nitrogen atom of adenine undergoes RNA methylation modification, which is involved in physiological and pathological processes in organisms,132 as illustrated in detail in Figure 1B.
Transforming growth factor(TGF)/signal transducer protein(SMAD) signaling to intra-articular chondrocytes is one of the important pathological mechanisms of OA.87 Lasman et al’s team134 and Bertero et al’s team135 concluded that the TGF-β/signal transducer protein 2/3 (SMAD2/3) signaling pathway interacts with the m6A methyltransferase complexes, eg, methyltransferase-like 3 (METTL3) and METTL14, to regulate the intracellular SMAD2/3 m6A levels, leading to the regulation of mRNA processing, modification, and degradation. m6A affects the level of inflammatory expression in chondrocytes, thus exerting a regulatory effect on OA. Studies have shown that the m6A methyltransferase METTL3 mediates the inflammatory response in chondrocytes by regulating the levels of inflammatory factors such as IL-8, IL-6, IL-12, and TNF-α. Inhibition of METTL3 leads to an increase in the expression of collagen type II (COI II) as well as a decrease in the extracellular matrix degrading enzyme, MMP-13.55 HE et al’s team82 found that METTL3 inhibited chondrocyte apoptosis and autophagy induced by TNF-α stimulation in vitro. Conversely, the METTL3 inhibitor S-adenosylhomocysteine (SAH) promotes apoptosis and autophagy in in vitro-cultured inflammatory chondrocytes. In vivo, in a temporomandibular joint (TMJ) OA mouse model induced by monosodium iodoacetate (MIA), SAH exacerbates the degradation of chondrocytes and subchondral bone. Shi et al56 reported that in OA cartilage, the expression of RPL38 is upregulated while that of SOCS2 is downregulated. RPL38 directly interacts with METTL3 to inhibit SOCS2 expression. Both silencing RPL38 and overexpressing SOCS2 can mitigate IL-1β induced chondrocyte apoptosis, inflammatory cytokine secretion, and ECM degradation. These findings indicate that RPL38 regulates chondrocyte inflammation and apoptosis in OA by modulating METTL3-mediated m6A modification of SOCS2, as illustrated in detail in Figure 2B.
Xiong et al’s team93 demonstrated that low METTL3 expression suppresses chondrocyte death. The underlying mechanism involves METTL3-mediated regulation of NIMA-associated kinase 7 (NEK7) m6A modification. Specifically, METTL3 promotes NEK7 expression, which in turn drives chondrocyte death, thus facilitating OA progression. Furthermore, NEK7 binds to NLRP3, triggering the activation of the NLRP3 inflammasome. This activation process entails the recruitment of apoptosis-associated apoptosis-associated speck-like protein containing a CARD (ASC) and the formation of inflammasome complexes via caspase-1 cleavage.71,72 As a result, it impacts pyroptosis, an inflammatory form of programmed cell death, as as illustrated in detail in Figure 3B.
The relationship between m6A modification and cellular autophagy is bidirectional. For instance, Fat mass and obesity-associated protein (FTO) removes m6A methylation from the ULK1 gene transcript (a key regulator of autophagy), thereby promoting cellular autophagy.83 Sang et al’s team84 investigated an IL-1β induced mouse model of chondrocyte degeneration and found that the mRNA level of METTL3 increased significantly, while the mRNA levels of ALKBH5, FTO, and METTL14 remained unchanged. This indicates that METTL3 plays a prominent role in OA. He et al’s team91 demonstrated that METTL3 exacerbates subchondral bone degeneration in OA. It does so by inhibiting TNF-α induced chondrocyte apoptosis and autophagy through the m6A/YTH domain-containing family protein 1 (YTHDF1)/Bcl2 axis, as illustrated in detail in Figure 4B. Chen et al’s team136 observed impaired autophagy in OA fibroblast - like synoviocytes (FLS). This impairment was attributed to METTL3-mediated m6A modification, which reduced the expression of ATG7 by decreasing its RNA stability.137 These findings suggest that METTL3-mediated m6A modification downregulates autophagy-related proteins by diminishing their RNA stability. Conversely, inhibiting METTL3 enhances autophagic flux in FLS, suppresses the expression of senescence associated secretory phenotypes (SASP), and ultimately accelerates cellular senescence and OA progression.
The m6A methylation modification plays a crucial role in regulating cellular ferroptosis and can either inhibit or promote tumor proliferation and migration in vivo. For example, miR-4443 can suppress ferroptosis by reducing the m6A modification level of FSP1 through METTL3.100 In cellular tumors, the expression of the methylase METTL3 is significantly upregulated. This upregulation promotes the m6A modification of SLC7A11 mRNA, enhancing its stability and translation efficiency. As a result, it inhibits cellular ferroptosis and promotes tumor cell proliferation and migration.94 In cancer cells, the human YTH structural domain protein 2 (YTHDC2) acts as an endogenous promoter of ferroptosis. It reduces the stability of HOXA13 mRNA by recognizing its m6A site, decreasing the expression of Soluble Transporter Protein Family 3 Member 2 (SLC3A2) (involved in the YTHDC2 regulated ferroptosis pathway). This further impairs the antioxidant capacity of cancer cells. In addition, YTHDC2 induced iron death by modifying m6A to inhibit the expression of SLC7A11. AKT inhibitors increased GPX4 m6A levels and promoted YTHDF2-mediated GPX4 mRNA decay by decreasing FTO. It was found that METTL3 promotes ferritin (FTH) m6A methylation and enhances its mRNA stability in a YTHDF1-dependent manner, in which YTHDF1 inhibits iron death by up-regulating FTH, as illustrated in detail in Figure 5B. In other diseases, m6A-modified circSAV1 increases the protein level of iron - responsive element - binding protein 2 (IREB2), disrupting iron homeostasis. This disruption leads to the accumulation of Lymphocytic Interstitial Pneumonia (LIPs) and lipid peroxidation, triggering ferroptosis and facilitating disease progression.95 In summary, m6A modification mitigates the pathogenesis of OA by inhibiting PCD. Proteases associated with the m6A RNA methylation process regulate PCD related proteins, ultimately suppressing PCD.
It has been shown that interfering with METTL3 and METTL14 attenuates the pathological process of vascular smooth muscle cell (VSMC) necrosis, the inflammatory response104 Enhanced METTL3-mediated modification of m6A inhibits tumor necrosis factor receptor-associated factor 5 (TRAF5)-mediated necrotic apoptosis, as well as TRAF5 hypoxia significantly increases the number of oxygen-induced necrotic cells, as illustrated in detail in Figure 6B.
Copper can promote lactate modification at the K229 site of METTL16, which increases the level of m6A modification of FDX1, promotes FDX1 expression, and ultimately induces copper dystrophy in GCs,107 as illustrated in detail in Figure 6A.
Histone Modification Regulates Programmed Chondrocyte Death
In KOA, increased histone deacetylation correlates with chondrocyte apoptosis, which can be slowed by inhibiting histone deacetylating agents.
Histone acetylation: acetylation is mediated by histone acetyltransferases, which act on specific lysine residues at the N-terminal end of histones, loosening the DNA structure of histones and prompting the opening of the transcription machinery,138 as illustrated in detail in Figure 1C.
Histone methylation: methylation of histone lysine or arginine residues is catalyzed by histone methyltransferases and protein arginine methyltransferases, and the action of demethylases can reverse one or more methyl groups regulating the transcription process,139 as illustrated in detail in Figure 1D.
Histone methyltransferases, like Enhancer of Zeste Homolog 2 (EZH2), and histone demethylases, such as Histone Lysine Specific Demethylase 1 (LSD1), can modulate apoptosis-related genes. For instance, genes within the BCL-2 family encode proteins that are pivotal in apoptosis regulation, while the p53 gene functions as a tumor suppressor, promoting apoptosis during cellular stress. Moreover, histone methylation can indirectly impact chondrocyte apoptosis by altering the expression of inflammatory factors and extracellular matrix -degrading enzymes. Inflammatory cytokines, including IL-1β and TNF-α, activate the NF-κB pathway, induce histone methylation modifications, and thereby promote chondrocyte apoptosis.139,140 Notably, in IL-1β treated chondrocytes, a reduction in apoptosis and levels of chondrolysis-related metabolic factors indicates that depletion of EZH2 may alleviate OA. Additionally, EZH2 promotes H3K27 methylation of the miR-138 promoter, suppressing miR-138 expression. This suppression upregulates the expression of its target gene, multiligand proteoglycan 1 (SDC1), increases the expression of cartilage matrix - degrading enzyme - like genes, and ultimately accelerates cartilage degeneration.57 In IL-1β treated human OA chondrocytes, overexpression of EZH2 exacerbates the cellular response to IL-1β. This leads to increased expression of inflammation and pain related genes, including NO, PEG2, IL6, and NGF, as well as catabolism - related genes such as MMPs. Conversely, treatment with the EZH2 inhibitor EPZ-6438 mitigates the effects of IL-1β and reduces IL-1β induced cartilage degeneration.57 During OA pathogenesis, decreased histone deacetylase 4 (HDAC4) levels in articular cartilage weaken its inhibitory effect on ATF4. This results in continuous activation of the ATF4 endoplasmic reticulum stress (ERS) signaling pathway, triggering massive chondrocyte apoptosis and promoting OA related articular cartilage degeneration. In a rat OA model, HDAC4 inhibits ATF4-mediated ERS to reduce chondrocyte apoptosis. Additionally, it promotes the synthesis of COI II, thereby delaying the degeneration of OA articular cartilage.58 Furthermore, the N-terminus of HDAC4 induces chondrocyte apoptosis. The underlying mechanism may involve upregulating pro-apoptotic factors Caspase-3 and Caspase-12. As a key component of the ERS apoptotic pathway, Caspase-12 can translocate from the endoplasmic reticulum to the cytosol during ERS, where it activates Caspase-3 and initiates the apoptosis process,59 as illustrated in detail in Figure 2C.
In cartilage tissue, abnormal histone methylation may be linked to cartilage pyroptosis. In both cellular pyroptosis and OA, a multitude of inflammatory cytokines, including IL-1β, IL-18, TNF-α, and TGF-β, are released. Key players in the cellular pyroptosis associated signaling pathway, such as NLRP3, caspase-1, IL-18, and IL-1β, contribute significantly to these processes. Research has demonstrated73 that histone methylation can influence cartilage pyroptosis by regulating the expression of inflammation and cell death related genes. For instance, specific histone methyltransferases like SETDB1 and histone demethylases such as LSD1 can modulate the expression of genes involved in pyroptosis, including those encoding the NLRP3 inflammasome and caspase-1. The NLRP3 inflammasome, a pivotal component of the pyroptosis signaling pathway, gets activated upon recognizing DAMPs and pathogen associated molecular patterns (PAMPs), thereby initiating the inflammatory response and the pyroptotic process.74 Inflammatory vesicles activate caspase-1 and cleave GSDMD (usually the N-terminal structural domain of GSDMD) to induce cellular death. In addition, caspase-8 and caspase-3 can directly cleave GSDMD to induce cell death. He et al’s team74 conducted in vitro experiments and found that nicotine inhibits chondrocyte differentiation and triggers pyroptosis. Using siRNA to interfere with NLRP3 expression revealed that pyroptosis mediates nicotine induced suppression of Wharton’s jelly derived mesenchymal stem cells (WJ-MSCs) differentiation into cartilage. Additionally, the study showed that the α7-nicotinic acetylcholine receptor (α7-nAChR) antagonist α-BTX reversed nicotine induced upregulation of NLRP3 and P300. Moreover, siRNA targeting p300 reversed nicotine induced increases in NLRP3 mRNA expression and histone acetylation levels in the NLRP3 promoter region. These findings indicate that nicotine exposure can disrupt chondrogenesis. The underlying mechanism is that nicotine exposure increases histone acetylation in the NLRP3 promoter region via the α7-nAChR/P300 pathway, leading to elevated NLRP3 expression. Subsequently, NLRP3 mediates the inhibition of chondrogenic differentiation by activating cellular pyroptosis, as illustrated in detail in Figure 3C.
Wei et al’s team85 discovered that lysine demethylase 1A (KDM1A) negatively regulates the autophagy process via the mTOR signaling pathway. Paradoxically, it promotes starvation and rapamycin induced autophagy. Inhibiting KDM1A activity or knocking down its gene expression leads to increased LC3-II protein levels, enhanced autophagosome formation, and stimulated autophagic flux. KDM4A serves dual roles: it acts as a negative transcriptional regulator of ATG genes and suppresses autophagy induction under nutrient rich conditions. Wang et al’s team62 demonstrated that KDM4A negatively regulates ATG gene expression, including ATG7, ATG8, ATG9, and ATG14, by preventing the recruitment of transcriptional activators to their promoter regions. Specifically, KDM4A modulates autophagy through regulating ATG7 gene transcription. Sun et al’s team86 showed that the acetylation of H4K16 is regulated by histone acetyltransferase Human Males absent on the first (hMOF) and histone deacetylase silent mating type information regulation 2 homolog (Sirt1). A reduction in H4K16 acetylation initiates cellular autophagy. During autophagy, as H4K16 acetylation decreases, histone deacetylation restores the positive charge of histones, enabling them to bind tightly to negatively charged DNA. This impedes transcriptional regulatory elements from accessing the promoter region, inhibiting gene transcription and reducing the expression of autophagy associated genes. Both hMOF and Sirt1 target H4K16 to control and maintain its acetylation status, thereby regulating cellular autophagy and influencing cell survival or death. Additionally, the histone 3 acetylation modification level (AcH3) primarily regulates the transcriptional activity of autophagy related genes and promotes the activation of the autophagy pathway,141 as illustrated in detail in Figure 4C.
Sui et al’s team96 discovered that the histone methyltransferase G9a can inhibit ferroptosis. It achieves this by catalyzing the methylation of lysine 9 on histone H3, which subsequently up-regulates the expression of ferroptosis related genes GPX4, SLC7A11, and SLC3A2. Zhang et al’s team97 found that trimethylation of histone H3 at lysine 4 (H3K4me3) increases GPX4 expression in cancer cells. This suggests that H3K4me3 may participate in the epigenetic regulation of ferroptosis by modulating GPX4 expression. Moreover, simultaneous mutation of acetylation deficient P53 at lysine sites K117, K161, and K162 may reduce glutathione (GSH) production. Additionally, the histone H3K9 demethylase lysine demethylase 3B (KDM3B), as a potential epigenetic regulator of ferroptosis, can inhibit ferroptosis by upregulating the expression of the SLC7A11 gene.142 This occurs through the indirect inhibition of cellular cysteine uptake, which leads to the accumulation of lipid ROS and ultimately induces ferroptosis.143 In cancer tissues, the acetylation of histone H3 at lysine 27 promotes high expression of GPX4, endowing cells with resistance to ferroptosis.144 G9a-catalyzed demethylation of histone H3 lysine 9 (H3K9me2) inhibits iron death triggered by GPX4 transcription and is a potential therapeutic target against inflammation-induced neurodegenerative diseases. JQ1 is an inhibitor of bromodomain protein 4 (BRD4) and inhibits BRD4 expression by activating SIRT1-mediated deacetylation of BRD4 histone. Impaired BRD4 downregulates the expression of GPX4, SLC7A11 and SLC3A2 in cancer cell lines and promotes iron death.96 The transcription factor Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) recruits P300/CBP-associated factor (PCAF) to increase H3K9ac levels of NRF2 and thus iron death in mesenchymal fibrosis. Ketamine, an inhibitor of lysine acetyltransferase 5 (KAT5), inhibits GPX4 by decreasing histone H3 lysine 27 acetylation (H3K27ac) levels, leading to iron death,98 as illustrated in detail in Figure 5C.
Non-Coding RNAs Regulate Programmed Chondrocyte Death
Non-coding RNAs are mainly MicroRNAs (miRNAs) and Long non-coding RNA (lncRNAs); specific miRNAs are expressed at significantly higher levels in KOA chondrocytes and contribute to the activation of apoptotic genes, and some lncRNAs have been found to interact with key transcription factors to regulate chondrocyte survival.145 The miRNA gene is first transcribed in the nucleus by RNA polymerase II to generate primary miRNA (pri - miRNA), which can be up to several thousand bases in length. pri - miRNA is processed in the nucleus by the nucleic acid enzyme Drosha and its cofactors to form a hairpin structure of about 70–100 nucleotides, ie, precursor miRNA (pre - miRNA). pre - miRNA is transported to the cytoplasm and further cleaved to form mature miRNA duplexes under the action of the nucleic acid enzyme Dicer. lncRNA is transcribed similarly to miRNA. miRNA is transported to the cytoplasm, where it is further cleaved to form mature miRNA duplexes under the action of the nuclease Dicer, as illustrated in detail in Figure 1E. lncRNA transcription is similar to that of mRNA, and is mainly catalyzed by RNA polymerase II, using DNA as a template for synthesis. The transcription process is more complex, involving a variety of transcription factors and regulatory elements. lncRNA genes have some differences in their transcription start sites, promoter regions, and so on, from mRNAs, as illustrated in detail in Figure 1F.
miRNAs play crucial roles in the proliferation and apoptosis of OA chondrocytes. For instance, one study revealed that silencing miR-34a effectively prevents IL-1β induced downregulation of COL2a1 and upregulation of iNOS, thereby inhibiting chondrocyte apoptosis.60 Overexpression of miRNA-337-3p suppresses PTEN mRNA and protein expression, promotes the phosphorylation of downstream effectors in the PTEN/AKT pathway, stimulates chondrocyte proliferation, enhances chondrocyte activity, and reduces apoptosis.61 Wang et al’s team62 demonstrated that miR-98 may contribute to chondrocyte apoptosis and cartilage degradation in OA by downregulating Bcl-2 expression, indicating its potential as a therapeutic target for OA. Gu et al’s team63 found that upregulating miR-199-3p or downregulating DNMT3A promotes the proliferation and inhibits the apoptosis of KOA chondrocytes. Wang et al’s team146 showed that miR-21-5p significantly influences the expression of matrix synthesis genes and the proliferation and apoptosis of chondrocytes. Zhao et al’s team64 reported that miR-146a-5p inhibits cell proliferation and promotes apoptosis in OA cartilage injury by regulating TXNIP, thereby affecting the inflammatory response. In addition, the long non-coding RNA (lncRNA) HOX transcriptional antisense intergenic RNA (HOTAIR) increases chondrocyte apoptosis by suppressing miR-130a-5p expression.65 Conversely, PACER reduces chondrocyte apoptosis by inhibiting HOTAIR expression.147 An increasing number of studies have demonstrated that lncRNAs play pivotal roles in the development and progression of OA. They influence cartilage extracellular matrix degradation, chondrocyte apoptosis, synovitis, and microangiogenesis.148 LncRNA-RMRP drives OA development. In an in vitro OA model of IL-1β induced human chondrocyte C28/I2, lncRNA-RMRP exhibits dual effects: it accelerates OA chondrocyte apoptosis and inhibits cell proliferation. The expression levels of lncRNA-RMRP in OA chondrocytes are negatively correlated with those of miR-206. By targeting miR-206, lncRNA-RMRP suppresses chondrocyte proliferation and promotes apoptosis.149 Chen et al’s team66 discovered that lncRNA HOTAIR is involved in OA pathogenesis. Contrary to some lncRNAs, overexpression of HOTAIR significantly reduces IL-1β induced chondrocyte apoptosis and extracellular matrix degradation. High expression of lncRNA FAS-AS1 in osteoarthritic cartilage tissues promotes chondrocyte apoptosis and extracellular matrix degradation. Zhu et al’s team67 found that lncRNA PART1 exacerbates OA progression by promoting chondrocyte apoptosis and extracellular matrix degradation. It does so by targeting miR-373-3p to regulate the expression of SRY-box transcription factor 4, as illustrated in detail in Figure 2D and Table 1.
The teams led by Iliopoulos D65 and Nicolas FE135 discovered that increased miR-22 expression, or siRNA mediated inhibition of PPARα or BMP, leads to elevated IL-1β and MMP-13 protein levels. Conversely, suppressing endogenous miR-22 in OA chondrocytes increases the expression of PPARα and BMP-7. This, in turn, inhibits IL-1β and MMP-13 expression, thereby reducing cellular pyroptosis and increasing the levels of cartilage - protective proteoglycans. Liu et al’s team150 demonstrated that miR-223 directly binds to the 3’UTR of NLRP3 mRNA, effectively inhibiting cellular pyroptosis. Sun et al’s team77 found that lncRNA MALAT1 negatively regulates the levels of miRNA-124-3p. Through the lncRNA MALAT1/miRNA-124-3p pathway, it inhibits cell proliferation and promotes chondrocyte apoptosis. Studies have shown that lncRNAs can mediate the chondrocyte pyroptosis response, influencing the development of OA. lncRNA MALAT1, a non-coding RNA highly expressed in cancer cells, is involved in regulating the ERS of OA chondrocytes. The viability of OA chondrocytes is closely related to the expression of lncRNA MALAT1; an up regulation of lncRNA MALAT1 expression decreases the viability of OA chondrocytes.151 Conversely, low expression of lncRNA MALAT1 inhibits PCD regulated by cellular pyroptosis and significantly impacts the NLRP3/caspase-1/IL-1β signaling pathway.152 This indicates that high lncRNA MALAT1 expression triggers cellular pyroptosis by increasing NLRP3 and caspase-1 levels. The team led by Feng et al’s team78 discovered that lncRNA MALAT1 negatively regulates miRNA-124-3p levels. Through the lncRNA MALAT1/miRNA-124-3p pathway, it suppresses cell proliferation. Additionally, miRNA-124 significantly reduces cellular pyroptosis by decreasing the levels of caspase-1, GSDMD, IL-1β, and IL-18, while promoting chondrocyte apoptosis, as illustrated in detail in Figure 3D and Table 2.
In KOA, autophagy serves as an anti inflammatory mechanism in chondrocytes. MiRNAs play diverse roles in regulating autophagy within these cells. miRNAs such as miR-34a-5p, miR-107, miR-335-5p, miR-140-5p, and miR-146a promote autophagy,78,151,152 while miR-206, miR-375, miR-411, and miR-449 inhibit it.77,151,153 Although autophagy is essential for maintaining chondrocyte homeostasis, abnormal autophagy can influence KOA progression. Sun et al’s team87 demonstrated that miR-375 suppresses the expression of chondrocyte ATG2B, inhibits autophagy, and promotes ERS. Jia et al’s team88 found that miR-146a-5p negatively regulates autophagy through the SDF-1/CXCR4 signaling axis, thereby inhibiting chondrocyte autophagy and delaying the pathological development of KOA. lncRNAs are also involved in autophagy regulation. They can modulate the function and activity of autophagy associated DNA, RNA, or proteins, or influence autophagy related stress factors and energy receptors. Liu et al’s team89 discovered that the expression of certain lncRNAs significantly inhibits autophagy, showing a negative correlation between the two. These findings indicate that lncRNAs play a role in regulating cellular autophagy, with some acting as inhibitors. Prominent autophagy inhibiting lncRNAs include UCA1, CAIF, and HAGLROS. For instance, UCA1 inhibits autophagy by competitively binding to miR-184, which in turn promotes the expression of osgin1, a growth inhibitory factor that suppresses autophagy. lncRNA H19 exhibits a dual regulatory function in cellular autophagy. It can inhibit autophagy by silencing the expression of DIEAS3. Conversely, it can promote autophagy through mechanisms such as enhancing Beclin 1 expression or inhibiting mTOR phosphorylation. As Xu et al’s team90 demonstrated, high levels of lncRNA H19 activate the PI3K/Akt pathway, reduce mTOR phosphorylation, and ultimately promote autophagy by inhibiting the mTOR pathway, as illustrated in detail in Figure 4D and Table 3.
Doll et al’s team154 discovered that miR-672-3p is aberrantly expressed in spinal cord injury and FSP1 targeted cells. In vitro cellular experiments confirmed that miR-672-3p regulates ferroptosis by targeting FSP1. Additionally, GPX4 reduces lipid peroxidation through the human fibroblast specific protein 1 (FSP1)/CoQ10 axis. Studies have shown that lncP53RRA interacts with Ras GTPase-activated protein-binding protein 1 (G3BP1) in the cytoplasm. lncP53RRA decreases p53 binding to G3BP1 in the cytoplasm and increases p53 accumulation in the nucleus to promote SLC7A11 transcription and inhibit iron death.99 lncMT1DP regulates erastin-induced iron death in non-small-cell lung cancer by stabilizing miR-2a-365p and inhibiting NF-E2 p45-related factor 2 (NRF2) MT1DP regulates erastin-induced iron death in non-small cell lung cancer by increasing the abundance of ROS, MDA and Fe2+. Cancer-associated fibroblasts inhibit iron death in gastric cancer cells by exosomally secreting miR-522 to target ALOX15 and prevent lipid ROS accumulation.100 Gomaa et al’s team101 found that miRNA-4715-3p increases UGC cell death by inhibiting GPX4 expression, indicating its potential involvement in ferroptosis regulation. Previous studies have shown that lncRNAs can modulate cellular oxidative stress and trigger ferroptosis. Wang et al’s team102 reported that overexpression of the long non-coding RNA LINC00618 not only elevates lipid ROS and lipid levels but also enhances the expression of ACSL4, a key ferroptosis inducing factor. This evidence suggests that LINC00618 promotes cellular ferroptosis. Moreover, LINC00336 promotes cellular ferroptosis by inhibiting miR6852 expression, which in turn affects intracellularGSH production,103 as illustrated in detail in Figure 5D and Table 4.
Studies have demonstrated that miR-155 prevents cell necrosis by directly targeting Receptor-interacting protein kinase 1 (RIP1).105 miR-499 inhibits calmodulin neural phosphatase mediated dephosphorylation of Dynamin-related protein 1 (Drp1), thereby preventing Drp1 accumulation in mitochondria and Drp1 mediated mitochondrial fission.155 The key deubiquitinating enzyme CLYD in the apoptosis/necrosis pathway is directly targeted by miR-181b-1 and miR-19. This targeting leads to overactivation of the NF-κB signaling pathway, resulting in a highly inflammatory state in cancer cells.156,157 Moreover, miR-873 promotes necroptosis by targeting caspase-8, a crucial regulator of the transition between apoptosis and necroptosis. PACER plays a pivotal role in regulating necroptotic cell death signaling pathways. Its tumor promoting activity may account for the absence of necroptotic signaling in cancer cells. Additionally, RIP3 kinase is an essential component associated with PACER mediated necrosis.106 Activated RIPK1 can trigger necrotic apoptotic cell death by promoting RIPK3 oligomerization and activation, which in turn phosphorylates MLKL. Oligomerization and translocation of phosphorylated MLKL to the plasma membrane promotes cell lysis. Necroptosis occurs when extracellular signals, such as death receptor binding, or intracellular triggers like microbial nucleic acids, inhibit apoptosis. This process involves a series of phosphorylation events that ultimately lead MLKL to form pore complexes at the plasma membrane. These pore complexes trigger the release of damage-associated molecular patterns (DAMPs), which in turn drive cellular self-destruction. Key hallmarks of necroptosis include organelle swelling, cell membrane rupture, and the disintegration of the cytoplasm and nucleus. The primary molecular players in necroptosis are RIPK1, RIPK3, and MLKL. Additionally, mediators such as TNF, members of the Toll-like receptor (TLR) family, and interferons have been identified as crucial factors in the necroptotic process, as illustrated in detail in Figure 6D and Table 5.
Copper death-associated lncRNA LINC00853 significantly enhances glycolysis and cell proliferation in cancer cells through PFKFB3 and increases the level of cellular mitochondrial respiration and tumor growth rate.157 lncRNAs may also serve as novel markers for guiding the prognosis and the immune microenvironment of osteosarcoma, as illustrated in detail in Figure 6C and Table 6.
 |
Table 7 Monomer Modulation of PCD in KOA Chondrocytes |
Monomer Active Ingredients of Traditional Chinese Medicine
Flavonoids
Quercetin (QUE), a flavonoid abundantly present in vegetables and fruits, is a potent free - radical scavenging compound. It exhibits multiple anti oxidative stress and anti inflammatory properties, effectively reducing the risk of oxidative stress related OA.158 QUE not only curbs the progression of KOA through its antioxidant and anti inflammatory actions but also directly impacts KOA by modulating the epigenetic modifications involved in the molecular pathological processes of articular cartilage. The team led by Burdeos GC159 discovered that QUE inhibits the abnormal activation of pro inflammatory genes. It achieves this by regulating the overall DNA methylation levels, specifically through tissue specific upregulation of the mRNA and protein expression of DNMT1. The team led by Liu160 found that in a nickel induced oxidative stress model, QUE reduces the activity of hepatic DNA methyltransferases (DNMTs). This action decreases the DNA methylation in the promoter region of the Nrf2 gene, facilitating its nuclear translocation and promoting the expression of downstream antioxidant genes, such as HO-1. This mechanism may potentially counteract chondrocyte oxidative damage in KOA via a similar pathway. IL-1β, a key proinflammatory cytokine, has been demonstrated to induce chondrocyte apoptosis both in vivo and in vitro. This process results in the degradation of the chondrogenic matrix and arthritic inflammation, ultimately contributing to the progression of KOA.161,162 The team led by Taganov KD163 discovered that QUE derivatives significantly upregulate the expression of miR-146a. By inhibiting key mediators of the TLR4/NF-κB signaling pathway, these derivatives reduce the release of pro-inflammatory factors like IL-1β and TNF-α. Epigenetic research indicates that the promoter methylation status of miR-146a directly influences its expression. QUE may enhance miR-146a’s negative regulation of inflammation by reversing transcriptional repression through demethylation. QUE alleviates ERS in chondrocytes via the SIRT1/AMPK pathway. As a class III histone deacetylase (HDAC), SIRT1 activation modulates histone acetylation levels, thereby impacting the transcription of MMP-13.164 Additionally, QUE inhibits IL-1β induced NF-κB activation. This action likely blocks the epigenetic activation of inflammation - associated genes (such as COX-2 and iNOS) by regulating the balance of histone acetyltransferases (HATs) and HDACs.165 In KOA chondrocytes, QUE, activated through the Nrf2/ROS/BAX/Bcl-xl axis, mitigates oxidative stress. It also maintains chondrocyte survival by modulating the epigenetic silencing of BAX (for example, through methylation or miRNA targeting). Moreover, QUE’s inhibition of extracellular matrix (ECM) degradation is closely linked to the epigenetic regulation of matrix - degrading enzyme genes by DNMTs and miRNAs.
Icariin (ICA), extracted from the dried stems and leaves of Arrowleaf Epimedium, Pilose Epimedium, Wushan Epimedium, and Korean Epimedium, is a major component of Epimedium. It exhibits diverse beneficial effects, including immunity enhancement, anti - inflammation, anti - aging, and cardiovascular protection. As the primary active ingredient of Epimedium, ICA not only has anti inflammatory, antioxidant, and pro - cartilage repair properties but also intervenes in the key pathological processes of KOA by regulating epigenetic modifications.226 The team led by ZU226 demonstrated that ICA inhibits chondrocyte pyroptosis. It achieves this by downregulating the activation of NLRP3 inflammasomes through inhibition of the MALAT1/miR-124-3p axis. As a lncRNA, MALAT1 can be regulated by epigenetic mechanisms (such as recruiting methylation enzymes or histone modifying factors) to modulate miR-124-3p expression. ICA likely attenuates the inflammatory response by interfering with this epigenetic regulation process. IL-1β is a crucial proinflammatory factor in KOA. It activates NF-κB and promotes the release of matrix - degrading enzymes like MMP-3.227 ICA directly inhibits the IL-1β/p-PI3K/Akt/mTOR signaling axis. Additionally, it may reduce the expression of inflammatory factors by regulating the epigenetic silencing of miRNAs, such as miR-146a and miR-21.228 Moreover, ICA promotes chondrocyte autophagy by activating the PI3K/AKT/mTOR pathway, which leads to upregulation of Beclin-1 and LC3 expression.229 Recent research indicates that the mTOR signaling pathway can modulate the epigenetic status of autophagy related genes by regulating HDACs and DNMTs. ICA may enhance autophagy and slow down cartilage degeneration through a comparable mechanism. ICA inhibits synoviocyte ferroptosis by activating the system xc⁻/GPX4 axis.229 The expression of GPX4 is regulated by Nrf2, whose transcriptional activity depends on the DNA methylation and histone acetylation status of its promoter region. ICA might alleviate oxidative damage in KOA by decreasing the methylation level of the Nrf2 gene, enhancing its histone acetylation, and thereby promoting the body’s antioxidant defenses. Furthermore, ICA counteracts IL-1β induced overexpression of MMP-3 and stimulates type II collagen synthesis.228 The transcription of MMPs is regulated by DNA methylation and microRNAs (such as miR-140), while collagen gene expression can be influenced by HDACs. By regulating these epigenetic mechanisms, ICA likely maintains the homeostasis of the cartilage ECM.
Baicalein (BAI), an important active ingredient derived from the root of Scutellaria baicalensis, exhibits a wide range of therapeutic effects, including anti-inflammation, antifibrosis, anti-apoptosis, and anti-tumor activities.166 The team led by Farooqi AA166 discovered that BAI relieves the transcriptional repression of PTEN (Phosphatase and Tension Protein Homologue) by down regulating miR-424-3p. This action inhibits the activation of the PI3K/AKT signaling pathway. As a crucial tumor suppressor, PTEN is epigenetically regulated, for example, through promoter methylation or miRNA targeting. BAI may restore PTEN expression by modulating DNA methylation or miRNA levels, potentially inhibiting IL-1β induced MMP-3/9 expression and delaying the degradation of the cartilage ECM.167 BAI also inhibits IL-1β induced COX-2 expression, reducing the production of the inflammatory mediator PGE2. This effectively blocks the activation of matrix MMPs and cartilage catabolism.167 Given that COX-2 transcription is regulated by histone acetylation (such as H3K27ac) and DNA methylation, BAI likely suppresses COX-2 expression by interfering with these epigenetic modifications. The team led by Jiang168 demonstrated that BAI significantly inhibits the activities of DNA 5mC and RNA m6A modifying enzymes, including METTL3, which targets the m6A site (2854) of the HKDC1 gene. This inhibition affects glucose metabolism and oxidative stress responses. In KOA, m6A modifications can regulate the mRNA stability of genes like IL-1β or GPX4. BAI may indirectly protect chondrocytes from injury by interfering with these m6A modifications. BAI enhances GPX4 expression and suppresses Erastin - induced ferroptosis by upregulating the SLC7A11/GPX4 axis or activating the Wnt/β - catenin pathway.169 Since GPX4 expression is regulated by Nrf2, and Nrf2’s transcriptional activity is influenced by DNA methylation and histone modifications in its promoter region, BAI may activate the antioxidant defense system through epigenetic mechanisms. BAI inhibits the PI3K/AKT/mTOR signaling pathway by upregulating PTEN, subsequently regulating chondrocyte autophagy and apoptosis. As PTEN expression can be modulated by miR-424-3p, BAI restores PTEN function via epigenetic reprogramming to promote chondrocyte survival. Furthermore, BAI suppresses pro - inflammatory transcription factors like NF-κB. It likely achieves this by regulating the activity of HDACs or EZH2. By inhibiting the activation of pro - inflammatory transcription factors, BAI reduces the expression of MMPs and COX-2.
The total flavonoids of Rhizoma Drynariae (AFDR), a key component of Rhizoma Drynariae in the Daphniaceae family, exerts potent anti inflammatory effects. AFDR significantly curbs the IL-1β induced expression of inflammatory factors. It inhibits the transcription of pro-inflammatory cytokines such as TNF-α and IL-6 by downregulating the NF-κB signaling pathway. Additionally, AFDR modulates the activity of DNMTs, thereby influencing the methylation status of inflammation related genes, including iNOS.170 Moreover, AFDR plays a crucial role in regulating chondrocyte apoptosis to delay the onset of KOA. It affects the methylation status of the Bax gene promoter by regulating the Bcl-2/Bax expression balance. Through miRNAs like miR-15a, AFDR targets the stability of Bcl-2 mRNA, which in turn impacts the Bax gene promoter methylation. This mechanism inhibits Caspase-3 activation. Furthermore, AFDR regulates H3K27 acetylation (H3K27ac) to modulate the expression of apoptosis related genes, ultimately suppressing chondrocyte apoptosis and retarding KOA progression.102,171
Chrysin, a flavonoid extracted from wood butterflies in the Zygophyllaceae family, exhibits anti inflammatory and antioxidant properties. It protects chondrocytes from IL-1β induced damage by upregulating the long non-coding RNA SNHG9. Chrysin competitively binds to miR-184, thereby relieving the inhibitory effect of miR-184 on genes essential for chondrocyte survival. In essence, chrysin safeguards chondrocytes against IL-1β damage by increasing SNHG9 levels, which subsequently reduces miR-184 expression.174 Raina R’s team173 discovered that chrysin exerts a profound regulatory influence on chromatin - modifying enzymes. It inhibits the activity of DNMTs, reducing 5-methylcytosine (5mC) levels in the promoter regions of tumor suppressor genes. Concurrently, chrysin promotes the expression of TET-family demethylases, downregulates histone methyltransferases (HMTs) and HDACs, and upregulates the activity of HATs. These actions enable chrysin to regulate the acetylation and methylation modification patterns of H3 and H4 histones. It should be noted that the statement about paederin in the original text seems out of place as it is not related to the previous content about chrysin. If it is relevant, more context and connection need to be provided. If not, it can be removed to make the text more coherent.174
Sweet orange flavonoid (sinensetin), a flavonoid widely found in citrus plants, can inhibit the release of ECM and inflammatory factors by inhibiting p-NF-κB and thereby reducing the release of NLRP3, effectively alleviating chondrocyte pyroptosis caused by IL-1β, and improving the survival rate of OA cells.230 The JI’s team175 demonstrated that Sinensetin could protect chondrocytes by inhibiting VEGF expression through enhancing miR-374c-5p expression down-regulating the expression of hypoxia-inducible factors, decreasing the phosphorylation of VEGFR2 and inhibiting the AKT signaling pathway, modulating the DNA methylation pattern of AKT genes, and affecting the histone acetylation state of key nodes of the AKT signaling pathway, thereby promoting chondrocyte apoptosis.
Licorice chalcone A is a flavonoid extracted from the legume licorice. Licorice chalcone A targets and inhibits the expression of ADAM9, a metalloproteinase, by up-regulating miR-1270, thereby blocking the activation of the Akt/NF-κB signaling pathway.231 The transcription of ADAM9 may be regulated by DNA methylation in its promoter region or by H3K27me3, whereas licorice chalcone A may indirectly affect the epigenetic silencing of ADAM9. Licorice chalcone A inhibits the nuclear translocation of NF-κB p65, which may alleviate LPS-induced chondrocyte pyroptosis by reducing the release of the inflammatory vesicle NLRP1 by modulating the H3K9ac or DNA methylation status of its gene locus.232 In addition, Licochalcone A, by inhibiting the Wnt/β-catenin signaling pathway, reduces IL-1β mediated cartilage degradation.233
Kaempferol, a flavonoid abundantly present in various Chinese medicines and foods, exhibits multiple physiological functions, including anti - inflammatory, antioxidant, and antitumor activities.176 Studies by Panahi et al177 and Huang et al178 have shown that kaempferol protects rat chondrocytes from OA. It achieves this by inhibiting the mitogen - activated protein kinase associated extracellular signaling regulated kinases and the P38 signaling pathway, thereby suppressing IL-1β induced inflammatory responses. ROS play a dual role in cartilage. While ROS act as important intracellular second messengers that maintain cartilage function by regulating cartilage homeostasis and chondrocyte differentiation,179 excessive accumulation of ROS triggers oxidative stress. This is associated with cellular dysfunction, apoptosis, ECM disruption, cell death, and ultimately, cartilage degradation.180 Ying et al’s team181 discovered that the body’s inflammatory responses are closely linked to the ROS/TXNIP pathway. A massive production of ROS upregulates TXNIP expression, which in turn activates NLRP3 inflammasomes and increases the secretion of inflammatory factors, resulting in uncontrolled inflammation, cell death, and cartilage degradation. Notably, the activity of HDACs influences the level of H3K27me3 modification at the miR-146a locus, potentially linking epigenetic regulation to the complex biological processes related to cartilage health and disease. The DNA methylation status of the promoter region of the miR-146a gene is regulated by kaempferol. In addition, the stability of core proteoglycan mRNA may be regulated by METTL3-mediated m6A modification. Wang et al. Team182 found that kaempferol inhibited the activation of the ROS/TXNIP pathway and ameliorated oxidative and inflammatory damage and cartilage degradation in chondrocytes of KOA rats. Jiang team experimentally found that kaempferol inhibited the transcriptional inhibition of core proteoglycan (Decorin) through the down-regulation of miR-146a and deregulating its transcriptional repression of core proteoglycan (Decorin), thereby inhibiting the over-activation of the PI3K/AKT/mTOR signaling pathway.183
Phenols
Curcumin, a natural compound derived from turmeric root, exhibits potent antioxidant, antimicrobial, anti-inflammatory, and anticancer properties. In chondrocytes, curcumin inhibits the damage to articular cartilage caused by inflammatory factors by blocking the NF-κB mediated IL-1β/TNF signaling pathway.184 Through the JNK pathway, it suppresses the expression of MMP-1 and MMP-3, effectively reducing immune cell infiltration, synovial membrane hyperplasia, and cartilage destruction.185 In KOA, excessive ROS production disrupts intracellular signaling pathways, alters the cartilage cell life cycle, and impairs cartilage matrix metabolism, ultimately leading to synovial inflammation and subchondral bone dysfunction.186–188 Curcumin significantly inhibits IL-1β/TNF induced chondrocyte apoptosis and promotes cell proliferation by upregulating miR-1227.189 Epigenetically, curcumin may modulate the DNA hypomethylation status in the miR-1227 gene promoter region. It can also inhibit HDACs or activate HATs, enhancing the H3K27 acetylation (H3K27ac) modification at the miR-1227 gene locus. These mechanisms suggest that curcumin protects chondrocytes from apoptosis and stimulates their proliferation by upregulating miR-1227 expression. Furthermore, curcumin promotes autophagy by regulating the AKT/mTOR signaling pathway, thereby exerting beneficial effects against osteoarthritis.190 The QIU’s team191 found that curcumin-treated MSC exosomes restored down-regulated miR-143 and miR-124 in OA cells, which in turn inhibited the expression of NF-κB and ROCK1. The mechanism involves selective packaging of exosomal miRNAs (mediated by curcumin-regulated RNA-binding proteins) with altered DNA methylation or histone deacetylation status of target genes (eg, ROCK1).191 Curcumin-pretreated MSC exosomes (Cur-EVs) significantly up-regulated the expression of BCL2, ACAN, SOX9, and COL2A1, and in addition, curcumin down-regulated inflammatory genes such as IL-1β, IL-6, and MMP13 by a mechanism that may involve exosome-borne miR-143 targeting MMP13 mRNA and exosomal lncRNA modulating histone methylation (eg, H3K4me3) of the COL2A1 gene.192
Polydatin, a natural plant ingredient, is used in the treatment of many diseases.193 Wu et al. Team193 found that polydatin significantly down-regulated the ratios of P-PI3K/PI3K and p-AKT/AKT by a mechanism that may involve the regulation of the DNA methylation status of the promoter regions of PI3K and AKT genes (eg, through the activation of the TET demethylase enzyme) by polydatin. Inhibition of DNMTs activity by thujaplicins reverses aberrant methylation silencing of pro-inflammatory genes. Silymarin inhibited the secretion of pro-inflammatory chemokines and enhanced chondrocyte viability and proliferation. This suggests that thujaplicin restores chondrocyte autophagy and attenuates joint damage by inhibiting PI3K/AKT signaling.
Chalcones
Safflower yellow pigment (SY), a chalcone compound extracted from safflower petals, exhibits analgesic, emmenagogue, and blood activating properties.171,234 SY protects chondrocytes by influencing downstream factors of the NF-κB pathway, namely AMPK/SIRT1. It inhibits TNF-α induced NF-κB activation and ERS by promoting the expression of P-AMPK and SIRT1, thereby preventing cartilage degeneration. In vitro studies show that SY promotes the expression of miR-140-5p in osteoarthritic chondrocytes. When miR-140-5p expression is downregulated, SY’s beneficial effects are reversed, indicating that SY promotes autophagy, reduces apoptosis, and inhibits the secretion of inflammatory factors. SY also counteracts the changes induced by OA. It restores the TNF-α induced upregulation of IL-1β, PTGS2, and MMP-13, as well as the downregulation of COL2A1 and ACAN. Additionally, SY can inhibit TNF-α to restore cell proliferation. By reducing MMP-13 expression and targeting COX-2 to decrease PGE2 release, SY minimizes cartilage catabolism and protects the cartilage and its matrix.171
Saponins
Panax ginseng saponin (PNS) is an active component of the traditional Chinese medicine Panax ginseng. ZHANG et al’s team204 found that PNS could elevate the expression levels of autophagy-related proteins and anti-apoptotic protein Bcl-2 in OA chondrocytes, and inhibit OA chondrocyte senescence and apoptosis. The mechanism is to protect chondrocytes and delay the degradation of articular cartilage by inhibiting the PI3K-AKT-mTOR signaling pathway. PNS was able to inhibit the expression of TLR4, NLRP3, and Caspase-1 proteins, which suggests that PNS can reduce OA chondrocyte scorched death by inhibiting the activation of TLR4/NLRP3/Caspase-1 signaling pathway. IL-1β and IL-8 have been shown to enhance cartilage catabolism and are able to promote cartilage extracellular matrix degradation and ultimately to OA disease progression by decreasing proteoglycan and type II collagen expression.195 The HU’s team195 demonstrated that Panax ginseng saponin reduced miR-27a expression, which negatively regulates SOX8 expression. In addition, activation of SOX8 upregulates β-catenin expression, which inhibits chondrocyte apoptosis, thereby suppressing cartilage matrix degradation and joint inflammation, and subsequently OA progression.
Astragaloside is one of the main active ingredients contained in Astragalus. Astragaloside can activate chondrocyte autophagy through the miR-21-mediated PTEN/PI3K/AKT/mTOR pathway to restore joint homeostasis and slow down OA progression.235 Astragaloside can reduce gene expression of RI3K and AKT, decrease the expression of Bax and Caspase-3, and promote the expression of Bcl-2, and increase the expression of type II collagen in degenerated chondrocytes, which suggests that astragaloside promotes chondrocyte proliferation and reduces apoptosis through the RI3K/AKT pathway.236
Panax quinquefolium saponin (PQS) is the main active ingredient of American ginseng. PQS decreased the levels of CHOP and caspases-3 in rat cartilage and reduced apoptosis in rat chondrocytes.196 PQS treatment protected chondrocytes from ERS and IL-1β induced associated apoptosis. PQS also could further attenuate triglyceride (TG) induced ERS and associated apoptosis. In addition, PQS may act as an inhibitor of apoptosis by inhibiting the ERS-activated NF-κB pathway and the associated inflammatory response in chondrocytes.
Achyranthes bidentata Bl Saponin (ABS), the primary active component of Achyranthes bidentata, exhibits anti inflammatory, antioxidant, and anti apoptotic properties.197 ABS effectively alleviates synovial inflammation in the knee joint. It reduces the levels of inflammatory factors in synovial tissues and fluids, mitigates the infiltration of synovial inflammatory cells, inhibits vascular proliferation, and relieves congestion and edema in synovial tissues.198,199 Studies have shown that the alcohol extract of Achyranthes bidentata, which mainly contains saponins and polysaccharides, can effectively reduce weight bearing pain and decrease joint effusion in patients with KOA.199,210 ABS specifically inhibits the IL-1β induced expression of COX-2 in chondrocytes, thereby safeguarding joint tissues.200 In OA patients, joint tissues have elevated COX-2 levels. The PGE2 synthesized by COX-2 promotes cartilage matrix degradation, chondrocyte apoptosis, inflammatory factor production, and vascular proliferation, and activates osteoclasts, ultimately leading to the destruction of cartilage and bone.201
Terpenes
Paeoniflorin, a monoterpene glycoside derived from white peony, exerts remarkable effects on chondrocytes. Following paeoniflorin intervention, damaged autophagy proteins in chondrocytes are restored to some extent. Paeoniflorin increases the miR-124 level and suppresses the expression of p-PI3K, p-AKT, TNF-α, and IL-6 in cells. By inhibiting the PI3K/AKT signaling pathway, it curbs the activity of inflammatory cytokines, enhances chondrocyte autophagy, and mitigates the inflammatory response and chondrocyte injury. Previous studies have indicated that paeoniflorin protects human chondrocytes from IL-1β induced inflammatory damage. It inhibits NF-κB activation in chondrocytes and reduces chondrocyte apoptosis through the circ-PREX1-miR-140-3p-WNT5B pathway.202,203 The research by the Wu team demonstrated that after paeoniflorin treatment, the expressions of LC3 II and Beclin 1 proteins increase. This suggests that paeoniflorin enhances the autophagy capacity of chondrocytes in arthritis, reduces cellular damage, and plays a crucial role in the prevention and treatment of related conditions.204
Loganin, a cyclic enol ether terpene glycoside extracted from herbs such as Cornus officinalis and Strychnos species, protects chondrocytes by inhibiting IL-1β induced apoptosis and reducing the release of catabolic enzymes and ECM degradation. Strychnoside, another compound, exerts chondroprotective effects through the activation of the PI3K/AKT signaling pathway. Epigenetically, it may regulate the DNA methylation status of the PTEN gene promoter, decreasing its methylation level, and influence the histone modification pattern of the AKT gene locus, increasing the activation mark of H3K4me3. These actions enable strychnoside to inhibit apoptosis, suppress ECM degradation, and partially mitigate cartilage degeneration.205 Moreover, strychnoside has multiple beneficial effects in OA. It inhibits OA related cartilage degeneration, suppresses chondrocyte pyroptosis, and blocks the activation of the NF-κB signaling pathway. Additionally, it restrains OA associated bone remodeling and reduces abnormal blood vessel formation in the subchondral bone.206
Gentiopicroside (GPS) can effectively balance the synthesis and degradation of ECM to achieve protection of osteoarticular tissues, and its mechanism of action may be related to the reduction of inflammatory factors in vivo and the promotion of MMP degradation of type II collagen.207 GPS works by inhibiting the phosphorylation of p-P38/p-ERK/p-JNK.220 The potential epigenetic mechanisms of GPS include: regulating the DNA methylation status (eg, DNMT1-mediated hypermethylation) of the promoter regions of MAPK pathway genes (eg, MAPK1/3), decreasing the level of H3K27ac at the COX-2 gene locus, decreasing the production of PGE2, and decreasing the expression of MMP-1, MMP-3, and MMP-13 for the purpose of inhibiting the degradation of type II collagen, which is of a certain degree of chondrogenic protective effect on chondrocytes.208
Aucubin is found to have diverse biological activities such as anti-inflammatory, antimicrobial, antioxidant, anti-aging, anti-tumor, and anti-apoptotic.209,210 Members of the Bcl-2 family play a crucial role in regulating chondrocyte survival and apoptosis, and can trigger the caspase cascade. Inhibiting Caspase-3 activation is a key factor in reducing chondrocyte apoptosis. The Bcl-2 family consists of pro-apoptotic proteins like Bax, which accelerates apoptosis, and anti apoptotic proteins such as Bcl-2, which suppresses it.211,212 Studies indicate that Bcl-2 levels are lower in OA cartilage compared to healthy cartilage, affecting chondrocyte survival. Wang’s team8 discovered that myricetin can combat KOA by modulating the anti - apoptotic effects of key mediators, including Bcl-2, Bax, Caspase-9, and Caspase-3, on chondrocytes. In vitro, myricetin reduces IL-1β induced expression of inflammatory factors in chondrocytes. In vivo, it delays OA progression, inhibits chondrocyte apoptosis, and reduces inflammatory factor levels in articular cartilage. Similarly, Coriolus versicolor also demonstrates significant effects. In vitro, it reduces IL-1β induced inflammatory factor expression in chondrocytes, while in vivo, it slows down OA progression and inhibits chondrocyte apoptosis and inflammatory factor production in articular cartilage.213 Moreover, Coriolus versicolor inhibits IL-1β induced chondrocyte apoptosis by modulating the miR-140/CREB1 pathway, thereby providing chondroprotection for OA chondrocytes. It should be noted that myricetin and aucubin are two different compounds. The initial description may have a mix-up. If aucubin was intended, the content should be adjusted accordingly to accurately reflect its properties and mechanisms.
Artemisinin (AT) is a sesquiterpene lactone isolated from the medicinal plant Artemisia annua. It has been demonstrated that artemisinin significantly inhibits IL-1β-induced phosphorylation of PI3K/AKT/mTOR pathway substrates, including p-PI3K, p-AKT, and p-mTOR, while activating mitochondria in a dose-dependent manner.240 It has been demonstrated that AT down-regulates the levels of cartilage degradation-associated proteins, MMP3, MMP13, and ADAMTS5, in OA. and alleviates OA by targeting TNFSF11, activating mitochondrial autophagy, inhibiting PI3K/AKT/mTOR signaling, activating mitochondrial autophagy, and thereby attenuating cartilage degradation and defects.
Atractylodes macrocephala extract (ATR) promotes chondrocyte autophagy and inhibits apoptosis by activating the AMPK/SIRT1 signaling pathway, IL-1β-induced autophagic vesicle levels in chondrocytes, and significantly elevated levels of LC3II/LC3I, Beclin-1, and Atg5 protein expression.227
Cardamom extract (CAD) is a compound extracted from Zingiber officinale. CAD regulates iron death and improves OA cartilage degradation through the P53 signaling pathway.239 CAD modulates iron death by up-regulating p53 expression, which reduces the expression of MMP13, iNOS, and COX2. The potential mechanisms include modulation of the DNA hypomethylation state (TETase-mediated demethylation) in the promoter region of the p53 gene, enhancement of histone acetylation (H3K27ac) modification of the p53 gene locus, and further inhibition of the negative regulators of the SLC7A11/GPX4 axis through miR-34a (a downstream target of p53). In addition, CAD inhibits NLRP3 inflammatory vesicles via Nrf2 and attenuates IL-1β-stimulated inflammation and oxidative stress in OA chondrocytes. The mechanisms include modulation of DNA demethylation of the Nrf2 gene (inhibition of DNMT1 activity) and increased histone methylation (H3K4me3) modification of Nrf2 target gene (eg, HO-1) loci.239
Clematichinenoside AR, (C-AR), a triterpenoid saponin derived from Wei Ling Xian, exhibits anti inflammatory and analgesic properties.99 Li et al’s team demonstrated that C-AR reduces the secretion of IL-6 and IL-8 and the production of MMPs in MH7A cells stimulated by TNF-α.214 It effectively counteracts TNF-α induced inflammation and cytotoxicity. In addition, when applied to cultured chondrocytes, C-AR increases the expression of autophagy related proteins Beclin1 and LC3B, as well as the LC3BII/LC3BI ratio. These findings indicate that C-AR alleviates inflammation and oxidative stress, inhibits apoptosis, and promotes autophagy in in vitro cultured knee osteoarthritis (KOA) chondrocytes. The Wang team215 combined bioinformatics prediction with experimental verification and found that circPTN promotes the expression of frizzled-4 (FZD4) by acting as a sponge for miR-145-5p. This activation of FZD4 then triggers the Wnt/β-catenin pathway. These results suggest that the circPTN/miR-145-5p/FZD4 signaling axis is implicated in the pathogenesis of rheumatoid arthritis.
Naphthoquinones
Shikonin, a natural naphthoquinone component extracted from traditional Chinese medicine comfrey, has a broad range of biological activities. It significantly inhibits the serum levels of IL-1β, TNF-α, and iNOS in OA model rats, markedly reduces the expression of COX-2 and the activity of Caspase-3, significantly restores p-AKT, and suppresses the inflammatory response of KOA and chondrocyte apoptosis via the modulation of the PI3K/AKT signaling pathway.46 ZHANG’s team216 found that comfreyin inhibited the expression of DNMT1, decreased the methylation of PTEN gene, increased the expression of PTEN protein, and inhibited the PI3K/AKT/mTOR pathway as a way to inhibit the inflammatory response of KOA and the apoptosis of chondrocytes.
Anthraquinones
Emodin reduces ROS production, enhances antioxidant function, reduces the level of oxidative stress in osteoarthritic chondrocytes, enhances their survival and proliferation, and inhibits apoptosis by up-regulating the expression of SIRT1 while attenuating p-mTOR, which ultimately exerts a chondroprotective effect.217 Its epigenetic mechanisms include the regulation of DNA hypomethylation (TETase-mediated demethylation) of the SIRT1 gene promoter region, enhancement of histone deacetylation (H3K27ac reduction) modification of SIRT1 gene loci, and the enhancement of SIRT1 gene promoter region by miR-34a (SIRT1 gene promoter region). Methylation status (TETase-mediated demethylation), enhancement of histone deacetylation (H3K27ac reduction) modification at the SIRT1 gene locus, and deregulation of SIRT1 mRNA by down-regulation of miR-34a (SIRT1-targeting miRNA).
Phenylpropanoids
Cinnamaldehyde, an aldehydic organic compound, is present in plants like cinnamon. Sheng et al’s team238 discovered that IL-1β-induced chondrocytes treated with varying concentrations of trans-cinnamaldehyde exhibited significantly lower protein levels of IL-8, TNF-α, and PGE2. Additionally, the gene expression levels of MMP-13, iNOS, COX-2, and ADAMTS-5 in chondrocytes decreased as the cinnamaldehyde concentration increased, and the expressions of p-AKT and p-PI3K also decreased. Trans-cinnamaldehyde inhibits IL-1β-induced inflammatory responses via the PI3K/AKT pathway, indicating that cinnamaldehyde could potentially serve as a therapeutic agent for OA treatment. Trans-cinnamaldehyde down-regulates miR-155 to mediate autophagy through the PI3K/Akt pathway as a way to protect chondrocytes.
Polysaccharides
Angelica polysaccharides (AP) up-regulates the expressions of chondrocyte autophagy proteins LC3II, Beclin-1, and ATG5, as well as the anti-apoptotic protein Bcl-2, while down-regulating the pro-apoptotic protein Bax, thereby reducing apoptosis. At the same time, it activates the ERK1/2 signaling pathway to promote autophagy expression and diminish the impact of apoptosis on KOA. Moreover, by activating the SIRT1/AMPK signaling pathway, its ERS is inhibited, the number of chondrocyte apoptosis is decreased, and cartilage degeneration in knee osteoarthritis is slowed.218
Astragalus polysaccharide (APS), the primary active component of the traditional Chinese medicine Astragalus, is composed of dextran, glucose, rhamnose, etc. It exhibits multiple pharmacological effects, including anti - inflammatory, antioxidant, and anti apoptotic properties.219–221 The team led by Gao et al222 discovered that APS intervention significantly upregulates the expression of p-JAK2 and p-STAT3 in KOA chondrocytes and increases the ratios of p-JAK2/JAK2 and p-STAT3/STAT3, indicating that APS can activate the JAK2/STAT3 pathway. Additionally, Gao et al’s team222 found that APS intervention remarkably enhances the proliferative activity and reduces the apoptotic rate of KOA chondrocytes. It upregulates the expression of cyclin D1 and Bcl-2 while downregulating the expression of Bax, cleaved Caspase-3, and increases the Bcl-2/Bax ratio. The potential mechanisms involve DNA hypermethylation of the Bax gene promoter, mediated by DNMT1, and the adsorption of pro-apoptotic miR-675 via the lncRNA H19 sponge, suggesting that APS promotes the proliferation and inhibits the apoptosis of KOA chondrocytes. The experimental research by the Liu team223 demonstrated that APS induces DNA methylation of the promoters of genes related to calcium homeostasis, osteoblast/osteoclast homeostasis, Wnt signaling, and hormone related processes.
Alkaloids
Sinomenine, an alkaloid monomer extracted from the traditional Chinese medicine Qingfeng vine. The Zheng et al’s team224 discovered that medium and high doses of sinomenine significantly up-regulated the expressions of autophagy-related proteins Atg-5, Atg-12, LC3-II, and Beclin-1, while down-regulating the expressions of key molecules like PI3K, AKT, and mTOR in the articular cartilage of OA model rabbits. This indicates that medium and high doses of sinomenine can promote autophagy in rabbit articular cartilage of the OA model by inhibiting the PI3K/AKT/mTOR signaling pathway. DONG team225 found that cytarabine treatment down-regulated inflammatory cytokine levels and protein expression of the inflammatory vesicle component of NLRP3 and up-regulated miR-223-3p expression in OA mice and IL-1β-stimulated chondrocytes. In vitro, we found that NLRP3 was a direct target of miR-223-3p, and overexpression of miR-223-3p blocked IL-1β-induced apoptosis and chondrocyte inflammatory responses. The detailed mechanisms of epigenetic-based regulation of programmed chondrocyte death by the above herbal monomers are shown in Figure 7 and Table 7.
 |
Figure 7 Diagram of the molecular mechanism by which monomers regulate PCD in KOA chondrocytes. By Figdraw. |
Compounding
Pubescent Angelica and Loranthus Decoction
Pubescent Angelica and Loranthus Decoction, from “Bijie Qianjin Yaofang”, consists of 15 traditional Chinese medicines such as Duhuo, Xinxin, Cinnamon, Gentian Macrophyllae, Fangfeng, Sangsusheng, Eucommia, Cortex Eucommiae, Cow’s Knee, Angelica Sinensis, Paeonia lactiflora, Dihuang, Rhizoma Ligustici Chuanxiong, Radix et Rhizoma Ginseng, Poria, and Radix Glycyrrhizae. It is mainly used to treat paralysis due to liver and kidney deficiencies and insufficient qi and blood, and shows good efficacy in KOA, lumbar intervertebral disc herniation, etc.241,242 The mechanism of action of Dokuto Parasite Soup in treating KOA includes inhibition of inflammatory factors and cellular pyroptosis, promotion of chondrocyte proliferation, and inhibition of cartilage matrix degradation. Degradation of cartilage matrix will destroy the cartilage structure and lead to the occurrence of KOA. Dysregulation of SDF-1/CXCR4 and Wnt/β-catenin signaling pathway will activate MMP-3 and MMP-13, which will specifically break down type II collagen and proteoglycans, leading to the degradation of cartilage matrix.243,244 The experimental study by the Cao team revealed that MiR-214-3p exerts a protective effect on chondrocytes. When MiR-214-3p is overexpressed, it reduces the expression of MMP-3 and MMP-13 while increasing the expression of COL2A1. This inhibition of apoptosis helps prevent cartilage degradation.245 The Xu team246 discovered that the Dokuto parasitic tonic upregulates MiR-214-3p, effectively inhibiting chondrocyte ferroptosis. Chen et al247 found that activation of the NLRP3/NF-κB signaling pathway increases the levels of inflammatory factors such as TNF-α, IL-1β, and IL-6 in the joint fluid and serum of KOA model rats. In contrast, the Dokuto parasitic soup significantly reduces the number of inflammatory cells and decreases the levels of these inflammatory factors in KOA rats’ joint fluid and serum. Jia et al248 demonstrated that the expression of BMP7 and SIRT1 in knee joint chondrocytes is positively correlated with the serum dose of Dokwo Sangsang Soup, while the expression of MMP-13 and MMP-3 is negatively correlated with it. These findings suggest that the serum containing Dokwo Sangsang Tang can promote chondrocyte regeneration by regulating the expression of BMP-7 and SIRT1, thereby delaying the progression of KOA.
Aconiti Decoction
Aconiti Decoction, from “The Essentials of the Golden Chamber”, is a commonly used formula in clinical treatment of knee osteoarthritis. It has the functions of supporting the righteous, dispelling evil spirits, expelling cold and dampness, and facilitating joints. Comprising systematic Chuanwu, ephedra, astragalus, white peony, and roasted licorice.249 Wu-tou Tang can inhibit oxidative stress in KOA chondrocytes by regulating the Nrf2/Keap1 pathway. Lin et al250 team found that aconite soup could significantly reduce the expression levels of NLRP3, caspase-1, GSDMD, IL-1β, IL-18 and increase the expression of miR-124. This suggests that aconite soup formula may mediate lncRNAMALAT1/miR-124 to regulate the NLRP3/caspase-1/GSDMD signaling pathway associated with cellular pyroptosis to play a role in delaying chondrocyte degeneration, which could be a good example for aconite soup formula to improve OA progression.252 Liu et al’s team251 found that aconite soup may activate SIRT1 signaling, inhibit FOXO1 acetylation induced downregulation of TNF-α and MMP-13 expression and inhibited apoptosis of OA chondrocytes.
Huanglian Jiedu Decoction
Huanglian Jiedu Decoction (HJD) is composed of four herbs: Huanglian Jiedu, Gardenia jasminoides, Scutellaria baicalensis, and Phellodendron amurense.252 The treatment of OA with HJD mainly pertains to various aspects such as chondrocyte growth and apoptosis, the expression of pro-inflammatory factors and inflammatory genes, and osteoblast differentiation and metabolism.253 HJD contains quercetin, phellodendron, and kaempferol, which can promote osteoarthritic chondrocyte proliferation, inhibit apoptosis, enhance cell healing ability, increase the expression of SOX9, ACAN, COL2A1, and Bcl-2, while reducing the expression of COL10A1, MMP3, MMP13, and Bax, thereby exerting a protective effect on chondrocytes.254 PI3K-Akt mainly regulates the cell growth process by participating in apoptosis in multiple ways such as mitochondria, death receptors, and ERS-mediated. HJD treatment for OA can inhibit the inflammatory response by modulating the PI3K-Akt signaling pathway, the AGE-RAGE system, the TNF signaling pathway, the IL-17 signaling pathway, and the Apoptosis pathway, regulating the cell cycle and controlling the metabolic response of osteoblasts, thereby alleviating or preventing the degeneration of articular cartilage. Studies have shown that HJD regulation of lncRNA MALAT1 is closely related to the expression of NLRP3, Caspase-1, IL-1β, and IL-18. LIN team255 experiments found that the protein content expression of NLRP3, Caspase-1, IL-1β, and IL-18 was significantly reduced in the experimental group intervened by HJD, and the level of lncRNA MALAT1 was significantly increased. levels were significantly higher, and overexpression of MALAT1 could improve cell viability and inhibit apoptosis and pyroptosis, which also indicated that HJD could inhibit cartilage matrix degradation and joint inflammation caused by inflammatory factors by up-regulating lncRNA MALAT1, thus inhibiting the development of KOA.
Guilu Erxian Gum
Guilu Erxian Gum contains tortoise shell, antler, ginseng, and wolfberry, mainly comprising collagen, amino acids, trace elements, ginseng saponin, Lycium berry polysaccharides, and fatty acids. It has anti-inflammatory, analgesic, and immune regulatory effects. Gelatin can promote the proliferation of degenerated chondrocytes by activating autophagy in the AMPK/mTOR/ULK1 pathway, thereby reducing chondrocyte apoptosis. The Wu et al’s team256 found that turtle deer Erxian gum could inhibit DNMTs, reduce the methylation level of AMPK promoter region, promote AMPK expression, activate autophagy initiation complex ULK1 to inhibit ERS reaction in degenerated chondrocytes, regulate the balance of ECM catabolism and synthesis, and improve the ER homeostasis of chondrocytes, which could reduce chondrocyte apoptosis.
Modified Danggui Sini Tang
Modified Danggui Sini Tang is composed of Angelica sinensis, Gui Zhi, Paeonia lactiflora, Huai Niujia, Sichuan Mucuna pruriens, Boneset, Bone Marrow, Safflower, Honeysuckle, Tongcao, Jujubes, and Roasted Glycyrrhiza glabra. Modified Danggui Sini Tang can reduce PI3K overexpression by inhibiting DNMT3B, lowering the methylation level of CpG island in the promoter region of PI3K gene, blocking the abnormal activation of AKT/mTOR signaling, inhibiting the PI3K/AKT/mTOR pathway, and inducing the expression of autophagy genes Beclin 1 and LC3 to elevate the level of autophagy of osteoarthritic chondrocytes of knee osteoarthritis in order to alleviate the KOA Articular cartilage degeneration.257
Modified Yanghe Soup
Modified Yanghe Soup, a renowned prescription, is the distillation of over 40 years of medical practice experience by Professor Dong Jianwen. It has shown satisfactory clinical results in treating OA. It consists of ripened dihuang, cinnamon, deer antler gelatin, haitengteng, chickweed vine, ephedra, Sichuan cow’s knee, and baihuanzhi.258 Modified Yanghe Soup inhibited DNMT1, decreased the CpG methylation level in the promoter region of Nrf2 gene, promoted Nrf2 transcriptional activity, enhanced the expression of antioxidant enzymes (SOD, HO-1), and inhibited the transduction of NF-κB signaling pathway, scavenging of ROS and MDA, and up-regulated the expression of SOD, which effectively reduced the oxidative stress damage, cellular senescence, and inflammation in the chondrocytes, and reduced the cartilage It effectively reduced the oxidative stress damage of chondrocytes, cellular senescence and inflammation, reduced the apoptosis of chondrocytes and improved the function of extracellular matrix secretion. In addition, the serum of Jiawei Yanghe Tang may inhibit the apoptosis of chondrocytes by regulating the expression of Bcl-2/Bax protein. A number of studies have shown that the escape of ROS from mitochondria to the cytoplasm is closely related to the alteration of mitochondrial membrane permeability by Bax, and that excessive ROS directly damage cells and activate related regulators that regulate the expression of Bcl-2 and Bax.259,260 This suggests that the serum in Yanghe Tang with Jiaweiyanghe inhibits apoptosis by lowering the level of ROS, which affects the expression of both Bcl-2 and Bax.
Modified Buyang Huanwu Tang
Modified Buyang Huanwu Tang consists of ingredients such as Tonifying Yang Huanwu Tang plus Danshen, Dangshen, Hyssop, Coix lacryma-jobi, Henbane, Zeilan, Epimedium, Bacopa monnieri, and Cistanchis. Li et al’s team261 demonstrated that the combination of Astragalus and Angelica sinensis can act on apoptosis-related factors, thus indirectly participating in apoptotic activities. Cistanchis, hyssop, and bacopa monnieri have also been shown to inhibit apoptosis.262,263 In addition, studies related to the mechanism of action of tonifying yang and returning wu tang have found that the formula can down-regulate the expression of Bax protein and up-regulate the expression of Bcl-2 protein, which has anti-apoptotic effects. The mechanism of adding tonifying Yang Huiwu Tang has a certain protective effect on the articular cartilage of KOA, which reduces the degree of damage and improves the pathological changes of cartilage, and the mechanism may be related to the reduction of apoptosis of chondrocytes.264 In addition, ZHANG team265 found that the experimental finding that Modified Buyang Huanwu Tang and returning five soup gavage experimental group nerve function recovery, promote neuron regeneration, which suggests that the addition of Modified Buyang Huanwu Tang and returning five soup can up-regulate the expression of miR-26a-5p, regulate the PTEN/PI3K/Akt signaling pathway and activate the autophagy of chondrocytes to restore the joint homeostasis and slow down the progress of OA.
Yougui Pill
Yougui Pill was first published in the Ming Dynasty by Zhang Jingyue “Jingyue Quanshu”, from the “Jin Gui” Kidney Qi Pills minus Poria, Mudan Pi, Ze Xie this “three diarrhea”, plus antler gelatin, Cortex Eucommiae, Cuscuta chinensis, Chinese wolfberries and other large teams of tonic drugs, tailor-made, specializing in warming, warm tonic for one of the representatives of the formula of tonifying the Kidney-Yang.266 In Yougui Pill, Radix Rehmanniae Praeparata and Rhizoma Coptidis have the effects of anti-aging, reduction of oxygen free radicals, and enhancement of body immunity, etc. Deer antler gelatin contains peptides, amino acids, trace elements and other effective components that can play a promotional role in the proliferation of chondrocytes and inhibit apoptosis, accelerating the repair of damaged cartilages, and at the same time, it has an anti-inflammatory and analgesic effect that can inhibit the local inflammation and edema.267–269 Dulcimer, in addition to anti-inflammatory and anti-aging, has a better In addition to anti-inflammatory and anti-aging, Cortex Eucommiae also has a good effect on regulating bone metabolism, while ferulic acid and phospholipids in Angelica sinensis can improve microcirculation, increase the activity of antioxidant enzymes in the organism, and reduce apoptosis of chondrocytes, which can in turn alleviate OA.270 Yougui Pill can play a therapeutic role in KOA by regulating the release of inflammatory factors, reducing the inflammatory response, slowing down the degeneration of articular cartilage, inhibiting chondrocyte apoptosis, inhibiting the degradation of ECM, and regulating the metabolism of Ca2+ to slow down the degenerative damage of articular cartilage. Yougui Pill inhibits DNMT3A, reduces CpG hypermethylation in the promoter region of NF-κB p65 gene, blocks NF-κB nuclear translocation, reduces the release of pro-inflammatory factors such as TNF-α and IL-1β, promotes the restoration of the dynamic equilibrium between MMPs and inhibitors of metalloproteinases (TIMPs) that are broken by inflammatory factors, adjusts the degradation of cartilage matrix, inhibits the abnormal degradation of cartilage, and protects the chondrocytes. cells, slowing down the process of joint degeneration and KOA development.270
Gui Zhi Paeoniae Zhimu Tang
Guizhi Shaoyao Zhimu Tang (from Zhang Zhongjing’s “The Essentials of the Golden Chamber”) is composed of Guizhi, Zhimu, Atractylodes macrocephala, Paeonia lactiflora, Fenghuang, ginger, Ephedra, Prepared Epilobium, and Glycyrrhiza Uralensis. It has the effect of promoting yang to relieve paralysis, dispelling wind and expelling dampness, which is highly consistent with the pathogenesis of KOA.272 Gavage of Guizhi Paeoniae Zhimu Tang was able to inhibit DNA methyltransferase DNMT3B, reduce the level of CpG methylation in the promoter region of the PI3K gene, lift the episodic silencing, promote PI3K transcription and AKT/mTOR signaling activation, and enhance the autophagy of chondrocytes, as well as reduce the detachment of the cartilage layer and the loss of chondrocytes, and decrease the expression of pro-inflammatory factors at the detachment site, with a marked improvement in the pathologic morphology, and a significant improvement in the KOA symptoms were significantly relieved.272
Tao Ren Knee Kang Pills
Tao Ren Knee Kang Pills can increase ULK1 and Beclin-1 protein expression and LC-II/I ratio in cartilage tissues, lower the expression of PI3K, AKT, and mTOR genes, and reduce the ratios of p-PI3K, p-AKT, and p-mTOR proteins to the total. By inhibiting the PI3K/AKT/mTOR signaling pathway, it boosts chondrocyte autophagy and slows cartilage degeneration.273 Tao Ren Knee Kang Pills can increase ULK1 and Beclin-1 protein expression and LC-II/I ratio in cartilage tissues, reduce the expression level of PI3K, AKT and mTOR genes, and reduce the ratio of p-PI3K, p-AKT and p-mTOR proteins to the corresponding total proteins, and enhance chondrocyte autophagy and delay cartilage degeneration through the inhibition of PI3K/AKT/mTOR signaling pathway.273 Some studies have found that Tao Ren Knee Kang Pills can play a role in inhibiting chondrocyte ECM by inhibiting MMP-9 generation and activation, and at the same time can also inhibit M1 macrophage inflammatory factors by inhibiting M1 macrophages. It has been found that Tao Ren Knee Kang Pills can inhibit chondrocyte ECM by inhibiting the generation and activation of MMP-9, and at the same time, Tao Ren Knee Kang Pills can inhibit inflammation by inhibiting the generation of inflammatory factors in M1 macrophages, which can play a role in the treatment of OA. In addition, Tao Ren Knee Kang Pills can prevent the activation of Caspase-3 cleavage, which has a significant inhibitory effect on the apoptosis of chondrocytes.274
Anti-Swelling and Analgesic Combinations
The anti-swelling and analgesic combinations, comprising Angelica sinensis, Radix Rehmanniae Praeparata, Rhizoma Ligustici Chuanxiong, Radix Paeoniae Alba, Radix et Rhizoma Polygoni Multiflori, Safflower, Panax Ginseng, Cortex Pseudostemonis Macrocephalae, Mullein, and Zelandra, is effective in dispelling wind, removing dampness, activating blood circulation, and relieving pain. It is mainly used to treat bone paralysis of the liver and kidney deficiency and tendon and vein stagnation type. This anti-swelling and analgesic combination can reduce the expression of PI3K, AKT, and Caspase-3 genes and proteins, significantly decrease the number of inflammatory cytokines in the serum and cartilage of KOA model rats, and inhibit chondrocyte catabolism while enhancing their anabolism via the regulation of the PI3K/AKT/mTOR signaling pathway, thereby exerting a certain repairing effect on cartilage damage and slowing down articular cartilage degradation.36 Li et al team275 found that after treatment with the swelling-relieving and pain-relieving combination, the serum IL-6, MMP-3, COX-2 levels of KOA rats were reduced, the expression of LC3, Beclin1, p-AMPK was increased, and the expression of caspase-9, mTOR was reduced, indicating that the swelling-relieving and pain-relieving combination may be closely related to the p-AMPK/mTOR signaling pathway. It prevents KOA-induced cartilage damage by up-regulating the autophagy level of chondrocytes, which in turn promotes the repair of KOA articular cartilage damage, delays articular cartilage degeneration, and exerts a certain protective effect on cartilage. The DU team276 found that the swelling-relieving and pain-relieving synthetics were able to inhibit the expression changes of the lncRNA MALAT1-ERK/p38MAPK-AQP-4 axis. Pharmacological intervention reduces the expression of AQP-4, ERK, MALAT1, and p38MAPK genes in spinal cord tissues, thereby protecting against osteoarthritis.
Bone Paralysis Formula
The bone paralysis formula, which is a modified version of Gui Zhi Poria Pill, has functions like tonifying the kidney, resolving blood stasis, dispelling wind and dampness, and clearing collaterals to relieve pain. It consists of Gui Zhi, Poria, Dampy, Paeonia lactiflora, Peach kernel, Turmeric, Eucommia, Sequelia, Scorpion, and Roasted Licorice.277 Bone paralysis formula can enhance the activity of AKT, reduce the expression of Bax, enhance the activation of the downstream target protein Bcl-2, inhibit the DNA methyltransferase DNMT3B, reduce the level of CpG methylation in the promoter region of the PI3K gene, lift the apparent silencing, promote the activation of the PI3K transcription and the AKT/mTOR signaling, reduce the apoptosis of the KOA cartilage, improve the tissue morphology of the knee joint, and protect the articular cartilage.278 Quercetin, kaempferol, and β-sitosterol in Bone Paralysis Formula are likely to act on IL-17 signaling pathway, TNF signaling pathway, and NF-κB signaling pathway through IL-6, TNF, and PTGS2 targets to exert the therapeutic effect of KOA.279
Rongxin and Pain Relief Formula
Rongxin and Pain Relief Formula is from Chen Keji’s “Qing Palace Formulas Integration”, which consists of Cow Knee, Angelica Sinensis, Fenghuang, Qiangwu, Duhuo, etc. It has the effect of tonifying the liver and kidneys, dispelling wind-dampness, and removing paralysis and pain in the treatment of OA.280 FU and other teams found that Rongjin Fengyuan can inhibit DNA methyltransferase DNMT1, reduce CpG hypermethylation in the promoter region of PERK gene, reverse PERK over-activation, reduce the expression of pro-apoptotic factors, such as ATF4, GADD153 and so on, and reduce the apoptosis rate of chondrocytes, which can slow down chondrocyte degeneration process. Rongjianfenqian can mediate lncRNA MGC-Mirg to regulate ERS-related signaling molecules (PERK signaling pathway) to play the role of delaying chondrocyte degeneration, which can be a role for Rongjianfenqian for the treatment of OA.280
Warming Meridian and Collaterals Soup
Warming Meridian and Collaterals Soup consists of Chuan Guizhi, Zedoary, Yanhuisuo, Chenpi, Centipede, Scorpion, Prepared Epiphyllum, Huai Niu Knee, Radix Rehmanniae Praeparata, Gallus gallus, Glycyrrhiza glabra, and Poria cocos. Warming Meridian and Collaterals Soup can markedly reduce IL-1β-induced chondrocyte apoptosis. Its mechanism might lie in regulating apoptotic factor release by up-regulating the PI3K/AKT signaling pathway, thereby exerting a protective effect on chondrocytes.281 Xu et al’s team282 found that Wenjing Tongluo Tang may play a therapeutic role by reducing cartilage matrix degradation through the VEGF/VEGFR2/ERK1/2 signaling pathway, attenuating cartilage defects, and slowing down the process of OA.
Zhuanggu Huoxue Tang
Zhuanggu Huoxue Tang is composed of Radix Rehmanniae Praeparata, Rhizoma Coptidis, Radix Achyranthis Bidentatae, Cortex Eucommiae, Cornu Cervi Pantotrichum, Radix Salviae Miltiorrhizae, Cortex Eucommiae, and Rhizoma Saffron. Zhuanggu Huoxue Tang can down-regulate PERK/eIF2α expression, further inhibit CHOP transcription and ERS, and reduce caspase 12 expression, effectively reducing chondrocyte apoptosis. Study283 by Cui et al team found that Zhuanggu Huoxue Tang modulated the PERK/Bip signaling pathway, inhibited ERS, decreased the expression of PERK, Bip, eIF-2α, ATF-4, GADD153, Caspase-9, and Caspase-3, and reduced the apoptosis of chondrocytes.
Active Knee Soup
Active Knee Soup is a clinical experience formula of Prof. Shao Xianfang, a renowned national veteran TCM practitioner, consisting of Duhuo, Sangsheng, Xu Duan, Eucommia, Boneset, Chuan Niu Xi, Gentian Macrophyllae, Poria, Hoshi, and Glycyrrhiza glabra.284 Some studies have indicated that it may inhibit Caspase-1-mediated chondrocyte pyroptosis via the CCL2/chemokine (C-C motif) receptor 2 (CCR2)/NF-κB signaling pathway. Xie et al team285 experiments applied the living knee soup intervention in rats cartilage degeneration was significantly improved, the expression level of CCL2, CCR2, Caspase-1, p-p65 decreased significantly, which indicates that the living knee soup through the inhibition of DNA methyltransferase DNMT3A, reduce the promoter region of the CCL2 gene CpG hypermethylation, reversing the overexpression of CCL2, blocking the monocyte chemotactic infiltration, inhibiting the activation of NF-κB signaling pathway, regulating Caspase-1-mediated chondrocyte death, and slowing down the degeneration of knee cartilage in rats. This suggests that the serum containing Living Knee Soup may down-regulate Caspase-1-mediated chondrocyte pyroptosis by inhibiting the CCL2/CCR2/NF-κB signaling axis.
Bushen Huoxue Formula
The Bushen Huoxue formula (BSHXF), a classic one for treating musculoskeletal pain and weakness, is based on the theories of “the Kidney governing the bones and producing marrow” and “when blood flows, blood stasis disappears”. BSHXF has the effect of tonifying the kidney, activating blood circulation, and removing blood stasis. It consists of Radix Rehmanniae Praeparata, Cortex Eucommiae, Fructus Lycii, Radix Bupleurum Chinense, Semen Cuscutae, Rhizoma Pinelliae, Rhizoma Myrrhizoma, Radix Angelicae Sinensis, Cornu Cervi Pantotrichum, and Herba Cistanches. Liu et al’s team286 and Li et al’s team287 showed that BSHXF could slow down cartilage damage, alleviate joint pain and swelling, prevent cartilage matrix destruction, and improve the metabolic environment within the articular cartilage. Liu et al’s team288 found that BSHXF significantly reduced the serum levels of IL-6, iNOS, and COX2 inflammatory factors in KOA mice, which suggests that its alleviation of the disease progression is related to the inhibition of inflammatory factor expression. Progression mechanism is related to the inhibition of inflammatory factor expression. Meanwhile, BSHXF was also found to inhibit DNMT3B, reduce CpG hypermethylation in the promoter region of the NF-κB p65 gene, block p65 nuclear translocation, reduce the expression of pro-inflammatory factors, such as IL-6 and COX-2, and inhibit the apoptosis of chondrocytes and the degradation of the extracellular matrix, thus alleviating the progression of KOA disease.
Peony Licorice and Fuzi Decoction
Peony Licorice and Fuzi Decoction has the functions of tonifying the liver and kidney, warming the meridians, clearing the veins, and balancing yang and yin. It is a classic formula for treating KOA.289 In the “Treatise on Typhoid Fever”, this soup consists of three herbs: Paeonia lactiflora for invigorating blood, softening the liver, and unclogging veins; Glycyrrhiza for tonifying the spleen and nourishing blood; and epimedium for warming the kidney and aiding yang. Bao et al’s team290 found that Paeonia lactiflora Glycyrrhiza appendiculata soup can inhibit the DNA methyltransferase DNMT3B by increasing the expression of Becbin1, lowering the level of CpG methylation in the promoter region of the PI3K gene, lifting the episodic silencing, and promoting the PI3K transcription and the activation of the AKT/mTOR signaling, which can increase the level of autophagy in chondrocytes, decrease the apoptosis of chondrocytes, and improve the patient’s condition and pain symptoms.
New Stop Bone Enlargement Pill
The New Stop Bone Enlargement Pill consists of Radix Rehmanniae Praeparata, Radix Bonesetae, Rhizoma Polygoni Multiflori, Hai Tong Pi, Cistanchiae Cistanches, Liu Shun Nu, Radix Achyranthis Bidentatae, and Ginger Sinensis, etc. Jin et al’s team291 found that Neoosteoporosis Pill reduced IL-6 and TNF-α secretion by inhibiting JAK2 and JAK2 phosphorylation as well as regulating the expression of SOCS1, which affected the JAK/STAT signaling pathway, and played a therapeutic role in the treatment of OA. Ma et al’s team292 found that Neoosteoporosis Pill could activate the regulation of the TGF-β/SMAD signaling pathway by activating miR-146a, thereby reducing the expression of IL-1β in rat knee cartilage and inhibiting inflammation and cartilage matrix degradation, thereby inhibiting the development of KOA. TGF-β/SMAD signaling pathway through activation of miR-146a, thus reducing the expression of IL-1β in the cartilage of rat knee joints, inhibiting inflammatory response and cartilage matrix degradation, and thus inhibiting the development of KOA. In addition, Bone Enhancement Pill can activate the TGF-β/SMAD signaling pathway, improve the expression of SMAD4 and TGF-β1, and promote the repair of damaged chondrocytes, which effectively treats the damage of articular cartilage.
Snow Lotus Strong Muscle and Bone Formula
The Snow Lotus Strong Muscle and Bone Formula consists of Snow Lotus, Prepared Rehmannia Root, Chinese Yam Rhizome, Osteophora Rhizoma, Pinellia Tuber, White Peony Root, Black Root, Ophiopogon Root, and Dillon Root. Zhao et al’s team293 found that snow lotus strong bone formula can block the p38MAPK signaling pathway in KOA chondrocytes. The mechanism is to inhibit DNA methyltransferase DNMT3B, reduce CpG hypermethylation in the promoter region of MAPK14 (p38α) gene, reverse p38MAPK overexpression, and block downstream pro-inflammatory signaling, which in turn leads to the loss and destruction of cartilage components and the generation of the corresponding clinical symptoms.294 At the same time, in the inflamed cartilage matrix, induction of MMP-13 expression through p38MAPK signaling pathway can lead to the loss of type II collagen. MMP-13 expression can lead to progressive degradation of type II collagen and accelerate cartilage destruction.295,296 Numerous studies have also demonstrated that chondrocyte apoptosis and degeneration can be suppressed by inhibiting p38MAPK activity.297
Ant Dragon Tongbi Decoction
Ant Dragon Tongbi Decoction is composed of edible ants, Prepared Chuanwu, Whole Scorpion, Ground Dragon, Boneset, and Black Bean. Wang et al’s teamteam298 found that after the application of ant dragon paralysis soup, it can inhibit DNMT3B, reduce the CpG hypermethylation in the promoter region of IL-1β gene, reverse the abnormally high expression of IL-1β, block NF-κB inflammatory signaling, and then inhibit the apoptosis of chondrocytes through the regulation of the ratio of Bcl-2/Bax expression, delay the degradation of cartilage matrix, and prevent the OA from further development of OA. The detailed mechanisms of epigenetic-based modulation of programmed chondrocyte death by the above herbal compounds are shown in Figure 8 and Table 8.
 |
Table 8 Compound Modulation of PCD in KOA Chondrocytes |
 |
Figure 8 Diagram of the molecular mechanism of complex regulation of KOA chondrocytes. By Figdraw. |
Conclusion and Outlook
Epigenetics offers a crucial scientific basis for understanding PCD of chondrocytes and its regulatory mechanisms in KOA. Through multiple regulatory mechanisms, including DNA methylation, histone modification, and non-coding RNAs, epigenetic alterations can profoundly influence the balance between chondrocyte survival and apoptosis. In KOA, the PCD of chondrocytes is often over-activated, leading to cartilage tissue degeneration and exacerbating the progression of the disease. In recent years, the integration of TCM and epigenetics has shown unique therapeutic advantages and promising development prospects. This interdisciplinary combination not only provides a novel scientific foundation for elucidating the “multi-component-multi-target” mechanism of TCM but also introduces a new therapeutic perspective of “epigenetic regulation.” By intervening in epigenetic processes like DNA methylation and histone modification, TCM can dynamically and reversibly regulate key disease pathways, such as the PCD of chondrocytes. This approach not only circumvents the ethical risks associated with gene therapy but also aligns with the body’s inherent regulatory principles. However, current research on epigenetics and herbal medicine for KOA still has significant knowledge gaps. Firstly, the systematic regulation of epigenetic mechanisms in KOA remains unclear. Specifically, there is insufficient evidence regarding cell - type - specific epigenetic reprogramming and the dynamic regulation of the chronic inflammation microenvironment. Secondly, the molecular mechanism underlying the synergistic effects of multiple components in Chinese herbal medicines through epigenetic targets lacks quantitative analysis. Moreover, the lack of standardization in herbs and preclinical modeling results in inconsistent therapeutic efficacy. Future research should integrate multi - omics technologies, such as epigenomics and metabolomics, and develop dynamic pathology models, including 3D organoids and single - cell epigenetic analysis. Additionally, it is essential to establish a precise delivery system for herbal medicines based on epigenetic biomarkers, such as cell - free DNA (cfDNA) methylation and miRNA profiles. These efforts will help uncover the cascading regulatory mechanism of the “component - epigenetic target - pathology network”, promoting the transition of combined Chinese and Western medicine treatment from an experience based approach to evidence based design. Ultimately, this will provide more effective treatment strategies for KOA patients.
Abbreviations
ABS, Achyranthes bidentata Bl Saponin; ACAN, aggregated proteoglycans; AcH3, acetylation modification level of histone 3; ACSL4, long-chain acyl-CoA synthetase 4; AFDR, The total flavonoids of Rhizoma Drynariae; AKT, protein kinase B; ALKBH5, alkylation repair homologue 5; AMPK, AMP-dependent protein kinase; AP, Angelica polysaccharides; APS, Astragalus polysaccharide; ASC, apoptosis-associated speck-like protein containing a CARD; AT, Artemisinin; ATF4, Activating Transcription Factor 4; Atg, target gene; ATR, Atractylodes macrocephala extract; AZA, 5-aza-2-deoxycytidine; BAI, Baicalein; BCL-2, B-cell lymphoma-2; BMP-7, bone morphogenetic protein-7; BMSCs, bone marrow mesenchymal stem cells; BRD4, bromodomain protein 4; BSHXF, Bushen Huoxue formula; CAD, Cardamom extract; C-AR, clematichinenoside AR; Caspase, cysteinyl aspartate specific proteinase; CCR2,chemokine (C-C motif) receptor 2; CoA, coenzyme A; COI II, collagen type II; COL10A1, type X collagen α1 chain; COL2A1, type II collagen fiber α1 gene; COL9A1, type IX collagen α1; COX-2, cyclooxygenase-2; CpG, Central pattern generators; CtBP, C-terminal binding protein; CXCR4, C-X-C chemokine receptor type 4; DAMPs, damage-associated molecular patterns; DAPK, death-associated protein kinase; DLAT, Dihydrolipoyl Transacetylase; DLD, dihydrolipoamide dehydrogenase; DLST, Dihydrolipoamide succinyltransferase; DNMT, DNA methyltransferase; DNMT1, DNA (cytosine-5)-methyltransferase 1; DNMT3A, DNA methyltransferase 3 alpha; DNMT3B, DNA methyltransferase 3B; Drp1, Dynamin-related protein 1; ECM, Extracellular matrix; EGLN2, prolyl hydroxylase 2; EMT, Epithelial-mesenchymal transition; ER, endoplasmic reticulum; ERS, reticulum stress; EWAS, Epigenomic linkage analysis; EZH2, Enhancer of Zeste Homolog 2; FADS2, fatty acid desaturase 2; FDX1, Ferredoxin 1; Fer-1, Ferrostatin-1; FLS, fibroblast-like synoviocytes; FPN1, Membrane iron transport protein; FSP1, Ferroptosis-suppressor protein 1; FTH, ferritin; FTO, Fat mass and obesity-associated protein; GDF5, growth differentiation factor 5; GLUT1, glucose transporter protein 1; GPX4, Glutathione Peroxidase 4; GSDMD, Gasdermin D; GSH, glomerular-stimulating hormone; H3K27ac, histone H3 lysine 27 acetylation; H3K4me3, histone H3 at lysine 4; HATs, histone acetyltransferases; HDAC4, recombinant histone deacetylase 4; HDACs, histone deacetylases; HDM, human histone demethylase; HJD, Huanglian Jiedu Decoction; Hmof, Human Males absent on the first; HOTAIR, HOX transcript antisense RNA; HOXA13, Homeobox A13; HMTs, histone methyltransferases; ICA, Icariin; IL1RN, interleukin 1 receptor antagonist; IL-1β, Interleukin-1β; iNOS, inducible nitric oxide synthase; INSR, insulin receptor; IREB2, iron-responsive element-binding protein 2; JAK2, Janus Kinase 2; KAT5, K(lysine) acetyltransferase 5; KDM1, histone lysine demethylase 1; KDM1A, lysine demethylase 1A; KDM3B, lysine demethylase 3B; KLF4; Kruppel-like factor −4; KOA, Knee osteoarthritis; LC3, light chain 3; LIAS, lipoic acid synthetase; LIP, Lymphocytic Interstitial Pneumonia; LIPT1, lipoyltransferase 1; lncRNA, Long non-coding RNA; lncRNA-RMRP, RNA component of mitochondrial RNA processing endoribonuclease; LSD1, Histone Lysine Specific Demethylase 1; m6A, N6-methyladenosine; MALAT1, metastasis associated lung adenocarcinoma transcript 1; MAPK, mitogen-activated protein kinase; MDA, Propylene Glycol; METTL14; Methyltransferase-like 14; METTL3; Methyltransferase-like 3; MiRNAs, MicroRNAs; MIA, monosodium iodoacetate; MLKL, Mixed Lineage Kinase Domain-Like; MMP13, matrix metalloproteinase 13; MMP3, matrix metalloproteinase 3; mTOR, Mammalian target of rapamycin; mTORC1, Mechanistic target of rapamycin complex 1; NAD+, nicotinamide adenine dinucleotide+; Nec-1, Necrostatin-1; NF-κB, Nuclear factor kappa-B; NLRP3, NOD-like receptor heat protein structural domain protein 3; NOR1, neuron-orphanin receptor 1; NRF2, Nuclear Factor Erythroid 2-Related Factor 2; NST-1s, Necrostatin-1s; OA, osteoarthritis; P53, Tumor Protein 53; PACER, Programmed Automatic Cardiac Pacing; PAMPs, pathogen associated molecular patterns; PCAF, P300/CBP-associated factor; PCD, programmed cell death; PCDHB14, procalcitonin β 14; PDHA1, Pyruvate dehydrogenase alpha 1; PDHB, Pyruvate Dehydrogenase Beta; PDK1, Pyruvate Dehydrogenase Kinase Isozyme 1; PDTC, Ammonium pyrrolidinedithiocarbamate; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PGE2, Prostaglandin E2; P, Phosphorylated; PI3K, Phosphatidylinositol 3-kinase; Pink1, phosphatase gene-induced kinase 1; PNE, nicotine exposure; PNS, Panax ginseng saponin; PPARα, Peroxisome Proliferator-Activated Receptor alpha; PPARγ, peroxisome proliferator-activated receptor γ; PQS, Panax quinquefolium saponin; PTEN, phosphatase and tensin homolog deleted on chromosome ten; PTGS2, prostaglandin-endoperoxide synthase 2; PTM, post-translational modification; QUE, Quercetin; RIP1, Receptor Interacting Protein 1; RIPK1, Receptor Interacting Protein Kinase 1; ROS, oxygen species; RUNX2, Runt-related transcription factor 2; SAH, S-adenosylhomocysteine; SASPs, senescence-associated secretory phenotypes; SCD1, stearoyl coenzyme A desaturase 1; SDC1, Recombinant Syndecan 1; SDF-1, Stromal Cell Derived Factor 1; SETDB1, SET domain, bifurcated 1; SIRT1, silent mating type information regulation 2 homolog; SLC3A2, Soluble Transporter Protein Family 3 Member 2; SLC31A1, Solute Carrier Family 31 Member 1; SLC7A11, Solute carrier family 7 member 11; SMAD2/3, Mothers against decapentaplegic homolog 2/3; SOCS2, Suppressors Of Cytokine Signaling 2; SOD, Superoxide Dismutase; SOD2, Recombinant Superoxide Dismutase 2, Mitochondrial; SOST, sclerostin; SOX4,sex-determiningregionY-relatedhighmobilitygroupbox4; SOX9, sex determining region Y-box 9; STAT3, Signal transducer and activator of transcription 3; SY, Safflower yellow pigment; TCM, traditional Chinese medicine; TET, Test and Evaluation Technology; TET2, 10-11 translocation 2; TGF, Transforming growth factor; TGF-β2, Transforming growth factor beta-2; TSG, tumor suppressor gene; TLR4, Toll-like receptor 4; TMJ, temporomandibular joint; TNF, tumor necrosis factor; TNF-α, tumor necrosis factor-α; TOLLR, Toll-like receptor; TRAF5, Recombinant TNF Receptor Associated Factor 5; TSC1, Tuberous Sclerosis Complex 1; TXNIP, thioredoxin interacting protein; UCA1, urothelial carcinoma associated 1; UGC, Upper Gastrointestinal Cancer; ULK1, Unc-51-like autophagy-activated kinase 1; VSMC, vascular smooth muscle cells; Wnt, Wingless-Type MMTV Integration Site Family; WNT11, MMTV integration site family member 11; YTHDC2, YTH structural domain’s protein 2; YTHDF1, YTH domain-containing family protein 1; ZEB1, zinc finger E-box binding protein 1.
Data Sharing Statement
The data used and analyzed in this study are included within the article.
Ethical Approval
This study did not involve human or animal subjects, and thus, no ethical approval was required. The study protocol adhered to the guidelines established by the journal.
Consent to Publish
All authors have carefully read and revised the full text and agreed to its publication.
Acknowledgement
Graphical abstract is drawn by Figdraw.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Self funded scientific research project of Guangxi Administration of Traditional Chinese Medicine (GXZYA20230146) and Guangxi Traditional Chinese Medicine Suitable Technology Development and Promotion Project(GZSY2024043).
Disclosure
All authors declare no financial or non-financial conflicts of interest related to the materials or methods described in this manuscript. The product/therapy mentioned in this study is cited for academic purposes only, and no endorsement or commercial association should be inferred.
References
1. Sharma L, Solomon CG. Osteoarthritis of the Knee. N Engl J Med. 2021;384(1):51–59. doi:10.1056/NEJMcp1903768
2. Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–1121. doi:10.1038/s41423-020-00630-3
3. Zhang RN, Sun ZJ, Zhang L. Pyroptosis in inflammatory bone diseases: molecular insights and targeting strategies. FASEB J. 2022;36(12):e22670. doi:10.1096/fj.202201229R
4. Esteller M. Cancer Epigenetics for the 21st Century: what’s Next? Genes Cancer. 2011;2(6):604–606. doi:10.1177/1947601911423096
5. Wang M, Liu L, Zhang CS, et al. Mechanism of Traditional Chinese Medicine in Treating Knee Osteoarthritis. J Pain Res. 2020;13:1421–1429. doi:10.2147/JPR.S247827
6. Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813(4):521–531. doi:10.1016/j.bbamcr.2010.12.019
7. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. doi:10.1038/nrm3722
8. Wang BW, Jiang Y, Yao ZL, Chen PS, Yu B, Wang SN. Aucubin Protects Chondrocytes Against IL-1β-Induced Apoptosis In Vitro And Inhibits Osteoarthritis In Mice Model. Drug Des Devel Ther. 2019;13:3529–3538. doi:10.2147/DDDT.S210220
9. Li D, Ni S, Miao KS, Zhuang C. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones. 2019;24(1):195–202. doi:10.1007/s12192-018-0956-4
10. Werry F, Mazur E, Theyse LFH, Edlich F. Apoptosis Regulation in Osteoarthritis and the Influence of Lipid Interactions. Int J Mol Sci. 2023;24(17):13028. doi:10.3390/ijms241713028
11. Peng Z, Sun H, Bunpetch V, et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268:120555. doi:10.1016/j.biomaterials.2020.120555
12. Kulkarni P, Martson A, Vidya R, Chitnavis S, Harsulkar A. Pathophysiological landscape of osteoarthritis. Adv Clin Chem. 2021;100:37–90.
13. Tateiwa D, Yoshikawa H, Kaito T. Cartilage and Bone Destruction in Arthritis: pathogenesis and Treatment Strategy: a Literature Review. Cells. 2019;8(8):818. doi:10.3390/cells8080818
14. Park DR, Kim J, Kim GM. Osteoclast-associated receptor blockade prevents articular cartilage destruction via chondrocyte apoptosis regulation. Nat Commun. 2020;11(1):4343. doi:10.1038/s41467-020-18208-y
15. Jimi E, Fei H, Nakatomi C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int J Mol Sci 20. 2019;20(24):6275. doi:10.3390/ijms20246275
16. Murahashi Y, Yano F, Kobayashi H, et al. Intra-articular administration of IκBα kinase inhibitor suppresses mouse knee osteoarthritis via downregulation of the NF-κB/HIF-2α axis. Sci Rep. 2018;8(1):16475. doi:10.1038/s41598-018-34830-9
17. Ma ZJ, Ma ML, Dong H, Yang B, Wang YX. Effects of cellular pyroptosis on bone homeostasis and prospects. Chin J Osteoporosis. 2022;28:922–926.
18. Zhang JW, Wang L, Zhao M, et al. Mechanisms of nucleotide-binding oligomerization structural domain-like receptor protein 3-mediated cellular focal death in gouty arthritis. Shanxi Med J. 2022;51:561–565.
19. Huang SS, Xiang XN, Yu X, He J. Mechanisms and effects of platelet-rich plasma-derived exosomes in the treatment of osteoarthritis of the knee. Chinese J Tissue Eng Res. 2023;27:2420–2426.
20. Li XY, Chen YY, Zhou MB, Wu KD, Jiang XC. The regulatory effects and mechanisms of chrysanthemum on cerebral ischemia-reperfusion injury in rats based on the classical pathway of NLRP3/Caspase-1 cell death. Acta Chinese Med. 2024;52:5–10.
21. Ding T, Yun S, Zhou YP, Yang WQ. miR-137 effects on IL-1β-induced inflammatory response and autophagy in chondrocytes and study of the mechanism. Chin J Immunol. 2019;35:2832–2836.
22. Benner RW, Shelbourne KD, Bauman SN, Norris A, Gray T. Knee Osteoarthritis: alternative Range of Motion Treatment. Orthop Clin North Am. 2019;50(4):425–432. doi:10.1016/j.ocl.2019.05.001
23. Zhao LR, Xing RL, Wang PM, et al. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP‑induced pyroptosis in knee osteoarthritis. Mol Med Rep. 2018;17(4):5463–5469. doi:10.3892/mmr.2018.8520
24. Jiang XY, Qian YH, J.l Z, et al. Effects of TCDCA on PGE2 secretion in fibroblast-like synoviocytes of AA rats stimulated with IL-1β. Livest Feed Sci. 2021;42:1–5.
25. Prein, C., Beier, F., 2019. ECM signaling in cartilage development and endochondral ossification. Curr Top Dev Biol 133, 25–47.
26. Zheng W, Li X, Liu D, et al. Mechanical loading mitigates osteoarthritis symptoms by regulating endoplasmic reticulum stress and autophagy. FASEB j. 2019;33(3):4077–4088. doi:10.1096/fj.201801851R
27. Duan R, Xie H, Liu ZZ. The Role of Autophagy in Osteoarthritis. Front Cell Dev Biol. 2020;8:608388. doi:10.3389/fcell.2020.608388
28. Xu Y, Wan W. Acetylation in the regulation of autophagy. Autophagy. 2023;19(2):379–387. doi:10.1080/15548627.2022.2062112
29. Jakhar R, Paul S, Bhardwaj M, Kang SC. Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis. Cancer Lett. 2016;372(1):89–100. doi:10.1016/j.canlet.2015.12.024
30. Sun K, Luo J, Guo F, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2020;28(4):400–409. doi:10.1016/j.joca.2020.02.027
31. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi:10.1038/nrm.2017.95
32. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi:10.1038/ncb2152
33. Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801. doi:10.1002/art.27305
34. Barranco C. Osteoarthritis: activate autophagy to prevent cartilage degeneration? Nat Rev Rheumatol. 2015;11(3):127. doi:10.1038/nrrheum.2015.12
35. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282. doi:10.1038/s41580-020-00324-8
36. Jing SW, Liu T, Song Y, Li L, Zhou WW, Liu PL. Experimental study on the effect of the swelling-eliminating and pain-relieving combination on the PI3K/Akt signaling pathway in the cartilage of rat osteoarthritis of the knee. Lishizhen Med Mater Med Res. 2019;30:40–42.
37. Sun Y, Chen P, Zhai B, et al. The emerging role of ferroptosis in inflammation. Biomed Pharmacother. 2020;127:110108. doi:10.1016/j.biopha.2020.110108
38. Yao X, Sun K, Yu S, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Translat. 2021;27:33–43. doi:10.1016/j.jot.2020.09.006
39. Zhou X, Zheng Y, Sun W, et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner. Cell Prolif. 2021;54(11):e13134. doi:10.1111/cpr.13134
40. Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity. 2017;47(1):51–65.e57. doi:10.1016/j.immuni.2017.06.001
41. Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28(1):9–21. doi:10.1038/cr.2017.133
42. Jeon J, Noh HJ, Lee H, et al. TRIM24-RIP3 axis perturbation accelerates osteoarthritis pathogenesis. Ann Rheum Dis. 2020;79(12):1635–1643. doi:10.1136/annrheumdis-2020-217904
43. Zheng X, Qiu J, Pan W, et al. Selumetinib - a potential small molecule inhibitor for osteoarthritis treatment. Front Pharmacol. 2022;13:938133. doi:10.3389/fphar.2022.938133
44. Zhang C, Lin S, Li T. Mechanical force-mediated pathological cartilage thinning is regulated by necroptosis and apoptosis. Osteoarthritis Cartilage. 2017;25(8):1324–1334. doi:10.1016/j.joca.2017.03.018
45. Liang S, Lv ZT, Zhang JM, et al. Necrostatin-1 Attenuates Trauma-Induced Mouse Osteoarthritis and IL-1β Induced Apoptosis via HMGB1/TLR4/SDF-1 in Primary Mouse Chondrocytes. Front Pharmacol. 2018;9:1378. doi:10.3389/fphar.2018.01378
46. Tsvetkov P. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;378:eadf5804.
47. Chang B, Hu Z, Chen L, Jin Z, Yang Y. Development and validation of cuproptosis-related genes in synovitis during osteoarthritis progress. Front Immunol. 2023;14:1090596. doi:10.3389/fimmu.2023.1090596
48. Zhu Y, Wu G, Yan W, Zhan H, Sun P. miR-146b-5p regulates cell growth, invasion, and metabolism by targeting PDHB in colorectal cancer. Am J Cancer Res. 2017;7(5):1136–1150.
49. Park S, Baek IJ, Ryu JH, Chun CH, Jin EJ. PPARα-ACOT12 axis is responsible for maintaining cartilage homeostasis through modulating de novo lipogenesis. Nat Commun. 2022;13(1):3. doi:10.1038/s41467-021-27738-y
50. Schamel M, Bernhardt A, Quade M, et al. Cu(2+), Co(2+) and Cr(3+) doping of a calcium phosphate cement influences materials properties and response of human mesenchymal stromal cells. Mater Sci Eng C Mater Biol Appl. 2017;73:99–110. doi:10.1016/j.msec.2016.12.052
51. Young DA, Barter MJ, Soul J. Osteoarthritis year in review: genetics, genomics, epigenetics. Osteoarthritis Cartilage. 2022;30(2):216–225. doi:10.1016/j.joca.2021.11.004
52. Simon TC, Jeffries MA. The Epigenomic Landscape in Osteoarthritis. Curr Rheumatol Rep. 2017;19(6):30. doi:10.1007/s11926-017-0661-9
53. Dou P, He Y, Yu B, Duan J. Downregulation of microRNA-29b by DNMT3B decelerates chondrocyte apoptosis and the progression of osteoarthritis via PTHLH/CDK4/RUNX2 axis. Aging (Albany NY). 2020;13(5):7676–7690. doi:10.18632/aging.103778
54. Zhang X, Zhao S, Yuan Q, et al. TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression. Cell Death Dis. 2021;12(7):642. doi:10.1038/s41419-021-03930-2
55. Liu Q, Li M, Fu B, Jiang R, Fu B. METTL3 promotes experimental osteoarthritis development by regulating inflammatory response and apoptosis in chondrocyte. Biochem Biophys Res Commun. 2019;516(1):22–27. doi:10.1016/j.bbrc.2019.05.168
56. Shi L, Hu H, Sun P, et al. RPL38 knockdown inhibits the inflammation and apoptosis in chondrocytes through regulating METTL3-mediated SOCS2 m6A modification in osteoarthritis. Inflamm Res. 2022;71(7–8):977–989. doi:10.1007/s00011-022-01579-x
57. Barter MJ, Bui C, Young DA. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis Cartilage. 2012;20(5):339–349. doi:10.1016/j.joca.2011.12.012
58. Guo L, Zhuo X, Lu C, et al. The N-terminal fragment of histone deacetylase 4 (1-669aa) promotes chondrocyte apoptosis via the p53-dependent endoplasmic reticulum stress pathway. J Cell Mol Med. 2024;28(20):e70135. doi:10.1111/jcmm.70135
59. Gu X, Li F, Che X, Li P, Li P. HDAC4 represses ER stress induced chondrocyte apoptosis by inhibiting ATF4 and attenuates cartilage degeneration in an osteoarthritis rat model. BMC Musculoskelet Disord. 2024;25(1):467. doi:10.1186/s12891-024-07578-9
60. Abouheif MM, Nakasa T, Shibuya H, Niimoto T, Kongcharoensombat W, Ochi M. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro. Rheumatology (Oxford). 2010;49(11):2054–2060. doi:10.1093/rheumatology/keq247
61. Huang Z, Zhang N, Ma W, Dai X, Liu J. MiR-337-3p promotes chondrocytes proliferation and inhibits apoptosis by regulating PTEN/AKT axis in osteoarthritis. Biomed Pharmacother. 2017;95:1194–1200. doi:10.1016/j.biopha.2017.09.016
62. Wang B, Fan X, Ma C, Lei H, Long Q, Chai Y. Downregulation of KDM4A Suppresses the Survival of Glioma Cells by Promoting Autophagy. J Mol Neurosci. 2016;60(2):137–144. doi:10.1007/s12031-016-0796-6
63. Gu W, Shi Z, Song G, Zhang H. MicroRNA-199-3p up-regulation enhances chondrocyte proliferation and inhibits apoptosis in knee osteoarthritis via DNMT3A repression. Inflamm Res. 2021;70(2):171–182. doi:10.1007/s00011-020-01430-1
64. Zhao G, Gu W. Effects of miR-146a-5p on chondrocyte interleukin-1 β-induced inflammation and apoptosis involving thioredoxin interacting protein regulation. J Int Med Res. 2020;48(11):300060520969550. doi:10.1177/0300060520969550
65. He B, Jiang D. HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol Int. 2020;44(2):524–535. doi:10.1002/cbin.11253
66. Chen Y, Zhang L, Li E, et al. Long-chain non-coding RNA HOTAIR promotes the progression of osteoarthritis via sponging miR-20b/PTEN axis. Life Sci. 2020;253:117685. doi:10.1016/j.lfs.2020.117685
67. Zhu YJ, Jiang DM. LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. Eur Rev Med Pharmacol Sci. 2019;23(19):8175–8185. doi:10.26355/eurrev_201910_19124
68. Song M, Li J, Sun J, et al. DNMT1-mediated DNA methylation in toll-like receptor 4 (TLR4) inactivates NF-κB signal pathway-triggered pyroptotic cell death and cellular inflammation to ameliorate lipopolysaccharides (LPS)-induced osteomyelitis. Mol Cell Probes. 2023;71:101922. doi:10.1016/j.mcp.2023.101922
69. Sun X, Xiao L, Chen J, et al. DNA methylation is involved in the pathogenesis of osteoarthritis by regulating CtBP expression and CtBP-mediated signaling. Int J Biol Sci. 2020;16(6):994–1009. doi:10.7150/ijbs.39945
70. Xiong X, Xiong H, Peng J, Liu Y, Zong Y. METTL3 Regulates the m(6)A Modification of NEK7 to Inhibit the Formation of Osteoarthritis. Cartilage. 2023;2023:19476035231200336.
71. Sharif H, Wang L, Wang WL, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570(7761):338–343. doi:10.1038/s41586-019-1295-z
72. Andreeva L, Rawson S, Rawson S, et al. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell. 2021;184(26):6299–6312.e6222. doi:10.1016/j.cell.2021.11.011
73. Xu H, Xu B. BMSC-Derived Exosomes Ameliorate Osteoarthritis by Inhibiting Pyroptosis of Cartilage via Delivering miR-326 Targeting HDAC3 and STAT1//NF-κB p65 to Chondrocytes. Mediators Inflamm. 2021;2021:9972805. doi:10.1155/2021/9972805
74. He H, Chen J, Hua Y, et al. α7-nAChR/P300/NLRP3-regulated pyroptosis mediated poor articular cartilage quality induced by prenatal nicotine exposure in female offspring rats. Chem Biol Interact. 2024;400:111183. doi:10.1016/j.cbi.2024.111183
75. Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3:e3740. doi:10.1371/journal.pone.0003740
76. Nicolas FE, Pais H, Schwach F, et al. mRNA expression profiling reveals conserved and non-conserved miR-140 targets. RNA Biol. 2011;8(4):607–615. doi:10.4161/rna.8.4.15390
77. Sun H, Li JJ, Feng ZR, Liu HY, Meng AG. MicroRNA-124 regulates cell pyroptosis during cerebral ischemia-reperfusion injury by regulating STAT3. Exp Ther Med. 2020;20(6):227. doi:10.3892/etm.2020.9357
78. Feng L, Feng C, Wang CX, et al. Circulating microRNA let‑7e is decreased in knee osteoarthritis, accompanied by elevated apoptosis and reduced autophagy. Int J Mol Med. 2020;45(5):1464–1476. doi:10.3892/ijmm.2020.4534
79. Portal-Núñez S, Esbrit P, Alcaraz MJ, Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol. 2016;108:1–10. doi:10.1016/j.bcp.2015.12.012
80. Degtyarev M, De Mazière A, Orr C, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183(1):101–116. doi:10.1083/jcb.200801099
81. Arico S, Petiot A, Bauvy C, et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276(38):35243–35246. doi:10.1074/jbc.C100319200
82. He Y, Wang W, Xu X, et al. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through mediating Bcl2 stability via Ythdf1-mediated m(6)A modification. Bone. 2022;154:116182. doi:10.1016/j.bone.2021.116182
83. Meyer KD, Jaffrey SR. Rethinking m 6 A Readers, Writers, and Erasers. Annu Rev Cell Dev Biol. 2017;33(1):319–342. doi:10.1146/annurev-cellbio-100616-060758
84. Sang W, Xue S, Jiang Y, et al. METTL3 involves the progression of osteoarthritis probably by affecting ECM degradation and regulating the inflammatory response. Life Sci. 2021;278:119528. doi:10.1016/j.lfs.2021.119528
85. Wei Y, Han T, Wang R, et al. LSD1 negatively regulates autophagy through the mTOR signaling pathway in ovarian cancer cells. Oncol Rep. 2018;40(1):425–433. doi:10.3892/or.2018.6432
86. Shu F, Xiao H, Li QN, et al. Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):32. doi:10.1038/s41392-022-01300-8
87. Sun X, Mi L, Du G, Sun C, He S. Platelet-rich plasma treatment alleviates osteoarthritis-related pain, inflammation, and apoptosis by upregulating the expression levels of microRNA-375 and microRNA-337. Immunopharmacol Immunotoxicol. 2022;44(1):87–98. doi:10.1080/08923973.2021.2007263
88. Jia D, Li Y, Han R, et al. miR‑146a‑5p expression is upregulated by the CXCR4 antagonist TN14003 and attenuates SDF‑1‑induced cartilage degradation. Mol Med Rep. 2019;19(5):4388–4400. doi:10.3892/mmr.2019.10076
89. Liu A, Liu S. Noncoding RNAs in Growth and Death of Cancer Cells. Adv Exp Med Biol. 2016;927:137–172.
90. Xu J, Xia Y, Zhang H, Guo H, Feng K, Zhang C. Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomed Pharmacother. 2018;101:691–697. doi:10.1016/j.biopha.2018.02.134
91. Zeng Z, Li T, Liu X, et al. DNA dioxygenases TET2 deficiency promotes cigarette smoke induced chronic obstructive pulmonary disease by inducing ferroptosis of lung epithelial cell. Redox Biol. 2023;67:102916. doi:10.1016/j.redox.2023.102916
92. Liu Y, Ouyang L, Mao C, et al. PCDHB14 promotes ferroptosis and is a novel tumor suppressor in hepatocellular carcinoma. Oncogene. 2022;41(27):3570–3583. doi:10.1038/s41388-022-02370-2
93. Liu L, He J, Sun G, et al. The N6-methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin Transl Med. 2022;12(5):e778. doi:10.1002/ctm2.778
94. Deng L, Di Y, Chen C, et al. Depletion of the N(6)-Methyladenosine (m6A) reader protein IGF2BP3 induces ferroptosis in glioma by modulating the expression of GPX4. Cell Death Dis. 2024;15(3):181. doi:10.1038/s41419-024-06486-z
95. Xia H, Wu Y, Zhao J, et al. N6-Methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023;30(5):1293–1304. doi:10.1038/s41418-023-01138-9
96. Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019;10(5):331. doi:10.1038/s41419-019-1564-7
97. Zhang X, Sui S, Wang L, et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J Cell Physiol. 2020;235(4):3425–3437. doi:10.1002/jcp.29232
98. Li H, Liu W, Zhang X, Wu F, Sun D, Wang Z. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Biochem Biophys Res Commun. 2021;585:111–116. doi:10.1016/j.bbrc.2021.11.029
99. Koppula P, Zhuang L, Gan B, et al. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599–620. doi:10.1007/s13238-020-00789-5
100. Zhang H, Deng T, Liu R, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19(1):43. doi:10.1186/s12943-020-01168-8
101. Gomaa A, Peng D, Chen Z, et al. Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers. Sci Rep. 2019;9(1):16970. doi:10.1038/s41598-019-53174-6
102. Wang Z, Chen X, Liu N, et al. A Nuclear Long Non-Coding RNA LINC00618 Accelerates Ferroptosis in a Manner Dependent upon Apoptosis. Mol Ther. 2021;29(1):263–274. doi:10.1016/j.ymthe.2020.09.024
103. Wang M, Mao C, Ouyang L, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26(11):2329–2343. doi:10.1038/s41418-019-0304-y
104. Xu Y, Xia C, Xu Y, Xia C. PLCγ1 inhibition combined with inhibition of apoptosis and necroptosis increases cartilage matrix synthesis in IL-1β-treated rat chondrocytes. FEBS Open Bio. 2021;11(2):435–445. doi:10.1002/2211-5463.13064
105. Liu J, van Mil A, Vrijsen K, et al. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J Cell Mol Med. 2011;15(7):1474–1482. doi:10.1111/j.1582-4934.2010.01104.x
106. Bozgeyik E, Bagis H, Bozgeyik I, Kocahan S. The roles of long non-coding RNAs in the necroptotic signaling of colon cancer cells. Mol Biol Rep. 2023;50(6):5021–5028. doi:10.1007/s11033-023-08441-1
107. Sun L, Zhang Y, Yang B, et al. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14(1):6523. doi:10.1038/s41467-023-42025-8
108. Chen L, He H, Shao F, Gao Y, He J. Systemic Analyses of Cuproptosis-Related lncRNAs in Pancreatic Adenocarcinoma, with a Focus on the Molecular Mechanism of LINC00853. Int J Mol Sci. 2023;24:1.
109. Cai Z, Long T, Zhao Y, Lin R, Wang Y. Epigenetic Regulation in Knee Osteoarthritis. Front Genet. 2022;13:942982. doi:10.3389/fgene.2022.942982
110. Park CS, Shen Y, Lewis A, Lacorazza HD. Role of the reprogramming factor KLF4 in blood formation. J Leukoc Biol. 2016;99(5):673–685. doi:10.1189/jlb.1RU1215-539R
111. Cota P, Helmi SA, Hsu C, Rancourt DE. Cytokine Directed Chondroblast Trans-Differentiation: JAK Inhibition Facilitates Direct Reprogramming of Fibroblasts to Chondroblasts. Cells. 2020;9(1):191. doi:10.3390/cells9010191
112. Cheng Z, Zou X, Jin Y, et al. The Role of KLF(4) in Alzheimer’s Disease. Front Cell Neurosci. 2018;12:325. doi:10.3389/fncel.2018.00325
113. Chang SF, Huang KC, Chang HI, Lee KC, Su YP, Chen CN. 2 dyn/cm 2 shear force upregulates kruppel-like factor 4 expression in human chondrocytes to inhibit the interleukin-1β-activated nuclear factor-κB. J Cell Physiol. 2018;234(1):958–968. doi:10.1002/jcp.26924
114. Pöschl E, Fidler A, Schmidt B, Kallipolitou A, Schmid E, Aigner T. DNA methylation is not likely to be responsible for aggrecan down regulation in aged or osteoarthritic cartilage. Ann Rheum Dis. 2005;64(3):477–480. doi:10.1136/ard.2004.022509
115. Moazedi-Fuerst FC, Hofner M, Gruber G, et al. Epigenetic differences in human cartilage between mild and severe OA. J Orthop Res. 2014;32(12):1636–1645. doi:10.1002/jor.22722
116. Kim KI, Park YS, Im GI. Changes in the epigenetic status of the SOX-9 promoter in human osteoarthritic cartilage. J Bone Miner Res. 2013;28(5):1050–1060. doi:10.1002/jbmr.1843
117. Reynard LN, Bui C, Canty-Laird EG, Young DA, Loughlin J. Expression of the osteoarthritis-associated gene GDF5 is modulated epigenetically by DNA methylation. Hum Mol Genet. 2011;20(17):3450–3460. doi:10.1093/hmg/ddr253
118. Murayama A, Sakura K, Nakama M, et al. A specific CpG site demethylation in the human interleukin 2 gene promoter is an epigenetic memory. EMBO j. 2006;25(5):1081–1092. doi:10.1038/sj.emboj.7601012
119. de Andrés MC, Imagawa K, Hashimoto K, et al. Suppressors of cytokine signalling (SOCS) are reduced in osteoarthritis. Biochem Biophys Res Commun. 2011;407(1):54–59. doi:10.1016/j.bbrc.2011.02.101
120. Chubinskaya S, Otten L, Soeder S, et al. Regulation of chondrocyte gene expression by osteogenic protein-1. Arthritis Res Ther. 2011;13(2):R55. doi:10.1186/ar3300
121. Zhu X, Chen F, Lu K, Wei A, Jiang Q, Cao W. PPARγ preservation via promoter demethylation alleviates osteoarthritis in mice. Ann Rheum Dis. 2019;78(10):1420–1429. doi:10.1136/annrheumdis-2018-214940
122. Kihara A, Kabeya Y, Yoshimori T, Yoshimori T. Beclin–phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2(4):330–335. doi:10.1093/embo-reports/kve061
123. Zhong Y, Wang QJ, Li X, et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11(4):468–476. doi:10.1038/ncb1854
124. Li Z, Chen B, Wu Y, Jin F, Xia Y, Liu X. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010;10(1):98. doi:10.1186/1471-2407-10-98
125. Wesselborg S, Alessi DR, Stork B. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy. 2011;7(7):696–706. doi:10.4161/auto.7.7.15451
126. Li W, Li X, Wang W, et al. NOR1 is an HSF1- and NRF1-regulated putative tumor suppressor inactivated by promoter hypermethylation in nasopharyngeal carcinoma. Carcinogenesis. 2011;32(9):1305–1314. doi:10.1093/carcin/bgr174
127. Jiang Y, Mao C, Yang R, et al. EGLN1/c-Myc Induced Lymphoid-Specific Helicase Inhibits Ferroptosis through Lipid Metabolic Gene Expression Changes. Theranostics. 2017;7(13):3293–3305. doi:10.7150/thno.19988
128. Xuan Y, Wang H, Yung MM, et al. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics. 2022;12(7):3534–3552. doi:10.7150/thno.70194
129. Kim DH, Kim WD, Kim SK, Moon DH, Lee SJ. TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis. 2020;11(5):406. doi:10.1038/s41419-020-2618-6
130. Hasegawa M, Takahashi H, Rajabi H, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7(11):11756–11769. doi:10.18632/oncotarget.7598
131. Du Y, Bu N, Zhan S, Bu N, Zhan S, Bu N. Downregulation of ELAVL1 attenuates ferroptosis-induced neuronal impairment in rats with cerebral ischemia/reperfusion via reducing DNMT3B-dependent PINK1 methylation. Metab Brain Dis. 2022;37(8):2763–2775. doi:10.1007/s11011-022-01080-8
132. Zhou Y, Fan W, Fan W, et al. Principles of RNA methylation and their implications for biology and medicine. Biomed Pharmacother. 2020;131:110731. doi:10.1016/j.biopha.2020.110731
133. Hu S, Zhao X, Mao G, et al. MicroRNA-455-3p promotes TGF-β signaling and inhibits osteoarthritis development by directly targeting PAK2. Exp Mol Med. 2019;51(10):1–13. doi:10.1038/s12276-019-0322-3
134. Lasman L, Hanna JH. m(6)A deposition: a boost from TGFβ. Cell Res. 2018;28(5):505–506. doi:10.1038/s41422-018-0037-3
135. Bertero A, Brown S, Madrigal P, et al. The SMAD2/3 interactome reveals that TGFβ controls m(6)A mRNA methylation in pluripotency. Nature. 2018;555(7695):256–259. doi:10.1038/nature25784
136. Chen X, Gong W, Shao X, et al. METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann Rheum Dis. 2022;81(1):87–99. doi:10.1136/annrheumdis-2021-221091
137. Li R, Guo LX, Li Y, et al. Dose-response characteristics of Clematis triterpenoid saponins and clematichinenoside AR in rheumatoid arthritis rats by liquid chromatography/mass spectrometry-based serum and urine metabolomics. J Pharm Biomed Anal. 2017;136:81–91. doi:10.1016/j.jpba.2016.12.037
138. Lee DY, Teyssier C, Strahl BD, Stallcup MR. Role of protein methylation in regulation of transcription. Endocr Rev. 2005;26(2):147–170. doi:10.1210/er.2004-0008
139. Monteagudo S, Cornelis FMF, Aznar-Lopez C, et al. DOT1L safeguards cartilage homeostasis and protects against osteoarthritis. Nat Commun. 2017;8(1):15889. doi:10.1038/ncomms15889
140. Jiang Y, Song S, Liu J, et al. Epigenetic regulation of programmed cell death in hypoxia-induced pulmonary arterial hypertension. Front Immunol. 2023;14:1206452. doi:10.3389/fimmu.2023.1206452
141. Cai JY. Histone acetylation modification regulates the mechanism of autophagy in traction stress-mediated osteogenesis of bone seam stem cells. J Med Biomech. 2024;39:246.
142. Sun L, Dong H, Zhang W, et al. Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms. DNA Cell Biol. 2021;40(2):172–183. doi:10.1089/dna.2020.5730
143. Liu Q, Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43(11):1245–1256. doi:10.1002/cbin.11121
144. Chu B, Kon N, Chen D, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21(5):579–591. doi:10.1038/s41556-019-0305-6
145. Zheng J, Liu H, Guo HY, Pan SJ, Zhang PF. Epigenetic regulation and progress in osteoarthritis research. Chinese J Pain Med. 2014;20:665–667+670.
146. Wang XB, Zhao FC, Yi LH, et al. MicroRNA-21-5p as a novel therapeutic target for osteoarthritis. Rheumatology. 2019;58(8):1485–1497. doi:10.1093/rheumatology/kez102
147. Jiang M, Liu J, Luo T, Chen Q, Lu M, Meng D. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci Rep. 2019;39(6). doi:10.1042/BSR20190404
148. Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol 220. 2021;220(2). doi:10.1083/jcb.202009045
149. Lu JF, Qi LG, Zhu XB, Shen YX. LncRNA RMRP knockdown promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-206/CDK9 axis. Pharmazie. 2020;75(10):500–504. doi:10.1691/ph.2020.0591
150. Liu W, Liu A, Li X, et al. Dual-engineered cartilage-targeting extracellular vesicles derived from mesenchymal stem cells enhance osteoarthritis treatment via miR-223/NLRP3/pyroptosis axis: toward a precision therapy. Bioact Mater. 2023;30:169–183. doi:10.1016/j.bioactmat.2023.06.012
151. Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. miRNA-335-5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci. 2019;226:164–172. doi:10.1016/j.lfs.2019.03.071
152. Tian F, Wang J, Zhang Z, Yang J. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol Res. 2020;53(1):9. doi:10.1186/s40659-020-00275-6
153. Yu Q, Zhao B, He Q, Zhang Y, Peng XB. microRNA-206 is required for osteoarthritis development through its effect on apoptosis and autophagy of articular chondrocytes via modulating the phosphoinositide 3-kinase/protein kinase B-mTOR pathway by targeting insulin-like growth factor-1. J Cell Biochem. 2019;120(4):5287–5303. doi:10.1002/jcb.27803
154. Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi:10.1038/s41586-019-1707-0
155. Wang JX, Jiao JQ, Li Q, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17(1):71–78. doi:10.1038/nm.2282
156. Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39(4):493–506. doi:10.1016/j.molcel.2010.07.023
157. Ye H, Liu X, Lv M, et al. MicroRNA and transcription factor co-regulatory network analysis reveals miR-19 inhibits CYLD in T-cell acute lymphoblastic leukemia. Nucleic Acids Res. 2012;40(12):5201–5214. doi:10.1093/nar/gks175
158. Basu A, Schell J, Scofield RH. Dietary fruits and arthritis. Food Funct. 2018;9(1):70–77. doi:10.1039/C7FO01435J
159. Burdeos GC, Blank R, Wolffram S. Influence of quercetin on the global DNA methylation pattern in pigs. Food Funct. 2020;11(9):7421–7426. doi:10.1039/D0FO00896F
160. Liu CM, Ma JQ, Xie WR, et al. Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-κB pathway. Food Chem Toxicol. 2015;82:19–26. doi:10.1016/j.fct.2015.05.001
161. Shakibaei M, John T, Seifarth C, Mobasheri A. Resveratrol Inhibits IL-1β–Induced Stimulation of Caspase-3 and Cleavage of PARP in Human Articular Chondrocytes in Vitro. Ann N Y Acad Sci. 2007;1095(1):554–563. doi:10.1196/annals.1397.060
162. Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26(12):1643–1648. doi:10.1002/jor.20683
163. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi:10.1073/pnas.0605298103
164. Qiu L, Luo Y, Chen X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed Pharmacother. 2018;103:1585–1591. doi:10.1016/j.biopha.2018.05.003
165. Lepetsos P, Papavassiliou KA, Papavassiliou AG. Redox and NF-κB signaling in osteoarthritis. Free Radic Biol Med. 2019;132:90–100. doi:10.1016/j.freeradbiomed.2018.09.025
166. Farooqi AA, Kapanova G, Kalmakhanov S, et al. Regulation of Cell Signaling Pathways and Non-Coding RNAs by Baicalein in Different Cancers. Int J Mol Sci. 2022;23:2.
167. Li Y, Wang J, Song X, et al. Effects of baicalein on IL-1β-induced inflammation and apoptosis in rat articular chondrocytes. Oncotarget. 2017;8(53):90781–90795. doi:10.18632/oncotarget.21796
168. Jiang H, Yao Q, An Y, Fan L, Wang J, Li H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m(6)A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase3 pathway. Phytomedicine. 2022;94:153823. doi:10.1016/j.phymed.2021.153823
169. Zhang X, Zhu Y, Chen X, et al. Baicalein ameliorates inflammatory-related apoptotic and catabolic phenotypes in human chondrocytes. Int Immunopharmacol. 2014;21(2):301–308. doi:10.1016/j.intimp.2014.05.006
170. Zhou RK, Chen CH, Li H. Clinical observation on oral treatment of patients with osteoarthritis of the knee with total flavonoids of osteoclasts. China Med Her. 2011;8:77–78.
171. Jin H. Observations on the efficacy of saffron yellow pigment combined with low molecular heparin calcium in the treatment of acute cerebral infarction. Chin Folk Ther. 2016;24:57–58.
172. Yang M, Zhang F, Pang T, Han J, Zhang RK. Protective effect of baiyangin on IL-1β-induced chondrocyte damage by regulating SNHG9/miR-184. Chinese J Gerontol. 2023;43:1974–1980.
173. Raina R, Almutary AG, Bagabir SA, et al. Chrysin Modulates Aberrant Epigenetic Variations and Hampers Migratory Behavior of Human Cervical (HeLa) Cells. Front Genet. 2021;12:768130. doi:10.3389/fgene.2021.768130
174. Yang Y, Shi YC, Shu JW, Yu L, Hu W. Paederin inhibits LPS-induced autophagy in chondrocytes via the PI3K/AKT signaling pathway. Chinese Pharmacol Bull. 2021;37:662–668.
175. Ji T, Ye L, Xi E, Liu Y, Wang X, Wang S. Sinensetin Inhibits Angiogenesis in Lung Adenocarcinoma via the miR-374c-5p/VEGF-A/VEGFR-2/AKT Axis. Cell Biochem Biophys. 2024;82(3):2413–2425. doi:10.1007/s12013-024-01352-3
176. Imran M, Rauf A, Shah ZA, et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: a comprehensive review. Phytother Res. 2019;33(2):263–275. doi:10.1002/ptr.6227
177. Panahi Y, Alishiri GH, Bayat N, Hosseini SM, Sahebkar A. Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: a randomized controlled trial. Excli J. 2016;15:203–210. doi:10.17179/excli2015-639
178. Huang X, Pan Q, Mao Z, et al. Kaempferol inhibits interleukin‑1β stimulated matrix metalloproteinases by suppressing the MAPK‑associated ERK and P38 signaling pathways. Mol Med Rep. 2018;18(3):2697–2704. doi:10.3892/mmr.2018.9280
179. Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016;2016(1):1245049. doi:10.1155/2016/1245049
180. Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–591. doi:10.1016/j.bbadis.2016.01.003
181. Yin SY, Wang DS, Yan LY, Wang SB, G.h D. Total flavonoids of Cow’s Tongue inhibit inflammatory vesicle activation against myocardial ischemia-reperfusion injury via ROS/TXNIP/NLRP3. Chinese J Pharmacol Toxicol. 2021;35:802.
182. Wang F, Mei QC, Feng J, Li HJ, Wang BY, Li HZ. Ameliorative effects of kaempferol modulating ROS/TXNIP pathway on chondrocyte oxidation and inflammatory injury in rats with knee osteoarthritis. Chinese J Gerontol. 2024;44:229–233.
183. Jiang R, Hao P, Yu G, et al. Kaempferol protects chondrogenic ATDC5 cells against inflammatory injury triggered by lipopolysaccharide through down-regulating miR-146a. Int Immunopharmacol. 2019;69:373–381. doi:10.1016/j.intimp.2019.02.014
184. Jiang CX, Yu CM, Li ZK. Curcumin analogs inhibit ERK/JNK and NF-κB signaling pathways to exert anti-inflammatory activity. Chin Herb Med. 2016;47:2871–2876.
185. Kim JM, Noh EM, Kwon KB, et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKCα-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine. 2012;19(12):1085–1092. doi:10.1016/j.phymed.2012.07.002
186. Bolduc JA, Loeser RF, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82. doi:10.1016/j.freeradbiomed.2018.08.038
187. Li XS, Chen H, Zhen P, et al. JAK2/STAT3 signal pathway mediating curcumin in cartilage cell metabolism of osteoarthritis. Zhongguo Gu Shang. 2016;29(12):1104–1109. doi:10.3969/j.issn.1003-0034.2016.12.008
188. Yang Y, Duan W, Liang Z, et al. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cell Signal. 2013;25(3):615–629. doi:10.1016/j.cellsig.2012.11.025
189. Guo K, Yue XX, Jiang JY, et al. Effects of curcumin on proliferation and apoptosis of osteoarthritic chondrocytes by regulating miR-1227 expression. Chinese J Gerontol. 2024;44:3808–3813.
190. Han G, Zhang Y, Li H, Cordani M. The Combination Treatment of Curcumin and Probucol Protects Chondrocytes from TNF- α Induced Inflammation by Enhancing Autophagy and Reducing Apoptosis via the PI3K-Akt-mTOR Pathway. Oxid Med Cell Longev. 2021;2021(1):5558066. doi:10.1155/2021/5558066
191. Qiu B, Xu X, Yi P, Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020;24(18):10855–10865. doi:10.1111/jcmm.15714
192. Li S, Lukas C, Lukas C, et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res Ther. 2021;12(1):252. doi:10.1186/s13287-021-02317-6
193. Wu Z, Zhang X, Zhang X, et al. Retraction Note: chondro-protective effects of polydatin in osteoarthritis through its effect on restoring dysregulated autophagy via modulating MAPK, and PI3K/Akt signaling pathways. Sci Rep. 2020;10:11511. doi:10.1038/s41598-020-68880-9
194. Zhang Y, Cai W, Han G, et al. [Corrigendum] Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K‑AKT‑mTOR pathway in osteoarthritic chondrocytes. Int J Mol Med. 2022;49(3). doi:10.3892/ijmm.2022.5085.
195. Hu Y, Wu L, Jiang L, et al. Notoginsenoside R2 reduces A β 25-35-induced neuronal apoptosis and inflammation via miR-27a/SOX8/ β -catenin axis. Hum Exp Toxicol. 2021;40:S347–s358. doi:10.1177/09603271211041996
196. Xie JJ, Guo SK, Guo S-K, et al. Panax quinquefolium saponin inhibits endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and attenuates the progression of osteoarthritis in rat. Biomed Pharmacother. 2018;97:886–894. doi:10.1016/j.biopha.2017.10.068
197. Lin PD, Weng XP, Li XH, Ye HZ. Exploration of the mechanism of action of the active components of hyssop against osteoarthritis. Arthritis Rheum-Us. 2015;4:56–59.
198. Gao K, Zhang Y, Chen DY, et al. Total saponins of bovine knee intervene in the expression of synovial fluid-derived cytokines in rabbit knee osteoarthritis. Chinese J Tissue Eng Res. 2019;23:5317–5321.
199. Yu T, Peng LP, Ma DJ, et al. Effects of total saponins of cow’s knee on synovial tissue of experimental rabbit knee osteoarthritis. Chinese J Trad Med Traumatol Orthod. 2017;25:1–5.
200. Na S, Duan Chen FY, Wang L, Chen GL. Preventive effect and mechanism of total saponins from cow’s knee on acute gouty arthritis in rats. Chinese J Clin Pharmacol Therapeut. 2017;22:966–971.
201. Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003;33(3):155–167. doi:10.1016/S0049-0172(03)00134-3
202. Guo P, Guo X. Effects of Paeonia lactiflora glycosides on gene expression of interleukins and their receptors in bone marrow cells of mice with radiation-induced blood deficiency. Pharmacol Clin Chinese Mater Med. 2009;25:25–27.
203. Hu PF, Sun FF, Jiang LF, Bao JP, Wu LD. Paeoniflorin inhibits IL-1β-induced MMP secretion via the NF-κB pathway in chondrocytes. Exp Ther Med. 2018;16(2):1513–1519. doi:10.3892/etm.2018.6325
204. Wu L, Tang R, Xiong W, Song S, Guo Q, Zhang Q. Paeoniflorin shows chondroprotective effects under IL-1β stress by regulating circ-PREX1/miR-140-3p/WNT5B axis. J Orthop Surg Res. 2023;18(1):766. doi:10.1186/s13018-023-04238-x
205. Wan H, Li C, Yang Y, Chen D. Loganin attenuates interleukin-1 β -induced chondrocyte inflammation, cartilage degeneration, and rat synovial inflammation by regulating TLR4/MyD88/NF-κB. J Int Med Res. 2022;50(8):3000605221104764. doi:10.1177/03000605221104764
206. Zheng X, Qiu J, Gao N, et al. Paroxetine Attenuates Chondrocyte Pyroptosis and Inhibits Osteoclast Formation by Inhibiting NF-κB Pathway Activation to Delay Osteoarthritis Progression. Drug Des Devel Ther. 2023;17:2383–2399. doi:10.2147/DDDT.S417598
207. Liu PJ, Tang KH, Sun L, Chen ZF. Effect of gentianoside on extracellular matrix of cartilage in osteoarthritis. World J Trad Chinese. 2024;19:957–961.
208. Ye J. Protective effects and mechanisms of total cycloenol ether terpene glycosides and gentian bitter glycosides in yellow pipe gentiana on IL-1β-induced chondrocyte damage in rats. GSUTCM. 2014.
209. Li CX, Zhang LZ, et al. Peach coralloid glycosides inhibit apoptosis of cardiomyocytes through activation of the ERβ pathway. Chinese Pharmacol Bull. 2021;37:68–74.
210. Cao JT, Dong YR, Chou CC, Jiang Y, Zhao LG. Enzymatic preparation of coralline sapogenins from Peach Leaf Coral and its stability. For Chem Ind. 2021;41:33–39.
211. Sun XM, Bratton SB, Butterworth M, Cohen GM, Cohen GM. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J Biol Chem. 2002;277(13):11345–11351. doi:10.1074/jbc.M109893200
212. van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16(2):203–213. doi:10.1038/sj.cr.7310028
213. Luan YJ, Wang L, Jia W. Experimental study of peach leaf coral glycosides inhibiting interleukin-1β-induced chondrocyte apoptosis and slowing down osteoarthritis disease progression in mice in vitro. Shanxi Med J. 2022;51:1055–1059+1065.
214. Li NP, Tu DX, Kuang GY, et al. Effects of lingxian xinoside on apoptosis and autophagy of chondrocytes in knee osteoarthritis. Chinese J Inform Trad Chinese Med. 2023;30:96–101.
215. Wang X, Zhou D, Zhou W, et al. Clematichinenoside AR inhibits the pathology of rheumatoid arthritis by blocking the circPTN/miR-145-5p/FZD4 signal axis. Int Immunopharmacol. 2022;113:109376. doi:10.1016/j.intimp.2022.109376
216. Zhang Y, Sun B, Huang Z, Zhao DW, Zeng Q. Shikonin Inhibites Migration and Invasion of Thyroid Cancer Cells by Downregulating DNMT1. Med Sci Monit. 2018;24:661–670. doi:10.12659/MSM.908381
217. Chen C, Zheng RQ, Zhang GC. Effects of rhodopsin on proliferation, apoptosis and oxidative stress in osteoarthritic chondrocytes. Chinese J Tissue Eng Res. 2024;28:4528–4534.
218. Wu YZ, Yao Y, Yang WX, Wu N, Zhou M, Huang ZF. Investigation of the effects of Angelica sinensis polysaccharides on LPS-mediated osteoarthritis in SD rats based on NF-κB signaling pathway. Chinese Arch Trad Chinese Med. 2024;42:136–140+284–288.
219. Qin Y, Cao F. Astragalus polysaccharide reduces brain injury in SAH rats by inhibiting inflammatory response. Cardiovasc Dis J Integr Trad Chinese West Med. 2020;8:65–67.
220. Sun QL, Ju J, Wang H, et al. Experimental study on the intervention of astragalus polysaccharide in diabetic myocardial oxidative stress. Chinese J Integr Med. 2020;40:196–203.
221. Zhao JY, Zhu XL, et al. Effects of astragalus polysaccharide on apoptosis induced by high glucose peritoneal dialysis solution in HMrSV5 cells. Chinese Arch Trad Chinese Med. 2020;38:113–117+280–281.
222. Gao WJ, Lin PC. Astragali polysaccharides regulate the proliferation and apoptosis of human knee osteoarthritic chondrocytes through the JAK2/STAT3 pathway. J Med Res. 2022;51:67–71+91.
223. Liu J, Liu J, Duan S, Liu L, Zhang G, Peng X. Reprogrammed Epigenetic Landscape-Prophesied Functions of Bioactive Polysaccharides in Alleviating Diseases: a Pilot Study of DNA Methylome Remodeling in Astragalus Polysaccharide (APS)-Improved Osteoporosis in a Rat Model. J Agric Food Chem. 2020;68(52):15449–15459. doi:10.1021/acs.jafc.0c06483
224. Zheng J, Yuan PW, Zhao LP, Bian MJ, Kang WL. Study on the mechanism of cymbidium alkaloids regulating the level of cartilage autophagy in knee OA rabbits through PI3K/AKt-mTOR signaling pathway. J Liaoning Univ Trad Chin Med. 2019;21:30–33.
225. Dong HC, Li PN, Chen CJ, et al. Sinomenine Attenuates Cartilage Degeneration by Regulating miR-223-3p/NLRP3 Inflammasome Signaling. Inflammation. 2019;42(4):1265–1275. doi:10.1007/s10753-019-00986-3
226. Zu Y, Zhang ST, Zhang ST, Yan HJ, Yan H-J. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res. 2019;14(1):307. doi:10.1186/s13018-019-1307-6
227. Zhao W, Yu HH, Meng WW, et al. Icariin restrains NLRP3 inflammasome-mediated Th2 immune responses and ameliorates atopic dermatitis through modulating a novel lncRNA MALAT1/miR-124-3p axis. Pharm Biol. 2023;61(1):1249–1259. doi:10.1080/13880209.2023.2244004
228. Fu Y, Lei J, Zhuang Y, Zhang K, Lu D. Overexpression of HMGB1 A-box reduced IL-1β-induced MMP expression and the production of inflammatory mediators in human chondrocytes. Exp Cell Res. 2016;349(1):184–190. doi:10.1016/j.yexcr.2016.10.014
229. Tang Y, Li Y, Xin D, Chen L, Xiong Z, Yu X. Icariin alleviates osteoarthritis by regulating autophagy of chondrocytes by mediating PI3K/AKT/mTOR signaling. Bioengineered. 2021;12(1):2984–2999. doi:10.1080/21655979.2021.1943602
230. Liu ZD. Role and mechanism of sweet Orange flavonoids in delaying knee osteoarthritis by regulating SERPINA3. 2023. doi:10.27441/d.cnki.gyzdu.2023.000083
231. Yan Z, Qi W, Zhan J, et al. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J Cell Mol Med. 2020;24(22):13046–13057. doi:10.1111/jcmm.15905
232. Pan C, Chen H, Yang B. Licochalcone A Inhibits Proliferation and Metastasis of Colon Cancer by Regulating miR-1270/ADAM9/Akt/NF-κB axis. Iran J Public Health. 2023;52(9):1962–1972. doi:10.18502/ijph.v52i9.13578
233. Hu CN. Modulatory effects and mechanisms of licorice chalcone A on rat chondrocytes mimicking the OA process. ZJU. 2016.
234. Wang C, Gao Y, Zhang Z, et al. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-κB/SIRT1/AMPK signaling pathways. Phytomedicine. 2020;78:153305. doi:10.1016/j.phymed.2020.153305
235. Yan CL, An FY, Jin H, et al. Effects of selenium-enriched Astragalus saponin on the mTOR signaling pathway in IL-1β-induced degenerating chondrocytes in rats. Chinese J Osteoporosis Bone Miner Res. 2021;14:382–388.
236. Xia PF, Yan CL, An FY, et al. Effects of selenium-enriched astragali saponin on the proliferation of degenerated chondrocytes in rats and its mechanism. Chinese J Clin Pharmacol. 2020;36:639–642.
237. Shen XJ, Cheng L, Liu L. Effects of cangrelorin-regulated adenylate-activated protein kinase/silent information regulatory factor 1 signaling pathway on autophagy and apoptosis induced by interleukin-1β in chondrocytes. Anhui Med. 2024;28:899–903+1064.
238. Sheng Y, Zhai R, Li S, et al. Enhanced understanding of cinnamaldehyde’s therapeutic potential in osteoarthritis through bioinformatics and mechanistic validation of its anti-apoptotic effect. Front Med (Lausanne). 2024;11:1448937. doi:10.3389/fmed.2024.1448937
239. Gong Z, Wang Y, Qiu B, Hu Y, Qiu B, Hu Y. Cardamonin alleviates chondrocytes inflammation and cartilage degradation of osteoarthritis by inhibiting ferroptosis via p53 pathway. Food Chem Toxicol. 2023;174:113644. doi:10.1016/j.fct.2023.113644
240. Li J, Jiang M, Yu Z, et al. Artemisinin relieves osteoarthritis by activating mitochondrial autophagy through reducing TNFSF11 expression and inhibiting PI3K/AKT/mTOR signaling in cartilage. Cell Mol Biol Lett. 2022;27(1):62. doi:10.1186/s11658-022-00365-1
241. Wu SJ, Tseng WC. Progress of clinical application of Duiyu parasitic soup in orthopedics and traumatology. Massage Rehabil Med. 2022;13:72–75.
242. Wei Y, Liang W, Liu S, Wang ZG, Rong B, S.q C. Efficacy of Dui Wu Sheng Tang in treating patients with knee osteoarthritis of liver and kidney deficiency type and its effect on SDF-1/CXCR4 signaling pathway in serum and joint fluid. Pharmacol Clini Chinese Mater Med. 2019;35:171–174.
243. He YH, Li YL, Xiang YY, Chen YJ, Yang S. Role of SDF-1/CXCR4 and downstream signaling pathways in the course of osteoarthritis. J Pract Med. 2019;35:3563–3567.
244. Cao Y, Tang S, Nie X, et al. Decreased miR-214-3p activates NF-κB pathway and aggravates osteoarthritis progression. EBioMedicine. 2021;65:103283. doi:10.1016/j.ebiom.2021.103283
245. Xu LM, Dong DY, Wang YZ, Huang XY. Regulation of miR-214-3p by Dok-Ho Parasite Soup inhibits iron death in chondrocytes. Journal of Chinese Medicine. 2024;39:2487–2492.
246. Zheng RX, Wu GW, Chen J, Qiu JQ. Effects of Dokou Sheng Tang on SDF-1/CXCR4 signaling pathway in rats with knee osteoarthritis. Asia Pac Trad Med. 2020;16:6–9.
247. Chen WJ. Mechanism study of regulating NLRP3/NF-κB signaling pathway in knee osteoarthritis treatment by sole living parasitic soup. NJUCM. 2018.
248. Jia J, Rong B, Li J, Sun P, Chen SQ. Effects of serum containing drug from Dokou Sangsheng Tang on chondrocyte metabolism,BMP-7 and SIRT1 expression in rats with osteoarthritis of the knee. Chin J Exp Tradit Med Form. 2017;23:159–165.
249. Chen CX, Zhong WH, Jin LL, et al. Inhibition of oxidative stress in knee osteoarthritis chondrocytes by aconite soup. Arthritis Rheum-Us. 2022;11:1–4+15.
250. Fu YM, Li CL, Tu YY, Chen HH, Zhong Y. Mechanism study on the inhibition of chondrocyte pyroptosis by modulation of lncRNA MALAT1/miR-124 pathway by Wu-Tou Tang. Trad Chinese Med Rehabil. 2024;1:9–15.
251. Liu LM, Peng W, Wu MQ, Tu W. Mechanism of the effect of aconite soup on the expression of matrix metalloproteinase 13 and tumor necrosis factor-α in osteoarthritic chondrocytes. Chinese J Trad Med Traumatol Orthod. 2024;32:12–19.
252. Takano S, Uchida K, Shoji S, et al. Vascular Endothelial Growth Factor Is Regulated by the Canonical and Noncanonical Transforming Growth Factor-β Pathway in Synovial Fibroblasts Derived from Osteoarthritis Patients. Biomed Res Int. 2019;2019:6959056. doi:10.1155/2019/6959056
253. Sanajou D, Ghorbani Haghjo A, Argani H, Aslani S. AGE-RAGE axis blockade in diabetic nephropathy: current status and future directions. Eur J Pharmacol. 2018;833:158–164. doi:10.1016/j.ejphar.2018.06.001
254. Ci YY. Exploring the effector substances and mechanism of action of Huanglian Xieyu Tang in the treatment of osteoarthritis based on network pharmacology-metabolomics. 2022. doi:10.27162/d.cnki.gjlin.2022.005004
255. Lin ZY, Wen MM, T.z X. Experimental study on the regulation of lncRNA MALAT1 by Huang Lian Xie-Tu Tang to reduce the inflammatory death of abscess wound in mice. Fujian Traditional Chinese Medicine. 2024;55:17–20.
256. Wu WX, Zheng ZP, Gu FC, et al. Effects of tortoise and deer erxian gum on endoplasmic reticulum homeostasis of degenerated chondrocytes from rats with osteoarthritis of the knee based on ATF6/GRP78/CHOP signaling pathway. Pharmacol Clini Chinese Mater Med. 2024;40:33–40.
257. Wang YT, Xie YZ, Fan XH, Yu Y. Effects of adding flavor of Angelica Sinensis Siwei Tang on cartilage degeneration in rats with knee osteoarthritis and its mechanism of action. Chinese J Trad Med Traumatol Orthod. 2022;30:7–12.
258. Xiao YP, Zeng J, Jiao LN, Xu XY. Progress in the treatment of osteoporosis and its signaling pathway regulation by kidney tonic herbs. Chin J Chin Mater Med. 2018;43:21–30.
259. Collins JA, Wood ST, Nelson KJ, et al. Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes. J Biol Chem. 2016;291(13):6641–6654. doi:10.1074/jbc.M115.693523
260. Rodier F, Coppé JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi:10.1038/ncb1909
261. Li XQ, Gao Y, Qin XM. Study on the molecular mechanism of “Astragalus and Angelica sinensis” in Astragalus-containing formulas and the core drugs. Chin Herb Med. 2019;50:5273–5281.
262. Huang T, Zhang GL, Li N. Effects of bacopa monnieri polysaccharides on apoptosis of rabbit chondrocytes cultured in vitro. J Beijing Sport Univ. 2010;33:56–61.
263. Ma DJ, Peng LP, Cao YF, et al. Experimental study on the proliferation and apoptosis of chondrocytes in osteoarthritis in vitro by total saponin-containing joint fluid from cow’s knee. Chinese J Trad Med Traumatol Orthod. 2019;27:1–5.
264. Cai KR, Liu ZX, Sun XD, Yan L. Protective effects and mechanisms of Cistanchia polysaccharides on cerebral neuronal cells in D-galactose-induced failure mice. Chinese J Gerontol. 2018;38:4732–4734.
265. Zhang XH, Fu KL, Lin K, Li N, Yao ML, Zheng GY. Buyang Huanwu Decoction attenuates cerebral ischemia-reperfusion injury in rats via miR-26a-5p mediated PTEN/PI3K/Akt signaling pathway. Zhongguo Zhong Yao Za Zhi. 2024;49(15):4197–4206. doi:10.19540/j.cnki.cjcmm.20240515.501
266. Yang YJ, Ren YL, Zheng Q, et al. Progress of the mechanism of action of Diqui Pill in the treatment of osteoarthritis of the knee. Chinese Arch Trad Chinese Med. 2022;40:59–62.
267. Shao LM, Xu SW. Advances in chemical composition and modern pharmacological studies of yam. Acta Chinese Med. 2017;45:125–127.
268. Bao Y, Gao JT, Sun JM, Zhang H. Progress in the study of Chinese herbal antler gum. Jilin Chinese Med. 2016;36:173–175+204.
269. Zhang HQ, Xiao M, Liu Y. Progress in the treatment of osteoarthritis with single-flavored traditional Chinese medicine. Chinese J Trad Med Traumatol Orthod. 2012;20:65–67.
270. Zhang B. Mechanistic study on the MEK/ERK signaling pathway-based mediation of cartilage proliferation and differentiation in osteoarthritis of the knee by Deguiwan. 2023. doi:10.27213/d.cnki.glnzc.2023.000381
271. Xia CM, Xu B, Li G, et al. Exploring the molecular mechanism of Guizhi, Paeoniae, and Zhimu Tang in the treatment of osteoarthritis based on network pharmacology. Chinese Arch Trad Chinese Med. 2018;36:2681–2684.
272. Zhang FM, Zhang DY. Study on the mechanism of action of Gui Zhi Paeoniae Zhimu Tang in treating rats with knee osteoarthritis model based on P13K/Akt/mTOR signaling pathway. J Hubei Univ Chin Med. 2019;21:21–25.
273. Qin N, Wei LW, Zhang H. Mechanism of activation of chondrocyte autophagy against osteoarthritis of the knee by Tao Ren Knee Kang Wan through PI3K/AKT/mTOR signaling pathway. Pharmacol Clini Chinese Mater Med. 2021;37:165–169.
274. Chen BL, Cheng Y, Ling CY, Luo YG. Effects of Tao Ren Knee Kang Wan on osteoarthritis-related cytokines and chondrocyte apoptosis. Clin J Chin Med. 2024;16:71–76.
275. Li JP, Liu T, He ZJ, Li Y, Chen W, Song Y. Experimental study on the effect of the swelling-eliminating and pain-relieving combination on the AMPK/mTOR signaling pathway in the cartilage of rat osteoarthritis of the knee. Chin J Osteoporosis. 2020;26:231–235.
276. Du KR. Protective effect of Longzhong Swelling and Pain Relief Combination on secondary spinal cord injury and expression of LncRNAMALAT1-ERKp38MAPK-AQP4 axis. 2021. doi:10.27026/d.cnki.ggszc.2021.000114
277. Li N, Lin CS, Yang WY, Liu MY. Clinical study of Bone Paralysis Formula for the treatment of knee osteoarthritis. Lishizhen Med Mater Med Res. 2016;27:120–122.
278. He XJ, Wang L, Wang RR, Qiu YJ. Effects of Bone Paralysis Formula on PI3K/AKt Signaling Pathway and Tissue Morphology in Rat Knee Osteoarthritis. Journal of Nanjing University of Traditional Chinese Medicine. 2018;34:157–161.
279. Liu DK, Liu MY, Liu XB, et al. Exploration of the network pharmacological mechanism of Bone Paralysis Formula for the treatment of knee osteoarthritis. Trad Chin Drug Res Clin Pharmacol. 2020;31:306–314.
280. Fu CL, Xie XY, Qiu ZW, et al. Rong-Jin-Feng-Pin formula modulates lncRNA MGC-Mirg to alleviate endoplasmic reticulum stress in osteoarthritic chondrocytes. Chin J Trad Chin Med Pharm. 2023;38:2561–2566.
281. Xu Q, Xu WM, Zhao PR, et al. Effects of Wenjing Tongluo Tang-containing serum on IL-1β-induced chondrocyte apoptosis via PI3K/AKT signaling pathway. Lishizhen Med Mater Med Res. 2022;33:2583–2587.
282. Xu Q, Qian JJ, Xu WM, et al. Effects of Wen Jing Tongluo Tang on VEGF/VEGFR2/ERK1/2 signaling pathway in mice with knee osteoarthritis model. Chin J Exp Tradit Med Form. 2021;27:28–34.
283. Cui HX, Zheng ZY, Guo JY, Cao XY, Liu YF, Guo ML. Mechanisms of endoplasmic reticulum stress-mediated apoptosis of human chondrocytes mediated by Strong Bone and Blood Tang in the prevention of osteoarthritis of the knee joint. Chin J Trad Chin Med Pharm. 2022;37:4814–4818.
284. Appleton CT, McErlain DD, Pitelka V, et al. Forced mobilization accelerates pathogenesis: characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9(1):R13. doi:10.1186/ar2120
285. Xie F, Liu YL, Li SD, et al. Mechanisms of the action of the Living Knee Soup on the death of chondrocytes in a rat model of osteoarthritis of the knee. J Trad Chin Med Univ Hunan. 2023;43:225–231.
286. Liu W, Wu YH, Liu XY, Xue B, Shen W, Yang K. Metabolic regulatory and anti-oxidative effects of modified Bushen Huoxue decoction on experimental rabbit model of osteoarthritis. Chin J Integr Med. 2013;19(6):459–463. doi:10.1007/s11655-011-0727-x
287. Li X, Xiao FJ, Li Z, et al. Mechanisms of cartilage repair in mice with knee osteoarthritis intervened by a formula of tonifying the kidney and activating blood. Chin Trad Pat Med. 2022;44:582–586.
288. Liu XL, Zhang Z, Yao JL, Mao T. Effects of tonifying the kidney and activating blood formula modulating the NF-κB signaling pathway on inflammation and apoptosis in cartilage tissues of osteoarthritic mice with knee osteoarthritis. Cent S Pharm. 2024;22:1725–1731.
289. Chen R, Lin DY, Fu YQ, Guan QZ, Gu S. Immunomodulatory effects of Paeoniae Lactiflorae, Glycyrrhiza Uralensis and Fructus Schisandrae chinensis Soup on rheumatoid arthritis rats. Chinese Arch Trad Chinese Med. 2008;2008:1081–1083.
290. Bao DS, Guo XG, Qin B, Liu G, Zeng SQ. Clinical study on regulation of chondrocyte apoptosis and autophagy by Paeoniaeum, Glycyrrhiza glabra and Epiphyllum Tang in the treatment of osteoarthritis of the knee. Henan Trad Chin Med. 2021;41:1495–1499.
291. Jin LF, Shi J, Wang XT, Ma N, Chen H, Liu CY. Effects of Xinzhi Osteoproliferative Pill on JAK2, P-JAK2 expression and SOCS1 negative feedback in JAK-STAT signaling pathway of osteoarthritic chondrocytes of knee. J Liaoning Univ Trad Chin Med. 2020;22:4–8.
292. Ma N. Exploration of the mechanism of the new stop bone augmentation pill for the treatment of KOA rat model based on the regulation of TGF-β/SMAD signaling pathway by miR-146a. 2022. doi:10.27213/d.cnki.glnzc.2022.000120
293. Zhao YS, Li FD, Yang XC, et al. Effects of Snow Lotus Strengthening Tendons and Bones Formula on p38 mitogen-activated protein kinase signaling pathway in osteoarthritic chondrocytes of the knee. J Trad Chin Orthod Traumatol. 2018;30:1–5.
294. Chen S, Wu S, Wang H, Liang FX. advances in the study of P38 signaling pathway and disease spectrum. J Hubei Univ Chin Med. 2017;19:110–113.
295. Hamamura K, Goldring MB, Yokota H. Involvement of p38 MAPK in regulation of MMP13 mRNA in chondrocytes in response to surviving stress to endoplasmic reticulum. Arch Oral Biol. 2009;54(3):279–286. doi:10.1016/j.archoralbio.2008.11.003
296. Long DL, Loeser RF. p38gamma mitogen-activated protein kinase suppresses chondrocyte production of MMP-13 in response to catabolic stimulation. Osteoarthritis Cartilage. 2010;18(9):1203–1210. doi:10.1016/j.joca.2010.05.016
297. Lim H, Kim HP. Matrix metalloproteinase-13 expression in IL-1β-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch Pharm Res. 2011;34(1):109–117. doi:10.1007/s12272-011-0113-4
298. Wang GF, Li XJ, Yang DH, Zhang JC. Effects of Antlong Tongpao Tang on the expression of genes regulating chondrocyte apoptosis in rabbit knee osteoarthritis. Chin J Exp Tradit Med Form. 2013;19:246–250.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Acupotomy Ameliorates KOA Related Chondrocyte Premature Senescence Through YAP/FOXD1 Pathway
Ma Y, Hu T, Liu N, Guo C, Xing L, Ma W, Cui Y, Chen X
Journal of Pain Research 2025, 18:2011-2023
Published Date: 12 April 2025