Back to Journals » International Journal of Nanomedicine » Volume 20
Targeted Nanoparticle-Based Therapies for Nasopharyngeal Carcinoma: Enhancing Radiosensitization, Photothermal Therapy, and Diagnostics
Authors Wang T, Wu H, Wang L, Lou N, Guo L
Received 17 February 2025
Accepted for publication 12 May 2025
Published 30 May 2025 Volume 2025:20 Pages 7021—7035
DOI https://doi.org/10.2147/IJN.S523213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Tianzhu Wang,1 Huanliang Wu,1 Liang Wang,1 Nana Lou,1 Lijuan Guo2
1The Department of Radiation Oncology, Hainan Cancer Hospital, Haikou, 570311, People’s Republic of China; 2Department of Radiotherapy, The First Affiliated Hospital of Hainan Medical University, Haikou, 570102, People’s Republic of China
Correspondence: Lijuan Guo, Department of Radiotherapy, The First Affiliated Hospital of Hainan Medical University, Haikou, 570102, People’s Republic of China, Email [email protected]
Abstract: Nasopharyngeal carcinoma (NPC) remains a major clinical challenge due to its high resistance to conventional therapies such as chemotherapy and radiotherapy. Nanoparticle (NP)-based technologies have emerged as promising tools to enhance the efficacy of NPC treatment through mechanisms like radiosensitization and photothermal therapy. This review discusses the potential of NPs, particularly metal-based nanoparticles (gold and iron oxide), in improving the therapeutic outcomes of radiotherapy by overcoming tumor hypoxia and increasing radiation absorption. Additionally, we explore the application of NPs in photothermal therapy, wherein nanoparticles absorb light and generate localized heat to target tumor cells with precision. NPs are also playing an increasingly vital role in early diagnosis and real-time imaging, enabling more effective monitoring and personalized treatment. Despite the promising potential, challenges as nanoparticle biocompatibility, toxicity, and efficient targeting remain obstacles for clinical translation. In this review, we aim to provide a comprehensive summary of the current state of targeted nanoparticle-based interventions in NPC therapy and to outline the potential for these technologies to improve therapeutic outcomes.
Keywords: nasopharyngeal carcinoma, nanomedicine, nanoparticle, treatment, nanotechnology
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy originating in the epithelial cells of the nasopharynx, a region at the back of the nasal cavity. Although relatively rare globally, NPC exhibits a notably higher incidence in certain regions, including Southeast Asia, the Mediterranean, and parts of North Africa, where the incidence rates far exceed those observed in Western countries.1–3 Early-stage NPC often presents with subtle symptoms, such as nasal obstruction, recurrent ear infections, and persistent sore throat. However, by the time more conspicuous signs, such as lymphadenopathy and distant metastases, become apparent, the disease is frequently diagnosed at an advanced stage, complicating treatment and diminishing efficacy.4,5
Epstein-Barr virus (EBV) plays a critical role in NPC development by promoting oncogenic signaling, immune evasion, and epigenetic modifications.6 EBV latent proteins such as LMP1 and EBNA1 activate pathways like NF-κB and JAK/STAT, driving tumor progression. Additionally, genetic mutations in TP53, PI3K/AKT, and DNA repair genes further contribute to tumor aggressiveness.7 The NPC tumor microenvironment (TME) is highly immunosuppressive, with tumor-associated macrophages, regulatory T cells, and myeloid-derived suppressor cells inhibiting immune responses.8 Hypoxia-induced factors enhance angiogenesis, metastasis, and therapy resistance, while cancer-associated fibroblasts secrete growth factors that promote tumor invasion.
The management of NPC has progressed over time, with a combination of radiotherapy and chemotherapy forming the cornerstone of treatment, particularly for locally advanced and metastatic disease.2,9 Early-stage NPC is typically treated with radiation therapy alone, owing to the tumor’s high sensitivity to radiation.10 In more advanced stages, chemotherapy is added to reduce tumor burden and control systemic spread.1 Despite these interventions, the prognosis for patients with advanced or metastatic NPC remains poor, primarily due to the propensity of the disease to relapse and metastasize to distant organs such as the lungs, liver, and bones. Moreover, the severe side effects of aggressive treatment regimens significantly impact patients’ quality of life.10
Recent advances in the integration of innovative technologies, particularly nanoparticles (NPs), have garnered significant attention in the battle against NPC.11 NPs hold considerable promise in overcoming many of the limitations associated with conventional therapies. These nanoscale particles, typically ranging from 1 to 100 nm in size, possess distinctive physical and chemical properties that enable them to interact with biological systems in ways that traditional drugs cannot.12,13 Specifically, NPs facilitate targeted drug delivery to tumor sites, minimize systemic toxicity, and enhance the pharmacokinetics of therapeutic agents. Their diminutive size promotes deeper penetration into tumor tissues, while their surface characteristics can be engineered to selectively target cancer cells.11,14
Despite existing literature has explored the use of nanoparticles for cancer treatment in general, very few reviews specifically focus on their potential role in NPC, particularly in the context of overcoming tumor hypoxia and enhancing radiotherapy effectiveness. Additionally, while individual therapies like radiosensitization and photothermal therapy have been investigated in NPC, there is a lack of integrated discussions on how these strategies can be synergistically combined with diagnostic tools to provide personalized and more effective treatment regimens. This review fills this gap by reviewing recent advancements in nanoparticle formulations and their applications, thus providing a comprehensive overview of both therapeutic and diagnostic strategies for NPC.
Types and Mechanisms
Classification of NPs
NPs, owing to their nanoscale size, exhibit a range of unique physical, chemical, and biological properties that can be utilized for both therapeutic and diagnostic purposes in cancer treatment, including NPC.15 The small size of NPs allows them to interact with biological systems at the molecular level, enabling enhanced drug delivery, improved bioavailability, and targeted treatment with minimal off-target effects.16,17 Additionally, their surface can be engineered to optimize interactions with specific cells or tissues, further increasing their therapeutic potential. Various types of NPs, including liposomes, polymeric nanoparticles, and metallic nanoparticles, have been developed, each offering distinct advantages tailored to specific clinical needs.18–20 For example, liposomes are particularly effective for improving the solubility and stability of hydrophobic drugs, while polymeric nanoparticles can be engineered for controlled release and sustained drug delivery.21 Metallic nanoparticles, such as gold or silver nanoparticles, are often utilized in imaging and diagnostic applications due to their strong optical properties.22 The versatility of these nanoparticles makes them a promising tool in the fight against NPC, with ongoing research focusing on optimizing their design for enhanced efficacy and safety. (Table 1).
 |
Table 1 Different Types of NPs Used in NPC Therapy |
Basic Properties of NPs
The efficacy of NPs in cancer therapy is largely dependent on several critical properties, including size, surface charge, biocompatibility, and drug-loading capacity. Firstly, the size of NPs plays a crucial role in their ability to penetrate the tumor tissue and interact with cancer cells.23 Smaller NPs are more likely to escape from blood vessels and accumulate in tumor tissues through the enhanced permeability and retention (EPR) effect.24 Notably, size affects the ability of NPs to be cleared from the body, with smaller particles generally being excreted more rapidly via the renal system.25 Secondly, the surface charge of NPs influences their interaction with biological membranes, their stability in circulation, and their ability to be internalized by cells.26 Positively charged NPs tend to interact more readily with the negatively charged cell membranes, facilitating cellular uptake. However, surface charge should be carefully optimized to avoid toxicity or premature clearance by the immune system.27 Thirdly, one of the key considerations in the design of NPs is their biocompatibility, which ensures that they do not elicit harmful immune responses or cause toxicity to healthy tissues.28 Biocompatible NPs are typically made from materials that are naturally occurring or have been extensively tested for safety in clinical applications. Surface modification with biocompatible coatings, such as polyethylene glycol (PEG), can further improve circulation time and reduce immune clearance.29 Lastly, NPs should have sufficient drug-loading capacity to carry therapeutic agents in a way that maximizes their effectiveness.30 Depending on the type of nanoparticle, drugs can be loaded into the core (for liposomes and dendrimers), adsorbed onto the surface (for inorganic NPs), or encapsulated within the polymer matrix (for polymeric NPs).31,32 High drug-loading capacity ensures that NPs can deliver adequate amounts of the therapeutic agent to the target site, improving therapeutic outcomes.
Targeted NPs Delivery to NPC Cells
NP delivery to NPC cells requires functionalization strategies that exploit specific tumor markers. Among these, EBV antigens and NPC-specific receptors are crucial for ensuring selective targeting.33 EBV infection is a hallmark of NPC, making viral proteins such as latent membrane proteins (LMP1 and LMP2) and Epstein-Barr nuclear antigen 1 (EBNA1) attractive targets.34 Functionalized NPs conjugated with monoclonal antibodies or aptamers specific to these antigens facilitate enhanced accumulation in NPC cells. Additionally, ligand-based targeting strategies utilize overexpressed receptors like epidermal growth factor receptor (EGFR) and transferrin receptor (TfR) by functionalizing NPs with EGFR-specific ligands or TfR-targeting peptides, improving selective uptake by NPC cells.35,36 Given the acidic and hypoxic TME of NPC, pH-sensitive polymers and hypoxia-responsive moieties enable controlled drug release, enhancing therapeutic efficacy. Although various modification methods allow us to construct nanoparticles that target NPC cells (including organic, inorganic, and organic-inorganic hybrids), the accuracy of these particles in vivo still needs to be further improved. Additionally, potential damage to other normal cells and side effects should be carefully monitored, with efforts to maximize their safety and efficacy in applications. At the same time, the metabolism and residual presence of inorganic nanoparticles in the body is another critical issue that requires focused research and improvement in the future.
Metal-Based NPs for Enhanced Radiotherapy in NPC
Metal-based NPs, particularly gold (AuNPs) and iron oxide (Fe3O4 NPs), enhance radiotherapy by amplifying the radiation dose and increasing DNA damage within tumor cells.37,38 High atomic number elements like gold absorb X-rays efficiently, enhancing the photoelectric effect and increasing secondary electron production, which induces DNA damage. Additionally, gold NPs generate Auger electrons, causing localized DNA strand breaks. Iron oxide NPs contribute to increased secondary radiation through Compton scattering, further augmenting radiation exposure in hypoxic tumor regions where radiotherapy is typically less effective. To counteract hypoxia-induced radioresistance, oxygen-carrying NPs or oxygen-generating NPs improve oxygenation within NPC tumors, thereby enhancing radiotherapy outcomes.39 For a recent example, Zhang et al developed a hypoxia-targeted nanozyme by loading platinum NPs into ferritin, which selectively recognizes hypoxic NPC areas through TfR1.36 The nanozyme exhibits enhanced catalase-like activity, alleviating tumor hypoxia in NPC xenografts. Combined with radiotherapy, it significantly inhibits tumor growth and prolongs survival, outperforming the clinically used radiosensitizer sodium glycididazole. These findings suggest that TfR1-targeting nanozymes could improve radiotherapy efficacy for hypoxic NPC through an in situ oxygen-generation mechanism (Figure 1).
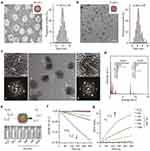 |
Figure 1 Characterization of Pt-HFn Structure and Catalase-Like Activity. (a) Left: TEM image of Pt-HFn with negative staining, highlighting the morphology of the HFn shell. Right: Particle size distribution of HFn derived from TEM analysis. Scale bar, 10 nm. (b) Left: TEM image of Pt-HFn without negative staining, revealing the morphology of the Pt core. Right: Size distribution of the Pt core from TEM analysis. Scale bar, 10 nm. (c) High-resolution TEM image of the Pt core within Pt-HFn, with insets showing atomic-level images of selected areas and their corresponding fast Fourier transform (FFT) patterns. Scale bar, 2 nm. (d) TEM-EDS spectrum of Pt-HFn, with the inset displaying the mass and atomic percentages of the elements present in the sample. (e) Upper: Diagram illustrating the catalase-like activity of Pt-HFn in decomposing H2O2 to produce O2. Lower: Photographs showing the generation of O2 bubbles in H2O2 solutions for each group. (f) Absorbance changes at 240 nm, indicating H2O2 reduction in solutions for each group (n = 4 independent experiments). (g) Changes in dissolved oxygen levels demonstrate increased O2 content in H2O2 solutions for each group (n = 3 independent experiments). Data are presented as mean ± SEM. Reproduced from Zhang R, Shen Y, Zhou X, et al. Hypoxia-tropic delivery of nanozymes targeting transferrin receptor 1 for nasopharyngeal carcinoma radiotherapy sensitization. Nat Commun. 2025;16(1):890. Copyright © 2025, The Author(s). This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.36 |
Metal-based NPs have shown significant potential for enhancing radiotherapy in NPC, primarily by improving tumor radiosensitivity. However, several challenges limit their clinical application. One major issue is the difficulty in precisely targeting hypoxic tumor regions, which can lead to suboptimal accumulation of NPs in the tumor tissue. Additionally, concerns about the toxicity and side effects of metal NPs persist, as high doses could harm healthy tissues and organs. Long-term biocompatibility and the potential for NPs accumulation in the body also remain significant issues, requiring further investigation. Notably, the complexity and high cost associated with the synthesis and scaling of metal-based NPs for clinical use could impede their widespread adoption.
Photothermal Therapy Using Gold and Carbon-Based NPs
Gold NPs and carbon-based nanomaterials, such as carbon nanotubes and graphene oxide, are widely utilized for photothermal therapy (PTT) due to their efficient light-to-heat conversion properties.40 Gold NPs exhibit strong near-infrared (NIR) absorption via surface plasmon resonance (SPR), allowing deep tissue penetration and localized hyperthermia. Carbon-based nanomaterials, including carbon nanotubes and graphene, have broad NIR absorption spectra and high photothermal conversion efficiencies, leading to enhanced tumor cell death through hyperthermia.8,41 The heat generated by these NPs raises tumor temperatures to 42–45°C, disrupting protein structures and inducing apoptosis or necrosis, thereby increasing therapeutic efficacy.12 By integrating PTT with conventional treatments, NPC therapy can achieve improved tumor ablation with minimal damage to surrounding healthy tissues. However, several issues should be paid enough attention. First, the potential for limited tissue penetration of NIR light can restrict the treatment’s effectiveness, especially for deep tumors. Second, the potential for nanotoxicity, including inflammation and immune responses, poses a challenge for long-term safety. Additionally, the stability and biodegradability of gold and carbon-based NPs in the body require careful evaluation to avoid accumulation and potential toxicity. Another concern is the need for precise and efficient NPs targeting to ensure minimal damage to healthy tissues. Further research is needed to address these limitations and improve the clinical viability of PTT with these NPs.
NPs Engineering for NPC TME Modulation
The NPC TME is characterized by hypoxia, acidity, and an immunosuppressive milieu, which can hinder conventional therapies.42,43 NPs can be engineered to respond to these factors for enhanced therapeutic efficacy. pH-responsive NPs, designed with pH-sensitive polymers such as poly(β-amino ester), allow for preferential drug release in the acidic NPC TME.44 Hypoxia-responsive NPs carrying prodrugs like tirapazamine become activated under low oxygen conditions, selectively targeting hypoxic NPC regions that are resistant to standard treatments. Furthermore, immune-modulating NPs, including anti-PD-L1-conjugated NPs, can reverse immune evasion mechanisms and enhance anti-tumor immunity.45
Inorganic NPs play a significant role in modulating the TME and enhancing treatment efficacy in NPC through targeted drug delivery, imaging, PTT/PDT, immune modulation, and hypoxia regulation.46 As drug carriers, NPs such as mesoporous silica, gold, and magnetic NPs improve drug solubility, stability, and targeted delivery, reducing systemic toxicity. Their unique optical and magnetic properties enhance imaging techniques like MRI, CT, and fluorescence imaging, improving tumor detection and monitoring. Gold nanorods and titanium dioxide enable PTT/PDT by generating localized heat or reactive oxygen species upon light activation, selectively destroying NPC cells.47 Additionally, immune-modulating NPs, including iron oxide and cerium oxide, activate macrophages and dendritic cells, enhancing tumor antigen presentation and reversing the immunosuppressive TME. Oxygen-releasing NPs, such as manganese dioxide, alleviate hypoxia, thereby enhancing radiotherapy and PDT efficacy by increasing oxygen availability and reactive oxygen species production.46,48 These multifunctional properties position inorganic NPs as powerful theranostic agents, offering precise, multimodal strategies for NPC treatment.
NPs engineering for modulation of the TME in NPC holds significant promise but also faces challenges. First, the ability to precisely target the unique features of the NPC TME, such as hypoxia and acidic conditions, is crucial for effective therapeutic outcomes. Engineering NPs with specific ligands or responsive properties can help overcome the selective targeting challenge, improving the delivery and efficacy of treatment. Second, the modulation of the TME to enhance drug delivery or radiosensitivity is another critical area. By designing NPs that can regulate tumor oxygenation, neutralize acidic pH, or break down extracellular matrix barriers, the therapeutic potential of conventional treatments like radiotherapy or chemotherapy could be significantly improved. However, achieving a balance between enhancing treatment efficacy and minimizing off-target effects remains a challenge. Finally, long-term safety and biocompatibility are major concerns. While NPs can modify the TME for better treatment, their accumulation in non-target tissues or organs may lead to unintended toxicity. Continued research on the biodegradability, stability, and immunological impact of engineered NPs is essential to ensure their clinical feasibility and reduce potential side effects.
NPs-Based Therapeutics in NPC
NPs have shown considerable promise in enhancing the effectiveness of traditional treatment modalities for NPC, such as chemotherapy and radiotherapy. By improving the targeted delivery and controlled release of therapeutic agents, NPs can help overcome many of the limitations associated with conventional treatments, including toxicity and poor drug bioavailability.
Enhanced Drug Effectiveness and Reduced Side Effects
Chemotherapy is a cornerstone in the management of advanced NPC, but its effectiveness is often compromised by issues such as low drug specificity, resistance, and significant side effects.49 The systemic nature of chemotherapy results in drugs being distributed throughout the body, affecting not only cancerous cells but also healthy tissues, leading to toxicities like nausea, immunosuppression, and hair loss.50
NPs, due to their small size and unique physicochemical properties, can significantly improve the delivery of chemotherapy agents directly to the tumor site. This targeted delivery minimizes the exposure of healthy tissues to the toxic effects of chemotherapy, reducing side effects and improving the quality of life for patients.51 NPs can encapsulate a wide range of chemotherapeutic agents, including hydrophobic drugs that may otherwise be difficult to deliver effectively.52 The surface of NPs can be modified with specific ligands that recognize and bind to overexpressed receptors on NPC cells, ensuring that the drug is selectively released at the tumor site. In a prior study, the researchers reported a novel targeted lipid nanoparticle, α-NTP-LNs, to enhance the treatment efficacy of NPC.51 By incorporating a fusion peptide α-NTP, which combines the nanostructure-controlling ability of the α-peptide and the NPC-specific therapeutic peptide NTP, α-NTP-LNs not only accelerated NPC cell uptake (4.8-fold increase) but also exerted coordinated cytotoxic effects by inducing apoptosis and autophagy. In vivo and ex vivo imaging showed efficient accumulation of α-NTP-LNs in NPC tumors with clear contrast between tumor and normal tissues, and flow cytometry confirmed a 13-fold greater uptake in tumor cells compared to hepatocytes. Furthermore, α-NTP-LNs demonstrated more than 85% tumor growth inhibition in NPC xenografts (5–8F cells) and significantly improved survival in an NPC lung metastasis model (Figure 2).
 |
Figure 2 Schematic diagrams of α-NTP and α-NTP-LNs illustrate the targeting mechanism. α-NTP-LNs were shown to specifically target NPC, demonstrating the combined synergistic effect of the α-peptide and NTP within the α-NTP fusion peptide for enhanced targeting. Reproduced from Luo H, Lu L, Yang F, et al. Nasopharyngeal cancer-specific therapy based on fusion peptide-functionalized lipid nanoparticles. ACS Nano. 2014;8(5):4334–4347.51 Copyright 2014, American Chemical Society. |
In addition to improving drug delivery, NPs can also enhance the drug’s therapeutic effectiveness. Their surface can be engineered to release the encapsulated drug in a controlled manner, prolonging the drug’s action and maintaining therapeutic levels within the tumor for extended periods. This sustained release can help overcome issues like drug resistance by providing continuous exposure to the chemotherapeutic agent, ensuring better control of tumor growth and potentially reducing the risk of metastasis.
NPs for Radiosensitization and Photothermal Therapy in NPC Treatment
In addition to chemotherapy, radiotherapy remains an essential treatment modality for NPC, particularly for localized tumors. However, the effectiveness of radiotherapy is limited by factors such as tumor hypoxia, which reduces the sensitivity of cancer cells to radiation. NPs offer an innovative solution to overcome these challenges, particularly in the areas of radiosensitization and photothermal therapy.53
NPs can be designed to enhance the effect of radiation therapy by making tumor cells more sensitive to radiation, a phenomenon known as radiosensitization. This can be achieved in several ways. For example, NPs containing metals such as gold or iron oxide can increase the local dose of radiation delivered to the tumor.54 These metal NPs can absorb radiation and generate secondary radiation, which increases the energy deposition within the tumor, thus amplifying the therapeutic effect. Furthermore, NPs can be engineered to accumulate in hypoxic regions of the tumor, areas that are typically resistant to radiation, thereby enhancing the overall response to treatment.55 For instance, Wang et al identified the circular RNA circADARB1 as a key player in NPC radiotherapy resistance, with its upregulation linked to poor prognosis. CircADARB1 inhibits ferroptosis, promoting resistance to radiotherapy by stabilizing proteins like SLC7A11 and GPX4, which protect cells from oxidative damage. To counter this, they developed a biomimetic nanomaterial that delivers siRNA targeting circADARB1 and iron ions to NPC cells, enhancing ferroptosis and improving radiosensitivity.11 These findings highlight the potential of combining nanotechnology and RNA interference to overcome radiotherapy resistance in NPC, offering promising therapeutic strategies for precision cancer treatment (Figure 3).
 |
Figure 3 Enhancement of Radiosensitivity in NPC Using Fe@Pdots-siRNA Nanomaterials. (A) Schematic illustration of the synthesis process for nanomaterials, which are encapsulated in NPC cell membrane cores composed of iron-doped semiconducting polymer nanoparticles (Fe@Pdot). These nanoparticles carry siRNA targeting circADARB1 or a scrambled siRNA as a negative control (NC), referred to as Fe@Pdots-siRNA and Fe@Pdots-siNC, respectively. (B) Representative transmission electron microscopy (TEM) images of Pdots, Fe@Pdots, Fe@Pdots-siNC, and Fe@Pdots-siRNA. (C) Particle size distribution of the four iron-loaded nanomaterials: Pdots, Fe@Pdots, Fe@Pdots-siNC, and Fe@Pdots-siRNA. (D) Fourier-transform infrared (FTIR) spectroscopy analysis of the chemical composition and molecular structure of the four nanomaterials. (E) In vivo fluorescence imaging was performed on tumor-bearing nude mice intravenously injected with saline, Fe@Pdots, Fe@Pdots-siNC, or Fe@Pdots-siRNA (10 mg/kg body weight). Imaging was conducted at 0, 6, 12, and 24 hours post-injection to track the distribution and accumulation of the nanomaterials in tumor tissues. The yellow circles represent the sites of tumor inoculation. (F) After 12 hours, the mice were euthanized, and organs including the heart, liver, spleen, lungs, kidneys, and tumor tissues were collected. The biodistribution of the nanomaterials in these organs and NPC xenografts was further assessed through in vivo imaging. (G) To establish a tumor-bearing model, nude mice were subcutaneously injected with NPC cells (CNE2) into the right dorsal flank. After 14 days, when xenografts reached 100–150 mm³, mice were randomly divided into three groups (n = 12). On days 14 and 20, each group received intravenous injections of saline, Fe@Pdots-siNC, or Fe@Pdots-siRNA (10 mg/kg body weight). Half of the mice in each group (n = 6) received 6 Gy X-ray irradiation at the tumor site (IR). Tumor sizes were measured every 3 days, and mice were euthanized on day 29. (H) At the end of the study, xenograft tumors were excised, and their volumes and weights were measured. **p < 0.01, ****p < 0.0001. Reproduced from Wang D, Tang L, Chen M, et al. Nanocarriers targeting circular RNA ADARB1 boost radiosensitivity of nasopharyngeal carcinoma through synergically promoting ferroptosis. ACS Nano. 2024;18(45):31055–31075..11 Copyright 2024, American Chemical Society. |
Another application of NPs in NPC treatment is their potential in photothermal therapy. In this approach, NPs, particularly those made from materials like gold, silica, or carbon, are designed to absorb light and convert it into heat.12 When exposed to near-infrared (NIR) light, these NPs generate localized heat that can effectively destroy tumor cells by inducing thermal damage. The advantage of photothermal therapy is its ability to target tumors with high precision, as the NPs can be guided to specific tumor sites using external imaging or surface modifications.12 Additionally, photothermal therapy can be used in conjunction with other therapies, such as chemotherapy or radiotherapy, to enhance the overall therapeutic outcome.56
NPs in Diagnostic Applications for NPC
NPs are not only transforming the therapeutic landscape of NPC but are also playing an increasingly significant role in its early diagnosis and imaging.57 Early detection of NPC is critical for improving patient outcomes, enabling timely interventions and more effective treatment strategies.58 The unique physicochemical properties of NPs position them as ideal candidates for enhancing imaging modalities, detecting specific biomarkers, and advancing the field of personalized medicine.
MRI, Fluorescence, and Bioimaging
The application of NPs in medical imaging has shown great promise in improving the sensitivity and accuracy of diagnostic techniques, facilitating the early detection of NPC. Magnetic resonance imaging (MRI), fluorescence imaging, and bioimaging are three primary imaging modalities that can benefit from the use of NPs.59–61
Firstly, MRI is widely used in cancer diagnostics, but its resolution can be limited when detecting small or early-stage tumors. NPs, especially those made from iron oxide or other magnetic materials, can be used as contrast agents to enhance the imaging of tumors.60 These magnetic NPs improve the signal-to-noise ratio in MRI, allowing for more precise visualization of tumor tissues. In NPC, magnetic NPs can target specific tumor cells or areas of metastasis, offering a more detailed view of the disease’s progression.61
Secondly, fluorescence imaging is another powerful diagnostic tool, especially for visualizing cancer cells in real time.62 Fluorescent NPs, such as quantum dots or organic fluorophores, can be functionalized to target NPC-specific biomarkers or receptors on cancer cells. Once these NPs bind to the tumor cells, they emit fluorescent signals that can be captured using a fluorescence microscope or imaging system.63 This technique allows for non-invasive, high-resolution imaging of tumor location and size, enabling early detection of NPC and monitoring of treatment response. For instance, a prior study focused on the development of a polyethylene glycol-coated ultrasmall superparamagnetic iron oxide nanoparticle-coupled sialyl Lewis X (USPIO-PEG-sLex) nanotheranostic platform for the diagnosis and treatment of NPC.64 The USPIO-PEG-sLex nanoparticles exhibit excellent photothermal conversion properties, with temperature increases proportional to nanoparticle concentration and near-infrared (NIR) power density at 808 nm. In vitro experiments revealed that the viability of NPC 5–8F cells decreased significantly as the concentration of USPIO-PEG-sLex nanoparticles increased, demonstrating their potential for photothermal therapy (PTT). Additionally, fluorescence imaging showed that these nanoparticles effectively targeted tumor tissue and reduced the T2 values on MRI, indicating successful tumor targeting. Tumor growth was significantly inhibited in the photothermal therapy group, with a notable decrease in tumor volume compared to control and untreated groups. Overall, the USPIO-PEG-sLex nanotheranostic platform shows promising potential as a novel therapeutic strategy for NPC, offering both targeted treatment and imaging capabilities for precise therapy (Figure 4).
 |
Figure 4 Characterization of USPIO-PEG-sLex nanoparticles. (A) Transmission electron microscopy (TEM) image of USPIO-PEG-sLex nanoparticles, revealing predominantly square and polygonal shapes, with a few spherical particles. (B) Particle size distribution of USPIO-PEG and USPIO-PEG-sLex. (C) Zeta potential measurements of USPIO-PEG (a) and USPIO-PEG-sLex (b). (D) Fourier-transform infrared (FTIR) spectra of USPIO-PEG and USPIO-PEG-sLex. Reproduced from Liu Q, Liu L, Mo C, et al. Polyethylene glycol-coated ultrasmall superparamagnetic iron oxide nanoparticles-coupled sialyl Lewis X nanotheranostic platform for nasopharyngeal carcinoma imaging and photothermal therapy. J Nanobiotechnology. 2021;19(1):171. © The Author(s) 2021. Creative Commons Attribution 4.0 International License.64 |
Moreover, bioimaging techniques, which combine various imaging modalities with advanced nanoparticle-based systems, enable real-time tracking of NPs as they move through the body.65 These systems allow for the observation of nanoparticle accumulation in tumor sites, providing important insights into the pharmacokinetics and biodistribution of nanoparticle-based therapeutics.66 By integrating NPs into bioimaging techniques, it is possible to visualize tumors more effectively, identify small or deep-seated tumors, and monitor the effectiveness of treatments.
NPC Biomarkers
Biomarkers are essential for early diagnosis, prognosis, and treatment monitoring in NPC. NPs can be employed to develop highly sensitive biosensors that detect these biomarkers with unprecedented accuracy and speed.67 These nanoparticle-based biosensors offer several advantages over traditional methods, such as higher sensitivity, quicker results, and the ability to detect biomarkers at very low concentrations.68
NPs can be functionalized with antibodies, aptamers, or other receptor molecules that specifically bind to NPC biomarkers, such as EBV antigens or genetic mutations associated with NPC.69 Once the biomarker is detected, the biosensor generates a measurable signal, often through a color change, fluorescence, or electrochemical response. This process can be used for both the diagnosis of NPC and the monitoring of treatment responses, allowing for personalized treatment adjustments based on real-time biomarker analysis.70
In addition, nanoparticle-based biosensors can be designed for point-of-care diagnostics, enabling rapid, on-site detection of NPC biomarkers in clinical settings.71 This could reduce the need for more invasive diagnostic procedures, provide early detection opportunities, and allow for better monitoring of disease progression and recurrence.
NPC Precision Medicine
The integration of nanotechnology into the diagnostics of NPC is progressing rapidly, offering promising prospects for more precise and personalized cancer care. The ability to engineer NPs with modified targeting capabilities provides new avenues for precision medicine in NPC. By designing NPs that selectively recognize and bind to the molecular signatures of NPC, clinicians may achieve earlier and more accurate diagnoses, thereby enhancing treatment outcomes.72
Recent advancements in nanotechnology have led to the development of multifunctional NPs that combine diagnostic and therapeutic functions.73 Theranostic NPs, for instance, can simultaneously deliver imaging agents and therapeutic payloads within a single platform, enabling both diagnosis and treatment in parallel. This dual approach facilitates real-time monitoring of therapeutic efficacy and tumor response, allowing for more personalized treatment regimens based on the individual characteristics of each patient.74
Moreover, innovations in nanotechnology have yielded more functional nanoparticle-based imaging agents, such as those utilizing surface-enhanced Raman scattering (SERS) or advanced fluorescent probes.75 These cutting-edge technologies enhance the sensitivity and specificity of NPC detection and offer deeper insights into the tumor microenvironment, including critical factors such as oxygenation, acidity, and other biochemical properties that influence treatment responses.76
Challenges in Nanoparticle-Based NPC Therapy
Despite the promising potential of NPs in enhancing the treatment of NPC, there are several significant challenges that need to be addressed before these therapies can be widely adopted in clinical practice. These challenges include across concerns related to biocompatibility, toxicity, NPs stability, and the effectiveness of targeted delivery.
Biocompatibility and Toxicity Concerns
The long-term safety of inorganic NPs remains a major concern due to potential cytotoxicity and accumulation in organs such as the liver, spleen, and kidneys. Some NPs, such as quantum dots and certain metal oxides, can release toxic ions or generate excessive reactive oxygen species (ROS), leading to oxidative stress, DNA damage, and inflammation.40 The composition of NPs—whether they are organic, inorganic, or a combination—can impact their toxicity profiles. Materials such as gold, silica, and certain metals can be toxic if they persist in the body for extended periods, leading to organ dysfunction or inflammation.77,78 Ensuring the use of biocompatible coatings and biodegradable NPs is crucial to minimizing adverse effects.
Stability and Controlled Release Mechanisms
For NPs to be effective in treating NPC, they should maintain their stability and integrity in the bloodstream, where they are subject to various biological processes, such as enzymatic degradation and protein adsorption. Nanoparticle instability can lead to premature drug release, compromising the therapeutic effect, or it can result in the aggregation of NPs, which may hinder their ability to target tumors effectively.79
Ensuring the controlled release of therapeutic agents at the tumor site remains a challenge. Premature drug leakage during circulation can reduce drug concentration at the tumor, while poor NP stability in physiological conditions may lead to aggregation or degradation. Stimuli-responsive NPs, activated by pH, temperature, or enzymes within the TME, offer a promising solution for controlled drug release. While significant progress has been made in developing smart NPs with stimuli-responsive release mechanisms, optimizing these systems for reliable and predictable drug release remains a major hurdle in their clinical application.
Targeted Delivery to NPC Cells and Blood-Tumor Barrier
The ability to deliver NPs specifically to NPC cells, while avoiding healthy tissues, is a key advantage of nanoparticle-based therapies. However, achieving effective targeted delivery is complicated by several factors. One of the primary challenges is the blood-tumor barrier—a complex and often dysfunctional network of blood vessels that surrounds tumors.80 Tumor blood vessels are typically leaky and irregular, which can make it difficult for NPs to penetrate deeply into the tumor tissue. Additionally, the rapid growth of tumor cells often leads to a hypoxic and acidic environment that can hinder nanoparticle diffusion and accumulation in the tumor.58
Another factor complicating targeted delivery is the need for NPs to be effectively “directed” to NPC cells. Tumor-specific targeting often requires functionalizing NPs with ligands that bind to specific receptors overexpressed on the surface of NPC cells. However, the identification of such tumor-specific markers that are uniquely expressed on NPC cells and absent in normal cells is still an area of active research. Additionally, tumor heterogeneity—where different areas of the same tumor express different biomarkers—further complicates the precision of nanoparticle targeting.79,80
Immune Response and Clearance
The body’s immune system may recognize NPs as foreign entities, leading to rapid clearance by macrophages and the mononuclear phagocyte system (MPS). This reduces NP circulation time and limits their therapeutic efficacy. Strategies such as surface PEGylation or biomimetic coatings can help evade immune detection and prolong circulation. However, excessive immune suppression may also disrupt normal immune functions, posing additional risks.
Conclusion
NPs-based therapeutics offer significant potential to enhance the treatment of NPC. These multifunctional nanoplatforms enhance therapeutic efficacy through radiosensitization, PTT, and tumor-targeted drug delivery while also improving imaging capabilities for early detection and treatment monitoring. By serving as radiosensitizers, NPs such as gold and hafnium dioxide enhance radiation therapy efficacy by increasing DNA damage within tumor cells. In PTT, gold nanorods and titanium dioxide efficiently convert light energy into localized heat, selectively inducing cancer cell apoptosis with minimal harm to surrounding healthy tissues. Additionally, functionalized NPs enable precise drug delivery, improving bioavailability and reducing systemic toxicity. Beyond therapy, NPs contribute to advanced imaging modalities, including MRI, CT, and fluorescence imaging, facilitating accurate tumor localization and disease progression monitoring.
Despite these developments, several gaps remain in the existing literature. The long-term biocompatibility and potential toxicity of NPs require further investigation, particularly concerning their accumulation in organs and effects on systemic health. Additionally, optimizing NP penetration within the TME remains challenging due to physical and biological barriers that hinder deep tumor infiltration. Further research is needed to refine NP design, ensuring controlled drug release and minimizing premature degradation in circulation. Moreover, clinical translation is hindered by inconsistencies between in vitro, in vivo, and clinical trial outcomes, necessitating standardized protocols and large-scale studies. Future studies should focus on developing biodegradable, immune-evasive NPs with enhanced tumor specificity and long-term safety profiles. Addressing these challenges will be critical for realizing the full potential of NPs-based strategies in advancing NPC diagnosis and treatment.
Abbreviations
NPs, nanoparticles; MRI, Magnetic Resonance Imaging.
Acknowledgments
We deeply appreciate the support from all participants. This work was supported by the Hainan Province Clinical Medical Center.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
2. Lee AWM, Ng WT, Chan JYW, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat Rev. 2019;79:101890. doi:10.1016/j.ctrv.2019.101890
3. Su ZY, Siak PY, Lwin YY, Cheah SC. Epidemiology of nasopharyngeal carcinoma: current insights and future outlook. Cancer Metastasis Rev. 2024;43(3):919–939. doi:10.1007/s10555-024-10176-9
4. Bossi P, Chan AT, Licitra L, et al; E.G.C.E.a. [email protected], Euracan. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up(dagger). Ann Oncol. 2021;32(4):452–465. doi:10.1016/j.annonc.2020.12.007
5. Bossi P, Gurizzan C, Chan A. Immunotherapy for nasopharyngeal carcinoma: the earlier the better. JAMA. 2023;330(20):1954–1955. doi:10.1001/jama.2023.22465
6. Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160270. doi:10.1098/rstb.2016.0270
7. Xu M, Yao Y, Chen H, et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat Genet. 2019;51(7):1131–1136. doi:10.1038/s41588-019-0436-5
8. Zhong Y, Bejjanki NK, Miao X, et al. Synthesis and photothermal effects of intracellular aggregating nanodrugs targeting nasopharyngeal carcinoma. Front Bioeng Biotechnol. 2021;9:730925. doi:10.3389/fbioe.2021.730925
9. Huang H, Yao Y, Deng X, et al. Immunotherapy for nasopharyngeal carcinoma: current status and prospects (Review. Int J Oncol. 2023;63(2). doi:10.3892/ijo.2023.5545
10. Jiang W, Lv JW, Tang LL, Sun Y, Chen YP, Ma J. Enhancing efficacy and reducing toxicity: therapeutic optimization in locoregionally advanced nasopharyngeal carcinoma. Cell Rep Med. 2024;5(6):101594. doi:10.1016/j.xcrm.2024.101594
11. Wang D, Tang L, Chen M, et al. Nanocarriers targeting circular RNA ADARB1 boost radiosensitivity of nasopharyngeal carcinoma through synergically promoting ferroptosis. ACS Nano. 2024;18(45):31055–31075. doi:10.1021/acsnano.4c07676
12. Lin Y, Qiu T, Lan Y, et al. Multi-modal optical imaging and combined phototherapy of nasopharyngeal carcinoma based on a nanoplatform. Int J Nanomedicine. 2022;17:2435–2446. doi:10.2147/IJN.S357493
13. Wen Z, Liu H, Qiao D, et al. Nanovaccines fostering tertiary lymphoid structure to attack mimicry nasopharyngeal carcinoma. ACS Nano. 2023;17(8):7194–7206. doi:10.1021/acsnano.2c09619
14. Guo M, Duan X, Peng X, et al. A lipid-based LMP2-mRNA vaccine to treat nasopharyngeal carcinoma. Nano Res. 2023;16(4):5357–5367. doi:10.1007/s12274-022-5254-x
15. Zhang Y, Song J, Wu C, Deng G. Lutein-loaded lotus root starch nanoparticles: preparation, release, and in vitro anti-inflammatory activity. Int J Biol Macromol. 2025;140785. doi:10.1016/j.ijbiomac.2025.140785
16. Kakkar P, Kakkar T, Nampi PP, Jose G, Saha S. Upconversion nanoparticle-based optical biosensor for early diagnosis of stroke. Biosens Bioelectron. 2025;275:117227. doi:10.1016/j.bios.2025.117227
17. Zhang S, Huang L, Chen W, et al. Piezoelectric hydrogel with self-powered biomechanical stimulation enhances bone regeneration. Acta Biomater. 2025;195:117–133.
18. Dong H, He Z, Cai S, et al. Methylprednisolone substituted lipid nanoparticles deliver C3 transferase mRNA for combined treatment of spinal cord injury. J Nanobiotechnology. 2025;23(1):98. doi:10.1186/s12951-025-03153-z
19. Khursheed R, Dua K, Vishwas S, et al. Biomedical applications of metallic nanoparticles in cancer: current status and future perspectives. Biomed Pharmacother. 2022;150:112951. doi:10.1016/j.biopha.2022.112951
20. Parkinson SJ, Tungsirisurp S, Joshi C, et al. Polymer nanoparticles pass the plant interface. Nat Commun. 2022;13(1):7385. doi:10.1038/s41467-022-35066-y
21. Zhang L, Shi J, Zhu MH, et al. Liposomes-enabled cancer chemoimmunotherapy. Biomaterials. 2025;313:122801. doi:10.1016/j.biomaterials.2024.122801
22. Kesharwani P, Ma R, Sang L, et al. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol Cancer. 2023;22(1):98. doi:10.1186/s12943-023-01798-8
23. Alamdari SG, Amini M, Jalilzadeh N, et al. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J Control Release. 2022;349:269–303. doi:10.1016/j.jconrel.2022.06.050
24. Amreddy N, Babu A, Muralidharan R, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–170.
25. Dang BN, Kwon TK, Lee S, Jeong JH, Yook S. Nanoparticle-based immunoengineering strategies for enhancing cancer immunotherapy. J Control Release. 2024;365:773–800. doi:10.1016/j.jconrel.2023.12.007
26. Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(3):670–680. doi:10.1002/anie.201804882
27. Guo Z, Zhu AT, Fang RH, Zhang L. Recent developments in nanoparticle-based photo-immunotherapy for cancer treatment. Small Methods. 2023;7(5):e2300252. doi:10.1002/smtd.202300252
28. Gupta J, Safdari HA, Hoque M. Nanoparticle mediated cancer immunotherapy. Semin Cancer Biol. 2021;69:307–324. doi:10.1016/j.semcancer.2020.03.015
29. Liu J, Zhang R, Xu ZP. Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small. 2019;15(32):e1900262. doi:10.1002/smll.201900262
30. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196. doi:10.1038/s41573-018-0006-z
31. Yoo YJ, Lee CH, Park SH, Lim YT. Nanoparticle-based delivery strategies of multifaceted immunomodulatory RNA for cancer immunotherapy. J Control Release. 2022;343:564–583. doi:10.1016/j.jconrel.2022.01.047
32. Zhuang J, Holay M, Park JH, Fang RH, Zhang J, Zhang L. Nanoparticle delivery of immunostimulatory agents for cancer immunotherapy. Theranostics. 2019;9(25):7826–7848. doi:10.7150/thno.37216
33. Kanekiyo M, Bu W, Joyce MG, et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell. 2015;162(5):1090–1100. doi:10.1016/j.cell.2015.07.043
34. Wu M, Hau PM, Li L, et al. Synthetic BZLF1-targeted transcriptional activator for efficient lytic induction therapy against EBV-associated epithelial cancers. Nat Commun. 2024;15(1):3729. doi:10.1038/s41467-024-48031-8
35. Liu D, Chen C, Hu G, et al. Specific targeting of nasopharyngeal carcinoma cell line CNE1 by C225-conjugated ultrasmall superparamagnetic iron oxide particles with magnetic resonance imaging. Acta Biochim Biophys Sin. 2011;43(4):301–306. doi:10.1093/abbs/gmr010
36. Zhang R, Shen Y, Zhou X, et al. Hypoxia-tropic delivery of nanozymes targeting transferrin receptor 1 for nasopharyngeal carcinoma radiotherapy sensitization. Nat Commun. 2025;16(1):890. doi:10.1038/s41467-025-56134-z
37. Bejjanki NK, Xu H, Xie M. GSH triggered intracellular aggregated-cisplatin-loaded iron oxide nanoparticles for overcoming cisplatin resistance in nasopharyngeal carcinoma. J Biomater Appl. 2021;36(1):45–54. doi:10.1177/0885328220982151
38. Xu G, Zhang H, Li Z, et al. Effect of HIF-1alphasiRNA-linked AuNRs on radiotherapy of nasopharyngeal carcinoma. Cell Mol Biol. 2020;66(5):185–190. doi:10.14715/cmb/2020.66.5.31
39. Wu N, Tu Y, Fan G, et al. Enhanced photodynamic therapy/photothermo therapy for nasopharyngeal carcinoma via a tumour microenvironment-responsive self-oxygenated drug delivery system. Asian J Pharm Sci. 2022;17(2):253–267. doi:10.1016/j.ajps.2022.01.002
40. Fang L, Dai J, Wang X, et al. Glutathione-driven disassembly of planar organic phototherapeutic agents to enhance photodynamic-photothermal therapy performance for nasopharyngeal carcinoma. Small. 2025;21(6):e2409196. doi:10.1002/smll.202409196
41. Li Z, Lin Y, Qiu T, et al. Noninvasive photothermal therapy of nasopharyngeal cancer guided by high efficiency optical-absorption nanomaterial enhanced by NIR-II photoacoustic imaging. Int J Nanomedicine. 2024;19:7817–7830. doi:10.2147/IJN.S457069
42. Gong L, Kwong DL, Dai W, et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat Commun. 2021;12(1):1540. doi:10.1038/s41467-021-21795-z
43. Liu H, Tang L, Li Y, et al. Nasopharyngeal carcinoma: current views on the tumor microenvironment’s impact on drug resistance and clinical outcomes. Mol Cancer. 2024;23(1):20. doi:10.1186/s12943-023-01928-2
44. Huang J, Huang W, Zhang Z, et al. Highly uniform synthesis of selenium nanoparticles with EGFR targeting and tumor microenvironment-responsive ability for simultaneous diagnosis and therapy of nasopharyngeal carcinoma. ACS Appl Mater Interfaces. 2019;11(12):11177–11193. doi:10.1021/acsami.8b22678
45. Huang Y, Li Y, He R, Dong S, Zhao Z, Jiao X. Cancer immunogenic cell death via pyroptosis with CXCR4-targeted nanotoxins in hepatocellular carcinoma. Front Bioeng Biotechnol. 2024;12:1433126. doi:10.3389/fbioe.2024.1433126
46. Nag S, Mitra O, Tripathi G, et al. Nanomaterials-assisted photothermal therapy for breast cancer: state-of-the-art advances and future perspectives. Photodiagnosis Photodyn Ther. 2024;45:103959. doi:10.1016/j.pdpdt.2023.103959
47. Mohanto S, Biswas A, Gholap AD, et al. Potential biomedical applications of terbium-based nanoparticles (TbNPs): a review on recent advancement. ACS Biomater Sci Eng. 2024;10(5):2703–2724. doi:10.1021/acsbiomaterials.3c01969
48. Nag S, Kar S, Mishra S, et al. Unveiling green synthesis and biomedical theranostic paradigms of selenium nanoparticles (SeNPs) - A state-of-the-art comprehensive update. Int J Pharm. 2024;662:124535. doi:10.1016/j.ijpharm.2024.124535
49. Guan S, Wei J, Huang L, Wu L. Chemotherapy and chemo-resistance in nasopharyngeal carcinoma. Eur J Med Chem. 2020;207:112758. doi:10.1016/j.ejmech.2020.112758
50. Lv J, Wei Y, Yin JH, et al. The tumor immune microenvironment of nasopharyngeal carcinoma after gemcitabine plus cisplatin treatment. Nat Med. 2023;29(6):1424–1436. doi:10.1038/s41591-023-02369-6
51. Luo H, Lu L, Yang F, et al. Nasopharyngeal cancer-specific therapy based on fusion peptide-functionalized lipid nanoparticles. ACS Nano. 2014;8(5):4334–4347. doi:10.1021/nn405989n
52. Ye H, Wei X, Meng C, et al. Mechanism of action of periplogenin on nasopharyngeal carcinoma based on network pharmacology and experimental study of vitamin e coupled with periplogenin self-assembled nano-prodrug for nasopharyngeal carcinoma. J Biomed Nanotechnol. 2020;16(9):1406–1415. doi:10.1166/jbn.2020.2978
53. Bejjanki NK, Zhong Y, Liu J, et al. Surface charge transition nano-theranostics based on ultra-small Fe(3)O(4) nanoparticles for enhanced photodynamic and photothermal therapy against nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2021;557:240–246. doi:10.1016/j.bbrc.2021.03.168
54. Movahedi MM, Mehdizadeh A, Koosha F, et al. Investigating the photo-thermo-radiosensitization effects of folate-conjugated gold nanorods on KB nasopharyngeal carcinoma cells. Photodiagnosis Photodyn Ther. 2018;24:324–331. doi:10.1016/j.pdpdt.2018.10.016
55. Zhao C, Liu Z, Chang CC, et al. Near-infrared phototheranostic iron pyrite nanocrystals simultaneously induce dual cell death pathways via enhanced Fenton reactions in triple-negative breast cancer. ACS Nano. 2023;17(5):4261–4278. doi:10.1021/acsnano.2c06629
56. Wang W, Cheng Z, Xing H, et al. Red cell membrane-coating Prussian blue for combined photothermal and NO gas therapy for nasopharyngeal carcinoma. J Mater Chem B. 2024;12(6):1579–1591. doi:10.1039/D3TB02444J
57. Gong R, Yang C, Abbas G, et al. Diagnosis of nasopharyngeal carcinoma using an ultrasensitive immunoassay method based on nanoparticles. Nanoscale. 2023;15(7):3475–3481. doi:10.1039/D2NR05848K
58. Lu J, Yu C, Du K, Chen S, Huang S. Targeted delivery of cisplatin magnetic nanoparticles for diagnosis and treatment of nasopharyngeal carcinoma. Colloids Surf B Biointerfaces. 2025;245:114252. doi:10.1016/j.colsurfb.2024.114252
59. Cui Y, Zhang C, Luo R, et al. Noninvasive monitoring of early antiangiogenic therapy response in human nasopharyngeal carcinoma xenograft model using MRI with RGD-conjugated ultrasmall superparamagnetic iron oxide nanoparticles. Int J Nanomed. 2016;11:5671–5682. doi:10.2147/IJN.S115357
60. Liu HQ, Wu XD, Fang XW, et al. Tumor-targeted magnetic micelles for magnetic resonance imaging, drug delivery, and overcoming multidrug resistance. ACS Omega. 2024;9(50):49566–49579. doi:10.1021/acsomega.4c07132
61. Yang D, Chen Q, Zhang M, et al. Drug-loaded acoustic nanodroplet for dual-imaging guided highly efficient chemotherapy against nasopharyngeal carcinoma. Int J Nanomedicine. 2022;17:4879–4894. doi:10.2147/IJN.S377514
62. Li Z, Yang S, Xiao H, et al. Lysosome-targeted and pH-activatable phototheranostics for NIR-II fluorescence imaging-guided nasopharyngeal carcinoma phototherapy. Bioconjug Chem. 2024;35(7):1015–1023. doi:10.1021/acs.bioconjchem.4c00225
63. Yang Q, Guo Y, Zhou Y, et al. Multifunctional nanotheranostics for dual-modal imaging-guided precision therapy of nasopharyngeal carcinoma. Mol Pharm. 2023;20(9):4743–4757. doi:10.1021/acs.molpharmaceut.3c00491
64. Liu Q, Liu L, Mo C, et al. Polyethylene glycol-coated ultrasmall superparamagnetic iron oxide nanoparticles-coupled sialyl Lewis X nanotheranostic platform for nasopharyngeal carcinoma imaging and photothermal therapy. J Nanobiotechnology. 2021;19(1):171. doi:10.1186/s12951-021-00918-0
65. Cai J, Miao YQ, Li L, Fan HM. Facile preparation of gold-decorated Fe(3)O(4) nanoparticles for CT and MR dual-modal imaging. Int J Mol Sci. 2018;19(12). doi:10.3390/ijms19124049
66. Liu D, Cao F, Xu Z, et al. Selective organ-targeting hafnium oxide nanoparticles with multienzyme-mimetic activities attenuate radiation-induced tissue damage. Adv Mater. 2024;36(19):e2308098. doi:10.1002/adma.202308098
67. Lin D, Hsieh CL, Hsu KC, et al. Geometrically encoded SERS nanobarcodes for the logical detection of nasopharyngeal carcinoma-related progression biomarkers. Nat Commun. 2021;12(1):3430. doi:10.1038/s41467-021-23789-3
68. Zheng Y, Liu Y, Jin H, et al. Scavenger receptor B1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by HDL-mimetic nanoparticles. Theranostics. 2013;3(7):477–486. doi:10.7150/thno.6617
69. Li T, Li F, Guo X, et al. Anti-Epstein-Barr virus BNLF2b for mass screening for nasopharyngeal cancer. N Engl J Med. 2023;389(9):808–819. doi:10.1056/NEJMoa2301496
70. Wang KMSD, Chen ZMSD, Long LMSD, et al. iTRAQ-based quantitative proteomic analysis of differentially expressed proteins in chemoresistant nasopharyngeal carcinoma. Cancer Biol Ther. 2018;19(9):809–824. doi:10.1080/15384047.2018.1472192
71. Wu L, Zheng K, Yan C, et al. Genome-wide study of salivary microRNAs as potential noninvasive biomarkers for detection of nasopharyngeal carcinoma. BMC Cancer. 2019;19(1):843. doi:10.1186/s12885-019-6037-y
72. Zhu QY, Zhao GX, Li Y, et al. Advances in pathogenesis and precision medicine for nasopharyngeal carcinoma. MedComm. 2021;2(2):175–206. doi:10.1002/mco2.32
73. Suryani L, Lee HPY, Teo WK, Chin ZK, Loh KS, Tay JK. Precision medicine for nasopharyngeal cancer-a review of current prognostic strategies. Cancers. 2024;16(5). doi:10.3390/cancers16050918
74. Chen YP, Yin JH, Li WF, et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020;30(11):1024–1042. doi:10.1038/s41422-020-0374-x
75. Feng S, Li Z, Chen G, et al. Ultrasound-mediated method for rapid delivery of nano-particles into cells for intracellular surface-enhanced Raman spectroscopy and cancer cell screening. Nanotechnology. 2015;26(6):065101. doi:10.1088/0957-4484/26/6/065101
76. Hong Q, Chen W, Zhang Z, et al. Nasopharyngeal carcinoma cell screening based on the electroporation-SERS spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2024;308:123747. doi:10.1016/j.saa.2023.123747
77. Oyewumi MO, Yokel RA, Jay M, Coakley T, Mumper RJ. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice. J Control Release. 2004;95(3):613–626. doi:10.1016/j.jconrel.2004.01.002
78. Sun X, Kou B. Biocompatibility and potential anticancer activity of gadolinium oxide (Gd(2)O(3)) nanoparticles against nasal squamous cell carcinoma. BMC Biotechnol. 2024;24(1):53. doi:10.1186/s12896-024-00877-y
79. Sun S, Han R, Sun Y, et al. A minimalist cancer cell membrane-shielded biomimetic nanoparticle for nasopharyngeal carcinoma active-targeting therapy. Colloids Surf B Biointerfaces. 2024;238:113909. doi:10.1016/j.colsurfb.2024.113909
80. Ding Y, Xiao X, Zeng L, et al. Platinum-crosslinking polymeric nanoparticle for synergetic chemoradiotherapy of nasopharyngeal carcinoma. Bioact Mater. 2021;6(12):4707–4716. doi:10.1016/j.bioactmat.2021.05.010
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis
Dessale M, Mengistu G, Mengist HM
International Journal of Nanomedicine 2022, 17:3735-3749
Published Date: 26 August 2022
Research Progress of Nanomedicine-Based Mild Photothermal Therapy in Tumor
He X, Zhang S, Tian Y, Cheng W, Jing H
International Journal of Nanomedicine 2023, 18:1433-1468
Published Date: 23 March 2023
The Role of Education in Nanomedicine as a Current Need for Academic Programs Related to the Healthcare Field: A Scoping Review
Huertas JD, Fuentes YV, Garcia JC, Bustos RH
Advances in Medical Education and Practice 2024, 15:65-74
Published Date: 27 January 2024

