Back to Journals » International Journal of Nanomedicine » Volume 20
Tailored Extracellular Vesicles from Dental Stem Cells: Advances in Specific Modifications for Enhanced Therapeutic Applications
Authors Liu X , Li Z, Fu J, Wang R, He J , Yao J, Ye Q, He Y
Received 13 March 2025
Accepted for publication 10 June 2025
Published 26 June 2025 Volume 2025:20 Pages 8327—8341
DOI https://doi.org/10.2147/IJN.S528190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lijie Grace Zhang
Xiaojing Liu,1,2 Ziwei Li,3 Jiao Fu,1,2 RuoXuan Wang,1,2 Jihui He,1,2 Juming Yao,1,2 Qingsong Ye,3 Yan He1,2
1First Clinical College, Wuhan University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 2Institute of Regenerative and Translational Medicine, Tianyou Hospital, Wuhan University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 3Center of Regenerative Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China
Correspondence: Qingsong Ye, Center of Regenerative Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China, Email [email protected] Yan He, Institute of Regenerative and Translational Medicine, Tianyou Hospital, Wuhan University of Science and Technology, Wuhan, Hubei, People’s Republic of China, Email [email protected]
Abstract: Extracellular vesicles(EVs) derived from dental stem cells have emerged as a key focus in regenerative medicine, owing to their remarkable ability to promote tissue repair and regeneration. Recent advancements revealed that targeted modifications can significantly boost their functional properties, creating new possibilities for the regeneration field. This article provides an overview of the most recent progress in EVs derived from dental stem cells research, with a particular emphasis on diverse engineering strategies such as genetic, chemical, and physical techniques, and their role in enhancing therapeutic performance. Furthermore, the influence of these engineering methods on the yield of EVs is thoroughly examined, offering critical perspectives for improving large-scale manufacturing efficiency. Pretreatment-generated conditioned dental stem cells-derived EVs are also explored as an innovative approach, demonstrating superior biological functions and regenerative potential. By integrating contemporary findings, this review underscores the superior capabilities of modified EVs derived from dental stem cells in driving progress in regenerative medicine and lays the groundwork for future investigations focused on clinical applications and therapeutic innovation.
Keywords: extracellular vesicles, preconditioning, regeneration, hypoxia, inflammation, predifferentiation
Introduction
Dental stem cells possess exceptional proliferation ability and the potential to differentiate into multiple lineages, highlighting their importance in tissue regeneration. Dental stem cells comprise approximately eight types,1 with dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SHEDs), periodontal ligament stem cells (PDLSCs), and stem cells from apical papilla (SCAPs) being the most extensively studied. Notably, DPSCs exhibit excellent immunomodulatory capacity coupled with low immunogenicity.2 Furthermore, dental stem cells isolation procedures present significant advantages, as they neither require invasive surgical interventions nor raise ethical concerns associated with other stem cell sources. These unique advantages position them as highly promising candidates for regenerative medicine applications.
EVs are particles with a lipid bilayer membrane structure released by cells at the nanoscale.3 Previous studies have demonstrated that stem cells exert immunomodulation and tissue regeneration primarily through paracrine signaling, with EVs serving as key mediators (eg, VEGF, miR-34a).4,5 The enhanced ability to penetrate biological barriers, superior therapeutic efficacy6,7 and “cell-free” characteristics render EVs attractive alternatives for mitigating the risks associated with live cell therapy. There have been numerous literature reports on the role of odontogenic EVs in inhibiting inflammation,8,9 exerting antioxidant effects,4 promoting tissue regeneration (osteogenesis,10–12 nerve regeneration),13 and facilitating tissue function recovery.14–16 Additionally, parental dental stem cells can be tailored to yield EVs with improved therapeutic potential. To tailor such EVs, cells could be preconditioned through methods including hypoxia, gene modification, growth factors, anti-inflammatory or inflammatory mediator pretreatment, and so on.17 In this article, we offer a comprehensive review of the recent studies on tailored EVs derived from preconditioned dental stem cells for improved therapeutic outcomes (Figure 1).
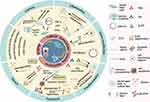 |
Figure 1 Preconditioning strategies and therapeutic applications of tailored extracellular vesicles. The schematic diagram reviews the therapeutic applications and pre-treatment strategies of dental stem cell-derived extracellular vesicles (EVs), which show significant potential in tissue recovery regeneration, anti-inflammation, and antioxidative effects. Key pre-treatment methods to enhance EVs efficacy include: 1. Hypoxia Preconditioning (via hypoxic culture or HIF-1α transfection) to boost pro-angiogenic and osteogenic potential. 2. Inflammatory Stimulation (using LPS, TNF-α, IFN-γ) to enhance immunomodulatory and pro-angiogenic properties. 3. Genetic Modification (via viral plasmid transfection or electroporation) to overexpress specific molecules and tailor EVs functions. 4. Pre-differentiation (using osteogenic/odontogenic medium) to improve osteogenic and odontogenic differentiation. 5. Apoptotic Induction (via staurosporine or serum starvation) to promote vascularization and tissue regeneration. Additionally, TWS119 pretreatment enhances EVs’ neurogenic potential, while high glucose exposure hinders periodontal regeneration. In summary, these strategies collectively optimize the therapeutic potential of dental stem cell-derived EVs for regenerative medicine applications. Created in BioRender. Liu, x. (2025) https://BioRender.com/v43r072. Abbreviations: EVs, extracellular vesicles; OM, osteogenic differentiation medium; Dex, dexamethasone; AA, ascorbic acid; β-GP, β-glycerophosphate; HIF-1α, hypoxia inducible factor-1; LPS, Lipopolysaccharide; TNF-α, tumor necrosis factor-alpha; IFN-γ, interferon-gamma. |
Current studies exhibit considerable inconsistency in EVs nomenclature, with a notable absence of standardized terminology. While the term “exosome” is widely adopted, the MISEV2023 guidelines explicitly discourage its use due to the inability of most isolation methods to verify a pure exosomal origin.3 To ensure terminological consistency, this review adheres to the nomenclature convention established in the MISEV2023 guidelines by the International Society for Extracellular Vesicles (eg, using “EVs” instead of “exosomes” where applicable).
Hypoxia
The physicochemical properties and even physiological functions of EVs secreted by cells are highly sensitive to cell culture conditions, with oxygen tension being a particularly critical determinant: Oxygen is essential for biological survival and participates in various biological reaction processes.18 Physiological oxygen levels in most tissues range from 1% to 5%, significantly lower than the 21% O2 typically maintained in conventional cell culture systems. This physiological disparity may introduce significant experimental bias, particularly given the established capacity of hypoxic conditions to modify EVs contents19 and therapeutic efficacy.20
Of clinical relevance, EVs derived from dental stem cells subjected to oxygen restriction exhibit a stronger potential in promoting angiogenesis and immunomodulatory capacity, suggesting their utility as novel biologics for regenerative medicine applications.21
Liu et al21 reported that when SCAPs were cultured under hypoxic conditions (1% O2, 5% CO2 for 48 hours), the resulting EVs exhibited similar morphology to normoxic ones, but with markedly increased particle yield, protein content, and surface markers expression (Alix, Tsg101, CD81), suggesting that hypoxia stimulates EVs secretion from SCAPs. Functionally, hypoxic EVs demonstrated superior uptake by human umbilical vein endothelial cells (HUVECs), significantly promoting their proliferation, migration, and tube formation capacity in vitro. Mechanistically, this pro-angiogenic effect was attributed to the activation of the Notch/JAG1/VEGF signaling axis. Complementing these findings, Lin et al22 identified miR-126 as a key mediator transferred via hypoxia-primed SCAPs EVs to HUVECs, where it downregulated sprouty-related expression, EVH1 domain containing protein 1 (SPRED1) to activate the extracellular signal regulated kinase(ERK) signaling pathway. This mechanism was further validated in vivo, with miR-126-enriched EVs inducing robust CD31+ and α-SMA+ vasculature in matrigel plug assays. The enhanced vascularization not only provides critical oxygen and nutrient supply, a prerequisite for successful tissue regeneration, but also creates a favorable microenvironment for osteogenesis. This mechanistic link is exemplified in the work of Gao et al,23 authors demonstrated that EVs from hypoxia-preconditioned SHEDs, while morphologically unchanged, exhibited enhanced osteogenic and angiogenic capacities. Notably, when loaded into injectable porous poly lactide-co-glycolide microspheres with bioinspired polydopamine coating, EVs achieved sustained release and significantly improved skull regeneration, highlighting the synergy between EVs therapeutics and biomaterial engineering.23
The degree of hypoxia used in preconditioning protocols varies across studies, but even moderate hypoxia can significantly alter EVs function. Unlike the previous study that used 1% O2 for hypoxia, in the study of Li et al,24 DPSCs were treated with 21% O2 and 2% O2 for 48 hours to obtain normoxic and hypoxic EVs. Hypoxic EVs exhibited stronger angiogenic effects than normoxic EVs, promoting HUVECs proliferation, migration, and tube formation ability. This effect is mediated by upregulated VEGFA and SDF-1, with lysyl oxidase-like 2(LOXL2) identified as a critical cargo protein, silencing LOXL2 abolishes the pro-angiogenic capacity of hypoxic EVs.25 Similarly, SHEDs-derived hypoxic EVs showed increased size and secretion, likely due to Rab27A-mediated biogenesis. Their enhanced angiogenic activity is linked to let-7f-5p/AGO1/VEGF and miR-210-3p/ephrinA3 signaling.26 These findings suggest that hypoxic preconditioning enhances the functional effects of EVs, but the optimal oxygen level deserves further investigation.
As mentioned above, hypoxic cultivation of maternal cells has established hypoxia inducible factor-1 (HIF-1α) as a master regulator of cellular adaptation.27 Notably, under hypoxic conditions, HIF-1α exhibits stable expression and serves as a key regulator for a wide array of genes linked to cellular proliferation and apoptotic processes.28 Building on these findings, part of the research employed genetic engineering technology to directly regulate HIF-1α expression, thereby increasing the therapeutic potential. As reported in the study of Hernán Gonzalez-King et al,29 lentivirus-mediated overexpression of HIF-1α in DPSCs significantly increased the production of Jagged1-rich EVs. Such EVs also possessed a superior angiogenic capacity compared to general EVs. This approach presents a promising strategy for ischemic conditions. Further innovation comes from Marta Gómez-Ferrer et al,30 who developed immortalized HIF-1α/telomerase-overexpressing DPSCs by combining genetic engineering and pro-inflammatory stimulation. The resulting EVs exhibited not only improved yield and homogeneity but also enhanced immunomodulatory properties.
Hypoxic preconditioning effectively enhances EVs yield and angiogenic potential across dental stem cells, yet critical gaps persist: (1) Oxygen optimization: Cell-type-specific responses to hypoxia remain uncharacterized, with no consensus on maximal efficacy thresholds. (2) Clinical translation: Hypoxic preconditioning is cost-requiring, and its protocol remains unstandardized; (3) Safety concern: The potential for residual pro-inflammatory factors to induce adverse immune reactions remains an important safety consideration; HIF-1α editing offers precision but carries genomic instability risks.
Inflammation
Evidence from multiple studies indicates that the inflammatory microenvironment can exert relatively positive impacts on stem cells, improving their proliferation, differentiation, adhesion, and other capabilities to promote tissue repair. Researchers believe that this function involves intercellular information exchange mediated by EVs.31
Zhang et al31 observed an enhanced vascularization phenomenon in periodontitis, and further isolated PDLSCs from healthy and periodontitis patients’ teeth. The research results demonstrated that inflammation not only promoted the secretion of EVs by PDLSCs but also enhanced their angiogenic potential through miR-17-5p/VEGFA signaling.31 Similarly, Wang et al32 reported that EVs secreted by inflammatory PDLSCs isolated from teeth of periodontitis patients could regulate macrophage polarization and immune responses through the miR-143-3p-regulated PI3K/AKT/NF-κB pathway, presenting a promising therapeutic strategy for periodontitis.32
Beyond obtaining stem cells directly from inflamed tissues, corresponding inflammatory cytokines can also be used to stimulate stem cells to simulate an inflammatory environment. One commonly used inflammatory factor is Lipopolysaccharide (LPS), which is widely applied in experimental studies to induce inflammatory responses. LPS stimulation significantly augments the pro-angiogenic capacity of EVs secreted by DPSCs, as evidenced by the enhanced proliferation, migration, and tube formation ability of HUVECs. This functional enhancement is mediated through angiogenesis-related miRNA in LPS-preconditioned EVs.33 Notably, Li et al34 has reported that LPS-preconditioned DPSCs EVs exhibit a more pronounced ability to enhance proliferation, migration, and odontogenic differentiation of Schwann cells (SCs), making them highly valuable for tooth injury repair.
In fact, LPS preconditioning not only facilitates tissue regeneration but also confers robust immunomodulatory properties to EVs. Umar et al35 used LPS/IFN-γ to polarize primary bone marrow macrophages into M1-like cells and IL-4 stimulation to polarize them into repair phenotype M2-like cells as models in vitro. EVs derived from LPS-preconditioned DPSCs exhibited stronger anti-inflammatory activity, significantly suppressing pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) while elevating anti-inflammatory mediators (IL-10 and TGF-β). These effects are mediated through EVs-enriched miRNAs, particularly miR-320a-3p, which potentially modulate the TLR4/NF-κB signaling pathway. Other evidence further supports the immunomodulatory potential of LPS-preconditioned dental stem cell EVs. EVs derived from LPS-preconditioned dental follicle stem cells (DFSCs) demonstrated enhanced therapeutic potential for periodontitis treatment. While maintaining typical EVs morphology, the production increased significantly (1.5-fold) and exhibited potent immunomodulatory effects, including promoting macrophage M2 polarization, exerting antioxidant effects,36 and regulating apoptotic/anti-apoptotic gene expression.37 Notably, while most studies indicate that LPS stimulation enhances the ability of dental stem cells EVs to mitigate inflammation, this appears to be context-dependent. For instance, EVs derived from LPS-stimulated PDLSCs were reported to promote M1 polarization,32,38 whereas Zheng et al39 observed that EVs from LPS-preconditioned PDLSCs impaired immunomodulation capacity via the miR-155-5p/SIRT1 axis, disrupting T helper cell 17(Th17) /regulatory T cell(Treg) balance compared to general EVs.39 This conflicting finding prompts the reasonable assumption that the impact of LPS preconditioning on dental stem cells EVs’ effects may depend on their tissue origin, highlighting the need for tailored inflammatory preconditioning strategies to optimize the therapeutic potential of EVs.
TNF-α is also one of the inducers used to simulate inflammation. Notably, pretreatment with TNF-α not only increases EVs yield but also augments their immunomodulatory and regenerative capacities31 through distinct molecular mechanisms. Yuki Nakao et al40 demonstrated that EVs from TNF-α-pretreated gingiva-derived mesenchymal stem cells (GMSCs) exhibited a 2.7-fold increase in productivity while maintaining standard biophysical characteristics. These tailored EVs exerted enhanced anti-inflammatory effects by promoting M2 macrophage polarization through the miR-1260b/Wnt5a/RANKL axis. This mechanism was particularly effective for periodontitis treatment, as it concurrently inhibited osteoclastogenesis and bone resorption. Similarly, Yan et al41 reported that inflammatory priming doubled EVs production from DPSCs without altering vesicular morphology, with the miR-758-5p/LMBR1/BMP2/4 pathway accounting for their superior osteo/odontogenic differentiation capacity. Beyond dental applications, Yu et al42 applied the EVs secreted by TNF-α-stimulated GMSCs in the treatment of glaucoma based on previous research findings that conditioned medium of mesenchymal stem cells (MSCs) stimulated by TNF-α has the stronger anti-allergic ability.43 The study revealed that preconditioned EVs exerted neuroprotective effects in a glaucoma model by exerting anti-apoptotic effects on retinal ganglion cells and inhibiting neuroinflammation via the MEG3/miR-21-5p/PDCD4 cascade, effectively attenuating retinal ischemia-reperfusion injury.42 However, TNF-α is a well-characterized pro-inflammatory cytokine44 capable of inducing adverse effects at elevated concentrations or extended exposure periods, its biological impact is highly dependent on dosage and treatment duration. In the current study, the parameters (TNF-α at 10 ng/mL for 48 hours) have been established to induce beneficial immunomodulation.42
Furthermore, an investigation by Gratpain et al45 employed TNF-α and IFN-γ stimulation to characterize EVs modifications from SCAPs, revealing significant miRNA profile alterations without detectable lipidomic changes. However, these original or tailored EVs failed to attenuate inflammation in both the LPS-challenged BV2 cell and spinal cord sections models. Therefore, researchers do not believe that EVs play a key role in the reported effects of SCAPs.
In conclusion, while numerous studies demonstrate that pro-inflammatory priming enhances the anti-inflammatory potential of dental stem cell-derived EVs, the outcomes of such pretreatment exhibit significant variability (not always reproducible across models). Reported effects range from negligible to paradoxical, suggesting that subtle variations in experimental parameters may critically influence the results. Table 1 provides a systematic summary of the key inflammatory preconditioning parameters for comparative analysis.
 |
Table 1 Summary of Inflammation Preconditioning Parameters for Dental Stem Cells-Derived EVs |
Gene Modification
EVs have emerged as promising gene delivery tools owing to their inherent biocompatibility and biomimetic membrane properties. Through genetic modification of parental cells, researchers can generate engineered EVs enriched with therapeutic nucleic acid (eg miRNA). These bioengineered nanovesicles serve as targeted molecular delivery platforms, demonstrating superior therapeutic outcomes compared to conventional delivery systems. Current research on genetic modification of dental stem cell-derived EVs remains limited, with electroporation and viral transduction being the predominant delivery methodologies employed.
Lin et al46 used electroporation to transfect miR-140-5p mimetics into DPSCs, constructing DPSCs overexpressing miR-140-5p and isolating EVs overexpressing miR-140-5p. miR-140-5p-enriched EVs demonstrated superior efficacy in cartilage repair by mitigating inflammatory chondrocyte apoptosis, offering promise for osteoarthritis treatment. Faezeh Vakhshiteh et al47 used XMIRXpress-34a lentivectors to prepare DPSCs overexpressing miR-34a, producing miR-34a-enriched EVs. Notably, miR-34a modification not only improved EVs production yield but also endowed them with potent anti-tumor properties against breast cancer cells in terms of apoptosis, migration, and invasion. Beyond miRNA engineering, innovative gene therapy approaches have been applied. The yCD::UPRT retroviral system created DPSCs capable of producing EVs that combine gene therapy and chemotherapy.48 This dual-action platform converts the prodrug 5-fluorocytosine into cytotoxic 5-fluorouracil while simultaneously delivering chemotherapeutic agents, resulting in synergistic anti-tumor effects against pancreatic cancer at reduced drug doses. For inflammatory conditions, the P2X7 receptor (P2X7R) has proven valuable in protecting PDLSCs from inflammation-induced damage. P2X7R-modified PDLSCs secreted EVs that enhance osteogenic potential and mitigate inflammatory responses, suggesting applications in periodontal regeneration.49
From the perspective of transfection efficiency, viral vector transfection can increase the content of target genes in EVs by 15 times,47 which is significantly better than the 4-fold increase brought by electroporation.46 However, the potential risk of genomic integration associated with viral vectors remains a critical concern. Unfortunately, the aforementioned study did not conduct any relevant safety assessments. There are various ways of genetic modification, each offering distinct advantages in terms of cell type compatibility, expression durability, and operational feasibility. Importantly, while genetic modification enables precise functional modulation of EVs, it also introduces potential off-target effects, necessitating a careful balance between efficiency and safety in methodological selection. Researchers must establish robust risk assessment protocols to mitigate unintended consequences and ensure clinical translational viability.
Predifferentiation
The general EVs have limited capacity to direct cellular differentiation, whereas EVs secreted by specific cell types can induce stem cell-specific lineage commitment. Therefore, some scholars have achieved the expected results by inducing the differentiation of stem cells and harvesting conditioned EVs. For dental stem cells, due to their inherent osteogenic and odontogenic properties, researchers commonly use osteogenic differentiation induction protocols: using osteogenic culture medium (OM) supplemented with 100 nM dexamethasone, 10mmol/L β-glycerophosphate sodium, and 50 μmol/L ascorbic acid on the basis of proliferation medium for induction. The experiment confirmed that the EVs obtained through this treatment indeed exhibited stronger osteogenic and odontogenic efficacy.
When SHEDs were induced with dental induction medium containing dexamethasone, the resulting EVs (OM-EV) exhibited altered cargo profiles, potentially mediated by the AMPK-mTOR signaling pathway.50 Although OM-EV showed reduced proliferation and migration effects on DPSCs compared to normal culture-derived EVs, they significantly enhanced odontogenic differentiation, as evidenced by corresponding staining results. This potential was further confirmed using 5% GelMA hydrogel loaded with 500 μg/mL OM-EV in a root slice model, which promoted dentin formation and mineralization. Similar effects were observed with osteogenically differentiated SHEDs-derived EVs, which promoted PDLSCs proliferation in a dose-dependent manner and enhanced osteogenic differentiation compared to control EVs.51 These effects were associated with increased Wnt 3a and BMP 2 expression, activating Wnt/β-catenin and BMP/Smad signaling pathways. The therapeutic benefits of tailored EVs extend to DPSCs. When combining EVs derived from osteogenic-induced DPSCs with hydrogel scaffolds, significant enhancement in bone remodeling capacity is observed, marked by upregulated BMP2 and ALP expression and increased formation of calcium nodules.52 Similar effects have also been reflected in the research of Hu et al,53 authors attributed this enhanced osteogenic activity to miR-27a-5p, which is 11 times more abundant in the odontogenic differentiation-induced DPSCs EVs and promotes odontogenic differentiation of DPSCs through downregulation of LTBP-1 and activation of the TGFβ1/Smads pathway. It is worth noting that the regulatory effect of predifferentiated EVs exhibits directional precision. As evidenced by Xie et al,54 PDLSCs were cultured in differentiation medium for 5 days to obtain specific EVs, which promote osteoclastogenesis via the circ_0000722/NF-κB/AKT axis. This bidirectional regulatory ability (promoting both osteogenesis and osteoclastogenesis) provides a new strategy for bone regeneration, indicating that predifferentiated EVs have unique application value in bone tissue engineering.
Interestingly, the efficacy of predifferentiation exhibits temporal dependence, with prolonged differentiation periods yielding stronger effects. Liu et al55 demonstrated this phenomenon using EVs derived from PDLSCs induced by OM for 14 days, which showed superior ALP, ARS staining, and osteogenic genes expression in bone marrow mesenchymal stem cells (BMSCs) compared to earlier time points (3 days and 7 days). While miRNA profiling revealed osteogenesis-related pathway enrichment, the precise mechanisms require further investigation. This temporal pattern was corroborated by Zhang et al,56 who induced DPSCs for 4 and 7 days using OM to obtain corresponding EVs. Seven-day induced EVs significantly enhanced ALP activity and osteogenic markers expression (Runx 2, OCN, and CollAl) versus controls. Notably, when loaded onto modified titanium scaffolds, the loading capacity of EVs was increased to nearly 5 times, and the system promoted robust cranial bone regeneration with sustained kinetics. RNA sequencing implicated MAPK and AMPK pathways in this process, though functional validation remains pending.56
While EVs derived from predifferentiated dental stem cells demonstrate considerable therapeutic promise, critical translational challenges remain to be resolved. The process of physiological bone remodeling depends on the precise regulation of osteogenic and osteoclastic activities. However maintaining this balance in bone tissue regeneration presents a considerable challenge, current research often overlook the necessity for sophisticated delivery platforms capable of precise spatiotemporal modulation to prevent pathological osteoclast activation. Additionally, the use of dexamethasone as a key inducer in predefferentiation protocols raises concerns regarding the long-term biosafety of derived EVs, particularly with respect to their potential to induce epigenetic modifications that warrant thorough investigation.
Apoptosis
The traditional view holds that apoptosis is a silent form of cell death, but recent studies have found that apoptotic cells release signals that promote mitosis in neighboring cells, which is essential for tissue regeneration and maintenance of tissue homeostasis.57,58 EVs generated during apoptosis are important signal mediators, as biogenesis59 differs from conventional vesicles and therefore contains unique contents (eg cleaved Caspase 3).60 Previous studies have shown that they can play a better role in promoting regeneration.57,61,62 For the convenience of discussion, this review uniformly refers to such EVs as apoptotic EVs. Current in vitro apoptosis induction methods, including staurosporine (STS) treatment, inflammatory factors, H2O2 exposure, UVC irradiation, and serum-free culture,63 remain underutilized in dental stem cells research. Currently, STS and serum starvation induction are mainly used, and their efficacy in promoting angiogenesis and bone regeneration is mainly focused on, which may limit the comprehensive analysis of their apoptotic EVs characteristics.
Li et al64 confirmed the necessity of DPSCs apoptosis for pulp regeneration through a nude mouse model: the use of apoptosis inhibitor Z-VAD can block the regeneration process, while 0.5 μM STS-induced DPSCs apoptotic EVs can significantly promote pulp-like tissue and vascular formation. These effects were further confirmed in preclinical large animals (dogs) and coculturation with endothelial cells (ECs) in vitro. Mechanism studies have shown that apoptotic EVs regulate ECs autophagy and activate angiogenesis by transferring TUFM, ultimately promoting effective regeneration of dental pulp. Fei et al65 further revealed that apoptotic EVs derived from DPSCs could be internalized by HUVECs, significantly enhancing cell migration, tube formation ability, and VEGFA expression at a concentration below 25 μg/mL. Their effects are mediated through the PI3K/Akt/VEGF pathway, and both safety and efficacy are considered in vivo experiments. The vascular regeneration function of apoptotic EVs derived from dental stem cells has been extended. In a mouse oxygen-induced retinopathy (OIR) model, Jing et al66 demonstrated that SHEDs-derived apoptotic EVs exhibit dual therapeutic actions: suppression of pathological angiogenesis coupled with PD1/PDL1 mediated regulation of ECs glycolysis, which collectively promoted physiological vascular reconstruction and visual function recovery. Remarkably, unilateral administration induced bilateral therapeutic effects, indicating systemic bioavailability.
Similarly, Yang et al67 demonstrated that apoptotic EVs derived from STS-induced DPSCs were effectively internalized by BMSCs, enhancing osteogenic differentiation as evidenced by increased ALP and ARS staining positivity and higher expression of related bone markers (OCN, RUNX2, ALP, and COL1A1). It is worth mentioning that the authors have done more in-depth research on osteoporosis, which is a systemic bone disease and was divided into premenopausal osteoporosis and senile osteoporosis.67 In ovariectomy-induced osteoporosis models, apoptotic EVs restored bone mineral deposition rates to sham-operated levels, with comparable efficacy observed in 18-month-old aged osteoporotic mice. In addition, combining apoptotic EVs with polydopamine-coated PLGA scaffolds significantly augmented new bone formation with better bone morphology in rat calvarial defects. Mechanistically, these pro-osteogenic effects were mediated through ERK1/2 pathway activation.67
Instead of inducing apoptosis with chemicals, Sunartvanichkul et al68 successfully isolated SHEDs apoptotic EVs using a 3-week serum starvation protocol. Specifically, the SHEDs with an 80% fusion rate in normal culture were converted to the FBS-free medium for 3 weeks without replacement, and then the apoptotic EVs were isolated from the conditioned medium. Compared to conventional EVs, these apoptotic EVs demonstrated superior pro-angiogenic activity, enhancing both HUVECs proliferation migration rate and vascular network complexity (evidenced by elevated tube density, connectivity, and total length in the tube formation assay).
Despite demonstrating significant therapeutic potential in tissue regeneration (particularly angiogenesis and bone repair), the clinical translation of dental stem cell-derived apoptotic EVs faces substantial hurdles. A major limitation stems from the current reliance on only two induction approaches: STS treatment and serum deprivation, with insufficient comparative studies to standardize induction parameters. This methodological constraint impedes robust evaluation of apoptotic biological activity, mechanistic characterization, and cross-study preclinical validation, ultimately hindering clinical advancement.
Glycogen Synthase Kinase-3β Inhibitor and Glucose
In addition to the above common preconditioning methods in the field of EVs of odontogenic stem cells, there are also some studies using relatively unique pretreatment methods for modification. In Wang et al’s69 study, human SHEDs stimulated by glycogen synthase kinase-3β inhibitor TWS119 activate the Wnt signaling pathway, and its derived EVs have been shown to promote neural differentiation of embryonic cancer cell line P19 from aspects of neurofilament and synapse formation. Lu et al70 compared the ability of EVs produced by PDLSCs under normal and high glucose culture conditions to save alveolar bone loss in periodontitis, and found that EVs could promote periodontal regeneration by inhibiting osteoclast formation. Notably, the effect of EVs derived from high glucose-preconditioned PDLSCs is relatively weak, which may be explained by the diabetic hyperglycemic microenvironment that hinders periodontal repair. However, EVs still played a regulatory role in alveolar bone regeneration by targeting eNOS via miR-31-5p, providing a new idea for diabetic periodontitis.
Higher Extracellular Vesicles Production
A major challenge hindering the clinical translation of EVs is their inherently low bioproduction yield. Current evidence indicates that 106 cells can only produce 1 to 4 μg of EVs per day, which is far from being available for clinical application.71 As mentioned above, hypoxia,21,23,26,29 inflammation,31,36,40,41 differentiation50 and gene modification47 have all been reported not only to demonstrate superior performance in corresponding disease models but also have been mentioned to increase EVs production to varying degrees (Figure 2). Unfortunately, these studies do not seem to provide a more detailed explanation of the specific reasons, which may constitute a worthwhile area for researchers to further investigate and organize. In addition to these simulations of physiological stimuli mentioned in this article, 3D-culture72,73 is also a method to enhance EVs production.
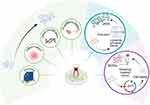 |
Figure 2 Methods to increase the production of vesicles secreted by dental stem cells. This figure outlines the pre-treatment methods employed to enhance production in the field of extracellular vesicles derived from dental stem cells. These methods include hypoxia, inflammation, gene modification, and pre-differentiation, which mimic physiological conditions. Furthermore, mechanical techniques such as extrusion are utilized to produce more high-yield and uniformly sized vesicle-like structures(eNVs), while repeated freeze-thaw cycles facilitate the acquisition of high-yield novel internal vesicles(ACDVs). Created in BioRender. Liu, x. (2025) https://BioRender.com/e89g046. Abbreviations: eNVs, extruded nanovesicles; ACDVs, a new type of artificial cell-derived vesicles. |
However, compared with these physiological simulation interventions, some mechanical methods may have more significant effects on production, such as microfluidic fabrication,74 sonication,75 and cellular-nanoporation method,76 they have become an alternative solution to the drawbacks of EVs’ low yield. However these methods rely on sophisticated and expensive instruments and associated operational costs present significant barriers to widespread implementation. In response to the demand for increased production of EVs from dental stem cells, recent research has shifted towards simplified alternative solutions, namely the preparation of engineered vesicles with EVs-like functions through extrusion and freezing-thawing cycling. These methods not only avoid the dependence on complex equipment but have also been proven to significantly improve vesicle yield, providing a feasible pathway for clinical translation.
Liang et al77 adopted the method of extrusion to get extruded nanovesicles, named as eNVs, specifically, creating by passing them through polycarbonate filter membranes using a liposome extrusion device. Structural analysis revealed that eNVs possess a typical phospholipid bilayer-enclosed vesicular architecture resembling EVs, both expressing specific EVs markers, but with more uniform size and higher protein production (136.8 times that of EVs) and higher particle count (41.1 times that of EVs). eNVs demonstrated a good effect in promoting periodontitis healing. In vitro, eNVs exhibited superior ability to promote PDLSCs migration and proliferation compared to EVs, while upregulating key osteogenic markers (ALP, CIL1, BMP2, and RUNX2) at the protein level. In the SD rat periodontal defect model, eNVs were delivered by matrix collagen and gathered at the periodontal defect, promoting diffuse new bone formation. Compared to the control group, treatment with eNVs resulted in elevated bone parameters, including bone surface area, bone trabecular number, and osteogenic markers such as RUNX2 and COL, although the effect was not superior to EVs. Collectively, the remarkable production yield of uniform eNVs and their comparable enhancement of osteogenic activity establish them as a promising EVs alternative.
In another research, Duan et al78 proposed a new class of vesicles, they obtained a new type of artificial cell-derived vesicles (ACDVs) by freezing-thawing multiple times. To be specific, the cells will be fully lysed through dilation with pure water and repeated freezing-thawing, and the remaining vesicles will be actively released in advance to obtain the cell lysate of DPSCs, then ACDVs will be isolated by ultracentrifugation. The novel ACDVs exhibited a significantly higher yield, approximately 16-fold greater, and superior purity in comparison to EVs. No notable differences were observed in size or cytokine composition between the two types, with both exhibiting a lipid bilayer spherical structure, comparable EVs marker expression, and similar protein secretion profiles. Regardless, certain protein components in ACDVs (eg BDNF) show higher levels than those in EVs. ACDVs exerted functional effects by stimulating HUVECs’ proliferation, migration, and vascularization, thereby effectively aiding wound healing. In a mouse burn model, ACDVs exhibited a wound-healing promotion similar to EVs, reducing the wound size to one-sixth of its initial area, half the size seen in the control group. Additionally, the levels of the anti-inflammatory factor and angiogenesis factor were elevated, while the inflammatory factor IL-1β was markedly reduced compared to the control group. Furthermore, the wound area displayed a more granulation tissue formation and increased regeneration of collagen and elastic fibers, emphasizing ACDVs’ role in enhancing wound healing. In conclusion, DUAN’s study introduces a novel class of ACDVs that exhibit comparable efficacy to EVs while offering a significantly higher yield, making them more suitable for clinical applications.
While engineered vesicles (eNVs and ACDVs) demonstrate substantial production advantages over natural EVs, their therapeutic performance remains merely comparable to or marginally superior to natural EVs. This efficacy-yield discrepancy raises critical concerns regarding their bioequivalence, suggesting the potential loss of crucial regulatory components during artificial fabrication. Additionally, as defined by MISEV2023 guidelines, both eNVs and ACDVs belong to EV mimetics(EVMs), representing vesicles passively released through pressure application, in contrast to natural EVs that undergo active cellular secretion. This fundamental distinction in biogenesis raises several safety considerations. Table 2 lists the pros and cons of eNVs and ACDVs for clinical translation.
 |
Table 2 Strengths and Limitations of eNVs and ACDVs for Clinical Applications |
Additionally, vesicle production varies substantially across intervention methods, with studies employing disparate quantification metrics (eg particle counts, protein concentration, or surface markers). These variations are comprehensively compared in Table 3.
 |
Table 3 Alterations in Vesicle Yield Resulting From Different Treatment Methods |
Conclusion
In summary, preconditioning strategies (hypoxia, inflammation, gene modification, etc) differentially enhance the therapeutic potential of dental stem cells-derived EVs, as systematically compared in Table 4. While no universal optimal strategy exists, selection criteria should align with target applications: hypoxia/apoptosis-primed EVs for angiogenesis, immunomodulatory-polarized EVs for inflammatory conditions, and genetically-modified/ predifferentiated EVs for osteo/odontogenic regeneration.
 |
Table 4 Comparative Analysis of EVs Preconditioning Strategies |
Current advances in preconditioning strategies for dental stem cells-derived EVs demonstrate significant therapeutic potential, yet critical challenges remain to be addressed for clinical translation: (1) Standardization challenges: Current studies exhibit substantial heterogeneity in processing protocols, necessitating the establishment of well-defined operational standards for clinical translation. Critical parameters (preconditioning duration, cytokine concentrations, etc) require systematic optimization and consensus guidelines. (2) Combinatorial strategies: Preconditioning approaches demonstrate synergistic potential when integrated with complementary modification techniques. For instance, coupling yield-enhancement methods (eg dental stem cells extrusion or freezing-thawing cycling) with functional preconditioning could generate engineered vesicles with both high productivity and disease-specific therapeutic properties. This dual-optimization paradigm addresses two major translational barriers: low EVs yield and limited targeting specificity, while enabling personalized therapeutic development for dental applications. (3) Safety considerations: Rigorous preclinical safety assessments are necessary, including comprehensive biodistribution analyses, target organ toxicity profiling, verification of delivery precision, and immunogenicity evaluation.
Collectively, all preconditioning strategies face scalability challenges requiring GMP-compliant optimization, and future efforts should prioritize combinatorial approaches to harmonize therapeutic efficacy with clinical translatability, thereby advancing the development of tailored EVs for regenerative medicine applications.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (grant number U22A20314), Wuhan University of Science of Technology startup fund (Chu Tian Scholars Program)(grant numberX22020024), the open laboratory fund of Hubei Province Key Laboratory of Oral and Maxillofacial Development and Regeneration (grant number 2022kqhm005), the Hubei Provincial Health Commission Research Project (grant number WJ2023M121).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ma S, Jiang Y, Qian Y, et al. The emerging biological functions of exosomes from dental tissue-derived mesenchymal stem cells. Cell Reprogram. 2023;25(2):53–64. doi:10.1089/cell.2022.0147
2. Bai X, Cao R, Wu D, Zhang H, Yang F, Wang L. Dental pulp stem cells for bone tissue engineering: a literature review. Stem Cells Int. 2023;2023:7357179. doi:10.1155/2023/7357179
3. Welsh JA, Goberdhan DCI, O’Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. doi:10.1002/jev2.12404
4. Hua S, Bartold PM, Gulati K, Moran CS, Ivanovski S, Han P. Periodontal and dental pulp cell-derived small extracellular vesicles: a review of the current status. Nanomaterials. 2021;11(7):1858. doi:10.3390/nano11071858
5. Xie M, Xiong W, She Z, et al. Immunoregulatory effects of stem cell-derived extracellular vesicles on immune cells. Front Immunol. 2020;11:13. doi:10.3389/fimmu.2020.00013
6. Costamagna A, Pasquino C, Lamorte S, et al. Human liver stem cells and derived extracellular vesicles protect from sepsis-induced acute lung injury and restore bone marrow myelopoiesis in a murine model of sepsis. Intensive Care Med Exp. 2024;12(1):111. doi:10.1186/s40635-024-00701-z
7. Wu L, Zhang L, Huang M, et al. Mesenchymal stem cell-derived exosomes: emerging as a promising cell-free therapeutic strategy for autoimmune hepatitis. Biomolecules. 2024;14(11):1353. doi:10.3390/biom14111353
8. Yu S, Chen X, Liu Y, et al. Exosomes derived from stem cells from the apical papilla alleviate inflammation in rat pulpitis by upregulating regulatory T cells. Int Endod J. 2022;55(5):517–530. doi:10.1111/iej.13721
9. Li L, Ge J. Exosome‑derived lncRNA‑Ankrd26 promotes dental pulp restoration by regulating miR‑150‑TLR4 signaling. Mol Med Rep. 2022;25(5). doi:10.3892/mmr.2022.12668
10. Qiao X, Tang J, Dou L, et al. Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenesis in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int J Nanomed. 2023;18:4683–4703. doi:10.2147/IJN.S420967
11. Fu Y, Cui S, Zhou Y, Qiu L. Dental pulp stem cell-derived exosomes alleviate mice knee osteoarthritis by inhibiting TRPV4-mediated osteoclast activation. Int J Mol Sci. 2023;24(5). doi:10.3390/ijms24054926
12. Zhuang X, Ji L, Jiang H, et al. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cells Int. 2020;2020:5816723. doi:10.1155/2020/5816723
13. Chai Y, Liu Y, Liu Z, et al. Study on the role and mechanism of exosomes derived from dental pulp stem cells in promoting regeneration of myelin sheath in rats with sciatic nerve injury. Mol Neurobiol. 2024;61(9):6175–6188. doi:10.1007/s12035-024-03960-9
14. Li S, Luo L, He Y, et al. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 2021;54(8):e13093. doi:10.1111/cpr.13093
15. Hu S, Chen B, Zhou J, et al. Dental pulp stem cell-derived exosomes revitalize salivary gland epithelial cell function in NOD mice via the GPER-mediated cAMP/PKA/CREB signaling pathway. J Transl Med. 2023;21(1):361. doi:10.1186/s12967-023-04198-0
16. Shi X, Yang G, Liu MY, Yuan MT, Wang D, Wang XF. Exosomes derived from human dental pulp stem cells increase flap survival with ischemia-reperfusion injuries. Regener Med. 2023;18(4):313–327. doi:10.2217/rme-2022-0206
17. Ala M. The beneficial effects of mesenchymal stem cells and their exosomes on myocardial infarction and critical considerations for enhancing their efficacy. Ageing Res Rev. 2023;89:101980. doi:10.1016/j.arr.2023.101980
18. Filippopoulou C, Thomé CC, Perdikari S, et al. Hypoxia-driven deSUMOylation of EXOSC10 promotes adaptive changes in the transcriptome profile. Cell Mol Life Sci. 2024;81(1):58. doi:10.1007/s00018-023-05035-9
19. Yang X, Wu M, Kong X, et al. Exosomal miR-3174 induced by hypoxia promotes angiogenesis and metastasis of hepatocellular carcinoma by inhibiting HIPK3. iScience. 2024;27(2):108955. doi:10.1016/j.isci.2024.108955
20. Wang H, Zhao H, Chen Z, et al. Hypoxic bone mesenchymal stem cell-derived exosomes direct Schwann cells proliferation, migration, and paracrine to accelerate facial nerve regeneration via circRNA_Nkd2/miR-214-3p/MED19 Axis. Int J Nanomed. 2024;19:1409–1429. doi:10.2147/IJN.S443036
21. Liu D, Shi B, Zhou W, Tao G. Exosomes from hypoxia-conditioned apical papilla stem cells accelerate angiogenesis in vitro through Notch/JAG1/VEGF signaling. Tissue Cell. 2023;84:102197. doi:10.1016/j.tice.2023.102197
22. Lin X, Wang H, Wu T, Zhu Y, Jiang L. Exosomes derived from stem cells from apical papilla promote angiogenesis via miR-126 under hypoxia. Oral Dis. 2023;29(8):3408–3419. doi:10.1111/odi.14285
23. Gao Y, Yuan Z, Yuan X, et al. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact Mater. 2022;14:377–388. doi:10.1016/j.bioactmat.2022.01.041
24. Li B, Xian X, Lin X, et al. Hypoxia alters the proteome profile and enhances the angiogenic potential of dental pulp stem cell-derived exosomes. Biomolecules. 2022;12(4):575. doi:10.3390/biom12040575
25. Li B, Liang A, Zhou Y, et al. Hypoxia preconditioned DPSC-derived exosomes regulate angiogenesis via transferring LOXL2. Exp Cell Res. 2023;425(2):113543. doi:10.1016/j.yexcr.2023.113543
26. Liu P, Qin L, Liu C, et al. Exosomes derived from hypoxia-conditioned stem cells of human deciduous exfoliated teeth enhance angiogenesis via the transfer of let-7f-5p and miR-210-3p. Front Cell Develop Biol. 2022;10. doi:10.3389/fcell.2022.879877
27. Chen Y, Liu M, Niu Y, Wang Y. Romance of the three kingdoms in hypoxia: hIFs, epigenetic regulators, and chromatin reprogramming. Cancer Lett. 2020;495:211–223. doi:10.1016/j.canlet.2020.09.009
28. Wang B, He J, Cui Y, et al. The HIF-1α/EGF/EGFR signaling pathway facilitates the proliferation of yak alveolar type II epithelial cells in hypoxic conditions. Int J Mol Sci. 2024;25(3):1442. doi:10.3390/ijms25031442
29. Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35(7):1747–1759. doi:10.1002/stem.2618
30. Gómez-Ferrer M, Villanueva-Badenas E, Sánchez-Sánchez R, et al. HIF-1α and Pro-inflammatory signaling improves the immunomodulatory activity of MSC-derived extracellular vesicles. Int J Mol Sci. 2021;22(7):3416. doi:10.3390/ijms22073416
31. Zhang Z, Shuai Y, Zhou F, et al. PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int J Med Sci. 2020;17(5):558–567. doi:10.7150/ijms.40918
32. Wang Y, Zhang X, Wang J, et al. Inflammatory periodontal ligament stem cells drive M1 macrophage polarization via exosomal miR-143-3p-mediated regulation of PI3K/AKT/NF-κB signaling. Stem Cells. 2023;41(2):184–199. doi:10.1093/stmcls/sxac087
33. Huang X, Qiu W, Pan Y, et al. Exosomes from LPS-stimulated hDPSCs activated the angiogenic potential of HUVECs in vitro. Stem Cells Int. 2021;2021:6685307. doi:10.1155/2021/6685307
34. Li J, Ju Y, Liu S, Fu Y, Zhao S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect Tissue Res. 2021;62(3):277–286. doi:10.1080/03008207.2019.1694010
35. Umar S, Debnath K, Leung K, et al. Immunomodulatory properties of naïve and inflammation-informed dental pulp stem cell derived extracellular vesicles. Front Immunol. 2024;15:1447536. doi:10.3389/fimmu.2024.1447536
36. Huang Y, Liu Q, Liu L, Huo F, Guo S, Tian W. Lipopolysaccharide-preconditioned dental follicle stem cells derived small extracellular vesicles treating periodontitis via reactive oxygen species/mitogen-activated protein kinase signaling-mediated antioxidant effect. Int J Nanomed. 2022;17:799–819. doi:10.2147/IJN.S350869
37. Huang Y, Li M, Liu Q, et al. Small extracellular vesicles derived from lipopolysaccharide-preconditioned dental follicle cells inhibit cell apoptosis and alveolar bone loss in periodontitis. Arch Oral Biol. 2024;162:105964. doi:10.1016/j.archoralbio.2024.105964
38. Kang H, Lee MJ, Park SJ, Lee MS. Lipopolysaccharide-preconditioned periodontal ligament stem cells induce M1 polarization of macrophages through extracellular vesicles. Int J Mol Sci. 2018;19(12):3843. doi:10.3390/ijms19123843
39. Zheng Y, Dong C, Yang J, et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. 2019;234(11):20662–20674. doi:10.1002/jcp.28671
40. Nakao Y, Fukuda T, Zhang Q, et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–324. doi:10.1016/j.actbio.2020.12.046
41. Yan C, Li N, Xiao T, et al. Extracellular vesicles from the inflammatory microenvironment regulate the osteogenic and odontogenic differentiation of periodontal ligament stem cells by miR-758-5p/LMBR1/BMP2/4 axis. J Transl Med. 2022;20(1):208. doi:10.1186/s12967-022-03412-9
42. Yu Z, Wen Y, Jiang N, et al. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials. 2022;284:121484. doi:10.1016/j.biomaterials.2022.121484
43. Su W, Wan Q, Huang J, et al. Culture medium from TNF-α-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136(2):423–432.e8. doi:10.1016/j.jaci.2014.12.1926
44. Alymatiri CM, Gkegka GT, Gavriatopoulou M, et al. Association of −308G/A, −238G/A TNF-α polymorphisms with multiple myeloma risk and survival: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2022;22(2):e96–e115. doi:10.1016/j.clml.2021.08.010
45. Gratpain V, Loriot A, Bottemanne P, et al. Influence of a pro-inflammatory stimulus on the miRNA and lipid content of human dental stem cell-derived extracellular vesicles and their impact on microglial activation. Heliyon. 2024;10(5):e27025. doi:10.1016/j.heliyon.2024.e27025
46. Lin T, Wu N, Wang L, Zhang R, Pan R, Chen YF. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR‑140‑5p‑overexpressing human dental pulp stem cells. Int J Mol Med. 2021;47(3):7. doi:10.3892/ijmm.2020.4840
47. Vakhshiteh F, Rahmani S, Ostad SN, Madjd Z, Dinarvand R, Atyabi F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: a new approach for drug delivery. Life Sci. 2021;266:118871. doi:10.1016/j.lfs.2020.118871
48. Klimova D, Jakubechova J, Altanerova U, et al. Extracellular vesicles derived from dental mesenchymal stem/stromal cells with gemcitabine as a cargo have an inhibitory effect on the growth of pancreatic carcinoma cell lines in vitro. Mol Cell Probes. 2023;67:101894. doi:10.1016/j.mcp.2023.101894
49. Xu XY, Tian BM, Xia Y, et al. Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl Med. 2020;9(11):1414–1430. doi:10.1002/sctm.19-0418
50. Lu H, Mu Q, Ku W, et al. Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration. J Nanobiotechnology. 2024;22(1):265. doi:10.1186/s12951-024-02542-0
51. Wang M, Li J, Ye Y, He S, Song J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation. 2020;111:1–11. doi:10.1016/j.diff.2019.10.003
52. Wang L, Wei X, He X, et al. Osteoinductive dental pulp stem cell-derived extracellular vesicle-loaded multifunctional hydrogel for bone regeneration. ACS Nano. 2024;18(12):8777–8797. doi:10.1021/acsnano.3c11542
53. Hu X, Zhong Y, Kong Y, Chen Y, Feng J, Zheng J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res Ther. 2019;10(1):170. doi:10.1186/s13287-019-1278-x
54. Xie L, Ren X, Yang Z, et al. Exosomal circ_0000722 derived from periodontal ligament stem cells undergoing osteogenic differentiation promotes osteoclastogenesis. Int Immunopharmacol. 2024;128:111520. doi:10.1016/j.intimp.2024.111520
55. Liu T, Hu W, Zou X, et al. Human periodontal ligament stem cell-derived exosomes promote bone regeneration by altering MicroRNA profiles. Stem Cells Int. 2020;2020:8852307. doi:10.1155/2020/8852307
56. Zhang S, Wang S, Chen J, et al. Human dental pulp stem cell-derived exosomes decorated titanium scaffolds for promoting bone regeneration. Colloids Surf B Biointerfaces. 2024;235:113775. doi:10.1016/j.colsurfb.2024.113775
57. Liu H, Liu S, Qiu X, et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy. 2020;16(12):2140–2155. doi:10.1080/15548627.2020.1717128
58. Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol. 2012;4(8):a008797. doi:10.1101/cshperspect.a008797
59. Lin R, Zhang T, Gao J. Apoptotic vesicles of MSCs: the natural therapeutic agents and bio-vehicles for targeting drug delivery. Small. 2023;19(47):e2301671. doi:10.1002/smll.202301671
60. Zhang X, Tang J, Kou X, et al. Proteomic analysis of MSC-derived apoptotic vesicles identifies Fas inheritance to ameliorate haemophilia a via activating platelet functions. J Extracell Vesicles. 2022;11(7):e12240. doi:10.1002/jev2.12240
61. Xin L, Wei C, Tong X, et al. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: a therapy for intrauterine adhesions. Bioact Mater. 2022;12:107–119. doi:10.1016/j.bioactmat.2021.10.025
62. Gregory CD, Dransfield I. Apoptotic tumor cell-derived extracellular vesicles as important regulators of the onco-regenerative niche. Front Immunol. 2018;9:1111. doi:10.3389/fimmu.2018.01111
63. Wang R, Fu J, He J, et al. Apoptotic mesenchymal stem cells and their secreted apoptotic extracellular vesicles: therapeutic applications and mechanisms. Stem Cell Res Ther. 2025;16(1):78. doi:10.1186/s13287-025-04211-x
64. Li Z, Wu M, Liu S, et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Mol Ther. 2022;30(10):3193–3208. doi:10.1016/j.ymthe.2022.05.006
65. Fei Y, Ling Z, Tong Q, Wang J. Apoptotic extracellular vesicles from supernumerary tooth-derived pulp stem cells transfer COL1A1 to promote angiogenesis via PI3K/Akt/VEGF pathway. Int J Nanomed. 2024;19:6811–6828. doi:10.2147/IJN.S466136
66. Jing Y, Zhao W, Zhou Z, et al. Apoptotic vesicles modulate endothelial metabolism and ameliorate ischemic retinopathy via PD1/PDL1 axis. Adv Healthc Mater. 2024;13(17):e2303527. doi:10.1002/adhm.202303527
67. Yang K, Zhu Y, Shao Y, et al. Apoptotic vesicles derived from dental pulp stem cells promote bone formation through the ERK1/2 signaling pathway. Biomedicines. 2024;12(4):730. doi:10.3390/biomedicines12040730
68. Sunartvanichkul T, Chaweewannakorn C, Tabtimmai L, et al. Apoptosis-induced exosomes from human exfoliated deciduous teeth enhance angiogenesis in human umbilical vein endothelial cells. Sci Rep. 2024;14(1):27921. doi:10.1038/s41598-024-79692-6
69. Wang X, Mi S, Gao J, Liu Y, Qi Z. Functionalized exosomes promote neural differentiation of P19 cells by activating the wnt signaling pathway. Curr Stem Cell Res Ther. 2023. doi:10.2174/1574888X18666230525141905
70. Lu J, Yu N, Liu Q, Xie Y, Zhen L. Periodontal ligament stem cell exosomes key to regulate periodontal regeneration by miR-31-5p in mice model. Int J Nanomed. 2023;18:5327–5342. doi:10.2147/IJN.S409664
71. Pang L, Jin H, Lu Z, et al. Treatment with mesenchymal stem cell-derived nanovesicle-containing gelatin methacryloyl hydrogels alleviates osteoarthritis by modulating chondrogenesis and macrophage polarization. Adv Healthc Mater. 2023;12(17):e2300315. doi:10.1002/adhm.202300315
72. Yuan X, Sun L, Jeske R, et al. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J Extracell Vesicles. 2022;11(6):e12235. doi:10.1002/jev2.12235
73. Jalilian E, Massoumi H, Bigit B, et al. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res Ther. 2022;13(1):425. doi:10.1186/s13287-022-03128-z
74. Jo W, Jeong D, Kim J, et al. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14(7):1261–1269. doi:10.1039/c3lc50993a
75. Go G, Lee J, Choi DS, Kim SS, Gho YS. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Adv Healthc Mater. 2019;8(4):e1801082. doi:10.1002/adhm.201801082
76. Yang Z, Shi J, Xie J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69–83. doi:10.1038/s41551-019-0485-1
77. Liang L, Wang L, Liao Z, et al. High-yield nanovesicles extruded from dental follicle stem cells promote the regeneration of periodontal tissues as an alternative of exosomes. J Clin Periodontol. 2024;51(10):1395–1407. doi:10.1111/jcpe.14036
78. Duan X, Zhang R, Feng H, et al. A new subtype of artificial cell-derived vesicles from dental pulp stem cells with the bioequivalence and higher acquisition efficiency compared to extracellular vesicles. J Extracell Vesicles. 2024;13(7):e12473. doi:10.1002/jev2.12473
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Long-Term Effects of Severe Burns on the Kidneys: Research Advances and Potential Therapeutic Approaches
Yang G, Tan L, Yao H, Xiong Z, Wu J, Huang X
Journal of Inflammation Research 2023, 16:1905-1921
Published Date: 1 May 2023
Exosomes as Vehicles for Noncoding RNA in Modulating Inflammation: A Promising Regulatory Approach for Ischemic Stroke and Myocardial Infarction
Lai Z, Ye T, Zhang M, Mu Y
Journal of Inflammation Research 2024, 17:7485-7501
Published Date: 21 October 2024
Extracellular Vesicles in Periodontitis: Pathogenic Mechanisms and Therapeutic Potential

Zhang L, Li X, Zhang B, Li R
Journal of Inflammation Research 2025, 18:1317-1331
Published Date: 28 January 2025
Cucurbitacin IIa Alleviates Colitis via Promoting the Release of Host-Derived Extracellular Vesicles Encapsulating microRNA-30b-5p

Zhao Y, Jiang B, Zuo S
Journal of Inflammation Research 2025, 18:1447-1458
Published Date: 31 January 2025
Mesenchymal Stem Cell-Derived Exosomes Hold Promise in the Treatment of Diabetic Foot Ulcers

Wang H, Wu S, Bai X, Pan D, Ning Y, Wang C, Guo L, Guo J, Gu Y
International Journal of Nanomedicine 2025, 20:5837-5857
Published Date: 6 May 2025

