Back to Journals » International Journal of Nanomedicine » Volume 20
Polymer Nanoparticles Advancements for Gynecological Cancers
Authors Lin X , Li Z, Huang Y, Li Y , Li Y, Zhang L , Wu M
Received 8 March 2025
Accepted for publication 12 May 2025
Published 26 May 2025 Volume 2025:20 Pages 6721—6742
DOI https://doi.org/10.2147/IJN.S527023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Dong Wang
Xiaorui Lin,1,2 Zihan Li,1,2 Yibao Huang,1,2 Yinuo Li,1,2 Yuting Li,1,2 Ling Zhang,1,2 Mingfu Wu1,2
1National Clinical Research Center for Obstetrical and Gynecological Diseases, Department of Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, People’s Republic of China; 2Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education, Wuhan 430030, People’s Republic of China
Correspondence: Mingfu Wu, Email [email protected]
Abstract: Gynecological cancers represent one of the leading causes of death in women and pose a critical global health challenge. While surgery and chemotherapy remain the first-line therapies for gynecological cancers, the persistently high morbidity and mortality rates have driven the urgent exploration of novel theranostic strategies. In recent years, polymer nanoparticles (PNPs) have gained increasing attention in the diagnosis and treatment of cancer due to their superior targeting ability and delivery efficiency. This review provides an overview of PNPs and their role in tumor diagnosis and treatment, with a strategic focus on their utility in gynecological cancers. It covers drug delivery, imaging, combination therapy, and theranostic integration in gynecological cancers, and summarizes the composition, principles and characteristics of diverse polymers and their cargoes. Furthermore, this work highlights innovative applications of PNPs in gynecological cancers management, spanning chemotherapy, immunotherapy, PARPi therapy, phototherapy and other therapies. Despite promising preclinical advancements in PNPs, formidable challenges persist in their clinical translation. This review serves as a comprehensive resource for researchers and clinicians aiming to optimize gynecological cancers theranostics as well as accelerate the development and clinical translation of PNPs.
Keywords: polymer nanoparticles, gynecological cancer, theranostics, nanoparticles, ovarian cancer, cervical cancer, endometrial cancer, nanomedicine
Introduction
Nanoparticles (NPs) are solid colloidal particles, usually between 10–1000 nm in size,1 which can be composed of lipids, metals, metal oxides, polymers and other materials.2 Polymer nanoparticles (PNPs) is a collective term for nano-sized particles made of polymer,3 with the optimal size of 10–200 nm for their in vivo application.4 PNPs have superior biocompatibility and drug-loading flexibility compared to other NPs. PNPs are mainly prepared by self-assembly, nanoprecipitation, dialysis, solvent evaporation, salting-out, based on preformed polymers or on monomers.1,3–5 The circulation time of PNPs in vivo is determined by their physicochemical properties, while their controllable shape, size, and surface modifications allow them to be a promising therapeutic carrier.4 PNPs can encapsulate various cargoes to improve their solubility, bioavailability, biocompatibility, half-life, etc.6 Then they deliver and release the cargoes precisely to exert therapeutic or diagnostic effects. Therefore, PNPs have undergone extensive research in the treatment and prevention of tumors,7–9 cardiovascular diseases10,11, and infectious diseases.12,13
Cancer is one of the most important risk factors for human mortality in modern society. According to Global Cancer Observatory, about 9.7 million people died of cancer worldwide in 2022.4 Gynecological cancers, including ovarian, cervical, endometrial, vulvar, and fallopian tube cancers, are the leading causes of death in women.14 For now, the major therapies for gynecological cancers are still surgery and chemotherapy.15–17 However, the recurrence, metastasis, chemoresistance, and the still-growing global burden of gynecological cancers highlight the urgent need to develop novel precision-based strategies. Recently, polymeric nanosystems for combination therapy and diagnostic and therapeutic integration have been developed to increase the therapeutic efficiency of gynecological cancers. Particularly, the potential of PNPs in gynecological cancers therapy is gradually being realized. Since different gynecological cancers vary significantly in their pathogenesis, target expression, drug sensitivity, etc, the design of PNPs needs to be differentiated according to the cancers. Researchers have designed many novel PNPs to deliver therapeutic agents or adjuvants required for chemotherapy, immunotherapy, phototherapy, gene therapy in gynecological cancers.
Although many studies are underway, very few PNPs have been successfully translated into clinical practice. The clinical application of these PNPs still faces many challenges, such as biosafety, heterogeneity of clinical effects, targeted delivery efficiency, and scale-up production.18
The Mechanism of PNPs in Cancer Diagnosis and Treatment
Cargoes Loaded with PNPs
PNPs produce effects in the diagnosis and treatment of tumors primarily by loading different cargoes. Among them, chemotherapeutic agents (CTAs) are one of the most common cargoes. Many PNPs have been developed to address their drawbacks such as premature drug release, difficulty in targeted delivery, low bioavailability, side effects and toxicity, and drug resistance19,20 enhancing the efficacy of oncology chemotherapy. Other strategies with PNPs including the application of prodrugs,21 the combination of two drugs,22 and synergistic treatment with other therapies23,24 are constantly being investigated.
RNA-based drugs such as small interfering RNAs (siRNA), mRNAs, antisense oligonucleotides, microRNAs, etc, are increasingly explored for tumor therapy.25 mRNA-based therapies deliver genetic instructions for therapeutic protein production without nuclear entry, minimizing genotoxicity risks compared to DNA-based approaches.26,27 However, siRNA silences oncogenes via RNA interference but faces challenges like poor stability, rapid renal clearance, and inefficient cellular uptake in its free form.28,29 The protection of the polymer keeps these nucleic acids from being degraded during delivery to tumor cells and promotes cellular uptake.30
PNPs can also deliver phototherapeutic agents such as photothermal agents (PTA) for photothermal therapy (PTT) and photosensitizers (PS) for photodynamic therapy (PDT)4 as well as contrast agents for magnetic resonance imaging (MRI),31,32 photoacoustic imaging (PAI),33 etc. This capability enhances both therapeutic precision and diagnostic accuracy. Compared to conventional therapies, phototherapy (including PTT and PDT) offers non-invasive treatment with minimal trauma.23 PTT generates localized heat to eliminate cancer cells34 and remodel the TME,35 while PDT relies on light-activated PS to produce cytotoxic reactive oxygen species (ROS), inducing tumor cell apoptosis or necrosis.23,36,37
Targeted Drug Delivery
Targeted drug delivery of PNPs is categorized as active targeting and passive targeting. Controllable surface modifications are the basis for active targeting. Active targeting mainly occurs through the binding of ligands on the surface of PNPs to receptors on the surface of tumor cells or tumor vascular endothelial cells. Therefore, the tumor cell receptors selected for designing PNPs are supposed to be specifically overexpressed on the tumor surface but not in healthy cells.4 Commonly overexpressed target receptors in tumors include human epidermal growth factor receptor 2 (HER2), CD44 receptor, folic acid (FA) receptor, vascular endothelial growth factor (VEGF) receptor, and others.30,38 For example, Sader et al39 utilized dermatan sulfate, which can target the CD44 receptor, to design PNPs capable of actively targeting triple-negative breast cancer. The site-specific binding of the ligands to the receptors decreases the probability of off-targeting during delivery.
However, passive targeting is achieved by enhanced permeation and retention (EPR) effects and does not require ligand-receptor binding. Nano-sized particles preferentially enter tumor cells but are difficult to exit due to leaky vasculature and poorly developed lymphatic drainage of the tumor,6 which allows them to accumulate and prolongs their sustained release within the tumor cells.40 (Figure 1) Although the theory of the EPR effect is widely recognized, it has not been successful in clinical translation. That might be related to the heterogeneity of the EPR effect in different tumors or individuals. Thus several researchers are exploring this area and trying to guide the application of nanomedicines through patient stratification.41
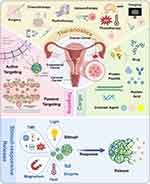 |
Figure 1 The mechanism of PNPs in the theranostics of gynecological cancers. Created in BioRender. Lin, X. (2025) https://BioRender.com/wlprr14. Abbreviations: PNPs, polymer nanoparticles; PDT, photodynamic therapy; PTT, photothermal therapy; ROS, reactive oxygen species; TME, tumor microenvironment; GSH, glutathione. |
Stimuli-Responsive Release
The tumor microenvironment (TME) is remarkably different from the environment in which healthy cells live. Acidic pH, high levels of glutathione (GSH), specific enzymes, ROS, hypoxia, and other stimuli unique to TME offer opportunities to design stimuli-responsive PNPs.42–44 In addition, in vitro physical stimuli such as light, heat, ultrasound, magnetic fields and so on can also mediate drug release.43 PNPs can release the cargoes they carried when they encounter the specific stimulus in the target site. This process is known as stimuli-responsive release. For example, as an important reducing agent in tumors, GSH can break disulfide bonds in the backbone of redox-responsive polymers, leading to the degradation of the polymer and the release of its loaded drug.45,46 This capacity to locally release the drug only in response to the specific stimulus effectively minimizes the loss of the drug in the circulation and the side effects of systemic administration (Figure 1).
Commonly Used Polymers
As the core of PNPs, the characteristics of the various polymers themselves cannot be ignored. The polymers used to design PNPs for drug delivery share the general characteristic of biocompatibility, which can be classified into natural and synthetic polymers47,48 (Table 1)
 |
Table 1 Commonly Used Polymers |
Natural Polymers
Chitosan
Chitosan (CS) is the most widely used natural polymer as a drug carrier which is found in large quantities in nature.48 CS can be extracted from crustacean shells or fungi,54 and it is a copolymer of β-(1,4)-linked D-glucosamine and N-acetyl-D-glucosamine.50 The cationic nature is one of the advantages of CS, which facilitates its binding to anionic molecules such as nucleic acids and cell surface macromolecules, and also enhances adhesion to negatively charged mucous membranes.49,50 Other advantages of CS include lower toxicity and immunogenicity, excellent biocompatibility and biodegradability, and potential for antimicrobial, antioxidant and anticancer activity.49,54,59 Ali et al60 designed chitosan/tannic acid nanoparticles (CS/TAN NPs) for loading loratadine (LOR), which exhibited stronger cytotoxicity than free loratadine, to enhance the anticancer activity of the antihistaminic agent LOR against MCF-7 breast cancer. Together with the ability of tannic to promote apoptosis in MCF-7 breast cancer cells, this demonstrates the great potential of LOR-CS/TAN NPs as a novel antiproliferative agent for breast cancer therapy.
The disadvantages of CS are also well defined, such as poor water solubility and inefficient endosomal escape ability,49 which prevent it from achieving clinical translation. In this regard, scientists have explored multiple approaches to overcome its shortcomings by adding various structural modifications. Among them, polyethylene glycolylation (PEGylation) is one of the reliable strategies to improve CS nanoparticles (CS-NPs) drug delivery.61,62 Coating the surface of CS-NPs with PEG, covalent conjugation of CS with PEG to form self-assembled NPs, or linking CS-NPs with drugs via PEG-linker are all used as means to form PEGylated CS-NPs. PEGylation improves CS solubility, inhibits mononuclear phagocytic system (MPS) clearance to prolong plasma circulation time, and enhances CS stability and anticancer ability in biological fluids.63
Albumin
Albumin is a protein-based polymer that has also been used to engineer PNPs. Albumin NPs are considered as excellent drug carriers due to their biological origin, lack of toxicity and immunogenicity, good water solubility, and the ability to accumulate at the tumor site. According to its source, albumin can be classified as human serum albumin, bovine serum albumin (BSA), etc.48 Its most classical application case is albumin-bound paclitaxel (Abraxane). Paclitaxel (PTX) is a Taxanes with well-defined tumor inhibitory properties, however, high insolubility has hindered its clinical development. Earlier, researchers had developed solvent-based PTX (Taxol®) with Cremophor EL as an excipient, but the risk of inducing hypersensitivity reactions, neutropenia and peripheral neuropathy was identified in subsequent clinical applications. In order to avoid the Cremophor EL-related toxicities, researchers developed Abraxane.64,65 Abraxane avoids solvent-related toxicity and has better response rates, tumor uptake, and tolerability.64 Clinical trials have shown that Abraxane can significantly improve the toxicity and efficacy of the drug, with higher volume of distribution and clearance, as well as significant linear pharmacokinetics compared to Taxol®.66
Other Natural Polymers
Owing to their natural abundance, low toxicity, biodegradability and biocompatibility, these natural polymers, including polysaccharide-based polymers (CS, hyaluronic acid (HA), alginate, starch, agarose, etc) and protein-based polymers (albumin, gelatin, collagen, etc), are widely used for drug delivery.48 For example, gelatin was used to load PTX for EPR effect and slow release of the drug.52 In addition, some teams have also designed PNPs with natural polyphenol Gossypol as the polymer backbone to promote anti-tumor effects utilizing its pro-apoptotic ability.67
Synthetic Polymers
Poly (Ethylene Glycol)
Poly (ethylene glycol) (PEG) is a hydrophilic polymer with high stealth properties in vivo and is the most common polymer used to produce PNPs.4 PEG shows excellent biocompatibility, drug encapsulation efficiency and non-toxicity.68 What’s more, it has the capacity to escape recognition and clearance by MPS, therefore breaking the limitation of circulation time.53 In addition, using PEG as a surface coating for NPs, PEGylation can also prolong its in vivo circulation time.53 However, other studies have reported that in vivo PEG antibody production and pre-existing PEG antibodies can induce accelerated blood clearance, negatively affecting the safety and efficacy of PEGylated drugs.69
Poly (Lactic‐co‐Glycolic Acid)
Poly (Lactic‐co‐Glycolic Acid) (PLGA), a polyester polymer approved for medical applications by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), has received extensive attention in the design of PNPs.70 PLGA is hydrolyzed in vivo to lactic acids and glycolic acids, which are degradable metabolite monomers that can be metabolized in the tricarboxylic acid cycle, thus making PLGA a polymeric material with minimal toxicity.56,70 The physicochemical properties such as hydrophobicity, mechanical strength, and degradation speed can be controlled by adjusting the ratio of lactic acid and glycolic acid content in PLGA.54,55 This allows PLGA to be utilized better for encapsulation of different drugs as well as treatment of different diseases.
Poly‐ε‐Caprolactone
Poly‐ε‐Caprolactone (PCL) is also a polyester polymer approved by the FDA that can be hydrolyzed or enzymatically cleaved to caproic acid and excreted in urine or feces without accumulation in the body,6,71 so it is considered a reliable synthetic polymer. PCL is a hydrophobic polymer that degrades slowly in vivo,56 and the encapsulated drug can be released slowly and gradually once it is applied.6 Some teams have utilized its controlled-release property to design drugs72,73 to prolong the duration of effects or to achieve long-term administration in vivo.56,71,74 Abriata et al75 utilized polycaprolactone (PCL) as a delivery vehicle for PTX and demonstrated its potential to optimize treatment protocols for ovarian adenocarcinoma in vitro.
Other Synthetic Polymers
Similar to PLGA and PCL, Poly(lactic acid) (PLA) is also one type of the hydrophobic polyesters, yet water molecules can break its ester bonds leading to hydrolysis.57 Its degradation product, lactic acid, can be metabolized by the body, making PLA also a suitable polymer for the production of PNPs.76,77 Polyethyleneimine (PEI) is a cationic synthetic polymer that encapsulates negatively charged DNA to protect it from degradation.58 It also has great transfection ability and has been extensively studied as a gene delivery carrier.4,26 However, its non-degradability can lead to cytotoxic effects,58 which limits its application. Therefore, researchers have attempted to synthesize some biodegradable polymers with similar functions to PEI. For example Poly(β-amino esters) (PBAE) synthesized by Jordan J Green et al78 for the delivery of DNA for the treatment of glioblastoma. Additionally, disulfide-bonded derivatives of PEI (l-PEIS) synthesized by Yan Lee et al79 which showed high transfection efficiency and low toxicity.
Combining the advantages of different polymers to form new copolymers is also an important research trend. For example, the PLGA introduced previously is a commonly used copolymer. The amphiphilic PNPs prepared using PEG-PCL featured both PCL as the hydrophobic core and PEG as the hydrophilic shell, which enhances the water solubility while protecting the physicochemical properties of the cargo.33 The mPEG-PEI copolymer NPs were utilized for the delivery of short hairpin RNA (shRNA) for the treatment of chemoresistant prostate cancer, taking advantage of the high transfection efficiency of PEI while incorporating PEG to reduce the toxicity of the NPs and enhance their stability.80 Coating CS and PEG on the outside of PLA-based NPs can inhibit the phagocytosis of PNPs by the reticuloendothelial system, facilitate the penetration of macromolecules across the mucosal surface and form a hydrated shell to prolong the in vivo circulation time of PNPs.81
Polymer-Hybrid Nanoparticles
In order to overcome the disadvantages of homopolymer, another strategy is to combine it with other kinds of materials (such as lipids, metals, inorganic compounds, etc) to form new polymer-hybrid nanoparticles (PHNPs).82 Farinha et al83 designed a novel targeted hybrid nanosystem consisting of a pH-sensitive lipid bilayer and a PLGA core in order to improve the effects of targeted therapeutics selumetinib and perifosine in combination with the tumor suppressor gene transgene for the treatment of hepatocellular carcinoma (HCC). They endowed this nanosystem with many advantages, including the capability of PLGA to load different therapeutic molecules, easy adjustment of the degradation rate, the stimulus responsiveness of lipids and reduced therapy-related side effects. Kong et al84 also designed a lipid-PHNP using PLGA with the incorporation of redox-responsive polymer for the delivery of mRNA encoding the tumor suppressor p53, to treat the patients with p53-deficient HCC and non-small cell lung cancer. Le’s team85 utilized PBAE combined with bioactive lipids, forming PBAE-lipid hybrid NPs that encapsulate the mRNA encoding bevacizumab, which is preferentially targeted to the pulmonary endothelial cells in systemic administration. There are many other studies that have demonstrated that the application of PHNPs can contribute to improving the blood circulation time of NPs, increasing the encapsulation rate, and optimizing the specific release.82
Novel Polymer Nanoparticles for Drug Delivery in Gynecological Cancers
Focusing on the field of gynecological cancers, although surgery and chemotherapy remain the mainstream treatment options, scientists are also attempting to leverage the unique advantages of PNPs to provide new strategies for gynecological cancers theranostics (Table 2)
 |
Table 2 Novel Polymer Nanoparticles for Gynecological Cancers |
Ovarian Cancer
Ovarian cancer (OC) is a common malignant tumor of the female reproductive system that lacks effective diagnostic strategies in the early stages, therefore most of the patients are already in advanced stages when they are diagnosed.116 According to the survey data of the World Health Organization (WHO), in 2022, there were 6.65 new cases of OC and 3.97 deaths due to OC per 100,000 women, and the incidence and mortality rates of ovarian cancer ranked 7th and 6th among women.117 OC is the most lethal gynecological cancer, while surgery and chemotherapy are the major therapies for OC. However, most patients relapse after chemotherapy or surgery.116 The high invasiveness, metastases and drug resistance of OC result in high mortality rates.102,118–120 Therefore new OC treatments still urgently need to be developed. In recent years, with the development of pharmaceutical research, the status of new modalities, such as immunotherapy, targeted therapy and hormonal therapy, has been rising in the management of OC. Notably, the development of PNPs has also provided an efficient strategy for the application of these new therapeutic molecules.
Chemotherapy
Chemotherapy is one of the most common treatments for OC. Neoadjuvant chemotherapy and postoperative adjuvant chemotherapy both belong to the first-line chemotherapy; while second-line approaches are usually applied in patients with OC recurrence or deterioration during the first-line chemotherapy.121
PTX has been the first-line drug for OC treatment in recent decades. Several formulations, such as Abraxane, have been put into use to deal with the problem of adverse reactions or low solubility of PTX.122 However, how to overcome its short half-life in vivo remains a focus of scientific inquiry, and PNPs have certain advantages in this field.123 Xiang Sun et al86 designed HA-PLA-PTX NPs, which took advantage of HA to bind to CD44 overexpressed on the surface of OC, used HA to surface-modify PNPs synthesized by PLA and PTX. They also PEGylated the surfaces of the PNPs in the process of preparation. The HA on the surface of HA-PLA-PTX NPs provides the ability to actively target OC, and the PEGylation provides the stealth ability to avoid being cleared by MPS. This facilitates the solution of the premature release of PTX and the improvement of the therapeutic targeting and killing efficiency of ovarian tumors in vivo.
Platinum is another chemotherapy drug that is frequently used. Banik’s team21 reported a novel platinum (IV) prodrug, Platin-C, a combination of curcumin and cisplatin, which is recognized as a promising drug to enhance the cytotoxic activity of platinum drugs. In order to deliver Platin-C to mitochondria directly, they used triphenyl phosphonium (TPP) cation-modified PLGA-b-PEG as a carrier for PNPs and validated its mitochondria-targeting activity in the cisplatin chemotherapy-resistant human OC cell line A2780/CP70.
Immunotherapy
Immunotherapy achieves its anti-tumor effects through enhancing the immune response.124 Interaction of programmed death 1 (PD-1) protein overexpressed in OC cells with its ligand PD-L1 decreases T-cell responsiveness in epithelial OC. Therefore, using siRNA to inhibit PD-1 expression is a viable immunotherapy for OC. However, PD-1 can also be expressed in healthy cells, so it is necessary to improve the specificity of siRNA delivery. Teo et al87 designed PNPs that use FA and PEG to modify the surface of PEI bound to siRNA, which can specifically bind to the FA receptors overexpressed in epithelial OC cells to enhance its uptake. The combination of PEI with siRNA can improve its stability by protecting siRNA from enzymatic degradation through the “proton sponge hypothesis”.
Tumor-associated macrophages (TAM) have anti-tumor properties when they express the M1-like phenotype. However TAMs usually express the M2-like phenotype, which means it has a pro-tumorigenic effect to reduce inflammation and promote tissue repair, leading to tumor progression, metastasis, and drug resistance.125,126 For reprogramming TAM into the M1-like, Zhang’s team88 developed IRF5/IKKβ NPs choosing mRNA-PBAE as the core of PNPs. In order to enable mRNA-PBAE to specifically target TAM and improve its stability, Di-mannitol-functionalized polyglutamic acid was coated on the outer side, and in vitro-transcribed mRNA that encodes the M1-polarizing transcription factors were delivered to M2-like macrophages. Through experiments in OC, glioblastoma and melanoma models, they confirmed the efficacy of IRF5/IKKβ NPs in inducing TAM reprogramming to antitumor, as well as the safety of repeated administration.
Tumor-derived Prostaglandin E2 (PGE2), along with its receptors EP2 and EP4, forms a PGE2-EP2/4 signaling axis, which has been proven to affect the immune function of dendritic cells (DCs) in the tumor microenvironment.127 Clinical trials have been conducted to study the efficacy of EP2/EP4 antagonists (aEP2/4) in the treatment of cancer.128 In order to address the possible off-target effects and premature degradation, Jorge Cuenca-Escalona et al89 used PLGA to encapsulate aEP2/4, which can be phagocytosed by DCs and inhibit the signal transduction involved in PGE2 to protect conventional type 2 DCs from inhibition and promote anti-tumor immune responses of DCs. This concept was validated in experiments with ascites from OC patients.
PARP Inhibitor
Poly (adenosine diphosphate ribose) polymerase inhibitor (PARPi) induces DNA strand breaks and shows synthetic lethality in homologous recombination deficiency (HRD) cells to exert anti-tumor effects.129,130 Clinical evidence supports PARPi’s role in improving overall survival (OS), particularly in patients with newly diagnosed OC and BRCA gene mutations,131,132 leading to FDA approval for OC maintenance therapy.129 However, PARPi also faces problems of poor long-term tolerability as well as acquired resistance.92
DNA repair consists of both single-stranded and double-stranded, which are handled by two enzymes, PARP and BRCA, separately. Therefore, the use of PARPi in BRCA-mutated HRD OCs results in the failure of both types of repair. In this case, tumor cells are unable to repair themselves in the event of genetic errors, inhibiting cell proliferation.90 The low rates of BRCA gene mutations in OC and the limited effects of PARPi in promoting apoptosis in non-HRD cells have restricted the clinical benefit of PARPi in the treatment of OC. Hence, a new combination strategy is needed to improve the sensitivity of OC to PARPi. Yangyang Li et al90 utilized gallium(III), which can disrupt cellular iron homeostasis and thus inhibit DNA, in combination with PARPi to improve the therapeutic efficacy of PARPi. However, gallium (III) ions (Ga3+) have low solubility and bioavailability in vivo and are associated with a risk of nephrotoxicity, so they developed olaparib-Ga nanomedicines composed of NPs with an average size of ~7 nm to deliver PARPi and Ga3+ simultaneously. Firstly, BSA-Ga3+ was formed based on the natural polymer BSA, then gallic acid (GA) was mixed to form stable GA-Ga3+, and then hydrophobic PARPi (olaparib) was attached to BSA and self-assembled to form pH-responsive releasing nanodrugs. The synergistic therapeutic effect of gallium (III) and olaparib was demonstrated in SKOV3 and OVCAR3 homologous recombination-proficient (HR-proficient) OC cells, and its inhibitory effect on tumor growth was proved in vivo in xenograft models.
The combination of PARPi and WEE1 inhibitor (WEE1i) can provide more effective inhibition of ovarian tumor growth, but it is not widely used in clinical practice because it is poorly tolerated.133 Therefore, Chaoyang Sun’s team91 designed a type of PNPs called TPNPs based on mesoporous polydopamine NPs modified with a tumor-targeting peptide (TMTP1), for the delivery of WEE1i (adavosertib) and PARPi (olaparib), reducing the side effects of the combination through precise delivery of PNPs.
It has been reported that the bromodomain and extra terminal inhibitors (BETi) can specifically regulate oncogene expression and enhance the therapeutic efficacy of PARPi when co-applied with olaparib in HR-proficient OC.134 Juan et al92 utilized PLA-based NPs encapsulating JQ1, a BETi, to form JQ1-NPs. The use of JQ1-NPs together with olaparib in OVCAR8 and SKOV3 OC cell lines showed an enhanced synergistic interaction as well as stronger ability to induce cell death than free JQ1. In addition, JQ1-NPs used alone also showed similar or slightly stronger antiproliferative effects than free JQ1.
Other Therapeutic Modalities and Medications
There are many signaling pathways dysregulated in OC. The widely recognized ones include the VEGFR and LPA pathways, whose dysregulation can stimulate tumor growth, adhesion, migration, invasion and angiogenesis and also lead to chemoresistance.93,135–137 VEGF and lysophosphatidic acid (LPA) secreted by OC cells are important signaling molecules involved in the above-mentioned signaling pathways. Ozel et al93 designed a targeted polymer-drug conjugate nanoparticles loaded with LPA receptor inhibitor (Ki16425) and VEGFR inhibitor (CBZ), using O-(2-Carboxyethyl) polyethylene glycol (PEG5000) as a carrier to inhibit the growth of OC.
As a natural product, propolis has been reported to have anticancer effects in OC, including inhibition of tumor angiogenesis, metastasis, as well as inhibition of anti-apoptotic proteins and activation of cysteine-containing aspartic protein hydrolases caspases that promote apoptosis.138 Justino’s team94 encapsulated Brazilian red propolis extract, which shows cytotoxicity against drug-resistant OC cells,139 in PLA to form PNPs for the treatment of OC. The use of polymer for loading this promising natural antitumor drug overcomes the disadvantages of its instability, low solubility and low bioavailability. Patra et al95 designed folate-targeted PLGA nanoparticles for delivery of Genistein (GEN), which has therapeutic potential for OC, to improve GEN’s water solubility and bioavailability, as well as to overcome its disadvantages that lack of targetability and rapid in vivo metabolism.
Cervical Cancer
Data from WHO surveys indicate that cervical cancer (CC) is the fourth most common cancer in women, with approximately 14.12 and 7.08 new cases or deaths per 100,000 women worldwide in 2022 respectively.117 CC is an infection-associated cancer and the majority of CC is caused by human papillomavirus (HPV) infection, while about 5% of cases are HPV-independent, and the latter is often associated with a poor prognosis.140 As for treatment, regular therapy for CC includes surgery, radiotherapy, chemotherapy, or a combination of these therapies. For recurrent or metastatic CC, first-line therapy involves platinum-containing combination regimens of chemotherapy as well as pembrolizumab in combination with chemotherapy. While for patients with progression after first-line therapy, other regimens like immunotherapy, pembrolizumab, tisotumab vedotin-tftv and cemiplimab are available as second-line treatment. However, the response rate is low, with a median progression-free survival (PFS) of only 3–6 months.16 Therefore, a number of PNPs for the treatment of CC are also being developed.
Chemotherapy
Platinum-containing chemotherapy is the standard regimen for CC treatment, but the risk of tumor progression and drug resistance should not be ignored.16,141 The use of synchronized radiotherapy is considered to bring additional clinical benefits,16,142 while there are many teams attempting to optimize CTAs and their carriers to improve the chemotherapeutic efficacy in CC.
Ir (III) complex is an antitumor agent that has received widespread attention for its excellent photophysical and photochemical properties as well as its ability to overcome the drawbacks of the commonly used CTA cisplatin. However, many studies have shown unsatisfactory therapeutic efficacy and severe side effects of Ir (III) complexes, which are related to their poor accumulation in tumors.96,143,144 Therefore Liang et al96 designed NPIr@Bp, a PNPs formed by self-assembly of Ir(III), dipyrrometheneboron difluoride (Bp) (a type of near-infrared PS) added to polymeric gemini NPs, and terminally capped PNPs with PEG. NPIr@Bp generates single-linear oxygen under near-infrared (NIR) light, which triggers the dissociation of nanostructures and activates the prodrug to achieve mitochondrial targeting. The CTA Ir accumulates in the mitochondria and thus achieves induction of apoptosis. In the cisplatin-resistant human CC mouse model, the application of 808 nm light irradiation of NPIr@Bp shows 95% tumor inhibition.
HA-modified PNPs can target CD44 receptors overexpressed on the surface of solid tumors, and the strong attraction of HA to CD44 promotes the internalization and retention of PNPs within tumor cells.97 And as a multifunctional transmembrane cell surface adhesion receptor, CD44 is involved in tumor progression by regulating signal transduction pathways and enhanced invasion.145 Anjum et al97 utilized HA-coated CS NPs loaded with CTA adriamycin (DOX) to overcome the systemic toxicity, lack of targeting, and other drawbacks of free DOX as well as to optimize the efficacy of DOX in CC therapy. Aluri’s team98 developed an l-tyrosine-based PNPs, which encapsulated DOX and camptothecin utilizing l-tyrosine-based amphiphilic poly(ester-urethane) self-assembled enzyme-responsive NPs. It was confirmed in CC HeLa cells that such l-tyrosine PNPs increased drug uptake and internalization by tumor cells compared to free DOX. Polydimethylsiloxane (PDMS) is one of polymers with organelle-targeting properties. Maparu et al99 proposed a novel strategy to use PDMS to produce soft NPs with a size of about 30 nm. They successfully utilized these NPs to deliver DOX to the nucleus and mitochondria of CC cells, and the IC50 value was reduced by more than four times compared with that of free DOX. The anticancer potential of PDMS NPs to specifically deliver drugs to the mitochondria and nucleus was also demonstrated by a series of experiments.
Immunotherapy
As an emerging therapeutic approach, some studies have suggested that immunotherapy has superior effect to surgery and radiotherapy in recurrent CC, however, the low tumor immunogenicity and targeting efficiency pose challenges for the application of immunotherapy in CC.146
Oncolytic virotherapy is a promising immunotherapy for CC. To address the rapid clearance of the virus by immune-neutralization in vivo, Kousar et al100 used thiolated CS NPs to encapsulate Newcastle disease virus (NDV) and modified the surface of the PNPs to attain active targeting of CD44 on the surface of CC cells. They found that such PNPs not only actively targeted cargo delivery, but also kept NDV away from the immune system and prolonged the release of NDV in the TME.
Therapeutic vaccines are also recognized as a promising therapy for HPV infection and CC. HPV E6 and E7 are ideal antigens to activate cell-mediated immune responses, but such peptide vaccines usually require adjuvants to attain the ideal efficacy.147 It has been reported that extracellular adenosine triphosphate (ATP) promotes DC recruitment as well as antigen uptake and presentation. And the binding of ATP to receptors on the surface of DCs can lead to the release of immunomodulators. Besides, ATP facilitates the maturation and homing of DCs. All of the above suggests that ATP has the potential to act as an adjuvant for therapeutic vaccines.101 In addition, particles with a size <500 nm are more easily internalized by DCs and more likely to stimulate CD8+ T cell responses.148 Therefore, Qishu Zhang et al101 used PLGA to encapsulate E7 peptide and carry the adjuvant ATP to form ATP-adjuvanted PNPs vaccine, which showed potent anti-tumor cellular immunity. The NPs formed by polymer PLGA in this nanovaccine play an important role in enhancing the stability, DC uptake, and lymph node accumulation of E7 peptide.
Polystyrene nanoparticles (PSNPs) are polymeric carriers with self-adjuvanting properties that induce antigen-specific CD8+ and CD4+ T cells. Moreover, it does not induce inflammation, pro-inflammatory cytokines, and expansion of inflammation reactive regulatory T cells, and can show superiority over conventional pro-inflammatory adjuvants in delivering protein-based antigens. Xiang et al102 utilized PSNPs to study peptide-based nanovaccines for CC and OC therapy.
Other Therapeutic Modalities, Medications
α-Mangostin is a natural product that derives from pericarps of mangosteen and is cytotoxic to a wide range of cancer cells. However, it has poor water solubility as well as a risk of oxidative degradation surrounding solid tumors. Therefore Suttithumsatid et al103 used TEMPO (a nitrogen oxide) with antioxidant properties as the side chain of amphiphilic copolymers and added silanol moiety to improve the encapsulation efficiency, self-assembled to form NanoAOX NPs for the delivery of α-Mangostin-rich extract (AME). It was proved that this PNPs could effectively inhibit the growth of HeLa cell lines and had a better safety profile than free AME.
In an attempt to develop anticancer drugs with higher efficacy and fewer side effects, six coumarin derivatives were designed by Arvas et al.104 And PLGA NPs were utilized as carriers to deliver compound 21 which has the lowest IC50 value among the six derivatives. The use of PLGA carriers provided better drug loading and controlled release of compound 21.
Curcumin is a plant-derived chemical with anticancer activity, but its drawback of poor water solubility should not be ignored. Poly(glycerol sebacate) (PGS) is another FDA-approved polymer made from the condensation of sebacic acid and glycerol and is biodegradable. Massironi et al105 encapsulated curcumin in PGS NPs to improve its bioavailability and absorption by oral administration, which showed higher ability to anti-HPV, cytotoxicity and activation of apoptosis in HeLa cells. Instead, Kavya et al106 utilized poly(methacryloyl beta-alanine) (PMBA) as the carrier to deliver curcumin. In this study, PMBA was polymerized from radicals in supercritical CO(2) and treated with FA. Both curcumin and Bcl2 siRNA were encapsulated in PMBA to form Poly@Cur-FA NPs for co-delivery of drugs and genes. Poly@Cur-FA stimulates autophagy and inhibits the growth of CC cells through activation of Bcl2 and multiple other signaling pathways, which may provide an idea for overcoming tumor drug resistance.
Ahmed et al107 studied CS and butyraldehyde together to form new modified CS derivative NPs called CS-3NPs in the presence of cross-linking agents. This new CS derivative has a size of less than 100 nm with a wide range of applications, an ideal stability with a zeta potential of 20±5.98 mV, an improved crystallinity, as well as the ability to induce apoptosis in Hela cells to achieve antitumor effects. And it shows therapeutic potential for CC.
Zhu et al108 developed gene-targeting technology-based PNPs for the treatment of CC. They utilized shRNA and CRISPR/Cas targeting to silence and knockdown the HPV16 E7 gene and delivered the associated nucleic acids using PBAE NPs. Their PNPs are intended for vaginal administration for the treatment of HPV infections, providing new ideas for the prevention and treatment of CC.
Endometrial Cancer
Endometrial cancer (EC) or more broadly known as carcinoma of the uterine corpus17 is an epithelial malignant tumor that occurs in the endometrium, which is also one of the three most common malignant tumors of the female reproductive system. The incidence of EC has been on the rise in recent years, with 420,368 newly diagnosed patients worldwide in 2022.117,149 As far as therapy is concerned, EC is usually treated with surgery, supplemented by a comprehensive approach of radiotherapy, chemotherapy and hormone therapy. It has also been reported that adding immunotherapy to chemotherapy for advanced or metastatic EC is beneficial for improving OS in patients.150
Earlier, Changyan Liang et al109 created a folate-modified PLGA NPs using PEG as a coupling agent to connect folate with PLGA, and encapsulated the CTA PTX in it. The modification of folate allows this PNPs to be internalized into EC cells through a folate receptor-mediated mechanism. And it was experimentally confirmed that PTX-loaded folate-targeted PNPs showed higher anti-tumor efficacy than free PTX, and the tumor inhibition rate of targeted PNPs was higher than that of non-targeted ones. Subsequently, there have also been many studies combining PNPs with CTAs used to improve the therapeutic efficacy of EC.
Jie Ding et al110 combined the CTA DOX with an anti-apoptotic gene Bcl-2 inhibitor (navitoclax) encapsulated in pH-sensitive poly(ethylene glycol)-poly(diisopropylamino)diethyl methacrylate (PEG-PDPA) NPs (NP@DOX/Nav) for the treatment of EC and delivered them to the tumor site through enhanced permeability and TME effects. And the NP@DOX/Nav showed a higher level of pro-apoptotic effects in the Ishikawa xenograft model than DOX or Nav alone.
Loss of function (LOF) p53 occurs in 80% of type II EC, may lead to EC resistance to chemotherapy. Recent studies have shown that CIP2b, a derivative of the fluoroquinolone antibiotic ciprofloxacin (CIP), exhibits cytotoxicity against various cancer cells. Its combination with PTX increases the accumulation of PTX in tumors and inhibits tumor growth.151 Naguib’s team111 designed CIP2b-NPs that encapsulate CIP2b in PLGA and use TPGS containing hydrophilic PEG chains as a surfactant, which aggregates at the tumor site through the EPR effect. The CIP2b-NPs were confirmed to increase the accumulation and cytotoxicity of PTX in Hec50co cells of EC. Moreover, the CIP2b-NPs were able to maintain a higher concentration in tumors for a longer period of time than soluble CIP2b alone, demonstrating its ability to synergize with PTX in the treatment of LOF p53 type II EC and to reduce chemotherapy resistance.
Kareem Ebeid’s team22 developed a PNP utilizing the combination of PTX and a tyrosine kinase inhibitor to synergistically induce LOF p53 cancer cell death by abrogating the G2/M checkpoint. The PNP was PLGA-based and carried PTX and the triple angiokinase molecular inhibitor (BIBF) to achieve co-delivery and increase the synthetically lethal to uterine serous carcinomas (USC) (a type II EC with low differentiation) cells. The application of these PNP carriers overcame the drawbacks of the low water solubility of PTX and BIBF, reduced the side effects due to off-target effects, and improved the pharmacokinetics.
Jiaolin Yang’s team112 designed NPs (CRZ@GEM-NPs) of a PCL-PEG-PCL triblock copolymer coupled with the CTA gemcitabine (GEM) and the protease inhibitor crizotinib (CRZ) to reduce the side effects of chemotherapy and improve their anti-tumor capabilities.
Hyperglycemia is associated with EC invasion and progression.152 Xiao Yang et al113 found that a high glucose environment could promote glucose metabolism reprogramming in EC cells by promoting the expression of pyruvate dehydrogenase kinase 1 (PDK1), while down-regulation of PDK1 significantly inhibited EC proliferation and invasion. Therefore, a reduction-sensitive polymer (P1) encapsulating a PDK1 inhibitor (JX06) was designed to form PNPs called JX06-NPs. JX06-NPs deliver small molecules of JX06 with limited water solubility to the tumor site via GSH-triggered release in TME. JX06 synergistically enhances antitumor effects with metformin, providing a novel adjuvant for the management of patients with both diabetes and EC.
Other Gynecological Cancers
Uterine sarcoma (US) is a very rare gynecological cancer that develops in the uterus. Compared to other uterine tumors, it has a poorer prognosis.153 Mostoufi et al114 designed a type of PTX-PNPs for multidrug-resistant US. They utilized the pH-responsive polymer poly(l-glutamic acid), the hydrophobic polymer poly(l-leucine), and PEG to form pH-sensitive copolymer NPs. This PNPs showed a 10-fold lower IC50 than free PTX in multidrug-resistant US cell line, as well as the ability to inhibit drug efflux and induce lysosomal membrane permeability.
Choriocarcinoma is a gynecological cancer that occurs after childbirth, miscarriage or gravida. Chemotherapy remains its first-line therapy, yet the non-selective drug distribution leads to serious side effects.153,154 The HCG81-NP created by Cong et al154 was aimed at solving this problem. The characteristic of choriocarcinoma overexpressing human chorionic gonadotropin (HCG) receptor was utilized by them to achieve targeted delivery of PNPs. They loaded CTA methotrexate using PEG-PLA and modified the PNPs with HCG. It was confirmed that the uptake of this PNPs was significantly increased in choriocarcinoma cell lines and showed higher inhibition of cell proliferation.
Polymer Drug Delivery Systems for Combination Therapy
In addition to the development of novel medicines, how existing therapies and drugs can be combined and reused is also an important research area. Evidence suggests that combination therapies work better against advanced tumors than single drugs or sequential drug combinations. On top of that, the heavy financial burden of new drug innovation and the uncertainty of efficacy have shifted our focus to the development of combination therapy strategies, even though this may contradict the interests of pharmaceutical companies.155,156 Although combination therapy is not a totally novel concept, its progress has been hampered in the past because of non-specific delivery of drugs, different pharmacokinetics of various agents, and other problems.157 With the increasing development of nanomedicine, chemotherapy, phototherapy, immunotherapy and other proven effective tumor therapies are combined to form new dual-mode or multi-mode therapeutic nano-systems, and the synergistic effects of tumor therapy are significantly improved.158 In the field of gynecological cancers treatment, several teams have created nanoplatforms for combination therapy such as chemotherapy and immunotherapy,159 chemotherapy and PDT160 or two-drug combination161, etc. Similarly, polymer-based nanosystems have been studied for gynecological cancers therapy, most of which are synergistic drug combinations,162 while several studies have involved combinations of diverse therapies.
High levels of nitric oxide (NO) gas on the one hand can react with ROS to produce tumor-killing reactive nitrogen species, and on the other hand may increase tumor sensitivity to chemotherapy, which provides a novel idea for synergistic treatment with chemotherapy and NO therapy.163–165 Based on the above, Guang Li’s team165 designed PSSP@ART-ISMN PNPs using GSH-responsive PNPs loaded with isosorbide 5-mononitrate (ISMN), a NO donor that can release NO, as well as a traditional Chinese medicine extract that promotes apoptosis and increases the concentration of ROS called Artesunate (ART). The synergistic therapeutic effect of PSSP@ART-ISMN was confirmed via in vivo experiments in SKOV3 tumor-bearing mice, which provides an innovative combination therapy strategy for OC.
PDT can be difficult to attain satisfactory efficacy because of insufficient ROS generation due to hypoxia in the TME. In this situation, it is necessary to combine PDT with other therapies, such as cold atmospheric plasma (CAP) therapy, to enhance the efficacy.36 CAP is an ionized gas near room temperature.166 Its antitumor effects through oxidative damage to DNA and cell membranes are most likely related to high concentrations of ROS and reactive nitrogen.36 Some studies have reported that CAP has a promising future in the treatment of gynecologic tumors.167,168 Ha and Kim36 prepared a PNPs named PPHE, which was formed by self-assembly of (Pheo a)-conjugated poly(γ-glutamic acid) and mPEG-PLGA to form the core of the PNP, and encapsulated the core with targeting ligand-modified HA (HA-EAE7). PPHE was released more readily in the acidic pH of the cellular lysosome than in the physiologic pH environment of blood, and showed enhanced phototoxicity against the CC CaSki cell line. They subsequently applied this HER3/CD44 dual-targeting PPHE in an in vitro experiment with combined PDT/CAP treatment, which showed enhanced ability to induce apoptosis in HPV-positive CC cells compared to free Pheo a or PDT alone.
Synthesis of Functional Polymers for Tumor Imaging and Therapy
Theranostics derives from the words diagnostic and therapeutic.169 Progress in nanomedicine has also made it possible to integrate the diagnosis and therapy of diseases on a single platform. In the last section, it was introduced that PNPs achieve synergistic treatment by carrying multiple therapeutic cargoes; similarly, co-delivery of therapeutic agents with contrast agents allows the integration of tumor treatment and imaging, as well as the simultaneous monitoring of treatment efficacy.158 The characteristics of PNPs, which can be easily modified, and their high drug-carrying capacity enable them to be a promising carrier for tumor theranostics. The strategy of integrating therapy with diagnosis is expected to provide novel personalized solutions with higher efficacy and safety for the treatment of tumors.170
In recent years, PAI has gained increasing attention in tumor detection due to its superior contrast, temporal and spatial resolution.171 The combination of phototherapy and PAI is one of the most widely studied strategies for the integration of diagnosis and therapy. PAI often can be applied concurrently with PTT, and PTA is usually suitable for PAI as well.
In PTT, the protective mechanisms of cancer cells are triggered at high temperatures. Therefore, to achieve cancer cell killing, heating above 50°C is required, but this can easily damage normal tissues. However, the milder conditions of 40–45°C may also increase the risk of tumor recurrence and spread.172 The efficacy of PTT is also influenced by the ability of the laser to penetrate tissues. The near-infrared-I (NIR-I) window (650–950 nm) has been studied a lot; while the near-infrared-II (NIR-II) window (1000–1700 nm) laser has attracted attention due to its deep-tissue penetration and less damage to normal tissues, and many related PTAs have been developed.35,173,174 In addition to the deep tumor treatment, NIR-II fluorescence imaging (FL) can also guide PTT through precise imaging.172
Lorenz’s team33 designed a novel PNP carried contrast agent to increase the diagnostic accuracy of PAI. They encapsulated two dyes that show excellent photoacoustic peaks in specific NIR regions in PEG-PCL NPs, which were intravenously injected and delivered to primary and metastatic lesions of OC. These PNPs produced well-separated absorption peaks when irradiated with 770 nm and 860 nm laser light, which could clearly distinguish the tumor from other tissues in the background; and when irradiated with 808 nm NIR laser light, PNPs can heat up the tumor to ≈49°C and kill the tumor, which enabled both PAI diagnosis and PTT treatment can be realized. Similarly, the PD-FA NPs prepared by Qiu et al175 enabled both NIR imaging and PAI-guided PTT.
Indocyanine Green (ICG) is an FDA-approved PTA as well as an excellent NIR fluorescent contrast agent, yet its poor stability limits its clinical application. Nuernisha’s team176 encapsulated ICGs with amphiphilic poly(styrene-co-maleic anhydride) (PSMA) to form PNPs and demonstrated its favorable biocompatibility and enhanced PTT efficiency in CC Hela cells. (Figure 2) Another study from this team177 utilized PSMA to encapsulate a new ICG (IR-820) that acts as a PTA and NIR fluorescent probe to enhance its biocompatibility. This IR-820@PSMA NP was confirmed to be uptaken by Hela cells under confocal microscopy and obtained a PTT efficiency of 77%. Their study suggests that theranostics PNPs can also be prepared with a single multifunctional cargo.
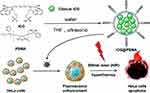 |
Figure 2 Schematic illustration of the synthesis process and PTT effect. Reprinted with permission from Chen S, Zhu L, Du Zet al Polymer encapsulated clinical ICG nanoparticles for enhanced photothermal therapy and NIR fluorescence imaging in cervical cancer. RSC Adv. 2021;11(34):20,850–20858. Copyright 2021 Royal Society of Chemistry.176 |
Apart from integration with phototherapy, PNPs have been developed in relation to other therapies. Guang Li’s team67 designed a nanoparticle called HA@PFG NPs, whose core is a PNP composed of Gossypol, a type of natural polyphenols, together with Fe3+ and Pt-COOH, a prodrug of the CTA cisplatin; and coated the outside of the PNP with HA, which can target tumor cells. When HA@PFG NPs were delivered into the TME, the acidic environment stimulated the breakage of pH-sensitive linkages to release Fe3+ and Pt-COOH. HA@PFG NPs exert cytotoxicity to suppress tumors through chemotherapy, Fe3+-induced iron death, and promotion of apoptosis by Gossypol. Fe3+ can also act as a contrast agent to enhance MRI visualization, allowing HA@PFG NPs to play a role in both diagnosis and treatment for OC.
Dragulska’s team178 has also developed a PNP that combines diagnostic imaging with targeted therapy, called the RGDFFF-CA4 NP. This PNP has a PLGA core, carries the vascular disrupting agent combretastatin A4 (CA4) used for OC treatment. Additionally, replaces the popular PEG coating on the surface of the PNPs with a short arginine-glycine-aspartic acid-phenylalanine x3 (RGDFFF) peptide to diminish immunogenicity and achieve targeting. What’s more, they also modified the surface of PNPs with NIR fluorophore label Cy7 for tumor imaging.
Challenges in Clinical Translation of PNPs
For clinical translation, in addition to Abraxane, which has been approved by the FDA for oncology treatment,179 few PNPs have been in clinical practice. For example, Paclical is approved by the EMA for OC therapy, as well as Genexol-PM is approved in South Korea for the treatment of breast cancer and non-small cell lung cancer.4 Besides, only a limited number of studies that have entered clinical trials, for example, CRLX101, a cyclodextrin-based PNP, whose first Phase 1/2a clinical trial results in OC were published in 2013,180 with several subsequent clinical trials completed alone or in combination.181,182 A systematic review showed that CRLX101 may bring higher efficacy and lower systemic toxicity to cancer therapy, but its safety and efficacy are still influenced by multiple factors.183 While another meta-analysis showed that CRLX101 improved patients’ median PFS compared to the control group, but delivered poorer median OS.184 Issues such as biological barriers, plasma stability, and immune clearance preventing the drug from reaching the desired therapeutic dose at the lesion site;185 as well as challenges in synthesizable scale-up such as production stability, green nanotechnology,18 are the core barriers faced by the clinical translation of PNPs.
Conclusions and Future Prospect
In conclusion, polymer is a promising material to prepare nanoparticles for tumor theranostics. The preparation of novel PNPs involves not only the modification and combination of the polymers themselves to optimize the circulation time, release mechanism and action site of the PNPs in vivo; but also the use of the polymers’ advantages to carry the freshly developed cargoes for improving the water solubility, biocompatibility, bioavailability and other properties of the cargoes. These advantages of PNPs could meet the urgent need for precision strategies in the management of gynecologic cancers. Especially in chemotherapy and immunotherapy for gynecological cancers, lots of novel regimens of PNPs have been proposed, which provide possibilities to better the prognosis of advanced gynecological cancers. In addition, PNPs can realize co-delivery by storing different agents in different layers/chambers in the particles respectively. These strategies for combination therapy as well as diagnostic and therapeutic integration will contribute to higher efficiency and lower economic burden for gynecological cancers theranostics.
Despite demonstrated preclinical success of several PNPs in cellular and animal models, their clinical translation remains challenging, with few candidates progressing to human trials. To bridge this translational gap, critical parameters must be addressed during PNP design and development: (1) Biosafety assurance and scalable synthesis protocols; (2) Wider therapeutic window and lower dosing frequency; (3) Tumor-selective targeting with mitigated off-tissue effects.
Future target of design PNPs could be shifted to the early diagnosis of tumors, especially for OC, which is diagnosed at advanced stages in most patients.186 PNPs carry contrast agents to precisely target tiny malignant lesions and provide imaging evidence for very early diagnosis of gynecological cancers. In terms of gynecological cancers, the female reproductive tract, as a unique route of administration, perhaps can replace the traditional ones to enhance gynecological cancers therapeutic efficiency. For example, the intrauterine device (IUD) is a common controlled release device for drugs used in ovarian endocrine disorders. Corrie et al187 proposed the concept of controlled release of PS-loaded PNPs through IUDs for PDT and FL. Similarly, such integration of gynecological cancers-targeted PNPs with IUD-based delivery platforms may establish a clinically viable strategy for precision intervention. Furthermore, the versatile surface functionalization capacity of PNPs provides a robust foundation for the further development of personalized therapy and intelligent NPs.
Acknowledgments
The authors thank all the reviewers for their constructive evaluations which have improved this manuscript.
Funding
This research was funded by National Key Research and Development Program of China (2023YFC2706003 and 2023YFC2706001).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36(7):887–913. doi:10.1016/j.progpolymsci.2011.01.001
2. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
3. Lu XY, Wu DC, Li ZJ, Chen GQ. Polymer nanoparticles. Prog Mol Biol Transl Sci. 2011;104:299–323. doi:10.1016/B978-0-12-416020-0.00007-3
4. Beach MA, Nayanathara U, Gao Y, et al. Polymeric nanoparticles for drug delivery. Chem Rev. 2024;124(9):5505–5616. doi:10.1021/acs.chemrev.3c00705
5. Crucho CIC, Barros MT. Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater Sci Eng C. 2017;80:771–784. doi:10.1016/j.msec.2017.06.004
6. Manning AN, Rowlands CE, Saindon H, Givens BE. Tuning the emulsion properties influences the size of poly(caprolactone) particles for drug delivery applications. AAPS J. 2023;25(6):100. doi:10.1208/s12248-023-00869-4
7. Sun H, Zhong Z. Bioresponsive Polymeric Nanoparticles: from Design, Targeted Therapy to Cancer Immunotherapy. Biomacromolecules. 2025;26(1):33–42. doi:10.1021/acs.biomac.4c01257
8. Wahengbam GS, Nirmal S, Nandwana J, et al. Polymeric nanoparticles revolutionizing brain cancer therapy: a comprehensive review of strategies and advances. Crit Rev Ther Drug Carrier Syst. 2025;42(2):73–106. doi:10.1615/CritRevTherDrugCarrierSyst.2024051822
9. Fang G, Hao P, Qiao R, et al. Stimuli-responsive chitosan based nanoparticles in cancer therapy and diagnosis: a review. Int J Biol Macromol. 2024;283(Pt 3):137709. doi:10.1016/j.ijbiomac.2024.137709
10. Soumya RS, Raghu KG. Recent advances on nanoparticle-based therapies for cardiovascular diseases. J Cardiol. 2023;81(1):10–18. doi:10.1016/j.jjcc.2022.02.009
11. Smith BR, Edelman ER. Nanomedicines for cardiovascular disease. Nat Cardiovasc Res. 2023;2(4):351–367. doi:10.1038/s44161-023-00232-y
12. Dong C, Wang Y, Zhu W, et al. Polycationic HA/CpG nanoparticles induce cross-protective influenza immunity in mice. ACS Appl Mater Interfaces. 2022;14(5):6331–6342. doi:10.1021/acsami.1c19192
13. Lin LCW, Chattopadhyay S, Lin JC, Cmj H. Advances and opportunities in nanoparticle- and nanomaterial-based vaccines against bacterial infections. Adv Healthcare Mater. 2018;7(13):1701395. doi:10.1002/adhm.201701395
14. McCluggage WG, Singh N, Gilks CB. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology. 2022;80(5):762–778. doi:10.1111/his.14609
15. Liu J, Berchuck A, Backes FJ, et al. NCCN guidelines® insights: ovarian cancer/fallopian tube cancer/primary peritoneal cancer, version 3.2024. J Natl Compr Canc Netw. 2024;22(8):512–519. doi:10.6004/jnccn.2024.0052
16. Abu-Rustum NR, Yashar CM, Arend R, et al. NCCN guidelines® insights: cervical cancer, version 1.2024. J Natl Compr Canc Netw. 2023;21(12):1224–1233. doi:10.6004/jnccn.2023.0062
17. Abu-Rustum N, Yashar C, Arend R, et al. Uterine neoplasms, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21(2):181–209. doi:10.6004/jnccn.2023.0006
18. Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M. Scale-up polymeric-based nanoparticles drug delivery systems: development and challenges. OpenNano. 2022;7:100048. doi:10.1016/j.onano.2022.100048
19. Cukierman E, Khan DR. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem Pharmacol. 2010;80(5):762–770. doi:10.1016/j.bcp.2010.04.020
20. Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(1):179–192. doi:10.1016/S0378-5173(01)00986-3
21. Banik B, Ashokan A, Choi JH, Surnar B, Dhar S. Platin-C containing nanoparticles: a recipe for the delivery of curcumin-cisplatin combination chemotherapeutics to mitochondria. Dalton Trans. 2023;52(12):3575–3585. doi:10.1039/d2dt03149c
22. Ebeid K, Meng X, Thiel KW, et al. Synthetically lethal nanoparticles for treatment of endometrial cancer. Nat Nanotechnol. 2018;13(1):72–81. doi:10.1038/s41565-017-0009-7
23. Yang T, Guo L. Advancing gastric cancer treatment: nanotechnology innovations and future prospects. Cell Biol Toxicol. 2024;40(1):101. doi:10.1007/s10565-024-09943-9
24. Liu Y, Xue Y, Tang J, et al. Porphyrin–camptothecin (CPT) grafted polyoxazoline amphiphiles for tumor photodynamic–chemotherapy combination treatment. ACS Appl Mater Interfaces. 2024;16(47):64617–64627. doi:10.1021/acsami.4c17267
25. El Moukhtari SH, Garbayo E, Amundarain A, et al. Lipid nanoparticles for siRNA delivery in cancer treatment. J Control Release. 2023;361:130–146. doi:10.1016/j.jconrel.2023.07.054
26. Parayath NN, Stephan SB, Koehne AL, Nelson PS, Stephan MT. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11(1):6080. doi:10.1038/s41467-020-19486-2
27. Li Z, Amaya L, Pi R, et al. Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism. Nat Commun. 2023;14(1):6983. doi:10.1038/s41467-023-42672-x
28. Hu B, Zhong L, Weng Y, et al. Therapeutic siRNA: state of the art. Sig Transduct Target Ther. 2020;5(1):1–25. doi:10.1038/s41392-020-0207-x
29. Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14(12):843–856. doi:10.1038/nrd4685
30. Gralewska P, Gajek A, Marczak A, Rogalska A. Targeted nanocarrier-based drug delivery strategies for improving the therapeutic efficacy of PARP inhibitors against ovarian cancer. Int J Mol Sci. 2024;25(15):8304. doi:10.3390/ijms25158304
31. Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. IJN. 2015;10(1):1727–1741. doi:10.2147/IJN.S76501
32. Mao X, Xu J, Cui H. Functional nanoparticles for magnetic resonance imaging. WIREs Nanomed Nanobi. 2016;8(6):814–841. doi:10.1002/wnan.1400
33. St Lorenz A, Moses AS, Mamnoon B, et al. A photoacoustic contrast nanoagent with a distinct spectral signature for ovarian cancer management. Adv Healthc Mater. 2023;12(9):e2202946. doi:10.1002/adhm.202202946
34. Shi M, Li Y, Pan W, et al. A BRD4-targeting photothermal agent for controlled protein degradation. Angew Chem Int Ed. 2024;63(29):e202403258. doi:10.1002/anie.202403258
35. Pu Y, Wu W, Zhou B, et al. Starvation therapy enabled “switch-on” NIR-II photothermal nanoagent for synergistic in situ photothermal immunotherapy. Nano Today. 2022;44:101461. doi:10.1016/j.nantod.2022.101461
36. Ha JH, Kim YJ. Photodynamic and cold atmospheric plasma combination therapy using polymeric nanoparticles for the synergistic treatment of cervical cancer. Int J Mol Sci. 2021;22(3):1172. doi:10.3390/ijms22031172
37. Gugu Nkosi PW, Chandran R, Abrahamse H. Hypocrellin: a natural photosensitizer and nano-formulation for enhanced molecular targeting of PDT of Melanoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024;16(6):e1997. doi:10.1002/wnan.1997
38. Domínguez-Ríos R, Sánchez-Ramírez DR, Ruiz-Saray K, et al. Cisplatin-loaded PLGA nanoparticles for HER2 targeted ovarian cancer therapy. Colloids Surf B. 2019;178:199–207. doi:10.1016/j.colsurfb.2019.03.011
39. Sader D, Zlotver I, Moya S, Calabrese GC, Sosnik A. Doubly self-assembled dermatan sulfate/chitosan nanoparticles for targeted siRNA delivery in cancer therapy. J Colloid Interface Sci. 2025;680(Pt B):763–775. doi:10.1016/j.jcis.2024.11.132
40. Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C. 2019;98:1252–1276. doi:10.1016/j.msec.2019.01.066
41. Liu D, Lu N, Zang F, et al. Magnetic resonance imaging-based radiogenomic analysis reveals genomic determinants for nanoparticle delivery into tumors. ACS Nano. 2024;18(51):34615–34629. doi:10.1021/acsnano.4c09387
42. Abed HF, Abuwatfa WH, Husseini GA. Redox-responsive drug delivery systems: a chemical perspective. Nanomaterials. 2022;12(18):3183. doi:10.3390/nano12183183
43. Cao Z, Li W, Liu R, et al. pH- and enzyme-triggered drug release as an important process in the design of anti-tumor drug delivery systems. Biomed Pharmacother. 2019;118:109340. doi:10.1016/j.biopha.2019.109340
44. Lu Q, Kou D, Lou S, et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J Hematol Oncol. 2024;17(1):16. doi:10.1186/s13045-024-01535-8
45. Wei D, Sun Y, Zhu H, Fu Q. Stimuli-responsive polymer-based nanosystems for cancer theranostics. ACS Nano. 2023;17(23):23223–23261. doi:10.1021/acsnano.3c06019
46. Wang Y, Jiang Y, Wei D, et al. Nanoparticle-mediated convection-enhanced delivery of a DNA intercalator to gliomas circumvents temozolomide resistance. Nat Biomed Eng. 2021;5(9):1048–1058. doi:10.1038/s41551-021-00728-7
47. Yu L, Luo Z, Chen T, et al. Bioadhesive nanoparticles for local drug delivery. Int J Mol Sci. 2022;23(4):2370. doi:10.3390/ijms23042370
48. George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: a review. Int J Pharm. 2019;561:244–264. doi:10.1016/j.ijpharm.2019.03.011
49. Zhang E, Xing R, Liu S, Qin Y, Li K, Li P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019;222:115004. doi:10.1016/j.carbpol.2019.115004
50. Jean M, Alameh M, De Jesus D, et al. Chitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetes. Eur J Pharm Sci. 2012;45(1):138–149. doi:10.1016/j.ejps.2011.10.029
51. Liu X, Guo Y, Pan J, et al. Nanoparticles constructed from natural polyphenols are used in acute kidney injury. J Mater Chem B. 2024;12(36):8883–8896. doi:10.1039/d4tb00837e
52. Lee EJ, Lim KH. Hardly water-soluble drug-loaded gelatin nanoparticles sustaining a slow release: preparation by novel single-step O/W/O emulsion accompanying solvent diffusion. Bioprocess Biosyst Eng. 2017;40(11):1701–1712. doi:10.1007/s00449-017-1825-8
53. Zalba S, ten Hagen TLM, Burgui C, Garrido MJ. Stealth nanoparticles in oncology: facing the PEG dilemma. J Control Release. 2022;351:22–36. doi:10.1016/j.jconrel.2022.09.002
54. Zhang W, Mehta A, Tong Z, Esser L, Voelcker NH. Development of polymeric nanoparticles for blood–brain barrier transfer—strategies and challenges. Adv. Sci. 2021;8(10):2003937. doi:10.1002/advs.202003937
55. Boltnarova B, Kubackova J, Skoda J, et al. PLGA based nanospheres as a potent macrophage-specific drug delivery system. Nanomaterials. 2021;11(3):749. doi:10.3390/nano11030749
56. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B. 2010;75(1):1–18. doi:10.1016/j.colsurfb.2009.09.001
57. Casalini T, Rossi F, Castrovinci A, Perale G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front Bioeng Biotechnol. 2019;7. doi:10.3389/fbioe.2019.00259.
58. Thomas TJ, Tajmir-Riahi HA, Pillai CKS. Biodegradable polymers for gene delivery. Molecules. 2019;24(20):3744. doi:10.3390/molecules24203744
59. Younes I, Hajji S, Frachet V, Rinaudo M, Jellouli K, Nasri M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int J Biol Macromol. 2014;69:489–498. doi:10.1016/j.ijbiomac.2014.06.013
60. Ali IH, Al-Tabakha MM, Khalil IA. Loratadine loaded chitosan tannic acid nanoparticles as anti-proliferative agent against breast cancer: in-silico, in-vitro and cell studies. Int J Nanomed. 2024;19:12483–12504. doi:10.2147/IJN.S483667
61. Helmi O, Elshishiny F, Mamdouh W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int J Biol Macromol. 2021;184:325–338. doi:10.1016/j.ijbiomac.2021.06.014
62. Mujtaba MA, Alotaibi NM, Alshehri SM, et al. Novel therapeutic approach in pegylated chitosan nanoparticles of apigenin for the treatment of cancer via oral nanomedicine. Polymers. 2022;14(20):4344. doi:10.3390/polym14204344
63. Al-Shadidi JRMH, Al-Shammari S, Al-Mutairi D, Alkhudhair D, Thu HE, Hussain Z. Chitosan nanoparticles for targeted cancer therapy: a review of stimuli-responsive, passive, and active targeting strategies. Int J Nanomed. 2024;19:8373–8400. doi:10.2147/IJN.S472433
64. Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170(3):365–372. doi:10.1016/j.jconrel.2013.05.041
65. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. JCO. 2005;23(31):7794–7803. doi:10.1200/JCO.2005.04.937
66. Spada A, Emami J, Tuszynski JA, Lavasanifar A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol Pharm. 2021;18(5):1862–1894. doi:10.1021/acs.molpharmaceut.1c00046
67. Li G, Shi S, Tan J, et al. Highly efficient synergistic chemotherapy and magnetic resonance imaging for targeted ovarian cancer therapy using hyaluronic acid-coated coordination polymer nanoparticles. Adv Sci. 2024;11(41):e2309464. doi:10.1002/advs.202309464
68. Dai J, Dong X, Wang Q, Lou X, Xia F, Wang S. PEG-polymer encapsulated aggregation-induced emission nanoparticles for tumor theranostics. Adv Healthcare Mater. 2021;10(24):2101036. doi:10.1002/adhm.202101036
69. Chen BM, Cheng TL, Roffler SR. Polyethylene glycol immunogenicity: theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano. 2021;15(9):14022–14048. doi:10.1021/acsnano.1c05922
70. Guo X, Zuo X, Zhou Z, et al. PLGA-based micro/nanoparticles: an overview of their applications in respiratory diseases. Int J Mol Sci. 2023;24(5):4333. doi:10.3390/ijms24054333
71. Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27(9):1735–1740. doi:10.1016/j.biomaterials.2005.09.019
72. Ma Y, Zheng Y, Zeng X, et al. Novel docetaxel-loaded nanoparticles based on PCL-Tween 80 copolymer for cancer treatment. Int J Nanomed. 2011;6:2679–2688. doi:10.2147/IJN.S25251
73. Boyacıoğlu Ö, Varan C, Bilensoy E, et al. A novel injectable nanotherapeutic platform increasing the bioavailability and anti-tumor efficacy of Arachidonylcyclopropylamide on an ectopic non-small cell lung cancer xenograft model: a randomized controlled trial. Int J Pharm. 2025;670:125153. doi:10.1016/j.ijpharm.2024.125153
74. Li L, Gatto GJ, Brand RM, et al. Long-acting biodegradable implant for sustained delivery of antiretrovirals (ARVs) and hormones. J Control Release. 2021;340:188–199. doi:10.1016/j.jconrel.2021.10.021
75. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347–355. doi:10.1016/j.msec.2018.11.035
76. Antonio E, Dos Reis Antunes Junior O, Rgdjv M, et al. Chitosan modified poly (lactic acid) nanoparticles increased the ursolic acid oral bioavailability. Int J Biol Macromol. 2021;172:133–142. doi:10.1016/j.ijbiomac.2021.01.041
77. Coolen AL, Lacroix C, Mercier-Gouy P, et al. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials. 2019;195:23–37. doi:10.1016/j.biomaterials.2018.12.019
78. Tzeng ST, Guerrero-Cázares H, Martinez EE, Sunshine JC, Quiñones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on poly(β-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–5410. doi:10.1016/j.biomaterials.2011.04.016
79. Lee Y, Mo H, Koo H, et al. Visualization of the degradation of a disulfide polymer, linear poly(ethylenimine sulfide), for gene delivery. Bioconjugate Chem. 2007;18(1):13–18. doi:10.1021/bc060113t
80. Wu Y, Yu J, Liu Y, et al. Delivery of EZH2-shRNA with mPEG-PEI nanoparticles for the treatment of prostate cancer in vitro. IntJ Mol Med. 2014;33(6):1563–1569. doi:10.3892/ijmm.2014.1724
81. Pillai SC, Borah A, Jindal A, Jacob EM, Yamamoto Y, Kumar DS. BioPerine encapsulated nanoformulation for overcoming drug-resistant breast cancers. As J Pharm Sci. 2020;15(6):701–712. doi:10.1016/j.ajps.2020.04.001
82. Ferreira Soares DC, Domingues SC, Viana DB, Tebaldi ML. Polymer-hybrid nanoparticles: current advances in biomedical applications. Biomed Pharmacother. 2020;131:110695. doi:10.1016/j.biopha.2020.110695
83. Farinha D, Sarmento-Ribeiro AB, Faneca H. Combination of gene therapy and chemotherapy in a new targeted hybrid nanosystem to hepatocellular carcinoma. Int J Nanomed. 2024;19:12505–12527. doi:10.2147/IJN.S474665
84. Kong N, Tao W, Ling X, et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci, trans med. 2019;11(523):eaaw1565. doi:10.1126/scitranslmed.aaw1565
85. Le ND, Nguyen BL, Patil BR, et al. Antiangiogenic therapeutic mRNA delivery using lung-selective polymeric nanomedicine for lung cancer treatment. ACS Nano. 2024;18(11):8392–8410. doi:10.1021/acsnano.3c13039
86. Sun X, Zhao R, Zhao E, Wang Q, Lian W, Xiong J. Targeting CD44-positive ovarian cancers via engineered paclitaxel prodrug nanoparticles for enhanced chemotherapeutic efficacy. Biomed Pharmacother. 2022;154:113655. doi:10.1016/j.biopha.2022.113655
87. Teo PY, Yang C, Whilding LM, et al. Ovarian Cancer Immunotherapy Using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance t cell killing. Adv Healthcare Mater. 2015;4(8):1180–1189. doi:10.1002/adhm.201500089
88. Zhang F, Parayath NN, Ene CI, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10(1):3974. doi:10.1038/s41467-019-11911-5
89. Cuenca-Escalona J, Bödder J, Subtil B, et al. EP2/EP4 targeting prevents tumor-derived PGE2-mediated immunosuppression in cDC2s. J Leukoc Biol. 2024;116(6):1554–1567. doi:10.1093/jleuko/qiae164
90. Li Y, Cen Y, Fang Y, et al. Breaking the iron homeostasis: a “trojan horse” self-assembled nanodrug sensitizes homologous recombination proficient ovarian cancer cells to PARP inhibition. ACS Nano. 2022;16(8):12786–12800. doi:10.1021/acsnano.2c04956
91. Wang W, Xiong Y, Hu X, et al. Codelivery of adavosertib and olaparib by tumor-targeting nanoparticles for augmented efficacy and reduced toxicity. Acta Biomater. 2023;157:428–441. doi:10.1016/j.actbio.2022.12.021
92. Juan A, Del M N-LM, Bravo I, et al. Enhanced antitumoral activity of encapsulated BET inhibitors when combined with PARP inhibitors for the treatment of triple-negative breast and ovarian cancers. Cancers. 2022;14(18):4474. doi:10.3390/cancers14184474
93. Ozel B, Sanlier S, Gunduz C, Selvi Gunel N. Preparation of dual drug-loaded polymer nanoconjugate to enhance treatment efficacy for ovarian cancer cells. Eur J Pharm Biopharm. 2024;204:114526. doi:10.1016/j.ejpb.2024.114526
94. Justino IA, Marincek A, Ferreira IRS, et al. Brazilian red propolis extract free and encapsulated into polymeric nanoparticles against ovarian cancer: formulation, characterisation and biological assays in 2D and 3D models. J Pharm Pharmacol. 2023;75(6):806–818. doi:10.1093/jpp/rgad030
95. Patra A, Satpathy S, Naik PK, Kazi M, Hussain MD. Folate receptor-targeted PLGA-PEG nanoparticles for enhancing the activity of genistein in ovarian cancer. Artif Cells Nanomed Biotechnol. 2022;50(1):228–239. doi:10.1080/21691401.2022.2118758
96. Liang G, Montesdeoca N, Tang D, et al. Facile one-pot synthesis of Ir(III) Bodipy polymeric gemini nanoparticles for tumor selective NIR photoactivated anticancer therapy. Biomaterials. 2024;309:122618. doi:10.1016/j.biomaterials.2024.122618
97. Anjum S, Akhtar A, Aldaqal SM, et al. Enhanced targeted treatment of cervical cancer using nanoparticle-based doxycycline delivery system. Sci Rep. 2025;15(1):2318. doi:10.1038/s41598-024-84203-8
98. Aluri R, Jayakannan M. Development of l-tyrosine-based enzyme-responsive amphiphilic poly(ester-urethane) nanocarriers for multiple drug delivery to cancer cells. Biomacromolecules. 2017;18(1):189–200. doi:10.1021/acs.biomac.6b01476
99. Maparu AK, Singh P, Rai B, Sharma A, Sivakumar S. A simple, robust and scalable route to prepare sub-50 nm soft PDMS nanoparticles for intracellular delivery of anticancer drugs. Nanotechnology. 2022;33(49):495102. doi:10.1088/1361-6528/ac8d99
100. Kousar K, Naseer F, Abduh MS, Anjum S, Ahmad T. CD44 targeted delivery of oncolytic Newcastle disease virus encapsulated in thiolated chitosan for sustained release in cervical cancer: a targeted immunotherapy approach. Front Immunol. 2023;14:1175535. doi:10.3389/fimmu.2023.1175535
101. Zhang Q, Huang W, Yuan M, et al. Employing ATP as a new adjuvant promotes the induction of robust antitumor cellular immunity by a PLGA nanoparticle vaccine. ACS Appl Mater Interfaces. 2020;12(49):54399–54414. doi:10.1021/acsami.0c15522
102. Xiang SD, Wilson KL, Goubier A, Heyerick A, Plebanski M. Design of peptide-based nanovaccines targeting leading antigens from gynecological cancers to induce HLA-A2.1 restricted CD8+ T cell responses. Front Immunol. 2018;9:2968. doi:10.3389/fimmu.2018.02968
103. Suttithumsatid W, Toriumi T, Sukketsiri W, Nagasaki Y, Panichayupakaranant P. Enhanced stability of α-mangostin-rich extract and selective cytotoxicity against cancer cells via encapsulation in antioxidant nanoparticles (AME@NanoAOX). ACS Biomater Sci Eng. 2024;10(8):5027–5038. doi:10.1021/acsbiomaterials.4c00997
104. Arvas B, Ucar B, Acar T, et al. Synthesis of novel coumarin-triazole hybrids and first evaluation of the 4-phenyl substituted hybrid loaded PLGA nanoparticles delivery system to the anticancer activity. Nanotechnology. 2024;35(30):305602. doi:10.1088/1361-6528/ad403e
105. Massironi A, Marzorati S, Marinelli A, et al. Synthesis and characterization of curcumin-loaded nanoparticles of poly(glycerol sebacate): a novel highly stable anticancer system. Molecules. 2022;27(20):6997. doi:10.3390/molecules27206997
106. Kavya KV, Vargheese S, Shukla S, et al. A cationic amino acid polymer nanocarrier synthesized in supercritical CO2 for co-delivery of drug and gene to cervical cancer cells. Colloids Surf B Biointerfaces. 2022;216:112584. doi:10.1016/j.colsurfb.2022.112584
107. Ahmed ME, Mohamed MI, Ahmed HY, Elaasser MM, Kandile NG. Fabrication and characterization of unique sustain modified chitosan nanoparticles for biomedical applications. Sci Rep. 2024;14(1):13869. doi:10.1038/s41598-024-64017-4
108. Zhu D, Shen H, Tan S, et al. Nanoparticles based on poly (β-Amino Ester) and HPV16-Targeting CRISPR/shRNA as potential drugs for HPV16-related cervical malignancy. Mol Ther. 2018;26(10):2443–2455. doi:10.1016/j.ymthe.2018.07.019
109. Liang C, Yang Y, Ling Y, Huang Y, Li T, Li X. Improved therapeutic effect of folate-decorated PLGA-PEG nanoparticles for endometrial carcinoma. Bioorg Med Chem. 2011;19(13):4057–4066. doi:10.1016/j.bmc.2011.05.016
110. Ding J, Zhang X, Chen C, Huang Y, Yu X, Li X. Ultra pH-sensitive polymeric nanovesicles co-deliver doxorubicin and navitoclax for synergetic therapy of endometrial carcinoma. Biomater Sci. 2020;8(8):2264–2273. doi:10.1039/d0bm00112k
111. Naguib YW, Alhaj-Suliman SO, Wafa EI, et al. Ciprofloxacin derivative-loaded nanoparticles synergize with paclitaxel against type II human endometrial cancer. Small. 2024;20(41):e2302931. doi:10.1002/smll.202302931
112. Yang J, Guo H, Lei J, et al. Fabrication of polymer-based self-assembly nanocarriers loaded with a crizotinib and gemcitabine: potential therapeutics for the treatment of endometrial cancer. J Biomater Sci Polym Ed. 2022;33(1):20–34. doi:10.1080/09205063.2021.1974149
113. Yang X, Cheng Y, Zhou J, et al. Targeting cancer metabolism plasticity with JX06 nanoparticles via inhibiting PDK1 combined with metformin for endometrial cancer patients with diabetes. Adv Sci. 2022;9(8):2104472. doi:10.1002/advs.202104472
114. Mostoufi H, Yousefi G, Tamaddon A-M, Firuzi O. Tamaddon A mohammad, Firuzi O. Reversing multi-drug tumor resistance to Paclitaxel by well-defined pH-sensitive amphiphilic polypeptide block copolymers via induction of lysosomal membrane permeabilization. Colloids Surf B. 2019;174:17–27. doi:10.1016/j.colsurfb.2018.10.072
115. Li Y, Li Z, Li Y, et al. Genetics of female pelvic organ prolapse: up to date. Biomolecules. 2024;14(9):1097. doi:10.3390/biom14091097
116. Konstantinopoulos PA, Matulonis UA. Clinical and translational advances in ovarian cancer therapy. Nat Cancer. 2023;4(9):1239–1257. doi:10.1038/s43018-023-00617-9
117. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
118. Wang L, Wang X, Zhu X, et al. Drug resistance in ovarian cancer: from mechanism to clinical trial. Mol Cancer. 2024;23(1):66. doi:10.1186/s12943-024-01967-3
119. Bayraktar E, Chen S, Corvigno S, Liu J, Sood AK. Ovarian cancer metastasis: looking beyond the surface. Cancer Cell. 2024;42(10):1631–1636. doi:10.1016/j.ccell.2024.08.016
120. Dai W, Zhou J, Chen T. Unraveling the extracellular vesicle network: insights into ovarian cancer metastasis and chemoresistance. Mol Cancer. 2024;23(1):201. doi:10.1186/s12943-024-02103-x
121. Ovarian cancer/fallopian tube cancer/primary peritoneal cancer guidelines detail. NCCN. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1453.
122. Mosca L, Ilari A, Fazi F, Assaraf YG, Colotti G. Taxanes in cancer treatment: activity, chemoresistance and its overcoming. Drug Resist Updat. 2021;54:100742. doi:10.1016/j.drup.2020.100742
123. Ying N, Liu S, Zhang M, et al. Nano delivery system for paclitaxel: recent advances in cancer theranostics. Colloids Surf B Biointerfaces. 2023;228:113419. doi:10.1016/j.colsurfb.2023.113419
124. Morand S, Devanaboyina M, Staats H, Stanbery L, Nemunaitis J. Ovarian cancer immunotherapy and personalized medicine. Int J Mol Sci. 2021;22(12):6532. doi:10.3390/ijms22126532
125. Wang H, Yung MM, Xuan Y, et al. Polyunsaturated fatty acids promote M2-like TAM deposition via dampening RhoA-YAP1 signaling in the ovarian cancer microenvironment. Exp Hematol Oncol. 2024;13(1):90. doi:10.1186/s40164-024-00558-8
126. Schweer D, McAtee A, Neupane K, Richards C, Ueland F, Kolesar J. Tumor-associated macrophages and ovarian cancer: implications for therapy. Cancers. 2022;14(9):2220. doi:10.3390/cancers14092220
127. Thumkeo D, Punyawatthananukool S, Prasongtanakij S, et al. PGE2-EP2/EP4 signaling elicits immunosuppression by driving the mregDC-Treg axis in inflammatory tumor microenvironment. Cell Rep. 2022;39(10):110914. doi:10.1016/j.celrep.2022.110914
128. Hong DS, Parikh A, Shapiro GI, et al. First-in-human Phase I study of immunomodulatory E7046, an antagonist of PGE2-receptor E-type 4 (EP4), in patients with advanced cancers. J Immunother Cancer. 2020;8(1):e000222. doi:10.1136/jitc-2019-000222
129. Hage Chehade C, Gebrael G, Sayegh N, et al. A pan-tumor review of the role of poly(adenosine diphosphate ribose) polymerase inhibitors. Ca a Cancer J Clin. 2025;75:141–167. doi:10.3322/caac.21870
130. Hardesty MM, Krivak TC, Wright GS, et al. OVARIO Phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecologic Oncol. 2022;166(2):219–229. doi:10.1016/j.ygyno.2022.05.020
131. DiSilvestro P, Banerjee S, Colombo N, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 Trial. JCO. 2023;41(3):609–617. doi:10.1200/JCO.22.01549
132. Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2021;22(12):1721–1731. doi:10.1016/S1470-2045(21)00531-3
133. Fang Y, McGrail DJ, Sun C, et al. Sequential Therapy with PARP and WEE1 inhibitors minimizes toxicity while maintaining efficacy. Cancer Cell. 2019;35(6):851–867.e7. doi:10.1016/j.ccell.2019.05.001
134. Yang L, Zhang Y, Shan W, et al. Repression of BET activity sensitizes homologous recombination–proficient cancers to PARP inhibition. Sci, trans med. 2017;9(400):eaal1645. doi:10.1126/scitranslmed.aal1645
135. Wang Z, Chen W, Zuo L, et al. The Fibrillin‐1/VEGFR2/STAT2 signaling axis promotes chemoresistance via modulating glycolysis and angiogenesis in ovarian cancer organoids and cells. Cancer Commun. 2022;42(3):245–265. doi:10.1002/cac2.12274
136. Wang M, Yuan C, Wu Z, et al. Paris saponin VII reverses resistance to PARP inhibitors by regulating ovarian cancer tumor angiogenesis and glycolysis through the RORα/ECM1/VEGFR2 signaling axis. Int J Biol Sci. 2024;20(7):2454–2475. doi:10.7150/ijbs.91861
137. Chae CS, Sandoval TA, Hwang SM, et al. Tumor-derived lysophosphatidic acid blunts protective type I interferon responses in ovarian cancer. Cancer Discovery. 2022;12(8):1904–1921. doi:10.1158/2159-8290.CD-21-1181
138. Ali E, Helmy MW, Radwan EH, et al. Evaluation of the cytotoxic activity of chemically characterized propolis originating from different geographic regions and vitamin D co-supplementation against human ovarian cancer cells. J Ovarian Res. 2024;17(1):181. doi:10.1186/s13048-024-01500-6
139. Banzato TP, Gubiani JR, Bernardi DI, et al. Antiproliferative flavanoid dimers isolated from Brazilian red propolis. J Nat Prod. 2020;83(6):1784–1793. doi:10.1021/acs.jnatprod.9b01136
140. Fernandes A, Viveros-Carreño D, Hoegl J, Ávila M, Pareja R. Human papillomavirus-independent cervical cancer. Int J Gynecological Cancer. 2022;32(1):1–7. doi:10.1136/ijgc-2021-003014
141. Tewari KS, Monk BJ, Vergote I, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med. 2022;386(6):544–555. doi:10.1056/NEJMoa2112187
142. Chen YY, Huang YT. Comparison of two platinum-containing chemotherapy regimens in the treatment of advanced cervical cancer with concurrent chemoradiotherapy. Ann Med. 2024;56(1):2371008. doi:10.1080/07853890.2024.2371008
143. Paprocka R, Wiese-Szadkowska M, Janciauskiene S, Kosmalski T, Kulik M, Helmin-Basa A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022;452:214307. doi:10.1016/j.ccr.2021.214307
144. He L, Tan C, Cao Q, Mao Z. Application of phosphorescent cyclometalated iridium(III) complexes in cancer treatment. Progress in Chemistry. 2018;30(10):1548–1556. doi:10.7536/PC180610
145. de Sousa C, Eksteen C, Riedemann J, Engelbrecht AM. Highlighting the role of CD44 in cervical cancer progression: immunotherapy’s potential in inhibiting metastasis and chemoresistance. Immunol Res. 2024;72(4):592–604. doi:10.1007/s12026-024-09493-6
146. Zhou X, Lian H, Li H, Fan M, Xu W, Jin Y. Nanotechnology in cervical cancer immunotherapy: therapeutic vaccines and adoptive cell therapy. Front Pharmacol. 2022;13:1065793. doi:10.3389/fphar.2022.1065793
147. Azmi F, Ahmad Fuaad AAH, Skwarczynski M, Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vaccin Immunother. 2014;10(3):778–796. doi:10.4161/hv.27332
148. Kim H, Niu L, Larson P, et al. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials. 2018;164:38–53. doi:10.1016/j.biomaterials.2018.02.034
149. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–1428. doi:10.1016/S0140-6736(22)00323-3
150. Bogani G, Monk BJ, Powell MA, et al. Adding immunotherapy to first-line treatment of advanced and metastatic endometrial cancer. Ann Oncol. 2024;35(5):414–428. doi:10.1016/j.annonc.2024.02.006
151. Alhaj-Suliman SO, Naguib YW, Wafa EI, et al. A ciprofloxacin derivative with four mechanisms of action overcomes paclitaxel resistance in p53-mutant and MDR1 gene-expressing type II human endometrial cancer. Biomaterials. 2023;296:122093. doi:10.1016/j.biomaterials.2023.122093
152. Byrne FL, Martin AR, Kosasih M, Caruana BT, Farrell R. The role of hyperglycemia in endometrial cancer pathogenesis. Cancers. 2020;12(5):1191. doi:10.3390/cancers12051191
153. Zhang Y, Tian TJ. Strategies, challenges, and prospects of nanoparticles in gynecological malignancies. ACS Omega. 2024;9(36):37459–37504. doi:10.1021/acsomega.4c04573
154. Cong Q, Lin L, Qi B, Xu C, Zhang X. Human Chorionic Gonadotropin Polypeptide Nanoparticle Drug Delivery System Improves Methotrexate Efficacy in Gestational Trophoblastic Neoplasia in vitro. Cancer Manag Res. 2021;13:1699–1708. doi:10.2147/CMAR.S279831
155. Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology—patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12(12):732–742. doi:10.1038/nrclinonc.2015.169
156. He C, Lu J, Lin W. Hybrid nanoparticles for combination therapy of cancer. J Control Release. 2015;219:224–236. doi:10.1016/j.jconrel.2015.09.029
157. Shrestha B, Tang L, Romero G. Nanoparticles-mediated combination therapies for cancer treatment. Adv Ther. 2019;2(11):1900076. doi:10.1002/adtp.201900076
158. Kong D, Zheng X, Ding K, et al. Multi-chambered core/shell supraparticles for real-time, full-time diagnosis and treatment integration of tumors. Adv Healthcare Mater. 2025;14(1):2401749. doi:10.1002/adhm.202401749
159. Wu X, Wen X, Lin X, et al. pH/glutathione-responsive theranostic nanoprobes for chemoimmunotherapy and magnetic resonance imaging of ovarian cancer cells. Colloids Surf B Biointerfaces. 2024;241:114053. doi:10.1016/j.colsurfb.2024.114053
160. Ali S, Amin MU, Tariq I, et al. Lipoparticles for synergistic chemo-photodynamic therapy to ovarian carcinoma cells: in vitro and in vivo assessments. Int J Nanomed. 2021;16:951–976. doi:10.2147/IJN.S285950
161. Wu S, Yang X, Lu Y, et al. A green approach to dual-drug nanoformulations with targeting and synergistic effects for cancer therapy. Drug Deliv. 2017;24(1):51–60. doi:10.1080/10717544.2016.1228716
162. Levit SL, Tang C. Polymeric nanoparticle delivery of combination therapy with synergistic effects in ovarian cancer. Nanomaterials. 2021;11(4):1048. doi:10.3390/nano11041048
163. Wan SS, Zeng JY, Cheng H, Zhang XZ. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials. 2018;185:51–62. doi:10.1016/j.biomaterials.2018.09.004
164. Deng Y, Wang Y, Jia F, et al. Tailoring supramolecular prodrug nanoassemblies for reactive nitrogen species-potentiated chemotherapy of liver cancer. ACS Nano. 2021;15(5):8663–8675. doi:10.1021/acsnano.1c00698
165. Li G, Ling M, Yu K, et al. Synergetic delivery of artesunate and isosorbide 5-mononitrate with reduction-sensitive polymer nanoparticles for ovarian cancer chemotherapy. J Nanobiotechnology. 2022;20(1):471. doi:10.1186/s12951-022-01676-3
166. Yan D, Sherman JH, Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8(9):15977–15995. doi:10.18632/oncotarget.13304
167. Li Y, Ho Kang M, Sup Uhm H, Joon Lee G, Ha Choi E, Han I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci Rep. 2017;7:45781. doi:10.1038/srep45781
168. Weiss M, Barz J, Ackermann M, et al. Dose-dependent tissue-level characterization of a medical atmospheric pressure argon plasma jet. ACS Appl Mater Interfaces. 2019;11(22):19841–19853. doi:10.1021/acsami.9b04803
169. Gupta N, Malviya R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188532. doi:10.1016/j.bbcan.2021.188532
170. Fang C, Zhang M. Nanoparticle-based theragnostics: integrating diagnostic and therapeutic potentials in nanomedicine. J Control Release. 2010;146(1):2–5. doi:10.1016/j.jconrel.2010.05.013
171. Kim J, Park S, Jung Y, et al. Programmable real-time clinical photoacoustic and ultrasound imaging system. Sci Rep. 2016;6:35137. doi:10.1038/srep35137
172. Huang W, Jin B, Gong H, et al. A tumor microenvironment-triggered protein-binding near-infrared-II theranostic nanoplatform for mild-temperature photothermal therapy. J Colloid Interface Sci. 2025;680:375–388. doi:10.1016/j.jcis.2024.11.049
173. Bian S, Zheng X, Liu W, et al. pH-Responsive NIR-II phototheranostic agents for in situ tumor vascular monitoring and combined anti-vascular/photothermal therapy. Biomaterials. 2023;303:122380. doi:10.1016/j.biomaterials.2023.122380
174. Zhang Z, Du Y, Shi X, et al. NIR-II light in clinical oncology: opportunities and challenges. Nat Rev Clin Oncol. 2024;21(6):449–467. doi:10.1038/s41571-024-00892-0
175. Qiu T, Lan Y, Wei Z, et al. In vivo multi-scale photoacoustic imaging guided photothermal therapy of cervical cancer based on customized laser system and targeted nanoparticles. Int J Nanomed. 2021;16:2879–2896. doi:10.2147/IJN.S301664
176. Chen S, Zhu L, Du Z, et al. Polymer encapsulated clinical ICG nanoparticles for enhanced photothermal therapy and NIR fluorescence imaging in cervical cancer. RSC Adv. 2021;11(34):20850–20858. doi:10.1039/d1ra02875h
177. Zhu L, Chen J, Yan T, et al. Near-infrared emissive polymer-coated IR-820 nanoparticles assisted photothermal therapy for cervical cancer cells. J Biophotonics. 2021;14(11):e202100117. doi:10.1002/jbio.202100117
178. Dragulska SA, Poursharifi M, Chen Y, et al. Engineering and validation of a peptide-stabilized poly(lactic-co-glycolic) acid nanoparticle for targeted delivery of a vascular disruptive agent in cancer therapy. Bioconjugate Chem. 2022;33(12):2348–2360. doi:10.1021/acs.bioconjchem.2c00418
179. Gawde KA, Sau S, Tatiparti K, et al. Paclitaxel and di-fluorinated curcumin loaded in albumin nanoparticles for targeted synergistic combination therapy of ovarian and cervical cancers. Colloids Surf B. 2018;167:8–19. doi:10.1016/j.colsurfb.2018.03.046
180. Weiss GJ, Chao J, Neidhart JD, et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest New Drugs. 2013;31(4):986–1000. doi:10.1007/s10637-012-9921-8
181. Krasner CN, Campos SM, Young CL, et al. Sequential Phase II clinical trials evaluating CRLX101 as monotherapy and in combination with bevacizumab in recurrent ovarian cancer. Gynecologic Oncol. 2021;162(3):661–666. doi:10.1016/j.ygyno.2021.07.002
182. Duska LR, Krasner CN, O’Malley DM, et al. A phase Ib/II and pharmacokinetic study of EP0057 (formerly CRLX101) in combination with weekly paclitaxel in patients with recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer. Gynecologic Oncol. 2021;160(3):688–695. doi:10.1016/j.ygyno.2020.12.025
183. Serrano-Martínez A, Victoria-Montesinos D, García-Muñoz AM, Hernández-Sánchez P, Lucas-Abellán C, González-Louzao R. A systematic review of clinical trials on the efficacy and safety of CRLX101 cyclodextrin-based nanomedicine for cancer treatment. Pharmaceutics. 2023;15(7):1824. doi:10.3390/pharmaceutics15071824
184. Shamaeizadeh N, Sadeghi E, Varshosaz J. Clinical outcomes and effectiveness of CRLX101 for solid tumors: a systematic review and meta-analysis. Curr Med Chem. doi:10.2174/0109298673263933231206101556
185. Bazzazan MA, Fathollazadeh P, Keshavarz shahbaz S, Rezaei N. Polymeric nanoparticles as a promising platform for treating triple-negative breast cancer: current status and future perspectives. Int J Pharm. 2024;664:124639. doi:10.1016/j.ijpharm.2024.124639
186. Nash Z, Menon U. Ovarian cancer screening: current status and future directions. Best Pract Res Clin Obstet Gynaecol. 2020;65:32–45. doi:10.1016/j.bpobgyn.2020.02.010
187. Corrie L, Kommineni N, Kaur J, Awasthi A, Gundaram R, Kukati L. In situ photo responsive biodegradable nanoparticle forming intrauterine implant for drug delivery to treat ovarian diseases: a rationale-based review. Curr Radiopharm. 2024;17(4):313–319. doi:10.2174/0118744710258313231105072931
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

