Back to Journals » Cancer Management and Research » Volume 17
Modulation of Chronic Cytokine Dysregulation in Cervical Cancer: Potential Biomarkers and Therapeutic Targets
Authors Obeagu EI
Received 12 March 2025
Accepted for publication 11 June 2025
Published 13 June 2025 Volume 2025:17 Pages 1113—1126
DOI https://doi.org/10.2147/CMAR.S527913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Seema Singh
Emmanuel Ifeanyi Obeagu1,2
1Department of Biomedical and Laboratory Science, Africa University, Mutare, Zimbabwe; 2Department of Medical Laboratory Science, Kampala International University, Kampala, Uganda
Correspondence: Emmanuel Ifeanyi Obeagu, Department of Biomedical and Laboratory Science, Africa University, Mutare, Zimbabwe, Email [email protected]
Abstract: Cervical cancer progression is not solely driven by persistent human papillomavirus (HPV) infection but is profoundly influenced by the local immune microenvironment, particularly chronic cytokine imbalances. Unlike the acute cytokine storms observed in infections or sepsis, cervical cancer is characterized by a persistent, low-grade, “smoldering inflammatory response” that fuels tumor initiation, progression, and immune evasion. Pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α, and IL-8 sustain a tumor-supportive milieu, promoting angiogenesis, epithelial-mesenchymal transition, and resistance to apoptosis, while immunosuppressive cytokines like IL-10 and TGF-β dampen anti-tumor immune responses and facilitate immune escape. This review explores chronic cytokine dysregulation in cervical cancer, examining how the prolonged, dysregulated cytokine network shapes the tumor microenvironment, remodels stromal interactions, and influences immune cell recruitment and function. We highlight key cytokines involved in these processes and discuss their clinical significance as potential diagnostic, prognostic, and predictive biomarkers. Understanding these sustained inflammatory processes is critical because they represent a distinct biological landscape compared to acute inflammatory reactions and offer unique windows for therapeutic intervention. The paper reviewed emerging therapeutic strategies targeting these chronic cytokine pathways, including cytokine blockade, immune modulation, and combination approaches integrating immunotherapies or nanomedicine. Addressing chronic cytokine dysregulation holds promise for improving cervical cancer management and patient outcomes.
Keywords: cytokine storm, cervical cancer, inflammation, therapeutic targets, biomarkers
Introduction
Cervical cancer is the fourth most common cancer among women worldwide, with an estimated 604,000 new cases and 342,000 deaths annually, particularly in low-resource settings.1 Persistent infection with high-risk human papillomavirus (HPV) is the primary etiological factor for cervical cancer, initiating a cascade of molecular events that lead to cellular transformation.2 Chronic inflammation triggered by HPV infection plays a central role in the progression from normal cervical epithelium to precancerous lesions and, ultimately, invasive cervical cancer.3,4 While the immune system typically attempts to control and eliminate HPV infection, prolonged exposure to the virus often results in immune dysregulation, fostering an environment conducive to tumorigenesis. One of the key manifestations of this immune dysregulation is the cytokine storm, an excessive and uncontrolled release of pro-inflammatory cytokines that contributes significantly to the progression of cervical cancer.5,6 Cytokines are signaling proteins produced by various immune and non-immune cells that regulate the immune response and mediate cell signaling. In the context of cervical cancer, cytokines such as IL-6, IL-1β, TNF-α, and IL-8 play essential roles in driving inflammation and immune responses. When released in excessive amounts, these cytokines can promote tumor cell proliferation, survival, and invasion. The tumor microenvironment (TME) in cervical cancer is often characterized by a sustained inflammatory response, which is not only driven by HPV but also by the immune cells infiltrating the tumor. Cytokine storm in this context fosters an environment that enhances tumor progression by inducing angiogenesis, facilitating epithelial-to-mesenchymal transition (EMT), and promoting immune evasion mechanisms. This persistent inflammatory response creates a vicious cycle that accelerates the development and spread of the cancer.7–10
Cervical cancer arises primarily due to persistent infection with high-risk human papillomavirus (HPV) types, notably HPV-16 and HPV-18. While the viral oncogenes E6 and E7 disrupt p53 and Rb tumor suppressor pathways, the local immune environment, shaped by chronic inflammation, critically modulates disease progression. Cytokines — small signaling proteins orchestrating immune responses — become persistently dysregulated in the cervical microenvironment, tipping the balance towards tumor-promoting inflammation, immune escape, and angiogenesis.1 It is crucial to distinguish the chronic inflammatory milieu of cancer from the acute cytokine storms seen in severe infections or sepsis. Acute cytokine storms are rapid, systemic, and overwhelming immune responses marked by surges of IL-6, TNF-α, and other mediators, often leading to multiorgan failure and death. In contrast, cervical cancer is characterized by a persistent, low-level, “smoldering” inflammatory response, where chronic, localized cytokine imbalances subtly reprogram the tumor microenvironment over time. This chronic state drives long-term processes such as angiogenesis, immune evasion, and metastasis, shaping a fundamentally different biological and therapeutic landscape than acute inflammation.11–13
Recent research has identified cytokine storm as a central factor in the development and metastasis of cervical cancer. Cytokine storm is often associated with adverse outcomes in various cancers, including cervical cancer. High levels of cytokines like IL-6 and TNF-α have been linked to poor prognosis, tumor invasiveness, and resistance to therapy. Moreover, the dysregulated cytokine production in the TME can suppress anti-tumor immunity, leading to immune escape. Tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs) are recruited to the tumor site by cytokines, further enhancing immune suppression and tumor survival. This results in a microenvironment that supports not only the growth of cervical cancer cells but also their ability to metastasize to distant organs.11–13 Cytokine storm in cervical cancer is also intricately linked with the epithelial-to-mesenchymal transition (EMT), a process that allows tumor cells to acquire migratory and invasive properties. This transition is strongly influenced by inflammatory cytokines, particularly IL-6 and IL-8, which activate the Janus kinase/signal transducer and activator of transcription (JAK/STAT) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways. These pathways induce the expression of various genes that promote cell mobility and invasion, allowing cancer cells to escape the primary tumor site and establish secondary metastases. As a result, cytokine storm not only accelerates local tumor growth but also enhances the ability of cervical cancer cells to spread to other organs, further complicating treatment efforts.14–17 The recognition of cytokine storm as a significant player in cervical cancer pathogenesis has opened new avenues for therapeutic intervention. Targeting specific cytokines or their signaling pathways represents a promising strategy to disrupt the inflammatory cascade that sustains tumor growth and metastasis. Several cytokine inhibitors are already in clinical use or under investigation for their potential in treating cancer. For example, monoclonal antibodies such as tocilizumab, which targets IL-6, and infliximab, which targets TNF-α, have shown promise in reducing inflammation and improving outcomes in various cancers. Additionally, small molecule inhibitors targeting the JAK/STAT and NF-κB pathways are being explored for their ability to modulate cytokine production and inhibit tumor growth.18
Despite significant progress in understanding cytokine dysregulation in cervical cancer, several critical questions remain unanswered. Identifying the optimal biomarkers for early detection of chronic cytokine imbalances is still a major challenge, as current markers lack sensitivity and specificity for the subtle, smoldering inflammatory state characteristic of tumor progression. Additionally, personalizing cytokine-targeting therapies to accommodate the diverse cytokine profiles and immune landscapes across patient subsets remains an unmet need, requiring more refined diagnostic tools and stratification strategies. Furthermore, the dynamic interplay between HPV integration and host cytokine signaling over time is poorly understood—specifically how viral oncogenes modulate cytokine networks to promote immune evasion and tumor progression. Addressing these gaps through longitudinal studies, advanced molecular profiling, and integrated multi-omics approaches will be essential for translating cytokine biology into effective, personalized interventions in cervical cancer care.
Aim
The aim of this review is to explore the mechanisms underlying cytokine storm in cervical cancer, its impact on tumor progression and metastasis, and to identify potential biomarkers and therapeutic targets for modulating the inflammatory response.
Review Methods
The review on cytokine storm modulation in cervical cancer involved a comprehensive, systematic approach to gathering and analyzing relevant scientific literature. The methodology adopted for this review was designed to ensure that a wide range of sources were evaluated, including studies on the mechanisms of cytokine storms, their role in tumor progression, immune modulation, and therapeutic strategies. The review was conducted by reviewing primary research articles, clinical trials, meta-analyses, and review articles published in reputable journals. The overall aim was to provide an in-depth understanding of the cytokine storm’s impact on cervical cancer and its potential as a therapeutic target.
Literature Search Strategy
The literature search was conducted using electronic databases such as PubMed, Google Scholar, and Scopus. Relevant keywords and search terms such as “cytokine storm”, “cervical cancer”, “tumor progression”, “immune dysregulation”, “biomarkers”, “therapeutic targets”, and “immune checkpoint inhibition” were used to retrieve studies published between 2000 and 2024. Studies were selected based on their relevance to the topic, focusing on cervical cancer and the inflammatory cytokine network. Articles that explored cytokine storm mechanisms in other cancers or immune diseases were also included if they provided valuable insights applicable to cervical cancer.
Inclusion and Exclusion Criteria
The inclusion criteria for the review consisted of peer-reviewed articles that:
- Investigated the role of cytokine storm in cervical cancer, including mechanisms of inflammation, immune dysregulation, and tumor progression.
- Explored therapeutic strategies targeting cytokine storm, including immunotherapies and other cytokine-modulating treatments.
- Provided data on the effects of cytokines and immune cells in the cervical cancer microenvironment, as well as their correlation with clinical outcomes.
- Included original research, clinical trials, and meta-analyses.
Exclusion criteria involved:
- Studies unrelated to cervical cancer or cytokine modulation in cancer.
- Articles published in non-peer-reviewed journals.
- Studies that did not provide original data or lacked clinical relevance.
Mechanisms of Cytokine Storm in Cervical Cancer
Cytokine storm is a pathological phenomenon characterized by an overwhelming and uncontrolled release of pro-inflammatory cytokines into the bloodstream, resulting in widespread tissue inflammation and immune dysregulation.19 In the context of cervical cancer, cytokine storm plays a central role in tumor progression, metastasis, and immune evasion. Persistent infection with high-risk human papillomavirus (HPV), particularly types 16 and 18, is the primary cause of cervical cancer, and the resulting chronic inflammation is a driving force behind the inflammatory microenvironment that fuels tumor growth. This inflammatory response is often exacerbated by an imbalance in immune signaling, triggering a cascade of cytokine release that can lead to a cytokine storm.20–22 The cytokines most implicated in this process are interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-8 (IL-8). These cytokines are produced by various immune and non-immune cells, such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and even the tumor cells themselves. In cervical cancer, the HPV-driven chronic inflammation creates an environment where these cytokines are perpetually upregulated, contributing to tumor progression by promoting various pro-tumorigenic processes.23,24 IL-6 is a key driver of cytokine storm in cervical cancer. It is produced by tumor cells, macrophages, and other stromal cells in the tumor microenvironment (TME). IL-6 signals through its receptor, IL-6R, activating the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, leading to the activation of STAT3. The STAT3 pathway is critical in cervical cancer progression, as it promotes cell proliferation, survival, and resistance to apoptosis. IL-6 also contributes to tumor angiogenesis by upregulating vascular endothelial growth factor (VEGF), further supporting tumor growth. Additionally, IL-6 induces the differentiation of MDSCs and Tregs, immune cells that contribute to immune suppression in the TME. This enhances the tumor’s ability to evade immune surveillance, creating a favorable environment for tumor survival.25–29
TNF-α is another pro-inflammatory cytokine that plays a critical role in the development of cytokine storm in cervical cancer. TNF-α is produced by both immune cells and cancer cells within the TME and binds to its receptors (TNFR1 and TNFR2) on target cells. The activation of TNFR1 triggers the nuclear factor-kappa B (NF-κB) pathway, which leads to the transcription of genes that promote inflammation, cell survival, and invasion. NF-κB also induces the production of matrix metalloproteinases (MMPs), which degrade the extracellular matrix and facilitate tumor cell invasion and metastasis. Additionally, TNF-α promotes the recruitment of neutrophils and macrophages to the tumor site, further intensifying the inflammatory response. This persistent inflammatory environment aids tumor progression and immune evasion by suppressing anti-tumor immunity and enhancing tumor cell plasticity.30–35 IL-1β also plays a significant role in cytokine storm in cervical cancer. It is a potent pro-inflammatory cytokine released primarily by activated macrophages in response to danger signals such as HPV infection. IL-1β activates the NF-κB and mitogen-activated protein kinase (MAPK) pathways, which contribute to the inflammatory microenvironment of the TME. Elevated IL-1β levels are associated with increased tumor cell proliferation, invasion, and resistance to apoptosis. Additionally, IL-1β induces the secretion of other pro-inflammatory cytokines and chemokines that further recruit immune cells to the tumor site, perpetuating the inflammatory cycle. This cytokine not only contributes to local tumor growth but also facilitates distant metastasis by altering the behavior of tumor cells and enhancing their invasive potential (Table 1).36–38
 |
Table 1 Summary of Key Cytokines, Their Roles, and Related Clinical Applications in Cervical Cancer |
IL-8, another key player in cytokine storm, is predominantly produced by tumor cells, macrophages, and endothelial cells in the TME. IL-8 is a potent chemokine that recruits neutrophils, monocytes, and other immune cells to the tumor site, contributing to the chronic inflammatory response. IL-8 also promotes angiogenesis by activating the NF-κB and mitogen-activated protein kinase (MAPK) pathways, leading to the upregulation of VEGF. The increased blood supply supports tumor growth and provides a route for metastasis. Furthermore, IL-8 has been shown to enhance the migration and invasion of cervical cancer cells, supporting the epithelial-to-mesenchymal transition (EMT) process, which is a critical step in cancer metastasis.39–41 The combination of these cytokines creates a positive feedback loop that perpetuates the cytokine storm and exacerbates the inflammatory tumor microenvironment. Tumor cells, along with infiltrating immune cells, continuously produce cytokines that not only sustain the inflammatory response but also induce immune suppression. Regulatory T cells (Tregs) and MDSCs, recruited to the TME by cytokines, inhibit the activity of cytotoxic T lymphocytes and natural killer (NK) cells, both of which are essential for anti-tumor immunity. This immune suppression promotes the survival and expansion of cancer cells, further contributing to tumor progression and metastasis.42 Moreover, cytokine storm in cervical cancer contributes to the disruption of the extracellular matrix (ECM), which facilitates the invasion and migration of tumor cells. This is particularly significant in the context of EMT, a process that allows epithelial cells to acquire mesenchymal characteristics, including increased motility and invasiveness. Pro-inflammatory cytokines, such as IL-6, IL-8, and TNF-α, activate signaling pathways that induce EMT, enabling cervical cancer cells to migrate from the primary site and invade surrounding tissues. The cytokine-mediated remodeling of the ECM also creates pathways for tumor cells to enter the bloodstream and lymphatic system, facilitating metastasis to distant organs (Figure 1).43,44
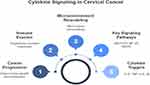 |
Figure 1 Cytokine Signaling in Cervical Cancer. |
Dual Roles and Context-Dependent Functions of Cytokines in Cervical Cancer
Cytokines in cervical cancer often exhibit dual and context-dependent roles that complicate their biological interpretation and therapeutic targeting. Many cytokines traditionally classified as pro-inflammatory or anti-inflammatory can paradoxically promote or inhibit tumor progression depending on the tumor stage, cellular context, and microenvironmental cues. For example, TNF-α, while known for its capacity to induce tumor cell death and stimulate antitumor immunity, can also enhance tumor survival by promoting chronic inflammation, angiogenesis, and metastatic potential in cervical cancer. Similarly, TGF-β plays a complex role; it acts as a tumor suppressor in early stages by inhibiting cell proliferation but switches to a tumor-promoting factor in advanced disease through inducing epithelial-mesenchymal transition (EMT), immune suppression, and stromal remodeling. IL-6, a major driver of chronic inflammation, can promote tumor growth and immune evasion, yet it also supports acute immune activation under different conditions. This functional plasticity underscores the importance of understanding the temporal and spatial dynamics of cytokine signaling within the tumor microenvironment. Such duality presents a significant challenge for therapeutic development, as indiscriminate cytokine blockade might disrupt beneficial immune functions or fail to account for shifting cytokine roles during disease progression. Therefore, cytokine-targeting strategies must be carefully designed with context-specific insights, potentially combining inhibitors with immune checkpoint modulators or personalized approaches based on cytokine profiling. Ultimately, deciphering these nuanced roles is critical for leveraging cytokines as both biomarkers and therapeutic targets in cervical cancer (Figure 2).40–44
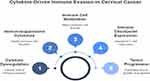 |
Figure 2 Cytokine- Driven Immune Evasion in Cervical Cancer. |
Role of Cytokine Storm in Tumor Progression and Metastasis in Cervical Cancer
The cytokine storm in cervical cancer plays a critical role in tumor progression and metastasis by promoting a chronic inflammatory environment within the tumor microenvironment (TME). In this condition, an excessive release of pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-8 (IL-8), triggers a cascade of molecular events that foster tumor growth, immune evasion, and metastasis. The sustained inflammatory response is a key factor in the progression of cervical cancer, especially in cases driven by persistent infection with high-risk strains of human papillomavirus (HPV), which are the primary cause of this malignancy. Chronic HPV-induced inflammation creates an ideal environment for the activation of pro-tumorigenic signaling pathways that support tumor cell proliferation, survival, and resistance to therapy.39,40 One of the central roles of cytokine storm in cervical cancer is the enhancement of angiogenesis, the process through which new blood vessels are formed to supply the growing tumor. This is primarily driven by IL-6 and IL-8, two potent cytokines involved in the recruitment of endothelial cells to the tumor site. IL-6 is known to activate the JAK/STAT pathway, resulting in the upregulation of angiogenic factors such as vascular endothelial growth factor (VEGF). VEGF induces endothelial cell proliferation and the formation of blood vessels, providing tumors with the oxygen and nutrients necessary for continued growth. Similarly, IL-8, through its action on endothelial cells, stimulates angiogenesis and increases vascular permeability, contributing to the structural changes in the tumor vasculature that promote tumor expansion. These enhanced blood supply pathways not only support the tumor’s metabolic demands but also facilitate the spread of tumor cells to distant organs, further promoting metastasis.41,42
Cytokine storm in cervical cancer also significantly impacts tumor invasion and metastasis through its influence on the extracellular matrix (ECM). The ECM provides structural support to tissues and regulates cell behavior; however, its disruption is a hallmark of metastatic cancer. Pro-inflammatory cytokines such as TNF-α and IL-1β play a crucial role in the degradation of the ECM by upregulating matrix metalloproteinases (MMPs). MMPs are enzymes that break down the components of the ECM, enabling cancer cells to invade surrounding tissues and migrate into the bloodstream or lymphatic vessels. This process, known as epithelial-to-mesenchymal transition (EMT), is essential for the ability of cervical cancer cells to detach from the primary tumor and invade distant tissues. Cytokines like IL-6 and TNF-α activate signaling pathways that induce EMT, leading to enhanced tumor cell motility and invasiveness. As a result, tumor cells become more adept at spreading to distant sites, particularly to the lungs, liver, and lymph nodes.43,44
Moreover, cytokine storm contributes to tumor progression by modulating the immune response within the TME. In the context of cervical cancer, cytokines such as IL-6 and IL-10 promote the recruitment and activation of immune cells that support tumor growth and suppress anti-tumor immunity. Regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) are key immune cells recruited by cytokines that play a pivotal role in immune suppression. Tregs inhibit the function of cytotoxic T cells and natural killer (NK) cells, both of which are essential for the immune system’s ability to target and eliminate tumor cells. MDSCs, on the other hand, suppress the activity of both T cells and NK cells and promote tumor cell proliferation. By expanding the population of these immunosuppressive cells, cytokine storm reduces the effectiveness of the immune system’s surveillance and response to the tumor. This immune evasion is a key factor in cervical cancer progression, allowing the tumor to grow unchecked and resist treatment.37,38 Additionally, the inflammatory environment induced by cytokine storm in cervical cancer supports tumor cell survival and resistance to apoptosis. Pro-inflammatory cytokines activate anti-apoptotic signaling pathways that protect tumor cells from programmed cell death. For example, IL-6 activates the JAK/STAT3 pathway, leading to the upregulation of survival factors such as Bcl-2 and Mcl-1, which inhibit apoptotic processes. IL-6 also increases the expression of anti-apoptotic proteins like survivin, further enhancing the survival of cervical cancer cells. By suppressing apoptosis, cytokine storm ensures the survival of tumor cells, even in the face of therapeutic interventions such as chemotherapy or radiation therapy. This resistance to cell death contributes to the persistence and progression of the disease.36,45 Cytokine storm is also involved in the alteration of immune cell function in cervical cancer. The release of IL-1β, TNF-α, and IL-6 leads to the activation of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), which in turn inhibit the activity of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. These immune cells play crucial roles in identifying and destroying tumor cells. However, when they are suppressed by MDSCs and Tregs, the immune system’s ability to effectively eliminate tumor cells is compromised, further enabling tumor progression and metastasis. The excessive inflammatory environment also hinders the ability of dendritic cells to effectively present antigens to T cells, further dampening the immune response. Consequently, the failure of immune surveillance allows the tumor to persist and spread to other parts of the body (Figure 3).43,44
 |
Figure 3 Cytokine Balance in Cervical Cancer Dictates Tumor Fate. |
Potential Biomarkers of Cytokine Storm in Cervical Cancer
Identifying biomarkers associated with cytokine storm is essential for improving early diagnosis, monitoring disease progression, and predicting therapeutic outcomes in patients with cervical cancer. Various cytokines, signaling pathways, and immune cell populations contribute to the pathophysiology of cervical cancer and serve as potential biomarkers for the cytokine storm phenomenon. One of the most studied cytokines in cervical cancer and cytokine storm is Interleukin-6 (IL-6). This cytokine plays a central role in inflammation, immune regulation, and cancer progression. Elevated levels of IL-6 in the serum of cervical cancer patients have been correlated with poor prognosis, advanced tumor stage, and increased risk of metastasis. IL-6 activates the JAK/STAT3 signaling pathway, which promotes tumor cell survival, angiogenesis, and immune evasion. Moreover, high levels of IL-6 can enhance the expression of other pro-inflammatory cytokines and promote the recruitment of immune cells that contribute to the tumor’s inflammatory microenvironment. As a biomarker, IL-6 levels could potentially help monitor the intensity of the cytokine storm, the response to treatment, and overall patient outcomes. Furthermore, IL-6 could be a therapeutic target, as inhibition of IL-6 signaling has shown promise in preclinical models of cervical cancer.28,29,46 Tumor Necrosis Factor-alpha (TNF-α) is another important cytokine involved in the cytokine storm in cervical cancer. TNF-α is primarily known for its role in promoting inflammation and immune responses, but it also plays a crucial role in the induction of apoptosis, cell proliferation, and the regulation of the immune microenvironment. In the context of cervical cancer, elevated levels of TNF-α have been found to correlate with aggressive tumor behavior, poor response to therapy, and enhanced tumor metastasis. The cytokine contributes to the activation of several key signaling pathways, including NF-kB, which controls the expression of genes involved in inflammation, immune cell recruitment, and tumor survival. High levels of TNF-α in patients may serve as a predictive biomarker for poor prognosis and disease progression, particularly in advanced stages of cervical cancer. Inhibiting TNF-α could provide a promising approach to modulating the cytokine storm and halting tumor progression.47–49
Interleukin-8 (IL-8) is another cytokine whose elevated expression plays a significant role in both the cytokine storm and tumor metastasis in cervical cancer. IL-8 is a potent chemokine that plays an essential role in angiogenesis, inflammation, and immune cell recruitment. It is known to promote the recruitment of neutrophils and endothelial cells to the tumor site, thereby enhancing angiogenesis and facilitating tumor growth. Additionally, IL-8 can trigger the activation of matrix metalloproteinases (MMPs), which degrade the extracellular matrix and enable cancer cells to invade surrounding tissues and migrate to distant organs. The expression of IL-8 in cervical cancer has been linked to lymph node metastasis and poor clinical outcomes. Serum levels of IL-8 may be useful as a diagnostic and prognostic biomarker, helping to monitor disease progression and predict the likelihood of metastasis.39,40,50,51 Another important biomarker in the cytokine storm of cervical cancer is C-reactive protein (CRP). CRP is an acute-phase reactant produced by the liver in response to inflammation. It is a non-specific marker of systemic inflammation, but elevated CRP levels have been strongly associated with the presence of chronic inflammation and tumor progression. In cervical cancer, CRP levels are often elevated and correlate with advanced stages of disease, poor treatment response, and shorter overall survival. High CRP levels in cervical cancer patients may reflect the systemic inflammatory environment created by the cytokine storm and serve as an indicator of the aggressive nature of the tumor. CRP can also be a useful biomarker for monitoring the effectiveness of anti-inflammatory or immunomodulatory therapies aimed at controlling cytokine-induced inflammation in cancer patients.52–57
Soluble Programmed Cell Death-Ligand 1 (sPD-L1) is an emerging biomarker in cervical cancer that is linked to the cytokine storm. PD-L1 is a cell surface protein that inhibits T cell-mediated immune responses by binding to the PD-1 receptor on T cells, thereby inducing immune tolerance and promoting immune evasion. In cervical cancer, high expression of PD-L1 on tumor cells and immune cells within the tumor microenvironment has been associated with poor prognosis and resistance to immune checkpoint inhibitors. Soluble PD-L1 (sPD-L1), which is released into the bloodstream, has been identified as a potential biomarker for assessing immune dysregulation in cervical cancer patients undergoing immune checkpoint blockade therapy. Elevated sPD-L1 levels in the blood can reflect immune evasion mechanisms promoted by the cytokine storm and may predict the response to immunotherapies. Furthermore, measuring sPD-L1 could help identify patients who may benefit from immune checkpoint inhibitors or other therapies targeting immune dysregulation in cervical cancer.58 Finally, Neutrophil-to-Lymphocyte Ratio (NLR) has gained attention as a promising biomarker of inflammation and immune response in cervical cancer. The NLR reflects the balance between neutrophils, which are involved in the inflammatory response, and lymphocytes, which are key players in immune surveillance and tumor defense. An elevated NLR is associated with poor prognosis, more advanced disease, and increased metastatic potential. In the context of the cytokine storm, the NLR may serve as an indicator of the immune system’s dysregulated response to the tumor and the extent of the inflammatory microenvironment. As a relatively simple and inexpensive biomarker, the NLR may be useful for predicting outcomes, monitoring treatment responses, and assessing the efficacy of therapeutic strategies aimed at reducing inflammation and restoring immune balance in cervical cancer patients.59–64
Therapeutic Targets for Modulating Cytokine Storm in Cervical Cancer
Cytokine storms are central to the pathophysiology of cervical cancer, driving inflammation, tumor progression, immune evasion, and metastasis. As such, targeting the cytokine storm presents a promising strategy for improving the prognosis of patients with cervical cancer. Numerous cytokines, immune cells, and signaling pathways play pivotal roles in the inflammatory response within the tumor microenvironment, and therapeutic modulation of these factors has the potential to mitigate the deleterious effects of excessive cytokine release. This section discusses the key therapeutic targets that can be used to modulate cytokine storms in cervical cancer, focusing on cytokines, immune checkpoints, immune cell populations, and signaling pathways.25
Targeting IL-6 and IL-6 Receptor Signaling
One of the most well-established cytokines implicated in the cytokine storm of cervical cancer is Interleukin-6 (IL-6). Elevated IL-6 levels are strongly associated with disease progression, poor prognosis, and increased metastasis in cervical cancer patients. IL-6 contributes to the inflammation within the tumor microenvironment by activating several pro-inflammatory pathways, such as the JAK/STAT3 signaling pathway. This pathway promotes tumor cell survival, angiogenesis, and immune evasion. Inhibiting IL-6 signaling has emerged as a potential therapeutic strategy in several cancers, including cervical cancer. Several approaches can be taken to target IL-6 signaling, including the use of monoclonal antibodies against IL-6 or its receptor (IL-6R). For instance, Tocilizumab, a humanized monoclonal antibody that blocks the IL-6 receptor, has been used in clinical trials to treat diseases with excessive IL-6 activity, such as rheumatoid arthritis. In cervical cancer, targeting IL-6 or IL-6R could potentially reduce inflammation, suppress tumor growth, and improve the response to other therapies. Additionally, small molecules that inhibit the JAK/STAT3 pathway, which is downstream of IL-6 signaling, are also being explored as therapeutic agents. By blocking this key signaling pathway, these inhibitors can disrupt tumor cell proliferation, induce apoptosis, and limit immune evasion mechanisms.27,65
TNF-α Inhibition
Tumor Necrosis Factor-alpha (TNF-α) is another critical cytokine involved in cervical cancer’s inflammatory microenvironment. TNF-α is a potent pro-inflammatory cytokine that plays a role in cell survival, immune response regulation, and tumor progression. It also activates the NF-kB pathway, which promotes the expression of genes involved in inflammation, cell proliferation, and survival. In cervical cancer, elevated TNF-α levels correlate with advanced tumor stages, lymph node metastasis, and poor prognosis. Targeting TNF-α has become an attractive therapeutic strategy, and several agents have been developed to block TNF-α activity. Etanercept and Infliximab are examples of biologic agents that neutralize TNF-α and are primarily used in autoimmune diseases. These agents have been evaluated in clinical trials for cancer treatment, and while their use has shown promise in reducing inflammation and suppressing tumor progression, their direct effects in cervical cancer remain under investigation. Moreover, inhibiting the TNF-α/NF-kB signaling axis could restore immune surveillance and enhance the effectiveness of immune therapies, making TNF-α a potential therapeutic target for modulating cytokine storms in cervical cancer.35,66
Modulation of Immune Checkpoints: PD-1/PD-L1 Blockade
The immune checkpoint pathway involving Programmed Cell Death-1 (PD-1) and its ligand PD-L1 has been a major target in cancer immunotherapy. PD-1 is a receptor expressed on the surface of activated T cells, and its interaction with PD-L1, which is expressed on tumor cells and immune cells within the tumor microenvironment, leads to immune suppression and tumor immune evasion. In cervical cancer, elevated PD-L1 expression is associated with a poor prognosis and resistance to immune therapies. The engagement of PD-1 with PD-L1 contributes to the immune dysregulation seen in the cytokine storm. Therapeutic strategies that inhibit PD-1 or PD-L1 can potentially restore T-cell-mediated immunity and prevent the suppressive effects of the PD-1/PD-L1 axis in the tumor microenvironment. Pembrolizumab and Nivolumab are immune checkpoint inhibitors targeting PD-1 that have shown clinical success in several cancers, including cervical cancer. These agents have been associated with improved survival outcomes and may reduce the inflammatory effects of cytokine storms. Additionally, targeting the PD-L1/PD-1 interaction in combination with other immune-modulating therapies, such as cytokine inhibitors, could further enhance the antitumor immune response and prevent excessive inflammation.67–69
Targeting IL-8 for Tumor Metastasis and Angiogenesis
Interleukin-8 (IL-8) is a chemokine that promotes inflammation, angiogenesis, and metastasis in cervical cancer. IL-8 is involved in the recruitment of neutrophils and endothelial cells to the tumor site, facilitating the formation of new blood vessels (angiogenesis) and enhancing cancer cell migration. Additionally, IL-8 promotes the activation of matrix metalloproteinases (MMPs), which degrade the extracellular matrix and allow tumor cells to invade surrounding tissues and migrate to distant sites. High levels of IL-8 have been associated with lymph node metastasis, poor prognosis, and resistance to therapy in cervical cancer. Therapeutic strategies aimed at targeting IL-8 in cervical cancer have been under exploration. Monoclonal antibodies against IL-8 or its receptor (CXCR1/CXCR2) could prevent the recruitment of immune cells and endothelial cells to the tumor, thereby reducing angiogenesis and metastasis. Moreover, small molecule inhibitors targeting the IL-8/CXCR2 signaling pathway have shown promise in preclinical studies, and their clinical application in cervical cancer could suppress tumor progression and mitigate the cytokine storm. Combining IL-8 blockade with other therapies, such as immune checkpoint inhibitors or anti-angiogenic agents, may provide a synergistic effect in modulating tumor growth and metastasis.70–72
Targeting the NLRP3 Inflammasome
The NLRP3 inflammasome plays a critical role in the initiation of the cytokine storm in cervical cancer by activating caspase-1, which leads to the production of pro-inflammatory cytokines, including IL-1β and IL-18. These cytokines contribute to inflammation, tumor growth, and immune suppression. The activation of the NLRP3 inflammasome is implicated in various cancers, including cervical cancer, where it may exacerbate the inflammatory environment and promote tumor progression. Targeting the NLRP3 inflammasome presents a novel therapeutic strategy for controlling the cytokine storm. Several approaches to inhibit the NLRP3 inflammasome are being investigated, including the use of small molecule inhibitors and monoclonal antibodies that target key components of the inflammasome complex, such as NLRP3 itself or caspase-1. Agents like MCC950 have shown promise in preclinical models by blocking NLRP3 activation and reducing IL-1β production. In cervical cancer, inhibiting NLRP3 activation could attenuate the inflammatory responses that drive tumor progression, improve the response to other treatments, and restore immune balance. This approach could be particularly valuable in combination with other therapies targeting cytokine pathways.73–75
Challenges in Translating Cytokine-Targeting Strategies to the Clinic for Cervical Cancer
Despite promising insights into the role of chronic cytokine dysregulation in cervical cancer, translating cytokine-targeting strategies into effective clinical therapies faces several challenges. One major hurdle is the complexity and redundancy of cytokine networks; targeting a single cytokine, such as IL-6 or TGF-β, may not be sufficient because multiple, overlapping pathways can compensate, diminishing therapeutic impact. Moreover, chronic inflammation in cervical cancer involves not just tumor cells but also stromal cells, immune cells, and endothelial cells, creating a highly interconnected microenvironment where interventions can have unpredictable effects.73 Another significant challenge lies in patient heterogeneity. The cytokine profiles of cervical tumors vary widely between individuals, influenced by factors such as HPV genotype, genetic background, tumor stage, and prior treatments. This variability complicates the identification of universal biomarkers or one-size-fits-all therapies, emphasizing the need for personalized approaches and robust patient stratification. Additionally, while preclinical models have provided valuable mechanistic insights, many fail to fully replicate the chronic, smoldering inflammation and immune interactions observed in human tumors, limiting their predictive power for clinical outcomes.74 Safety concerns remain a key barrier. Cytokines play essential roles in normal immune homeostasis, tissue repair, and defense against infections. Broad cytokine blockade risks causing unintended immunosuppression or tissue damage, raising the importance of carefully designing targeted or combinatorial strategies with acceptable toxicity profiles. Overcoming these challenges will require integrating precision diagnostics, combination therapies, and improved clinical trial designs to successfully harness cytokine modulation as a therapeutic strategy in cervical cancer.75
Conclusion
Cytokine storm plays a pivotal role in the progression and metastasis of cervical cancer, driving inflammation, immune dysregulation, and tumor growth. The complex network of pro-inflammatory cytokines and immune cells within the tumor microenvironment not only exacerbates the disease but also contributes to poor prognosis and resistance to conventional therapies. Targeting the key components of the cytokine storm—such as IL-6, TNF-α, IL-8, immune checkpoint pathways, and the NLRP3 inflammasome—offers a promising therapeutic strategy to mitigate its harmful effects and enhance treatment efficacy. Therapeutic agents that target these cytokines and pathways, including monoclonal antibodies, small molecules, and immune checkpoint inhibitors, have shown potential in preclinical and clinical settings. These strategies can restore immune function, reduce inflammation, and prevent tumor progression, ultimately improving the prognosis for patients with cervical cancer. Furthermore, combining cytokine modulation with existing therapies such as chemotherapy and immunotherapy could lead to synergistic effects, resulting in more effective and personalized treatment options. Future therapeutic strategies should not consider cytokine modulation in isolation but rather in combination with established treatments such as chemotherapy, radiotherapy, and immune checkpoint inhibitors, to optimize patient responses and prevent therapeutic resistance.
Disclosure
The author reports no conflict of interest in this work.
References
1. Mbodi L, Bassa S, Kgoebane-Maseko M, Adeola HA, Mehrotra R, Dlamini Z. Merging cyberspace with physical space to improve cervical cancer management and women’s health in lower-middle-income countries. In: Society 5.0 and Next Generation Healthcare: Patient-Focused and Technology-Assisted Precision Therapies. Cham: Springer Nature Switzerland; 2023:131–154.
2. Ojha PS, Maste MM, Tubachi S, Patil VS. Human papillomavirus and cervical cancer: an insight highlighting pathogenesis and targeting strategies. Virusdisease. 2022;33(2):132–154. doi:10.1007/s13337-022-00768-w
3. Sadri Nahand J, Moghoofei M, Salmaninejad A, et al. Pathogenic role of exosomes and microRNAs in HPV‐mediated inflammation and cervical cancer: a review. Int J Cancer. 2020;146(2):305–320. doi:10.1002/ijc.32688
4. Kumari S, Bhor VM. Association of cervicovaginal dysbiosis mediated HPV infection with cervical intraepithelial neoplasia. Microb Pathogenesis. 2021;152:104780. doi:10.1016/j.micpath.2021.104780
5. Obeagu EI. From inflammation to invasion: neutrophils in cervical cancer pathogenesis. Ann Med Surg. 2024;2024:10–97.
6. Obeagu EI, Mahmoud SA. Monocytes and cervical ripening: a narrative review of prolonged labor pathophysiology. Ann Med Surg. 2025;87:10–97. doi:10.1097/MS9.0000000000002774
7. Kumar N, Vyas A, Agnihotri SK, Chattopadhyay N, Sachdev M. Small secretory proteins of immune cells can modulate gynecological cancers. In: Seminars in Cancer Biology. Vol. 86. Academic Press; 2022:513–531.
8. Aghbash PS, Rasizadeh R, Nahand JS, Baghi HB. The role of immune cells and inflammasomes in Modulating cytokine responses in HPV-Related cervical cancer. Int Immunopharmacol. 2025;145:113625. doi:10.1016/j.intimp.2024.113625
9. de Moura EL, Dos Santos AC, da Silva DM, et al. Association of polymorphisms in cytokine genes with susceptibility to precancerous lesions and cervical cancer: a systematic review with meta-analysis. Immunol invest. 2021;50(5):492–526. doi:10.1080/08820139.2020.1778023
10. Wang Q, Steger A, Mahner S, Jeschke U, Heidegger H. The formation and therapeutic update of tumor-associated macrophages in cervical cancer. Int J Mol Sci. 2019;20(13):3310. doi:10.3390/ijms20133310
11. Xu HH, Xie YY, Yang Z, Han QY, Han Q-Y. Dynamic changes of soluble HLA-G and cytokine plasma levels in cervical cancer patients: potential role in cancer progression and immunotherapy. J Cancer Res Clin Oncol. 2023;149(8):4195–4204. doi:10.1007/s00432-022-04331-4
12. Madeddu C, Sanna E, Nemolato S, et al. Pathogenic and prognostic roles of paraneoplastic leukocytosis in cervical cancer: can genomic-based targeted therapies have a role? A literature review and an emblematic case report. Diagnostics. 2022;12(8):1910. doi:10.3390/diagnostics12081910
13. Tang Y, Zhang AX, Chen G, Wu Y, Gu W. Prognostic and therapeutic TILs of cervical cancer—Current advances and future perspectives. Mol Ther Oncolytics. 2021;22:410–430. doi:10.1016/j.omto.2021.07.006
14. Kałafut J, Czerwonka A, Anameriç A, et al. Shooting at moving and hidden targets—tumour cell plasticity and the notch signalling pathway in head and neck squamous cell carcinomas. Cancers. 2021;13(24):6219. doi:10.3390/cancers13246219
15. Sekimata M, Kinjo Y, Tohyama A, et al. Cytokine release syndrome induced by immune checkpoint inhibitor treatment for uterine cervical cancer recurrence: a case report. Oncol Lett. 2024;28(1):331. doi:10.3892/ol.2024.14463
16. Watkins DE, Craig DJ, Vellani SD, et al. Advances in targeted therapy for the treatment of cervical cancer. J Clin Med. 2023;12(18):5992. doi:10.3390/jcm12185992
17. Geller MA, Cooley S, Argenta PA, et al. Toll-like receptor-7 agonist administered subcutaneously in a prolonged dosing schedule in heavily pretreated recurrent breast, ovarian, and cervix cancers. Cancer Immunol Immunother. 2010;59:1877–1884. doi:10.1007/s00262-010-0914-1
18. Yi M, Li T, Niu M, et al. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct Target Ther. 2024;9(1):176. doi:10.1038/s41392-024-01868-3
19. Kaur H, Ghorai SM. Role of cytokines as immunomodulators. In: Immunomodulators and Human Health. Singapore: Springer Nature Singapore; 2022:371–414.
20. Chen HC, Schiffman M, Lin CY, et al. CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J National Cancer Inst. 2011;103(18):1387–1396. doi:10.1093/jnci/djr283
21. Ferenczy A, Franco E. Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol. 2002;3(1):11–16. doi:10.1016/S1470-2045(01)00617-9
22. Shi N, Lu Q, Zhang J, et al. Analysis of risk factors for persistent infection of asymptomatic women with high-risk human papilloma virus. Hum Vaccines Immunother. 2017;13(6):1404–1411. doi:10.1080/21645515.2016.1239669
23. Luo JH, Zhang CY, Lu CY, Guo GH, Tian YP, Li YL. Serum expression level of cytokine and chemokine correlates with progression of human ovarian cancer. Eu J Gynaecol Oncol. 2017;38(1):33–39.
24. Korbecki J, Bosiacki M, Barczak K, et al. Involvement in tumorigenesis and clinical significance of CXCL1 in reproductive cancers: breast cancer, cervical cancer, endometrial cancer, ovarian cancer and prostate cancer. Int J Mol Sci. 2023;24(8):7262.
25. Zhou ZW, Long HZ, Xu SG, et al. Therapeutic effects of natural products on cervical cancer: based on inflammatory pathways. Front Pharmacol. 2022;13:899208. doi:10.3389/fphar.2022.899208
26. Cai C, Peng X, Zhang Y. Serum IL-6 level predicts the prognosis and diagnosis in cervical cancer patients. Int J Women’s Health. 2022;Volume 14:655–663. doi:10.2147/IJWH.S347740
27. Song Z, Lin Y, Ye X, et al. Expression of IL-1α and IL-6 is associated with progression and prognosis of human cervical cancer. Med Sci Monit. 2016;22:4475. doi:10.12659/MSM.898569
28. Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 in cervical cancer: the relationship with vascular endothelial growth factor. Gynecologic Oncol. 2001;82(1):49–56. doi:10.1006/gyno.2001.6235
29. Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22(10):1517–1527. doi:10.1038/sj.onc.1206226
30. Liu L, Yang X, Chen X, et al. Association between TNF-α polymorphisms and cervical cancer risk: a meta-analysis. Mol Biolo Rep. 2012;39:2683–2688. doi:10.1007/s11033-011-1022-9
31. Barbisan G, Pérez LO, Contreras A, Golijow CD. TNF-α and IL-10 promoter polymorphisms, HPV infection, and cervical cancer risk. Tumor Biol. 2012;33:1549–1556. doi:10.1007/s13277-012-0408-1
32. Li J, Zhang Y, Chen L, et al. Cervical cancer HeLa cell autocrine apoptosis induced by coimmobilized IFN-γ plus TNF-α biomaterials. ACS Appl Mater Interfaces. 2018;10(10):8451–8464. doi:10.1021/acsami.7b18277
33. Zhang J, Wu H, Li P, Zhao Y, Liu M, Tang H. NF-κB-modulated miR-130a targets TNF-α in cervical cancer cells. J Transl Med. 2014;12:1–4. doi:10.1186/1479-5876-12-155
34. Roszak A, Misztal M, Sowińska A, Jagodziński PP. TNF-α− 308 G/A as a risk marker of cervical cancer progression in the polish population. Mol Diagn Ther. 2015;19:53–57. doi:10.1007/s40291-015-0130-y
35. Deshpande A, Nolan JP, White PS, et al. TNF-α promoter polymorphisms and susceptibility to human papillomavirus 16–associated cervical cancer. J Infect Dis. 2005;191(6):969–976. doi:10.1086/427826
36. Al-Tahhan MA, Etewa RL, El Behery MM. Association between circulating interleukin-1 beta (IL-1β) levels and IL-1β C–511T polymorphism with cervical cancer risk in Egyptian women. Mol Cell Biochem. 2011;353:159–165. doi:10.1007/s11010-011-0782-9
37. Tao L, Liu S, Xiong J, et al. IL-1β promotes cervical cancer through activating NF-κB/CCL-2. Int J Clin Exp Pathol. 2021;14(4):426.
38. Matamoros JA, Da Silva MI, De Moura PM, Leitão MD, Coimbra EC. Reduced expression of IL-1β and IL-18 proinflammatory interleukins increases the risk of developing cervical cancer. Asian Pac J Cancer Prev. 2019;20(9):2715. doi:10.31557/APJCP.2019.20.9.2715
39. Jia L, Li F, Shao M, et al. IL-8 is upregulated in cervical cancer tissues and is associated with the proliferation and migration of HeLa cervical cancer cells. Oncol Lett. 2018;15(1):1350–1356. doi:10.3892/ol.2017.7391
40. Shang WQ, Li H, Liu LB, et al. RANKL/RANK interaction promotes the growth of cervical cancer cells by strengthening the dialogue between cervical cancer cells and regulation of IL-8 secretion. Oncol Rep. 2015;34(6):3007–3016. doi:10.3892/or.2015.4303
41. Chen RJ, Chen SU, Chou CH, Lin MC. Lysophosphatidic acid receptor 2/3‐mediated IL‐8‐dependent angiogenesis in cervical cancer cells. Int J Cancer. 2012;131(4):789–802. doi:10.1002/ijc.26476
42. Balode E, Pilmane M, Rezeberga D, Jermakova I, Kroica J. Interleukin IL-1α, IL-6, IL-8, IL-10 expression in different staging of cervical intraepithelial neoplasia. Acta Chirurgica Latviensis. 2017;17(1):8. doi:10.1515/chilat-2017-0010
43. Yadav VK, Lee TY, Hsu JB, Huang HD, Yang WC, Chang TH. Computational analysis for identification of the extracellular matrix molecules involved in endometrial cancer progression. PLoS One. 2020;15(4):e0231594. doi:10.1371/journal.pone.0231594
44. Poltavets V, Kochetkova M, Pitson SM, Samuel MS. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front Oncol. 2018;8:431. doi:10.3389/fonc.2018.00431
45. Qian N, Chen X, Han S, et al. Circulating IL-1β levels, polymorphisms of IL-1B, and risk of cervical cancer in Chinese women. J Cancer Res Clin Oncol. 2010;136:709–716. doi:10.1007/s00432-009-0710-5
46. Srivani R, Nagarajan B. A prognostic insight on in vivo expression of interleukin-6 in uterine cervical cancer. Int J Gynecological Cancer. 2003;13(3):331–339. doi:10.1136/ijgc-00009577-200305000-00012
47. Wang Y, Yang J, Huang J, Tian Z. Tumor necrosis factor-α polymorphisms and cervical cancer: evidence from a meta-analysis. Gynecol Obstet Invest. 2020;85(2):153–158. doi:10.1159/000502955
48. Du GH, Wang JK, Richards JR, Wang JJ. Genetic polymorphisms in tumor necrosis factor alpha and interleukin-10 are associated with an increased risk of cervical cancer. Int Immunopharmacol. 2019;66:154–611. doi:10.1016/j.intimp.2018.11.015
49. Jin Y. Association of single nucleotide polymorphisms in tumor necrosis factor-alpha with cervical cancer susceptibility. Cell Biochem Biophys. 2015;71:77–84. doi:10.1007/s12013-014-0165-4
50. Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60(10):2632–2635.
51. Wu S, Shang H, Cui L, et al. Targeted blockade of interleukin-8 abrogates its promotion of cervical cancer growth and metastasis. Mol Cell Biochem. 2013;375:69–79. doi:10.1007/s11010-012-1529-y
52. Polterauer S, Grimm C, Tempfer C, et al. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecologic Oncol. 2007;107(1):114–117. doi:10.1016/j.ygyno.2007.06.001
53. Polterauer S, Grimm C, Zeillinger R, et al. Association of C-reactive protein (CRP) gene polymorphisms, serum CRP levels and cervical cancer prognosis. Anticancer Res. 2011;31(6):2259–2264.
54. Yang S, Zhang Z, Shen L. Prognostic significance of C-reactive protein in patients with cervical cancer: a meta-analysis. Front Oncol. 2023;13:1232409. doi:10.3389/fonc.2023.1232409
55. He X, Li JP, Liu XH, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer. 2018;9(10):1877. doi:10.7150/jca.23320
56. Guo S, Yang B, Liu H, et al. Serum expression level of squamous cell carcinoma antigen, highly sensitive C-reactive protein, and CA-125 as potential biomarkers for recurrence of cervical cancer. J Cancer Res Ther. 2017;13(4):689–692. doi:10.4103/jcrt.JCRT_414_17
57. Buyukbayram ME, Hannarici Z, Turhan A, et al. A novel prognostic biomarker in progression free survival for patients with cervical cancer, glucose to c-reactive protein ratio (GCR). BMC Cancer. 2024;24(1):626. doi:10.1186/s12885-024-12347-x
58. Alrehaili AA, Gharib AF, Almalki A, et al. Soluble programmed death-ligand 1 (sPD-L1) as a promising marker for head and neck squamous cell carcinoma: correlations with clinical and demographic characteristics. Cureus. 2023;15(8):1.
59. Prabawa IP, Bhargah A, Liwang F, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as a predictive value of hematological markers in cervical cancer. Asian Pac J Cancer Prev. 2019;20(3):863. doi:10.31557/APJCP.2019.20.3.863
60. Trinh H, Dzul SP, Hyder J, et al. Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT). Clin. Chim. Acta. 2020;510:711–716. doi:10.1016/j.cca.2020.09.008
61. Li YX, Chang JY, He MY, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and monocyte‐to‐lymphocyte ratio (MLR) predict clinical outcome in patients with stage IIB cervical cancer. J Oncol. 2021;2021(1):2939162. doi:10.1155/2021/2939162
62. Tas M, Yavuz A, Ak M, Ozcelik B. Neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in discriminating precancerous pathologies from cervical cancer. J Oncol. 2019;2019(1):2476082. doi:10.1155/2019/2476082
63. Wu J, Chen M, Liang C, Su W. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in cervical cancer: a meta-analysis and systematic review. Oncotarget. 2017;8(8):13400. doi:10.18632/oncotarget.14541
64. Huang QT, Man QQ, Hu J, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in cervical cancer: a systematic review and meta-analysis of observational studies. Oncotarget. 2017;8(10):16755. doi:10.18632/oncotarget.15157
65. Miao JW, Liu LJ, Huang J. Interleukin-6-induced epithelial-mesenchymal transition through signal transducer and activator of transcription 3 in human cervical carcinoma. Int J Oncol. 2014;45(1):165–176. doi:10.3892/ijo.2014.2422
66. Lee KH, Lee MH, Kang YW, Rhee KJ, Kim TU, Kim YS. Parkin induces apoptotic cell death in TNF-α-treated cervical cancer cells. BMB Reports. 2012;45(9):526–531. doi:10.5483/BMBRep.2012.45.9.104
67. Ge Y, Zhang Y, Zhao KN, Zhu H. Emerging therapeutic strategies of different immunotherapy approaches combined with PD-1/PD-L1 blockade in cervical cancer. Drug Des Devel Ther. 2022;Volume 16:3055–3070. doi:10.2147/DDDT.S374672
68. Tuyaerts S, Van Nuffel AM, Naert E, et al. PRIMMO study protocol: a Phase II study combining PD-1 blockade, radiation and immunomodulation to tackle cervical and uterine cancer. BMC Cancer. 2019;19:1. doi:10.1186/s12885-019-5676-3
69. Chitsike L, Duerksen-Hughes P. The potential of immune checkpoint blockade in cervical cancer: can combinatorial regimens maximize response? A review of the literature. Curr Treat Options Oncol. 2020;21(12):95. doi:10.1007/s11864-020-00790-4
70. Fujimoto J. Novel strategy of anti-angiogenic therapy for uterine cervical carcinomas. Anticancer Res. 2009;29(7):2665–2669.
71. Xiong Y, Xu X, Zhou X, Tong Y, Yu C. Anlotinib inhibits cervical cancer cell proliferation and invasion by suppressing cytokine secretion in activated cancer-associated fibroblasts. Front Oncol. 2024;14:1412660. doi:10.3389/fonc.2024.1412660
72. Franciosi ML, Do Carmo TI, Zanini D, Cardoso AM. Inflammatory profile in cervical cancer: influence of purinergic signaling and possible therapeutic targets. Inflammation Res. 2022;71(5):555–564. doi:10.1007/s00011-022-01560-8
73. Yu S, Zhao N, He M, Zhang K, Bi X. MiRNA-214 promotes the pyroptosis and inhibits the proliferation of cervical cancer cells via regulating the expression of NLRP3. Cell Mol Biol. 2020;66(6):59–64. doi:10.14715/cmb/2020.66.6.11
74. Missiroli S, Perrone M, Boncompagni C, et al. Targeting the NLRP3 inflammasome as a new therapeutic option for overcoming cancer. Cancers. 2021;13(10):2297. doi:10.3390/cancers13102297
75. Liu X, Xie X, Li Q, et al. KIF23 promotes cervical cancer progression via inhibiting NLRP3‐mediated pyroptosis. FASEB J. 2024;38(10):e23685. doi:10.1096/fj.202400281R
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

