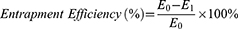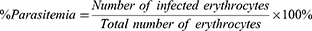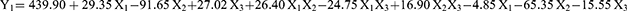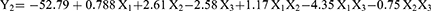Back to Journals » International Journal of Nanomedicine » Volume 19
Fabrication of Phytosome with Enhanced Activity of Sonneratia alba: Formulation Modeling and in vivo Antimalarial Study
Authors Dewi MK , Muhaimin M , Joni IM, Hermanto F, Chaerunisaa AY
Received 7 March 2024
Accepted for publication 31 July 2024
Published 11 September 2024 Volume 2024:19 Pages 9411—9435
DOI https://doi.org/10.2147/IJN.S467811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Mayang Kusuma Dewi,1 Muhaimin Muhaimin,2 I Made Joni,3,4 Faizal Hermanto,5 Anis Yohana Chaerunisaa6
1Doctoral Program in Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, Indonesia; 2Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, Indonesia; 3Functional Nano Powder University Center of Excellence (FiNder U CoE), Universitas Padjadjaran, Sumedang, Indonesia; 4Department of Physics, Faculty of Mathematics and Natural Science, Universitas Padjadjaran, Sumedang, Indonesia; 5Department of Pharmacology and Toxicology, Faculty of Pharmacy, Universitas Jenderal Achmad Yani, Cimahi, Indonesia; 6Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, Indonesia
Correspondence: Anis Yohana Chaerunisaa, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, Indonesia, Email [email protected]
Introduction: Sonneratia alba extract exhibits antimalarial activity, mainly due to its secondary metabolites—naphthoquinones, flavonoids, tannins, and saponins—where naphthoquinone is the primary active component. However, its low bioavailability limits its effectiveness. To improve this, a phytosome-based vesicular system was proposed. This study focused on formulating a phytosome with S. alba and developing a predictive model to enhance its antimalarial activity.
Methods: Phytosomes were produced using antisolvent precipitation and optimized with 3-factor, 3-level Box-behnken model. Particle size, zeta potential, and entrapment efficiency were assessed. The optimized phytosomes were characterized by their physical properties and release profiles. Their antimalarial activity was tested in white BALB/c mice infected with Plasmodium berghei using Peter’s 4-day suppressive test.
Results: The optimal phytosome formulation used a phospholipid-to-extract ratio of 1:3, reflux temperature of 50°C, and a duration of 2.62 hours. The phytosomes had a particle size of 471.8 nm, a zeta potential of − 54.1 mV, and an entrapment efficiency (EE) of 82.4%. In contrast, the phytosome-fraction showed a particle size of 233.4 nm, a zeta potential of − 61.5 mV, and an EE of 87.08%. TEM analysis confirmed both had a spherical shape. In vitro release rates at 24 hours were 86.2 for the phytosome-extract and 95.9% for the phytosome-fraction, compared to 46.9% and 37.7% for the extract and fraction alone. Overall, the phytosome formulation demonstrated good stability. The actual experimental values closely matched the predicted values from the Box–Behnken model, indicating a high degree of accuracy in the model. Additionally, the phytosomes exhibited significantly greater antimalarial activity than the S. alba extract and fraction alone.
Conclusion: The findings indicated that the vesicular formulation in phytosomes can enhance the antimalarial activity of S. alba extract and fraction.
Keywords: antimalarial S.alba, phytosome, box-behnken, extract, fraction
Introduction
Lipid drug delivery systems (LDDS) are gaining significant attention from researchers in formulation development due to their potential to improve drug efficacy and safety.1 LDDS is a novel technology designed to address challenges such as the solubility and bioavailability of poorly water-soluble drugs.2 Many active substances with promising therapeutic potential can have their physical properties enhanced through an appropriate drug delivery system.3
Herbal remedies have been extensively examination by researchers worldwide for their clinical effectiveness, serving as a potential substitute for synthetic pharmaceuticals. These remedies offer notable advantages, such as significant therapeutic efficacy, minimal adverse effects, and cost-effectiveness compared to synthetic medications.4 However, a major challenge in pharmaceutical formulation is the limited bioavailability of these compounds due to their inherent low solubility in aqueous solutions.5 Phytosome—a type of nanoparticulate system—can enhance the aqueous solubility of phytoconstituents, thereby increasing their bioavailability and therapeutic activity. Phytosomes are vesicular delivery systems composed of natural active substances and phospholipids.6,7 Phospholipids—a class of lipids with amphiphilic characteristics—facilitate the formation of lipid bilayer.6–8 The primary goal of the phytosome delivery system is to improve the bioavailability of active ingredients, making them more easily absorbed.7,9,10 Research has demonstrated that phytosomes effectively enhance membrane stability and permeability. Recent studies have shown that this technology can significantly improve the activity of various phytoconstituents,11 such as sinigrin,12 apigenin,13 quercetin,14 gingerol,15 Lantana camara extract,16 and Murraya koenigii extract.17
Phytosomes can be prepared using several methods, such as solvent evaporation, antisolvent precipitation, freeze-drying co-solvency, and salting-out techniques.18 In the antisolvent precipitation technique for phytosome production, the plant extract or active ingredient is dissolved with phospholipids in a solvent. This mixture is then refluxed at a specific temperature for a predetermined time to form a transparent solution. Next, n-hexane is added as an antisolvent to precipitate the phytosomes.19 Figure 1 illustrates the phytosome preparation using the antisolvent precipitation method.
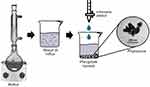 |
Figure 1 Phytosome fabrication process using the antisolvent precipitation method. |
S. alba is a mangrove plant traditionally used by Indonesian coastal communities to treat wounds, diarrhea, and fever.20,21 The plant’s antimalarial activity is attributed to secondary metabolites, such as alkaloids, flavonoids, quinones, and triterpenoids.22,23 Specifically, naphthoquinone has been identified as the key component responsible for its antimalarial properties.22 However, naphthoquinone exhibits low bioavailability due to its poor solubility in water and rapid elimination from the body.24–26 This limitation poses a significant challenge to the effective use of herbal medicine for health promotion.27 Lipid-based drug delivery systems have been shown to enhance the activity and physicochemical stability of bioactive compounds.28–30 Therefore, employing such systems offers a promising approach to enhancing the antimalarial activity and physical properties of S. alba.
The challenge of controlling malaria has become increasingly complex, primarily due to widespread drug resistance.31 This escalating resistance renders many antimalarial drugs ineffective, resulting in higher morbidity and mortality rates.32 Consequently, there is a growing interest in herbal medicine as an alternative therapeutic approach. However, the inherent issues of low bioavailability and solubility of herbal active compounds highlight the need for research into improved delivery systems. Nanotechnology, with its potential to enhance drug safety and efficacy, offers promising avenues for addressing these challenges.27
To achieve the desired product quality, careful considerations of formulation ingredients and process parameters is essential.33 Employing a systematic, scientific, and risk-based approach in the design and development of formulations and manufacturing processes ensures the quality of the final product.34–36 Design of experiments (DOE) is a valuable statistical tool for establishing correlation among key independent variables that influence production.37 This tool allows for the analysis of interaction between variables and respond parameters, facilitating interpretation and prediction based on input parameters. Experimental design enables the prediction of nanoparticles with optimal particle size, zeta potential, and entrapment efficiency (EE).38 The Box-Behnken design—a statistical method—enables the simultaneous analysis of multiple parameters, minimizing the need for experiments in settings that pose risks or uncertainties.39
The primary objective of this research was to develop a predictive formulation model for S.alba phytosomes as lipid-based delivery system using the Box-Behnken design. This included studying the effect of independent variables (phospholipid-to-extract ratio, reflux temperature, and reflux time) on the responses such as particle size, zeta potential, and entrapment efficiency (EE). Additionally, the optimized phytosome formulations were evaluated for their in vivo antimalarial activity.
Materials and Methods
Instruments
Particle size analyzer (PSA) and zeta potential characterization were conducted using the Horiba Scientific SZ-100. Electronic spectra were obtained using a Specord 200 UV-Vis spectrophotometer across the wavelength range of 200 to 800 nm. The range was selected to identify the peak wavelength of the active compound, naphthoquinone, which literature gests to be at 269 nm.40 Confirmation of this wavelength was achieved through measurement, indicating a specific peak at 268 nm within the 200–800 nm range. Transmission electron microscopy (TEM) images were captured using a JEOL JEL-1400 Transmission electron microscope operating at 100 kV.
Materials
The materials utilized in this study included standardized S. alba extract sourced from Teluk Majelis Village in East Tanjung Jabung Regency (Jambi Province, Indonesia), confirmed through plant identification conducted by Drs. Joko Kusmoro, M.P., from the Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Indonesia, in 2022, based on designation No. 115/LBM/IT/12/2022. Chemicals included 2-Hydroxy-1,4-naphthoquinone (purity >99.9%) (Sigma-Aldrich), soy lecithin (Archer Daniels Midlands), dichloromethane (Merck), n-hexane (Merck), methanol (Merck), ethyl acetate, 0.5% PGA suspension, Plasmodium berghei, artemisinin (Sigma Aldrich), heparin, Phosphate Buffered Saline (PBS), and Giemsa dye. All other chemicals and reagents used were of analytical grade.
Animals
Healthy male BALB/c mice weighing 20–30 grams and aged 6–8 weeks were used for the antimalarial activity tests. The experimental animals were sourced from PT. Biopharma, Indonesia, and all procedures were conducted following the ethical standards established by the Research Ethics Committee of Universitas Padjadjaran, Bandung. The committee adheres to globally recognized guidelines, including the Eighth Edition of the Guide for the Care and Use of Laboratory Animals (NRC, 2011), and follows the principles of the 3Rs (reduction, refinement, and replacement) as outlined by William Russell and Rex Burch (1959) for animal experimentation decision-making.41,42 Ethical approval for all experiments conducted in this study was granted by the Research Ethics Committee at Universitas Padjadjaran Bandung under Ethical Approval No. 243/UN6.KEP/EC/2023.
Method
Quantification of Naphthoquinone by UV-Vis Spectrophotometer
The levels of naphthoquinone—a marker compound for antimalarial activity—in the extracts and fractions from previous study were determined using UV-Vis spectrophotometry (Spectrophotometer UV-Vis Specord 200). The external standard method was employed, and absorbance were measured at the maximum wavelength of 268 nm.43 The naphthoquinone content in 30 ppm of extract and 30 ppm of S. alba fraction was calculated.
Development of Phytosome Formula Prediction Using Box-Behnken Design
A factorial design with three factors and three levels was used to optimize essential characteristics impacting the response variable.44 The critical material attributes (CMAs) for this study were phospholipid-to-extract ratio (X1), reflux temperature (oC; X2), and reflux time (hour; X3). These attributes were evaluated at three levels: low, medium, and high, as shown in Table 1. The critical quality attributes (CQAs) assessed in this study included particle size (nm; Y1), zeta potential (mV; Y2), and entrapment efficiency (%; Y3) (Table 2). A total of 15 batches were prepared to determine the optimal formula for the phytosome complex. The data obtained was entered into the Design-Expert® software (Version 13.0.5.0; Stat-Ease Inc., Minneapolis, MN). The most appropriate model for the measured response was selected based on the predicted and adjusted coefficients of determination. Adequate precision was also calculated to ensure model fit.45,46 The equation representing the model with the best fit was derived. The statistical technique of analysis of variance (ANOVA) was utilized to assess the significance of the researched variables by analyzing the measured response, with a significance level of p < 0.05.45 Response surface analysis was performed, and contour plots, as well as 3D response surface plots, were constructed to elucidate the relationships and interaction between the variables.46
 |
Table 1 Independent and Dependent Variables in Box-Behnken Design for Phytosome-Extract |
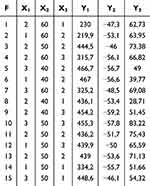 |
Table 2 Phytosome-Extract Formulations Prepared According to the Box-Behnken Design |
Preparation of Phytosomes Loaded with S. Alba Extract
Phytosomes were prepared using the antisolvent precipitation technique. A total of 300 mg of S. alba leaf extract and the corresponding quantity of phospholipids, as indicated in Table 2 (ratios of 1:1, 1:2, and 1:3, equivalent to 300, 600, 900 mg), were added into a 100 mL round-bottom flask. The mixture was subjected to reflux using 20 mL of dichloromethane at a temperature range of 40°C, 50 oC, or 60°C for a duration of 1, 2, or 3 hours. The mixture was concentrated to 5–10 mL. Subsequently, 20 mL n-hexane was added with constant stirring to precipitate the phytosomes, which were then filtered and collected. The precipitate was stored in a desiccator overnight. The resulting phytosome powder was placed in amber-coloured glass vials and stored at room temperature.45
Evaluation of Phytosome
Determination of Particle Size and Zeta Potential
The particle size and zeta potential of the phytosomes were determined using Particle Size Analyzer (PSA) with the dynamic light scattering (DLS) method. The measurements were performed using Horiba Scientific SZ-100, with the sample diluted 10 times in aqueous medium at room temperature.47,48
Entrapment Efficiency
The entrapment efficiency of naphthoquinone in the phytosomes was assessed by determining the amount of unentrapped naphthoquinone.49 The phytosome were diluted with methanol and centrifuged at 13,000 rpm for 45 minutes at −4°C using a refrigerated centrifuge. The supernatant was collected, and the concentration of free naphthoquinone was measured using a UV/Vis spectrophotometer at a wavelength of 268 nm.50 The entrapment efficiency of the phytosome was calculated using the following equation.51
In the formula, E0 represents the total amount of naphthoquinone added to the phytosomes and E1 represents the amount of free naphthoquinone.
Morphological Study by Transmission Electron Microscopy (TEM)
The morphological features of the phytosomes were assessed using a transmission electron microscope (JEOL JEL-1400). The samples were diluted with distilled water at a ratio of 1:20 and subjected to sonication for 10 minutes. A droplet of the phytosome solution was placed on a carbon-coated copper grid. A 2% uranyl acetate solution was then added to provide negative staining of the samples. The samples were allowed to dry naturally at room temperature for 15 minutes before imaging. The phytosomes were imaged using TEM at an acceleration voltage of 100 kilovolts (kV).45,52
In vitro Release Studies
In vitro drug release from extracts, fractions, phytosome-extracts, and phytosome-fractions was conducted using dialysis bag technique.53 Release studies were performed at intestinal pH (7.4) using phosphate-buffered saline (PBS) at pH 7.4. In the dialysis bag method, pre-weighed samples of extracts/fractions and phytosome-extracts/phytosome-fractions (equivalent to 2.5 mg of extracts/fractions) were placed in pre-soaked dialysis bags (molecular weight cut-off (MWCO) 14 KDa; Sigma-Aldrich, St. Louis, MO, USA). The release was carried out in 100 mL of PBS pH 7.4 at 37 ± 0.5°C with constant stirring at 100 rpm using a magnetic stirrer. Samples were collected at the following time points: 0, 0.25, 0.50, 0.75, 1, 1.25, 1.75, 2.5, 4, 5.5, 8, 12, 16, 20 and 24 hours. An equivalent volume of fresh media was added to replaced the aliquot removed.54 The samples were analyzed using a UV-Vis spectrophotometer to determine the naphthoquinone content.
Stability Studies
The stability of the phytosomes was evaluated under two different storage conditions. Room temperature stability studies were conducted at 25°C ± 2°C and 60% ± 5% relative humidity, while accelerated stability studies were performed at 40°C ± 2°C and 75% ± 5% relative humidity. Samples were taken at intervals of 0, 30, 60, to 90 days. Particle size and zeta potential were measured to assess the stability profile, and entrapment efficiency was evaluated after the stability testing. Additionally, a visual inspection was performed to check for any discoloration of the formulation.10,55–58
Antimalarial Activity Study
Animal Preparation
The experimental subjects were male BALB/c strain white mice, weighing between 20 and 30 grams, and aged 6 to 8 weeks, obtained from PT. Biofarma, Indonesia. The mice were acclimatized for seven days in a controlled environment with 12-hour light/dark cycle. The temperature was maintained at 25 ± 3°C and the humidity was kept between 50% and 60%. During this acclimatization period, the mice had ad libitum access to food and clean water. Plasmodium berghei strain ANKA was sourced from the local malaria laboratory Indonesia. Donor mice were intraperitoneally infected with blood containing P. berghei. When the parasitemia in the donor mice reached 20–30%, blood samples were collected directly from the heart.59
Animal Grouping and Dosing
The mice were randomly assigned to six groups, each consisting of four animals. Group 1 (the negative control group) received a 0.5% PGA solution. Group 2 (the positive control group) was administered artemisinin at 36.4 mg/kg body weight. Groups 3 and 4 were treated with individual extracts and fractions at a dosage of 95.28 mg/kg body weight, suspended in a 0.5% PGA solution. Groups 5 and 6 received phytosome-extract and phytosome-fraction, respectively, at a dose equivalent to 95.28 mg/kg body weight of the extract or fraction, also suspended in 0.5% PGA. All treatments were administered via oral gavage.
Rodent Malaria Parasite
The Plasmodium berghei strain ANKA was obtained from Universitas Jenderal Ahmad Yani, Cimahi, Indonesia. Cryopreserved parasite stocks were thawed and administered intraperitoneally to the mice, with each mice receiving 0.2 mL containing 1×107 infected erythrocytes. Parasitic levels were monitored daily by examining Giemsa-stained thin blood smears, collected from the tail vein, under a microscope.60
Calculation of Parasitemia
Parasitemia in mice was assessed using thin blood smears prepared from tail blood. A drop of blood was placed on a glass side, spread with a cover slip, and allowed to air dry. The blood film was then fixed in absolute methanol for approximately 1 second and air-dried. Following this, the slide was stained with 10% Giemsa dye for 10 minutes at room temperature, gently rinsed under running water, and air-dried. Infected erythrocytes were counted under a light microscope ata 1000x magnification using an oil immersion lens. The percentage of parasitemia was calculated using the following formula.60
Four-Day Parasite Suppression Test
Peter’s suppressive test was conducted over four consecutive days to evaluate antimalarial activity. Initially, all mice were intraperitoneally injected with 0.2 mL of a P. berghei suspension, containing approximately 107 parasites. Treatments with the test samples (extracts, fractions, or phytosomes) were administered orally 2 hours post-infection (Day 0). The mice received daily oral treatment on Days 1, 2, and 3 at the specified dosages. On Day 4, blood samples were collected from the tail, prepared as thin blood smears, and fixed in methanol. The smears were stained with a 10% Giemsa dye solution and examined under61 light microscope (Olympus, model CX-31, Japan) at 100x magnification.61 The percentage of parasitemia suppression was calculated using the formula.
In the formula, A represents the mean parasitemia of the negative control group on day 4, and B denotes the mean parasitemia of the test group on day 4.
Results
Quantification of Naphthoquinone in the Extracts and Fractions
Naphthoquinone—a key phytoconstituent in S. alba responsible for its antimalarial activity—was used as a marker for quantitative analysis in the development of S.alba phytosomes. In this study, it was determined that 30 ppm of S. alba extract contained 4.036 ppm of naphthoquinone, whereas 30 ppm of the fraction contained 5.243 ppm of naphthoquinone.
Modelling the Formula for Phytosome Containing S. Alba Extract
The Box-Behnken design was employed to evaluate the effects of various formulation parameters on the phytosome development process. Specifically, the study investigated the impact of the phospholipid-to-extract ratio (X1), reflux temperature (°C; X2), and reflux time (hours; X3). On the formulations outcomes. The responses evaluated for the formulations prepared using the Box-Behnken design are outlined in Table 2.
Table 3 presents the statistical analysis of the optimal model for the measured responses. Figure 2 compares the observed experimental results with the predicted values for particle size (A), zeta potential (B), and entrapment efficiency (C). The close alignment between the actual and predicted values demonstrates the robustness of the chosen design approach in modelling the formulation to meet specific criteria.45
 |
Table 3 Statistical Analysis of Responses Y1, Y2, and Y3 for the Optimal Model |
 |
Figure 2 Comparison of predicted and actual values for (A) particle size (nm), (B) zeta potential (mV), and (C) entrapment efficiency (%) of phytosomes containing S. alba. |
Effect of Variables on Response
The optimized method elucidated the effects of formulation variables—phospholipid-to-extract concentration ratio (X1), reflux temperature (oC; X2), and reflux time (hours; X3) on the particle size (nm; Y1), zeta potential (mV; Y2), and entrapment efficiency (%; Y3) of phytosomes. The particle size, zeta potential, and entrapment efficiency of the phytosome-extract range from 230 to 466.7 nm, −59.2 to −46 mV, and 39.8 to 83.2%, respectively. The model equations derived from the response surface analysis are as follows:
Analysis of variance (ANOVA) using quadratic model for particle size response indicated significant effect of linear terms X1 (phospholipid-to-extract ratio), X2 (reflux temperature), and X3 (reflux time) at p < 0.05. Additionally, interactions between X1 and X2, X1 and X3, and X2 and X3 significantly impacted particle size. The squared terms of X22 and X32 also demonstrated a significant on particle size. Figure 3A illustrates the effects of these independent factors on particle size. A negative slope for variable X2 indicates an inverse relationship between reflux temperature and particle size. Conversely, a positive correlation was observed between X1 and X3, suggesting that an increased phospholipid-to-extract ratio and longer reflux time are associated with larger particle sizes. In contrast, higher reflux temperatures resulted in smaller particles. Positive coefficients for X1 and X3, along with a negative coefficient for X2, corroborate these observations.
 |
Figure 3 Effect of phospholipid-to-extract ratio (X1), reflux temperature (X2), and reflux time (X3) on the response: (A) particle size, (B) zeta potential, and (C) entrapment efficiency. |
The zeta potential response, as modeled by the 2FI (two-factor interaction) model, is significantly influenced by the linear terms X2 (reflux temperature) and X3 (reflux time) at p < 0.05. Additionally, the interaction between X1 (phospholipid-to-extract ratio) and X3 has a significant impact on zeta potential. The 2FI model specifically examines the interaction between pairs of factors, focusing on how the interaction between these two factors affects the response. Figure 3B illustrates the effects of the independent variables on zeta potential. Analysis of the data revealed a positive correlation with variable X2 indicating that an increase in the phospholipid-to-extract ratio is associated with a higher zeta potential. In contrast, a negative correlation was observed with variable X3, suggesting that longer reflux times are associated with a decrease in zeta potential.
The entrapment efficiency was significantly influenced by the linear terms X1 (phospholipid-to-extract ratio), X2 (reflux temperature), and X3 (reflux time), as determined by the quadratic model with a significance level of p < 0.05. Additionally, the interaction between X2 and X3 had a notable effect on entrapment efficiency. The quadratic terms X22 and X32 also exhibited significant influence on this response. Data analysis revealed a positive correlation with variable X1, indicating that a higher phospholipid-to-extract ratio is associated with increased entrapment efficiency. Both variables X2 and X3 demonstrated positive slopes up to the midpoint, suggesting that optimal reflux temperature and reflux time lead to higher entrapment efficiency. However, beyond this midpoint, entrapment efficiency decreased with increasing reflux temperatures and longer reflux times. Figure 3C illustrates the effects of these independent variables on entrapment efficiency.
The ideal phytosome characteristics include particle size in the nanometer range, zeta potential within a stable range, and maximized entrapment efficiency. To assess these parameters, the effects of factors X1, X2, and X3 on particle size (refer to Figure 4), zeta potential (refer to Figure 5), and entrapment efficiency (refer to Figure 6) were evaluated. Contour plots, both 2D and 3D, were used to visualize the impact of these factors.
 |
Figure 4 Contour plots (A–C) and surface plots (D–F) illustrating the impact of variables (X1–X3) on the particle size of phytosomes. |
 |
Figure 5 Contour plots (A–C) and surface plots (D–F) depicting the influence of variables (X1–X3) on the zeta potential of phytosomes. |
 |
Figure 6 Contour plots (A–C) and surface plots (D–F) demonstrating the effect of variables (X1–X3) on the entrapment efficiency of phytosomes. |
Contour plots represent a two-dimensional surface where points with identical responses are connected to form lines indicating consistent response levels. In contrast, 3D-surface plots provide a more detailed perspective on the response surface, offering a clearer understanding of the interactions between variables and their effects.
The goal of the optimization was to achieve phytosomes with particle sizes in the nanometer range, zeta potentials indicating good stability, and maximum entrapment efficiency. The optimized phytosome-extract formula was then applied to phytosome-fraction formulations. The physical characteristics of both the optimized phytosome-extract and phytosome-fraction are summarized in Table 4.
 |
Table 4 Physical Parameters of Optimized Phytosome Formulations |
Surface Morphology of Phytosomes
The surface morphology of the phytosome-extract and phytosome-fraction from the optimized formulations was examined using transmission electron microscopy (TEM) 100 kV. As depicted in Figure 7, the TEM images revealed that both S. alba phytosome-extracts and phytosome-fractions exhibited a spherical shape with smooth with smooth surface characteristics.
 |
Figure 7 Surface morphology of optimized phytosome-extract (A) and phytosome-fraction (B) from S. alba at 10000x magnification. |
In vitro Release Studies
In vitro dissolution studies at pH 7.4, shown in Figure 8, demonstrated that the drug release percentages from phytosomes were significantly higher compared to those from extracts and fractions. This enhanced release can be attributed to the improved solubilization and complete release of the active constituents from the phytosome matrix. Over 24 hours, the percentage of drug released was as follows: extract = 37.7%, fraction = 46.9%, phytosome-extract = 86.2%, and phytosome-fraction = 95.9%.
 |
Figure 8 Drug release profiles of extract, fraction, phytosome extract, and phytosome fraction at pH 7.4. |
Stability Studies of Phytosomal Formulation
Stability studies were conducted monthly over three months to assess the particle size and zeta potential of phytosomal formulations. These evaluations were performed under two conditions: at room temperature (25 ± 2°C) and under accelerated conditions (40 ± 2°C). The variations in particle size and zeta potential are detailed in Table 5 and Table 6. Additionally, the entrapment efficiency of naphthoquinone was assessed at the end of the stability period and is presented in Table 7. The results indicate that, overall, the phytosome formulations maintained stability at room temperature.
 |
Table 5 Changes in Particle Size and Zeta Potential at Room Temperature Over Three Months |
 |
Table 6 Changes in Particle Size and Zeta Potential at 40°C in Three Months |
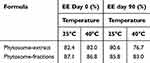 |
Table 7 Stability Studies of Naphthoquinone at Different Temperatures |
Antiplasmodium Activity
Previous research has established that the effective dose (ED50) of S. alba ethanol extract for antimalarial activity is 95.28 mg/kg body weight (BW) mice.62 In this study, the same dosage was administered to test groups, which included both extracts and fractions at 95.28 mg/kg BW. For phytosomes-extracts and phytosomes-fractions, the doses were adjusted to be equivalent to 95.28 mg/kg BW of the respective extracts or fractions. The antimalarial activity against Plasmodium berghei is illustrated in Figures 9 and 10, showing percentage parasitemia percentage inhibition, respectively. The negative control group exhibited the highest parasitemia percentage (25.7%), followed by extracts (11.95%), fractions (10.12%), phytosome-extracts (5.12%), phytosome-fractions (3.98%), and the positive control (0%). In terms of suppression percentage, the positive control achieved the highest suppression (100%), followed by phytosome-fraction (84.51%), phytosome-extract (80.12%), fraction (60.54%), extract (53.38%), and the negative control (0%).
 |
Figure 9 Percentage of parasitemia in negative control, positive control, ethanol extract, ethyl acetate fraction, phytosome-extract, and phytosome-fraction of S. alba leaves. |
 |
Figure 10 Percentage suppression of parasitemia on day 4 for negative control, positive control, ethanol extract, ethyl acetate fraction, phytosome-extract, and phytosome-fraction of S. alba leaves. |
Discussion
Phytosomes are vesicular delivery systems created through the interaction between phytoconstituents and phosphatidylcholine in phospholipids, resulting the formation of hydrogen bonds between the two components.63 This innovative approach enhances the efficacy of bioactive compounds by integrating lipid molecules, thereby increasing the lipophilicity and improving bioavailability.
Compared to other lipid-based drug delivery systems, phytosomes offer distinct advantages in enhancing the bioavailability of phytochemical extracts. They form molecular complexes with phospholipids, which help protect active ingredients from degradation and increase their permeability across cell membranes.64–67 This interaction improves the solubility of poorly soluble phytoconstituents, thereby enhancing their absorption and bioavailability.68,69 The chemical bonds formed between phospholipid head and the phytoconstituents ensure the stability of the complex during storage and use.68,70 Furthermore, phytosomes demonstrate higher entrapment efficiency, indicating that the active compounds are effectively encapsulated within the system.71
In this study, phytosomes were prepared using the antisolvent precipitation method. This approach demonstrated superior entrapment efficiency compared to other methods, highlighting its effectiveness in the process.67 Specifically, the antisolvent precipitation method outperformed the rotary evaporation technique in terms of entrapment efficiency, as evidenced by the investigation into phytosome-naringin systems.72 Additionally, the antisolvent precipitation method is advantageous for large-scale production due to its simplicity, cost-effectiveness, and ease of using available equipment.67,73 Importantly, this method yields phytosomes in powder form, which is crucial for maintaining stability—a key factor in scaling up production for commercial use.
Sonneratia alba was selected as the primary plant extract for this study based on several criteria, including its traditional use, phytochemical composition, and availability. S. alba—a mangrove species—has been traditionally employed by Indonesian coastal communities for treating wounds, diarrhea, and fever.20,21 Phytochemical analyses have identified secondary metabolites in S. alba, such as quinones, triterpenoids, alkaloids, and flavonoids, which are known for their antimalarial properties.22 Previous research has demonstrated that ethanol extracts of S. alba leaves exhibit significant antimalarial activity against P. berghei ANKA—the malaria parasite.62 This highlights S. alba as a promising natural resource for developing antimalarial agents. Additionally, S. alba’s widespread presence across various regions of Indonesia enhances its accessibility for both research and commercial applications.
In this study, a loading dose of 300 mg of S. alba was selected based on preliminary experimental optimizations. Loading doses exceeding 300 mg resulted in lower entrapment efficiency (EE), likely due to the phytosomes’ limited capacity to encapsulate the drug effectively.
The design of experiments (DOE) is a systematic approach used to evaluate the interactions among various factors affecting formulation process and the quality of the final product.74 By providing a framework to assess how independent variables influence the responses, DOE enhances the understanding of the relationships within the system and supports predictive modeling. In this study, a three-level Box-Behnken design (BBD) was employed to formulate and optimize the phytosome-extract derived from S. alba. BBD is advantageous for its precision and efficacy in analyzing and optimizing process parameters across various applications and research scenarios. Analysis of variance (ANOVA) was utilized as part of this methodological approach to ensure the robustness and accuracy of the results. ANOVA applied to the Box–Behnken design is a statistical method used to determine significant differences among variations tested in an experiment. In the context of phytosome formulation, variations such as phospholipid concentration, reflux temperature, and reflux time were examined for their impact on phytosome particle size. The primary goals were to achieve a nanoscale particle size, a stable zeta potential, and maximum entrapment efficiency. The independent variables investigated have been previously recognized as crucial factors in phytosome formulation.75 The experimental results indicated that a quadratic model best described the responses for particle size (Y1) and entrapment efficiency (Y3), while a two-factor interaction (2FI) model was most suitable for the zeta potential (Y2).75
The models were selected due to their insignificant lack of fit (LOF) values (refer to Table 3). The term “lack of fit” describes a situation where the mathematical model does not adequately represent the relationship between the variable and the responses. LOF assesses the model adequacy by measuring the discrepancy between observed and predicted values of the response variable. A statistically significant LOF indicates that the model does not effectively capture data variation, while an insignificant LOF value suggests a good fit76 and provides further validation of the model accuracy.76,77 The validity of the models was confirmed through various statistical tests and diagnostic plots to ensure they accurately represents the relationship between the variables and the response.78
To select the most appropriate model, it is essential to evaluate it based on the coefficient of determination (R2). To ensure the model’s validity, the discrepancy between the adjusted R2 and the predicted R2 should remain below 0.2.76 This criterion is crucial for developing reliable predictions and optimizing processes or systems. The experimental design demonstrated its efficacy in predicting the optimum formulation through the conducted trials, utilizing the selected independent variables.
By employing the Box-Behnken Design (BBD), the relationship between actual and predicted values can be described by a mathematical model derived from the experiment. This model should accurately reflect the data measured at specific experimental points. A well-developed model will yield predictions that closely match the actual value, as illustrated in Figure 2. In this figure, actual values (represented by bullets points) are in proximity to the predicted values (depicted as lines generated by the mathematical functions).
The interaction graph in the Box-Behnken design (refer to Figure 3) visually represents the interplay between independent variables and the responses measured in the experiment. This graph aids in understanding how variations in one factor can influence the responses. By analyzing interaction graphs in Box-Behnken designs, researchers can make more informed and relevant decisions regarding the effects of the factors on their experimental outcomes. A positive coefficient indicates a direct correlation between the factors and the response variable, meaning that as the factor increases, the response also increases. Conversely, a negative coefficient implies an inverse relationship, suggesting that an increase in the factor leads to a decrease in the response.5 The equation reveals a positive relationship between the independent variable (phospholipid-to-extract ratio [X1]), and the three responses variables (particle size [Y1], zeta potential [Y2], and entrapment efficiency [Y3]. The reflux temperature (X2) positively influence zeta potential (Y2) and EE (Y3), but negatively affects particle size (Y1). Conversely, reflux time (X3), has positive impact on particle size (Y1) and EE (Y3), while its effect on zeta potential (Y2) is negative. The response surface graph, illustrated in Figure 3, provides a clearer depiction of the relationships between these factors and their respective responses.
The specific levels for the independent variables—phospholipid-to-extract ratio, reflux temperature, and reflux time—in the Box-Behnken design for phytosome formulation using the antisolvent precipitation method were selected based on extensive literature reviews.5,19,45,50,79,80 These levels were chosen to provide a thorough understanding of how independent variables affect phytosome formulation and to optimize the formulation through the Box-Behnken design. This approach helps reduce both the cost and time of experimentation while enhancing the accuracy and reliability of predictions. The Box-Behnken analysis identified the main influential factors and their impact on the responses, including particle size, zeta potential, and entrapment efficiency, which are affected by the tested variables (phospholipid-to-extract ratio, reflux temperature, and reflux time) as depicted in Figures 4–6. The optimal formulation was identified as having a phospholipid-to-extract ratio (1:3), a reflux temperature of 50°C, and a reflux time of 2.62 hours. Higher temperatures have been associated with adverse changes in chemical reactions and molecular structures, potentially leading to the formation of unintended compounds not present in the original extract.81 The particle size, zeta potential, and EE of the phytosome-extract optimum formulation were found to be 471.8 nm, −54.1 mV, and 82.4%, respectively. When this optimal formulation was applied to phytosome-fractions, it yielded particle size, zeta potential, and EE values of 233.4 nm, −61.5 mV, and 87.08%, respectively.
Contour plots and three-dimensional (3D) response surface plots are instrumental in elucidating both main and interaction effects while keeping other factors constant. Contour plots offer a two-dimensional representation of the response surface, illustrating the interaction between two independent variables and their impact on the response variable. They are useful for identifying optimal formulation parameters by examining the relationship among independent variables (ie, phospholipid-to-extract ratio, reflux temperature, and reflux time) and response variables (such as particle size <500 nm, zeta potential <-30 mV, and high EE). The Box-Behnken design provides prediction points to determine the optimal formulation with expected outcomes. Subsequently, the predicted model is validated through laboratory experiments, and the results are compared with the predicted outcomes to obtain actual results.82 Plot surfaces, offer a more detailed visualization by presenting the response surface in three-dimensional space. This approach enhances the understanding of the relationship between independent variables and the response variables, facilitating the identification of optimal operating conditions.82 These visual representations are crucial for optimizing processes and formulations, as they help pinpoint critical factors and their optimal levels, ultimately leading to the desired responses.83
Figure 4A and D illustrate the effects of phospholipid concentration and reflux temperature on phytosome particle size. An increase phospholipid concentration was associated with larger particle sizes. Conversely, higher reflux temperatures were linked to smaller particle sizes. Generally, an increase in phospholipid concentration and longer the reflux time tend to increase particle size, as shown in Figure 4B and E. However, when the reflux time exceeded 2.5 hours, a reduction in particle size was observed. An increase in phospholipid concentration typically results in larger phytosome sizes. This phenomenon can be attributed to the higher phospholipid content, which increases viscosity and promotes aggregation, leading to larger phytosomes. This observation aligns with the findings of Song et al, who reported a positive correlation between lipid content and particle size in the production of phytosomes-breviscapine with various drug-to-lipid ratios (1:1, 1:2, and 1:3). Their study demonstrated that an increase in lipid content corresponds to a rise in particle size.84 The higher number of phospholipid molecules contributes to aggregation, which in turn increases the particle size.85 In the preparation of phytosome-sinigrin, it was observed that higher phospholipid concentrations resulted in larger particles.75 Similarly, the formation of Icariin phytosomes showed that phospholipid concentration significantly affects vesicle size, with higher concentration leading to increased particle size.45
The results indicated that higher reflux temperatures led to smaller the particle size (refer to Figure 4C and F), while increased reflux times resulted in larger particle sizes. This observation was visually confirmed by color gradient in the plots, where the blue-red spectrum represented particle sizes ranging from 219.9 to 467 nm. Specifically, red hues indicated large particle sizes, while the blue hues denoted smaller particle sizes. These findings are consistent with previous research, which reported that increased reflux temperature can decreased particle size. Higher temperatures tend to accelerate the solvent evaporation rate, thereby facilitating the formation of smaller particles.67 Another study indicated that increased temperature can enhance complexation and tight integration between phytoconstituents and phospholipids, resulting in particles with smaller diameters.85 However, this relationship is not universally applicable and may vary depending on the specific phytosome formulation and solvent system employed. For instance, in the formation of Icariin phytosomes, reflux temperature significantly influenced vesicle size, with a negative slope reflecting an inverse relationship between reflux temperature and particle size.45 Other research has suggested that extended reflux times can lead to increased particle aggregation, which results in larger phytosome particle sizes when prepared using the antisolvent precipitation method.18,74,86
Figure 5A and D illustrate the relationship between phospholipid concentration and reflux temperature on the zeta potential of phytosomes. The data show that increased phospholipid concentration results in a linear increase in zeta potential values. Generally, the negativity zeta potential is attributed to the negative phosphate groups in phospholipids, which are oriented toward the outer layer of the phytosomal complex.87 As phospholipid concentration rises, the zeta potential becomes more negative, reflecting an increase in surface charge. A zeta potential value greater than ± 30 mV is indicative of good suspension stability.88,89 High zeta potential values suggest stronger repulsive force between particles, reducing the likelihood of aggregation during storage. Conversely, low zeta potential values can lead to particle attraction and aggregation, potentially resulting in flocculation and reduced stability of the suspension.90 A study on phytosomes preparation using extracts from ginger rhizomes and rosehips confirmed that phytosomes exhibited a negative charged, with zeta potential values becoming increasingly negative as the phospholipid concentration increased.52
Higher temperatures can cause phospholipids to assume a more disordered conformation, which may result in less negative zeta potential due to the reduced exposure of the negative phosphate groups.91 This phenomenon is illustrated by the color gradient in the plots, where the zeta potential ranges from −59.2 to −46 mV.
Figure 5B and E illustrate the effects of phospholipid concentration and reflux time on zeta potential. High zeta potential values were observed with high phospholipid concentrations and low reflux time, as indicated by the red areas on the contour plots. This suggests that phytosomes prepared under these conditions may aggregate more easily during storage. To achieve lower zeta potential values, two approaches can be considered: using high phospholipid concentration with extended reflux times or maintaining both parameters at low values, as shown by the blue areas on the contour plots. The influence of phospholipids concentration on zeta potential is not solely linear; other factors, such as reflux time, also play a significant role.52 In this study, the effect of reflux time was more pronounced, resulting in a significant increase in zeta potential values.
Figure 6A and D illustrate the relationship between phospholipid concentration and reflux temperature on the entrapment efficiency of phytosomes. The elliptical shape of the contour plots indicates that optimal entrapment efficiency is achieved under specific conditions: high phospholipid concentration combined with intermediate reflux temperatures. Additionally, higher entrapment efficiency is observed when using formulations with elevated phospholipid concentration and reflux times exceeding three hours (refer to Figure 6B and E).
It is crucial to recognize that entrapment efficiency in phytosomes is influenced by several factors, including the stability and formation of the phytosome structure. Increased phospholipid concentration typically enhances entrapment efficiency, as phospholipids are fundamental to the formation and stability of phytosomes.75 A higher concentration of phospholipids provides more molecules available for encapsulating active compounds, thereby improving entrapment efficiency.91 This relationship between phospholipid concentration and entrapment efficiency has been consistently observed in studies studies.91–93
Increased entrapment efficiency was also observed with adjustments to reflux temperature and reflux time (refer to Figure 6C and F). The elliptical contour plots indicated that the highest efficiency was achieved with reflux times of 2–3 hours at specific reflux temperatures ranging from 45–60°C.
Higher reflux temperatures can enhance the solubility of the phytochemicals, potentially leading to improved encapsulation efficiency.18 However, excessive high temperatures may also degrade phytochemicals or phospholipids, which could negatively impact entrapment efficiency.18 Elevated temperatures may alter the solubility of the components, affecting the formation and stability of the phytosomes and, consequently, their entrapment efficiency.94 The effect of reflux temperature on entrapment efficiency is complex and depends on the specific formulation. Optimizing reflux temperature is crucial for each phytosome formulation to achieve the desired entrapment efficiency. Additionally, longer reflux times facilitate the complete encapsulation of active compounds within the phytosomes, leading to higher entrapment efficiency.94,95
To ensure the reproducibility and consistency of phytosome formulations across batches, several measures should be implemented. One of them is to standardized the phytosome preparation method and replicate it consistently to maintain result uniformity. This involves controlling materials, and process parameters (ie, temperature, reflux time, and stirring speed), while ensuring these parameters are maintained consistently throughout each batch. At the manufacturing scale, quality control measures should be applied to verify the quality and consistency of raw materials and final products. The design of experiments (DOE), specifically the Box–Behnken design, can be utilized to optimize process parameters and enhance reproducibility. Additionally, routine batch-to-batch characterization of phytosome products is essential to confirm that the formulation process consistently produces uniform results across different production batches.
Transmission electron microscopy (TEM) is a powerful technique for visualizing and analyzing the morphology and size of phytosomes.18 TEM images of S. alba phytosome-extracts and phytosome-fractions revealed that the particles are spherical and exhibit a smooth surface (refer to Figure 7). These observations confirm the successful preparation of phytosomes and provides valuable insights into their morphology and structure. The spherical vesicles observed are characteristic of phytosome structures, consisting of a phospholipid bilayer encapsulating the active compound.96,97 The TEM images corroborate the particle size analysis result obtained from dynamic light scattering technique. Key features observed in the TEM images (eg, the spherical shape of the phytosomes) are crucial for their stability and performance.74,88,98 The images show that the phytosomes are of consistent size and fall within the designated range, indicating successful formulation.74,88 Additionally, the smooth surface morphology of the phytosomes, as depicted in the TEM images, is essential for their interaction with the surrounding environment and for maintaining stability.74,88 Importantly, the absence of aggregation in the TEM images is crucial for ensuring long-term stability and preventing particle growth over time.74,88
In vitro release results demonstrated that phytosomes significantly enhance the solubilization of naphthoquinone—a marker compound of S. alba. The release of the drug from phytosomes was twice as high compared to that from extracts or fractions. Phytosomes, as lipid-based nanocarriers, effectively encapsulate hydrophobic compounds within their lipid bilayer. The encapsulation of the extract in phospholipids, improved the solubility of the extract, facilitating better dissolution and release of the active compounds.99,100 This enhancement contributes to a more pronounced in vitro release profile and potentially improved in vivo antimalarial activity.99,101 The phospholipid layer of phytosomes protect the extract, preventing the degradation of active substance. This protective layer preserves the integrity of the extract and enhances its release, resulting in an elevated in vitro release profile.102 After 24 hours, the release of naphthoquinone from phytosomes approached nearly 100%, whereas the release from pure extracts or fractions was less than 50% (refer to Figure 8). Soya lecithin—phospholipid— enhances the solubility of naphthoquinone by improving its wettability, which leads to increased solubilization in the release medium. Previous studies have also reported the ability of soya lecithin to enhance the solubility of poorly soluble drugs.103 Additionally, the nanometer size of the phytosomes likely contributes to the enhanced release of naphthoquinone through the dialysis membrane. Smaller particles typically exhibit faster diffusion across semipermeable membranes due to their higher surface area-to-volume ratios, facilitating their passage through pores.18
Stability testing of powdered phytosomes was conducted to simulate real storage conditions. The study revealed that during long-term physical stability testing, there were no alterations in particle size or zeta potential. Maintaining consistency in these parameters ensures that the formulation remains stable during storage, thereby offering sustained pharmacological activity.49 Furthermore, the study on the entrapment efficiency of phytosome during stability testing indicated that the phytosomes exhibited chemical stability, with no degradation or deterioration. This stability guarantees the preservation bioavailability and effectiveness of the formulation over time.52 The findings suggest that the production of phytosomes by antisolvent precipitation technique results in excellent stability. The powdered form of these phytosomes can be stored for extended periods without deterioration, thus ensuring long-term stability and efficacy. The powdered phytosome product is not only well-suited for long-term storage but also versatile in its applications, such as serving as an alternative treatment for malaria. While the particle size and zeta potential of phytosomes showed slight changes, these variations were not significant. Notably, particle size variation was relatively greater at 40°C compared to 25°C, as shown in Tables 3 and 4. The stability of phytosomes can be influenced by temperature. Higher temperatures increase the kinetic energy of the molecules within the phytosome, potentially causing vesicles coalescence and changes in particle size and zeta potential.74,104,105 Therefore, maintaining temperature control is crucial for ensuring the stability of phytosome.
The entrapment efficiency of phytosome showed a slight decrease after storage for three months. It was further concluded that at a room temperature of 25°C the amount of active substances in phytosomes was more stable than at 40°C. Visual inspection revealed no significant color change in the formulation after three months at either 25°C or 40°C. Phytosomes can be an effective formulation with good stability, and they are best stored at room temperature (25°C). In the stability studies, the decrease in entrapment efficiency was attributed to vesicle leakage, which is caused by the high flexibility of the lipid bilayer at elevated temperatures.93,106
In vivo antimalarial activity testing was conducted due to its practicality and efficiency compared to in vitro tests.107,108 The content of the extract in phytosomes was equivalent to the effective dose (ED50) of the extract as determined by prior study (95.28 mg/kg body weight of mice).62 The antimalarial activity test was conducted using the four-day suppression method—widely employed standard test for early infection and primary screening of antimalarial drugs. Substances with a percentage inhibition value of more than 30% are considered active.59,109 As shown in Figure 9, the percentage of parasitemia in the phytosome-extract group was lower than in the extract group.110 Meanwhile, in Figure 10, the percentage inhibition in all groups, except the negative control, exceeded 50%. This indicates that the samples administered to the tested groups effectively protected mice from P. berghei infection. The strongest parasite inhibition activity was observed on the fourth day of investigation, coinciding with the peak levels of the active constituent after continuous administration into the animal’s body system.61 Based on the results, the antimalarial activity of phytosomes was higher than that of the extract and fraction, likely due to several mechanisms: increased solubilization and enhanced bioavailability. Phospholipids in phytosomes form hydrogen bonds with water molecules, thereby increasing the solubility of naphthoquinone by facilitating its dissolution in aqueous media.111 Furthermore, phospholipids can deform cell membranes, allowing deeper penetration of naphthoquinone and thus increasing its bioavailability.111 Phytosomes also enhance the absorption of naphthoquinone by improving its solubility, which facilitates more efficient penetration into the bloodstream.111 The enhanced solubility and activity of naphthoquinone within the phytosome system make it a promising delivery method to improve therapeutic efficacy.112 This research highlights a significant novelty. Phytosomes—using the same dose of free extract from a previous study—demonstrated higher activity.62 Phospholipids, being amphiphilic, interact effectively with cell membrane, which are also composed of phospholipid.75 Active compounds encapsulated in phytosomes can cross the cell membrane more easily, thus enhancing the bioavailability of the active compounds and potentially reducing the risk of adverse side effects.113
A previous study by Muhaimin et al demonstrated the antimalarial properties of Sonneratia alba using Peter’s Test method on mice. The findings revealed that the ethanol extract derived from the leaves of S. alba inhibited parasitic growth in malaria-infected mice. Doses of 300, 150, and 75 mg/kg BW resulted in parasitemia suppression of 77.34%, 62.35%, and 43.53%, respectively.62 The current research is its continuation to which the delivery system of the active extract to enhance its antimalarial activity. It was found that using the same dose, the phytosome-formulated extract exhibited significantly higher antimalarial activity (80.12%) compared to the extract alone (53.38%).
Chloroquine is a commonly used antimalarial medication, exhibiting approximately 90% efficacy against malaria-causing parasites. However, its use is often limited due to drug resistance and adverse side effects. Phytosomes derived from S. alba extract demonstrated a significant antimalarial inhibition rate of 80%. Since these phytosomes are derived from a natural source, they may reduce the likelihood of adverse effects associated with synthetic antimalarial drugs. In summary, S. alba extract phytosomes exhibited significant efficacy, comparable to conventional drugs, in combating malaria while offering a safer option with fewer risks due to their natural origin. This suggests their potential as a natural alternative for malaria treatment.
A previous study by Muhaimin et al identified naphthoquinone as the compound responsible for the antimalarial activity of S. alba leaves.22,62 This activity is likely due to the inhibition of parasite growth by damaging mitochondrial function, which affects electron transport and prevents the formation of hemozoin.22,114 Naphthoquinones are important chemical compounds in the development of antiparasitic drugs.115 Buparvaquone, parvaquone, and atovaquone as well as lapachol and α- and β- lapachones, are naphthoquinones that have been investigated as potential antimalarial agents. These compounds have demonstrated a broad range of antimalarial activity against both chloroquine-sensitive and chloroquine-resistant strains.116
Considering the high resistance to current malaria drugs, the potential for S. alba phytosomes with antimalarial efficacy in clinical trials is significant. However, before clinical studies on humans, several factors must be carefully addressed. Potential negative effects from the extract, fraction, or phytosomes need to be considered, including allergies, systemic responses, or other complications from long-term administration. Monitoring and evaluating the dosage and administration of phytosomes is essential to understanding their biopharmaceutical behavior in the human body, as both excessive and insufficient doses can have serious repercussions. Additionally, a thorough toxicity assessment is required to compared the potential negative effects of phytosomes with those of the extract and fraction, ensuring that at an effective dose, the dosage form is safe for human use.
Conclusion
The primary factors influencing phytosomes production—particle size, zeta potential, and entrapment efficiency (EE)—were identified through Box-Behnken design analysis. The mathematical model generated relationships between actual and predicted values, offering high predictions accuracy. The optimized phytosome-extract formulation achieved particle size, zeta potential, and EE values of 471.8 nm, −54.1 mV, and 82.4%, respectively. When applied to phytosome-fractions, the formulation yielded particle size, zeta potential, and EE values of 233.4 nm, −61.5 mV, and 87.08%, respectively. Transmission electron microscopy (TEM) confirmed that both phytosome-extract and phytosome-fraction of S. alba exhibited spherical shape with smooth surface. Stability studies indicated that the phytosome formulation remained stable over time.
In vitro release studies demonstrated that phytosomes significantly enhance the solubilization of naphthoquinone—a marker compound with antimalarial activity in S. alba. The antimalarial efficacy of phytosomes showed marked improvement compared to the extract and fraction alone. These findings suggest that phytosomes enhance the effectiveness of phytoconstituents in S. alba extract and fraction antimalarial agents.
Acknowledgments
The authors gratefully acknowledge the support of the Directorate General of Higher Education, Ministry of Education and Culture, Republic of Indonesia, for funding this research through the Doctoral Dissertation Research Grant for year 2021.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Patel D, Patel B, Thakkar H. Lipid based nanocarriers: promising drug delivery system for topical application. Eur J Lipid Sci Technol. 2021;123(5). doi:10.1002/ejlt.202000264
2. Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. J Pharm. 2014;2014:1–10. doi:10.1155/2014/801820
3. Asadujjaman M, Mishuk AU. Novel approaches in lipid based drug delivery systems. JDDT. 2013;3(4). doi:10.22270/jddt.v3i4.578
4. Elkordy AA, Haj-Ahmad RR, Awaad AS, Zaki RM. An overview on natural product drug formulations from conventional medicines to nanomedicines: past, present and future. J Drug Deliv Sci Technol. 2021;63. doi:10.1016/j.jddst.2021.102459
5. Agarwal A, Kharb V, Saharan VA. Process optimisation, characterisation and evaluation of resveratrol-phospholipid complexes using Box-Behnken statistical design. ICPJ. 2014;3(7). doi:10.3329/icpj.v3i7.19079
6. Gandhi A, Dutta A, Pal A, Bakshi P. Recent trends of phytosomes for delivering herbal extract with improved bioavailability. J Pharmacogn Phytochem. 2012;1(4):1.
7. Sanjay Saha SASP and C. Phytosome: a brief overview. SAJP. 2013;2(1):12–20.
8. Yusuf NA, Abdassah M, Mauludin R, Joni IM, Chaerunisaa AY. Transfersome: a vesicular drug delivery with enhanced permeation. JAPER. 2021;11(2). doi:10.51847/vrYnt7vHhp
9. Singh A, Saharan VA, Singh M, Bhandari A. Phytosome: drug delivery system for polyphenolic phytoconstituents. Iran J Pharm Sci. 2011;7(4):1.
10. Rathee S, Kamboj A. Optimization and development of antidiabetic phytosomes by the Box–Behnken design. J Liposome Res. 2018;28(2). doi:10.1080/08982104.2017.1311913
11. Dewi MK, Chaerunisaa AY, Muhaimin M, Joni IM. Improved activity of herbal medicines through nanotechnology. Nanomaterials. 2022;12(22):4073. doi:10.3390/nano12224073
12. Mazumder A, Dwivedi A, Du Preez JL, Du Plessis J. In vitro wound healing and cytotoxic effects of sinigrin-phytosome complex. Int J Pharm. 2016;498(1–2):283–293. doi:10.1016/j.ijpharm.2015.12.027
13. Telange DR, Patil AT, Pethe AM, Fegade H, Anand S, Dave VS. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur J Pharm Sci. 2017;105:108. doi:10.1016/j.ejps.2016.12.009
14. El-Fattah AI A, Fathy MM, Ali ZY, El-Garawany AERA, Mohamed EK. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem Biol Interact. 2017;271. doi:10.1016/j.cbi.2017.04.026
15. Singh RP, Gangadharappa HV, Mruthunjaya K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: in vitro and in vivo evaluation. Eur J Pharm Sci. 2018;112:122. doi:10.1016/j.ejps.2018.06.028
16. Chime SA, Akpa PA, Onyishi IV, Ezenduka DE. phytosomes enhanced the antibacterial and antifungal properties of lantana camara. Innov J Ayurv Sci. 2020;8(1):1–5.
17. Rani A, Kumar S, Khar RK. Murraya koenigii extract loaded phytosomes prepared using antisolvent precipitation technique for improved antidiabetic and hypolidemic activity. IJPER. 2022;56(2s):s326–s338. doi:10.5530/ijper.56.2s.103
18. Barani M, Sangiovanni E, Angarano M, et al. Phytosomes as innovative delivery systems for phytochemicals: a comprehensive review of literature. Int J Nanomed. 2021;16:6983–7022. doi:10.2147/IJN.S318416
19. Shah B, Puranik SB, Raghuchandan HS. Preparation and evaluation of curcumin phytosomes. Int J Pharm Pharm Res. 2020;17(4):767–792.
20. Musa WJA, Bialangi N, Situmeang B, Silaban S. Triterpenoid compound from methanol extract of mangrove leaves (Sonneratia alba) and anti-cholesterol activity test. J Pendidikan Kimia. 2019;11(1):18–23. doi:10.24114/jpkim.v11i1.13124
21. Ragasa CY, Ebajo VD, De Los Reyes MM, Mandia EH, Brkljača R, Urban S. Triterpenes and sterols from sonneratia alba. IJPR. 2015;6(6):1.
22. Muhaimin M, Latief M, Putri RD, et al. Antiplasmodial activity of methanolic leaf extract of mangrove plants against plasmodium berghei. Pharmacogn J. 2019;11(5):929–935. doi:10.5530/pj.2019.11.148
23. Muhaimin M, Ramadhan DW, Latief M. Isolasi Senyawa Turunan Kuinon dari Ekstrak Aseton Daun Perepat (Sonneratia Alba) dan Uji Aktivitas Terhadap Staphylococcus Aureus. JISIC. 2022;14(1):44–56. doi:10.22437/jisic.v14i1.18213
24. Rajput RPS, Chakravarty C, Bhardwaj SK. Preparation and evaluation of phytosome of herbal plant of Lawsonia inermis l for topical application. JIPS. 2019;3(2):1.
25. Tambe V, Ditani A, Rajpoot K, Tekade RK. Pharmacokinetics aspects of structural modifications in drug design and therapy. Biopharmaceutics and Pharmacokinetics Considerations: Volume 1 in Advances in Pharmaceutical Product Development and Research. 2021. doi:10.1016/B978-0-12-814425-1.00014-0
26. Zulham ZZ, Wilar G, Susilawati Y, Subarnas A, Chaerunisaa AY. Microparticles of Herbal Extracts with Antioxidant Activity. Pharmacogn J. 2021;13(1):285–295. doi:10.5530/pj.2021.13.38
27. Harwansh RK, Deshmukh R, Rahman MA. Nanoemulsion: promising nanocarrier system for delivery of herbal bioactives. J Drug Deliv Sci Technol. 2019;51:224–233. doi:10.1016/j.jddst.2019.03.006
28. Assadpour E, Mahdi Jafari S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit Rev Food Sci Nutr. 2019;59(19):3129–3151. doi:10.1080/10408398.2018.1484687
29. Rostamabadi H, Falsafi SR, Jafari SM. Nanoencapsulation of carotenoids within lipid-based nanocarriers. JCR. 2019;298:38–67. doi:10.1016/j.jconrel.2019.02.005
30. Jafari SM, McClements DJ. Nanotechnology Approaches for Increasing Nutrient Bioavailability. In: Advances in Food and Nutrition Research. Elsevier; 2017. doi10.1016/bs.afnr.2016.12.008
31. Antoine T, Fisher N, Amewu R, O’Neill PM, Ward SA, Biagini GA. Rapid kill of malaria parasites by artemisinin and semi-synthetic endoperoxides involves ROS-dependent depolarization of the membrane potential. J Antimicrob Chemother. 2014;69(4):1005–1016. doi:10.1093/jac/dkt486
32. Newton PN, Caillet C, Guerin PJ. A link between poor quality antimalarials and malaria drug resistance? Expert Rev Anti Infect Ther. 2016;14(6):531–533. doi:10.1080/14787210.2016.1187560
33. Gurumukhi VC, Bari SB. Fabrication of efavirenz loaded nano-formulation using quality by design (QbD) based approach: exploring characterizations and in vivo safety. J Drug Deliv Sci Technol. 2020;56. doi:10.1016/j.jddst.2020.101545
34. Amasya G, Aksu B, Badilli U, Onay-Besikci A, Tarimci N. QbD guided early pharmaceutical development study: production of lipid nanoparticles by high pressure homogenization for skin cancer treatment. Int J Pharm. 2019;563. doi:10.1016/j.ijpharm.2019.03.056
35. Colobatiu L, Gavan A, Mocan A, Bogdan C, Mirel S, Tomuta I. Development of bioactive compounds-loaded chitosan films by using a QbD approach – a novel and potential wound dressing material. React Funct Polym. 2019;138. doi:10.1016/j.reactfunctpolym.2019.02.013
36. Pallagi E, Jójárt-Laczkovich O, Németh Z, Szabó-Révész P, Csóka I. Application of the QbD-based approach in the early development of liposomes for nasal administration. Int J Pharm. 2019;562. doi:10.1016/j.ijpharm.2019.03.021
37. Antoy J. Design of Experiments for Engineers and Scientists. Elsevier; 2014. doi:10.1016/C2012-0-03558-2
38. Wagner JR, Mount EM, Giles HF. Extrusion: The Definitive Processing Guide and Handbook. William Andrew; 2013. doi:10.1016/C2010-0-67040-4
39. Jazuli I, Annu, Nabi B, Nabi B, et al. Optimization of nanostructured lipid carriers of lurasidone hydrochloride using box-behnken design for brain targeting: in vitro and in vivo studies. J Pharm Sci. 2019;108(9):3082–3090. doi:10.1016/j.xphs.2019.05.001
40. Yang L, Cai T, Ding D, et al. Biodegradation of 2-hydroxyl-1,4 naphthoquinone (lawsone) by Pseudomonas taiwanensis LH-3 isolated from activated sludge. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-06338-1
41. Russell WMS, Burch RL. The Principles of Humane Experimental Technique. Methuen; 1959.
42. National Research Council, Division on Earth, Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care, Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2011.
43. Patel K, Patel J, Patel M, Rajput G. A validated method for development of atovaquone as API and tablet dosage forms by UV spectroscopy. Pharm Methods. 2010;1(1):61. doi:10.4103/2229-4708.72234
44. Arora D, Nanda S. Quality by design driven development of resveratrol loaded ethosomal hydrogel for improved dermatological benefits via enhanced skin permeation and retention. Int J Pharm. 2019;567. doi:10.1016/j.ijpharm.2019.118448
45. Alhakamy NA, Fahmy UA, Badr-Eldin SM, et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics. 2020;12(4):346. doi:10.3390/pharmaceutics12040346
46. Negi P, Singh B, Sharma G, Beg S, Raza K, Katare OP. Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: qbD-based development and evaluation. Drug Deliv. 2016;23(3):941–957. doi:10.3109/10717544.2014.923067
47. Sahibzada MUK, Sadiq A, Khan S, et al. Fabrication, characterization and in vitro evaluation of silibinin nanoparticles: an attempt to enhance its oral bioavailability. Drug Des Devel Ther. 2017:11. doi:10.2147/DDDT.S133806
48. Chaerunisaa AY, Dewi MK, Joni IM, Dwiyana RF. Development of cathelicidin in liposome carrier using thin layer hydration method. IJAP. 2022;14(4). doi:10.22159/ijap.2022v14i4.44480
49. Direito R, Reis C, Roque L, et al. Phytosomes with persimmon (Diospyros kaki l.) extract: preparation and preliminary demonstration of in vivo tolerability. Pharmaceutics. 2019;11(6):296. doi:10.3390/pharmaceutics11060296
50. Singh RP, Narke R. Preparation and evaluation of phytosome of lawsone. Int J Pharm Sci Res. 2015;6(12):5217–5226.
51. Peanparkdee M, Yooying R. Enhancement of solubility, thermal stability and bioaccessibility of vitexin using phosphatidylcholine-based phytosome. NFS J. 2023;31. doi:10.1016/j.nfs.2023.03.001
52. Deleanu M, Toma L, Sanda GM, et al. Formulation of phytosomes with extracts of ginger rhizomes and rosehips with improved bioavailability, antioxidant and anti-inflammatory effects in vivo. Pharmaceutics. 2023;15(4):1066. doi:10.3390/pharmaceutics15041066
53. Alfaifi MY, Shati AA, Elbehairi SEI, Fahmy UA, Alhakamy NA, Md S. Anti-tumor effect of PEG-coated PLGA nanoparticles of febuxostat on A549 non-small cell lung cancer cells. 3 Biotech. 2020;10(3). doi:10.1007/s13205-020-2077-x
54. Rasul A, Khan MI, Rehman MU, et al. In vitro characterization and release studies of combined nonionic surfactant-based vesicles for the prolonged delivery of an immunosuppressant model drug. Int J Nanomed. 2020:15. doi:10.2147/IJN.S268846
55. Jain P, Taleuzzaman M, Kala C, Kumar Gupta D, Ali A, Aslam M. Quality by design (Qbd) assisted development of phytosomal gel of aloe vera extract for topical delivery. J Liposome Res. 2021;31(4):381–388. doi:10.1080/08982104.2020.1849279
56. Taleuzzaman M, Sartaj A, Kumar Gupta D, Gilani SJ, Mirza MA. Phytosomal gel of Manjistha extract (MJE) formulated and optimized with central composite design of Quality by Design (QbD). J Dispers Sci Technol. 2023;44(2):236–244. doi:10.1080/01932691.2021.1942036
57. Shariare MH, Afnan K, Iqbal F, et al. Development and optimization of epigallocatechin-3-gallate (egcg) nano phytosome using design of experiment (DoE) and their in vivo anti-inflammatory studies. Molecules. 2020;25(22):5453. doi:10.3390/MOLECULES25225453
58. ICH. ICH Topic Q 1 A (R2) stability testing of new drug substances and products step 5 note for guidance on stability testing: stability testing of new drug substances and products; 2003. Available from: http://www.emea.eu.int.
59. Chaniad P, Phuwajaroanpong A, Plirat W, Techarang T, Chukaew A, Punsawad C. In vivo assessment of the antimalarial activity and acute oral toxicity of an ethanolic seed extract of Spondias pinnata (L.f.) Kurz. BMC Complement Med Ther. 2022;22(1). doi:10.1186/s12906-022-03546-9
60. Ounjaijean S, Romyasamit C, Somsak V, Tamadon A. Evaluation of antimalarial potential of aqueous crude gymnema inodorum leaf extract against plasmodium berghei infection in mice. Evid Based Complement Alternat Med. 2021;2021:1–7. doi:10.1155/2021/9932891
61. Udu R, Oyweri J, Gathirwa J, Yuste J. Antimalarial Activity of Nigella sativa L. Seed extracts and selection of resistance in plasmodium berghei ANKA in a mouse model. J Pathog. 2021;2021:1–10. doi:10.1155/2021/6165950
62. Muhaimin M, Latifah N, Chaerunisaa AY, Subarnas A, Susilawati Y, Hirzan R. Antiplasmodial activity of ethanol extract of sonneratia alba leaves. Trop J Nat Prod Res. 2024;8(4):6884–6890. doi:10.26538/tjnpr/v8i4.19
63. Damle M, Mallya R. Development and evaluation of a novel delivery system containing phytophospholipid complex for skin aging. AAPS Pharm Sci Tech. 2016;17(3):607–617. doi:10.1208/s12249-015-0386-x
64. Kumar A, Kumar B, Singh SK, Kaur B, Singh S. A review on phytosomes: novel approach for herbal phytochemicals. Asian J. Pharm. Clin. Res. 2017;10(10):41. doi:10.22159/ajpcr.2017.v10i10.20424
65. Kumar S, Baldi A, Sharma DK. Phytosomes: a modernistic approach for novel herbal drug delivery-enhancing bioavailability and revealing endless frontier of phytopharmaceuticals. J Dev Drugs. 2020;9(2):1.
66. Dhyani A. Phytosomes: an advanced herbal drug delivery system. Curr Trends Biomed Eng Biosci. 2017;3(5). doi:10.19080/ctbeb.2017.03.555621
67. Gaikwad SS, Morade YY, Kothule AM, Kshirsagar SJ, Laddha UD, Salunkhe KS. Overview of phytosomes in treating cancer: advancement, challenges, and future outlook. Heliyon. 2023;9(6):e16561. doi:10.1016/j.heliyon.2023.e16561
68. Awasthi R, Kulkarni GT, Pawar VK. Phytosomes: an approach to increase the bioavailability of plant extracts. Int J Pharm Pharm Sci. 2011;3(2):1.
69. Swati Verma AR; Ananta Choudhury*. Phytosome: a novel dosage form for herbal drug delivery. J Appl Pharmaceut Res. 2014;2(2348):1
70. Toma L, Deleanu M, Sanda GM, et al. Bioactive compounds formulated in phytosomes administered as complementary therapy for metabolic disorders. Int J Mol Sci. 2024;25(8):4162. doi:10.3390/ijms25084162
71. Khanzode MB, Kajale AD, Channawar MA, Gawande SR. Review on phytosomes: a novel drug delivery system. GSC Bio Pharmaceutical Sciences. 2020;13(1). doi:10.30574/gscbps.2020.13.1.0345
72. Jeevana Jyothi B, Mary Ragalatha P. Development and in vitro evaluation of phytosomes of naringin. Asian J Pharm Clin Res. 2019;15(1):280–9. doi:10.22159/ajpcr.2019.v12i9.34798
73. Sasikirana W, Annisaa’ E, Ekawati N. Phytosome as cytotoxic agent delivering system: a review. Gener J Res Pharm. 2021;1(2). doi:10.14710/genres.v1i2.10953
74. Sundaresan N, Kaliappan I. Development and characterization of a nano-drug delivery system containing vasaka phospholipid complex to improve bioavailability using quality by design approach. Res Pharm Sci. 2021;16(1). doi:10.4103/1735-5362.305193
75. Lu M, Qiu Q, Luo X, et al. Phyto-phospholipid complexes (phytosomes): a novel strategy to improve the bioavailability of active constituents. Asian J Pharm Sci. 2019;14(3):265–274. doi:10.1016/j.ajps.2018.05.011
76. Makkar R, Behl T, Singh S, Sharma N, Chauhan A, Tuli HS. Box behnken design based formulation optimization and characterization of gallic acid loaded phytosomes. Eur Chem Bull. 2023;12(4):2568–2586.
77. Gandhi T, Patel V, Dalwadi S, Thakkar V. Development and optimization of polyherbal formulation for antiurolithiatic activity using box behnken statistical design. Thai J Pharmac Sci. 2022;46(4):432–440. doi:10.56808/3027-7922.2625
78. HBM Prenscia Inc. Box-behnken designs: analysis results; 2017. Available from: https://help.synthesisplatform.net/weibull_alta11/box-behnken_designs__analysis_results.htm.
79. Alhakamy NA, Badr-Eldin SM, Fahmy UA, et al. Thymoquinone-loaded soy-phospholipid-based phytosomes exhibit anticancer potential against human lung cancer cells. Pharmaceutics. 2020;12(8):761. doi:10.3390/pharmaceutics12080761
80. Habbu P, Madagundi S, Kulkarni R, Jadav S, Vanakudri R, Kulkarni V. Preparation and evaluation of Bacopa-phospholipid complex for antiamnesic activity in rodents. Drug Invention Today. 2013;5(1):13–21. doi:10.1016/j.dit.2013.02.004
81. Maltese F, Van Der Kooy F, Verpoorte R. Solvent derived artifacts in natural products chemistry. Nat Prod Commun. 2009;4(3):447–454. doi:10.1177/1934578x0900400326
82. Minitab. Contour plots and 3D surface plots. Available from: https://support.minitab.com/en-us/minitab/21/help-and-how-to/statistical-modeling/using-fitted-models/supporting-topics/graphs/contour-plots-and-3d-surface-plots/.
83. Akram W, Garud N. Design expert as a statistical tool for optimization of 5-ASA-loaded biopolymer-based nanoparticles using Box Behnken factorial design. Futur J Pharm Sci. 2021;7(1). doi:10.1186/s43094-021-00299-z
84. Song Z, Yin J, Xiao P, et al. Improving breviscapine oral bioavailability by preparing nanosuspensions, liposomes and phospholipid complexes. Pharmaceutics. 2021;13(2):132. doi:10.3390/pharmaceutics13020132
85. Chen X, Fan X, Li F. Development and evaluation of a novel diammonium glycyrrhizinate phytosome for nasal vaccination. Pharmaceutics. 2022;14(10):2000. doi:10.3390/pharmaceutics14102000
86. Permana AD, Utami RN, Courtenay AJ, Manggau MA, Donnelly RF, Rahman L. Phytosomal nanocarriers as platforms for improved delivery of natural antioxidant and photoprotective compounds in propolis: an approach for enhanced both dissolution behaviour in biorelevant media and skin retention profiles. J Photochem Photobiol B. 2020;2020:205. doi:10.1016/j.jphotobiol.2020.111846
87. Demir B, Barlas FB, Guler E, et al. Gold nanoparticle loaded phytosomal systems: synthesis, characterization and in vitro investigations. RSC Adv. 2014;4(65):34687–34695. doi:10.1039/C4RA05108D
88. Shriram RG, Moin A, Alotaibi HF, et al. Phytosomes as a plausible nano-delivery system for enhanced oral bioavailability and improved hepatoprotective activity of silymarin. Pharmaceuticals. 2022;15(7):790. doi:10.3390/ph15070790
89. Anwar E, Farhana N. Formulation and evaluation of phytosome-loaded maltodextrin-gum Arabic microsphere system for delivery of camellia sinensis extract. J Young Pharm. 2018;10(2):S56–S62. doi:10.5530/jyp.2018.2s.11
90. Sharma S, Shukla P, Misra A, Mishra PR. Interfacial and colloidal properties of emulsified systems: pharmaceutical and biological perspective. Pharmaceutical and biological perspective. Coll Interf Sci Pharmac Res Develop. 2014. doi:10.1016/B978-0-444-62614-1.00008-9
91. Maurya SD, Dhakar RC, Aggarwal S, Tilak VK, Verma KK, Prajapati SK. Enhancement of transdermal permeation of indinavir sulfate via ethosomevesicles the African. J Pharmac Sci Pharm. 2011;2(2):33–47.
92. Were LM, Bruce BD, Davidson PM, Weiss J. Size, stability, and entrapment efficiency of phospholipid nanocapsules containing polypeptide antimicrobials. J Agric Food Chem. 2003;51(27):8073–8079. doi:10.1021/jf0348368
93. Ullmann K, Leneweit G, Nirschl H. How to achieve high encapsulation efficiencies for macromolecular and sensitive apis in liposomes. Pharmaceutics. 2021;13(5):691. doi:10.3390/pharmaceutics13050691
94. Moustafa AB, Sobh RA, Rabie AM, Nasr HE, Ayoub MMH. Differential microemulsion polymerization as a new root for entrapment of drugs. J Appl Polym Sci. 2013;127(6):4634–4643. doi:10.1002/app.38059
95. Khan I, Needham R, Yousaf S, et al. Impact of phospholipids, surfactants and cholesterol selection on the performance of transfersomes vesicles using medical nebulizers for pulmonary drug delivery. J Drug Deliv Sci Technol. 2021:66. doi:10.1016/j.jddst.2021.102822
96. Mazumder A, Dwivedi A, Fox LT, et al. In vitro skin permeation of sinigrin from its phytosome complex. J Pharm Pharmacol. 2016;68(12):1577–1583. doi:10.1111/jphp.12594
97. Dwivedi J, Sachan P, Wal P, Kosey S, Khan MMU. Progressive journey of phytosomes: preparation, characterization, patents, clinical trials & commercial products. J Res Pharm. 2023;27(5). doi:10.29228/jrp.457
98. Kumar DS, Deivasigamani K, Roy B. Development and optimization of phytosome for enhancement of therapeutic potential of epiyangambin in tinospora cordifolia extract identified by GC–MS and docking analysis. Pharmacogn Mag. 2023;19(2):371–384. doi:10.1177/09731296231157192
99. Kaur B, Saxena A, Roy K, Bhardwaj M, Bisht M. Development and in-vitro evaluation of phytosomes containing herbal extract of centella asiatica for antiulcer and antioxidant activity. J Adv Sci Res. 2023;14(03). doi:10.55218/jasr.2023140305
100. Solanki H, Malviya K, Soni P, Kumar P, Omray LK. Formulation and evaluation of phytosomes loaded with tabernaemontana divaricata leaf extract. J Popul Ther Clin Pharmacol. 2023;30:1810–1817. doi:10.53555/jptcp.v30i18.3357
101. Freag MS, Elnaggar YSR, Abdallah OY. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: optimization and ex vivo permeation. Int J Nanomed. 2013;8:2385–2397. doi:10.2147/IJN.S45231
102. Bepe N. Preparation, characterisation and evaluation of artemisia afra phytosomes with modified release properties; 2017. Available from: http://etd.uwc.ac.za/.
103. Nguyen TL, Nguyen TH, Nguyen DH. Development and in vitro evaluation of liposomes using soy lecithin to encapsulate paclitaxel. Int J Biomater. 2017;2017:1–7. doi:10.1155/2017/8234712
104. Chittasupho C, Chaobankrang K, Sarawungkad A, et al. Antioxidant, anti-inflammatory and attenuating intracellular reactive oxygen species activities of nicotiana tabacum var. Virginia leaf extract phytosomes and shape memory gel formulation. Gels. 2023;9(2):78. doi:10.3390/gels9020078
105. Human C, Aucamp M, de Beer D, van der Rijst M, Joubert E. Food‐grade phytosome vesicles for nanoencapsulation of labile C ‐glucosylated xanthones and dihydrochalcones present in a plant extract matrix—Effect of process conditions and stability assessment. Food Sci Nutr. 2023;11(12):8093–8111. doi:10.1002/fsn3.3730
106. Anderson M, Omri A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004;11(1):33–39. doi:10.1080/10717540490265243
107. Romanha AJ, de Castro SL, Soeiro de MNC, et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz. 2010;105(2):233–238. doi:10.1590/S0074-02762010000200022
108. Keiser J. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology. 2010;137(3):589–603. doi:10.1017/S0031182009991739
109. Gorobets N, Sedash YV, Singh BK, Rathi B. An overview of currently available antimalarials. Curr Top Med Chem. 2017;17(19). doi:10.2174/1568026617666170130123520
110. Jelveh K, Mottaghitalab M, Mohammadi M. Effects of green tea phytosome on growth performance and intestinal integrity under coccidiosis infection challenge in broilers. Poult Sci. 2023;102(5):102627. doi:10.1016/j.psj.2023.102627
111. Susilawati Y, Chaerunisa AY, Purwaningsih H. Phytosome drug delivery system for natural cosmeceutical compounds: whitening agent and skin antioxidant agent. J Adv Pharm Technol Res. 2021;12(4). doi:10.4103/japtr.JAPTR_100_20
112. Assadpour E, Jafari SM. An overview of lipid-based nanostructures for encapsulation of food ingredients. In: Lipid-Based Nanostructures for Food Encapsulation Purposes. The Nanoencapsulation in the Food Industry Series; 2019. doi10.1016/B978-0-12-815673-5.00001-5
113. Karataş A, Turhan F. Phyto-phospholipid complexes as drug delivery system for herbal extracts/molecules. Turk J Pharm Sci. 2015;12(1):1.
114. Hughes LM, Lanteri CA, Oneil MT, Johnson JD, Gribble GW, Trumpower BL. Design of anti-parasitic and anti-fungal hydroxy-naphthoquinones that are less susceptible to drug resistance. Mol Biochem Parasitol. 2011;177(1):12–19. doi:10.1016/j.molbiopara.2011.01.002
115. Moreira DRM, De Sá MS, Macedo TS, et al. Evaluation of naphthoquinones identified the acetylated isolapachol as a potent and selective antiplasmodium agent. J Enzyme Inhib Med Chem. 2015;30(4):615–621. doi:10.3109/14756366.2014.958083
116. Pérez-Sacau E, Estévez-Braun A, Ravelo ÁG, Gutiérrez Yapu D, Giménez Turba A. Antiplasmodial activity of naphthoquinones related to lapachol and β -lapachone. Chem Biodivers. 2005;2(2):264–274. doi:10.1002/cbdv.200590009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.