Back to Journals » Infection and Drug Resistance » Volume 18
Identification of Pathogens in HIV-Infected Patients Using Metagenomic Next-Generation Sequencing (mNGS) as Compared to Conventional Microbiological Tests (CMTs)
Authors Zhu X, Cao L, Wang J, Lu X, Huang Z, Wen X, Bian L, Wu C, Zou M
Received 19 September 2024
Accepted for publication 22 January 2025
Published 17 February 2025 Volume 2025:18 Pages 929—940
DOI https://doi.org/10.2147/IDR.S491946
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Xiaohong Zhu, Li Cao, Juan Wang, Xuefeng Lu, Zuoyu Huang, Xiaoping Wen, Lihong Bian, Congxia Wu, Meiyin Zou
The Third Affiliated Hospital, Nantong University, Nantong, Jiangsu, 223001, People’s Republic of China
Correspondence: Congxia Wu; Meiyin Zou, Email [email protected]; [email protected]
Background: The rapid and accurate identification of infectious pathogens in HIV-infected patients remains a challenge. Metagenomic next-generation sequencing (mNGS) is a panpathogen assay for rapid diagnosis of infectious diseases. Here, the diagnostic value of mNGS was evaluated in HIV-infected patients and compared with conventional microbiological tests (CMTs).
Methods: This study was conducted on 216 hIV-infected patients with suspected opportunistic infections. Infectious pathogen detection was done by mNGS and conventional microbiological tests, respectively.
Results: A total of 195 patients (90.2%) were positive for microbial pathogens by mNGS, while 135 patients (62.5%) were positive for microbial pathogens by CMTs. Mixed infection was identified in 92 patients by mNGS, and 41 patients were detected with mixed infection by CMTs. Fungi and virus mixed infection was the most frequent pattern detected by mNGS (32, 14.8%) and CMTs (22, 10.2%). The CD4+ T cell count in patients with mixed pathogens was significantly lower than that in patients infected with a single pathogen. Pathogens were quickly identified by mNGS in 151 patients (69.9%), and appropriate treatments were initiated. In 47 patients antibacterial agents were adjusted based on mNGS results, in 39 patients antifungal agents were changed, and 35 patients had antiviral agents added.
Conclusion: mNGS is a valuable tool and enhances rapid microbiological identification in HIV-infected patients. Combined with CMTs, mNGS may facilitate personalized antimicrobial treatment strategies and increase survival.
Keywords: metagenomic next-generation sequencing (mNGS), HIV, conventional microbiological tests (CMTs), antimicrobial treatment
Introduction
Human immunodeficiency virus-1 (HIV-1) attacks the immune system and leads to acquired immunodeficiency syndrome (AIDS).1 It has been reported that about 39 million people were living with HIV-1, 1.3 million people became newly infected with HIV-1, and 630,000 people died from AIDS-related disease across the globe in 2022.2 Therefore, HIV-1 infection is a major worldwide public health problem. Due to the damaged immune system in HIV-positive people, opportunistic infections occur more often and severely in HIV-positive people.3 In a cohort inpatients study, opportunistic infections were associated with mortality and morbidity for HIV-positive people.4 Early diagnosis of the etiological pathogens will improve appropriate treatment and decrease mortality rates.5 However, it is a great challenge clinically to identify the potential pathogens using conventional methods due to its broad spectrum.6 The gold standard microbiological identification method is pathogen culture, but it is time-consuming and needs special culture media. Serological tests identify pathogens by checking the presence of pathogen-specific antibodies and require prediction of the possible types of pathogens. PCR methods show low specificity and might amplify normal flora. Meanwhile, certain pathogens are difficult to detect by conventional methods.7 Empirical antibiotic treatment is often started when HIV-positive patients present with fever, cough, and other signs of infection, even though it has been shown it was not necessary.8 Inappropriate antibiotic treatment can lead to antibiotic resistance, disruption of gut microbiota, and prolonged hospital stays. Therefore, rapid and accurate identification of causative pathogens in HIV-positive people is imperative for guiding and optimizing antibiotic therapy strategies.
Clinical metagenomic next-generation sequencing (mNGS) recently has been successfully used to identify causative pathogens from patients’ specimens, such as pulmonary infections,9–11 bone infections,12,13 gastrointestinal tract infections,14,15 and CNS infections.16,17 This new high-throughput strategy characterizes the genome of pathogens by next-generation sequencing in a target-independent manner and provides rapid and accurate etiological diagnosis.18 Accumulating data have shown that mNGS plays an important role in identification of mixed infections and novel pathogens, providing evidence for proper antibiotic treatment strategies.19,20 The limitation of application of mNGS in HIV-positive people is the high cost.21 In this study, we evaluate the utility of mNGS for identification of potential pathogens in HIV-positive people by comparison with conventional microbiological tests.
Materials and Methods
Patient Population
This study was conducted on 216 hIV-infected patients who were hospitalized at the Third Affiliated Hospital, Nantong University due to suspected opportunistic infections from 2021 to 2023. Patients were enrolled according to the following criteria: age between 21 and 65 years old, HIV infection was confirmed by antibody testing, and opportunistic infections were suspected by symptoms or imaging studies. Ethical approval (No. EK2023129) was given by the Ethics Committee of the Third Affiliated Hospital, Nantong University. General clinical information such as age, sex, CBC, and laboratory results were obtained by reviewing patients’ charts. Clinical specimens were collected, and mNGS and conventional microbiological tests were performed, respectively.
Specimen Collections
Depending on the site of the suspected opportunistic infection, different specimens were collected from HIV-infected patients, including: whole blood (peripheral blood vessel), bronchoalveolar lavage fluid (BALF, bronchoscopy), sputum (deep cough), soft tissue (biopsy), cerebrospinal fluid (CSF, lumbar puncture), pus (soft tissue puncture), lymph node tissue (biopsy), pleural fluid (thoracic puncture), and bone marrow (bone marrow puncture).
Conventional Microbiological Testing (CMT)
Cultures of fungi, bacteria, and mycobacteria were performed on whole blood, bone marrow, BALF, CSF, and pleural fluid. Enzyme-linked immunosorbent assay (ELISA) was used to detect antibodies for Chlamydophila pneumoniae, Toxoplasma gondii, Mycoplasma pneumoniae, Syphilis, and human herpesvirus. Gold immunochromatographic assay was done to detect Cryptococcus antigen in serum and CSF. Real-time PCR was used for human gamma-herpesvirus and cytomegalovirus. The fungal cell wall component β-1,3-D-glucan (BDG) in serum was detected by ELISA kit. Aspergillus galactomannan antigen in BALF and serum was detected by double-sandwich ELISA assay. Xpert MTB/RIF (Xpert) and T-SPOT.TB assays were performed to detect Mycobacterium tuberculosis. The level of C-reactive protein and procalcitonin, and the number of CD4+ T lymphocyte cells and white blood cells are also evaluated.
Metagenomic Next-Generation Sequencing
Each specimen was mixed with glass beads and vortexed at 3000 rpm for 30 min. TIANamp Micro DNA kit (Tiangen Biotech, China) was used to extract DNA according to the manufacturer’s manual. DNA libraries were made by digesting the extracted DNA into fragments, followed by end-repair, A-tailing reactions, ligation of adapters, and PCR amplification. The concentration of the DNA library was measured using Qubit dsDNA HS assay Kit (ThermoFisher, USA) according to the manufacturer’s protocol. Agarose gel electrophoresis was performed to estimate the molecular size. The pooled libraries were sequenced on NextSeq 550 system (Illumina, USA) using a single-end sequencing kit (Illumina, USA). The quality control of the DNA library was measured by the Agilent 2100 Bioanalyzer system. The low-quality reads were removed by FastP.22 Bioinformatics data processing of FASTQ reads was performed as previously described.23,24 The sequencing files were aligned to microbial genome databases (https://www.ncbi.nlm.nih.gov/genomes). The reference database includes 6350 bacterial genomes, 4945 viral genome sequences, 1064 pathogenic fungi genomes, and 234 parasite genome sequences.
Antimicrobial Treatment
The patients’ antimicrobial treatment regimen was made at admission and adjustment according to imaging studies, clinical presentation, patients’ responses to the treatment, mNGS results, and CMT results.
Statistical Analysis
SPSS software was used for statistical analyses in this study. Chi-square test or Student’s t-test was performed for categorical variables or quantitative variables, respectively. Statistical significance was considered when p value is less than 0.05.
The sensitivity and specificity were calculated to compare mNGS and CMTs in detection of different pathogens.
Results
Distribution of Sample Types
A total of 275 specimens were collected from 216 hIV-infected patients (Table 1 and Figure 1) from different sites, including 75 samples of whole blood, 41 samples of BALF, 25 samples of sputum, 27 samples of soft tissue, 22 samples of CSF, 15 samples of pus, 19 samples of lymph node biopsies, 31 samples of pleural fluid, and 20 samples of bone marrow. The various sites of specimens provide a broader understanding of the application of mNGS and CMTs in detecting pathogens in clinical samples.
 |
Table 1 Specimen Types From HIV-Infected Patients |
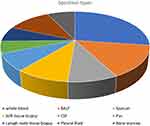 |
Figure 1 Specimen types from HIV-infected patients: various types of specimens from different sites were collected. |
Patient Characteristics
The clinical characteristics and lab results of the patients are shown in Table 2. The average age of the patients was 35 years old, and 165 were males (76.4%) and 51 (23.6%) females. A total of 125 patients (57.9%) were treated with antiretroviral therapy (ART). The most common clinical symptom was fever (95/216, 44.0%), followed by chest tightness (62/216, 28.7%) and cough/shortness of breath (56/216, 25.9%). Abnormal WBC counts were seen in 51 patients (23.6%), 38 patients (17.6%) had abnormal neutrophils ratio, 62 patients (28.7%) presented with abnormal C reactive protein, and 68 patients (31.2%) showed abnormal procalcitonin. A total of 153 patients (70.8%) had CD4+ T cell counts less than 50 cells/µL, 51 patients (23.6%) had CD4+ T cell counts between 50 and 100 cells/µL, and 12 patients (5.6%) had CD4+ T cell counts more than 100 cells/µL. Computer tomography (CT) abnormalities were found in 18 patients (8.3%), abnormal MRIs were seen in 22 patients (10.2%), and 113 patients (52.3%) showed abnormal chest X-rays. The majority of patients (93.1%) improved after treatment. The clinical characteristics of the patients confirmed the HIV status and clinical symptoms.
 |
Table 2 Patient Characteristics |
Distribution of Pathogens Detected by mNGS and CMTs
A total of 195 patients (90.2%) were positive for microbial pathogens by mNGS, 135 patients (62.5%) were positive for microbial pathogens by CMTs, and 67 patients (31.0%) showed positive results by both mNGS and CMTs. A total of 128 patients (59.3%) were positive only by mNGS, and no patients were positive only by CMTs. Negative results by both mNGS and CMTs were seen in 21 patients (9.7%) (Figure 2). Table 3 shows that mNGS detected more viruses compared with CMTs (p<0.05). Pneumocystis jiroveci, Streptococcus pneumoniae, and CMV were the most common pathogens identified by mNGS, followed by Mycobacterium intracellulare, Haemophilus, Pseudomonas aeruginosa, Actinomyces, Clostridium perfringens, human herpesviruses, JC virus, and Torque teno virus (TTV). mNGS showed significantly higher sensitivity and specificity than those in CMTs. Specimen types are also important in identification of pathogens. Our study showed that more pathogens were detected in whole blood (p=0.0197), followed by BALF (p=0.0115), CSF (p=0.0146), and pleural fluid (p=0.0109) (Figure 3A-C).
 |
Table 3 Microbial Pathogens Detected by CMTs and mNGS |
 |
Figure 2 The positivity of microbial pathogens detected by mNGS, compared with CMTs. |
Figure 4 shows that 98 patients (45.3%) were identified with mixed infection by mNGS and 60 patients (27.8%) were detected with mixed infection by CMTs. Fungi and virus mixed infection was the most frequent pattern detected by mNGS (32, 14.8%) and CMTs (22, 10.2%). Bacteria and virus mixed infection was found in 19 patients (8.8%) by mNGS and in 11 patients (5.1%) by CMTs. Fungi and bacteria mixed infection was detected in 30 patients (13.9%) by mNGS and in 17 patients (7.9%) by CMTs. Fungi, bacteria, and virus mixed infection was detected in 17 patients (7.9%) by mNGS and in 2 patients (0.9%) by CMTs. Toxoplasma gondii infection was detected in 6 patients by mNGS. The average turnaround time of CMTs was about 112 hours, and the average turnaround time of mNGS was about 32 hours (Figure 5).
 |
Figure 4 The mixed microbial pathogen infection detected by mNGS and CMTs. |
 |
Figure 5 The average turnaround time of mNGS and CMTs. |
CD4+ T Cell Count and Pathogen Detected by mNGS
In patients with CD4+ T cell count less than 50 cells/µL, mixed infection was detected in 72 patients, while 15 patients showed single pathogen. When CD4+ T cell count was between 50 and 100 cells/µL, 25 patients exhibited mixed infection, 21 patients showed single pathogen infection, and 1 patient was detected negative. When CD4+ T cell count was more than 100 cells/µL but less than 200 cells/µL, 31 patients showed mixed infection, 19 patients exhibited single pathogen infection, and 3 patients were detected negative. The CD4+ T cell count in patients with mixed pathogens was significantly lower than that in patients infected with a single pathogen (Figure 6).
 |
Figure 6 The relationship of CD4+ T cell count and microbial pathogen infection by mNGS. |
Treatment Adjustment Impacted by mNGS and CMTs
Empiric antibiotic treatment was started when patients presented with symptoms suspicious for microbial infection. A total of 199 patients (92.1%) received broad-spectrum antibiotic treatment before mNGS; however, only 89 patients (41.2%) continued broad-spectrum antibiotic treatment after mNGS. Pathogens were quickly identified by mNGS in 151 patients (69.9%), and the initial treatments were adjusted accordingly. As shown in Figure 7, in 47 patients antibacterial agents were adjusted based on mNGS results, 39 patients had their antifungal agents changed, and 35 patients had antiviral agents added. CMV was detected by mNGS in 25 patients, and acyclovir was given and symptoms improved quickly. Meanwhile, CMV was identified only in 8 patients by CMTs. Pyrimethamine sulfadiazine was given to 6 patients when mNGS helped identify Toxoplasma gondii. Pneumocystis jirovecii pneumonia is the most common opportunistic infection in HIV patients, and prophylactic treatment was started when patients presented with respiratory symptoms. Pneumocystis jiroveci was confirmed by mNGS in 52 patients, and trimethoprim/sulfamethoxazole (TMP/SMX) dosage was increased according to mNGS results. The proper treatment strategies were initiated by mNGS results.
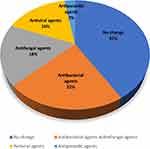 |
Figure 7 Treatment adjustment based on mNGS results. |
Discussion
mNGS helps quickly and accurately identify infectious pathogens in HIV patients. Our study showed significant benefits of using mNGS in identification of infectious pathogens in HIV-infected patients. mNGS demonstrated higher sensitivity compared with CMTs. All infectious pathogens identified by CMTs were also detected by mNGS.
Hui et al reported that the turnaround time of mNGS was significantly lower than that of CMTs in management of patients with bronchiectasis.25 Our study showed that mNGS significantly reduced turnaround time of pathogen identification. The average turnaround time of mNGS was about 32 hours, compared with 112 hours for CMTs. The quick identification of pathogens allows the proper treatment strategies to be initiated. Meanwhile, varieties of infectious pathogens were detected in different types of specimens. Pathogens were more often identified in whole blood, BALF, and CSF by mNGS. It is difficult to identify viruses by CMTs; however, our study showed that mNGS detected more viruses in a short turnaround time, compared with CMTs. Unnecessary antibacterial antibiotics were stopped based on mNGS results. Meanwhile, mNGS was able to uncover new infectious pathogens. In our study, mNGS detected Torque teno virus and Toxoplasma gondii, which were not identified by CMTs.
mNGS demonstrates distinctive advantages in identification of mixed and complex infections. Sun et al19 reported that mNGS showed distinct benefits in detecting mixed infections in patients with severe pneumonia. In our study, mNGS identified 45.3% of patients with mixed infection, while CMTs only identified 27.8% of patients with mixed infection. mNGS identified 14.8% of patients with fungi and virus mixed infection, whereas CMTs identified 10.2% of patients with fungi and virus mixed infection, which was the most frequent mixed infection pattern. Mixed infection is difficult to treat, and it is critical to identify the mixed pathologens as quickly as possible so that the correct treatment can be initiated.
By comparing with gene libraries, mNGS detected all pathogens rapidly and accurately and ensured appropriate application of antimicrobial agents. Hao et al26 showed that more patients with suspected lower respiratory tract infections had theirantibiotics treatment modified based on mNGS results and the majority of patients had a good response.
Zhang et al25 reported that about 50% of patients with suspected infections had their antibiotic treatment adjusted according to mNGS results. The modifications included escalation, discontinuation, and alteration in antibiotic regimen. In our study, 92.1% of patients received empiric broad-spectrum antibiotic treatment based on suspicious symptoms before lab testing. However, only 41.2% of patients continued broad-spectrum antibiotic treatment because mNGS results allowed initiation of proper treatment strategies. A total of 21.8% of patients had adjustment of antibacterial agents based on mNGS results, 18.1% patients had changes to antifungal agents, and in 16.2% patients antiviral agents were added. In particular, acyclovir was started in 11.6% of patients when CMV was detected by mNGS. Pyrimethamine sulfadiazine was started in 2.8% of patients when mNGS identified Toxoplasma gondii, and trimethoprim/sulfamethoxazole (TMP/SMX) dosage was increased when Pneumocystis jiroveci was confirmed by mNGS.
mNGS is not considered a routine clinical test due to high cost and high equipment requirements. The current agreement in China is that mNGS should be used for some target patient populations, such as immunodeficient patients, including HIV patients, cancer patients, and patients with chronic infections.
It is critical to rule out infection in HIV patients so that empirical antibiotic treatment can be stopped and unnecessary antibiotics treatment avoided. Studies27,28 have indicated when mNGS results suggested no pathogens were detected, the negative prediction accuracy rate is significant. Therefore, mNGS has a higher negative predictive value than CMTs. Our study showed that no infectious pathogens were detected in 21 patients by mNGSs, confirmed by CMTs. Antibiotic treatments were stopped, and these patients recovered after supportive care. Some limitations such as costs, quantity of pathogen DNA, sequencing depth, reference database might limit the integration of mNGS into large-scale clinical practice. For example, mNGS is a very sensitive test and might provide false-positive results by identification of microbial contamination from reagents. Therefore, proper negative controls are required in each test to avoid false-positive results. Meanwhile, advanced bioinformatic tools are required to analyze and interpret the large amount of data generated by mNGS. In daily clinical practice, the proper combination of CMTs and mNGS in target patient populations could dramatically improve diagnostic accuracy in life-threatening infections.
In summary, our study suggested that mNGS is a valuable tool and significantly enhanced pathogen identification in HIV patients, with a short turnaround time, and facilitated personalized antimicrobial treatment strategies.
Conclusion
mNGS is a valuable tool and enhances rapid microbiological identification in HIV-infected patients. Combined with CMTs, mNGS may facilitate personalized antimicrobial treatment strategies and increase survival.
Data Sharing Statement
The original data can be requested; requests should be directed to the corresponding authors.
Ethics Statement
Ethical approval (No. EK2023129) was given by the Ethics Committee of the Third Affiliated Hospital, Nantong University. And the written consents were obtained from all enrolled patients.
Author Contributions
XZ, LC, and XW collected patients’ clinical information; XZ, JW, XL, and ZH analyzed mNGS and CMTs data; LB analyzed the treatment strategies; and CW and MZ designed the project and drafted and revised the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by Jiangsu Province Government Scholarship for Medical Health (YSA2021000795). The funder had no role in project design, data collection and analysis, and preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boasso A, Shearer GM, Chougnet C. Immune dysregulation in human immunodeficiency virus infection: know it, fix it, prevent it? J Intern Med. 2009;265(1):78–96. doi:10.1111/j.1365-2796.2008.02043.x
2. Swinkels HM, Justiz Vaillant AA, Nguyen AD, Gulick PG. HIV and AIDS. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
3. Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34(8):1098–1107. doi:10.1086/339548
4. Ruiz GO, Herrera CFL, Bohórquez JAM, Betancur JE. Mortality in patients with acquired human immunodeficiency virus infection hospitalized in an intensive care unit during the period 2017-2019. Sci Rep. 2022;12(1):15644. doi:10.1038/s41598-022-19904-z
5. Xiao J, Du S, Tian Y, Su W, Yang D, Zhao H. Causes of death among patients infected with HIV at a tertiary care hospital in china: an observational cohort study. AIDS Res Hum Retroviruses. 2016;32(8):782–790. doi:10.1089/aid.2015.0271
6. Batool M, Galloway-Peña J. Clinical metagenomics-challenges and future prospects. Front Microbiol. 2023;14:1186424. doi:10.3389/fmicb.2023.1186424
7. Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. 2015;1(1):15035. doi:10.1038/nrdp.2015.35
8. Ohnuma T, Chihara S, Costin B, et al. Association of appropriate empirical antimicrobial therapy with in-hospital mortality in patients with bloodstream infections in the US. JAMA Network Open. 2023;6(1):e2249353. doi:10.1001/jamanetworkopen.2022.49353
9. Wu D, Wang W, Xun Q, et al. Metagenomic next-generation sequencing indicates more precise pathogens in patients with pulmonary infection: a retrospective study. Front Cell Infect Microbiol. 2022;12:977591. doi:10.3389/fcimb.2022.977591
10. Gao Q, Li L, Su T, et al. A single-center, retrospective study of hospitalized patients with lower respiratory tract infections: clinical assessment of metagenomic next-generation sequencing and identification of risk factors in patients. Respir Res. 2024;25(1):250. doi:10.1186/s12931-024-02887-y
11. Xu H, Hu X, Wang W, et al. Clinical application and evaluation of metagenomic next-generation sequencing in pulmonary infection with pleural effusion. Infect Drug Resist. 2022;15:2813–2824. doi:10.2147/IDR.S365757
12. Ramchandar N, Burns J, Coufal NG, et al. Use of metagenomic next-generation sequencing to identify pathogens in pediatric osteoarticular infections. Open Forum Infect Dis. 2021;8(7):ofab346. doi:10.1093/ofid/ofab346
13. Wang G, Long J, Zhuang Y, et al. Application of metagenomic next-generation sequencing in the detection of pathogens in spinal infections. Spine J. 2023;23(6):859–867. doi:10.1016/j.spinee.2023.02.001
14. Zhou Y, Wylie KM, El Feghaly RE, et al. Metagenomic approach for identification of the pathogens associated with diarrhea in stool specimens. J Clin Microbiol. 2016;54(2):368–375. doi:10.1128/JCM.01965-15
15. Kujiraoka M, Kuroda M, Asai K, et al. Comprehensive diagnosis of bacterial infection associated with acute cholecystitis using metagenomic approach. Front Microbiol. 2017;8:685. doi:10.3389/fmicb.2017.00685
16. Qu C, Chen Y, Ouyang Y, et al. Metagenomics next-generation sequencing for the diagnosis of central nervous system infection: a systematic review and meta-analysis. Front Neurol. 2022;13:989280. doi:10.3389/fneur.2022.989280
17. Liu Y, Zhu W, Jiao M, Guo W, Luo Y. Clinical application value of metagenomic next-generation sequencing in the diagnosis of central nervous system infections. Front Bioeng Biotechnol. 2023;11:885877. doi:10.3389/fbioe.2023.885877
18. Zhu Y, Xu M, Ding C, et al. Metagenomic next-generation sequencing vs. traditional microbiological tests for diagnosing varicella-zoster virus central nervous system infection. Front Public Health. 2021;9:738412. doi:10.3389/fpubh.2021.738412
19. Sun C, Zhou C, Wang L, et al. Clinical application of metagenomic next-generation sequencing for the diagnosis of suspected infection in adults: a cross-sectional study. Medicine. 2024;103(16):e37845. doi:10.1097/MD.0000000000037845
20. He Y, Geng S, Mei Q, et al. Diagnostic value and clinical application of metagenomic next-generation sequencing for infections in critically III patients. Infect Drug Resist. 2023;16:6309–6322. doi:10.2147/IDR.S424802
21. Olthoff M, Kobayashi T, Parsons MG, et al. Impact of metagenomic next-generation sequencing on clinical decision-making at an academic medical center, a retrospective study, Iowa, 2020-2022. Antimicrob Steward Healthc Epidemiol. 2024;4(1):e39. doi:10.1017/ash.2024.31
22. Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i90. doi:10.1093/bioinformatics/bty560
23. Yuan L, Zhu XY, Lai LM, Chen Q, Liu Y, Zhao R. Clinical application and evaluation of metagenomic next-generation sequencing in pathogen detection for suspected central nervous system infections. Sci Rep. 2024;14(1):25984. doi:10.1038/s41598-024-76648-8
24. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi:10.1038/nmeth.1923
25. Zhang H, Shen D, Zhou J, et al. The utility of metagenomic next-generation sequencing (mNGS) in the management of patients with bronchiectasis: a single-center retrospective study of 93 cases. Open Forum Infect Dis. 2023;10(8):ofad425.
26. Hao J, Li W, Wang Y, Zhao J, Chen Y. Clinical utility of metagenomic next-generation sequencing in pathogen detection for lower respiratory tract infections and impact on clinical outcomes in southernmost China. Front Cell Infect Microbiol. 2023;13:1271952. doi:10.3389/fcimb.2023.1271952
27. Parize P, Muth E, Richaud C, et al. Untargeted next-generation sequencing-based first-line diagnosis of infection in immunocompromised adults: a multicentre, blinded, prospective study. Clin Microbiol Infect. 2017;23(8):
28. Qian M, Zhu B, Zhan Y, et al. Analysis of negative results of metagenomics next-generation sequencing in clinical practice. Front Cell Infect Microbiol. 2022;12:892076. doi:10.3389/fcimb.2022.892076
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


