Back to Journals » Journal of Hepatocellular Carcinoma » Volume 12
In Situ Vaccination with Poly-ICLC Combined with Systemic Nivolumab for the Treatment of Unresectable Hepatocellular Carcinoma
Authors Liang JD, Liang PC, Shun CT , Chen CH , Wu YM, Hsu YC, Lee YT, Yang PM, Huang GT , Salazar AM, Lee HS, Sheu JC, Weng MT
Received 4 February 2025
Accepted for publication 12 June 2025
Published 14 June 2025 Volume 2025:12 Pages 1191—1204
DOI https://doi.org/10.2147/JHC.S520710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Jörg Trojan
Ja-Der Liang,1 Po-Chin Liang,2,3 Chia-Tung Shun,4 Chien-Hung Chen,1,5 Yao-Ming Wu,6,7 Yu-Chen Hsu,4,8 Ying-Te Lee,4 Pei-Ming Yang,1,4 Guan-Tarn Huang,1,9 Andres M Salazar,10 Hsuan-Shu Lee,1,4 Jin-Chuan Sheu,1,4 Meng-Tzu Weng1,11
1Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan; 2Department of Medical Imaging and Radiology, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan; 3Department of Medical Imaging, National Taiwan University Hospital, Hsin-Chu Branch, Hsin-Chu, Taiwan; 4Liver Disease Prevention and Treatment Research Foundation, Taipei, Taiwan; 5Department of Internal Medicine, National Taiwan University Cancer Center, Taipei, Taiwan; 6Department of Surgery, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan; 7Department of Surgical Oncology, National Taiwan University Cancer Center, Taipei, Taiwan; 8Department of Clinical Laboratory Science and Medical Biotechnology, College of Medicine, National Taiwan University, Taipei, Taiwan; 9Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan; 10Oncovir, Washington, DC, USA; 11Department of Medical Research, National Taiwan University Hospital, Hsin-Chu Branch, Hsin-Chu, Taiwan
Correspondence: Meng-Tzu Weng, Department of Internal Medicine, National Taiwan University Hospital, No. 7 Chung-Shan South Road, Taipei, Taiwan, Tel +886-2-23123456 ext 67266, Fax +886-2-23947927, Email [email protected]
Purpose: Unresectable hepatocellular carcinoma (HCC) presents significant therapeutic challenges. While immune checkpoint inhibitors (ICIs) are part of the current standard of care, combining poly-ICLC as an in situ vaccination with an ICI may enhance treatment efficacy. The study investigated the safety and therapeutic effects of combining poly-ICLC with nivolumab, an ICI, in patients with unresectable HCC.
Patients and Methods: Patients with unresectable HCC were enrolled to receive intratumoral and intramuscular poly-ICLC injections along in combination with nivolumab infusions. The primary endpoint was safety, and secondary endpoints included objective response as measured by mRECIST and changes in serum alpha-fetoprotein (AFP) levels. Gene expression profiling, pathway analysis, and immune cell type deconvolution were conducted using NanoString GeoMx Digital Spatial Profiling.
Results: Four patients were enrolled. The combination therapy was safe and well-tolerated. Among them, one patient achieved a complete response (CR), and another achieved a partial response (PR). Both responders showed significant declines in serum AFP levels. Notably, the patient with CR showed eradication of cancerous component of the portal vein thrombus, and an abscopal effect was observed in the patient with PR. Gene analysis indicated that interferon-gamma signaling was the most enriched pathway in tumors of the responders.
Conclusion: This combination therapy was safe and effective, with two out of four patients demonstrating objective responses. These preliminary findings warrant further investigation into larger clinical cohorts.
Keywords: Hepatocellular carcinoma, Poly-ICLC, In situ vaccination, immune checkpoint inhibitors, Portal vein thrombus, Digital spatial profiling
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide.1 Early-stage HCC can be effectively treated with surgical resection, radiofrequency ablation, or liver transplantation.2 However, despite the implementation of screening programs for high-risk populations, a significant proportion of patients are still diagnosed at an advanced stage.3 For these patients, systemic therapy is essential. The first major breakthrough in systemic therapy came in 2007 with the introduction of molecular targeted therapies, led by sorafenib. In 2011, the emergence of immune checkpoint inhibitors (ICIs) marked another important advance in HCC treatment. Nivolumab, an anti-PD1 antibody, was approved in 2017 as a second-line monotherapy for advanced HCC.4 Subsequent studies demonstrated that ICI-based combination therapies often outperform ICI monotherapy.5 Currently, the combination of atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) is considered the most effective first-line treatment for unresectable HCC, showing an objective response rate (ORR) of 30% and a median overall survival of 19.2 months—surpassing the results achieved with sorafenib.6 Nevertheless, there remains a need to further improve therapeutic efficacy, and numerous trials are ongoing to explore novel ICI-based combinations.4
Therapeutic cancer vaccines have emerged as promising candidates for combination with ICIs, given their complementary mechanisms of action in the cancer–immunity cycle. Cancer vaccines function by increasing the anti-tumor T cell repertoire: they promote antigen release from tumor cells and facilitate antigen presentation to the immune system. In contrast, ICIs restore T cell activity by blocking immune checkpoints that suppress T cell function.7 This mechanistic complementarity suggests a potential for synergistic therapeutic effects when the two are combined.8 Cancer vaccines can target a range of antigens, including tumor-specific antigens—such as viral antigens and neoantigens arising from non-synonymous somatic mutations—and tumor-associated antigens, which include tissue- or development-specific antigens.9 Despite decades of research, vaccines targeting tumor-associated antigens have yielded limited clinical success.10 More recently, interest has shifted toward personalized neoantigen vaccines.9 Although T cell responses have been elicited in clinical trials of neoantigen-based vaccines, these have not always translated into meaningful clinical benefit.11,12 However, promising developments have emerged. A recent clinical trial using an mRNA-based individualized neoantigen vaccine in patients with resected melanoma demonstrated that combining the vaccine with pembrolizumab (anti-PD1) significantly prolonged recurrence-free survival and reduced recurrence rates compared to pembrolizumab monotherapy.13 Another single-arm trial in HCC evaluated DNA plasmid-encoded neoantigens combined with plasmid-encoded interleukin-12 and pembrolizumab, reporting an ORR of 30.6%, with 8.3% of patients achieving a complete response (CR).14 These findings support further clinical investigation into vaccine-ICI combinations.
Nevertheless, neoantigen vaccine strategies face challenges, particularly due to tumor genetic heterogeneity, which is a key factor in resistance to cancer therapies.15,16 Neoantigens derived from one tumor region may not be expressed in others, limiting the effectiveness of such vaccines. Additionally, personalized neoantigen vaccine development is time-consuming and costly, requiring high-throughput sequencing, complex bioinformatics, antigenicity prediction, and customized peptide or mRNA synthesis. An alternative and potentially more practical strategy is in situ vaccination (ISV). ISV leverages therapy-induced immunogenic cell death (ICD) to trigger the release of tumor antigens, followed by activation of local antigen-presenting cells (APCs), which then stimulate a tumor-specific immune response.9,17 ISV-generated vaccines are both personalized and off-the-shelf, providing logistical advantages.18 Moreover, ISV enables repeated administration across multiple tumor sites or distinct tumors, potentially overcoming challenges posed by tumor heterogeneity.
One promising candidate for ISV is polyinosinic–polycytidylic acid–poly-L-lysine carboxymethylcellulose (poly-ICLC). This synthetic, virus-mimicking double-stranded RNA activates both Toll-like receptor (TLR) 3 and melanoma differentiation-associated gene 5, triggering robust immune responses.19 In our previous studies, in vitro incubation of poly-ICLC induced ICD in HCC cells, and intratumoral (IT) injection of poly-ICLC activated endogenous dendritic cells (DCs), the most potent APCs. Sequential IT followed by intramuscular (IM) poly-ICLC administration significantly suppressed HCC growth and enhanced infiltration of CD8⁺ T cells in two syngeneic murine HCC models.20 Furthermore, we demonstrated that combining either IT or IM administration—particularly IT—of poly-ICLC with systemic anti-PD1 therapy synergistically inhibited tumor growth in a mouse HCC model.21
A clinical trial has investigated the use of IT and IM poly-ICLC injections in combination with other therapeutic modalities, such as irradiation and local regional treatment, to treat patients with unresectable HCC in the pre-ICI era. Some patients demonstrated tumor responses and prolonged survival.22 Building on our previous animal study demonstrating the synergistic inhibitory effect of poly-ICLC combined with anti-PD1 on HCC growth,21 we conducted this clinical trial to evaluate the combination of poly-ICLC and nivolumab in patients with unresectable HCC.
Materials and Methods
Study Design
This open-label, single-arm clinical trial was conducted at National Taiwan University Hospital, Taipei, Taiwan. The study aimed to evaluate both safety and tumor response, as assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST).23 The trial investigated the combination of IT and IM poly-ICLC injections (Oncovir, Inc., Washington, DC, USA) with nivolumab (Bristol-Myers Squibb, Princeton, NJ) in patients with unresectable HCC.
In patients with multinodular HCC, a single tumor larger than 2 cm and accessible for ultrasound-guided IT injection was designated as the target lesion. A needle liver biopsy of the target tumor was performed within 28 days prior to initiating therapy. Patients received weekly IT injections of poly-ICLC (1.0 mg) for three weeks, followed by biweekly IM booster injections (20 µg/kg) for four weeks. This was succeeded by four additional cycles, each consisting of a three-week rest period and two biweekly IM injections. The IT and IM dosing regimens were based on previous studies.22 Nivolumab was administered intravenously at a dose of 3 mg/kg over 90 minutes every two weeks, beginning in week 2, for a total treatment duration of six months (see Figure 1). Following treatment completion, patients were monitored for up to two years or until withdrawal, death, or other study exit criteria were met. CT scans of the brain, chest, abdomen, and pelvis were conducted at baseline, as well as at 12 and 24 weeks post-treatment initiation. A radiologist (P.C.L). measured the CT attenuation values (in Hounsfield units, HU) of portal vein thrombosis (PVT) regions, calculating the contrast enhancement by comparing pre- and post-contrast images. All research procedures adhered to the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of National Taiwan University Hospital (approval number: 202104104MIND) and registered at ClinicalTrials.gov (identifier: NCT05281926).
 |
Figure 1 Schematic representations of treatment protocol. |
Patient Eligibility
Participants were eligible for enrollment if they met the following inclusion criteria: (1) histologically confirmed diagnosis of HCC, (2) age 20 years or older, (3) unresectable disease assessed by the surgeon Y.M.W., (4) radiologically measurable disease with a target tumor at least 2 cm in the longest dimension, (5) ECOG performance status of ≤ 2, (6) Child-Pugh classification A, (7) for patients with chronic hepatitis B (HBV) infection, long-term antiviral agents with a high barrier to resistance, (8) for patients with chronic hepatitis C (HCV) infection, achievement of sustained viral response with any direct-acting agent, (9) acceptable hematologic, renal, and liver function within 28 days before trial entry (absolute neutrophil count ≥ 1500/mm³, platelets ≥ 80,000/mm³, hemoglobin > 10.0 g/dL, creatinine ≤ 2.0 mg/dL, total bilirubin ≤ 1.5 mg/dL unless due to Gilbert’s syndrome, AST and ALT ≤ 2 times the upper limits, INR < 1.5, and (10) ability to provide informed consent.
Patients were excluded if they met any of the following conditions: (1) concurrent use of other investigational agents, biological agents, or anti-cancer medications, (2) uncontrolled intercurrent illnesses, including ongoing or active infection requiring antibiotics, symptomatic congestive heart failure, unstable angina pectoris, or psychiatric/social situations that would limit compliance with study requirements, (3) pregnancy or nursing, (4) diagnosis of primary immunodeficiency diagnosis or use of systemic steroids or other immunosuppressive therapy within 7 days prior to the first dose of trial treatment, (5) active autoimmune disease requiring systemic treatment in the past year, (6) HIV-positive patients with detectable viral load, those not on a stable HAART regimen, or with <200 CD4+ T cells/microliter in peripheral blood, (7) history of allogeneic hematopoietic cell transplantation or solid organ transplantation, and (8) documented allergy or hypersensitivity to any protein therapeutics.
Assessments of Tumor Response and Toxicity
Tumor response was evaluated via CT imaging using mRECIST criteria at screening, week 12, and at the end of treatment. Serum alpha-fetoprotein (AFP) levels were monitored every two months. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Dose-limiting toxicities (DLTs) related to poly-ICLC, specifically those classified as Grade 3 or 4, were closely monitored. If a DLT occurred, treatment was paused until the toxicity resolved to Grade 1 or baseline. Upon resolution, a reduced poly-ICLC dose (50%) was administered. If the DLT did not resolve, the patient was withdrawn from the study.
Slide Preparation and Staining
Formalin-fixed, paraffin-embedded biopsy tissues were serially sectioned at 5 µm thickness for 5 sections and mounted onto slides. The slides were then baked in a histology oven for 1 hour. After deparaffinization and rehydration in ethanol gradient, the first slide underwent hematoxylin and eosin (H&E) staining to serve as the reference slide. The diagnosis and assessment of HCC differentiation according to the Edmondson-Steiner grading system were conducted by pathologist C.T.S. The other slides underwent double immunofluorescence staining using primary fluorophore-conjugated antibodies targeting cytokeratin (CK) 8 (Novus Biologicals, NBP2-34655AF488, k8.8+DC10,1:100 dilution to yield green color), CD45 (Novus Biologicals, NBP2-34528AF594,2B11+PD7/26,1:100 dilution to yield red color) plus Syto 83 nuclei dye. One of the slides with best quality was selected for digital spatial profiling (DSP).
Selection and Segmentation of Regions of Interest for DSP
In the GeoMx DSP instrument, the selected slides of each sample were imaged at 20X magnification and 3 regions of interest (ROIs) of CK8+ tumor nests and 3 ROIs of CD45+ tumor microenvironment (TME) in the vicinity of the tumor nests were drawn with random shapes. In the special case of infiltrative-type HCC (Patient 1), the tumor nests (labeled as “T”) were interwoven with normal parenchyma (labeled as “NP”). Both regions were CK8+ and thus difficult to distinguish on the immunofluorescence-stained slide (Figure 2d). To avoid selecting CK8+ normal parenchyma, the CK8+ tumor nests (T1 and T2) were identified with the guidance of the H&E-stained reference slide (Figure 2a). Enlarged images of the T1 and T2 tumor nests in the H&E-stained slide are shown in Figures 2b and c, respectively. In these images, the tumor regions are clearly distinguishable from the normal parenchyma based on nuclear atypia, including enlarged nuclei, hyperchromasia, a higher nuclear-to-cytoplasmic ratio, and pleomorphism. The GeoMa software was then used to segment the tumor ROIs to red color CK8+ and neighboring green color CD45+ regions (Figure 2e and f). The ROIs were then subjective to UV illumination at 385 nm. The programmable digital micromirror device embedded in the Geo Mx DSP machine allows for selective illumination. UV cleavable oligonucleotides were released and collected for subsequent library preparation.
Library Preparation and Sequencing
Sequencing libraries were prepared via polymerase chain reaction using photo-released indexing oligonucleotides and area of illumination (AOI)-specific illumination adapter sequences. The resulting libraries were sequenced on an Illumina NovaSeq 6000 platform.
Analysis Description
Initial quality control checks were performed on the raw count data. Genes with expression levels comparable to the geometric mean of negative control probes in more than 95% of AOIs were filtered out. Following this step, 12,322 genes were retained from the original 18,676. The remaining data were normalized using the third quartile (Q3) normalization method. Differential expression analysis was conducted using an unpaired t-test with p-values adjusted by the Benjamini-Hochberg false discovery rate (FDR) method. Normalized gene expression data were compared based on AOI annotations. Pathway enrichment analysis was performed using the fGSEA package integrated into the NanoString DSP Analysis Suite. Pathways with an adjusted p-value ≤ 0.05 were considered significant and ranked by their Normalized Enrichment Score (NES). Cell type deconvolution was carried out using the Spatial Decon package, leveraging the Immune Tumor Safe TME signature profile.
Results
Patient Enrollment
Enrollment of eligible patients began in September 2022. At that time, Taiwan’s National Health Insurance (NHI) did not provide coverage of ICIs for patients with advanced HCC, making the provision of nivolumab in this trial a significant incentive for enrollment. However, in August 2023, Taiwan’s NHI began reimbursing the combination of atezolizumab and bevacizumab for these patients. Consequently, the trial was prematurely terminated due to the loss of this enrollment incentive and ethical concerns, as there remained no rationale to suggest the investigational regimen offered greater benefit than the now-available standard treatment.
Patient Characteristics
Only four patients were enrolled before the trial was terminated, with a mean age of 75.8 years (range 70–80), including three men and one woman. Two patients had HBV-related HCC and two had HCV-related HCC. Three patients had liver cirrhosis, all classified as Child–Pugh A. Pre-treatment albumin levels were normal in all patients, indicating preserved liver reserve. Based on the Barcelona Clinic Liver Cancer (BCLC) staging system, two patients were stage B and two were stage C. Three had a history of HCC treated with curative resection but had not received therapy for their recurrent disease prior to enrollment. The current tumors were infiltrative in one patient (Patient 1) and multinodular in the other three. Tumor differentiation, graded by the Edmondson-Steiner system, with three patients classified as grade 3 and one as grade 4. In the multinodular cases, the number of nodules ranged from 3 to over 10, with maximum diameters between 2 and 4.7 cm. Although CT scans revealed no extrahepatic spread, portal vein thrombosis (PVT) was present in two patients (Patient 1 and 4). Notably, these two patients with PVT also had serum AFP levels exceeding 400 ng/mL, suggesting that elevated AFP may correlate with more aggressive tumor characteristics in advanced HCC (Table 1).
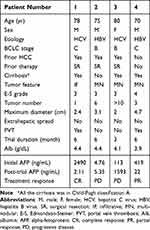 |
Table 1 Patient Characteristics, Tumor Features, and Outcomes |
Patient Outcomes Revealed Varied Responses
Three patients (Patients 1, 2, and 4) completed the full treatment course, while one patient (Patient 3) withdrew early due to rapid disease progression. At trial entry, Patient 3 had more than ten lesions and showed aggressive disease progression, prompting discontinuation of the study treatment after three months. She subsequently received one session of transarterial chemoembolization (TACE) and lenvatinib, but her condition continued to worsen, culminating in the development of ascites, hepatic encephalopathy, liver failure, and ultimately, death. Patient 2, who initially presented with six tumors, did not exhibit a treatment response (Table 1).
Of the three patients who completed the trial, two (Patients 1 and 4) demonstrated favorable responses. Both had PVT. Patient 1 had infiltrative HCC with bilateral PVT; following treatment, the infiltrative lesion resolved completely (Figure 3a). Patient 4 initially presented with three tumors and right-sided PVT. By the end of the study, the target lesion showed significant shrinkage and a marked reduction in contrast enhancement on CT imaging (yellow arrows, Figure 3b). Notably, one of the non-injected tumors also exhibited a decrease in both size and enhancement (red arrows, Figure 3b), while another increased in size and retained its enhancement (white arrows, Figure 3b). Although PVT persisted in both responders, their serum AFP levels declined markedly (Table 1; Figure 3a and b).
In the regions of PVT, the pre-treatment increase in CT attenuation from pre-contrast to post-contrast scans was 47.5 hU for Patient 1 and 49.8 hU for Patient 4. By the end of treatment, these values had dropped to 4.8 hU and 25.6 hU, respectively (Figure 4). Based on established criteria defining portal vein tumor thrombosis (PVTT) as a ≥20 hU increase,24,25 Patient 1’s PVT was classified as PVTT at baseline but was reclassified as bland post-treatment. In contrast, Patient 4’s PVT met the criteria for PVTT both before and after treatment; however, the reduced enhancement (from 49.8 to 25.6 hU) suggests a response of the PVTT to therapy.
In summary, Patient 1 achieved a CR, with the disappearance of infiltrative HCC and resolution of PVTT. Patient 4 achieved a partial response (PR), with more than a 30% reduction in total intrahepatic tumor diameter and decreased enhancement of residual PVTT, which remained neoplastic (Table 1).
No Severe Toxicities Were Reported After Treatment
AEs were generally mild. Patients 1, 2 and 4 experienced grade 1–2 fevers after IT injections of poly-ICLC, all of which resolved uneventfully with antipyretic treatment. No fever was reported after IM injections. Other treatment-related AEs—including skin rash, anorexia, abdominal pain, arthralgia, constipation, and elevated serum transaminases—were all grade 2 or lower in severity (Table 2).
 |
Table 2 Adverse Events |
Responders Exhibited Enriched Interferon Signaling and CD8 Memory T Cell–Rich Tumor Microenvironment
Pathway enrichment analysis of tumor cells revealed significant upregulation of both interferon-gamma (IFN-γ) and interferon-alpha/beta signaling in treatment responders (Figure 5a). Supporting this, a heatmap analysis showed elevated expression of interferon gamma-inducible protein 30 (IFI30) in the responder group (Figure 5b). Immune cell deconvolution further highlighted a predominance of CD8 memory T cells in the tumor microenvironment of responders, whereas non-responders showed greater infiltration by CD4 memory T cells and macrophages (Figure 5c).
Discussion
Despite the number of enrolled patients was low, this study demonstrated that the combination therapy of poly-ICLC and anti-PD1 was well tolerated and safe, with only low-grade AEs. Reported AEs included fever and skin rash (Patients 1, 2 and 4), anorexia (Patients 1, 3 and 4), abdominal pain (Patient 2), arthralgia (Patient 1), and constipation and elevated serum transaminases (Patient 4). All AEs were grade 2 or lower in severity (Table 2). Two patients showed promising responses to the therapy—one CR (Patient 1) and one PR (Patient 4) (Table 1)—highlighting the potential efficacy of this combination approach in treating HCC. All participants were over 70 years of age (Table 1), an age group commonly associated with immunosenescence, which can reduce the efficacy and safety of immunotherapies.26 However, such age-related limitations were not observed in this study. Whether younger patients might derive even greater benefit from this regimen remains an open question.
Lurje et al previously advocated for the use of ISV as a therapeutic strategy against HCC.17 ISV promotes systemic anti-tumor immunity by inducing ICD in tumor cells, which recruits DCs to the tumor site, facilitates antigen uptake, and activates DCs to present tumor neoantigens, thereby priming tumor-specific CD8⁺ T cells in the draining lymph nodes (dLNs).27 Modalities such as radiotherapy, chemotherapy, TACE, and targeted therapies have been explored for ISV,16 as they can induce ICD and remodel the TME, but their ability to recruit and activate DCs is often limited.17 Immunological adjuvants, particularly TLR ligands like poly(I:C), LPS, CpG, R848, and Pam3CSK4, have demonstrated greater potential in this regard.28 Our previous murine studies confirmed that poly-ICLC induced ICD on HCC cell lines and activated DCs in both the tumors20 and dLNs.21 Sequential IT followed by IM administration of poly-ICLC inhibited tumor growth and induced an abscopal effect.20 Additionally, combining poly-ICLC (via either IT or IM injection) with anti-PD1 therapy led to greater anti-tumor efficacy compared to anti-PD1 monotherapy, supporting the role of poly-ICLC as an immunostimulatory enhancer of checkpoint blockade.21 Although poly-ICLC had previously been evaluated in a Phase 1 clinical trial as an ISV agent before the widespread use of ICIs,22 its combination with anti-PD1 has not yet been clinically evaluated in HCC patients. This study represents a novel clinical approach by combining poly-ICLC with anti-PD1 for the treatment of unresectable HCC.
A hallmark of ISV is the abscopal effect, in which local radiotherapy induces antitumor responses at distant tumor sites. This phenomenon is thought to be mediated by circulating cytotoxic T lymphocytes (CTLs) and cytokines such as type I interferons, IL-6, IL-1α, tumor necrosis factor-α, reactive oxygen species, and nitric oxide.29 In this study, it is intriguing that Patient 4 exhibited shrinkage of a non-injected tumor, while another tumor remained unaffected following treatment. This differential response is unlikely to be due to the bystander effect, given the physical distance between the tumors. A more plausible explanation is intertumoral heterogeneity, which may have allowed the non-responsive tumor to evade recognition or destruction by tumor-specific CTLs.
Both intertumoral and intratumoral heterogeneity16,30,31 are common in HCC and pose significant challenges for treatment strategies, including targeted therapies, ICIs, and neoantigen-based vaccines.32,33 As an intratumoral agent, poly-ICLC offers the flexibility to be administered to multiple tumor sites during the course of treatment, potentially addressing intertumoral heterogeneity. Furthermore, its ability to reach various regions within a single large HCC lesion may also help overcome intratumoral heterogeneity.
Both Patient 1 and Patient 4 presented with PVT and underlying cirrhosis. In cirrhotic livers, bland PVT is not uncommon and may result from disrupted hemostasis and damage to the portal vein due to portal hypertension and bacterial translocation.34 In the context of HCC, however, PVT may represent PVTT, which is associated with poor prognosis.35,36 Differentiating bland PVT from PVTT is critical in cirrhotic patients with HCC. A post-contrast increase in CT attenuation of ≥20 hU is indicative of PVTT.24,25 At baseline, both patients were diagnosed with PVTT, but following treatment, Patient 1’s PVT appeared to transition to a bland thrombus, while Patient 4’s remained consistent with PVTT. This suggests that Patient 1’s PVT may have initially contained both bland and tumorous components, with the tumor component resolving post-treatment. This interpretation is supported by a substantial decline in serum AFP levels—from 2,490 ng/mL to 2.11 ng/mL—and the disappearance of intrahepatic lesions, meeting the criteria for a CR (Table 1). Notably, ICI treatment has been associated with a 52.9% objective response rate in PVTT.37
In this study, both responders had baseline AFP levels ≥400 ng/mL, whereas the two non-responders had levels ≤400 ng/mL (Table 1). In the IMbrave150 study, patients with baseline AFP ≤400 ng/mL had better overall and progression-free survival following treatment with atezolizumab plus bevacizumab.38 In contrast, the HIMALAYA39 and CheckMate 459 studies40 trials found that patients with AFP ≥400 ng/mL had improved survival following treatment with tremelimumab plus durvalumab and nivolumab, respectively. The results from our study are consistent with the latter findings. Albumin levels have been suggested as a prognostic biomarker in advanced cancer patients receiving ICIs.41 However, since all patients in our study had normal pre-treatment albumin levels, its utility in predicting treatment response could not be assessed.
As demonstrated by Patient 1, the spatial transcriptome technique is a powerful tool for investigating gene expression profiles in HCC, particularly in needle biopsy samples from infiltrative HCCs that display intertwined normal parenchyma and cancerous tissue. This approach enables precise isolation of tumor RNAs (Figure 2). Transcriptome analysis revealed that IFN-γ signaling was the most enriched pathway in the responders (Figure 5a). This finding aligns with a recent report highlighting the upregulation of IFN-γ signaling and major histocompatibility complex (MHC) II-related antigen presentation as a molecular marker of response to anti-PD1 therapy in advanced HCC.42 Notably, IFI30, one of the differentially expressed genes in responders (Figure 5b), is inducible by IFN-γ and plays a role in both MHC I-restricted antigen cross-presentation and MHC II-restricted antigen processing by decreasing the disulfide bonds of endocytosed proteins.43 Most tumor cells broadly express MHC-1, presenting tumor antigens that enable recognition and elimination by tumor-specific CD8+ T cells.44 However, a subset of tumors also expresses MHC-II, a molecule typically found on APCs. Recent studies have shown that MHC-II expression in tumor cells is linked to favorable outcomes and enhanced responses to immunotherapies.45 Intriguingly, in this study, CD8 memory cells in TME were more abundant in responders, whereas CD4 memory cells were more in non-responders (Figure 5c). The presence of resident CD8 memory (CD8 TRM) cells in tumor infiltration is correlated with better prognosis and improved response to current immunotherapies.46 In contrast, CD4 TRM cells are less well studied and characterized.47 This study did not differentiate between helper and regulatory T cells, raising a question of whether the HCC tissues from non-responders contained a higher proportion of regulatory CD4 TRM cells. Along with CD4 memory cells, macrophages were more abundant in the TME of non-responders (Figure 5c). Macrophages are a double-edged sword in the TME, depending on their M1 and M2 phenotypes. Recently, they have emerged as a key target for developing new cancer immunotherapies.48,49
This study has several limitations. First, the small sample size constrains the statistical power and generalizability of the findings. Second, the absence of a control arm precludes definitive attribution of observed effects to the investigational therapy. Third, all enrolled patients were over 70 years old, and those with decompensated liver cirrhosis were excluded. This selective inclusion criterion may impact the treatment’s efficacy, as older patients may respond differently to therapy, and excluding patients with more severe liver dysfunction limits the applicability of the results to a broader HCC population. Due to its premature termination, this study may pivot to exploring the combination of poly-ICLC with atezolizumab and bevacizumab in the future. To enhance the applicability, future studies should include a larger cohort encompassing a wider range of age groups and disease stages.
Conclusions
Although limited by the small sample size, our preliminary findings suggest that the combination therapy of poly-ICLC and nivolumab may be a potentially safe and effective option for patients with unresectable HCC. Further studies involving larger patient cohorts and exploring additional combinations, such as atezolizumab plus bevacizumab, are warranted to confirm and expand upon these observations.
Abbreviations
AE, Adverse events; CR, Complete response; dLNs, Draining lymph nodes; HCC, Hepatocellular carcinoma; ICIs, Immune checkpoint inhibitors; IM, Intramuscular; ISV, In situ vaccination; IT, Intratumoral; Mrecist, Modified Response Evaluation Criteria in Solid Tumors; ORR, Objective response rate; PD, Progressive disease; poly-ICLC, Polyinosinic–polycytidylic acid–poly-L-lysine carboxymethylcellulose; PR, Partial response; PVT, Portal vein thrombosis; TLR, Toll-like receptor.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the Research Ethics Committee of the National Taiwan University Hospital (approval number: 202104104MIND) and performed in accordance with the ethical standards as laid down in the Helsinki Declaration of 1975, as revised in 2008. All participants signed informed consent for the study. This study was registered at ClinicalTrials.gov (identifier: NCT05281926).
Acknowledgment
English writing was edited by ChatGPT.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Liver Disease Prevention and Treatment Research Foundation, Taipei, Taiwan.
Disclosure
Andres M. Salazar is the Chief Strategy Officer of Oncovir, Inc. In addition, Dr Andres M. Salazar has patents 7439349, 7834064, 8592391, EP 1778186 issued to Oncovir; patents 18/445640 & 18/445641 pending to Oncovir. The other authors have no conflicts of interest to declare for this work.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Villanueva A, Longo DL. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
3. Su TH, Wu CH, Liu TH, Ho CM, Liu CJ. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Taiwan perspective. Clin Mol Hepatol. 2023;29(2):230–241. doi:10.3350/cmh.2022.0421
4. Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20(4):203–222. doi:10.1038/s41575-022-00704-9
5. Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72(2):307–319. doi:10.1016/j.jhep.2019.09.025
6. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
7. Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity. 2023;56(10):2188–2205. doi:10.1016/j.immuni.2023.09.011
8. Oladejo M, Paulishak W, Wood L. Synergistic potential of immune checkpoint inhibitors and therapeutic cancer vaccines. Semin Cancer Biol. 2023;88:81–95. doi:10.1016/j.semcancer.2022.12.003
9. Lin MJ, Svensson-Arvelund J, Lubitz GS, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3(8):911–926. doi:10.1038/s43018-022-00418-6
10. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi:10.1038/nm1100
11. Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 547:217–221. doi:10.1038/nature22991
12. Cafri G, Gartner JJ, Zaks T, et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest. 2020;130(11):5976–5988. doi:10.1172/JCI134915
13. Weber JS, Carlino MS, Khattak A, et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet. 2024;403(10427):632–644. doi:10.1016/S0140-6736(23)02268-7
14. Yarchoan M, Gane EJ, Marron TU, et al. Personalized neoantigen vaccine and pembrolizumab in advanced hepatocellular carcinoma: a phase 1/2 trial. Nat Med. 2024;30(4):1044–1053. doi:10.1038/s41591-024-02894-y
15. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. doi:10.1038/nrclinonc.2017.166
16. Losic B, Craig AJ, Villacorta-Martin C, et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat Commun. 2020;11(1):291. doi:10.1038/s41467-019-14050-z
17. Lurje I, Werner W, Mohr R, Roderburg C, Tacke F, Hammerich L. In situ vaccination as a strategy to modulate the immune microenvironment of hepatocellular carcinoma. Front Immunol. 2021;12:650486. doi:10.3389/fimmu.2021.650486
18. Hammerich L, Binder A, Brody JD. In situ vaccination: cancer immunotherapy both personalized and off-the-shelf. Mol Oncol. 2015;9(10):1966–1981. doi:10.1016/j.molonc.2015.10.016
19. Sultan H, Salazar AM, Celis E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin Immunol. 2020;49:101414. doi:10.1016/j.smim.2020.101414
20. Weng MT, Yang SF, Liu SY, et al. In situ vaccination followed by intramuscular poly-ICLC injections for the treatment of hepatocellular carcinoma in mouse models. Pharmacol Res. 2023;188:106646. doi:10.1016/j.phrs.2023.106646
21. Liu SY, Hsu CL, Yang SF, Lee HS, Sheu JC, Weng MT. Intratumoral administration of poly-ICLC enhances the antitumor effects of anti-PD-1. J Hepatobiliary Pancreat Sci. 2025;32(2):139–150. doi:10.1002/jhbp.12088
22. de la Torre AN, Contractor S, Castaneda I, et al. A Phase I trial using local regional treatment, nonlethal irradiation, intratumoral and systemic polyinosinic-polycytidylic acid polylysine carboxymethylcellulose to treat liver cancer: in search of the abscopal effect. J Hepatocell Carcinoma. 2017;4:111–121. doi:10.2147/JHC.S136652
23. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
24. Canellas R, Mehrkhani F, Patino M, Kambadakone A, Sahani D. Characterization of portal vein thrombosis (Neoplastic Versus Bland) on CT Images using software-based texture analysis and thrombus density (Hounsfield Units). AJR Am J Roentgenol. 2016;207(5):W81–W87. doi:10.2214/AJR.15.15928
25. Bae JS, Lee JM, Yoon JH, et al. How to best detect portal vein tumor thrombosis in patients with hepatocellular carcinoma meeting the Milan criteria: gadoxetic acid-enhanced MRI versus contrast-enhanced CT. Liver Cancer. 2020;9(3):293–307. doi:10.1159/000505191
26. Lyu N, Yi JZ, Zhao M. Immunotherapy in older patients with hepatocellular carcinoma. Eur J Cancer. 2022;162:76–98. doi:10.1016/j.ejca.2021.11.024
27. Gong N, Alameh MG, El-Mayta R, Xue L, Weissman D, Mitchell MJ. Enhancing in situ cancer vaccines using delivery technologies. Nat Rev Drug Discov. 2024;23(8):607–625. doi:10.1038/s41573-024-00974-9
28. Ho NI, Huis T, Veld LGM, Raaijmakers TK, Adema GJ. Adjuvants enhancing cross-presentation by dendritic cells: the key to more effective vaccines? Front Immunol. 2018;9:2874. doi:10.3389/fimmu.2018.02874
29. Goto T. Radiation as an In situ auto-vaccination: current perspectives and challenges. Vaccines. 2019;7(3). doi:10.3390/vaccines7030100
30. Friemel J, Rechsteiner M, Frick L, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21(8):1951–1961. doi:10.1158/1078-0432.CCR-14-0122
31. Xue R, Li R, Guo H, et al. Variable intra-tumor genomic heterogeneity of multiple lesions in patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):998–1008. doi:10.1053/j.gastro.2015.12.033
32. Lu LC, Ch H, Cheng AL, Cheng A-L. Tumor heterogeneity in hepatocellular carcinoma: facing the challenges. Liver Cancer. 2016;5(2):128–138. doi:10.1159/000367754
33. Kalasekar SM, VanSant-Webb CH, Evason KJ. Intratumor heterogeneity in hepatocellular carcinoma: challenges and opportunities. Cancers. 2021;13(21). doi:10.3390/cancers13215524
34. Senzolo M, Garcia-Tsao G, Garcia-Pagan JC. Current knowledge and management of portal vein thrombosis in cirrhosis. J Hepatol. 2021;75(2):442–453. doi:10.1016/j.jhep.2021.04.029
35. Gavriilidis P, Pawlik TM, Azoulay D. Comprehensive review of hepatocellular carcinoma with portal vein tumor thrombus: state of art and future perspectives. Hepatobiliary Pancreat Dis Int. 2024;23(3):221–227. doi:10.1016/j.hbpd.2023.10.009
36. Sun J, Guo R, Bi X, et al. Guidelines for diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus in China (2021 Edition). Liver Cancer. 2022;11(4):315–328. doi:10.1159/000523997
37. Tsai HM, Han MZ, Lin YJ, et al. Real-world outcome of immune checkpoint inhibitors for advanced hepatocellular carcinoma with macrovascular tumor thrombosis. Cancer Immunol Immunother. 2021;70(7):1929–1937. doi:10.1007/s00262-020-02845-9
38. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
39. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus DURVALUMAB IN UNRESECTABLE HEPATOCELLULAR CARcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. doi:10.1056/EVIDoa2100070
40. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, Phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
41. Guven DC, Sahin TK, Erul E, et al. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Mol Biosci. 2022;9:1039121. doi:10.3389/fmolb.2022.1039121
42. Haber PK, Castet F, Torres-Martin M, et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. Gastroenterology. 2023;164(1):72–88e18. doi:10.1053/j.gastro.2022.09.005
43. Zhang S, Ren L, Li W, et al. Interferon gamma inducible protein 30: from biological functions to potential therapeutic target in cancers. Cell Oncol. 2024;47(5):1593–1605. doi:10.1007/s13402-024-00979-x
44. Lee MY, Jeon JW, Sievers C, Allen CT. Antigen processing and presentation in cancer immunotherapy. J Immunother Cancer. 2020;8(2):e001111. doi:10.1136/jitc-2020-001111
45. Axelrod ML, Cook RS, Johnson DB, Balko JM. Biological consequences of MHC-II expression by tumor cells in cancer. Clin Cancer Res. 2019;25(8):2392–2402. doi:10.1158/1078-0432.CCR-18-3200
46. Okla K, Farber DL, Zou W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J Exp Med. 2021;218(4). doi:10.1084/jem.20201605
47. Notarbartolo S, Abrignani S. Human T lymphocytes at tumor sites. Semin Immunopathol. 2022;44(6):883–901. doi:10.1007/s00281-022-00970-4
48. Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6(1):127. doi:10.1038/s41392-021-00506-6
49. Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21(11):799–820. doi:10.1038/s41573-022-00520-5
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





