Back to Journals » Drug Design, Development and Therapy » Volume 19
The Active Components of Traditional Chinese Medicines Regulate the Multi-Target Signaling Pathways of Metabolic Dysfunction-Associated Fatty Liver Disease
Authors Song Z , Bu S, Sang S, Li J, Zhang X, Song X, Ran Y
Received 26 December 2024
Accepted for publication 31 March 2025
Published 9 April 2025 Volume 2025:19 Pages 2693—2715
DOI https://doi.org/10.2147/DDDT.S514498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jianbo Sun
Zhicong Song,1 Shuai Bu,2 Suzhen Sang,3 Jie Li,4 Xihai Zhang,1 Xu Song,2 Yuqin Ran1
1First School of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan City, Shandong Province, People’s Republic of China; 2Department of Cardiology, The Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan City, Shandong Province, People’s Republic of China; 3Affiliated Hospital of Shandong Academy of Traditional Chinese Medicine, Jinan City, Shandong Province, People’s Republic of China; 4Scientific Research Office, Shandong University of Traditional Chinese Medicine, Jinan City, Shandong Province, People’s Republic of China
Correspondence: Shuai Bu, Department of Cardiology, The Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, No. 1 Jingba Road, Shizhong District, Jinan City, Shandong Province, 250001, People’s Republic of China, Email [email protected]
Abstract: Metabolic dysfunction-associated fatty liver disease (MAFLD), which is characterized by hepatocyte lipid accumulation driven by systemic metabolic dysregulation, represents a critical therapeutic challenge in the context of the global metabolic syndrome epidemic. The clinically recommended drugs for MAFLD mainly include antioxidants, hepatoprotective anti-inflammatory drugs, and weight-loss drugs. However, the mechanisms underlying the progression of MAFLD is characterized by nonlinearity, highlighting the urgent need for safer multi-target alternative therapies. Although existing single-target pharmacological interventions often show limited efficacy and adverse effects, the multi-component and multi-target nature of the active ingredients in traditional Chinese medicine (TCM) formulations represent new opportunities for systemic metabolic regulation. In this study, by searching PubMed and Web of Science, we identified 108 experimental studies. By evaluating multiple mechanisms, such as improving lipid metabolism and insulin resistance, alleviating oxidative stress damage, inhibiting liver inflammation, suppressing liver fibrosis, reducing endoplasmic reticulum stress, regulating hepatocyte autophagy, inhibiting hepatocyte apoptosis, improving mitochondrial dysfunction, and regulating the intestinal flora, we constructed a cross-scale regulatory network for the treatment of MAFLD by the active components of TCM. Subsequently, the dynamic target groups were screened, and a new paradigm of “mechanism-oriented and spatiotemporal-optimized” design for TCM compound prescriptions was proposed, providing a theoretical framework for the development of precise therapies that can improve liver lipid metabolism, block inflammation and fibrosis, and restore intestinal homeostasis.
Keywords: metabolic dysfunction-associated fatty liver disease, traditional Chinese medicine, active ingredient, lipid metabolism disorder, action mechanism
Introduction
With the growing prevalence of obesity and metabolic syndrome, metabolic dysfunction-associated fatty liver disease (MAFLD) has become the main cause of chronic liver disease worldwide. MAFLD affects 30% of adults globally, and its incidence is greater than 40% among individuals with diabetes and severe obesity. The prevalence is the highest in Asia-Pacific (32–35%), followed by the Middle East and Western nations. Over 20% of the cases of MAFLD show progression to advanced fibrosis within a decade, and these cases show a 5-fold higher risk of hepatocellular carcinoma (HCC).1 Moreover, MAFLD imposes a substantial economic burden, with annual direct medical costs exceeding $100 billion globally, which are primarily driven by cirrhosis management and HCC treatments. This factor highlights the increasing demand for cost-effective treatment of MAFLD.
MAFLD manifests as a spectrum of overlapping pathological states, including steatosis, inflammatory nonalcoholic steatohepatitis (NASH), fibrosis, and HCC, that progress through heterogeneous trajectories. Instead of showing a linear sequence, these states often coexist and interact through the crosstalk among metabolic dysfunction, oxidative stress, and chronic inflammation. Notably, fibrosis can develop independently of NASH in subsets of patients with specific genetic/metabolic risk profiles. Importantly, persistent inflammatory injury and advanced fibrosis (F3-F4) significantly elevate the risk of HCC, with epigenetic reprogramming and oncogenic signaling driving malignant transformation in a subset of MAFLD cohorts. This continuum underscores the need for personalized therapeutic strategies targeting multifactorial pathways, particularly interventions capable of interrupting the steatosis-inflammation-fibrosis-carcinogenesis axis.2 The progression of MAFLD and its nonlinear characteristics can be represented by the flowchart shown in Figure 1.
The pathogenesis of MAFLD is influenced by many genetic and environmental factors, and is not fully understood at present. The conceptualization of MAFLD pathogenesis has been profoundly shaped by two landmark theories. The first of these theories, the “two-hit” hypothesis, proposed a sequential mechanism wherein the first hit (hepatic lipid accumulation driven by insulin resistance [IR] and de novo lipogenesis) primes the liver for the second hit (oxidative stress and inflammatory cascades triggering NASH and fibrosis). This framework revolutionized the field in the late 1990s by shifting the paradigm from passive lipid storage to dynamic cellular injury processes, providing the first mechanistic roadmap for therapeutic development.3 Nevertheless, while the two-hit hypothesis yielded groundbreaking contributions, some critical limitations of this hypothesis gradually became evident. Clinical observations challenged its assumption of linear progression, since up to 28% of patients show advanced fibrosis without prior NASH, particularly those harboring PNPLA3 rs738409 polymorphisms. Subsequent studies demonstrated that lipid overload, mitochondrial dysfunction, and gut-derived endotoxins such as lipopolysaccharide (LPS) often coexist rather than follow a strict temporal sequence. Moreover, emerging evidence highlighted the role of extrahepatic crosstalk, wherein adipose tissue dysfunction and skeletal muscle IR independently exacerbate hepatic injury, mechanisms irreconcilable with the original two-step model.
These insights led to the “multiple parallel hit” theory, which emphasizes synchronized insults from metabolic dysregulation, epigenetic modifications, and microbiota-derived signals.4 This paradigm shift directly informs our study’s focus on traditional Chinese medicine (TCM) components with multiple targets, such as quercetin modulating the adenosine monophosphate (AMP)-activated protein kinase (AMPK)/sirtuin 1 (SIRT1)/farnesoid X receptor (FXR) pathways and berberine regulating proprotein convertase subtilisin/kexin type 9 (PCSK9)/nuclear factor (NF)-κB/liver X receptor (LXR) networks. The pleiotropic mechanisms underlying these effects inherently align with the need for combinatorial pathway modulation in MAFLD management.
MAFLD was originally known as nonalcoholic fatty liver disease. In 2020, a panel of experts from 22 countries proposed a new definition that is internationally independent of other liver diseases, renaming this condition as MAFLD to more accurately reflect its association with metabolic disorders.5 The newly proposed MAFLD framework also aligns with the holistic approach of TCM treatment. For example, berberine simultaneously improves insulin sensitivity, reduces hepatic fat synthesis, and regulates gut microbiota. Current first-line therapies, such as vitamin E and pioglitazone, have limited efficacy and pose risks of cardiovascular complications and osteoporosis with long-term use. Although dietary modification and exercise remain the cornerstones of MAFLD treatment and management, 80% of patients are unable to adhere to lifestyle interventions for more than six months. In contrast, the multi-target mechanisms and formulation flexibility of TCM can synergistically regulate pathways involved in lipid oxidation, inflammation, and fibrosis, reducing or even eliminating the occurrence of these adverse reactions and making TCM a viable option for patients who have difficulty adhering to dietary control. Considering the nonlinear progression, concurrent triggers, and organ interactions in the pathogenesis of MAFLD, the multi-target strategy of TCM can address the complexity of MAFLD more effectively than single-pathway drugs, highlighting the increasing importance of the TCM paradigm in metabolic disease research.
A growing number of experimental studies have confirmed the advantages of the active ingredients of TCM formulations in preventing and improving MAFLD.6 In the present study, we used keywords such as “MAFLD”, “traditional Chinese medicine”, and “active ingredient” to search for relevant English-language literature published on PubMed and Web of Science in the past decade. The inclusion criteria covered experimental studies and studies exploring therapeutic mechanisms, while case reports and irrelevant studies were excluded. This study investigated MAFLD by reviewing data obtained through phytochemical and pharmacological experimental designs and systematically constructed a cross-scale regulatory network of TCM active ingredients for treating MAFLD. Subsequently, a new paradigm of TCM compound design, “mechanism-oriented, spatiotemporal optimization”, was proposed by screening dynamic target populations. This model provides a theoretical framework for the development of precision treatment modalities that can simultaneously improve hepatic lipid metabolism, block inflammation and fibrosis, and restore gut homeostasis.
Common Mechanisms of Action of TCM Active Ingredients in the Treatment of MAFLD
Numerous experimental studies have evaluated the mechanisms of action of TCM active ingredients in the treatment of MAFLD. Relatively stable animal models of MAFLD have been established by feeding animals high-fat diets (HFDs) or methionine-choline deficient (MCD) diets. The active components of TCM can antagonize the occurrence and development of MAFLD at several interrelated levels. Their mechanisms of action are mainly related to improving lipid metabolism and IR, alleviating oxidative stress damage, inhibiting liver inflammation, inhibiting liver fibrosis, alleviating endoplasmic reticulum stress (ERS), regulating hepatocyte autophagy, inhibiting hepatocyte apoptosis, improving mitochondrial dysfunction, and regulating intestinal flora. The basis for classification of TCM active ingredients used for the treatment of MAFLD is presented in Figure 2. The mechanisms of action of these ingredients are summarized in the following paragraphs.
Improving Lipid Metabolism Disorders and IR
The liver is the most active organ in lipid metabolism in the body, and it participates in the digestion, absorption, transportation, catabolism and anabolism of fat. Disruptions in lipid metabolism may lead to increased synthesis and decreased breakdown of fat in the liver, and the resultant lipid overload in liver cells may eventually lead to MAFLD, which is closely related to IR and genetic susceptibility. Abnormal lipid metabolism, especially the metabolic imbalance of free fatty acids (FFA), low-density lipoprotein (LDL), triacylglycerol (TG), and total cholesterol (TC), can directly or indirectly lead to MAFLD.7 Therefore, targeting the overload of serum FFA, LDL, TG, TC and aspartate aminotransferase (AST) levels and increasing the production of high-density lipoprotein (HDL-C) can effectively improve liver lipid metabolism and reduce liver metabolic stress, thereby playing a positive therapeutic effect on MAFLD. AMPK is a key regulator of energy metabolism, and its phosphorylation can inhibit the biosynthesis of cholesterol, fatty acids, and triglycerides, thereby reducing lipid deposition in the liver.8 Various active ingredients of TCM formulations exert therapeutic effects by specifically regulating the AMPK pathway, including puerarin,9 alisol a,10,11 Lonicera caerulea extract,12 crocin,13 dihydromyricetin (DHM),14 betaine,15 Lycium barbarum polysaccharides (LBP),16 ursolic acid,17 Radix Hedysari polysaccharide,18 kaempferol (KAP),19 Leonurus ethanol extract,20 gardenin,21 Magnolia officinalis extract,22 ginsenoside Rb2,23 atractylenolide III,24 green tea extract,25 gastrodin,26 coix seed extract,27 licorice extract,28 and Scutellaria baicalensis.29 Crucially, the components of TCM harness the spatiotemporal regulatory plasticity of AMPK to achieve optimized effects in different tissues. For example, ginsenoside Rb2 enhances the phosphorylation of the AMPKα1-Ser485 site, promoting the translocation of glucose transporter 4 (GLUT4) without triggering inflammatory NF-κB feedback regulation like pan-AMPK activators do. Resveratrol synchronizes the circadian oscillations of AMPK with the rhythms of butyrate produced by gut microbiota, enhancing lipid oxidation through co-activation with peroxisome proliferator-activated receptor alpha (PPARα). This multi-tiered regulatory approach, which takes advantage of the isoform specificity of AMPK, the diversity of phosphorylation sites, and circadian dynamics, demonstrates how the inherent polypharmacology of TCM can address the complexity of MAFLD, which is beyond the capabilities of single-target drugs.30 Chlorogenic acid (CGA) is a natural polyphenol widely found in plants. It can regulate autophagy by specifically binding to AlkB homolog 5 (ALKBH5) to inhibit its m6A methylase activity and improve liver lipid deposition in HFD-fed mice by activating AMPK.31 Panpan Liu et al used network pharmacology methods and observed that KAP can prevent the occurrence and development of MAFLD through multiple targets such as inhibiting inflammation, improving IR, and reducing oxidative stress. Through cell and animal experiments, they also confirmed that KAP can inhibit the inflammatory response both in vitro and in vivo by suppressing the nuclear transcriptional activity of NF-κB, thereby preventing the occurrence of MAFLD.32 Baicalein has been confirmed to reduce hepatic fat accumulation by activating AMP-AMPK and inhibiting the cleavage of sterol regulatory element-binding protein 1 (SREBP1). This, in turn, suppresses the transcriptional activity of SREBP1 and the synthesis of hepatic fat in oleic acid-induced HepG2 cells and HFD-induced non-insulin-resistant mice. Moreover, baicalein can decrease TC and LDL-C levels while increasing HDL-C levels, thereby ameliorating the progression of MAFLD.33 Myricetin extracted from sea buckthorn can slow down the progression of MAFLD in HFD rats. Myricetin shows these effects by reducing the levels of TC, TG, alanine transaminase (ALT), and aspartate aminotransferase (AST) through multiple means, such as activating AMP-AMPK, improving the key gut microbiota system, and decreasing the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in the plasma, liver, and colon.34 In addition, regulation of the LXR and sterol regulatory element-binding protein 1c (SREBP1c) pathways by the components of TCM is equally crucial. The LXR is involved in regulating cholesterol balance, inhibiting inflammation and improving IR. Inhibition of LXR transcriptional activity can improve liver steatosis, reduce inflammation, and prevent the development of liver fibrosis.35 Ursolic acid,17 tanshinone IIA,36 and diosgenin37,38 can improve lipid accumulation in liver cells by antagonizing LXR alpha (LXRa). Leonurus ethanol extract can improve liver β-oxidation by upregulating the AMPK lipid metabolism signaling pathway and PPARα expression.20 Activation of AMPK negatively regulates SREBP-1c, thereby ameliorating lipid metabolism disorders. SREBP-1c, one of the components of sterol regulatory element-binding protein (SREBPs), is a key regulator of lipid and cholesterol metabolism in the liver, and is one of the important targets of the effective active ingredients of TCM in the treatment of MAFLD, such as Schisandra polysaccharides,39 alisol a,11 Sargassum fusiforme polysaccharide (SFPS),40 Ophiopogon polysaccharide (MDG),41,42 oroxin A,43 and oroxin B.44 Acerola polysaccharides (ACPs) are a potent active ingredient of conifers, and the results of the study showed that ACPs inhibited liver SREBP1-c levels in mice, significantly improving the accumulation of liver lipid in mice fed HFD.45 IR refers to a state characterized by reduced efficiency of insulin in promoting glucose uptake and utilization, resulting in a compensatory state of hyperinsulinemia. IR plays a central role in the mechanism and development of MAFLD by triggering disorders in glucose and lipid metabolism in the liver, as well as chronic inflammation and oxidative stress.46 TCM ingredients such as Angelica polysaccharide,47 emodin,48 and silibinin49 exhibit multi-target characteristics to improve IR. Berberine (BBR) is the active ingredient of Coptis chinensis. BBR preferentially activates the AMPK α2 isoform by phosphorylating the Thr172 site, suppressing SREBP-1c-mediated lipogenesis without affecting muscle glucose uptake.50 BBR may also inhibit liver fatty acid consumption by reducing the expression of SCD1, FABP1, CD36 and CPT1A, and activating AMPK phosphorylation to improve IR, thereby inhibiting lipid metabolism.51 The relevant active ingredients of TCM formulations and their mechanisms of action are shown in Table 1.
 |
Table 1 Summary of Experimental Studies on the Effects of Active Components of Traditional Chinese Medicine on the Treatment of MAFLD by Improving Lipid Metabolism Disorder and Insulin Resistance |
Relieving Oxidative Stress
The lipotoxic microenvironment caused by lipid metabolism disorders further exacerbates excessive production of reactive oxygen species (ROS). Oxidative stress refers to a state wherein excessive production of free radicals due to stimulation by mitochondrial ROS as a result of hyperglycemia, inflammatory signals, or exogenous toxins overwhelms the body’s ability to scavenge these free radicals, causing disorders of the body’s reduction-oxidation system. Oxidative stress is one of the important reasons for the occurrence and development of MAFLD, so alleviating oxidative stress-induced damage is a key objective of the treatment of MAFLD. Many active ingredients of TCM formulations have been shown to alleviate oxidative stress, including Codonopsis lanceolata polysaccharide (CLPS),63 salvianolic acid B,64 2,3,4′,5-tetrahydroxystilbene-2-0-β-
 |
Table 2 Summary of Experimental Studies on the Effects of Active Components of Traditional Chinese Medicine on MAFLD by Relieving Oxidative Stress |
Inhibiting Liver Inflammation
Excessive accumulation of ROS directly damages liver cells and also triggers a persistent inflammatory response by activating inflammatory signaling pathways. Liver inflammation plays a crucial role in the progression of MAFLD. This inflammatory state drives exacerbation of the disease through a dual mechanism: on one hand, pro-inflammatory factors such as TNF-α and IL-6 exacerbate intrahepatic lipid deposition; on the other hand, these factors promote collagen secretion by activating hepatic stellate cells, leading to the transition of simple fatty liver through the stages of steatohepatitis and fibrosis.74 At the level of inflammation regulation, the active ingredients of TCM formulations block this vicious cycle by targeting key signaling nodes. For example, LBP block the nuclear translocation of NF-κB by inhibiting the formation of the TLR4-MyD88 complex.75,76 Tetramethylpyrazine (TMP), on the other hand, simultaneously improves mitochondrial dysfunction and apoptosis by downregulating the phosphorylated NF-κB (p-NF-κB)/ROS signaling axis.77 Notably, andrographolide directly inhibits the phosphorylation cascade reaction of NF-κB by specifically binding to the kinase domain of inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ), and its inhibitory effect exhibits a dose-dependent characteristic.78 More recent studies have revealed the central role of the metabolism-immunity interaction in the inflammation of MAFLD. Metabolites such as succinate and palmitic acid can directly activate the NLRP3 inflammasome in macrophages. However, berberine breaks this association through a dual effect: it inhibits the signal transduction of succinate and reprograms the metabolic pattern of macrophages to be dominated by oxidative phosphorylation via the AMPK-peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) pathway. This metabolic remodeling reduces the release of pro-inflammatory factors and improves the intrahepatic lipid microenvironment by enhancing fatty acid β-oxidation.79 In addition, the multi-target characteristics of TCM components, such as LBP, paeonin,80 Poria cocos extract,81 Perilla oil supplementation (ALA),82 Tremella polysaccharide,83 nucifoline,84 Cassia semen ethanol extract,85 and gypenosides,86 demonstrate unique advantages in the complex inflammatory network. This network-based regulation mode breaks through the limitations of single-target drugs and provides new ideas for improving the long-term prognosis of patients with MAFLD. The relevant active ingredients of TCM and their mechanisms of action are shown in Table 3.
 |
Table 3 Summary of Experimental Studies on the Effects of Active Components of Traditional Chinese Medicine on MAFLD by Inhibiting Liver Inflammation |
Inhibiting Liver Fibrosis
A sustained inflammatory response promotes the activation of stellate cells, which is a key triggering factor for liver fibrosis. Liver fibrosis is a pathological process caused by various chronic liver injuries, and it is an important part of fatty liver disease. In this process, excessive deposition of collagen, mucin, and other extracellular matrix components in the liver and reduced degradation of these components result in abnormal liver structure and function. Liver fibrosis is considered to be a dynamic and reversible process. If the underlying cause is removed or controlled in time, liver fibrosis can be reversed to varying degrees. This finding provides a new direction for the treatment of MAFLD to slow or even reverse the progression of the disease by suppressing liver inflammation and improving liver fibrosis. The active ingredients of TCM formulations have shown obvious advantages in the treatment of liver fibrosis, including oxymatrine,88 andrographolide,78 and gypenoside LXXV.89 Precise interventions targeting the key nodes of fibrosis has achieved breakthroughs in recent studies. For example, the total glucosides of peony (TGP) can specifically bind to the promoter region of NLRP3 and inhibit its transcriptional activity by inducing the expression of the transcription factor FLI1. This epigenetic regulatory mechanism can alleviate hepatocyte apoptosis and block the progression of fibrosis by remodeling the immune microenvironment.90 In addition, glycyrrhizic acid (GA) shows unique multi-target effects: while inhibiting collagen deposition, it reduces de novo lipogenesis by downregulating the SREBP-1c/FAS pathway and activates PPARα to enhance fatty acid oxidation, achieving a triple synergy of anti-fibrosis, lipid metabolism-regulating, and anti-inflammatory effects.91 Notably, the spatiotemporal dynamic regulatory characteristics of TCM components represent an advantage in the management of complex pathological networks. For example, eugenol selectively inhibits the cyclooxygenase (COX)-2/prostaglandin E2 (PGE2) inflammatory pathway through a phase-separated mechanism,92 while schisandrin B enhances the antioxidant reserve of hepatocytes through the Nrf2-GSH axis.93 This hierarchical defense system can target the driving factors of fibrosis and create microenvironmental conditions facilitating hepatocyte repair. The relevant active ingredients of TCM and their mechanisms of action are shown in Table 4.
 |
Table 4 Summary of Experimental Studies on the Effects of Active Components of Chinese Medicine on MAFLD by Inhibiting Liver Fibrosis |
Relieving ERS
In addition to disrupting the structure of the liver, abnormal deposition of the extracellular matrix during the process of fibrosis also exacerbates metabolic imbalance by interfering with the functions of lipid synthesis and protein folding in the endoplasmic reticulum (ER). The “multiple-hit” theory proposed in recent years emphasizes that ERS is the core mechanism for the progression of MAFLD to NASH. By activating unfolded protein response (UPR) signals (such as the inositol-requiring enzyme 1α [IRE1α]–X-box binding protein 1 [XBP1] pathway), ERS induces the expression of inflammatory factors and inhibits the secretion of apolipoproteins, thus forming a vicious cycle of lipotoxicity and inflammation.95 At the level of ERS regulation, the active ingredients of TCM formulations restore ER homeostasis by targeting key nodes. Betulinic acid (BA) blocks the apoptosis signal-regulating kinase 1 (ASK1)- c-Jun N-terminal kinase (JNK) apoptotic signaling cascade by inhibiting the kinase activity of IRE1α.96 Resveratrol, on the other hand, alleviates the translational inhibition caused by the excessive activation of the protein kinase R-like ER kinase (PERK) pathway by enhancing the SIRT1-dependent deacetylation of eIF2α.97 Notably, catalpol can simultaneously down-regulate the expression levels of binding immunoglobulin protein (BiP) and IRE1α. Its dual inhibitory effect reduces the ERS markers in hepatocytes by more than 65% and significantly decreases the levels of serum TG/TC in HFD mice, demonstrating synergistic beneficial effects on ERS regulation and lipid metabolism. Catalpol is an active ingredient extracted from Rehmannia root. It shows many biological activities such as anti-inflammation and anti-apoptosis activities. Catalpol can significantly reduce the expression of key proteins involved ERS, such as BiP and IRE1α. By alleviating ERS, catalpol can reduce the expression of proteins associated with apoptosis, thereby inhibiting the apoptosis process of hepatocytes. In addition, catalpol can significantly reduce serum TG and TC levels in mice fed HFD, thereby improving the symptoms of MAFLD.98 The relevant active ingredients of TCM and their mechanisms of action are shown in Table 5.
 |
Table 5 Summary of Experimental Studies on the Effects of Active Components of Chinese Medicines on MAFLD by Alleviating Endoplasmic Reticulum Stress |
Regulating Autophagy of Hepatocytes
The alleviation of ERS creates the necessary microenvironmental conditions for the dynamic regulation of the autophagy system. MAFLD is characterized by hepatic lipid accumulation that exceeds the metabolic capacity of the liver, resulting in lipid overload within liver cells. In addition to affecting the normal functioning of the liver, this lipid overload also reduces fat autophagy, which, in turn, slows the self-degradation of lipids to create a vicious cycle. Therefore, regulation of hepatocyte autophagy is of great importance in the treatment of MAFLD. At the level of autophagy regulation, the active ingredients of TCM formulations restore the autophagic flux by targeting different links. Resveratrol promotes the formation of autophagosomes by activating the AMPK-Unc-51-like kinase 1 (ULK1) phosphorylation cascade.99 Puerarin, on the other hand, increases the biosynthesis of lysosomes by enhancing the nuclear translocation of transcription factor EB (TFEB), which improves the autophagic degradation efficiency by 2.3-fold.100 Notably, bergamot polyphenols (BPF) significantly accelerate the clearance of lipid droplets and damaged organelles by synergistically upregulating the levels of the LC3-II/Beclin1 complex and reducing the expression of p62. The effect of BPF in promoting the autophagic flux has been verified through liver ultrasound imaging and liver histological examinations.101 Considering the complexity of the autophagy regulation network, the components of TCM formulations exhibit the advantages of multi-dimensional intervention. Ginsenoside Rb2 enhances the assembly of the Atg12-Atg5 conjugate through a mammalian target of rapamycin (mTOR)-independent pathway,23 while capsaicin activates the CaMKKβ-AMPK signaling axis via the TRPV1 calcium ion channel.102 This multi-target characteristic enables the components of TCM formulations to simultaneously regulate the initiation, elongation, and termination stages of autophagy. In addition, certain components such as resveratrol can also selectively remove dysfunctional mitochondria through SIRT1-mediated mitophagy, blocking the excessive production of ROS at the source. The relevant active ingredients of TCM and their mechanisms of action are shown in Table 6.
 |
Table 6 Summary of Experimental Studies on the Effects of Active Components of Traditional Chinese Medicine on MAFLD by Regulating Hepatocyte Autophagy |
Inhibiting Hepatocyte Apoptosis
Precise regulation of autophagy activity directly affects the fate of hepatocytes, ie, whether they survive or undergo programmed cell death. It has been found that apoptosis is the most clearly defined and widely studied form of hepatocyte death in the MAFLD process.103 Apoptosis is a form of programmed cell death which occurs through the regulation of genes and their products in the cell. Hepatocyte apoptosis is usually induced by lipotoxic substances and causes hepatocyte injury through sublethal and lethal stress effects, inducing secondary liver injuries such as liver IR, inflammatory responses, and fibrosis and promoting the development of MAFLD.104 The active ingredients of Tuckahoe,105 Atractylodes rhizome,106 and Cordyceps flower107 have been confirmed to be involved in inhibiting the hepatocyte apoptosis pathway and thereby slowing down the progression of MAFLD. Considering the complexity of the apoptosis signaling network, the components of TCM formulations exhibit the advantages of spatiotemporal-specific regulation. LBP are the main active components of Lycium barbarum. LBP alleviate oxidative stress in MCD diet-fed mice by downregulating NF-κB expression, blocking inflammation, and inhibiting liver fibrosis, thereby inhibiting hepatocyte apoptosis.75 Gastrodia ethanol extract can significantly reduce TG and TC levels in serum, upregulate AMPK levels in liver and muscle, improve dyslipidemia, hypertension, IR, and vascular endothelial function impairment in rats fed HFD, upregulate the expression of anti-apoptotic factors, inhibit the expression of Bax protein, and thereby inhibit hepatocyte apoptosis.108 The relevant active ingredients of TCM and their mechanisms of action are shown in Table 7.
 |
Table 7 Summary of Experimental Studies on the Effect of Active Components of Chinese Medicine on MAFLD by Inhibiting Hepatocyte Apoptosis |
Improving Mitochondrial Dysfunction
The inhibition of apoptosis signals is closely related to stabilization of the mitochondrial membrane potential, and the two work together to maintain homeostasis of cellular energy metabolism. At present, the “multiple shock” theory is widely used to explain the pathogenesis of MAFLD, wherein dysfunction of liver mitochondria plays an important role.109 Elevated serum retinol-binding protein 4 levels can cause mitochondrial dysfunction and steatosis in the liver. Abnormal mitochondrial fatty acid oxidation, oxidative stress, or abnormal autophagy can induce liver inflammation and liver cell death, leading to the occurrence of MAFLD.110 Therefore, improving mitochondrial dysfunction is an effective approach to slow down the occurrence and development of MAFLD. TCM active ingredients as ACPs,45 quercetin,111 icariin,107 and puerarin100 have been shown to play roles in the treatment of MAFLD by improving mitochondrial dysfunction. Sagittaria sagittifolia polysaccharide can improve lipid metabolism disorder and oxidative stress in MAFLD mice, and can also significantly improve mitochondrial damage and restore mitochondrial adenosine triphosphate (ATP) content.68 The relevant active ingredients of TCM and their mechanisms of action are shown in Table 8.
 |
Table 8 Summary of Experimental Studies on the Effect of Active Ingredients of Chinese Medicine on MAFLD by Improving Mitochondrial Dysfunction |
Improving Intestinal Flora Imbalance
In addition to its regulatory effects within the liver, the restoration of mitochondrial function also indirectly affects the ecological balance of the gut microbiota through the gut-liver axis. The gut microbiota has been recognized as a key factor in the development of MAFLD. Imbalance of the intestinal flora affects the integrity of the intestinal mucosal barrier, triggers inflammation, produces bacterial metabolites, affects liver lipid metabolism and accelerates the occurrence and development of MAFLD.112 Therefore, intestinal microbiome-targeting therapeutic strategies for MAFLD hold much value.113 For repair of the intestinal barrier function, the synergistic effects of multiple components is particularly remarkable. Luteolin enhances tight junctions by upregulating the expression of zonula occludens 1 (ZO-1).114 BBR reduces bacterial translocation by activating the AMPK-occludin (OCLN) signaling axis, and its effect of inhibiting the entry of endotoxins into the liver leads to a 58% decrease in the serum LPS level.115 Artemisia polysaccharide, on the other hand, promotes the secretion of mucin by goblet cells through the IL-22/signal transducer and activator of transcription 3 (STAT3) pathway, forming a dual physical and chemical barrier.116 In the context of metabolism-immunity regulation, components of TCM break the association between inflammation and gut microbiota imbalance through multi-target intervention. Jade bamboo polysaccharides regulates the GPR43 receptor to balance the proportion of Th17/Treg cells.117 Oleanolic acid (OA) improves the enterohepatic circulation of bile acids through the FXR-FGF15 axis.118 This multi-level mode of action enables compound preparations such as Qingrequzhuo capsule to simultaneously correct gut microbiota dysbiosis, alleviate gut-derived inflammation, and improve lipid metabolism disorders. In addition, relevant studies have shown that the bioactive components of TCM drugs such as Walnut green husk polysaccharides (WGHP),119 Ganoderma lucidum mycelium polysaccharides,120 ferulic acid (FA),106 MP-A,54 Qingrequzhuo capsule,121 can improve the imbalance of the gut microbiota by improving the composition ratio of the gut microbiota. For instance, resveratrol alleviates the ecological imbalance of gut microbes, increases the abundance of Ruminococcaceae and Lachnospiraceae, reduces the abundance of Desulfovibrio (a class of anaerobic bacteria that reduce sulfate to produce H2S), reduces bacterial invasion and translocates, and thereby regulates the endocannabinoid system. It can maintains intestinal barrier integrity, inhibit intestinal inflammation, and improve intestinal flora disorders.122 The relevant active ingredients of TCM and their mechanisms of action are shown in Table 9.
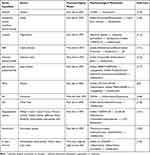 |
Table 9 Summary of Experimental Studies on the Effects of Active Ingredients of Chinese Medicine on MAFLD by Improving Intestinal Flora Imbalance |
Discussion
MAFLD has become an important cause of chronic liver disease worldwide. However, unless patients undergo specific tests to identify MAFLD, they remain unaware of the disease until the condition becomes severe or irreversible liver damage occurs.124 This study elucidates how the active ingredients of TCM formulations combat MAFLD through a multi-dimensional regulatory network encompassing lipid metabolism, oxidative stress, inflammation, and gut-liver crosstalk. Specifically, we attempted to identify the pharmacological models and mechanisms of action provided in the existing literature, and thereby reveal the common bioactive components in numerous TCM ingredients that show therapeutic effects on MAFLD. The specific pathways and targets are shown in Figure 3. An inductive summary based on the chemical structures and related targets is shown in Table 10.
 |
Table 10 Synergistic Targeting Analysis of Compound Classification in the Nonlinear Pathogenesis of MAFLD |
This study systematically constructed the first cross-scale regulatory network of the active ingredients of TCM formulations for the treatment of MAFLD. Based on the screening of the dynamic target population, a new “mechanism-oriented and spatiotemporal optimized” paradigm of TCM compound design has been proposed. However, the preclinical studies cited in this review had the following common limitations: (1) Male animals were used in 89% of the studies, and sex-related differences were not evaluated. (2) The methods of random grouping were not specified in 62% of the studies. (3) Only 12% of the studies conducted a blinded assessment of pathological endpoints, potentially affecting the generalizability of the conclusions. (4) Extrapolations of the findings of animal experimental results to humans are limited by the physiological and pathological differences between animals and humans, the variations in drug metabolism and response, as well as the inability of animal models to fully mimic the complex environmental and lifestyle factors involved in human diseases. Nevertheless, to maintain the integrity of the evidence, we have retained all the studies that met the inclusion criteria.
While the multi-target therapeutic effects of TCM ingredients offer advantages in MAFLD management, their potential hepatotoxicity and herb-drug interactions require rigorous evaluation. For instance, long-term administration of berberine (>500 mg/day) may alter CYP450 enzyme activity, and high-dose resveratrol supplementation (≥1 g/day) has been associated with mitochondrial membrane destabilization in hepatocytes. However, some of the compounds described in this paper have been successfully tested or evaluated in humans. For example, recent clinical evidence from a meta-analysis of 26 randomized trials (n = 2375) demonstrated that silymarin, a hepatoprotective botanical extract, significantly improved hepatic steatosis (OR = 3.25), reduced serum ALT/AST levels (SMD = −12.39/-10.97), and ameliorated lipid profiles in MAFLD patients, aligning with the multi-target therapeutic strategy proposed in this review.125 The crux of the matter is that the nonlinear progression of MAFLD, ie, the mutual amplification of metabolic dysfunction (AMPK suppression), oxidative damage (excessive production of ROS), and inflammation (activation of NLRP3/NF-κB), is driven by the interactions among these signaling pathways. The compound classification framework (Table 10) shows that flavonoids, alkaloids, and polysaccharides achieve synergistic effects by simultaneously targeting AMPK-mediated lipid oxidation, NLRP3-driven inflammation, and insulin sensitivity enhanced by PPARγ. This therapeutic breadth is unparalleled by single-target drugs such as vitamin E or pioglitazone. Therefore, in the future, multicenter trials can be designed to validate the synergistic effects of different classes of components. For example, combining quercetin with LBP can simultaneously improve hepatic steatosis and gut barrier function, which are key indicators for reversing MAFLD. Such combinations can also address the nonlinear characteristics of MAFLD by simultaneously inhibiting oxidative stress and interrupting the crosstalk between metabolism and inflammation. In addition, compound classes that have been proven to modulate the gut-liver axis (eg, polysaccharides such as Ganoderma lucidum polysaccharides and alkaloids like berberine) can be preferentially selected to prepare stable oral formulations for the development of standardized formulations. By jointly targeting NLRP3, PPARγ, etc., the linear progression of the AMPK-oxidative stress-inflammation axis can be broken, and the transition from various stages to an uncontrollable stage can be basically prevented.
Under the current trend of combination therapy, clarifying the mechanisms of action of different active ingredients can help address the challenges of patient compliance through sustained-release technologies, providing a practical alternative to lifestyle interventions, since 80% of patients are unable to adhere to lifestyle interventions for more than six months. Although lifestyle modification remains the cornerstone of MAFLD management, the multi-target adaptability and formulation flexibility of TCM make it a viable option for patients who experience difficulty in adhering to dietary control. By focusing on reproducible formulation engineering and clinically relevant endpoints, this field can transition from empirical practice to evidence-based precision therapy, ultimately achieving the integration of traditional wisdom and modern medicine.
Abbreviations
BA, Betulinic, acid; BiP, Binding, immunoglobulin, protein; ER, Endoplasmic, reticulum; ERS, Endoplasmic, reticulum, stress; FFA, Free, fatty, acids; FXR, Farnesoid, X, receptor; GA, Glycyrrhizic, acid; HCC, Hepatocellular, carcinoma; HFD, High-fat, diets; IR, Insulin, resistance; JNK, Jun, N-terminal, kinase; LBP, Lycium, barbarum, polysaccharides; LDL, Low-density, lipoprotein; LXR, Liver, X, receptor; MAFLD, Metabolic, dysfunction-associated, fatty, liver, disease; MCD, Methionine-choline, deficient; OA, Oleanolic, acid; ROS, Reactive, oxygen, species; SOD, Superoxide, dismutase; SREBP, Sterol, regulatory, element-binding, protein; TC, Total, cholesterol; TCM, Traditional, Chinese, medicine; TGP, Total, glucosides, of, peony; UPR, Unfolded, protein, response.
Acknowledgments
We are grateful to the sponsors of the Fund.
Author Contributions
All authors made significant contributions to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors also participated in drafting, revising, or critically reviewing the article; gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Key Research and Development Plan Project (Fund No.: 2023YFC3606203), Shandong Taishan Scholars Project Special Fund Project (Fund No.: ts201712097), and Shandong Medicine and Health Science and Technology Youth Project (Fund No.: 202303031041).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
2. Abdelmalek MF. Nonalcoholic fatty liver disease: another leap forward. Nat Rev Gastroenterol Hepatol. 2021;18(2):85–86. doi:10.1038/s41575-020-00406-0
3. Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi:10.1016/s0016-5085(98)70599-2
4. Tilg H, Adolph TE, Moschen AR. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: revisited after a decade. Hepatology. 2021;73(2):833–842. doi:10.1002/hep.31518
5. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
6. Leng YR, Zhang MH, Luo JG, Zhang H. Pathogenesis of NASH and promising natural products. Chin J Nat Med. 2021;19(1):12–27. doi:10.1016/S1875-5364(21)60002-X
7. Pei K, Gui T, Kan D, et al. An overview of lipid metabolism and nonalcoholic fatty liver disease. Biomed Res Int. 2020;2020:4020249. doi:10.1155/2020/4020249
8. Wang Q, Liu S, Zhai A, Zhang B, Tian G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol Pharm Bull. 2018;41(7):985–993. doi:10.1248/bpb.b17-00724
9. Kang OH, Kim SB, Mun SH, et al. Puerarin ameliorates hepatic steatosis by activating the PPARα and AMPK signaling pathways in hepatocytes. Int J mol Med. 2015;35(3):803–809. doi:10.3892/ijmm.2015.2074
10. Zhou X, Ren Q, Wang B, Fang G, Ling Y, Li X. Alisol a 24-acetate isolated from the alismatis rhizoma improves hepatic lipid deposition in hyperlipidemic mice by ABCA1/ABCG1 pathway. J Nanosci Nanotechnol. 2019;19(9):5496–5502. doi:10.1166/jnn.2019.16592
11. Ho C, Gao Y, Zheng D, et al. Alisol a attenuates high-fat-diet-induced obesity and metabolic disorders via the AMPK/ACC/SREBP-1c pathway. J Cell mol Med. 2019;23(8):5108–5118. doi:10.1111/jcmm.14380
12. Park M, Yoo JH, Lee YS, Lee HJ. Lonicera caerulea extract attenuates non-alcoholic fatty liver disease in free fatty acid-induced HepG2 hepatocytes and in high fat diet-fed mice. Nutrients. 2019;11(3):494. doi:10.3390/nu11030494
13. Luo L, Fang K, Dan X, Gu M. Crocin ameliorates hepatic steatosis through activation of AMPK signaling in db/db mice. Lipids Health Dis. 2019;18(1):11. doi:10.1186/s12944-018-0955-6
14. Yang Y, Qiu W, Xiao J, Sun J, Ren X, Jiang L. Dihydromyricetin ameliorates hepatic steatosis and insulin resistance via AMPK/PGC-1α and PPARα-mediated autophagy pathway. J Transl Med. 2024;22(1):309. doi:10.1186/s12967-024-05060-7
15. Seo J, Kwon D, Kim SH, Byun MR, Lee YH, Jung YS. Role of autophagy in betaine-promoted hepatoprotection against non-alcoholic fatty liver disease in mice. Curr Res Food Sci. 2024;8:100663. doi:10.1016/j.crfs.2023.100663
16. Li DD, Ma JM, Li MJ, et al. Supplementation of Lycium barbarum polysaccharide combined with aerobic exercise ameliorates high-fat-induced nonalcoholic steatohepatitis via AMPK/PPARα/PGC-1α pathway. Nutrients. 2022;14(15):3247. doi:10.3390/nu14153247
17. Lin YN, Wang CCN, Chang HY, et al. Ursolic acid, a novel liver X receptor α (LXRα) antagonist inhibiting ligand-induced nonalcoholic fatty liver and drug-induced lipogenesis. J Agric Food Chem. 2018;66(44):11647–11662. doi:10.1021/acs.jafc.8b04116
18. Sun WM, Wang YP, Duan YQ, Shang HX, Cheng WD. Radix hedysari polysaccharide suppresses lipid metabolism dysfunction in a rat model of non‑alcoholic fatty liver disease via adenosine monophosphate‑activated protein kinase pathway activation. mol Med Rep. 2014;10(3):1237–1244. doi:10.3892/mmr.2014.2327
19. Li N, Yin L, Shang J, et al. Kaempferol attenuates nonalcoholic fatty liver disease in type 2 diabetic mice via the Sirt1/AMPK signaling pathway. Biomed Pharmacother Biomedecine Pharmacother. 2023;165:115113. doi:10.1016/j.biopha.2023.115113
20. Lee MR, Park KI, Ma JY. Leonurus japonicus houtt attenuates nonalcoholic fatty liver disease in free fatty acid-induced HepG2 cells and mice fed a high-fat diet. Nutrients. 2017;10(1):20. doi:10.3390/nu10010020
21. Shen B, Feng H, Cheng J, et al. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J Cell mol Med. 2020;24(9):5097–5108. doi:10.1111/jcmm.15139
22. Lee JH, Jung JY, Jang EJ, et al. Combination of honokiol and magnolol inhibits hepatic steatosis through AMPK-SREBP-1 c pathway. Exp Biol Med. 2015;240(4):508–518. doi:10.1177/1535370214547123
23. Huang Q, Wang T, Yang L, Wang HY. Ginsenoside Rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of Sirt1 and activation of AMPK. Int J mol Sci. 2017;18(5):1063. doi:10.3390/ijms18051063
24. Li Q, Tan JX, He Y, et al. Atractylenolide III ameliorates non-alcoholic fatty liver disease by activating hepatic adiponectin receptor 1-mediated AMPK pathway. Int J Biol Sci. 2022;18(4):1594–1611. doi:10.7150/ijbs.68873
25. Santamarina AB, Oliveira JL, Silva FP, et al. Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by AMPK activation via LKB1 in mice fed a high-fat diet. PLoS One. 2015;10(11):e0141227. doi:10.1371/journal.pone.0141227
26. Wan J, Zhang Y, Yang D, et al. Gastrodin improves nonalcoholic fatty liver disease through activation of the adenosine monophosphate-activated protein kinase signaling pathway. Hepatology. 2021;74(6):3074–3090. doi:10.1002/hep.32068
27. Gu L, Zhang Y, Zhang S, et al. Coix lacryma-jobi seed oil reduces fat accumulation in nonalcoholic fatty liver disease by inhibiting the activation of the p-AMPK/SePP1/apoER2 pathway. J Oleo Sci. 2021;70(5):685–696. doi:10.5650/jos.ess20255
28. Liou CJ, Lee YK, Ting NC, et al. Protective effects of licochalcone a ameliorates obesity and non-alcoholic fatty liver disease via promotion of the sirt-1/AMPK pathway in mice fed a high-fat diet. Cells. 2019;8(5):447. doi:10.3390/cells8050447
29. Chen Q, Liu M, Yu H, et al. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J Nat Med. 2018;72(3):655–666. doi:10.1007/s11418-018-1199-5
30. Zhang W, Ho CT, Lu M. Piperine improves lipid dysregulation by modulating circadian genes Bmal1 and clock in HepG2 cells. Int J mol Sci. 2022;23(10):5611. doi:10.3390/ijms23105611
31. Meng F, Song C, Liu J, et al. Chlorogenic acid modulates autophagy by inhibiting the activity of ALKBH5 demethylase, thereby ameliorating hepatic steatosis. J Agric Food Chem. 2023;71(41):15073–15086. doi:10.1021/acs.jafc.3c03710
32. Liu P, Wu P, Yang B, et al. Kaempferol prevents the progression from simple steatosis to non-alcoholic steatohepatitis by inhibiting the NF-κB pathway in oleic acid-induced HepG2 cells and high-fat diet-induced rats. J Funct Foods. 2021;85:104655. doi:10.1016/j.jff.2021.104655
33. Sun W, Liu P, Wang T, Wang X, Zheng W, Li J. Baicalein reduces hepatic fat accumulation by activating AMPK in oleic acid-induced HepG2 cells and high-fat diet-induced non-insulin-resistant mice. Food Funct. 2020;11(1):711–721. doi:10.1039/c9fo02237f
34. Sun WL, Li XY, Dou HY, et al. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 2021;36(9):109641. doi:10.1016/j.celrep.2021.109641
35. Liu Y, Qiu DK, Ma X. Liver X receptors bridge hepatic lipid metabolism and inflammation. J Dig Dis. 2012;13(2):69–74. doi:10.1111/j.1751-2980.2011.00554.x
36. Gao WY, Chen PY, Hsu HJ, Lin CY, Wu MJ, Yen JH. Tanshinone IIA downregulates lipogenic gene expression and attenuates lipid accumulation through the modulation of LXRα/SREBP1 pathway in HepG2 cells. Biomedicines. 2021;9(3):326. doi:10.3390/biomedicines9030326
37. Cheng S, Liang S, Liu Q, et al. Diosgenin prevents high-fat diet-induced rat non-alcoholic fatty liver disease through the AMPK and LXR signaling pathways. Int J mol Med. 2018;41(2):1089–1095. doi:10.3892/ijmm.2017.3291
38. Yao H, Tao X, Xu L, et al. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway. Pharmacol Res. 2018;131:51–60. doi:10.1016/j.phrs.2018.03.017
39. Wang CM, Yuan RS, Zhuang WY, et al. Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice. Lipids Health Dis. 2016;15(1):195. doi:10.1186/s12944-016-0358-5
40. He D, Yan L, Zhang J, et al. Sargassum fusiforme polysaccharide attenuates high-sugar-induced lipid accumulation in HepG2 cells and drosophila melanogaster larvae. Food Sci Nutr. 2021;9(10):5590–5599. doi:10.1002/fsn3.2521
41. Wang X, Shi L, Wang X, Feng Y, Wang Y. MDG-1, an Ophiopogon polysaccharide, restrains process of non-alcoholic fatty liver disease via modulating the gut-liver axis. Int J Biol Macromol. 2019;141:1013–1021. doi:10.1016/j.ijbiomac.2019.09.007
42. Zhang L, Wang Y, Wu F, Wang X, Feng Y, Wang Y. MDG, an Ophiopogon japonicus polysaccharide, inhibits non-alcoholic fatty liver disease by regulating the abundance of akkermansia muciniphila. Int J Biol Macromol. 2022;196:23–34. doi:10.1016/j.ijbiomac.2021.12.036
43. Cai T, Xu X, Dong L, et al. Oroxin a from oroxylum indicum improves disordered lipid metabolism by inhibiting SREBPs in oleic acid-induced HepG2 cells and high-fat diet-fed non-insulin-resistant rats. Heliyon. 2024;10(7):e29168. doi:10.1016/j.heliyon.2024.e29168
44. Huang Y, Wang C, Wang M, et al. Oroxin B improves metabolic-associated fatty liver disease by alleviating gut microbiota dysbiosis in a high-fat diet-induced rat model. Eur J Pharmacol. 2023;951:175788. doi:10.1016/j.ejphar.2023.175788
45. Hu Y, Yin F, Liu Z, et al. Acerola polysaccharides ameliorate high-fat diet-induced non-alcoholic fatty liver disease through reduction of lipogenesis and improvement of mitochondrial functions in mice. Food Funct. 2020;11(1):1037–1048. doi:10.1039/c9fo01611b
46. Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of insulin resistance in MAFLD. Int J mol Sci. 2021;22(8):4156. doi:10.3390/ijms22084156
47. Wang K, Cao P, Wang H, et al. Chronic administration of angelica sinensis polysaccharide effectively improves fatty liver and glucose homeostasis in high-fat diet-fed mice. Sci Rep. 2016;6:26229. doi:10.1038/srep26229
48. Shen C, Pan Z, Wu S, et al. Emodin palliates high-fat diet-induced nonalcoholic fatty liver disease in mice via activating the farnesoid X receptor pathway. J Ethnopharmacol. 2021;279:114340. doi:10.1016/j.jep.2021.114340
49. Zhang Y, Hai J, Cao M, et al. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/akt pathway. Int Immunopharmacol. 2013;17(3):714–720. doi:10.1016/j.intimp.2013.08.019
50. Wang C, Jiang JD, Wu W, Kong WJ. The compound of mangiferin-berberine salt has potent activities in modulating lipid and glucose metabolisms in HepG2 cells. Biomed Res Int. 2016;2016:8753436. doi:10.1155/2016/8753436
51. Yu M, Alimujiang M, Hu L, Liu F, Bao Y, Yin J. Berberine alleviates lipid metabolism disorders via inhibition of mitochondrial complex I in gut and liver. Int J Biol Sci. 2021;17(7):1693–1707. doi:10.7150/ijbs.54604
52. Yan Z, Fan R, Yin S, et al. Protective effects of ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int J Biol Macromol. 2015;80:573–580. doi:10.1016/j.ijbiomac.2015.05.054
53. Song B, Sun Y, Chu Y, et al. Ginsenoside Rb1 alleviated high-fat-diet-induced hepatocytic apoptosis via peroxisome proliferator-activated receptor γ. Biomed Res Int. 2020;2020:2315230. doi:10.1155/2020/2315230
54. Wu J, Shao H, Zhang J, et al. Mussel polysaccharide α-D-glucan (MP-a) protects against non-alcoholic fatty liver disease via maintaining the homeostasis of gut microbiota and regulating related gut-liver axis signaling pathways. Int J Biol Macromol. 2019;130:68–78. doi:10.1016/j.ijbiomac.2019.02.097
55. Wu J, Li M, Huang N, et al. Curcumin alleviates high-fat diet-induced nonalcoholic steatohepatitis via improving hepatic endothelial function with microbial biotransformation in rats. J Agric Food Chem. 2023;71(27):10338–10348. doi:10.1021/acs.jafc.3c01067
56. Mu Q, Wang H, Tong L, et al. Betulinic acid improves nonalcoholic fatty liver disease through YY1/FAS signaling pathway. FASEB J off Publ Fed Am Soc Exp Biol. 2020;34(9):13033–13048. doi:10.1096/fj.202000546R
57. Han X, Cui ZY, Song J, et al. Acanthoic acid modulates lipogenesis in nonalcoholic fatty liver disease via FXR/LXRs-dependent manner. Chem Biol Interact. 2019;311:108794. doi:10.1016/j.cbi.2019.108794
58. Huang R, Guo F, Li Y, et al. Activation of AMPK by triptolide alleviates nonalcoholic fatty liver disease by improving hepatic lipid metabolism, inflammation and fibrosis. Phytomedicine Int J Phytother Phytopharm. 2021;92:153739. doi:10.1016/j.phymed.2021.153739
59. Ren R, Yang Z, Zhao A, et al. Sulfated polysaccharide from enteromorpha prolifera increases hydrogen sulfide production and attenuates non-alcoholic fatty liver disease in high-fat diet rats. Food Funct. 2018;9(8):4376–4383. doi:10.1039/c8fo00518d
60. Hong X, Tang H, Wu L, Li L. Protective effects of the alisma orientalis extract on the experimental nonalcoholic fatty liver disease. J Pharm Pharmacol. 2006;58(10):1391–1398. doi:10.1211/jpp.57.10.0013
61. Yang T, Wang Y, Cao X, et al. Targeting mTOR/YY1 signaling pathway by quercetin through CYP7A1-mediated cholesterol-to-bile acids conversion alleviated type 2 diabetes mellitus induced hepatic lipid accumulation. Phytomedicine Int J Phytother Phytopharm. 2023;113:154703. doi:10.1016/j.phymed.2023.154703
62. Tian X, Ru Q, Xiong Q, Wen R, Chen Y. Catalpol attenuates hepatic steatosis by regulating lipid metabolism via AMP-activated protein kinase activation. Biomed Res Int. 2020;2020:6708061. doi:10.1155/2020/6708061
63. Zhang Y, Wang H, Zhang L, Yuan Y, Yu D. Codonopsis lanceolata polysaccharide CLPS alleviates high fat/high sucrose diet-induced insulin resistance via anti-oxidative stress. Int J Biol Macromol. 2020;145:944–949. doi:10.1016/j.ijbiomac.2019.09.185
64. Wang YC, Kong WZ, Jin QM, Chen J, Dong L. Effects of salvianolic acid B on liver mitochondria of rats with nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21(35):10104–10112. doi:10.3748/wjg.v21.i35.10104
65. Xu J, Peng Y, Zeng Y, Hua YQ, Xu XL. 2, 3, 4’, 5-tetrahydroxystilbene-2-0-β-d glycoside attenuates age- and diet-associated non-alcoholic steatohepatitis and atherosclerosis in LDL receptor knockout mice and its possible mechanisms. Int J mol Sci. 2019;20(7):1617. doi:10.3390/ijms20071617
66. Sun W, Liu P, Yang B, et al. A network pharmacology approach: inhibition of the NF-κB signaling pathway contributes to the NASH preventative effect of an oroxylum indicum seed extract in oleic acid-stimulated HepG2 cells and high-fat diet-fed rats. Phytomedicine Int J Phytother Phytopharm. 2021;88:153498. doi:10.1016/j.phymed.2021.153498
67. Shen B, Zhao C, Wang Y, et al. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J Cell mol Med. 2019;23(6):4063–4075. doi:10.1111/jcmm.14293
68. Deng X, Ke X, Tang Y, et al. Sagittaria sagittifolia polysaccharide interferes with arachidonic acid metabolism in non-alcoholic fatty liver disease mice via Nrf2/HO-1 signaling pathway. Biomed Pharmacother Biomedecine Pharmacother. 2020;132. doi:10.1016/j.biopha.2020.110806
69. Hn L, Ll Z, Dy Z, Dq C. Ganoderma lucidum polysaccharides ameliorates hepatic steatosis and oxidative stress in db/db mice via targeting nuclear factor E2 (erythroid-derived 2)-related factor-2/heme oxygenase-1 (HO-1) pathway. Med Sci Monit Int Med J Exp Clin Res. 2020;26. doi:10.12659/MSM.921905
70. Li J, Wang T, Liu P, et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021;12(9):3898–3918. doi:10.1039/d0fo02736g
71. Zhao XJ, Yu HW, Yang YZ, et al. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124–137. doi:10.1016/j.redox.2018.07.002
72. Clare K, Dillon JF, Brennan PN. Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J Clin Transl Hepatol. 2022;10(5):939–946. doi:10.14218/JCTH.2022.00067
73. Aloui H, Baraket K, Sendon R, Silva AS, Khwaldia K. Untargeted metabonomics reveals intervention effects of chicory polysaccharide in a rat model of non-alcoholic fatty liver disease. Int J Biol Macromol. 2019;139:128. doi:10.1016/j.ijbiomac.2019.01.141
74. Petrescu M, Vlaicu SI, Ciumărnean L, et al. Chronic inflammation-a link between nonalcoholic fatty liver disease (NAFLD) and dysfunctional adipose tissue. Med Kaunas Lith. 2022;58(5):641. doi:10.3390/medicina58050641
75. Xiao J, Wang F, Liong EC, So KF, Tipoe GL. Lycium barbarum polysaccharides improve hepatic injury through NFkappa-B and NLRP3/6 pathways in a methionine choline deficient diet steatohepatitis mouse model. Int J Biol Macromol. 2018;120(Pt B):1480–1489. doi:10.1016/j.ijbiomac.2018.09.151
76. Xiao J, Xing F, Huo J, et al. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci Rep. 2014;4:5587. doi:10.1038/srep05587
77. Chen B, Ma Y, Xue X, Wei J, Hu G, Lin Y. Tetramethylpyrazine reduces inflammation in the livers of mice fed a high fat diet. mol Med Rep. 2019;19(4):2561–2568. doi:10.3892/mmr.2019.9928
78. Cabrera D, Wree A, Povero D, et al. Andrographolide ameliorates inflammation and fibrogenesis and attenuates inflammasome activation in experimental non-alcoholic steatohepatitis. Sci Rep. 2017;7(1):3491. doi:10.1038/s41598-017-03675-z
79. Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosh CHS. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7(7):e40156. doi:10.1371/journal.pone.0040156
80. Zhang L, Yang B, Yu B. Paeoniflorin protects against nonalcoholic fatty liver disease induced by a high-fat diet in mice. Biol Pharm Bull. 2015;38(7):1005–1011. doi:10.1248/bpb.b14-00892
81. He J, Yang Y, Zhang F, et al. Effects of poria cocos extract on metabolic dysfunction-associated fatty liver disease via the FXR/PPARα-SREBPs pathway. Front Pharmacol. 2022;13:1007274. doi:10.3389/fphar.2022.1007274
82. Chen T, Yuan F, Wang H, et al. Perilla oil supplementation ameliorates high-fat/high-cholesterol diet induced nonalcoholic fatty liver disease in rats via enhanced fecal cholesterol and bile acid excretion. Biomed Res Int. 2016;2016:2384561. doi:10.1155/2016/2384561
83. Khan TJ, Xu X, Xie X, et al. Tremella fuciformis crude polysaccharides attenuates steatosis and suppresses inflammation in diet-induced NAFLD mice. Curr Issues mol Biol. 2022;44(3):1224–1234. doi:10.3390/cimb44030081
84. Guo F, Yang X, Li X, et al. Nuciferine prevents hepatic steatosis and injury induced by a high-fat diet in hamsters. PLoS One. 2013;8(5):e63770. doi:10.1371/journal.pone.0063770
85. Meng Y, Liu Y, Fang N, Guo Y. Hepatoprotective effects of cassia semen ethanol extract on non-alcoholic fatty liver disease in experimental rat. Pharm Biol. 2019;57(1):98–104. doi:10.1080/13880209.2019.1568509
86. Li H, Ying H, Hu A, Hu Y, Li D. Therapeutic effect of gypenosides on nonalcoholic steatohepatitis via regulating hepatic lipogenesis and fatty acid oxidation. Biol Pharm Bull. 2017;40(5):650–657. doi:10.1248/bpb.b16-00942
87. Zheng T, Yang X, Li W, et al. Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid Med Cell Longev. 2018;2018:8597897. doi:10.1155/2018/8597897
88. Shi L, Juan SL, Song GY, et al. Oxymatrine attenuates hepatic steatosis in non-alcoholic fatty liver disease rats fed with high fructose diet through inhibition of sterol regulatory element binding transcription factor 1 (Srebf1) and activation of peroxisome proliferator activated receptor alpha (pparα). Eur J Pharmacol. 2013;714(1–3):89–95. doi:10.1016/j.ejphar.2013.06.013
89. Lee JH, Oh JY, Kim SH, et al. Pharmaceutical efficacy of gypenoside LXXV on non-alcoholic steatohepatitis (NASH). Biomolecules. 2020;10(10):1426. doi:10.3390/biom10101426
90. Zhang J, Fu Y, Yang B, Xiang X. Total glucosides of paeony inhibits liver fibrosis and inflammatory response associated with cirrhosis via the FLI1/NLRP3 axis. Am J Transl Res. 2022;14(6):4321–4336.
91. Wang C, Duan X, Sun X, et al. Protective effects of glycyrrhizic acid from edible botanical glycyrrhiza glabra against non-alcoholic steatohepatitis in mice. Food Funct. 2016;7(9):3716–3723. doi:10.1039/c6fo00773b
92. Jo HK, Kim GW, Jeong KJ, Kim DY, Chung SH. Eugenol ameliorates hepatic steatosis and fibrosis by down-regulating SREBP1 gene expression via AMPK-mTOR-p70S6K signaling pathway. Biol Pharm Bull. 2014;37(8):1341–1351. doi:10.1248/bpb.b14-00281
93. Kwan HY, Niu X, Dai W, et al. Lipidomic-based investigation into the regulatory effect of schisandrin B on palmitic acid level in non-alcoholic steatotic livers. Sci Rep. 2015;5:9114. doi:10.1038/srep09114
94. Rq W, Hm M, L H, Sx Z, Yh J, Ym N. Modulation of IKKβ/NF-κB and TGF-β1/smad via fuzheng huayu recipe involves in prevention of nutritional steatohepatitis and fibrosis in mice. Iran J Basic Med Sci. 2015;18(4).
95. Lachkar F, Papaioannou A, Ferré P, Foufelle F. ER stress and NAFLD. Biol Aujourdhui. 2020;214(1–2):15–23. doi:10.1051/jbio/2020007
96. Gu M, Zhao P, Zhang S, et al. Betulinic acid alleviates endoplasmic reticulum stress-mediated nonalcoholic fatty liver disease through activation of farnesoid X receptors in mice. Br J Pharmacol. 2019;176(7):847–863. doi:10.1111/bph.14570
97. Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS One. 2017;12(8):e0183541. doi:10.1371/journal.pone.0183541
98. Zhu HF, Shao Y, Qin L, et al. Catalpol enhances neurogenesis and inhibits apoptosis of new neurons via BDNF, but not the BDNF/trkb pathway. Drug Des Devel Ther. 2019;13:4145–4157. doi:10.2147/DDDT.S223322
99. Zhang Y, Chen ML, Zhou Y, et al. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. mol Nutr Food Res. 2015;59(8):1443–1457. doi:10.1002/mnfr.201500016
100. Fang X, Lan X, Zhu M, et al. Puerarin induces macrophage M2 polarization to exert antinonalcoholic steatohepatitis pharmacological activity via the activation of autophagy. J Agric Food Chem. 2024;72(13):7187–7202. doi:10.1021/acs.jafc.3c09601
101. Parafati M, Lascala A, Morittu VM, et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J Nutr Biochem. 2015;26(9):938–948. doi:10.1016/j.jnutbio.2015.03.008
102. Li Q, Li L, Wang F, et al. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. Pflugers Arch - Eur J Physiol. 2013;465(9):1303–1316. doi:10.1007/s00424-013-1274-4
103. Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15(12):738–752. doi:10.1038/s41575-018-0065-y
104. Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1049–1061. doi:10.1016/j.metabol.2016.02.014
105. Ye H, Ma S, Qiu Z, et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J Ethnopharmacol. 2022;296:115457. doi:10.1016/j.jep.2022.115457
106. Fu J, Yang J, He L, et al. Ferulic acid alleviates hepatic lipid accumulation and inflammation by improving proximal and distal intestinal barriers in NAFLD mice. Tohoku J Exp Med. 2023;260(2):149–163. doi:10.1620/tjem.2023.J023
107. Choi J, Choi H, Chung J. Icariin supplementation suppresses the markers of ferroptosis and attenuates the progression of nonalcoholic steatohepatitis in mice fed a methionine choline-deficient diet. Int J mol Sci. 2023;24(15):12510. doi:10.3390/ijms241512510
108. Kho MC, Lee YJ, Cha JD, Choi KM, Kang DG, Lee HS. Gastrodia elata ameliorates high-fructose diet-induced lipid metabolism and endothelial dysfunction. Evid-Based Compl Altern Med ECAM. 2014;2014:101624. doi:10.1155/2014/101624
109. Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155(3):629–647. doi:10.1053/j.gastro.2018.06.083
110. Liu Y, Mu D, Chen H, et al. Retinol-binding protein 4 induces hepatic mitochondrial dysfunction and promotes hepatic steatosis. J Clin Endocrinol Metab. 2016;101(11):4338–4348. doi:10.1210/jc.2016-1320
111. Jiang JJ, Zhang GF, Zheng JY, Sun JH, Ding SB. Targeting mitochondrial ROS-mediated ferroptosis by quercetin alleviates high-fat diet-induced hepatic lipotoxicity. Front Pharmacol. 2022;13:876550. doi:10.3389/fphar.2022.876550
112. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi:10.1016/j.jhep.2019.10.003
113. Ma J, Zhou Q, Li H. Gut microbiota and nonalcoholic fatty liver disease: insights on mechanisms and therapy. Nutrients. 2017;9(10):1124. doi:10.3390/nu9101124
114. Sun WL, Yang JW, Dou HY, et al. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg Chem. 2021;112:104966. doi:10.1016/j.bioorg.2021.104966
115. Cao Y, Pan Q, Cai W, et al. Modulation of gut microbiota by berberine improves steatohepatitis in high-fat diet-fed BALB/C mice. Arch Iran Med. 2016;19(3):197–203.
116. Li J, Pang B, Shao D, Jiang C, Hu X, Shi J. Artemisia sphaerocephala krasch polysaccharide mediates lipid metabolism and metabolic endotoxaemia in associated with the modulation of gut microbiota in diet-induced obese mice. Int J Biol Macromol. 2020;147:1008–1017. doi:10.1016/j.ijbiomac.2019.10.069
117. Wang Y, Fei Y, Liu L, et al. Polygonatum odoratum polysaccharides modulate gut microbiota and mitigate experimentally induced obesity in rats. Int J mol Sci. 2018;19(11):3587. doi:10.3390/ijms19113587
118. Xue C, Li Y, Lv H, et al. Oleanolic acid targets the gut-liver axis to alleviate metabolic disorders and hepatic steatosis. J Agric Food Chem. 2021;69(28):7884–7897. doi:10.1021/acs.jafc.1c02257
119. Wang G, Yang X, Wang J, et al. Walnut green husk polysaccharides prevent obesity, chronic inflammatory responses, nonalcoholic fatty liver disease and colonic tissue damage in high-fat diet fed rats. Int J Biol Macromol. 2021;182:879–898. doi:10.1016/j.ijbiomac.2021.04.047
120. Jin M, Zhu Y, Shao D, et al. Effects of polysaccharide from mycelia of ganoderma lucidum on intestinal barrier functions of rats. Int J Biol Macromol. 2017;94(Pt A):1–9. doi:10.1016/j.ijbiomac.2016.09.099
121. Lv S, Zhang Z, Su X, et al. Qingrequzhuo capsule alleviated methionine and choline deficient diet-induced nonalcoholic steatohepatitis in mice through regulating gut microbiota, enhancing gut tight junction and inhibiting the activation of TLR4/NF-κB signaling pathway. Front Endocrinol. 2022;13:1106875. doi:10.3389/fendo.2022.1106875
122. Chen M, Hou P, Zhou M, et al. Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin Nutr Edinb Scotl. 2020;39(4):1264–1275. doi:10.1016/j.clnu.2019.05.020
123. Han R, Qiu H, Zhong J, et al. Si miao formula attenuates non-alcoholic fatty liver disease by modulating hepatic lipid metabolism and gut microbiota. Phytomedicine Int J Phytother Phytopharm. 2021;85:153544. doi:10.1016/j.phymed.2021.153544
124. Duell PB, Welty FK, Miller M, et al. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American heart association. Arterioscler Thromb Vasc Biol. 2022;42(6):e168–e185. doi:10.1161/ATV.0000000000000153
125. Li S, Duan F, Li S, Lu B. Administration of silymarin in NAFLD/NASH: a systematic review and meta-analysis. Ann Hepatol. 2024;29(2):101174. doi:10.1016/j.aohep.2023.101174
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Research Progress on the Treatment of Related Diseases With Astragalus
Zhang X, Lin B, Wang X, Fanɡ N, Wu L, Wan H, Zhou H
Drug Design, Development and Therapy 2025, 19:2845-2862
Published Date: 12 April 2025




