Back to Journals » International Journal of Nanomedicine » Volume 20
How Traditional Chinese Medicine Can Play a Role In Nanomedicine? A Comprehensive Review of the Literature
Authors Wang C, Ren K , Yang M, Li X , Li N, Li P, Yang H, Zhang G , Wei X
Received 21 January 2025
Accepted for publication 13 May 2025
Published 20 May 2025 Volume 2025:20 Pages 6289—6315
DOI https://doi.org/10.2147/IJN.S518610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Farooq A. Shiekh
Chi Wang,1,2 Kaixiang Ren,1,2 Mei Yang,1,2 Xiang Li,3 Ningxi Li,4 Peng Li,1,2 Huang Yang,5,6 Guangjian Zhang,1,2 Xiaodan Wei1,2
1Department of Thoracic Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China; 2Key Laboratory of Enhanced Recovery After Surgery of Intergrated Chinese and Western Medicine, Administration of Traditional Chinese Medicine of Shaanxi Province, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China; 3Department of Ophthalmology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China; 4Mianyang Key Laboratory of Anesthesia and Neuroregulation, Department of Anesthesiology, Mianyang Central Hospital, Mianyang, 621000, People’s Republic of China; 5Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310003, People’s Republic of China; 6MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310003, People’s Republic of China
Correspondence: Xiaodan Wei; Guangjian Zhang, Department of Thoracic Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Email [email protected]; [email protected]
Abstract: Traditional Chinese medicine (TCM), a time-honored practice rooted in natural therapeutics, has served as a cornerstone in safeguarding human health across millennia, aiding in disease mitigation and life vitality preservation. However, many TCM active ingredients suffer from poor solubility, low bioavailability, uncertain toxicity and weak targeting ability. Nanomedicine represents a modern scientific frontier, emerging from the precise engineering of unique nanoscale characteristics, with extensive applications encompassing targeted therapeutic delivery and diverse biomedical fields. Although TCM and nanomedicine diverge fundamentally in historical origins and disciplinary foundations, growing investigations demonstrate their synergistic potential. In this review, nanosized TCM has been revealed as an innovative therapeutic strategy with significant clinical value. Based on the biological activities and structural characteristics of TCM active ingredients, we classify them into two categories: natural nanostructured formulations for TCM and nano-drug delivery systems for TCM. A systematic and comprehensive analysis of preparations specific and functions to two classes of TCM nanomedicines is highlighted. Insights into the advantage of TCM nanomedicines are also introduced. Subsequently, the applications of TCM nanomedicines in the biomedical treatment, including anti-cancer, anti-inflammation and anti-bacterial are summarized. Finally, challenges and future research directions are emphasized, aiming to offer guidance for the modernization of TCM nanomedicines.
Keywords: traditional Chinese medicine, active ingredients, drug delivery, biomedical treatment
Introduction
Traditional Chinese medicine (TCM), termed medicinal plants, has been of great importance in human health for thousands of years, serving as a complementary therapy for clinically treating diseases in China, South Korea, Japan, India and other countries. Specially, the World Health Organization has incorporated TCM into the supportive treatment system for cancer, allowing more countries in fully exploiting the therapeutic potential of TCM. A growing amount of research validates the diverse biological effects of TCM, such as anti-tumor,1 anti-bacterial,2 and anti-inflammatory3 properties. Furthermore, TCM extracts with unique structural skeleton and stereochemistry could effectively bind to drug targets, regulate physiological processes, and treat numerous diseases, providing extensive and valuable avenues for developing novel drugs.4 Many TCM-derived drugs are already in clinical use, including artemisinin extracted from sweet wormwood serves as a treatment for malaria,5 curcumin for anti-inflammation therapy,6 and quercetin as a tumor chemotherapy drug.7 Regrettably, most TCM extracts have poor water solubility, restricted bioavailability, weak targeting ability and low stability, which impedes several novel drugs from entering clinical application.8–10 Thus, fully harnessing the biological activity of TCM, and developing innovative drug products with less side effects than the prevalent drugs, is a significant challenge in current scientific research.
The deep interdisciplinary convergence across biomedicine, chemistry, and materials has driven paradigm-shifting innovations, offering groundbreaking theoretical foundations for state-of-the-art technological advancements. In recent decades, emerging nanotechnology has brought about a revolutionary change in the domain of medicine and healthcare. Particularly, nanomedicines have been intensively investigated for the prevention and therapeutic interventions against diverse pathologies, such as cancer and infectious diseases, Nano-drug delivery systems (NDDS) as the specific application play a crucial role in driving the progress of advancing nanomedicine due to their multiple beneficial characteristics, including the high-efficiency encapsulation of hydrophobic pharmaceuticals within amphiphilic systems, the preservation of therapeutic payloads against metabolic clearance, the superior targeting capabilities on diseased tissue, and so on.11–13 Integrating the cutting-edge nanotechnology with TCM exhibiting polypharmacological activities is a strategically pivotal direction in the current biomedical innovation. So far, significant breakthroughs and progress have been achieved in the field of TCM through nanotechnology implementation, which primarily involves natural nanostructured formulations and NDDS. Nanosized TCM improves drug solubility and stability, enhancing therapeutic efficacy and reducing toxicity.14 Meanwhile, NDDS enables precise drug delivery and controlled release, maximizing efficacy and minimizing side effects through synergistic interactions with carriers.15
Herein, we summarize the nanostructured formulations of TCM active ingredients, including natural nanostructured formulations for TCM (such as the self-assembly of natural compounds, carbon dots and extracellular vesicles derived from TCM) and exogenous NDDS for TCM (such as inorganic nanocarriers, organic nanocarriers, and so on) (Figure 1). We elucidate their nanostructures, functions, as well as pharmacological activities, and categorically introduce the biomedical treatment applications of TCM nanomedicines. Moreover, we explore the advantages and challenges associated with TCM nanomedicines, thereby gaining a profound comprehension of how nanotechnology functions in TCM and driving the modernization process of TCM nanomedicines.
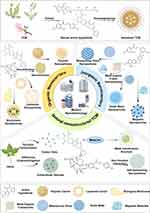 |
Figure 1 Engineering strategies for TCM nanomedicines. By Figdraw. https://www.figdraw.com. |
Where Do We Stand at TCM?
Plant resources and human medicine have evolved together throughout civilization, with their therapeutic practice extensively documented throughout human history. As a landmark treatise, the Compendium of Materia Medica (Bencao Gangmu) details rigorously the pharmacological properties of 1,892 medicinal plant species and their clinical indications. The bioactive potential of these TCM is originated from diverse components, encompassing leaves, flowers, buds, pollen, fruits, exocarps, seeds, roots, stems, bark, and entire plant organism, each endowing distinct therapeutic profiles. The advent of modern chemical methodologies enables the isolation and characterization of numerous bioactive natural products from TCM. German pharmacist Friedrich Serturner first isolated morphine from opium poppy, which are now extensively employed in clinical practice as a potent analgesic and anesthetic, thus inaugurating a new era of natural ingredients of TCM research. Subsequent pharmacological investigations have elucidated the clinical efficacy of various TCM-derived ingredients, solidifying their role as indispensable sources of modern therapeutics. Prominent examples include: artemisinin from the leaves of Artemisia annua leaves with remarkable antimalarial properties;5 baicalin from the roots of Scutellaria baicalensis with anti-inflammatory activity;16 camptothecin from the stem bark of camptotheca acuminata with potent antitumor activity.17 These findings demonstrate the enduring value of TCM-derived natural ingredients in drug discovery and development. Given their structural diversity and biological relevance, natural ingredients from TCM will certainly remain a cornerstone of pharmacological innovation, contributing significantly to global therapeutic advancement. To further exploit the therapeutic potential of TCM active ingredients, advanced nanoformulation-based delivery strategies have emerged as a pivotal research focus. This direction not only impacts the clinical translation efficiency of TCM, but also may redefining the theoretical framework for natural products in intelligent drug development. Based on numerous recent studies, this review provides a systematic overview of current classifications of nanosized TCM, their corresponding constituent formulations, and preparation methodologies (Table 1).
 |
Table 1 Classification and Fabrication Strategies of TCM Nanomedicines |
Natural Nanoformulations for TCM Active Ingredients
Self-Assembly Nanoengineering of TCM Active Ingredients
Metal Coordination Strategy
Metal-coordinated self-assembled nanoparticles (MSANs), as an emerging paradigm in nanomedicine, leverage the synergistic interplay of coordination bonds between metal ions and organic ligands to construct metal-supramolecular assemblies with precisely engineered topological architectures.54 In recent years, a growing number of researchers have strategically employed bioactive components derived from TCM as organic ligands to construct MSANs for disease therapeutics.55,56 Typically, TCM active ingredients with strong coordination capacity, such as polyphenols and polysaccharides, are selected as organic ligands.57,58 Through coordination-driven assembly with transition metal ions (eg, Fe³⁺, Zn²⁺, Cu²⁺) possessing multiple vacant electron orbitals, stable coordination bonds between metal ions and TCM active ingredients are formed, ultimately leading to the construction of corresponding nanoparticles.59
Conventional synthesis strategies predominantly utilize a direct blending protocol, wherein TCM-derived ligands and metal ion solutions are mixed under precisely controlled conditions to induce nanostructural self-assembly via metal-ligand coordination interactions.60 For instance, Yuan et al18 employed a direct blending method to synthesize curcumin-iron nanoparticles by coordinating curcumin with Fe³⁺ ions. Characterization results demonstrated the superior stability of the resulting nanosystem. Guo et al also fabricated Zn-Shik nanoparticles via this methodology using shikonin (Shik) and Zn²⁺ ions for anti-inflammatory therapy and reactive oxygen species (ROS) scavenging.19 Additionally, interfacial self-assembly also represents a critical methodology for fabricating TCM active ingredients-metal coordinated nanoparticles. Interfacial self-assembly refers to a nanostructural architecture strategy where molecules undergo self-organization at multiphase interfaces (eg, liquid-liquid, gas-liquid), demonstrating distinct advantages in the precise fabrication of complex nanostructures.61 Wang et al20 innovatively leveraged the solid-liquid interface formed by a choline and geranic acid ionic liquid as a spatially confined reaction environment to construct MSANs through coordination-driven assembly between quercetin and Fe³⁺, and systematically evaluating their antibacterial efficacy. In recent years, sacrificial template method (also referred to as the hard-particle directed self-assembly) has emerged as a novel strategy and has been successfully applied to the fabrication of TCM active ingredients-metal coordinated nanoparticles.62,63 The fundamental principle of this methodology involves the initial introduction of templating molecules to orchestrate coordination-driven assembly between TCM-derived ligands and metal ions. Following the completion of TCM active ingredients -metal coordination nanoparticle assembly, the templating agents are subsequently removed via selective separation and purification techniques.64,65 Qin et al21 successfully fabricated TA-Fe/Cu nanoparticles using zeolitic imidazolate framework-8 as a sacrificial template through coordination-driven assembly of tannic acid (TA) with Fe³⁺ and Cu²⁺. Compared to conventional MSANs with solid structures, the hollow architecture of TA-Fe/Cu demonstrates superior photothermal conversion efficiency. In vitro and in vivo results confirm that TA-Fe/Cu exhibit remarkable wound-healing efficacy through synergistic photothermal effects and ROS scavenging capability.
Nanoengineered TCM active ingredients-metal complexes exhibit multifaceted advantages in disease therapeutics. First, their dynamic metal-ligand coordination networks significantly address the inherent limitations of TCM active ingredients, such as low bioavailability and poor chemical stability.66 Second, the intrinsic functionalities of metal ions (eg, redox activity of Fe³⁺ and antimicrobial properties of Cu²⁺) combined with the programmable loading capacity for functional molecules endow these nanoparticles with versatile functionalization capabilities. Zhang et al leveraged this characteristic to conjugate photothermally active gold nanorods with TCM active ingredients-metal complexes, successfully constructing a novel and highly efficient antibacterial nanosystem that synergistically integrates photothermal therapy with conventional chemotherapy.22 This coordination chemistry-guided precision nanoengineering strategy not only enhances the targeted delivery efficiency of TCM components but also enables synergistic therapeutic effects, thereby systematically expanding the mechanistic research dimensions of TCM in disease treatment.
TCM Molecular Self-Assembly Strategy
Beyond the aforementioned strategies, another prevalent approach in the self-assembly of TCM active components involves endogenous self-assembly, where amphiphilic-structured TCM molecules autonomously facilitate nanoparticle formation through intermolecular interactions.67 The intermolecular interactions governing this self-assembly process are predominantly non-covalent, including electrostatic interactions, van der Waals forces, hydrogen bonding, hydrophobic interactions, and π-π stacking.68 Current research in the field predominantly focuses on the self-assembly of TCM active components, including polysaccharides, saponins, polyphenols, terpenoids, and alkaloids, owing to their hydrogen bond-rich structures and amphiphilic nature (A molecule exhibits both hydrophilicity and hydrophobicity), which confer superior self-assembly capability compared to other TCM constituents.69–72
The self-assembly process of TCM molecules encompasses multiple initiation strategies, including high-temperature heating, nano-coprecipitation, sonication treatment, and pH modulation methods. High temperature heating can promote non-covalent bonding processes between molecules and enhance the stability of reaction products.73 For instance, Nie et al successfully constructed novel self-assembled nanoparticles through high-temperature processing at 120°C using morusin and liquiritigenin, and characterization results indicated that the nanoparticles displayed excellent stability.23 The nanoprecipitation methodology, a classical approach in nanopharmaceutical fabrication, is primarily governed by two interrelated physicochemical processes: diffusion-controlled thermodynamic equilibration and aggregation-driven kinetic assembly.74 Furthermore, Gao et al synthesized berberine-hesperetin nanoparticles via nano-coprecipitation mediated self-assembly for ulcerative colitis therapy, demonstrating a novel preclinical treatment strategy.24 The underlying principle of sonication lies in its capacity to modulate molecular dimensions, thereby enhancing intermolecular interaction forces that facilitate molecular self-assembly.75 Leveraging this mechanochemical mechanism, Chang et al engineered resveratrol nanoparticles through 100 hz sonication-induced supramolecular organization, establishing a bioengineered platform for developing targeted respiratory syncytial virus infection therapeutics.25 The pH modulation strategy for optimizing the synthesis of self-assembled nanoparticles stems from the protonation-state-regulated mechanism under critical pH conditions, which substantially accelerates the reaction kinetics of molecular self-assembly processes.76,77 Therefore, Fu et al26 successfully induced the self-assembly of berberine and chlorogenic acid by precisely adjusting the pH of the reaction system to 7.0–7.5 using NaOH. Characterization results demonstrated that the resulting self-assembled nanoparticles exhibited excellent structural stability.
TCM molecular self-assembled particles are endowed with inherent biocompatibility and broad-spectrum pharmacological activity profiles via nature-inspired architectural strategies, thereby demonstrating low-risk therapeutic potential with broad-spectrum efficacy in oncology,78 inflammatory disorders,79 and bacterial infection.80 Furthermore, their intrinsic programmability enables precision functionalization modifications to achieve superior drug delivery performance through programmable supramolecular engineering.81
Carbon Dots Nanoengineering of TCM Active Ingredients
Carbon dots (CDs) are an emerging class of ultrasmall nanoparticles (with a size of less than 10 nm) that are primarily classified into four categories: graphene quantum dots (GQDs), carbon quantum dots (CQDs), carbon nanodots (CNDs), and carbon-based polymer dots (CPDs).82 Recently, with advancements in nanopharmaceutical preparation technologies and the deepening exploration of TCM, active ingredients derived from TCM have emerged as promising precursors for synthesizing novel CDs due to their unique natural architectures and biocompatibility.83 These nanomaterials, fabricated using extracts or bioactive molecules from TCM as carbon sources, are collectively termed TCM-CDs.84 Structurally, TCM-CDs can be categorized into two distinct classes: CQDs and CNDs.85,86
The synthesis strategies of CDs are primarily categorized into two major classes: the “top-down” approach and the “bottom-up” approach. The so-called “top-down” synthesis of CDs fundamentally relies on physicochemical techniques to disintegrate bulk carbon materials into CDs, including electrochemical exfoliation, ultrasonication, laser ablation, and arc discharge methods.87–89 However, the “top-down” strategy exhibits intrinsic drawbacks such as broad size distribution, pronounced structural heterogeneity, inadequate control over processing parameters, and inevitable impurity/defect incorporation.90 The “bottom-up” synthesis methodology relies on molecular-level assembly of precursors to enable the controlled fabrication of CDs,91 which demonstrates superior viability over the “top-down” approach for achieving large-scale and cost-effective production of CDs, primarily due to its inherent advantages in controllable molecular assembly and energy-efficient processing pathways.92 Consequently, the bottom-up approach has been established as the predominant methodology in CDs synthesis, particularly in the fabrication of TCM-CDs. The “bottom-up” synthesis strategy currently encompasses four principal methodologies for CDs fabrication: hydrothermal synthesis,93 pyrolytic carbonization,94 solvothermal synthesis,95 and microwave-assisted processing.96 The hydrothermal method represents an eco-friendly synthetic strategy, whose standardized protocol encompasses sequential steps including precursor dissolution, hydrothermal reaction under elevated temperature and pressure, controlled gradient cooling, centrifugal separation, membrane filtration purification, and terminal dialysis refinement, ultimately yielding CDs with well-defined structural characteristics.97 Xia et al27 engineered CDs with integrated fluorescence tracing and near-infrared photothermal conversion capabilities via hydrothermal synthesis using Rhein, which effectively alleviated dextran sulfate sodium (DSS)-induced ulcerative colitis through ROS scavenging and suppression of NF-κB-mediated inflammatory cascades. A representative pyrolytic carbonization strategy involves high-temperature carbonization of molecular precursors, followed by boiling and dialysis of the carbonized products to yield CDs.98 Wang et al implemented this strategy in fabricating Pollen Typhae (PT)-derived carbon dots (PT-CDs). Specifically, PT was subjected to gradient pyrolysis treatment (250–400°C) in a crucible, followed by sequential boiling and dialysis purification of the carbonized products, ultimately yielding PT-CDs with remarkable therapeutic efficacy in ameliorating acute kidney injury.28 The solvothermal method is fundamentally characterized by the employment of non-aqueous solvents (eg, ethanol, dimethylformamide) as reaction media, distinct from the hydrothermal approach that exclusively utilizes aqueous systems.99,100 This strategy achieves precise regulation of CDs synthesis through systematic modulation of solvent properties.101 Kasi and co-workers synthesized neuroregenerative CDs via a 180°C ethanol-mediated solvothermal strategy using quercetin as the carbon precursor. These CDs demonstrated significant enhancement of Schwann cell proliferative activity, thereby accelerating post-injury regeneration of peripheral nerve tissues.29 Microwave-assisted synthesis facilitates CDs preparation through dielectric heating-induced bond dissociation in molecular precursors, which drives cascade physicochemical transformations. This approach demonstrates superior precursor activation kinetics and precise control over CDs morphology and surface chemistry compared to conventional thermal methods.102 Utilizing citrus peel-derived biomass as a carbon precursor, Hu et al30 synthesized fluorescence-responsive functionalized CDs within 1 minute via a microwave-assisted approach. These CDs demonstrated sensitive detection of Escherichia coli in dairy matrices, highlighting the exceptional efficiency of microwave-driven synthesis in advancing green CD fabrication strategies.
The hallmark advantage of TCM-CDs lies in their environmentally benign synthesis, enabling energy-efficient fabrication under non-toxic reagent conditions.103 Their sub-10 nm nanostructure facilitates rapid renal clearance via glomerular filtration, significantly mitigating bioaccumulation risks and demonstrating superior biocompatibility.104 Furthermore, TCM-CDs inherently integrate fluorescence-based tracing capabilities with precise diagnostic functionalities, underscoring their versatility across diverse application scenarios.105
Extracellular Vesicles Derived from TCM
Extracellular vesicles (EVs) are phospholipid bilayer-enclosed structures secreted by cells, characterized by their low immunogenicity and high biocompatibility, which have positioned them as an emerging therapeutic strategy in disease treatment.106 TCM-EVs are naturally secreted nano-scale vesicles from herbal cells, encapsulating lipids, proteins, nucleic acids, and bioactive phytochemicals, demonstrating versatile therapeutic potential across multiple disease contexts.107
Currently, the primary preparation methods for TCM-EVs include differential ultracentrifugation, density gradient centrifugation, size-exclusion chromatography, polymer-based precipitation, and ultrafiltration. Differential ultracentrifugation sequentially removes particulate impurities through gradient centrifugal forces, followed by ultracentrifugation pelleting to isolate EVs within a target size range. This standardized protocol employs multi-step centrifugation for precise preparation of EV products.108 Density gradient ultracentrifugation represents a methodological refinement evolved from conventional differential ultracentrifugation protocols. Seo et al31 isolated ginseng-derived EVs utilizing a sucrose density gradient method. These EVs demonstrated efficacy in inhibiting osteoclast differentiation, positioning them as a potential therapeutic agent for osteoporosis. The core workflow comprises density gradient formation, isopycnic centrifugation, fractionation, and elution purification.109 Li et al employed iodixanol density gradient construction coupled with fractionation-based isolation to prepare Lonicera japonica-derived EVs, which alleviated DSS-induced colitis.32 Size-exclusion chromatography achieves size-dependent separation of EVs through molecular sieving via porous matrices (eg, agarose/dextran gels). The standardized protocol involves chromatographic column elution of the sample mixture at a constant flow rate to achieve target EVs isolation.110 Sánchez-López et al isolated EVs from punica granatum using this methodology, demonstrating wound-healing promotion, anti-inflammatory, and antioxidant properties.33 The principle of polymer precipitation involves competitive binding of hydrophilic polymer to water molecules in the hydration layer on the surface of EVs, disrupting their colloidal stability and thereby inducing precipitation for separation. The standardized protocol involves incubation of the sample with polymer solution followed by sequential centrifugation steps to achieve EVs isolation.111 Zhang et al utilized ExoQuick solution to isolate panax ginseng-derived EVs via polymer-based precipitation.34 Ultrafiltration achieves size-selective separation of EVs through molecular sieving effects generated by porous membranes with defined pore size distributions.112 Ham et al developed a novel molecular sieve membrane enabling high-efficiency isolation of allium cepa-derived EVs via ultrafiltration methodology.35
Exogenous NDDS for TCM Active Ingredients
Inorganic Nanocarriers
Mesoporous Silica Nanoparticles
Mesoporous Silica Nanoparticles (MSNs) are silicon-based nanoparticles with a porous structure. MSN encompasses multiple categories, including ordered MSN, hollow MSN, core-shell MSN, dendrimer-like MSN, and biodegradable MSN, among others.113 Currently, the primary methods for synthesizing MSNs include the sol-gel process and template-assisted approaches.114 The sol-gel synthesis method of MSNs involves the condensation reaction of the precursor within the micelles confined by the emulsion, followed by template extraction to generate an ordered mesoporous structure.115 The template-assisted method entails selecting a suitable templating agent and mixing it with the precursor (or having it adsorbed onto a specific substrate), allowing the precursor to react and grow following the template, and then removing the template through methods such as calcination and extraction to obtain the material.116
Due to their high specific surface area, ease of modification, adjustable pore size, and good biosafety, MSN have been widely utilized as a sophisticated inorganic nano-carrier for drug delivery in various biomedical applications, including targeted therapy for cancer.117 MSN is frequently utilized for the delivery of active ingredients of TCM that possess anti-cancer properties. Fan and his colleagues employed rod-shaped MSN to encapsulate bufalin for targeted therapy of colorectal cancer. Thanks to the exceptional EPR effect exhibited by MSN, the efficacy of bufalin in treating colorectal cancer was significantly enhanced.36 In recent years, functionalizing MSN to enhance its therapeutic effects has emerged as a new trend in research.118 Li and colleagues further utilized gold nanoparticles and folate-modified MSN for targeted delivery of berberine in the treatment of liver cancer. The gold nanoparticles endowed the nanoparticles with a photothermal effect, while the folate enabled active targeting and recognition of liver cancer cells by the nanoparticles. The high targeting efficiency, combined with the synergistic action of photothermal therapy (PTT) and chemotherapy, significantly enhanced the anticancer efficacy of berberine.37
Metal-Organic Frameworks
Metal-Organic Frameworks (MOFs) are a novel type of porous materials assembled through the coordination bonding of metal ions or metal clusters with organic ligands.119 The synthetic methodologies for MOFs encompass hydrothermal/solvothermal synthesis, microwave-assisted synthesis, mechanochemical approaches, electrochemical methods, ultrasound-mediated synthesis, and diffusion techniques.120
MOFs have been widely applied in the research and development of nanomedicines due to their adjustable porous structures, good biocompatibility, and ease of functionalization.121 Wang and his team have developed an iron-based MOFs nanocarrier for loading triptolide, targeting its application in the treatment of melanoma. This MOFs nanocarrier has undergone modifications with bovine serum albumin and folic acid, and possesses chemodynamic therapy (CDT) activity. These features not only enhance targeting efficacy but also achieve synergistic effects among multiple therapeutic approaches, thereby significantly improving the therapeutic efficacy of triptolide through various aspects.38 In another study, He and colleagues developed a cyclodextrin-based MOFs system for loading honokiol, targeting its application in breast cancer treatment. This MOFs system was also loaded with indocyanine green and encapsulated with the cell membrane of MCF-7 cells (a type of breast cancer cell). This innovative approach not only enhanced the stability and targeting ability of honokiol but also synergized it with PTT, thereby significantly boosting the therapeutic efficacy of honokiol.39
Noble Metal Nanoparticles
Noble metal nanoparticles refer to tiny particles with sizes typically not exceeding 100 nanometers, composed of precious metal elements such as gold, silver, platinum, and palladium. The synthetic methodologies for noble metal nanoparticles bear similarities to those employed in the fabrication of MOFs.
A distinct advantage of noble metal nanoparticles lies in their dual functionality: serving as nanocarriers for targeted delivery of TCM bioactive components while exerting therapeutic effects through their intrinsic physicochemical properties.122,123 For instance, gold nanoparticles (Au NPs) exhibit excellent photothermal effects. Taking advantage of this property, Zhang et al designed a functionalized Au NPs modified with AS1411 for targeted therapy of colorectal cancer. This nanoparticle demonstrates significant killing effects on colorectal cancer cells through the PTT effect.124 In terms of serving as nanocarriers, noble metal nanoparticles can enhance the efficacy of active ingredients in TCM by increasing their stability, solubility, and other properties. It is reported that researchers utilized silver nanoparticles encapsulated with olive leaf extract for the treatment of colorectal cancer. In vitro experiments demonstrated the nanoparticles remarkable inhibitory effect on HCT-116 cells (a type of colorectal cancer cell), thereby showcasing their exceptional potential for anticancer therapy.40 Furthermore, Xiang and his team have developed a metal-based nanoparticle system loaded with TA, utilizing platinum nanoparticles (Pt NPs) as the foundation. This system is designed to inhibit cancer progression by enhancing anti-cancer immune responses. As a nanocarrier, Pt NPs play a crucial role in significantly boosting the bioavailability and targeting efficiency of tannic acid, thereby prolonging its circulation time in the body and facilitating its accumulation at tumor sites. These improvements collectively enhance the anti-cancer activity of TA.41
Magnetic Nanoparticles
Mineral medicines are an important part of TCM in our country. Such as cinnabar, realgar, and magnetite, etc., they are known as “metal and stone medicines” and are widely used in traditional Chinese medicine. Some TCMs of inorganic mineral origin can be directly generated into nanomedicines through physical methods (mechanical crushing method, ball milling method) and chemical methods (precipitation method, sol-gel method, hydrothermal method). Similar to magnetite, magnetic nanoparticles (MNPs) are a type of nanoscale particles composed of magnetic elements (such as iron, nickel, cobalt) and their compounds, exhibiting unique properties including a high surface-to-volume ratio, magnetism, and high stability. These characteristics have led to their widespread application in the biomedical field.125
When loaded with active ingredients of TCM for cancer treatment, MNPs can enhance the efficacy of these ingredients through two distinct properties. Firstly, the magnetic guidance effect of MNPs is utilized to achieve targeted delivery of the active ingredients of TCM. In previous research, MNPs have been shown to accumulate specifically at tumor sites under the guidance of an external magnetic field, thereby increasing the drug concentration at those sites and ultimately enhancing the therapeutic effect.126 Li and his team have developed lipid-coated magnetic nanoparticles loaded with dihydroartemisinin (DHA), based on iron oxide, for the treatment of head and neck squamous cell carcinoma. Both in vitro and in vivo experiments have demonstrated that these nanoparticles can achieve targeted delivery of DHA under the influence of an external magnetic field, exhibiting significantly enhanced therapeutic efficacy compared to non-targeted DHA delivery.42 Furthermore, MNP can synergistically treat cancer along with the active ingredients of TCM through their magnetocaloric effect, thereby enhancing the therapeutic efficacy. Under the application of an external alternating magnetic field, MNPs can absorb magnetic energy to produce heat, thereby elevating the local temperature of tumor tissue to the point of eradicating tumor cells.127 Li and his team have developed a targeted therapy for colorectal cancer based on magnetic mesoporous silicon nanoparticles (MMSNPs) encapsulating 6-gingerol. Under the influence of an external alternating magnetic field, these MMSNPs synergistically induce colorectal cancer cell death through a combination of magneto-thermal effects, the stimulation of antitumor immunity, and oxidative stress. This approach has significantly enhanced the therapeutic efficacy of 6-gingerol and demonstrated potent anticancer activity.43
Organic Nanocarriers
Polymer Nanocarriers
Polymer nanocarriers are drug delivery systems constructed using one or more polymers, such as polyethylene glycol (PEG) and chitosan.128,129 In the preparation of polymeric nanocarriers, the most commonly employed methods are emulsification-solvent evaporation130 and nanoprecipitation,131 which have emerged as predominant techniques due to their high efficiency, scalability, and broad applicability.
Polymeric nanocarriers are widely used in the field of targeted drug delivery, especially in the delivery of active ingredients of TCM for cancer treatment, due to their advantages such as high biocompatibility, high stability, adjustable surface properties, versatility, good drug loading and release capabilities, and low immunogenicity and renal filtration rate.132 Researchers developed a polymeric nanocarrier based on β-cyclodextrin that loads quercetin and doxorubicin. This nanocarrier not only enhances the bioavailability of quercetin but also reverses the resistance of MCF-7 cells to doxorubicin, achieving an improved anticancer effect through synergistic therapy.44 In the treatment of cervical cancer, some researchers have developed a polymer nanoparticle based on poly (methacryloyl beta-alanine) for the targeted delivery of curcumin. This nanoparticle improves the targeting and therapeutic effect of curcumin by increasing the release rate of curcumin and guiding curcumin to act on the nucleus of HeLa cells (a type of cervical cancer cell), providing a new strategy for the clinical treatment of cervical cancer.45 Leveraging the advantage of easy surface modification of polymer-based nanocarriers, some researchers have further conducted surface engineering to endow them with additional functionalities, thereby enhancing their drug delivery efficiency and amplifying the therapeutic effects of the administered drugs. For example, Peng et al constructed a biomimetic polymer nanocarrier by coating poly (lactic-co-glycolic acid) (PLGA) nanocarriers with mannose-modified red blood cell membranes, and utilized it to deliver artesunate and chloroquine for targeted therapy of colorectal cancer. The mannose-modified red blood cell membranes enable the nanoparticles to have a longer circulation time in the body and to target and accumulate at tumor sites for effective action. In vitro and in vivo results indicate that this nanocarrier significantly enhances the anticancer therapeutic effects of artesunate and chloroquine.46
Lipid Nanoparticles
Lipid nanoparticles are vesicular nanostructures primarily composed of lipid molecules, encompassing various types such as liposomes, lipid nanoparticles (LNPs), solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs).133 In the preparation of lipid-based nanocarriers, the most commonly used methods are Thin-Film Hydration134 and Methanol/Ethanol Injection.135 These two methods have become the preferred strategies for synthesis due to their well-established technology, ease of operation, and broad applicability.
Lipid nanoparticles have been extensively utilized in drug delivery,136 biomedical imaging and diagnostics,137 as well as gene therapy,138 due to their advantages such as excellent controlled release properties, targeting capabilities, and enhanced drug bioavailability.139 The utilization of lipid nanocarriers to load active ingredients of TCM for cancer treatment is also a new trend in the current development of nanomedicine. Zhang and his team have devised a functionalized SLN carrier for loading curcumin, specifically tailored for the treatment of liver cancer. By employing hyaluronic acid for surface modification, this nanoparticle carrier acquires the capability to specifically target liver cancer cells. Experimental results indicate that curcumin loaded onto this SLN carrier exhibits a higher degree of accumulation at the tumor site compared to free curcumin, resulting in a marked enhancement of its anticancer therapeutic efficacy.47 In another study, Jing and colleagues utilized LNPs to encapsulate camptothecin for the treatment of liver cancer. These LNPs were also functionally modified, and innovatively co-delivered camptothecin along with a contrast agent. This enables these nanoparticles not only to possess targeted therapeutic capabilities against liver cancer but also to achieve synchronous in vitro MRI imaging, thereby integrating diagnosis and treatment. This not only broadens the application scenarios of active ingredients derived from TCM but also offers new insights into personalized cancer treatment.48
Nanoemulsion
Nanoemulsions are nanoscale dispersion systems composed of oil phase, aqueous phase, and emulsifiers. Their nanoscale droplet size and exceptional stability demonstrate broad applicability in drug delivery.140,141 TCM-derived nanoemulsions represent an advanced drug delivery platform wherein active ingredients from TCM are encapsulated via nanotechnology within emulsion-based systems, aiming to enhance bioavailability, stability, and targeted delivery capabilities. The oil phase is predominantly constituted by lipophilic (curcumin, paclitaxel, tanshinone, etc) or oleaginous (volatile oil of ligusticum chuanxiong, peppermint oil, zedoary oil, etc) TCM active ingredients.
The currently prevalent preparation techniques for TCM nanoemulsions primarily include high-pressure homogenization,142 ultrasonication,143 phase inversion temperature (PIT) method,144 and self-emulsifying drug delivery systems (SEDDS).145 The former two methods fall into high-energy preparation techniques, while the latter two belong to low-energy fabrication approaches.
In practical applications, nanoemulsions are extensively employed in the construction of nanosized TCM formulations owing to their high stability, enhanced bioavailability, and superior safety profiles.146 Liao et al developed a self-emulsifying nanodelivery system based on scutellarin to suppress excessive inflammatory responses. Characterization data demonstrated that the nanoemulsion exhibited excellent physical stability and achieved lymph node-specific accumulation in vivo, significantly mitigating LPS-induced systemic cytokine storms. This system markedly enhanced the anti-inflammatory efficacy of scutellarin. Biosafety evaluation further confirmed the absence of toxic effects on major organs (heart, liver, lungs, and kidneys) in experimental animals following administration of the nanoemulsion.49 In addition, R. M. El-Moslemany et al prepared a tanshinone IIA nanoemulsion via the ultrasonication emulsification method. This nanoemulsion exhibited significant bioactivity and demonstrated remarkable therapeutic efficacy in mitigating acute lung injury in vivo.50 Lei et al developed a pH-responsive sodium alginate hydrogel-encapsulated nanoemulsion co-loaded with curcumin and emodin via ultrasonication emulsification. This nanoemulsion demonstrated pH-triggered controlled drug release in the colon, effectively alleviating the inflammatory microenvironment through downregulation of pro-inflammatory cytokines (TNF-α, IL-6), upregulation of anti-inflammatory cytokine IL-10, scavenging of macrophage-derived ROS, and enhancement of intestinal mucosal tight junction protein expression.51
Biomimetic Nanoparticles
Biomimetic nanoparticles are a novel type of nanocarriers fabricated by coating the surfaces of traditional nanomaterials with cellular constituent (such as cell membranes, extracellular vesicles, etc.).147–149 The primary preparation strategies for membrane-coated biomimetic nanoparticles include membrane extrusion150,151 and electroporation.152 The detailed isolation and purification techniques for EVs (eg, differential centrifugation, size-exclusion chromatography, or ultracentrifugation) have been outlined above.
Compared with traditional nanocarriers, biomimetic nanocarriers exhibit better biocompatibility, higher targeting efficiency, and more superior abilities to penetrate biological membranes.153 Based on these advantages, an increasing number of researchers are combining them with active ingredients of TCM that have anticancer effects to develop novel nanomedicines with even stronger anticancer properties. In the research conducted by Zhang and his team, they utilized red blood cell membranes to encapsulate a PCL-PEG polymer carrier, thereby creating a novel biomimetic nanocarrier loaded with resveratrol for targeted therapy of colorectal cancer. By leveraging the properties of the red blood cell membranes, this nanocarrier can effectively evade clearance by the body’s immune system, leading to an extension of its circulation time within the body. This prolonged circulation enhances the chances of the drug reaching the disease site, ultimately boosting the therapeutic efficacy of resveratrol.52 Exosomes, a type of nanovesicles derived from cells, also serve as a type of biomimetic nanocarrier.154 They are capable of encapsulating drugs and achieving targeted delivery without the need to bind with other nanoparticles. A group of researchers has successfully developed a delivery system based on milk-derived exosomes encapsulating curcumin. This system efficiently delivers curcumin to breast cancer cells, enhancing its anticancer activity and providing a promising strategy for the treatment of breast cancer.53
Advantages of Nanosized TCM for Biomedical Applications
Compared with traditional TCM, nanosized TCMs exhibit a series of prominent advantages. They have better solubility, stronger permeability, more precise targeting, and can enable the combined application of multiple therapies. These advantages effectively enhance the pharmacological activity of nanosized TCMs, demonstrating greater potential in the treatment of disease.
Bioavailability
The bioavailability of active ingredients in TCM is generally not strong, primarily due to the intricate molecular architectures of these compounds, the high abundance of polar or hydrophobic functional groups, and hepatic first-pass metabolism. This poor bioavailability poses limitations on the clinical application of active ingredients in TCM. To address this issue, researchers have developed nanocarrier-based delivery systems for constructing nanosized TCM formulations to enhance their bioavailability155 (Figure 2). The first strategy involves encapsulating TCM active ingredients within amphiphilic nanocarriers to enhance hydrophilicity and solubility, thereby improving bioavailability. Yao et al developed an amphiphilic PEG-PLGA nanoparticle-based delivery system for encapsulating isoliensinine in hypertension treatment. Pharmacokinetic studies demonstrated that this nanocarrier significantly enhanced plasma drug concentration of isoliensinine and prolonged its systemic retention, thereby effectively improving bioavailability.156 The second strategy leverages the nanoscale size effect by reducing drug particle size to increase specific surface area, thereby accelerating dissolution kinetics and markedly enhancing drug solubility and bioavailability. Ge et al group engineered an ellagic acid-gallic acid-catechin (EA-GA-CA) sandwich-like nanostructure for antibacterial therapy. Structural characterization revealed that this nanostructure exhibited a remarkably high specific surface area, which facilitated a substantial enhancement in aqueous solubility and ultimately optimized bioavailability.157 Another strategy involves reducing hepatic clearance to prolong the systemic circulation time of the drug, thereby enhancing bioavailability. Zhou et al innovatively developed a biomimetic nanodelivery system utilizing erythrocyte membrane and PLGA for targeted shikonin delivery in colorectal cancer therapy. This bioinspired design, mimicking natural erythrocyte characteristics, effectively reduced hepatic metabolic clearance of the nanoparticles. Pharmacokinetic analysis demonstrated that the biomimetically engineered shikonin formulation significantly extended systemic circulation half-life compared to free drug, confirming the strategy’s efficacy in enhancing bioavailability.158
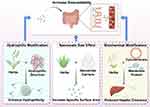 |
Figure 2 Strategies of nano-engineering for improving the bioavailability of active ingredients in TCM. By Figdraw. https://www.figdraw.com. |
Targeting
Nanosized TCM active ingredients can increases the dosage of drugs accumulated at the lesion site, thereby improving drug delivery efficiency and therapeutic efficacy, and reduces adverse reactions caused by drug-induced damage to other normal tissues in the body.159 The mechanisms for enhancing drug targeting can be divided into passive targeting, active targeting, and physicochemical targeting (Figure 3).
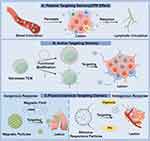 |
Figure 3 Schematic diagram of targeted delivery of nanosized TCM to pathological lesions. (A). Nanosized TCM achieves passive targeting to lesion sites via the EPR effect. (B). Nanosized TCM realizes active targeting to lesion sites through biomimetic modification or surface protein engineering. (C). Nanosized TCM enables physicochemical targeting to lesion sites by responding to endogenous or exogenous stimuli. By Figdraw. https://www.figdraw.com. |
The mechanism of passive targeting delivery by nanosized TCM primarily relies on the Enhanced Permeability and Retention (EPR) effect, which is closely related to the physicochemical properties of the nanocarriers, including particle size, morphology, molecular weight, charge properties, and charge amount.160,161 For example, Ji et al fabricated self-assembled nanoparticles based on honokiol via a hydrothermal method for colorectal cancer treatment. In vivo pharmacokinetic and biodistribution studies demonstrated that these nanoparticles could achieve targeted accumulation in tumor tissues through the EPR effect.162 Nanocarriers composed of PEG and PLGA can enhance drug stability and improve bioavailability.163 Based on this advantage, researchers have utilized PEG-PLGA nanocarriers to encapsulate honokiol for the treatment of breast cancer. Through the EPR effect, the passive targeted delivery of honokiol can be achieved, thereby improving its therapeutic efficacy.164 Specially, Zheng et al designed a self-assembling nanocarrier based on 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N- [maleimide (polyethylene glycol)-2000] (DSPE-PEG2000-Mal) and cholesterol for simultaneous targeted delivery of curcumin and resveratrol in the treatment of liver cancer. Both in vivo and in vitro experiments were conducted to verify its ability to enhance the anticancer efficacy of these drugs.165
Active targeting refers to a drug targeting delivery strategy that nanoparticles modified with specific ligands can be precisely internalized by target cells to exert therapeutic effects. The core of this strategy lies in the specific binding between ligands and receptors.166 This strategy not only specifically acts on target cells but can also bind to target organelles after cellular internalization, thereby improving the precision of treatment.167 Currently, a popular strategy for achieving active targeting is to modify the surface of nanoparticles with ligands that can specifically recognize certain overexpressed receptors on the surface of cancer cells, thereby enabling the active targeted delivery of active ingredients in TCMs.168 For instance, folate is a type of B vitamin, and its receptors are often overexpressed in cancer cells.169 Therefore, targeting folate receptors has become one of the strategies for active targeted delivery. Guo et al utilized folate-modified star polyester nanocarriers for targeted delivery of curcumin to treat breast cancer. This nanocarrier exhibited good targeting ability for the curcumin and significantly enhanced its therapeutic efficacy.170 Li et al also utilized folate-coated MSNs to load tanshinone IIA. This nanocarrier demonstrated good targeting toward promyelocytes both in vivo and in vitro, and exhibited superior therapeutic effects compared to MSNs carriers without folate coating.171 The asialoglycoprotein receptor is also one of the receptors that are overexpressed on the surface of cancer cells. Based on this, researchers have utilized galactose-modified PLGA nanocarriers to encapsulate dalbergin for the liver cancer treatment. This approach not only improves the bioavailability of dalbergin but also increases its distribution within liver cancer tissues, thereby achieving targeted therapy.172 Furthermore, Ling et al synthesized a polymer nanocarrier capable of targeted recognition of IL-13Rα2 (an IL-13 receptor that is highly expressed in glioblastoma multiforme, GBM), which was used to deliver resveratrol, thereby achieving precise treatment of GBM and enhancing the anticancer therapeutic effect of resveratrol.173,174 Guo et al engineered curcumin-derived extracellular vesicles with death receptor 5 (DR5) antibody functionalization. These nanoparticles achieved targeted delivery to tumor sites by specifically recognizing DR5 on senescent tumor cell surfaces, thereby overcoming the limited targeting capacity of curcumin. In vivo studies further demonstrated their potent tumor growth inhibitory efficacy.175
Physicochemical targeting primarily achieves targeted delivery of drugs through the utilization of endogenous physicochemical stimuli within the disease microenvironment or the influence of certain external physicochemical factors.176 For instance, hypoxia is a prominent characteristic of the tumor microenvironment.177 Based on this characteristic, Liu et al developed a kind of micelle based on angelica polysaccharide for the delivery of curcumin in the treatment of liver cancer. This micelle was modified with azobenzene, which enables it to stimulate the release of curcumin under hypoxic conditions in liver cancer, achieving targeted therapy.178 The pH value of tumor tissue is lower than that of normal tissue, exhibiting a weak acidity.179 Therefore, the development of NDDSs with pH-responsive properties is also one of the current strategies for achieving active targeted drug delivery. Researchers have developed an MSN modified with folic acid and polyacrylic acid for the delivery of chrysin in the treatment of breast cancer. Experiments have demonstrated that this nanocarrier can efficiently release chrysin under the pH conditions specific to the tumor environment, achieving pH-responsive targeted drug delivery.180 In addition to responding to internal stimuli within tumor tissues, researchers have also utilized strategies involving nano-carriers that respond to external stimuli to achieve physicochemical targeted delivery. S. Roy et al constructed a bioinspired magnetic nanocarrier system using Lactobacillus rhamnosus (Au-Lac)-derived biotemplates coupled with zinc ferrite, successfully achieving targeted delivery of quercetin – an anticancer bioactive compound from TCM. In vitro studies confirmed the nanoparticles’ magnetically guided directional migration into cervical cancer cells under external magnetic field control, demonstrating remarkable magnetic targeting properties.181
Multimodal Therapy
Nanosized TCM can achieve combined therapy against disease through multiple mechanisms, thereby significantly enhancing treatment efficacy (Figure 4). PTT is an emerging and highly precise cancer treatment method. It primarily involves the targeted delivery of nanoparticles with photothermal conversion effects to the tumor site. Under the illumination of an external light source, these nanoparticles convert light energy into thermal energy, raising the local temperature of the tumor tissue to kill tumor cells, thereby achieving the goal of anticancer treatment.182,183 Polydopamine (PDA) is an excellent photothermal agent, Su and his team utilized polydopamine that possessing great photothermal conversion effect to load camptothecin for the treatment of lung cancer, which exhibits superior inhibitory effects on lung cancer cells under the dual mechanisms of chemotherapy and PTT, demonstrating more significant therapeutic effects compared to free camptothecin.184 Molybdenum disulfide (MoS2) is also a material with a photothermal conversion effect.185 Chen et al utilized MoS2 to construct a nanocarrier for targeted co-delivery of curcumin and erlotinib in the treatment of lung cancer. Under the synergistic treatment of combined chemotherapy and PTT, these nanoparticles demonstrated favorable therapeutic effects on lung cancer both in vitro and in vivo.186 Photodynamic therapy (PDT) refers to an anticancer treatment strategy where nanoparticles with photosensitizing properties are delivered to the tumor site, and under the action of exogenous light and the participation of molecular oxygen in the tissue, a reaction occurs to produce cytotoxic substances, thereby inducing tumor cell death.187,188 In recent years, researchers have employed nanotechnology to process the active ingredients of TCM, granting them additional photosensitizing effects. For example, researchers encapsulated quercetin and the photosensitizer zinc phthalocyanine within lipid nanocarriers to create anticancer nanoparticles that leverage both PDT and chemotherapy effects. Both in vitro and in vivo experiments demonstrated the nanoparticles’ excellent anticancer efficacy.189,190 Fullerenes are nanoparticles capable of generating ROS under light illumination and are often utilized in PDT.191 Similarly, Zhang et al employed fullerene-based nanocarriers to deliver artesunate for PDT and chemotherapy synergistic treatment.192 In addition, in the latest research, researchers have utilized nanotechnology to develop nanoparticles with two-photon excitation effects based on the inherently photosensitizing active ingredients of TCM, such as curcumin and emodin. Compared to traditional PDT, these nanoparticles exhibit more efficient ROS generation capabilities and demonstrate more significant therapeutic effects against cancer.193,194 In addition, Wen et al successfully engineered photodynamic curcumin-derived carbon dots via a chitosan-hyaluronic acid functionalization strategy. In vitro antibacterial assays revealed that 405 nm light irradiation markedly triggered ROS generation in the composite system, conferring robust antimicrobial activity.195
 |
Figure 4 Phototherapy, chemodynamic therapy, and photodynamic therapy based on nanosized TCM. By Figdraw. https://www.figdraw.com. |
CDT refers to a novel anticancer treatment approach that employs nanoparticles to catalyze the Fenton reaction within the tumor microenvironment, generating hydroxyl radicals which subsequently induce the death of tumor cells.196 Metal particles (such as iron, copper, manganese, etc.) are commonly used as Fenton-like nanoparticles for CDT.197 Zhang and his colleagues developed a nano-hydrogel encapsulated with artesunate, utilizing Cu-Fe3O4 particles, for targeted therapy against osteosarcoma. Through the synergistic effects of CDT and chemotherapy, this nano-hydrogel demonstrated robust anticancer activity by inducing the generation of abundant radicals, triggering oxidative stress in tumor cells, and ultimately leading to cell death both in vitro and in vivo.198 In another research endeavor, Chen and his team leveraged hollow mesoporous Prussian Blue (HMPB) nanoparticles for targeted delivery of curcumin. By reacting with endogenous hydrogen sulfide at the disease site to generate radicals, these nanoparticles elicit oxidative stress, thereby inducing autophagy in colorectal cancer cells and exerting an anticancer effect. Experiments further revealed that the combined use of curcumin and HMPB nanoparticles exhibited higher anticancer activity than either free curcumin or free HMPB nanoparticles alone. This underscores the complementary nature of CDT and chemotherapy, which together enhance therapeutic efficacy, achieving a synergistic effect where the whole is greater than the sum of its parts.199
Therapeutic Applications of TCM Nanomedicines: From Mechanisms to Practice
Compared with conventional TCM formulations, TCM nanomedicines have demonstrated multifaceted therapeutic advantages and achieved broad clinical application across diverse pathological contexts. This review specifically elucidates the mechanistic foundations of TCM nanomedicines in three critical therapeutic domains – oncotherapy, inflammation modulation, and microbial eradication – through integrated analysis of nanostructured fabrication strategies and molecular targeting pathways (Figure 5).
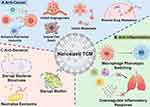 |
Figure 5 The applications of TCM nanomedicines in biomedical treatment field. (A). Anticancer mechanism mediated by nanosized TCM. (B). Anti-inflammation mechanisms exerted by nanosized TCM (C). Anti-bacterial mechanism of nanosized TCM. By Figdraw. https://www.figdraw.com. |
Anti-Cancer
At present, cancer is a serious global public health problem. According to the International Agency for Research on Cancer (IARC), there were 20 million new cases of cancer worldwide in 2022, while 9.7 million people deaths from cancer.200 High morbidity and mortality rates of cancer make it one of the major threats to human health worldwide. The development of nanotechnology-based TCM has opened innovative intervention approaches for malignant tumor treatment. Through features such as precise drug delivery, multi-target regulation, and biological barrier penetration, it overcomes the inherent limitations of TCM components and provides nanoscale solutions with TCM characteristics for precision cancer therapy.
At the molecular mechanistic level, nanosized TCM exerts antitumor efficacy through multi-pathway and multi-target synergistic effects. The primary mechanism involves enhancing the host’s antitumor immune response. Zhang et al developed a novel self-assembled TCM nanocomplex based on the molecular interaction characteristics of ursolic acid and epigallocatechin gallate, which was applied to precision therapy for Hepatocellular Carcinoma (HCC). Mechanistic studies revealed that this nanosystem could significantly enhance the anti-tumor response in the tumor immune microenvironment by stimulating anti-cancer cytokines (IL-12 and IFN-γ) and activating CD4⁺ T cells and CD8⁺ T cells.201 Liu et al constructed a self-assembled nanodelivery system using celastrol as a precursor molecule. In vitro and in vivo results demonstrated that this nanotherapeutic agent could significantly suppress the growth of subcutaneously xenografted tumors by trigger death of tumor cells.202 Furthermore, Zhou et al engineered erythrocyte membrane-coated biomimetic nanoparticles with piceatannol as the active pharmaceutical ingredient. Mechanistic studies confirmed their targeted inhibition of colorectal cancer metastasis through modulation of the Hippo/YAP1/SOX9 signaling axis.203 Additionally, it can effectively suppress tumor angiogenesis. Zhou et al engineered a coordination-driven metal-phenolic network architecture through curcumin complexation with Cu²⁺/Zn²⁺, subsequently integrating this system into a liposomal delivery platform for tumor-targeted delivery. In vitro validation confirmed that this nanotherapeutic potently suppressed tumor angiogenesis, thereby establishing a novel therapeutic strategy for clinical oncology.204 Moreover, nanosized TCM exerts therapeutic synergism by reversing tumor cell multidrug resistance, thereby significantly potentiating chemotherapeutic efficacy. Li et al engineered a hyaluronic acid-based nanodelivery system co-loaded with capsaicin and doxorubicin for combination chemotherapy in HCC. Experimental validation demonstrated that capsaicin reverses doxorubicin chemoresistance by modulating the Substance P/hepatic stellate cell axis.205
Anti-Inflammation
Inflammation is an evolutionarily conserved defense mechanism that orchestrates physiological responses to injury or infection by mediating pathogen eradication and tissue repair.206 However, pathological hyperactivation or chronic persistence of this process initiates autodestructive signaling cascades,206 culminating in irreversible organ dysfunction (eg, pulmonary fibrosis,207 hepatic cirrhosis,208 and renal failure209).Most active components of TCM possess excellent anti-inflammatory activity.210 Therefore, developing novel anti-inflammatory nano-formulations based on the active components of TCM to precisely regulate inflammatory responses is of great clinical application value.
In terms of mechanistic dimensions, nanosized TCM exhibits multi-targeted modulation of inflammatory responses. Primarily, it attenuates inflammatory responses through immunomodulatory axis by precisely regulating immune cell populations. Gao et al prepared curcumin-derived EVs for the treatment of ulcerative colon cancer. Mechanistic studies demonstrated that these EVs could promote the phenotypic transformation of macrophages from M1 to M2, thereby down-regulating the inflammatory response.211 Secondly, it exerts anti-inflammatory effects by inhibiting inflammatory cell infiltration and downregulating inflammatory cytokines. Zheng et al constructed a pulmonary-targeted anti-inflammatory NDDS by co-loading astragaloside IV and ligustrazine into PEG-PLGA nanocarriers, which could inhibit bleomycin-induced pulmonary inflammation by suppressing inflammatory cell infiltration and downregulating the release of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α.212
Anti-Bacterial
Bacterial infections, a significant public health issue threatening human health for millennia, witnessed a historic turning point in prevention and control with the clinical application of antibiotics. Although the discovery of antibiotics provided humanity with a temporary advantage in combating pathogenic bacteria, the evolution and spread of drug-resistant pathogens rapidly reversed this situation. WHO data reveal that global deaths caused by drug-resistant bacterial infections have surged to 1.27 million cases.213 Against this backdrop, the development of innovative therapeutic strategies targeting resistant pathogenic bacteria has become a critical research area requiring urgent breakthroughs in global clinical medicine. TCM has been extensively applied in the anti-bacterial field with demonstrated remarkable efficacy.214 With the continuous advancement of pharmaceutical technologies, researchers are integrating TCM with nanotechnology to develop novel anti-bacterial agents, providing innovative therapeutic strategies for the treatment of drug-resistant bacterial infections.
At the mechanistic level of antibacterial action, nanoscale TCM exerts bactericidal effects primarily through structural disruption of bacterial cell membranes. Ge et al engineered a self-assembled nanoparticle using ellagic acid, gallic acid, and catechin. Experimental studies demonstrated that this nanoparticle disrupts the cell membrane integrity of Staphylococcus aureus, inducing lytic rupture and exhibiting potent antibacterial efficacy.157 Beyond bactericidal activity, nanoscale TCM exerts antibacterial effects through a key mechanistic strategy: suppression of bacterial biofilm formation. Zhang et al engineered a quercetin-based nanomicelle system. Experimental validation demonstrated that this nanomicelle potently suppresses biofilm formation in Staphylococcus aureus.215 Another antibacterial therapeutic mechanism involves neutralizing bacterial exotoxins to mitigate pathogen-induced pathological damage at the infection site. Tan et al synthesized dandelion-derived EVs, which promoted infected wound healing through specific neutralization of Staphylococcus aureus exotoxins.216
The Future Perspectives
The emergence of nano-TCM has written a new chapter in the modern nanomedicine field. This convergence of traditional medicine and cutting-edge science has reignited the millennia-old herbal medicine heritage with renewed radiance. In recent years, with the extensive integration of nanotechnology into TCM, the biosafety evaluation and elucidation of pharmacological mechanisms of nano-engineered TCM formulations have emerged as critical scientific challenges impeding their clinical translation. Current research demonstrates that while nanoscale modifications can significantly enhance the bioavailability and targeted delivery efficiency of TCM, with preliminary validation of their biosafety in experimental animal models, their potential cytotoxicity in humans, alterations in metabolic pathways, and long-term biological effects still require further clinical validation through rigorous trials. Additionally, scalable production and cost-effective manufacturing of nano-engineered TCM is also a considerable problem. Established preparation techniques, including nano-co-precipitation and ultrasonic fragmentation, face significant industrialization barriers due to limitations in process complexity and equipment costs. The development of green and sustainable preparation processes represents a critical pathway to overcoming translational bottlenecks from laboratory-scale research to clinical applications.
Generally, TCM compound prescriptions serve as the primary modality and means in clinical TCM practice. As a modernized TCM form of TCM formulations, component-based Chinese medicine is systematically developed by combining bioactive constituents in accordance with the fundamental “monarch (Jun), minister (Chen), assistant (Zuo), and envoy (Shi)” compatibility principle of herbal prescription. Rooted in TCM theory while incorporating contemporary pharmacological evidence, these multi-component systems demonstrate characteristic polypharmacological synergism through precisely designed component interactions. However, current nanosized TCM research is predominantly focused on single-active compounds (Table 2). Developing advanced nanosized TCM technologies for multi-component TCM formulations necessitates immediate breakthroughs. Building on supramolecular assembly principles and natural drug compatibility theory, Qian et al developed a novel strategy to elucidate the anti-inflammatory mechanism of the classical Huanglian Wumei decoction, which features the rhizoma coptidis-fructus mume (RC-FM) pair as its signature combination. Self-assembled natural phytochemicals were obtained during the decoction process of HLWMD, then infrared/ultraviolet-visible spectroscopy revealed that berberine (BBR) and chlorogenic acid (CGA) are two main active constituents of RC-FM pair. Subsequently, BBR and CGA self-assemble into an amphiphilic spheroid macromolecule (BCS) with an average size of about 98.3 nm through electrostatic interactions, π-π stacking and hydrophobic interactions. In vitro results demonstrated that the stacking pattern and amphiphilic molecular structure of BCS significantly augmented the anti-inflammatory performance compared with that of BBR and CGA alone. This findings explained the scientific connotations of TCM compatibility from the perspective of self-assembly and synergistic effects, providing a novel insight and strategy for elucidating the mechanism of multi-component combinations of TCM.217 Emerging studies have reported that the presence of naturally occurring nanoparticles in certain TCM decoctions, where these nanoscale components exhibit enhanced therapeutic efficacy through their stabilized morphological characteristics.218 Zhang et al assessed the pharmacodynamic basis of the TCM formula T-QY305, which consists of five herbs: astragalus membranaceus, Lonicera japonica, angelica sinensis, licorice, and centipede. The study demonstrated that the T-QY305 spontaneously forms self-assembled nanostructures (N-QY305) during decoction. Through comprehensive characterization and animal experiments, N-QY305 was identified as the crucial bioactive component underlying the formula’s pharmacological effects.219 From this study, TCM research may further be considered as significantly related to nanomedicine, opening new avenues for nanotechnology-driven exploration. Collectively, developing compound-based nano-formulations aligned with the holistic principles of TCM and systematically establishing multi-component targeted co-delivery systems will emerge as a pivotal research direction for enhancing the clinical efficacy of nano-engineered TCM.
 |
Table 2 Clinical Translation Status of TCM Nanomedicines |
Acknowledgments
This work was supported, in part or in whole, by the Postdoctoral Fellowship Program (Grade C) of China Postdoctoral Science Foundation (Grant Number: GZC 20241369), Institutional Foundation of The First Affiliated Hospital of Xi’an Jiaotong University, Shaanxi Province Postdoctoral Science Foundation (2024BSHSDZZ040), Shaanxi Provincial Administration of Traditional Chinese Medicine Research Project (2023-ZDYJSL-004, 2022-SLRH-LJ-009).
Disclosure
The authors declare no conflict of interest.
References
1. Xi ZC, Dai RC, Ze YF, Jiang X, Liu MF, Xu HX. Traditional Chinese medicine in lung cancer treatment. Mol Cancer. 2025;24(1):57. doi:10.1186/s12943-025-02245-6
2. Yao CY, Yuan Y, Du GY, Li Q, Ji YT. Chinese herbal medicine-inspired construction of multi-component hydrogels with antibacterial and wound-healing-promoting functions. J Mater Chem B. 2025;13(8):2826–2833. doi:10.1039/d4tb02058h
3. Xu HY, Li SF, Liu JY, et al. Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19. Proc Natl Acad Sci U S A. 2023;120(18):e2301775120. doi:10.1073/pnas.2301775120
4. Fan WX, Fan LH, Wang ZY, et al. Rare ginsenosides: a unique perspective of ginseng research. J Adv Res. 2024;66:303–328. doi:10.1016/j.jare.2024.01.003
5. Chen J, Bai Y, He X, et al. The spatiotemporal transcriptional profiling of murine brain during cerebral malaria progression and after artemisinin treatment. Nat Commun. 2025;16(1):1540. doi:10.1038/s41467-024-52223-7
6. Liu XL, Chen BH, Chen JQ, et al. A cardiac-targeted nanozyme interrupts the inflammation-free radical cycle in myocardial infarction. Adv Mater. 2024;36(2):e2308477. doi:10.1002/adma.202308477
7. Das P, Ghosh S, Ashashainy V, Nayak B. Augmentation of anti-proliferative efficacy of quercetin encapsulated chitosan nanoparticles by induction of cell death via mitochondrial membrane permeabilization in oral cancer. Int J Biol Macromol. 2023;250:126151. doi:10.1016/j.ijbiomac.2023.126151
8. Peng Z, Lu J, Liu K, et al. Hypericin as a promising natural bioactive naphthodianthrone: a review of its pharmacology, pharmacokinetics, toxicity, and safety. Phytother Res. 2023;37(12):5639–5656. doi:10.1002/ptr.8011
9. Fang C, You Y, Luo F, et al. Silk fibroin encapsulated icariin nanoparticles mitigate bisphenol a-induced spermatogenesis dysfunction. Adv Healthc Mater. 2024;13(6):2302899. doi:10.1002/adhm.202302899
10. Gu W, Kong R, Qi S, et al. Sono-assembly of ellagic acid into nanostructures significantly enhances aqueous solubility and bioavailability. Food Chem. 2024;442:138485. doi:10.1016/j.foodchem.2024.138485
11. Zhuo Z, Guo K, Luo Y, et al. Targeted modulation of intestinal epithelial regeneration and immune response in ulcerative colitis using dual-targeting bilirubin nanoparticles. Theranostics. 2024;14(2):528–546. doi:10.7150/THNO.87739
12. Shao X, Zhao X, Wang B, Fan J, Wang J, An H. Tumor microenvironment targeted nano-drug delivery systems for multidrug resistant tumor therapy. Theranostics. 2025;15(5):1689–1714. doi:10.7150/THNO.103636
13. Switala L, Di L, Gao H, et al. Engineered nanoparticles promote cardiac tropism of AAV vectors. J Nanobiotechnol. 2024;22(1):223. doi:10.1186/s12951-024-02485-6
14. Qiang R, Huang H, Chen J, et al. Carbon quantum dots derived from herbal medicine as therapeutic nanoagents for rheumatoid arthritis with ultrahigh lubrication and anti-inflammation. Acs Appl Mater Inter. 2023;15(32):38653–38664. doi:10.1021/acsami.3c06188
15. Zhu W, Dong Y, Xu P, et al. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022;154:212–230. doi:10.1016/j.actbio.2022.10.038
16. Yang Y, Hu Q, Shao Q, et al. A baicalin-based functional polymer in dynamic reversible networks alleviates osteoarthritis by cellular interactions. Adv Sci. 2025;12(10):e2410951. doi:10.1002/advs.202410951
17. Hill SK, England RM, Perrier S. Modular design of cyclic peptide – polymer conjugate nanotubes for delivery and tunable release of anti-cancer drug compounds. J Control Release. 2024;367:687–696. doi:10.1016/j.jconrel.2024.01.023
18. Yuan RYK, Li YQ, Han S, et al. Fe-curcumin nanozyme-mediated reactive oxygen species scavenging and anti-inflammation for acute lung injury. Acs Central Sci. 2022;8(1):10–21. doi:10.1021/acscentsci.1c00866
19. Guo J, Miao Y, Nie F, et al. Zn-Shik-PEG nanoparticles alleviate inflammation and multi-organ damage in sepsis. J Nanobiotechnology. 2023;21(1):448. doi:10.1186/s12951-023-02224-3
20. Wang Y, Wang DY, Li ZB, et al. Liquid bacterial ablation: metal-phenolic interface enabled biological barrier breaking for efficient infection clearance and wound healing. Chem Eng J. 2025;506:160111. doi:10.1016/j.cej.2025.160111
21. Qin X, Tian R, Wang B, et al. Metal-phenolic nanocapsules with photothermal antibacterial and ros scavenging ability for diabetic wound healing. Adv Healthc Mater. 2024;13(10):e2303604. doi:10.1002/adhm.202303604
22. Zhang CY, Huang LJ, Sun DW, Pu HB. Interfacing metal-polyphenolic networks upon photothermal gold nanorods for triplex-evolved biocompatible bactericidal activity. J Hazard Mater. 2022;426:127824. doi:10.1016/j.jhazmat.2021.127824
23. Nie WL, Liu Y, Lan JS, et al. Self-assembled nanoparticles from xie-bai-san decoction: isolation, characterization and enhancing oral bioavailability. Int J Nanomed. 2024;19:3405–3421. doi:10.2147/IJN.S449268
24. Gao S, Zheng HC, Xu SJ, et al. Novel natural carrier-free self-assembled nanoparticles for treatment of ulcerative colitis by balancing immune microenvironment and intestinal barrier. Adv Healthc Mater. 2023;12(31):e2301826. doi:10.1002/adhm.202301826
25. Chang CQ, Lu C, Zheng Y, et al. Sonication-assisted self-assembled resveratrol nanoparticles with enhanced antiviral and anti-inflammatory activity against respiratory syncytial virus-induced pneumonia. Acs Appl Mater Inter. 2024;16(38):50442–50458. doi:10.1021/acsami.4c11525
26. Fu SY, Yi X, Li Y, et al. Berberine and chlorogenic acid-assembled nanoparticles for highly efficient inhibition of multidrug-resistant. J Hazard Mater. 2024;473:134680. doi:10.1016/j.jhazmat.2024.134680
27. Xia JS, Wang JY, Liu FY, et al. Red/NIR-I-fluorescence carbon dots based on Rhein with active oxygen scavenging and colitis targeting for UC therapeutics. Adv Healthc Mater. 2024;13(19):e2304674. doi:10.1002/adhm.202304674
28. Wang X, Wu T, Yang Y, et al. Ultrasmall and highly biocompatible carbon dots derived from natural plant with amelioration against acute kidney injury. J Nanobiotechnology. 2023;21(1):63. doi:10.1186/s12951-023-01795-5
29. Kasi PB, Opoku H, Novikova LN, et al. Quercetin-derived carbon dots promote proliferation and migration of Schwann cells and enhance neurite outgrowth. Colloid Surf B. 2025;251:114609. doi:10.1016/j.colsurfb.2025.114609
30. Hu X, Li Y, Xu Y, et al. Green one-step synthesis of carbon quantum dots from Orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021;339:127775. doi:10.1016/j.foodchem.2020.127775
31. Seo K, Yoo JH, Kim J, et al. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation. Nanoscale. 2023;15(12):5798–5808. doi:10.1039/d2nr07018a
32. Li W, Ding J, Chen S, et al. Alleviation of colitis by honeysuckle MIR2911 via direct regulation of gut microbiota. J Control Release. 2024;376:123–137. doi:10.1016/j.jconrel.2024.09.050
33. Sánchez-López CM, Manzaneque-López MC, Pérez-Bermúdez P, Soler C, Marcilla A. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 2022;13(24):12870–12882. doi:10.1039/d2fo01806c
34. Jang J, Jeong H, Jang E, et al. Isolation of high-purity and high-stability exosomes from ginseng. Front Plant Sci. 2023;13:1064412. doi:10.3389/fpls.2022.1064412
35. Ham YM, Kang Y, Kang SJ, Lee S, Lee JY, Rhee WJ. Advanced enrichment and separation of extracellular vesicles through the super absorbent polymer nanosieves. Acs Appl Mater Inter. 2024;16(48):65863–65876. doi:10.1021/acsami.4c14542
36. Fan YB, Zhang W, Iqbal Z, et al. Rod-shaped mesoporous silica nanoparticles reduce bufalin cardiotoxicity and inhibit colon cancer by blocking lipophagy. Lipids Health Dis. 2024;23(1):318. doi:10.1186/s12944-024-02301-y
37. Li XD, Wang Z, Wang XR, et al. Berberine-loaded Janus gold mesoporous silica nanocarriers for chemo/radio/photothermal therapy of liver cancer and radiation-induced injury inhibition. Int. J Nanomedicine. 2019;14:3967–3982. doi:10.2147/IJN.S206044
38. Wang S, Guo Q, Xu R, Lin P, Deng G, Xia X. Combination of ferroptosis and pyroptosis dual induction by triptolide nano-MOFs for immunotherapy of Melanoma. J Nanobiotechnology. 2023;21(1):383. doi:10.1186/s12951-023-02146-0
39. He Y, Guo J, Ding H, et al. Glutathione-responsive CD-MOFs co-loading honokiol and indocyanine green biomimetic active targeting to enhance photochemotherapy for breast cancer. Int J Pharm. 2024;660:124310. doi:10.1016/j.ijpharm.2024.124310
40. Mansour HMM, Shehata MG, Darwish AMG, et al. Antioxidant and anti-cancer potentials of Ag green-synthesized and encapsulated olive leaves particles on HCT-116 cells. Int J Biol Macromol. 2024;278:134776. doi:10.1016/j.ijbiomac.2024.134776
41. Xiang J, Zhang Y, Liu X, et al. Natural polyphenols-platinum nanocomplexes stimulate immune system for combination cancer therapy. Nano Lett. 2022;22(13):5615–5625. doi:10.1021/acs.nanolett.2c02161
42. Li H, Li X, Shi X, Li Z, Sun Y. Effects of magnetic dihydroartemisinin nano-liposome in inhibiting the proliferation of head and neck squamous cell carcinomas. Phytomedicine. 2019;56:215–228. doi:10.1016/j.phymed.2018.11.007
43. Li B, Zu M, Jiang A, et al. Magnetic natural lipid nanoparticles for oral treatment of colorectal cancer through potentiated antitumor immunity and microbiota metabolite regulation. Biomaterials. 2024;307:122530. doi:10.1016/j.biomaterials.2024.122530
44. Pawar CS, Rajendra Prasad N, Yadav P, et al. Enhanced delivery of quercetin and doxorubicin using β-cyclodextrin polymer to overcome P-glycoprotein mediated multidrug resistance. Int. J Pharm. 2023;635:122763. doi:10.1016/j.ijpharm.2023.122763
45. Kavya KV, Vargheese S, Shukla S, et al. A cationic amino acid polymer nanocarrier synthesized in supercritical CO for co-delivery of drug and gene to cervical cancer cells. Colloids Surf B Biointerfaces. 2022;216:112584. doi:10.1016/j.colsurfb.2022.1125842
46. Peng J, Zhou J, Sun R, et al. Dual-targeting of artesunate and chloroquine to tumor cells and tumor-associated macrophages by a biomimetic PLGA nanoparticle for colorectal cancer treatment. Int. J Biol Macromol. 2023;244:125163. doi:10.1016/j.ijbiomac.2023.125163
47. Zhang M, Liang J, Liang Y, Li X, Wu W. Efficient delivery of curcumin by functional solid lipid nanoparticles with promoting endosomal escape and liver targeting properties. Colloids Surf B Biointerfaces. 2024;244:114177. doi:10.1016/j.colsurfb.2024.114177
48. Rong J, Liu T, Yin X, et al. Co-delivery of camptothecin and MiR-145 by lipid nanoparticles for MRI-visible targeted therapy of hepatocellular carcinoma. J Exp Clin Cancer Res. 2024;43(1):247. doi:10.1186/s13046-024-03167-9
49. Liao H, Ye J, Gao Y, et al. Baicalein self-microemulsion based on drug-phospholipid complex for the alleviation of cytokine storm. Bioeng Transl Med. 2022;8(1):e10357. DOI:10.1002/btm2.10357
50. El-Moslemany RM, El-Kamel AH, Allam EA, Khalifa HM, Hussein A, Ashour AA. Tanshinone IIA loaded bioactive nanoemulsion for alleviation of lipopolysaccharide induced acute lung injury via inhibition of endothelial glycocalyx shedding. Biomed Pharmacother. 2022;155:113666. doi:10.1016/j.biopha.2022.113666
51. Lei F, Zeng F, Yu X, et al. Oral hydrogel nanoemulsion co-delivery system treats inflammatory bowel disease via anti-inflammatory and promoting intestinal mucosa repair. J Nanobiotechnology. 2023;21(1):275. doi:10.1186/s12951-023-02045-4
52. Zhang Z, Ji Y, Hu N, et al. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci. 2022;17(5):751–766. doi:10.1016/j.ajps.2022.07.006
53. González-Sarrías A, Iglesias-Aguirre CE, Cortés-Martín A, et al. Milk-derived exosomes as nanocarriers to deliver curcumin and resveratrol in breast tissue and enhance their anticancer activity. Int J Mol Sci. 2022;23(5):2860. doi:10.3390/ijms23052860
54. Xu J, Wang J, Ye J, et al. Metal-coordinated supramolecular self-assemblies for cancer theranostics. Adv Sci. 2021;8(16):e2101101. doi:10.1002/advs.202101101
55. Liu S, Xu X, Ye J, et al. Metal-coordinated nanodrugs based on natural products for cancer theranostics. Chem Eng J. 2023;456:140892. doi:10.1016/j.cej.2022.140892
56. Guan HQ, Harris C, Sun SH. Metal-ligand interactions and their roles in controlling nanoparticle formation and functions. Acc Chem Res. 2023;56(12):1591–1601. doi:10.1021/acs.accounts.3c00156
57. Li S, Zhao YQ, Ma W, et al. A multivalent polyphenol-metal-nanoplatform for cascade amplified chemo-chemodynamic therapy. Acta Biomater. 2024;173:389–402. doi:10.1016/j.actbio.2023.11.006
58. Li XB, Jiang FC, Liu MY, et al. Synthesis, characterization, and bioactivities of polysaccharide metal complexes: a review. J Agr Food Chem. 2022;70(23):6922–6942. doi:10.1021/acs.jafc.2c01349
59. Liu X, Liu S, Jin X, et al. An encounter between metal ions and natural products: natural products-coordinated metal ions for the diagnosis and treatment of tumors. J Nanobiotechnology. 2024;22(1):726. doi:10.1186/s12951-024-02981-9
60. Ye Y, Zheng Q, Wang Z, et al. Metal-phenolic nanoparticles enhance low temperature photothermal therapy for bacterial biofilm in superficial infections. J Nanobiotechnology. 2024;22(1):713. doi:10.1186/s12951-024-02985-5
61. Li Y, Liang D, Wang R, et al. Interfacial Self-Assembly Nanostructures. Constructions Applications Small. 2024;20(49):e2405318. doi:10.1002/smll.202405318
62. Zhang Z, Xie L, Ju Y, Dai Y. Recent advances in metal-phenolic networks for cancer theranostics. Small. 2021;17(43):2100314. doi:10.1002/smll.202100314
63. Liu K, Jin H, Huang L, et al. Puffing ultrathin oxides with nonlayered structures. Sci Adv. 2022;8(20):eabn2030. doi:10.1126/sciadv.abn2030
64. Lin ZX, Zhou JJ, Cortez-Jugo C, et al. Ordered mesoporous metal-phenolic network particles. J Am Chem Soc. 2020;142(1):335–341. doi:10.1021/jacs.9b10835
65. Xie L, Wang G, Sang W, et al. Phenolic immunogenic cell death nanoinducer for sensitizing tumor to PD-1 checkpoint blockade immunotherapy. Biomaterials. 2021;269:120638. doi:10.1016/j.biomaterials.2020.120638
66. Fu WY, Huang ZS, Li WQ, et al. Copper-luteolin nanocomplexes for Mediating multifaceted regulation of oxidative stress, intestinal barrier, and gut microbiota in inflammatory bowel disease. Bioact Mater. 2025;46:118–133. doi:10.1016/j.bioactmat.2024.12.004
67. Wang Y-L, Mu Y, Zhang Y-L, et al. Accessible and effective nanomedicines: self-assembly products from Chinese Herbal Medicines (CHMs). Adv Funct Mater. 2025;35(9):2416151. doi:10.1002/adfm.202416151
68. Hou Y, Chen MY, Ruan HA, et al. A new supramolecular natural product gel based on self-assembled pomolic acid from traditional Chinese medicine. Colloid Interface Sci Commun. 2022;46:100583. doi:10.1016/j.colcom.2021.100583
69. Hou Y, Zou LJ, Li QL, et al. Supramolecular assemblies based on natural small molecules: union would be effective. Mater Today Bio. 2022;15:100327. doi:10.1016/j.mtbio.2022.100327
70. Wang J, Wu X, Chen J, Gao T, Zhang YM, Yu N. Traditional Chinese medicine polysaccharide in nano-drug delivery systems: current progress and future perspectives. Biomed Pharmacother. 2024;173:116330. doi:10.1016/j.biopha.2024.116330
71. Li T, Wang P, Guo W, et al. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano. 2019;13(6):6770–6781. doi:10.1021/acsnano.9b01346
72. Qi YT, Li JR, Nie Q, et al. Polyphenol-assisted facile assembly of bioactive nanoparticles for targeted therapy of heart diseases. Biomaterials. 2021;275:120952. doi:10.1016/j.biomaterials.2021.120952
73. Yu LR, Meng QQ, Li MS, Tian L, Wu XG, Jie Y. Heating-driven self-assembled glycyrrhizin nanomicelles loading bisdemethoxycurcumin: preparation, characterization, and efficacy evaluation on experimental dry eye. Colloid Surf B. 2025;245:114206. doi:10.1016/j.colsurfb.2024.114206
74. Xu WZ, Chen Y, Yang RX, et al. “reaction”-like shaping of self-delivery supramolecular nanodrugs in the nanoprecipitation process. Acs Nano. 2023;17(18):18227–18239. doi:10.1021/acsnano.3c05229
75. Chen L, Zhang SB. Structural and functional properties of self-assembled peanut protein nanoparticles prepared by ultrasonic treatment: effects of ultrasound intensity and protein concentration. Food Chem. 2023;413:135626. doi:10.1016/j.foodchem.2023.135626
76. Fu XZ, Wu J, Li J, et al. Heavy-metal resistant bio-hybrid with biogenic ferrous sulfide nanoparticles: pH-regulated self-assembly and wastewater treatment application. J Hazard Mater. 2023;446:130667. doi:10.1016/j.jhazmat.2022.130667
77. Yuan YK, Ma MJ, Zhang SZ, Wang DF, Xu Y. pH-driven self-assembly of alcohol-free curcumin-loaded propylene glycol alginate nanoparticles. Int J Biol Macromol. 2022;195:302–308. doi:10.1016/j.ijbiomac.2021.12.025
78. Yu R, Jin L, Song Z, et al. A general strategy toward self-assembled nanovaccine based on cationic lentinan to induce potent humoral and cellular immune responses. Small. 2024;20(43):e2402792. doi:10.1002/smll.202402792
79. Li GJ, He F, Feng JB, et al. Injectable self-assembling procyanidin nanospheres for effective osteoarthritis treatment. Int J Nanomed. 2025;20:1133–1145. doi:10.2147/IJN.S496827
80. Ma DJ, Li TH, Yang SY, et al. Self-assembling Bletilla polysaccharide nanogels facilitate healing of acute and infected wounds via inflammation control and antibacterial activity. Int. J Biol Macromol. 2025;299:140125. doi:10.1016/j.ijbiomac.2025.140125
81. Cui J, Wang X, Li J, et al. Immune exosomes loading self-assembled nanomicelles traverse the blood–brain barrier for chemo-immunotherapy against glioblastoma. ACS Nano. 2023;17(2):1464–1484. doi:10.1021/acsnano.2c10219
82. Truskewycz A, Yin H, Halberg N, et al. Carbon dot therapeutic platforms: administration, distribution, metabolism, excretion toxicity, and therapeutic potential. Small. 2022;18(16):e2106342. doi:10.1002/smll.202106342
83. Zeng M, Wang Y, Liu M, et al. Potential efficacy of herbal medicine-derived carbon dots in the treatment of diseases: from mechanism to clinic. Int J Nanomed. 2023;18:6503–6525. doi:10.2147/IJN.S431061
84. Luo WK, Zhang LL, Yang ZY, et al. Herbal medicine derived carbon dots: synthesis and applications in therapeutics, bioimaging and sensing. J Nanobiotechnology. 2021;19(1):320. doi:10.1186/s12951-021-01072-3
85. Nair A, Haponiuk JT, Thomas S, Gopi S. Natural carbon-based quantum dots and their applications in drug delivery: a review. Biomed Pharmacother. 2020;132:110834. doi:10.1016/j.biopha.2020.110834
86. Wang RB, Zhang SL, Zhang J, et al. State-of-the-art of lignin-derived carbon nanodots: preparation, properties, and applications. Int J Biol Macromol. 2024;273:132897. doi:10.1016/j.ijbiomac.2024.132897
87. Maruthapandi M, Saravanan A, Das P, Luong JHT, Gedanken A. Microbial inhibition and biosensing with multifunctional carbon dots: progress and perspectives. Biotechnol Adv. 2021;53:107843. doi:10.1016/j.biotechadv.2021.107843
88. Jin Z, Liu M, Huang X, et al. Top-down rational engineering of heteroatom-doped graphene quantum dots for laser desorption/ionization mass spectrometry detection and imaging of small biomolecules. Anal Chem. 2022;94(21):7609–7618. doi:10.1021/acs.analchem.2c00802
89. Fan Y, Wang JH, Qian S, Xue HG, Tian JQ, Jiang TF. Assembling carbon nitride quantum dots into hollow fusiformis and loading CoP for photocatalytic hydrogen evolution. J Colloid Interf Sci. 2024;667:128–135. doi:10.1016/j.jcis.2024.04.066
90. Alafeef M, Srivastava I, Aditya T, Pan D. Carbon dots: from synthesis to unraveling the fluorescence mechanism. Small. 2024;20(4):e2303937. doi:10.1002/smll.202303937
91. Yang S, Li Y, Chen L, et al. Fabrication of carbon-based quantum dots via a “bottom-up approach: topology, chirality, and free radical processes in “building blocks”. Small. 2023;19(31):e2205957. doi:10.1002/smll.202205957
92. Xia C, Zhu S, Feng T, Yang M, Yang B. Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots. Adv Sci. 2019;6(23):1901316. doi:10.1002/advs.201901316
93. Pandey AK, Bankoti K, Nath TK, Dhara S. Hydrothermal synthesis of PVP-passivated clove bud-derived carbon dots for antioxidant, catalysis, and cellular imaging applications. Colloid Surf B. 2022;220:112926. doi:10.1016/j.colsurfb.2022.112926
94. Lin CJ, Chang L, Chu HW, et al. High amplification of the antiviral activity of curcumin through transformation into carbon quantum dots. Small. 2019;15(41):e1902641. doi:10.1002/smll.201902641
95. Wang PC, Yan Y, Zhang Y, et al. An improved synthesis of water-soluble dual fluorescence emission carbon dots from holly leaves for accurate detection of mercury ions in living cells. Int J Nanomed. 2021;16:2045–2058. doi:10.2147/IJN.S298152
96. Architha N, Ragupathi M, Shobana C, et al. Microwave-assisted green synthesis of fluorescent carbon quantum dots from Mexican Mint extract for Fe3+ detection and bio-imaging applications. Environ Res. 2021;199:111263. doi:10.1016/j.envres.2021.111263
97. Dong JX, Wang Q, Gu TT, et al. Rapamycin functionalized carbon Dots: target-oriented synthesis and suppression of vascular cell senescence. J Colloid Interf Sci. 2024;660:534–544. doi:10.1016/j.jcis.2024.01.032
98. Dong LY, Zhao YF, Luo J, et al. Carbon dots derived from curcumae radix and their heartprotective effect. Int J Nanomed. 2024;19:3315–3332. doi:10.2147/IJN.S444125
99. Zhang Y, Zhang ST, Tan BC, Guo L, Li HT. Solvothermal synthesis of functionalized carbon dots from amino acid as an eco-friendly corrosion inhibitor for copper in sulfuric acid solution. J Colloid Interf Sci. 2021;604:1–14. doi:10.1016/j.jcis.2021.07.034
100. Mauro N, Utzeri MA, Sciortino A, et al. Decagram-scale synthesis of multicolor carbon nanodots: self-tracking nanoheaters with inherent and selective anticancer properties. Acs Appl Mater Inter. 2022;14(2):2551–2563. doi:10.1021/acsami.1c19599
101. Chen L, Wang CF, Liu C, Chen S. Facile access to fabricate carbon dots and perspective of large‐scale applications. Small. 2022;19(31):e2206671. doi:10.1002/smll.202206671
102. Rani UA, Ng LY, Ng CY, Mahmoudi E. A review of carbon quantum dots and their applications in wastewater treatment. Adv Colloid Interface Sci. 2020;278:102124. doi:10.1016/j.cis.2020.102124
103. González-Reyna MA, Molina GA, Juarez-Moreno K, Rodríguez-Torres A, Esparza R, Estevez M. Green nanoarchitectonics of carbon quantum dots from cinchona pubescens Vahl as targeted and controlled drug cancer nanocarrier. Biomater Adv. 2023;153:213561. doi:10.1016/j.bioadv.2023.213561
104. Li YB, Bai GX, Zeng SJ, Hao JH. Theranostic carbon dots with innovative NIR-II emission for in vivo renal-excreted optical imaging and photothermal therapy. Acs Appl Mater Inter. 2019;11(5):4737–4744. doi:10.1021/acsami.8b14877
105. Hu YF, Chen YX, Chen SY. Green synthesis of near-infrared carbon dots as a novel aptamer-based fluorescence probe for the detection and imaging of alpha-fetoprotein. Microchim Acta. 2025;192(3):179. doi:10.1007/s00604-025-07046-8
106. Debbi L, Guo S, Safina D, Levenberg S. Boosting extracellular vesicle secretion. Biotechnol Adv. 2022;59:107983. doi:10.1016/j.biotechadv.2022.107983
107. Zhao B, Lin HJ, Jiang XC, et al. Exosome-like nanoparticles derived from fruits, vegetables, and herbs: innovative strategies of therapeutic and drug delivery. Theranostics. 2024;14(12):4598–4621. doi:10.7150/thno.97096
108. Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int J Nanomedicine. 2021;16:1575–1586. doi:10.2147/IJN.S293067
109. Jiang P, Ma X, Wang X, et al. Isolation and comprehensive analysis of cochlear tissue-derived small extracellular vesicles. Adv Sci. 2024;11(48):e2408964. doi:10.1002/advs.202408964
110. Lattmann E, Räss L, Tognetti M, et al. Size-exclusion chromatography combined with DIA-MS enables deep proteome profiling of extracellular vesicles from melanoma plasma and serum. Cell Mol Life Sci. 2024;81(1):90. doi:10.1007/s00018-024-05137-y
111. Martínez-García J, Fernández B, Álvarez-barrios A, Álvarez L, González-Iglesias H, Pereiro R. Determination of endogenous trace elements in extracellular vesicles secreted by an in vitro model of human retinal pigment epithelium under oxidative stress conditions using ICP-MS. Talanta. 2023;263:124693. doi:10.1016/j.talanta.2023.124693
112. Gao CY, Chen Y, Wen XY, et al. Plant-derived exosome-like nanoparticles in tissue repair and regeneration. J Mater Chem B. 2025;13(7):2254–2271. doi:10.1039/d4tb02394c
113. Godakhindi V, Tarannum M, Dam SK, Vivero-Escoto JL. Mesoporous silica nanoparticles as an ideal platform for cancer immunotherapy: recent advances and future directions. Adv Healthc Mater. 2024;13(20):e2400323. doi:10.1002/adhm.202400323
114. Feng Y, Liao Z, Li MY, et al. Mesoporous silica nanoparticles-based nanoplatforms: basic construction, current state, and emerging applications in anticancer therapeutics. Adv Healthc Mater. 2023;12(16):e2201884. doi:10.1002/adhm.202201884
115. Pinna A, Torki baghbaderani M, Vigil Hernández V, et al. Nanoceria provides antioxidant and osteogenic properties to mesoporous silica nanoparticles for osteoporosis treatment. Acta Biomater. 2021;122:365–376. doi:10.1016/j.actbio.2020.12.029
116. Enyu X, Xinbo L, Xuelian C, Huimin C, Yin C, Yan C. Construction and performance evaluation of pH-responsive oxidized hyaluronic acid hollow mesoporous silica nanoparticles. Int J Biol Macromol. 2024;257:128656. doi:10.1016/j.ijbiomac.2023.128656
117. Xu BL, Li SS, Shi R, Liu HY. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Trans Tar. 2023;8(1):435. doi:10.1038/s41392-023-01654-7
118. Barkat A, Beg S, Panda SK, Alharbi S, Rahman K, Ahmed M. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin Cancer Biol. 2021;69:365–375. doi:10.1016/j.semcancer.2019.08.022
119. Yang J, Yang YW. Metal-organic frameworks for biomedical applications. Small 2020;16(10):e1906846. doi:10.1002/smll.201906846
120. Lawson HD, Walton SP, Chan C. Metal-organic frameworks for drug delivery: a design perspective. ACS Appl Mater Interfaces. 2021;13(6):7004–7020. doi:10.1021/acsami.1c01089
121. Gao P, Chen YY, Pan W, Li N, Liu Z, Tang B. Antitumor agents based on metal-organic frameworks. Angew Chem Int Edit. 2021;60(31):16763–16776. doi:10.1002/anie.202102574
122. Khan FA, Albalawi R, Pottoo FH. Trends in targeted delivery of nanomaterials in colon cancer diagnosis and treatment. Med Res Rev. 2022;42(1):227–258. doi:10.1002/med.21809
123. Garcia-Peiro JI, Bonet-Aleta J, Santamaria J, Hueso JL. Platinum nanoplatforms: classic catalysts claiming a prominent role in cancer therapy. Chem Soc Rev. 2022;51(17):7662–7681. doi:10.1039/d2cs00518b
124. Zhang Y, Zhou L, Tan J, Liu J, Shan X, Ma Y. Laser-triggered collaborative chemophotothermal effect of gold nanoparticles for targeted colon cancer therapy. Biomed Pharmacother. 2020;130:110492. doi:10.1016/j.biopha.2020.110492
125. Rezaei B, Yari P, Sanders SM, et al. Magnetic nanoparticles: a review on synthesis, characterization functionalization, and biomedical applications. Small. 2024;20(5):e2304848. doi:10.1002/smll.202304848
126. Espinosa A, Reguera J, Curcio A, et al. Janus magnetic-plasmonic nanoparticles for magnetically guided and thermally activated cancer therapy. Small. 2020;16(11):e1904960. doi:10.1002/smll.201904960
127. Sun R, Chen H, Zheng J, et al. Composite scaffolds of gelatin and fe o nanoparticles for magnetic hyperthermia-based breast cancer treatment and adipose tissue regeneration. Adv Healthc Mater. 2023;12(9):e2202604. doi:10.1002/adhm.20220260434
128. Maleki Dana P, Hallajzadeh J, Asemi Z, Mansournia MA, Yousefi B. Chitosan applications in studying and managing osteosarcoma. Int. J Biol Macromol. 2021;169:321–329. doi:10.1016/j.ijbiomac.2020.12.058
129. Wei D, Tong Q, An Q, et al. Dual stimuli-responsive nanocarriers based on polyethylene glycol-mediated schiff base interactions for overcoming tumour chemoresistance. Colloids Surf B Biointerfaces. 2022;213:112408. doi:10.1016/j.colsurfb.2022.112408
130. Chen Y, Li R, Fu X, et al. “All in one” lipid-polymer nanodelivery system for gene therapy of ischemic diseases. Biomaterials. 2025;313:122799. doi:10.1016/j.biomaterials.2024.122799
131. Guerassimoff L, Ferrere M, Van Herck S, et al. Thermosensitive polymer prodrug nanoparticles prepared by an all-aqueous nanoprecipitation process and application to combination therapy. J Control Release. 2024;369:376–393. doi:10.1016/j.jconrel.2024.03.049
132. Song YH, De R, Lee KT. Emerging strategies to fabricate polymeric nanocarriers for enhanced drug delivery across blood-brain barrier: an overview. Adv Colloid Interface Sci. 2023;320:103008. doi:10.1016/j.cis.2023.103008
133. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
134. Andishmand H, Yousefi M, Jafari N, et al. Designing and fabrication of colloidal nano-phytosomes with gamma-oryzanol and phosphatidylcholine for encapsulation and delivery of polyphenol-rich extract from pomegranate peel. Int J Biol Macromol. 2024;256:128501. doi:10.1016/j.ijbiomac.2023.128501
135. Cao S, Zhang W, Pan H, et al. Bioactive lipid-nanoparticles with inherent self-therapeutic and anti-angiogenic properties for cancer therapy. Acta Biomater. 2023;157:500–510. doi:10.1016/j.actbio.2022.12.022
136. Salunkhe SA, Chitkara D, Mahato RI, Mittal A. Lipid based nanocarriers for effective drug delivery and treatment of diabetes associated liver fibrosis. Adv Drug Deliv Rev. 2021;173:394–415. doi:10.1016/j.addr.2021.04.003
137. Nel J, Franconi F, Joudiou N, Saulnier P, Gallez B, Lemaire L. Lipid nanocapsules as in vivo oxygen sensors using magnetic resonance imaging. Mater Sci Eng C Mater Biol Appl. 2019;101:396–403. doi:10.1016/j.msec.2019.03.104
138. Zhang Y, Luo J, Gui X, et al. Bioengineered nanotechnology for nucleic acid delivery. J Control Release. 2023;364:124–141. doi:10.1016/j.jconrel.2023.10.034
139. Rahman MM, Wang J, Wang G, et al. Chimeric nanobody-decorated liposomes by self-assembly. Nat Nanotechnol. 2024;19(6):818–824. doi:10.1038/s41565-024-01620-6
140. Geng M, Feng X, Wu X, et al. Characterization and utilization of soy protein isolate-(-)-epigallocatechin gallate-maltose ternary conjugate as an emulsifier for nanoemulsions: enhanced physicochemical stability of the β-carotene nanoemulsion. Food Chem. 2023;417:135842. doi:10.1016/j.foodchem.2023.135842
141. Chen Y, Wang J, Xu J, et al. Fabrication of a polysaccharide-protein/protein complex stabilized oral nanoemulsion to facilitate the therapeutic effects of 1,8-cineole on atherosclerosis. ACS Nano. 2023;17(10):9090–9109. doi:10.1021/acsnano.2c12230
142. Inthamat P, Siripatrawan U. Influence of chitosan encapsulation on functionality and stability of astaxanthin nanoemulsion fabricated using high pressure homogenization. Int J Biol Macromol. 2025;303:140379. doi:10.1016/j.ijbiomac.2025.140379
143. Liu M, Bai Y, Feng M, et al. The synergistic antibacterial effects of allicin nanoemulsion and ε-polylysine against Escherichia coli in both planktonic and biofilm forms. Food Chem. 2025;472:142949. doi:10.1016/j.foodchem.2025.142949
144. Liu J, Xu J, Jia L, et al. Pterostilbene nanoemulsion promotes Nrf2 signaling pathway to downregulate oxidative stress for treating Alzheimer’s disease. Int J Pharm. 2024;655:124002. doi:10.1016/j.ijpharm.2024.124002
145. Ding X, Li S, Tian M, et al. Facile preparation of a novel nanoemulsion based hyaluronic acid hydrogel loading with Poria cocos triterpenoids extract for wound dressing. Int J Biol Macromol. 2023;226:1490–1499. doi:10.1016/j.ijbiomac.2022.11.261
146. Sana SS, Chandel AKS, Raorane CJ, et al. Recent advances in nano and micro formulations of Ginsenoside to enhance their therapeutic efficacy. Phytomedicine. 2024;134:156007. doi:10.1016/j.phymed.2024.156007
147. Park JH, Dehaini D, Zhou J, Holay M, Fang RH, Zhang L. Biomimetic nanoparticle technology for cardiovascular disease detection and treatment. Nanoscale Horiz. 2020;5(1):25–42. doi:10.1039/c9nh00291j
148. Oroojalian F, Beygi M, Baradaran B, Mokhtarzadeh A, Shahbazi MA. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021;17(12):e2006484. doi:10.1002/smll.202006484
149. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
150. AlQahtani SA, Harisa GI, Alomrani AH, Alanazi FK, Badran MM. Improved pharmacokinetic and biodistribution of 5-fluorouracil loaded biomimetic nanoerythrocytes decorated nanocarriers for liver cancer treatment. Colloids Surf B Biointerfaces. 2021;197:111380. doi:10.1016/j.colsurfb.2020.111380
151. Zhang X, Liang L, Wang F, Jose PA, Chen K, Zeng C. Irisin-encapsulated mitochondria-targeted biomimetic nanotherapeutics for alleviating acute kidney injury. Adv Sci. 2024;11(38):e2402805. doi:10.1002/advs.202402805
152. Oieni J, Levy L, Letko Khait N, et al. Nano-Ghosts: biomimetic membranal vesicles, technology and characterization. Methods. 2020;177:126–134. doi:10.1016/j.ymeth.2019.11.013
153. Rawal S, Patel M. Bio-nanocarriers for lung cancer management: befriending the barriers. Nanomicro Lett. 2021;13(1):142. doi:10.1007/s40820-021-00630-6
154. Liang G, Zhu Y, Ali DJ, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18(1):10. doi:10.1186/s12951-019-0563-2
155. Qiu C, Zhang JZ, Wu B, et al. Advanced application of nanotechnology in active constituents of Traditional Chinese Medicines. J Nanobiotechnology. 2023;21(1):456. DOI:10.1186/s12951-023-02165-x
156. Yao M, Wu M, Yuan M, et al. Enhancing the therapeutic potential of isoliensinine for hypertension through PEG-PLGA nanoparticle delivery: a comprehensive in vivo and in vitro study. Biomed Pharmacother. 2024;174:116541. doi:10.1016/j.biopha.2024.116541
157. Ge C, Wei X, Xu Y, et al. Natural ellagic acid-polyphenol ″sandwich biscuit″ self-assembled solubilizing system for formation mechanism and antibacterial synergia. ACS Appl Mater Interfaces. doi:10.1021/acsami.5c00683
158. Zhou J, Jiang Z, Sun R, et al. Comparison of cell delivery and cell membrane camouflaged PLGA nanoparticles in the delivery of shikonin for colorectal cancer treatment. Colloids Surf B Biointerfaces. 2024;241:114017. doi:10.1016/j.colsurfb.2024.114017
159. Wei G, Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11(13):6370–6392. doi:10.7150/thno.57828
160. Passaro F, Tocchetti CG, Spinetti G, et al. Targeting fibrosis in the failing heart with nanoparticles. Adv Drug Deliv Rev. 2021;174:461–481. doi:10.1016/j.addr.2021.05.004
161. Al-Shadidi JRMH, Al-Shammari S, Al-Mutairi D, Alkhudhair D, Thu HE, Hussain Z. Chitosan nanoparticles for targeted cancer therapy: a review of stimuli-responsive, passive, and active targeting strategies. Int J Nanomed. 2024;19:8373–8400. doi:10.2147/IJN.S472433
162. Ji H, Wang W, Li X, et al. Natural small molecules enabled efficient immunotherapy through supramolecular self-Assembly in P53-mutated colorectal cancer. ACS Appl Mater Interfaces. 2022;14(2):2464–2477. doi:10.1021/acsami.1c16737
163. Zhang D, Liu L, Wang J, et al. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front Pharmacol. 2022;13:990505. doi:10.3389/fphar.2022.990505
164. Haggag YA, Ibrahim RR, Hafiz AA. Design, formulation and in vivo evaluation of novel honokiol-loaded PEGylated PLGA nanocapsules for treatment of breast cancer. Int J Nanomed. 2020;15:1625–1642. doi:10.2147/IJN.S241428
165. Zheng Y, Jia R, Li J, Tian X, Qian Y. Curcumin- and resveratrol-co-loaded nanoparticles in synergistic treatment of hepatocellular carcinoma. J Nanobiotechnology. 2022;20(1):339. doi:10.1186/s12951-022-01554-y
166. Ahmad A, Khan F, Mishra RK, Khan R. Precision cancer nanotherapy: evolving role of multifunctional nanoparticles for cancer active targeting. J Med Chem. 2019;62(23):10475–10496. doi:10.1021/acs.jmedchem.9b00511
167. Dutta B, Barick KC, Hassan PA. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv Colloid Interface Sci. 2021;296:102509. doi:10.1016/j.cis.2021.102509
168. Agwa MM, Elmotasem H, Elsayed H, et al. Carbohydrate ligands-directed active tumor targeting of combinatorial chemotherapy/phototherapy-based nanomedicine: a review. Int J Biol Macromol. 2023;239:124294. doi:10.1016/j.ijbiomac.2023.124294
169. Nawaz FZ, Kipreos ET. Emerging roles for folate receptor FOLR1 in signaling and cancer. Trends Endocrinol Metab. 2022;33(3):159–174. doi:10.1016/j.tem.2021.12.003
170. Guo F, Yu N, Jiao Y, et al. Star polyester-based folate acid-targeting nanoparticles for doxorubicin and curcumin co-delivery to combat multidrug-resistant breast cancer. Drug Deliv. 2021;28(1):1709–1721. doi:10.1080/10717544.2021.1960926
171. Li Z, Zhang Y, Zhu C, et al. Folic acid modified lipid-bilayer coated mesoporous silica nanoparticles co-loading paclitaxel and tanshinone IIA for the treatment of acute promyelocytic leukemia. Int J Pharm. 2020;586:119576. doi:10.1016/j.ijpharm.2020.119576
172. Gautam AK, Kumar P, Maity B, et al. Synthesis and appraisal of dalbergin-loaded PLGA nanoparticles modified with galactose against hepatocellular carcinoma: in-vitro, pharmacokinetic and in-silico studies. Front Pharmacol. 2022;13:1021867. doi:10.3389/fphar.2022.1021867
173. Brown CE, Rodriguez A, Palmer J, et al. Off-the-shelf, steroid-resistant, IL13Rα2-specific CAR T cells for treatment of glioblastoma. Neuro Oncol. 2022;24(8):1318–1330. doi:10.1093/neuonc/noac024
174. Lin XM, Shi XX, Xiong L, et al. Construction of IL-13 receptor α2-targeting resveratrol nanoparticles against glioblastoma cells: therapeutic efficacy and molecular effects. Int J Mol Sci. 2021;22(19):10622. doi:10.3390/ijms221910622
175. Guo Z, Zhang Y, Gong Y, et al. Antibody functionalized curcuma-derived extracellular vesicles loaded with doxorubicin overcome therapy-induced senescence and enhance chemotherapy. J Control Release. 2025;379:377–389. doi:10.1016/j.jconrel.2025.01.029
176. Li L, Zhang X, Pi C, et al. Review of curcumin physicochemical targeting delivery system. Int J Nanomed. 2020;15:9799–9821. doi:10.2147/IJN.S276201
177. Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):70. doi:10.1038/s41392-023-01332-8
178. Liu X, Wu Z, Guo C, et al. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022;29(1):138–148. doi:10.1080/10717544.2021.2021324
179. Ding H, Tan P, Fu S, et al. Preparation and application of pH-responsive drug delivery systems. J Control Release. 2022;348:206–238. doi:10.1016/j.jconrel.2022.05.056
180. Ghosh N, Kundu M, Ghosh S, et al. pH-responsive and targeted delivery of chrysin via folic acid-functionalized mesoporous silica nanocarrier for breast cancer therapy. Int J Pharm. 2023;631:122555. doi:10.1016/j.ijpharm.2022.122555
181. Roy S, Debasmita D, Dey U, Ghosh SS, Chattopadhyay A. Unveiling the cytotoxic potential of quercetin-loaded magnetic bacterial bots against cervical cancer. ACS Appl Mater Interfaces. 2025;17(4):5799–5812. doi:10.1021/acsami.4c17079
182. Liu Y, Zhu X, Wei Z, et al. Customized photothermal therapy of subcutaneous orthotopic cancer by multichannel luminescent nanocomposites. Adv Mater. 2021;33(30):e2008615. doi:10.1002/adma.202008615
183. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi:10.1038/s41571-020-0410-2
184. Su M, Chen Y, Jia L, Zhang Z. Camptothecin-loaded and manganese dioxide-coated polydopamine nanomedicine used for magnetic resonance imaging diagnosis and chemo-photothermal therapy for lung cancer. Int J Nanomed. 2022;17:6687–6705. doi:10.2147/IJN.S359300
185. Li K, Xu K, Liu S, et al. All-in-one engineering multifunctional nanoplatforms for sensitizing tumor low-temperature photothermal therapy in vivo. ACS Nano. 2023;17(20):20218–20236. doi:10.1021/acsnano.3c05991
186. Chen Z, Wei X, Zheng Y, et al. Targeted co-delivery of curcumin and erlotinib by MoS nanosheets for the combination of synergetic chemotherapy and photothermal therapy of lung cancer. J Nanobiotechnology. 2023;21(1):333. doi:10.1186/s12951-023-02099-42
187. Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva VMV IV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9(1):e001926. doi:10.1136/jitc-2020-001926
188. Ji B, Wei M, Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022;12(1):434–458. doi:10.7150/thno.67300
189. Thakur NS, Mandal N, Patel G, et al. Co-administration of zinc phthalocyanine and quercetin via hybrid nanoparticles for augmented photodynamic therapy. Nanomedicine. 2021;33:102368. doi:10.1016/j.nano.2021.102368
190. Luo T, Nash GT, Xu Z, Jiang X, Liu J, Lin W. Nanoscale metal-organic framework confines zinc-phthalocyanine photosensitizers for enhanced photodynamic therapy. J Am Chem Soc. 2021;143(34):13519–13524. doi:10.1021/jacs.1c07379
191. Bai X, Dong C, Shao X, Rahman FU, Hao H, Zhang Y. Research progress of fullerenes and their derivatives in the field of PDT. Eur J Med Chem. 2024;271:116398. doi:10.1016/j.ejmech.2024.116398
192. Zhang H, Hou L, Jiao X, Ji Y, Zhu X, Zhang Z. Transferrin-mediated fullerenes nanoparticles as Fe(2+)-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials. 2015;37:353–366. doi:10.1016/j.biomaterials.2014.10.031
193. Liu Y, Zhao J, Xu X, et al. Emodin-based nanoarchitectonics with giant two-photon absorption for enhanced photodynamic therapy. Angew Chem Int Ed Engl. 2023;62(33):e202308019. doi:10.1002/anie.202308019
194. Zhou J, Ji M, Yang Y, et al. Two-photon photodynamic therapy with curcumin nanocomposite. Colloids Surf B Biointerfaces. 2025;245:114306. doi:10.1016/j.colsurfb.2024.114306
195. Wen F, Su W, Cen L, et al. Natural fluorescent carbon quantum dots embedded polyvinyl alcohol/chitosan film with photoregulation and high antibacterial efficiency for infected wound healing. Int J Biol Macromol. 2025;306:141716. doi:10.1016/j.ijbiomac.2025.141716
196. Zhang L, Li CX, Wan SS, Zhang XZ. Nanocatalyst-mediated chemodynamic tumor therapy. Adv Healthc Mater. 2022;11(2):e2101971. doi:10.1002/adhm.202101971
197. Jia C, Guo Y, Wu FG. Chemodynamic therapy via fenton and fenton-like nanomaterials: strategies and recent advances. Small. 2022;18(6):e2103868. doi:10.1002/smll.202103868
198. Zhang Y, Zhang N, Xing J, et al. In situ hydrogel based on Cu-Fe3O4 nanoclusters exploits oxidative stress and the ferroptosis/cuproptosis pathway for chemodynamic therapy. Biomaterials. 2024;311:122675. doi:10.1016/j.biomaterials.2024.122675
199. Chen J, Xue F, Du W, et al. An endogenous HS-activated nanoplatform for triple synergistic therapy of colorectal cancer. Nano Lett. 2022;22(15):6156–6165. doi:10.1021/acs.nanolett.2c013462
200. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
201. Zhang B, Jiang J, Wu P, et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm Sin B. 2021;11(1):246–257. doi:10.1016/j.apsb.2020.07.026
202. Liu Y, Li J. Self-assembling nanoarchitectonics of size-controllable celastrol nanoparticles for efficient cancer chemotherapy with reduced systemic toxicity. J Colloid Interface Sci. 2023;636:216–222. doi:10.1016/j.jcis.2022.12.162
203. Zhou M, Niu H, Huang G, et al. Biomimetic nano-delivery of small-molecule piceatannol modulates tumor stemness and suppresses colorectal cancer metastasis via Hippo/YAP1/SOX9 Signaling. Small. 2025;21(2):e2407191. doi:10.1002/smll.202407191
204. Zhou S, Li J, Yu J, et al. Unique flower-like Cur-metal complexes loaded liposomes for primary and metastatic breast cancer therapy. Mater Sci Eng C Mater Biol Appl. 2021;121:111835. doi:10.1016/j.msec.2020.111835
205. Li Z, Wang F, Li Y, et al. Combined anti-hepatocellular carcinoma therapy inhibit drug-resistance and metastasis via targeting “substance P-hepatic stellate cells-hepatocellular carcinoma. Axis Biomaterials. 2021;276:121003. doi:10.1016/j.biomaterials.2021.121003
206. Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374(6571):1070–1075. doi:10.1126/science.abi5200
207. Hong SY, Lu YT, Chen SY, et al. Targeting pathogenic macrophages by the application of SHP-1 agonists reduces inflammation and alleviates pulmonary fibrosis. Cell Death Dis. 2023;14(6):352. doi:10.1038/s41419-023-05876-z
208. Zhang Z, Yuan Y, Hu L, et al. ANGPTL8 accelerates liver fibrosis mediated by HFD-induced inflammatory activity via LILRB2/ERK signaling pathways. J Adv Res. 2023;47:41–56. doi:10.1016/j.jare.2022.08.006
209. Tanaka S, Portilla D, Okusa MD. Role of perivascular cells in kidney homeostasis, inflammation, repair and fibrosis. Nat Rev Nephrol. 2023;19(11):721–732. doi:10.1038/s41581-023-00752-7
210. Lan T, Jiang S, Zhang J, et al. Breviscapine alleviates NASH by inhibiting TGF-β-activated kinase 1-dependent signaling. Hepatology. 2022;76(1):155–171. doi:10.1002/hep.32221
211. Gao C, Zhou Y, Chen Z, et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 2022;12(12):5596–5614. doi:10.7150/thno.73650
212. Zheng M, Liu K, Li L, Feng C, Wu G. Traditional Chinese medicine inspired dual-drugs loaded inhalable nano-therapeutics alleviated idiopathic pulmonary fibrosis by targeting early inflammation and late fibrosis. J Nanobiotechnology. 2024;22(1):14. doi:10.1186/s12951-023-02251-0
213. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi:10.1016/S0140-6736(21)02724-0.
214. Zou Q, Chen Y, Qin H, et al. The role and mechanism of TCM in the prevention and treatment of infectious diseases. Front Microbiol. 2023;14:1286364. doi:10.3389/fmicb.2023.1286364
215. Zhang Z, Chen M, Wang J, et al. Hyaluronic acid-modified micelles of azithromycin and quercetin against infections caused by methicillin-resistant staphylococcus aureus. Int J Nanomed. 2024;19:9637–9658. doi:10.2147/IJN.S476471
216. Tan S, Liu Z, Cong M, et al. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing Staphylococcus aureus exotoxins for the care of invasive wounds. J Control Release. 2024;368:355–371. doi:10.1016/j.jconrel.2024.02.045
217. Qian W, Zhang B, Gao M, et al. Supramolecular prodrug inspired by the rhizoma coptidis-fructus mume herbal pair alleviated inflammatory diseases by inhibiting pyroptosis. J Pharm Anal. 2025;15(2):101056. doi:10.1016/j.jpha.2024.101056
218. Zhang J, Hu K, Di L, et al. Traditional herbal medicine and nanomedicine: converging disciplines to improve therapeutic efficacy and human health. Adv Drug Deliv Rev. 2021;178:113964. doi:10.1016/j.addr.2021.113964
219. Zhang YL, Wang YL, Yan K, et al. Traditional Chinese medicine formulae QY305 reducing cutaneous adverse reaction and diarrhea by its nanostructure. Adv Sci. 2024;11(5):e2306140. doi:10.1002/advs.202306140
220. Chen J, Chen S, Luo H, Wan X, Wu W, Wang S. The complementary and alternative roles of elemene injection in cancer: an umbrella review. Pharmacol Res. 2023;198:107007. doi:10.1016/j.phrs.2023.107007
221. Sun YN, Zhang ZY, Zeng YC, Chi F, Jin XY, Wu R. Comparative efficacy of whole-brain radiotherapy with and without elemene liposomes in patients with multiple brain metastases from non-small-cell lung carcinoma. Curr Oncol. 2016;23(4):e377–e382. doi:10.3747/co.23.3183
222. Jinnouchi M, Miyahara T, Suzuki Y. Coix seed consumption affects the gut microbiota and the peripheral lymphocyte subset profiles of healthy male adults. Nutrients. 2021;13(11). doi:10.3390/nu13114079
223. Sasaki K, Kantarjian H, Wierda W, et al. Phase 2 study of hyper-CMAD with liposomal vincristine for patients with newly diagnosed acute lymphoblastic leukemia. Am J Hematol. 2020;95(7):734–739. doi:10.1002/ajh.25784
224. Rezaeimanesh N, Rafiee P, Saeedi R, et al. The effect of crocin-selenium nanoparticles on the cognition and oxidative stress markers of multiple sclerosis patients: a randomized triple-blinded placebo-controlled clinical trial. Biometals. 2024;37(2):305–319. doi:10.1007/s10534-023-00548-z
225. Vazirabadi SB, Mehrpouya M, Motallebi N, et al. A double-blind randomized split-mouth clinical trial on a hemostatic dental material containing aloe vera nanoparticles: the effects on pain and occurrence of dry socket after tooth extraction. Curr Pharm Biotechnol. 2025. doi:10.2174/0113892010363882250210054655
226. Pérez-Pacheco CG, Fernandes NAR, Primo FL, et al. Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: randomized, placebo-controlled, double-blind split-mouth clinical trial. Clin Oral Investig. 2021;25(5):3217–3227. doi:10.1007/s00784-020-03652-3
227. Jaafar HM, Ameen DMH, Mohammad TAM, Jafaar AM. The effects of nanocurcumin on immune-related factors in the ankylosing spondylitis patients: a double-blind, randomized, placebo-controlled clinical trial. Mol Biol Rep. 2025;52(1):324. doi:10.1007/s11033-025-10397-3
228. Sarwa KK, Mazumder B, Suresh PK, Kaur CD. Topical analgesic nanolipid vesicles formulation of capsaicinoids extract of bhut jolokia (Capsicum chinense Jacq): pharmacodynamic evaluation in rat models and acceptability studies in human volunteers. Curr Drug Deliv. 2016;13(8):1325–1338. doi:10.2174/1567201813666160614120809
229. Sanoff HK, Moon DH, Moore DT, et al. Phase I/II trial of nano-camptothecin CRLX101 with capecitabine and radiotherapy as neoadjuvant treatment for locally advanced rectal cancer. Nanomedicine. 2019;18:189–195. doi:10.1016/j.nano.2019.02.021
230. Schmidt KT, Karzai F, Bilusic M, et al. A single-arm phase II study combining NLG207, a nanoparticle camptothecin, with enzalutamide in advanced metastatic castration-resistant prostate cancer post-enzalutamide. Oncologist. 2022;27(9):718–e694. doi:10.1093/oncolo/oyac100
231. Casarin M, Pazinatto J, Oliveira LM, Souza ME, Santos RCV, Zanatta FB. Anti-biofilm and anti-inflammatory effect of a herbal nanoparticle mouthwash: a randomized crossover trial. Braz Oral Res. 2019;33:e062. doi:10.1590/1807-3107bor-2019.vol33.0062
232. Oliveira CA, Peres DD, Graziola F, et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur J Pharm Sci. 2016;81:1–9. doi:10.1016/j.ejps.2015.09.016
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

