Back to Journals » International Journal of Nanomedicine » Volume 20
Proteomic Characterization of Extracellular Vesicles from Human Neural Precursor Cells: A Promising Advanced Therapy for Neurodegenerative Diseases
Authors Stricker PEF , de Oliveira NB , Mogharbel BF , Irioda AC, da Rosa NN, Lührs L, Saçaki CS, Munhoz da Rocha I, Alves LR, Poubel SB, Cardoso da Silva J , Carvalho PC , Fischer JSG, de Carvalho KAT
Received 28 November 2024
Accepted for publication 1 May 2025
Published 25 May 2025 Volume 2025:20 Pages 6675—6699
DOI https://doi.org/10.2147/IJN.S502031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Priscila Elias Ferreira Stricker,1,* Nathalia Barth de Oliveira,1,* Bassam Felipe Mogharbel,1 Ana Carolina Irioda,1 Nádia Nascimento da Rosa,1 Larissa Lührs,1 Claudia Sayuri Saçaki,1 Isadora Munhoz da Rocha,2 Lysangela Ronalte Alves,2 Saloe Bispo Poubel,1 Julia Cardoso da Silva,3 Paulo Costa Carvalho,3 Juliana Saldanha da Gama Fischer,3 Katherine Athayde Teixeira de Carvalho1
1Pelé Pequeno Príncipe Research Institute, Child and Adolescent Health Research & Pequeno Príncipe Faculties, Advanced Therapy and Cellular Biotechnology in Regenerative Medicine Department, Curitiba, PR, Brazil; 2Gene Expression Regulation Laboratory, Carlos Chagas Institute, FIOCRUZ, Curitiba, PR, Brazil; 3Computational Mass Spectrometry Group, Carlos Chagas Institute, FIOCRUZ, Curitiba, PR, Brazil
*These authors contributed equally to this work
Correspondence: Katherine Athayde Teixeira de Carvalho, Pelé Pequeno Príncipe Research Institute, Child and Adolescent Health Research & Pequeno Príncipe Faculties, Av. Munhoz da Rocha, 490 - Juvevê, Curitiba, PR, 80035-000, Brazil, Tel +55-41-3310-1034, Email [email protected]
Background: The therapeutic effect of stem cells is attributed to their direct maturation into somatic cells and their paracrine effects, which influence the extracellular environment. One such component released is extracellular vesicles containing proteins and genetic materials with immunomodulatory functions and facilitating cell-to-cell communication.
Purpose: The study’s main objective was to characterize extracellular vesicles (EVs) from Human Neural Precursor Cells (hNPCs).
Methods: Wharton’s Jelly mesenchymal stem cells (WJ-MSCs) were isolated by explant technique and characterized by flow cytometry and trilineage differentiation. The hNPCs obtained from neurospheres were produced by seeding WJ-MSCs on a natural functional biopolymer matrix. EVs derived from WJ-MSCs and hNPCs were isolated by precipitation methodology and characterized by flow cytometry, nanoparticle tracking analysis (NTA), scanning electron microscopy (TEM), and proteomic.
Results: hNPCs expressed proteins and genes characteristic of neural precursor cells. The EVs were characterized by flow cytometry and showed varied expression for the markers CD63, CD9, and CD81, indicating different subpopulations based on their origin of formation. NTA and TEM of the EVs exhibited characteristic size, shape, and structural integrity consistent with the criteria established by the International Society for Extracellular Vesicles (ISEV). EV-hNPCs function enrichment analysis of the proteomic results showed that these vesicles presented abundant proteins directly involved in neuronal biological processes such as plasticity, transduction, postsynaptic density, and overall brain development.
Discussion: The results indicate that EVs derived from hNPCs maintain key neural precursor characteristics and exhibit marker variability, suggesting distinct subpopulations. Their structural integrity aligns with ISEV standards, supporting their potential as reliable biological entities. The proteomic analysis highlights their role in neuronal functions, reinforcing their applicability in neurodegenerative research and therapeutic strategies.
Conclusion: The EVs were successfully isolated from hNPCs with abundant proteins involved in neuronal processes, making them attractive for acellular therapies to treat neurodegenerative diseases.
Keywords: mesenchymal stem cell, Wharton’s Jelly, neurospheres, neural precursor cells, extracellular vesicles
Graphical Abstract:

Introduction
Biotechnology and regenerative medicine have been gaining space in pre-clinical and clinical studies due to the advancement of cell therapies and their application in cancer treatments and neurodegenerative diseases. According to the Alliance for Regenerative Medicine, an organization dedicated to promoting studies in advanced therapy and regenerative medicine, cell therapy has been applied worldwide in approximately 2093 clinical trials, of which 48% are sponsored by industry and 52% by non-industrial institutions (academics, for example).1,2 Considering this information, it is essential to carry out research aimed at developing good manufacturing practice (GMP) techniques to produce mesenchymal stem cells and their derivatives for therapeutic application in humans.
Evidence suggests that the therapeutic effect of stem cell (SC) transplantation is not only due to the cell itself but also to the paracrine effects that these cells can cause. These paracrine effects are known to be caused by what the cell releases into the extracellular environment.3–9 The released components are the extracellular vesicles with proteins and genetic materials (messenger RNA/mRNA, siRNA, long RNAs, and micro-RNA/miRNA) inside, with immunomodulatory and intercellular communication functions.10–18 Stem cell therapy is limited when it considers that these cells within the organism could have changes in their survival rate and differentiation capacity due to changes in their behavior in vitro and to be subject to the modifications and physiological responses from the host.19 One of the effects of cell transplantation is matched by what it releases into the extracellular environment, that is, the paracrine effects. Therefore, cellular products become strong candidates to replace therapy with cells giving rise to acellular biological therapy.16,18–20
One of the challenges for nervous system regeneration is finding a safe cellular type without induction factors or transfections that impose some risks as teratogenic or tumor cells. Several cell types have been considered for this purpose: i) induced pluripotent stem cells (iPSCs) that need the transfection of the somatic cells then to differentiate into pluripotent stem cells that can differentiate in various types of neurons;21,22 ii) the embryonic stem cells (ESCs) with have the capacity to differentiate into all cellular types, including extraembryonic tissues;23 iii) neural progenitors cells, derived from specific regions of the brain and spinal cord, but with ethical concerns similar to the ESCs, as they are obtained from fetal or postnatal neural tissue;24 and iv) mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells according to the International Society for Cellular Therapy (ISCT),25 which are multipotent, capable of differentiating into mesodermal and non-mesodermal lineages, such as neurons, also exhibit paracrine effects. MSCs are easily obtained from medical waste tissues such as adipose tissue and umbilical cord.26–28
This study aims to isolate and characterize extracellular vesicles (EVs) derived from Wharton jelly mesenchymal stem cells (WJ-MSCs) and human neural precursor cells (hNPCs) in vitro cultured medium. The MSCs can be the candidate for this aim because they are the origin of the neural crest in embryonic development.29–31 The caudal part of the neural tube and some MSCs have the properties to transit for epithelial cells derived from this MSCs population and constitute a subpopulation as human neural precursor cells or neural crest-derived stem cells.32,33 On the other side, the paracrine effects of the MSCs demonstrated that they were responsible for the beneficial effects of cells released in medium culture or host tissue after transplantation as a secretoma.34 For this reason, developing other therapies based on extracellular vesicles from hNPCs and their derivatives as proteins to treat neurological diseases will be interesting.
Materials and Methods
The study was conducted in four stages (Figure S1). First, WJ-MSCs were collected, isolated, and characterized by flow cytometry and trilineage differentiation. The second stage involved the production and characterization of hNPCs using Suppl immunocytochemistry and RT-PCR. In the third stage, EV isolation was standardized using a precipitation method for both WJ-MSC- and hNPC-derived EVs. Finally, EVs were characterized by flow cytometry with magnetic beads, NTA, and TEM.
Umbilical Cord-Ethical Donors
The Human Ethics Research Committee of Pequeno Príncipe Faculties (CEP-FPP) approved this study, numbered 3.288.297 (2019/04/26). Three human umbilical cord (HUC) samples were collected from healthy mothers who signed the Free and Informed Consent Form. The HUCs were obtained from healthy mothers at a maternity hospital in Curitiba, Paraná State, Brazil. The collections were performed at the time of birth, shortly after the placenta was discharged. The HUCs were processed within four hours.
Isolation of Wharton Jelly Mesenchymal Stem Cells
For Wharton’s Jelly Mesenchymal Stem Cell isolation, HUCs were washed with PBS (Sigma-Aldrich®) containing 3% antibiotic (Thermo Fisher®), sectioned, and had veins and arteries removed. The tissue was fragmented into 2×2 mm pieces, plated with DMEM/Ham-F12 (Sigma-Aldrich®) supplemented with 10% FBS (Gibco, Thermo Fisher®) and 1% antibiotic, then incubated at 37°C with 5% CO2. The medium was first replaced after five days and then every 72 hours until 85% confluency was reached (Figure S2).35
Mesenchymal Stem Cells Characterization
WJ-MSCs were characterized following the International Society of Cell Therapy guidelines. Immunophenotypic analysis used CD105, CD73, CD90, CD29, CD34, CD45, and 7-AAD (Becton Dickinson®), the latter for cell viability (Stricker et al, 2021). Pluripotency was assessed through adipogenic, osteogenic, and chondrogenic differentiation using StemPro kits (Gibco®, Thermo Fisher®). Differentiation was confirmed by specific staining: Alizarin Red and Oil Red O for osteogenic, and Alcian Blue for chondrogenic (Sigma-Aldrich®).
Productions and Characterization of Human Neural Precursor Cells
hNPCs were prepared as described by Stricker et al. Briefly, a natural functional biopolymer matrix (NFBX) was prepared at a dilution (v/v) of 1:2 in aqueous solution, pure water, followed by exposure to ultraviolet rays of laminar flow for overnight sterilization and then plated onto the culture flasks and developed the neurospheres when a density plating of 2×104 WJ-MSCs/mL and DMEM/Ham-F12, 100 UI/mL penicillin, 100 µg/mL streptomycin (Gibco®, Thermo Fisher, Waltham, Massachusetts, EUA) culture medium incubated with 20% FBS at 37°C and 5% CO2. Since this is a primary culture, the lifespan of the neurospheres is approximately 15 to 21 days, depending on the sample.28
Immunocytochemistry Characterization of Human Neural Precursors Cells
hNPCs were cultured in 24-well plates, fixed with 4% paraformaldehyde (Sigma-Aldrich®, San Luis, Missouri, USA), and washed with PBS. Cells were then permeabilized with 0.1% Triton X-100 (Sigma-Aldrich®) and 1% BSA, followed by overnight incubation at 4°C with primary antibodies against Nestin (S1409 - Sigma-Aldrich®), β-tubulin III (T8578 - Sigma-Aldrich®), and GFAP (G3893 - Sigma-Aldrich®). After washing, the cells were incubated with FITC anti-rabbit IgG, FITC anti-mouse IgG, and CY5 anti-mouse IgG (Sigma-Aldrich®) for one hour in the dark, then stained with Hoechst 33342 (Thermo Fisher®, Waltham, Massachusetts, USA). The experiment included WJ-MSCs and the NLP-ReNCell neural progenitor lineage (ReNcellTMVM Immortalized, SCC008, Sigma-Aldrich®). Fluorescence was analyzed using the IN-Cell Analyzer 2000 Imaging System (GE HealthcareTM, Illinois, Chicago, USA).
Qualitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
To assess the expression of neuronal progenitor-associated genes (NESTIN/NES, MAP2, GFAP, and β-TUBIII/TUBB3), RNA was extracted from WJ-MSCs (passage 3), hNPCs (passage 5), NLP-ReNCell (positive control), and CCD1059SK fibroblasts (negative control) using the Purelink kit (Thermo Fisher®, Waltham, Massachusetts, USA), following the manufacturer’s protocol. cDNA synthesis was performed using the Promega ImProm II Reverse Transcription System Kit (Promega®, Madison, USA). PCR was conducted in a 25μL reaction containing magnesium-free buffer, dNTPs, magnesium chloride, primers (forward and reverse), Taq polymerase (Thermo Fisher®), and 2μL of cDNA. Primer sequences, amplicon sizes, and annealing temperatures are detailed in Table 1.
 |
Table 1 Primers |
Standardization of Isolation of Extracellular Vesicles
The EV isolation assay was performed using the Total Exosome Isolation Kit (from cell culture media) (Thermo Fisher®, Waltham, Massachusetts, EUA), following the manufacturer’s instructions. The assay was performed by culturing cells in 225 cm2 culture flasks, the medium was collected, and the isolation was performed. Quantification of proteins for each assay was performed using the Bradford method. Tests were performed using DMEM Ham-F12 medium without phenol supplemented with 100 IU/mL penicillin: 0.1 mg/mL streptomycin.
Standardization of EVs Flow Cytometry Characterization
EVs were characterized using specific magnetic beads: Exosome-Human CD63 Isolation/Detection (CAT 10608D, Thermo Fisher®), Exosome-Human CD9 Flow Detection (CAT 10614D, Thermo Fisher®), and Exosome-Human CD81 Flow Detection (CAT 10616D, Thermo Fisher®). Beads were homogenized, mixed with 1% HSA PBS, incubated in a magnetic separator (Dynabeads-Sigma-AldrichTM), and combined with 100 µL of isolated EVs. After an overnight incubation at 2–8°C under rotation, the samples were washed, resuspended in 300 µL of PBS 1% HSA, and incubated with anti-CD9 (PE Cat. 555372-Becton Dickinson®), anti-CD81 (APC Cat. 555676-Becton Dickinson®), and anti-CD63 (FITC Cat. 10606D-Thermo Fisher®) antibodies. Isotype controls (PE, APC, FITC) were added separately. Following incubation on a plate shaker under light protection, samples were washed twice, resuspended in 200 µL of PBS 1% HSA, and analyzed by flow cytometry (FACS Calibur; Becton Dickinson®) using Infinicyt Flow CytometryTM version 1.6.0 software. Graphical data are shown in Figures S3–S5.
Characterization of EVs by Nanoparticle Tracking Analysis
Nanoparticle tracking analysis (NTA) aims to measure the size of EV based on Brownian motion. The particles are illuminated with a laser beam, and by scattering the light, a conventional optical microscope can capture the light reflected from the vesicles and measure the size of the particles. For this experiment, three samples of extracellular vehicles from WJ-MSCs and three samples of VEs from hNPCs were used. Each measurement was performed in triplicate using the Nanosight-NS500 (Malvern PanalyticalTM, Malvern, UK) instrument from Instituto Carlos Chagas, FIOCRUZ-PR, Curitiba, Brazil. Samples were diluted 50-fold in PBS, collected in a 1-mL syringe, and pumped into the instrument under constant pressure and temperature. After laser incidence, the concentration (in particles/mL) and size (nm) of the particles of each sample could be measured.
Characterization of EVs by Transmission Electron Microscopy (TEM)
An amount of 50 µL EVs from each sample was applied to microscopy grids containing formvar, and the grids were incubated for 1 hour at room temperature. After incubation, the grids were washed with PBS and fixed with Karnovisky for 10 min. They were then washed three times with 0.1 M cacodylate buffer and negatively contrasted with 5% uranyl for 2 minutes, followed by washing with 18.2 Ώ H2O. Gratings were coated with a carbon film (Leica EM ACE200-Leica MicrosystemsTM, Wetzlar, Germany), and readings were taken in a transmission electron microscope (Jeol -Jem-1400 – Plus – Hitachi High Technologies, Tokyo, Japan) at 100kV and a magnification of 20k to 25k, which is part of the technology platform of the Instituto Carlos Chagas, FIOCRUZ-PR, Curitiba, Brazil.
Proteomics
Sample Preparation
Proteins from extracellular vesicles were initially mechanically extracted using 0.1 mm zirconium beads. Then, chemical extraction was carried out using RapiGest detergent at a concentration of 0.1% in accordance with the manufacturer’s guidelines. Protein concentrations were determined using the Qubit platform’s fluorometric assay (Invitrogen). Proteins from four samples of extracellular vesicles from MSCs (EV-WJ-MSCs) and four samples of extracellular vesicles from NPCs (EV-hNPCs) were reduced with dithiothreitol (DTT-10 mM) for 30 minutes at 60°C. After cooling to room temperature, the samples were alkylated with iodoacetamide (30 mm) for 25 minutes at room temperature in the dark and ultimately digested with sequence-grade modified trypsin in a 1/50 (E/S) ratio for 20 hours at 37°C.36,37
Desalting and Sample Quantification
Subsequently, the enzymatic reaction was stopped by introducing trifluoroacetic acid (0.4% v/v final), and the peptides were further incubated for additional 40 minutes to degrade the RapiGest. Following this, the samples underwent centrifugation at 18,000× g for 10 minutes to eliminate any insoluble materials. Then, the peptides were quantified using the fluorometric assay (Qubit 2.0 - Invitrogen), as recommended by manufacturer. Each sample was desalted and concentrated using Stage-Tips (STop and Go-Extraction TIPs) in accordance with the method described by Rappsillber et al.36
Mass Spectrometry Analysis
The peptides underwent LC–MS/MS analysis using a Thermo ScientificTM Easy-nLC 1000 ultra-high-performance liquid chromatography (UPLC) system coupled with an LTQ Orbitrap XL mass spectrometer. The peptide mixtures were loaded onto an in-house-packed column (75 mm i.d., 30 cm long) with 3.2 μm ReproSil-Pur C18-AQ resin at a flow rate of 250 nL/min. Subsequently, elution occurred with a flow rate of 250 nL/min, transitioning from 5% to 40% acetonitrile (ACN) in 0.1% formic acid and 5% dimethyl sulfoxide (DMSO) over a 180-minute gradient.
The mass spectrometer operated in data-dependent mode, alternating between MS and MS/MS (MS2) acquisition. Survey full-scan MS spectra (from m/z 300–2000) were acquired in the Orbitrap analyzer at a resolution of R = 60,000 at m/z 400, following accumulation to a target value of 1,000,000 in the linear trap. The ten most intense ions were sequentially isolated and subjected to fragmentation in the linear ion trap using collision-induced dissociation with a normalized energy of 35%. Previously selected target ions for MS/MS were dynamically excluded for 90 seconds. The total cycle time was approximately 3 seconds.
General mass spectrometric conditions included a spray voltage of 2.4 kV, no sheath and auxiliary gas flow, an ion transfer tube temperature of 175°C, collision gas pressure of 1.3 mTorr, and normalized energy collision energy using wide-band activation mode set at 35% for MS2. Ion selection thresholds were established at 250 counts for MS2. MS2 acquisitions applied an activation q = 0.25 and an activation time of 30 ms. Each biological replicate was subjected to two technical replicates.
Data Analysis
A biological replicate for each sample (EV-WJ-MSCs and EV-hNPCs) were performed, with two technical replicates each. Quantitation followed the Normalized Ion Abundance Factors (NIAF) method from PatternLab, serving as a relative quantitation strategy, as outlined in our bioinformatics protocol. It is worth noting that NIAF is analogous to NSAF but is applied to extracted ion chromatograms (XIC).
Differentially proteins were identified using PatternLab’s TFold, comparing two samples, EV-WJ-MSCs and EV-hNPCs. Additionally, a T-Fold analysis was conducted comparing the two batches of biological replicates. The analysis was conducted considering proteins that are present in at least three samples from the same biological group. Finally, Enrichr tools were employed to aid in the interpretation of the data and discover in which signaling pathways the differentially expressed proteins are present.38–40 Database SynGO is used to analyze the synaptic pathway, which is a public knowledge base and online analysis platform for synapse research.41
Results
Flow Cytometry Analysis
The WJ-MSCs from all isolated samples had the characteristics the International Society of Cell Therapy recommended for mesenchymal stem cells. They were 99.25% positive for the markers CD73, CD90, and CD105 and negative for the hematopoietic markers CD45 and CD34. These cells showed low immunogenicity, 99.97% negative for the marker HLA-DR. Of these cells, 11.12% were 7AAD-positive cells, meaning they were not viable.
Trilineage Differentiation Test
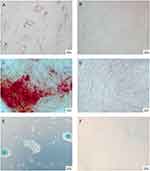
WJ-MSCs satisfactorily differentiated into three cell lines. In Figure 1A, cells differentiated into adipocytes, with lipid deposition detected by staining with Oil Red O (red dots in the cytoplasm of the cells). In Figure 1C, cells differentiate into osteocytes, with calcium deposition detected by staining with Alizarin Red (red dots on the cells). In Figure 1E, cells differentiate into chondrocytes, with proteoglycan deposition detected by staining with Alcian blue (blue/green deposition within cells with round morphology-chondrocytes). In Figure 1B, D and F WJ-MSCs- were not stimulated to adipogenic, osteogenic and chondrogenic differentiation, which is the negative control of this technique.
Production of Human Neural Precursor Cells
Human Neural precursor cells were obtained from WJ-MSCs and were satisfactory. In Figure 2, the process of formation of neurospheres on a natural functional biopolymer matrix (NFBX) without induction factors or gene transfections (A and B), the already formed neurospheres on the NFBX membrane (C and D) and the hNPCs after the expansion process of neurospheres (E and F) is shown.
Human Neural Precursor Cells Characterization
Immunocytochemistry
hNPCs showed protein expressions of Nestin, Beta Tubulin 3, and glial fibrillary acidic protein (GFAP), as shown in Figure 3.
Qualitative Reverse Transcription–Polymerase Chain Reaction
Both WJ-MSCs and Human neural precursor cells (hNPCs) showed expression for the NES, MAP2, GFAP, and TUBB3 genes. As well as the human neural progenitor cells from the ReNCell line (ReNcellTMVM Immortalized, SCC008, Sigma-Aldrich, San Luis, Missouri, EUA), which was used as a positive control, also showed expression for all amplified genes. Amplification of a constitutive gene, in this case β- ACTIN, was used as a control for the methodology performed and showed expression for all samples tested, as shown in Figure 4.
Isolation Method of Extracellular Vesicles
Three samples of EVs were isolated from WJ-MSCs and from their respective neural precursor cells. Table 2 shows protein yield and the number of cells from these isolations.
 |
Table 2 Protein Yield of Isolated EVs |
Extracellular Vesicles Characterization
Flow Cytometry Characterization
Flow cytometric characterization assay of EVs was performed with magnetic beads. The extracted EVs were characterized from three samples of mesenchymal stem cells from Wharton Jelly origin (4E, 5E, and 6E) and three respective samples of human neural precursor cells (4E, 5E, and 6E)—the results are represented in Table 3.
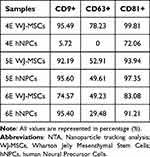 |
Table 3 Flow Cytometry Results |
Nanoparticle Tracking Analysis (NTA)
Isolation of EVs by the precipitation method resulted in vesicles with sizes between 100 and 200 nm for both particles of WJ-MSCs and hNPs (Figure 5). The concentration of the particles was varied, and no direct correlation with the concentration of cells or proteins could be established. In Table 4 show values of the modes in nanometers as well as the concentration of particles per milliliter of each sample detected by NTA.
 |
Table 4 NTA Results |
Transmission Electron Microscopy (TEM)
TEM confirms the results observed in the NTA graphs, concluding that EVs have sizes between 100 and 200 nm in Figure 6. Furthermore, Figure 6 shows the rounded structures with a lipid bilayer (arrows indicating the lipid bilayer in a rounded structure). The TEM results in Figure 6 below are for both EV-WJ-MSCs and EV-hNSCs of sample 6E.
Proteomic Characterization from EV-WJ-MSCs and EV-hNPCs
Proteomic analysis of EV-WJ-MSCs and EV-hNPCs identified a total of 982 proteins and 9586 peptides of which 8695 map to a single entry in the sequence database. Thirty-three were exclusive to EV-WJ-MSCs and six were exclusive to EV-hNPCs (Table S1 - Raw proteomic analysis data Supplementary Material). This article will discuss these differentially presented abundant proteins and the exclusive protein abundances from each source.
As we can observe in the vein diagram and volcano plot (Figure 7A and B), there is a significant resemblance between the two sources of extracellular vesicles (Correlation of 0.896 – Figure S6). In Figure 8A, the DEPs indicate that, while correlated, there are differences in the proteins expressed across each analyzed source of extracellular vesicles. This suggests that, even when similar, these vesicles demonstrate variations in their formation and signaling patterns. Figure 8B and C demonstrated that differentially expressed proteins are interconnected within signaling pathways in functional enrichment analysis.
Functional Analysis of EV-WJ-MSCs and EV-hNPCs
Gene Ontology (GO) functional annotation was performed for the total number of proteins, differentially expressed proteins (DEPs), and proteins exclusively identified from each source of extracellular vesicles, describing biological processes (BP), cellular components (CC), and molecular functions (MF). The results for the total number of proteins indicated that EV-WJ-MSCs and EV-hNPCs exhibit similar gene ontology function annotations, as illustrated in Figure 9. KEGG diagram from these proteins also shown similar pathways from EV-WJ-MSCs and EV-hNPCs, as seen in Figure 9.
The GO and KEGG pathway analyses for the DEPs from EV-WJ-MSCs and EV-hNPCs demonstrated that these proteins participate in different biological processes, cellular components, and molecular functions when compared, as can be seen in Figure 10.
The analysis of enrichment of proteins exclusively expressed in each of the vesicle sources demonstrated that EV-hNPCs is involved in various biological processes and neuronal cellular components such as Postsynaptic Process Involved In Chemical Synaptic Transmission (GO:0099565), Postsynapse to Nucleus Signaling Pathway (GO:0099527), postsynaptic Density, Intracellular Component (GO:0099092), Presynaptic Cytosol (GO:0099523), Postsynaptic Density (GO:0014069). The complete results of the analysis of enrichment of exclusive proteins are shown in Figure 11.
Discussion
MSC-WJ Culture and Characterization
MSCs have high regenerative capacity and differentiation potential, making them prominent in cell therapy studies. Data from 2020 show that research with MSCs has grown significantly, where more than a thousand clinical studies have been registered, 61% of which are already in Phase 2, with patients with diseases that can be treated with mesenchymal stem cell therapy.42 When compared WJ-MSCs with the MSCs originating from the bone marrow demonstrated, in addition to a greater capacity for proliferation and significant power of differentiation.43 In this study, MSCs derived from Wharton’s jelly were isolated and cultivated using the explant methodology, and an average yield of 8×106 cells was obtained—other methodologies were used for isolating WJ-MSCs, such as enzymatic digestion. However, the literature differs concerning the methodology that has the best performance. Some authors cite the methodology of enzymatic digestion as more profitable.44 This isolation technique relies on digestive enzymes such as trypsin and collagenase for tissue dissociation and cell cultivation. Other authors described the explant methodology with higher yield.45 This methodology is free of digestive or chemical agents; only tissue fragmentation is necessary for cell cultivation to be possible. Finally, other authors claim no difference in performance between the methodologies.46 When the intention is to use cells or their derivatives for therapy in humans, the intention is to use isolation methods with less interference as possible. The explant is a technique that minimally modifies the cells, not using digestive enzymes, providing more remarkable self-renewal and purity power.47
The characterization of WJ-MSCs followed the recommendations of the ISCT with adherence to a plastic substrate, differentiation potential (adipogenic, chondrogenic, and osteogenic), and proven specific cell surface markers.25,48 Considering the recommended criteria, the WJ-MSCs isolated in this work showed plasticity. They differentiated into three different cell lineages (osteocyte, chondrocyte, and adipocyte), and the surface markers were specific for mesenchymal lineages, with 99.25% of cells positive for CD73, CD105 and CD90 and negative for the evaluated hematopoietic markers, CD34 and CD45.
In this study, the low immunogenicity of the cultured WJ-MSCs was demonstrated with the low expression of HLA-DR; that is, 99.97% of the cells were negative for this marker in the flow cytometry methodology. This result demonstrates that WJ-MSCs are a source with safe characteristics for applicability in therapy, considering the low immunogenicity.49,50
Culture and Characterization of Neural Precursor Cells
This study aims to isolate the extracellular vesicles from hNPCs; therefore, their cultivation and characterization were conducted. First, the hNPCs were cultured according to the protocol developed by this research group.28 The principle of hNPCs production by this method is based on the plating of MSCs, independent of their origin, in a natural polymer, without chemical inducers or gene transfections. This differentiation is explained by epigenetics or, more precisely, by the process of mechanotransduction of YAP and AMOT proteins.51 Producing hNPCs is crucial for cultivating and expanding cells with neural characteristics. Usually, this culturing process is associated with chemical growth factors or gene transfections.52,53 Therefore, as it is a production technique without chemical interferences or gene transfection, the methodology used in this research to produce hNPCs makes the use of these cells, or their products, in cell therapy even more attractive.
The hNPCs produced were characterized and showed characteristics compatible with the neural precursors listed in the literature, such as the expression of genes characteristic of these lineages, such as NES, BTUB3, GFAP, and MAP2), and protein expression of Nestin, Beta Tubulin 3 and of glial fibrillary acidic protein (GFAP).52–54 As can be seen, the results of protein expression and gene expression for WJ-MSCs and hNPCs were positive for the proteins and genes evaluated. The results align with the literature when considering that WJ-MSCs have the capacity for spontaneous neuronal differentiation. Therefore, the expression of the Nestin, beta Tubulin 3, and GFAP proteins in WJ-MSCs and their hNPCs becomes evident and essential.55,56 In addition, sustaining this hypothesis, the NES, BTUB3, GFAP, and MAP2 genes’ expressions are demonstrated. To quantify the expression rate of these genes in different cell types and to verify whether they are differentially expressed in the two cell types, additional real-time PCR experiments will be necessary, which is one of the limitations of this study.
Isolation and Characterization of Extracellular Vesicles
The therapeutic effect of MSCs is well-defined and characteristic of these cells. The therapeutic effect of these cells is the result of what they can release into the extracellular environment, such as proteins, lipids, and genetic material, such as miRNAs.57 Therefore, EVs released by MSCs become a promisor product for therapeutic application.57,58 In addition, these vesicles show potential use in nanomedicine, being essential for communication between cells, biomarkers, and drug carriers, thus becoming structures applicable in therapies and diagnosis.59–61
Studies demonstrate the therapeutic anti-inflammatory action of EVs derived from MSCs for several diseases such as osteoarthritis62 pancreatitis,63 corneal inflammation,64 neuro inflammation,65 multiple sclerosis,66 spinal cord injury,67 and myocardial injury.68 These effects are, in part, mediated by bioactive molecules carried by EVs, including genetic material, lipids, proteins, and membrane-bound ecto-enzymes. In addition to proteins, lipids, and genetic material, ecto-enzymes play a crucial role in modulating the extracellular environment, influencing immune responses and tissue regeneration, further supporting the therapeutic potential of EVs in inflammatory and degenerative diseases.69–71
The isolation of EVs is a major challenge when considering the yield and purity of these structures. The isolation by precipitation, chosen for the development of this study, presented sufficient yield for the proposed characterizations. In addition, it is a faster technique when compared to ultracentrifugation, immunoaffinity, or density gradient centrifugation methodologies.72 On the other hand, Andreu et al compared different exosome isolation methods, such as ultracentrifugation and total exosome isolation (TEI) precipitation from various manufacturers, which were used according to their instructions. These authors concluded that the precipitation method is the most appropriate procedure for EV enrichment to incorporate into clinical practice. However, there has yet to be a consensus in the literature.
EVs from WJ-MSCs or hNPCs hold great promise for immunomodulation and regenerative effects, but the yield of these vesicles is a challenge.73 Regarding the yield found in this study and considering the total protein by the standardized method, a concentration similar to studies that used the same methodology was obtained.74 Cell cultivation conditions can influence EVs’ yield and final characterization.75 Therefore, standardization of cell cultivation and isolation of EVs should be considered for large-scale applications, considering the use of bioreactors.76,77
Characterization by flow cytometry of EVs originating from WJ-MSCs and hNPCs have shown controversial results. The presence of specific markers (CD9, CD63, and CD81) is not equally positive for the analyzed samples. While the WJ-MSCs 4E sample showed positivity for all markers, the hNPCs derived from the same sample lost CD63 expression and showed low expression for CD9. Despite the static analysis demonstrating that there was no statistical difference between the expression of the markers, it was possible to biologically observe the decrease in the expression of the CD63 marker in EVs-hNPCs.
This same pattern of decrease in the expression of the CD63 marker was also observed for samples 5E and 6E, considering the two origins WJ-MSCs and hNPCs. This result is understood with the acknowledgment that EVs are formed. Mammalian cells can release two types of extracellular vesicles derived from intracellular compartments (late endosomes or multivesicular bodies) after fusing with the plasma membrane or those derived directly from the plasma membrane.78 According to the authors Mathieu et al, the CD63 marker may be an essential clue to defining the origin of EVs.79
The results of the characterization of EVs from different samples show that there may be heterogeneity between them in forming the EVs found. Other authors have already identified these subpopulations through proteomics comparison, evidencing the possibility of subpopulations in small EV isolations.80 However, between the EV-WJ-MSCs and the EV-hNPCs, the probable existence of these subpopulations can be observed, which explains the origin of these vesicles’ formation, which probably has heterogeneity in the formation process.
Following a previous study, EVs isolated by precipitation technique showed sizes between 121.5 and 184.3 nm.74 Although methods of isolation of EVs by ultracentrifugation result in a purer population of vesicles considering the size, in this study, we can treat them as a population of small EVs.77,81 Considering the possibility of the presence of subpopulations of EVs to the presence or absence of the CD63 marker, the results of the NTA corroborate the identification of these subpopulations since the EVs originating from the plasma membrane can present sizes between 100 and 1000 nm.82 Furthermore, in the statistical analysis of vesicle sizes comparing the origins of EVs (WJ-MSCs and hNPCs), no difference was observed between them. Finally, the EVs were characterized according to their morphology using the TEM methodology. EVs from both origins (WJ-MSCs and hNPCs) showed spheroid morphology and the presence of the lipid bilayer characteristic of EVs, as recommended in the literature. In addition, the sizes of EVs evidenced by the NTA results followed the sizes described through the TEM technique at the 200 nm scale.77
Proteomic Characterization of Extracellular Vesicles
Proteomic characterization of EVs-WJ-MSCs and EV-hNPCs showed a similar profile of proteins with a correlation of 0.896. However, these proteins exhibited different expressions in each source. It is widely discussed that the therapeutic effects of stem cells are also mediated by paracrine signaling, with extracellular vesicles emerging as key candidates for conveying these effects.8,77,83–86 Consequently, these differences may relate to predicting variations in treatments using EVs from these two sources. Compared to cells, EVs exhibit advantages such as biocompatibility and low immunogenicity.
The proteomic profile of EVs-WJ-MSCs show that proteins CKAP4, H2BC21, H2BC26, H2BC3, H2BC17, H2BC11, H2BC18, H2BC12L, H2BC5, H2BC12, H2BC14, H2BC4, H2BC9, H2BC15, H2BC13, CCT6B, PDIA6, FKBP10, CALR and KRT19 are differently expressed, as seen in Figure 8A. Histones proteins are essential for chromatin arrangement and are related to gene expression regulation.87 This is an important function performed by extracellular vesicles.88–91 The presence of these proteins in EVs confers a signaling character for differentiation, as these proteins are directly related to the signaling of chromatin organization through their methylation.88 Histone proteins are reported to be important in neuronal differentiation signaling due to the post-translational modifications (PTM) that these proteins undergo.91,92 Defects in the pattern of PTM of histones are associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s.93,94
The proteins CKAP4, PDIA6, FKBP10, CALR, and KRT19 play roles in protein folding, cellular homeostasis, endoplasmic reticulum (ER) function, and cellular structure.95–97 All these proteins, except KRT19, are associated with the ER and collaborate in processes like protein folding, quality control, and responding to ER stress.95–99 PDIA6 and CALR are specifically involved in protein folding and quality control within the ER, potentially coordinating their activities with ER-resident proteins such as CKAP4 and FKBP10.100 FKBP10 plays a distinct role in collagen biosynthesis, which might intersect with other proteins participating in collagen maturation and secretion.101
Lastly, KRT19 is a structural protein that could interact with or be influenced by other proteins like CKAP4, which also plays a role in cytoskeletal organization.102
These proteins are essential for signaling cell differentiation, thus is expected to be present in EVs-WJ-MSCs. These findings corroborate with the results from GO, which indicated that the proteins are involved in biological processes such as extracellular matrix organization, cell adhesion, external encapsulating structure organization, and cytoskeleton organization; cellular components as collagen-containing extracellular matrix, focal adhesion, cell-substrate junction and extracellular matrix; and molecular functions such as structural constituent of cytoskeleton, extracellular matrix structural constituent, integrin binding, protein binding, signaling receptor binding. To finalize KEGG analysis demonstrated that EVs-WJ-MSCs proteome shown to be associated with pathways related to proteins differentially expressed, such as ECM-receptor interaction, tight junction, phagosome, and focal adhesion. Regarding the exclusive proteins found in EVs-WJ-MSCs, they are associated with intracellular homeostasis processes. Furthermore, they already show evidence of function in processes such as the synaptic vesicle cycle, as indicated by the analysis of KEGG and SynGO (Figure 11B and C), which is associated with neuronal communication. Li et al have already demonstrated that extracellular vesicles from umbilical cord-derived cells can regulate the synaptic vesicle cycle pathway and have shown through a preclinical model that these vesicles possess therapeutic potential.103
The proteomic profile of EV-hNPCs show proteins LTBP2, COL1A1, LAMA2, PFKL, COL4A2, GPC1, FBN2 and POSTN that are differently expressed, as seen in Figure 7B. It can be observed that the differentially expressed proteins in EV-hNPCs are distinct from those found in EVs-WJ-MSCs. These proteins, including LTBP2, COL1A1, COL4A2, FBN2, and POSTN, engage in ECM organization, maintenance, and remodeling.104,105 They may interact directly or indirectly through shared ECM components or signaling pathways.106 Proteins like LAMA2 and POSTN participate in cell adhesion and migration processes, which are essential for tissue development and repair.107 PFKL plays a role in glycolytic metabolism, which provides energy for various cellular processes, including ECM synthesis and remodeling.108,109 GPC1 and LTBP2 are involved in cell signaling pathways, potentially influencing cellular responses to growth factors and other signaling molecules.110 Six proteins are founded exclusively in EV-hNPCs: COL8A1, BMP10, ANOS1, BRCA2, ANKS1B, CEP250. Among these proteins, ANOS1 stands out as being involved in axonal guidance and migration of olfactory neurons.111,112 These ANOS1 gene mutation are associated with various neurodegenerative disease, such as Alzheimer and Parkinson.111 Bae et al demonstrated through the Xenopus embryos models that ANOS1 have a significant role in the development of craniofacial structures. The study shown that ANOS1 knockdown influences the development of derivatives from the adenohypophysis, olfactory, and optic placodes.113 This protein can be a crucial for signaling and improving neural development when present in the EV-hNPCs. Another important protein founded exclusively in EV-hNPCs is BMP10. BMP proteins are involved in various biological process as differentiation, immunosuppression, proliferation, tissue repair and apoptosis.114 Several studies show that BMPs are involved in the regulation and plasticity of brain development.115 Ogawa et al demonstrated that the BMP10 protein is widely expressed in the CNS of rats through immunocytochemistry, found in neurons, astrocytes, and ependymal cells.116 Thus, this protein also plays an important role in signaling for neuronal development. Protein ANKS1B (also known as AIDA or AIDA-1) is encoded by the gene ANKS1B and is also present in the normal development of the brain.117,118 ANKS1B is associated with tyrosine kinase signal transduction and post-synaptic density.119–121 Studies demonstrate that dysfunction of this protein contributes to non-health conditions such as schizophrenia,118 Alzheimer’s disease,122 and autism spectrum disorder.123 COL8A1, BRCA2, and CEP250 are proteins related to maintaining tissue integrity and potentially influencing cell signaling pathways,124,125 genomic stability,126 centrosome cohesion, and cell cycle,127 respectively.
These biological functions are imperative for neural development, and consequently, they are important findings in the characterization of EV-hNPCs. All this evidence corroborates with the results found in the functional enrichment analysis. The result, as shown in Figure 11, demonstrated that these proteins presented exclusively in EV-hNPCs are involved in biological processes as postsynaptic process involved in chemical synaptic transmission, and postsynapse to nucleus signaling pathway; and cellular component as postsynaptic density (intracellular component), presynaptic cytosol. This result demonstrates the signaling capacity of EVs for neuronal differentiation and functionality processes, making these vesicles an acellular material to be applied for the treatment of pre-clinical models of neurodegenerative diseases, such as Parkinson’s and Alzheimer’s, for example, considering that these cells have already been obtained free of the interferences and these hNPCs could differentiate into several neuronal lineages as described before128 as well been demonstrated their capacity to regenerate the nervous tissue in diabetic retinopathy.129
Research into preclinical applications of EV-hNPCs is essential to demonstrate the true therapeutic potential of these structures. Preliminary studies suggest significant promise, but it is imperative to conduct in-depth analysis in preclinical settings to validate their efficacy and safety. Additionally, the need for further characterizations, such as identifying the genetic material present in these vesicles, is crucial to fully understand their mechanisms of action and optimize their clinical application.
Conclusion
This study successfully obtained EVs from cultured human neural precursor cells without induction factors or gene transfections, making this product attractive for acellular therapies.
These EV-hNPCs show promising potential for therapeutic applications in neurodegenerative diseases, such as Parkinson’s and Alzheimer’s.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Human Ethics Research Committee of Pequeno Príncipe Faculties (CEP-FPP) and complies with the Declaration of Helsinki. Human umbilical cord (HUC) samples were collected from healthy mothers who signed a Free and Informed Consent Form. The study received approval, numbered 3.288.297, on April 26, 2019.
Acknowledgments
We want to thank the financial support of Araucaria Foundation (State of Paraná-Brazil), the Coordination for the Improvement of Higher Education Personnel – Brazil (Capes) by Scholarship for Post-Graduation Student of Pequeno Príncipe Faculties – Finance code 001.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by the National Council for Scientific and Technological Development (CNPq): Process: No. 442585/2020-9 of the Thematic Axis II—Development of Extensively Manipulated Products and Process: No. 406078/2024-6 of the Thematic Axis II Advanced Therapy Products, and By the Brazilian Funding Authority for Studies and Projects (FINEP), Process: No 0268/24.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Clarke D, Stanton J, Powers D, et al. Managing particulates in cell therapy: guidance for best practice. Cytotherapy. 2016;18(9):1063–1076. doi:10.1016/j.jcyt.2016.05.011
2. Majors S. Regenerative medicine: the pipeline momentum builds H1. Alliance Regener Med. 2022;2022:1–30.
3. Hu G, Drescher KM, Chen XM. Exosomal miRNAs: biological properties and therapeutic potential. Front Genet. 2012;3:56. doi:10.3389/fgene.2012.00056
4. Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5(1). doi:10.3402/jev.v5.31292
5. De Carvalho KAT, Steinhoff G, Chachques JC. Mesenchymal stem cell therapy in nonhematopoietic diseases. Stem Cells Int. 2015;2015:676903. doi:10.1155/2015/676903
6. Jimenez-Puerta GJ, Marchal JA, López-Ruiz E, Gálvez-Martín P. Role of mesenchymal stromal cells as therapeutic agents: potential mechanisms of action and implications in their clinical use. J Clin Med. 2020;9(2):445. doi:10.3390/jcm9020445
7. Yin L, Yang Z, Wu Y, et al. Label-free separation of mesenchymal stem cell subpopulations with distinct differentiation potencies and paracrine effects. Biomaterials. 2020;240:119881. doi:10.1016/j.biomaterials.2020.119881
8. Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic properties of mesenchymal stromal/stem cells: the need of cell priming for cell-free therapies in regenerative medicine. Int J Mol Sci. 2021;22(2):1–20. doi:10.3390/ijms22020763
9. Mishra VK, Shih HH, Parveen F, et al. Identifying the therapeutic significance of mesenchymal stem cells. Cells. 2020;9(5):1145. doi:10.3390/cells9051145
10. Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):1–20. doi:10.1186/s13287-015-0116
11. Fang S, Xu C, Zhang Y, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5(10):1425–1439. doi:10.5966/sctm.2015-0367
12. Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc. 2016;5(1). doi:10.1161/JAHA.115.002856
13. Moloudizargari M, Hekmatirad S, Mofarahe ZS, Asghari MH. Exosomal microRNA panels as biomarkers for hematological malignancies. Curr Probl Cancer. 2021;45(5):100726. doi:10.1016/j.currproblcancer.2021.100726
14. Zhang Y, Han T, Feng D, et al. Screening of non-invasive miRNA biomarker candidates for metastasis of gastric cancer by small RNA sequencing of plasma exosomes. Carcinogenesis. 2020;10(41):582–590. doi:10.1093/carcin/bgz186/5629159
15. Chen JJ, Yang G, Yan QQ, Zhao J, Li S. Exosome-encapsulated microRNAs as promising biomarkers for Alzheimer’s disease. Rev Neurosci. 2019;31(1):77–87. doi:10.1515/revneuro-2019-0001
16. Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14(1):66. doi:10.1186/s13287-023-03287-7
17. Xue Z, Liao Y, Li Y. Effects of microenvironment and biological behavior on the paracrine function of stem cells. Genes Dis. 2024;11(1):135–147. doi:10.1016/j.gendis.2023.03.013
18. Janockova J, Slovinska L, Harvanova D, Spakova T, Rosocha J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J Biomed Sci. 2021;28(1). doi:10.1186/s12929-021-00736-4
19. Luo T, von der Ohe J, Hass R. Msc-derived extracellular vesicles in tumors and therapy. Cancers. 2021;13(20):5212. doi:10.3390/cancers13205212
20. Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789–792. doi:10.1038/s41409-019-0616-z
21. Engle SJ, Blaha L, Kleiman RJ. Best practices for translational disease modeling using human iPSC-derived neurons. Neuron. 2018;100(4):783–797. doi:10.1016/j.neuron.2018.10.033
22. Jerber J, Seaton DD, Cuomo ASE, et al. Population-scale single-cell RNA-seq profiling across dopaminergic neuron differentiation. Nat Genet. 2021;53(3):304–312. doi:10.1038/s41588-021-00801-6
23. Chuang JH, Yang WC, Lin Y. Glutamatergic neurons differentiated from embryonic stem cells: an investigation of differentiation and associated diseases. Int J Mol Sci. 2021;22(9):4592. doi:10.3390/ijms22094592
24. Cope EC, Gould E. Adult neurogenesis, glia, and the extracellular matrix. Cell Stem Cell. 2019;24(5):690–705. doi:10.1016/j.stem.2019.03.023
25. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–1024. doi:10.1016/j.jcyt.2019.08.002
26. George S, Hamblin MR, Abrahamse H. Differentiation of mesenchymal stem cells to neuroglia: in the context of cell signalling. Stem Cell Rev Rep. 2019;15(6):814–826. doi:10.1007/s12015-019-09917-z
27. Zhang Z, Li Z, Deng W, et al. Ectoderm mesenchymal stem cells promote differentiation and maturation of oligodendrocyte precursor cells. Biochem Biophys Res Commun. 2016;480(4):727–733. doi:10.1016/j.bbrc.2016.10.115
28. Stricker PEF, de Souza Dobuchak D, Irioda AC, et al. Human mesenchymal stem cells seeded on the natural membrane to neurospheres for cholinergic-like neurons. Membranes. 2021;11(8):1–16. doi:10.3390/membranes11080598
29. Solis-Castro OO, Rivolta MN, Boissonade FM. Neural Crest-Derived Stem Cells (NCSCs) obtained from Dental-Related Stem Cells (DRSCs): a literature review on current knowledge and directions toward translational applications. Int J Mol Sci. 2022;23(5):2714. doi:10.3390/ijms23052714
30. Dupin E, Calloni GW, Coelho-Aguiar JM, Le Douarin NM. The issue of the multipotency of the neural crest cells. Dev Biol. 2018;444:S47–S59. doi:10.1016/j.ydbio.2018.03.024
31. Jayachandran J, Srinivasan H, Mani KP. Molecular mechanism involved in epithelial to mesenchymal transition. Arch Biochem Biophys. 2021;710:108984. doi:10.1016/j.abb.2021.108984
32. Battula VL, Evans KW, Hollier BG, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28(8):1435–1445. doi:10.1002/stem.467
33. Catala M. Overview of secondary neurulation. J Korean Neurosurg Soc. 2021;64(3):346–358. doi:10.3340/jkns.2020.0362
34. Martins LF, Costa RO, Pedro JR, et al. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-03592-1
35. Cho H, Seo YK, Jeon S, Yoon HH, Choi YK, Park JK. Neural differentiation of umbilical cord mesenchymal stem cells by sub-sonic vibration. Life Sci. 2012;90(15–16):591–599. doi:10.1016/j.lfs.2012.02.014
36. Camillo-Andrade AC, Santos MDM, Fischer JSG, et al. Proteomics reveals that quinoa bioester promotes replenishing effects in epidermal tissue. Sci Rep. 2020;10(1). doi:10.1038/s41598-020-76325-6
37. Carvalho PC, Lima DB, Leprevost FV, et al. Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0. Nat Protoc. 2016;11(1):102–117. doi:10.1038/nprot.2015.133
38. Xie Z, Bailey A, Kuleshov MV, et al. Gene set knowledge discovery with Enrichr. Curr Protoc. 2021;1(3). doi:10.1002/cpz1.90
39. Chen EY, Tan CM, Kou Y, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:1–4. doi:10.1186/1471-2105-14-S18-S1
40. Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(1):W90–W97. doi:10.1093/nar/gkw377
41. Koopmans F, van Nierop P, Andres-Alonso M, et al. SynGO: an evidence-based, expert-curated knowledge base for the synapse. Neuron. 2019;103(2):217–234.e4. doi:10.1016/j.neuron.2019.05.002
42. Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldaña HA. Mesenchymal stem cells current clinical applications: a systematic review. Arch Med Res. 2021;52(1):93–101. doi:10.1016/j.arcmed.2020.08.006
43. Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010;19(1):117–130. doi:10.1089/scd.2009.0177
44. Salehinejad P, Alitheen NB, Ali AM, et al. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. In Vitro Cell Dev Biol Anim. 2012;48:75–83. doi:10.1007/s11626-011-9480-x
45. Yoon JH, Roh EY, Shin S, et al. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. Biomed Res Int. 2013;2013:428726. doi:10.1155/2013/428726
46. Boey PYK, Lim SLD, Tang KF, et al. Comparative study of the methods of extracting mesenchymal stem cells from cryopreserved Wharton’s Jelly. J Stem Cells Regen Med. 2017;13(1):29–32. doi:10.46582/jsrm.1301005
47. Widowati W, Gunanegara RF, Rizal R, et al. Comparative analysis of Wharton’s Jelly Mesenchymal Stem Cell (WJ-MSCs) isolated using explant and enzymatic methods. J Phys Conf Ser. 2019;1374(1):012024. doi:10.1088/1742-6596/1374/1/012024
48. Dominici M, le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi:10.1080/14653240600855905
49. Liau LL, Ruszymah BHI, Ng MH, Law JX. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr Res Transl Med. 2020;68(1):5–16. doi:10.1016/j.retram.2019.09.001
50. Rizal, Syaidah R, Aqsha ZM, Josephin A, Pakpahan VM. Characterization, differentiation, and population doubling time of Wharton’s jelly mesenchymal stem cells (WJ-MSCs) in passage 5 and 8. AIP Conf Proc. 2021;2344. doi:10.1063/5.0047340
51. de Oliveira NB, Irioda AC, Stricker PEF, et al. Natural membrane differentiates human adipose-derived mesenchymal stem cells to neurospheres by mechanotransduction related to yap and amot proteins. Membranes. 2021;11(9):687. doi:10.3390/membranes11090687
52. Zhang HT, Chen H, Zhao H, Dai YW, Xu RX. Neural stem cells differentiation ability of human umbilical cord mesenchymal stromal cells is not altered by cryopreservation. Neurosci Lett. 2011;487(1):118–122. doi:10.1016/j.neulet.2010.10.008
53. Mukai T, Nagamura-Inoue T, Shimazu T, et al. Neurosphere formation enhances the neurogenic differentiation potential and migratory ability of umbilical cord-mesenchymal stromal cells. Cytotherapy. 2016;18(2):229–241. doi:10.1016/j.jcyt.2015.10.012
54. Zhang HT, Fan J, Cai YQ, et al. Human Wharton’s jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells. Differentiation. 2010;79(1):15–20. doi:10.1016/j.diff.2009.09.002
55. Dabrowska S, Sypecka J, Jablonska A, et al. Neuroprotective potential and paracrine activity of stromal vs. culture-expanded hMSC derived from Wharton Jelly under co-cultured with hippocampal organotypic slices. Mol Neurobiol. 2018;55(7):6021–6036. doi:10.1007/s12035-017-0802-1
56. Drela K, Lech W, Figiel-Dabrowska A, et al. Enhanced neuro-therapeutic potential of Wharton’s Jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy. 2016;18(4):497–509. doi:10.1016/j.jcyt.2016.01.006
57. Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9):3100–3110. doi:10.1111/cas.14563
58. Schepici G, Silvestro S, Mazzon E. Regenerative effects of exosomes-derived MSCs: an overview on spinal cord injury experimental studies. Biomedicines. 2023;11(1):201. doi:10.3390/biomedicines11010201
59. Wei W, Ao Q, Wang X, et al. Mesenchymal stem cell–derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2021;11. doi:10.3389/fphar.2020.590470
60. Racchetti G, Meldolesi J. Extracellular vesicles of mesenchymal stem cells: therapeutic properties discovered with extraordinary success. Biomedicines. 2021;9(6):667. doi:10.3390/biomedicines9060667
61. Forsberg MH, Kink JA, Hematti P, Capitini CM. Mesenchymal stromal cells and exosomes: progress and challenges. Front Cell Dev Biol. 2020;8(665):1–11. doi:10.3389/fcell.2020.00665
62. Jin Z, Ren J, Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int Immunopharmacol. 2020;78(155):105946. doi:10.1016/j.intimp.2019.105946
63. Luo P, Jiang C, Ji P, Wang M, Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. 2019;10(1):1–12. doi:10.1186/s13287-019-1341-7
64. Shojaati G, Khandaker I, Funderburgh ML, et al. Mesenchymal stem cells reduce corneal fibrosis and inflammation via extracellular vesicle-mediated delivery of miRNA. Stem Cells Transl Med. 2019;8(11):1192–1201. doi:10.1002/sctm.18-0297
65. Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther. 2019;10(1):1–16. doi:10.1186/s13287-019-1207-z
66. Laso-García F, Ramos-Cejudo J, Carrillo-Salinas FJ, et al. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS One. 2018;13(9):1–16. doi:10.1371/journal.pone.0202590
67. Huang JH, Yin XM, Xu Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J Neurotrauma. 2017;34(24):3388–3396. doi:10.1089/neu.2017.5063
68. Wei Z, Qiao S, Zhao J, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019;232:116632. doi:10.1016/j.lfs.2019.116632
69. Li M, Fang F, Sun M, Zhang Y, Hu M, Zhang J. Extracellular vesicles as bioactive nanotherapeutics: an emerging paradigm for regenerative medicine. Theranostics. 2022;12(11):4879–4903. doi:10.7150/thno.72812
70. Wu J, Chen LH, Sun SY, Li Y, Ran XW. Mesenchymal stem cell-derived exosomes: the Dawn of diabetic wound healing. World J Diabetes. 2022;13(12):1066–1095. doi:10.4239/wjd.v13.i12.1066
71. Trams EG, Lauter CJ, Salem N, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi:10.1016/0005-2736(81)90512-5
72. Zhou M, Weber SR, Zhao Y, Chen H, Sundstrom JM. Methods for exosome isolation and characterization. Exosomes. 2020;23–38. doi:10.1016/b978-0-12-816053-4.00002-x
73. Park KS, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10(1). doi:10.1186/s13287-019-1398-3
74. Patel GK, Khan MA, Zubair H, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-41800-2
75. Palviainen M, Saari H, Kärkkäinen O, et al. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J Extracell Vesicles. 2019;8(1). doi:10.1080/20013078.2019.1596669
76. Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol. 2020;8(149). doi:10.3389/fcell.2020.00149
77. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1). doi:10.1080/20013078.2018.1535750
78. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi:10.1038/nri2567
79. Mathieu M, Névo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1). doi:10.1038/s41467-021-24384-2
80. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–E977. doi:10.1073/pnas.1521230113
81. Witwer KW, van Balkom BWM, Bruno S, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1). doi:10.1080/20013078.2019.1609206
82. Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:1–8. doi:10.3410/B3-15
83. Costa-Ferrero ZSM, Rocha GV, Paredes BD, et al. Product characterization and preclinical evaluation of the biodistribution and safety of umbilical cord mesenchymal stromal cell-derived extracellular vesicles. 2023. doi:10.21203/rs.3.rs-2949774/v1.
84. van Balkom BWM, Gremmels H, Giebel B, Lim SK. Proteomic signature of mesenchymal stromal cell-derived small extracellular vesicles. Proteomics. 2019;19(1–2). doi:10.1002/pmic.201800163
85. Shin S, Lee J, Kwon Y, et al. Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and Wharton’s Jelly. Int J Mol Sci. 2021;22(2):1–17. doi:10.3390/ijms22020845
86. Zhang Z, Mi T, Jin L, et al. Comprehensive proteomic analysis of exosome mimetic vesicles and exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2022;13(1). doi:10.1186/s13287-022-03008-6
87. Fyodorov DV, Zhou BR, Skoultchi AI, Bai Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat Rev Mol Cell Biol. 2018;19(3):192–206. doi:10.1038/nrm.2017.94
88. Adams-Cioaba MA, Min J. Structure and function of histone methylation binding proteins. Biochem Cell Biol. 2009;87(1):93–105. doi:10.1139/O08-129
89. Zhao M, Liu S, Wang C, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS nano. 2020;15(1):1519–1538. doi:10.1021/acsnano.0c08947
90. Qian Z, Shen Q, Yang X, Qiu Y, Zhang W. The role of extracellular vesicles: an epigenetic view of the cancer microenvironment. Biomed Res Int. 2015;2015:1–8. doi:10.1155/2015/649161
91. Lomvardas S, Maniatis T. Histone and DNA modifications as regulators of neuronal development and function. Cold Spring Harb Perspect Biol. 2016;8(7):a024208. doi:10.1101/cshperspect.a024208
92. Geng H, Chen H, Wang H, Wang L. The histone modifications of neuronal plasticity. Neural Plast. 2021;2021:1–7. doi:10.1155/2021/6690523
93. Ramazi S, Allahverdi A, Zahiri J. Evaluation of post-translational modifications in histone proteins: a review on histone modification defects in developmental and neurological disorders. J Biosci. 2020;45(1). doi:10.1007/s12038-020-00099-2
94. Park J, Lee K, Kim K, Yi SJ. The role of histone modifications: from neurodevelopment to neurodiseases. Signal Transduct Target Ther. 2022;7(1). doi:10.1038/s41392-022-01078-9
95. Cai HQ, Zhang MJ, Cheng ZJ, et al. FKBP10 promotes proliferation of glioma cells via activating AKT-CREB-PCNA axis. J Biomed Sci. 2021;28(1). doi:10.1186/s12929-020-00705-3
96. Osugi Y, Fumoto K, Kikuchi A. CKAP4 regulates cell migration via the interaction with and recycling of integrin. Mol Cell Biol. 2019;39(16). doi:10.1128/mcb.00073-19
97. Tuffy KM, Planey SL. Cytoskeleton-associated protein 4: functions beyond the endoplasmic reticulum in physiology and disease. ISRN Cell Biol. 2012;2012:1–11. doi:10.5402/2012/142313
98. Yanagita K, Nagashio R, Jiang SX, et al. Cytoskeleton-associated protein 4 is a novel serodiagnostic marker for lung cancer. Am J Pathol. 2018;188(6):1328–1333. doi:10.1016/j.ajpath.2018.03.007
99. Araki K, Nagata K. Protein folding and quality control in the ER. Cold Spring Harb Perspect Biol. 2011;3(11):a007526–a007526. doi:10.1101/cshperspect.a007526
100. Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386(6627):843–847. doi:10.1038/386843a0
101. Alanay Y, Avaygan H, Camacho N, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86(4):551–559. doi:10.1016/j.ajhg.2010.02.022
102. Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129(6):705–733. doi:10.1007/s00418-008-0435-6
103. Li S, Zhang J, Liu X, et al. Proteomic characterization of hUC-MSC extracellular vesicles and evaluation of its therapeutic potential to treat Alzheimer’s disease. Sci Rep. 2024;14(1). doi:10.1038/s41598-024-56549-6
104. Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFβ and BMP signaling. Curr Opin Cell Biol. 2009;21(5):616–622. doi:10.1016/j.ceb.2009.05.005
105. Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2022;115(14):2817–2828. doi:10.1242/jcs.115.14.2817
106. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi:10.1126/science.1176009
107. Ceafalan LC, Dobre M, Milanesi E, et al. Gene expression profile of adhesion and extracellular matrix molecules during early stages of skeletal muscle regeneration. J Cell Mol Med. 2020;24(17):10140–10150. doi:10.1111/jcmm.15624
108. Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909.
109. Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579.
110. Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2009;9(5). doi:10.1186/gb-2008-9-5-224
111. Bhatia-Dey N, Heinbockel T. The olfactory system as marker of neurodegeneration in aging, neurological and neuropsychiatric disorders. Int J Environ Res Public Health. 2021;18(13). doi:10.3390/ijerph18136976
112. Kim SH, Hu Y, Cadman S, Bouloux P. Diversity in fibroblast growth factor receptor 1 regulation: learning from the investigation of Kallmann syndrome. J Neuroendocrinol. 2008;20(2):141–163. doi:10.1111/j.1365-2826.2007.01627.x
113. Bae CJ, Hong CS, Saint-Jeannet JP. Anosmin-1 is essential for neural crest and cranial placodes formation in Xenopus. Biochem Biophys Res Commun. 2018;495(3):2257–2263. doi:10.1016/j.bbrc.2017.12.127
114. Wozney JM. The bone morphogenetic protein family: multifunctional cellular regulators in the embryo and adult. Eur J Oral Sci. 1998;106(1 SUPPL):160–166. doi:10.1111/j.1600-0722.1998.tb02170.x
115. Jensen GS, Leon-Palmer NE, Townsend KL. Bone morphogenetic proteins (BMPs) in the central regulation of energy balance and adult neural plasticity. Metabolism. 2021;123. doi:10.1016/j.metabol.2021.154837
116. Ogawa C, Mikawa S, Li S, Hayashi Y, Masumoto K, Sato K. BMP10 expression in the adult rat central nervous system. J Chem Neuroanat. 2022;121:102084. doi:10.1016/j.jchemneu.2022.102084
117. Parra-Damas A, Saura CA. Synapse-to-nucleus signaling in neurodegenerative and neuropsychiatric disorders. Biol Psychiatry. 2019;86(2):87–96. doi:10.1016/j.biopsych.2019.01.006
118. Tindi JO, Chávez AE, Cvejic S, Calvo-Ochoa E, Castillo PE, Jordan BA. ANKS1B gene product AIDA-1 controls hippocampal synaptic transmission by regulating GluN2B subunit localization. J Neurosci. 2015;35(24):8986–8996. doi:10.1523/JNEUROSCI.4029-14.2015
119. Fu X, Mcgrath S, Pasillas M, Nakazawa S, Kamps MP. EB-1, a tyrosine kinase signal transduction gene, is transcriptionally activated in the t(1;19) subset of pre-B ALL, which express oncoprotein E2a-Pbx1. Oncogene. 1999;2(18). doi:10.1038/sj.onc.1202874
120. Jordan BA, Fernholz BD, Boussac M, et al. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3(9):857–871. doi:10.1074/mcp.M400045-MCP200
121. Enga RM, Rice AC, Weller P, et al. Initial characterization of behavior and ketamine response in a mouse knockout of the post-synaptic effector gene Anks1b. Neurosci Lett. 2017;641:26–32. doi:10.1016/j.neulet.2017.01.044
122. Ghersi E, Noviello C, D’Adamio L. Amyloid-β Protein Precursor (AβPP) intracellular domain-associated protein-1 proteins bind to AβPP and modulate its processing in an isoform-specific manner. J Biol Chem. 2004;279(47):49105–49112. doi:10.1074/jbc.M405329200
123. Carbonell AU, Cho CH, Tindi JO, et al. Haploinsufficiency in the ANKS1B gene encoding AIDA-1 leads to a neurodevelopmental syndrome. Nat Commun. 2019;10(1). doi:10.1038/s41467-019-11437-w
124. Gerth J, Cohen CD, Hopfer U, et al. Collagen type VIII expression in human diabetic nephropathy. Eur J Clin Invest. 2007;37(10):767–773. doi:10.1111/j.1365-2362.2007.01864.x
125. Hopfer U, Hopfer H, Meyer-Schwesinger C, et al. Lack of type VIII collagen in mice ameliorates diabetic nephropathy. Diabetes. 2009;58(7):1672–1681. doi:10.2337/db08-0183
126. O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–560. doi:10.1016/j.molcel.2015.10.040
127. Floriot S, Bellutti L, Castille J, et al. CEP250 is required for maintaining centrosome cohesion in the germline and fertility in male mice. Front Cell Dev Biol. 2022;9. doi:10.3389/fcell.2021.754054
128. De Dobuchak DS, Ferreira Stricker PE, Barth De Oliveira N, et al. The neural multilineage differentiation capacity of human neural precursors from the umbilical cord-ready to bench for clinical trials. Membranes. 2022;12:873. doi:10.3390/membranes12090873
129. Saçaki CS, Mogharbel BF, Stricker PEF, et al. Potential of human neural precursor cells in diabetic retinopathy therapeutics–preclinical model. Curr Eye Res. 2022;47(3):450–460. doi:10.1080/02713683.2021.2002909
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Mesenchymal Stem Cell-Derived Extracellular Vesicles: Emerging Therapies for Neurodegenerative Diseases
Chen P, Wang F, Ling B, Zhu Y, Lin H, Huang J, Wang X
International Journal of Nanomedicine 2025, 20:8547-8565
Published Date: 2 July 2025