Back to Journals » Advances in Medical Education and Practice » Volume 16
The Learning Curve for Laparoscopic Sacrocolpopexy Based on Dissection Skills if Structured Teaching and Standardized Surgery are Applied
Authors Studer AM , Krebs J , Brambs C, Christmann-Schmid C
Received 8 January 2025
Accepted for publication 8 May 2025
Published 24 May 2025 Volume 2025:16 Pages 917—925
DOI https://doi.org/10.2147/AMEP.S513699
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sateesh B Arja
Andreas Martin Studer,1 Jörg Krebs,2 Christine Brambs,1 Corina Christmann-Schmid1
1Department of Urogynecology, Cantonal Hospital of Lucerne, Lucerne, Switzerland; 2Neuro-Urology Research Group, Swiss Paraplegics Research, Nottwil, Switzerland
Correspondence: Andreas Martin Studer, Department of Urogynecology, Cantonal Hospital of Lucerne, Lucerne, Switzerland, Email [email protected]
Purpose: Teaching is an important but time- and resource-consuming process. Therefore, it is important to optimize and structure it effectively. The aim of this study was to evaluate if dry-lab suture and knotting training lead to stable theater performance. Consequently, the learning curve is supposed to be ascribed to only on-patient trainable skills like tissue preparation.
Patients and Methods: To test this hypothesis, a structured training and stepwise surgical program were implemented to perform laparoscopic sacrocolpopexy (SCP) for urogynecological fellows adapting existing literature.
The program was structured and recorded as following: dry-lab training weekly for knotting and suturing skills, assisting 20 SCP, stepwise overtaking defined steps under supervision including preparation of the dissecting planes anteriorly, posteriorly and at the level of the promontory, mesh fixation and retroperitoneal closure. All women opting for sacrocolpopexy as pelvic organ prolapse repair and being treated by one designated fellow were included without any specific selection. The only exclusion criterium was repeat sacrocolpopexy.
Results: Within 45 procedures, the fellow reached a plateau of 80 minutes surgical time for SCP (excluding port-entry/-closure or concomitant interventions like hysterectomy or adnexectomy), with a complication rate of 11.1%. A high subjective and objective success rate was reported post-operatively. Differences in overall time were clearly correlated with overall dissection time.
Conclusion: We demonstrated that a stepwise, structured, and standardized intraoperative surgical program facilitated safe and efficient laparoscopic SCP performance in the analyzed situation. Off-patient trainable skills like suturing and knotting led to consistent mesh fixation times from the start of the qualification process and variation arose due to in situ learnable factors.
Keywords: education, urogynecology, pelvic organ prolapse, teaching
Introduction
Teaching young gynecologists, in a situation where efficient and high-quality surgery is needed to reduce expanding economic costs of healthcare, is a challenging burden for senior staff members. A stepwise and structured surgical program can facilitate surgical skill training.1 To date, there have been multiple well-implemented surgical workshops that have been integrated into fellowship programs.2,3 However, the acquired skills must be transferred to theater practice.
Pelvic organ prolapse is a widespread benign condition that can severely restrict quality of life.4 Laparoscopic sacrocolpopexy (SCP) is the gold standard procedure for surgically managing apical and multi-compartment prolapse.5 This mostly standardized and well-implemented procedure therefore needs to be part of the skill portfolio obtained during a urogynecology fellowship program.
Based on the existing literature and adapting to our center, we implemented a training program for laparoscopic sacrocolpopexy and we sought to evaluate in the study if it led to sustainable and safe surgical expertise. In the existing literature about 20–60 cases are described to reach such expertise.6–9 The vast majority of analysis focusing on overall surgical time and complication rate. We aspired on one hand to replicate the steep learning curve of the programs but also determine which area contributes the most to a stable and reduced surgical time. Assuming that trainable skills such as suturing and knotting only have minor influence on the progress over time if trained prior to theater exposure. To differentiate which area improved the most during training, we zoomed in on pre-defined surgical sub-steps and timed them individually.
Materials and Methods
To test the above stated hypothesis, we focused this study on one surgeon passing through the initiated learning program. At baseline, the trainee had performed about 70 laparoscopic hysterectomies, 100 therapeutic laparoscopies (including ectopic pregnancies, adnexal mass-removal, adnexectomy) and had only minor exposure to SCP, assisting about 20 procedures during residency. The educational setting was a urogynecological referral center consisting of one head, a consultant, two fellows and a rotation resident. As a center, about 100 SCPs are performed a year. Included in this analysis from July 2019 to February 2023 are all women with an apical or multi-compartment prolapse ≥ stage 2 according to the International Continence Society undergoing a laparoscopic SCP performed by one designated fellow.10 Patents were arbitrarily selected based on personal contact in consultation. The only exclusion from this study was women who planned to undergo a repeat SCP.
Routinely a medical and surgical history was obtained. Subjective outcome measures were administered by a German translated validated female pelvic floor questionnaire (GFPFQ) and Patient Global Impression of Improvement (PGI-I).11–13 To assess the success rate, clinical examination included assessment of the pelvic floor utilizing the Pelvic Organ Prolapse Quantification system (POP-Q).14 An ultrasound evaluation of the pelvic floor completed the objective work-up to determine the post-operative mesh position, mesh lay on the vagina, hematoma, or other pathologies. All pre- and post-operative assessments were carried out by one of the team members, mainly the assessed fellow.
As general entry criteria for the training program, it was required for participants to have either intermediate surgical skill level of GESEA MIGS or Swiss national general gynecological surgery specialty training qualifying them to independently perform a laparoscopic hysterectomy.2 Basic training started with a weekly two-hour dry-lab practice for six months using a pelvic trainer, foam pads and equal needle holders, as in theater. The goal was to perform an intracorporeal stitch and knot with a monofilament, including introducing and extracting the needle as well as cutting the thread in under three minutes using the techniques described in GESEA. The nerve-sparing sacrocolpopexy technic was divided into sub-steps, including knowing the pitfalls and how to prevent them.15–17
On-patient training in theater was chronologically structured as follows:
1. Assisting 20 procedures carried out by a senior urogynecologist, followed by stepwise taking on the defined steps under supervision.
2. Four procedures involving dissecting the planes for the mesh-inlay anteriorly and posteriorly, the longitudinal ligament at sacral promontory, and closure of the peritoneum.
3. Four interventions were then performed under supervision relating to additional fixation of the mesh anteriorly by the fellow.
4. Subsequently, five complete laparoscopic nerve-sparing SCPs (including posterior mesh suturing and fixation at the promontory by a taker system) were conducted together with the senior surgeon.
5. Completing the procedure independently, having a senior surgeon on call (outside of theater), assisted by residents or junior fellows (less experienced than the trainee).
6. After the fifth and fifteenth (consecutively) independently performed SCP, performance was assessed by the senior surgeon (head of department) in theater.
To evaluate the progress of the performance we used different parameters, including the overall surgical time which we limited to the SCP itself. Time for installing and closing port sites and handling of unexpected complications and additional time for concomitant interventions, such as hysterectomy or adnexectomy, were recorded separately. Intraoperatively predefined sub-steps were timed as follows: dissection of the vesicovaginal space, recto-vaginal space, longitudinal ligament at the promontory, peritoneal mesh pocket, fixation of the mesh with intracorporeal sutures anteriorly and posteriorly (each separately), mesh (EndoGYNious, A.M.I.® GmbH, Feldkirch, Austria) fixation with the taker system (ProTack™, Covidien™, Dublin, Ireland) at the level of the sacral promontory, and closure of peritoneum by continuous suture. Timing was recorded from the beginning of the sub-step concerned until the start of the next one by the surgical theater assistant, and post-operative double-checking took place by reviewing intraoperative videos.
Further intraoperative need for support, complications according to ClassIntra 1.0 and Dindo-Clavien classification, respectively, and blood loss were noted separately.18,19
Based on their estimated overall influence on performance, we ranked different parameters according to the performance index shown in Table 1. The possible values of the performance index range from 3 to −10. Positive results were ranked as good performance.
 |
Table 1 Measurement Parameters for Performance Index |
As the primary outcome, we defined stable suturing times from the start. Secondarily, we wanted to demonstrate decreasing dissection time over the course of the training, to reach the overall goal of the implemented training program achieving a stable overall operation time of <80min after 30 interventions (supervised and independent combined).
Statistical analysis Due to the sample size and single surgeon setup statistical analysis is constrained to Pearson correlation coefficient and descriptive interpretation. Correlation of overall surgical time, dissection and suturing time was too strong to carry out a regression analysis.
Ethics Statement
The local ethics committee “Ethikkommission Nordwest- und Zentralschweiz” (EKNZ Req-2024-00001) approved the study to be in line with ethical considerations. Because the analyzed data consist of measurement concerning the author and patient data comprise generally registered history and clinical practice covered by signed general consent, the ethics committee decided that the project does not fall under the scope of the Human Research Act.
Results
In this analysis, 45 laparoscopic nerve-sparing SCPs carried out by one designated urogynecology fellow from July 2019 to February 2023 were assessed and analyzed. Table 2 summarizes demographics and previous pelvic floor interventions.
 |
Table 2 Demographics and Previous Pelvic Floor Interventions |
The time taken for SCPs performed completely by the trainee (excluding the first eight only partially performed SCPs) without port-entry and port-closure but including management of occurring complications was 76 min median (ranging from 51 to 137 min). This is visualized over time in Figure 1.
The sub-steps required 2 to 43 minutes and are plotted in Figure 2. Despite closure of the peritoneum, suturing sub-steps demonstrated significantly less variability than dissecting sub-steps.
Dissection of the surgical planes (anterior and posterior vagina, peritoneal mesh pocket and promontory together) had a strong correlated of 0.834 (p < 0.00001) to the total surgical time (Figure 3) and required 34 minutes on average (SD 13.5 minutes). Cumulative mesh suturing time, both anterior and posterior, was consistent, averaging 18 minutes, with a standard deviation of 4.7 minutes. Also, peritoneal closure time was fairly consistent, at 20 minutes on average, but had five outliers of over 25 minutes. Four of these were assisted by residents and one was due to a teared thread when tying the finishing knot. Correlation off total suturing time, cumulative mesh suturing and peritoneal closure, to overall operative time was with 0.554 (p = 0.0004) distinctively lower than towards dissection time.
Further, weak correlation was found between operative time of 0.41 (p = 0.005) and dissection total of 0.31 (p = 0.038) to body-mass-index. Although there was no correlation of suturing times to body-mass-index (p = 0.2).
The development of the performance index is plotted in Figure 4 and demonstrates more consistent performance with less variance over time. Calls for surgical help were requested in four cases, and verbal advice was requested in seven cases, outside of the first 13 cases planned with the senior surgeon. In the last 15 surgeries, only once was there a need for a call for help.
 |
Figure 4 Performance index per case over time – cases assisted by a resident marked by *; red colored background indicates cases assisted by senior surgeon. |
All women were seen six to eight weeks post-operatively and reported positive subjective feedback on PGI-I. Table 3 shows the objective success post-operatively at six to eight weeks. A persistent prolapse stage II was found in 15% (7/45) anteriorly. One woman had apical prolapse stage I and two posterior prolapse stage II. Out of these persistent prolapses, two anterior ones and an apical one occurred under supervision by a senior urogynecologist. The other anterior persistence and the posterior prolapses appeared in non-supervised situations.
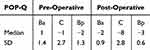 |
Table 3 Pre- and Post-Operative POP-Q Values |
Perineal and transvaginal ultrasound revealed desired mesh position at the level of the bladder neck anteriorly and posteriorly one to two centimeters from the perineal body, flat mesh on the vaginal cuff and no pathologies. Three months after surgery five out of 45 women reported bulging symptoms in the German female pelvic floor questionnaire (Question No. 28)11.
Intraoperative complications occurred in 11.1% (five out of 45) of cases, including two bladder injuries, one vaginal and one sigmoid serosal laceration and one omental bleeding caused by port-entry. Overall blood loss, except in the case of omental bleeding, was reported to be below 50mL.
Discussion
In contrast to the existing literature focusing on overall surgical time in SCPs, we wanted to determine the underlying factors that lead to improved performance over time.6,8,9 Like Claerhout et al, we also analyzed sub-steps of the procedure and demonstrated that structured surgical fellowship training results in safe and sustainable expertise.7 In comparison to the previous cited publication, in which the trainee was assisted by an experienced surgeon, in our case the observed fellow was assisted by less experienced staff than himself unless previously planned (as for the first 13 cases and assessment surgeries in cases 19/20 and 36/37). The significantly decreasing need for support, with consistently stable performance, suggests the conclusion that about 30–35 interventions are needed to reach a satisfactory level of expertise. This is slightly higher than the best results of other studies of around 20 interventions6,9 and is most likely due to long case-to-case intervals in the initial training. The long period required to reach 45 interventions as well as initial expanded case-to-case interval is explainable by a nine-month restriction on elective surgeries due to the COVID-19 pandemic, four-month traumatic injury-related incapacity to work, and ongoing on-duty work, which resulted in 20 training surgeries taking place per year only after the restrictions were lifted and medical recovery took place. A more intensive training rhythm is theoretically associated with a faster learning curve but can sometimes be difficult to implement depending on the daily routine of the clinic and other circumstances.
Restricting the analyzed time for SCP, excluding port-entry and port-closure, as well as concomitant interventions, was employed because most of these aspects are performed by residents or different trainees for educational purposes.
There was no selection of patients for the training program and demographics did not vary from our general population included in previous studies published by Christmann et al.15 Persistent prolapses and occurrence of complications arose in teaching scenarios as well as in non-teaching scenarios and were similar to the previously mentioned teaching programs for laparoscopic SCP.
Under the assumption that variable and prolonged time for surgical sub-steps results from difficulties, eg, finding the correct dissection plane after hysterectomy or unexpected excessive scarring, we demonstrated that the challenging surgical steps arise essentially from only in situ trainable factors, like tissue preparation and anatomical distinction, as previously reported by Claerhout et al.7 Affirmed by the stronger correlation of the dissection time to the total time compared to all other variables and the low variability of suturing times.
The variability in peritoneal closure time arises from our three-handed suturing technic, involving the first assistant being a surgically unexperienced junior resident in some cases marked in Figure 1 compared to the mesh attachment, which the surgeon performed on his own. Hence, resulting differences in total time were essentially effects of disparity in preparation of the dissection planes, which underlines the importance of combining on- and off-patient training.
Concerning satisfaction, we achieved a high subjective and objective success rate that is not different to published data on overall success rates for laparoscopic SCP.20–22
The perception of the performance index revealed a simplified display of subjective and objective awareness of difficult preparation and complications. As expected, the performance initially de-creased in independent surgeries, but variation reduced from approximately 20 autonomous interventions (28 interventions overall) onwards. The performance index confirms in a numeric value an outcome parameter ranging from acceptable deviation from the goal if the value is positive to indicating intermediate to severe adverse variance in negative values. This is a simplified method to objectify the outcome, and further evaluation would be needed if performance index wanted to be applied on a bigger scale.
The complication rate was also comparable to similar training programs.23–25
The main limitation of this study is its reliance on the experience of a single center and a single surgeon, but it indicates that it is advisable to establish a stepwise and structured learning and teaching environment to achieve a steep and consistent learning curve. We encourage further studies and different teaching programs to evaluate the surgical sub-steps, instead of overall surgical time with unclear intraoperative contribution factors like assisting surgeon or anatomical alterations.
Conclusion
Finally, we conclude that structured intraoperative training and standardized surgery can enhance the learning process in advanced surgical procedures such as laparoscopic SCP. We demonstrated that 30 to 35 interventions are required to accomplish a level of independence in regard to carrying out these operations. Organized teaching is not replacing or reaching the level of expertise gained by multiple years of experience, but empowers the fact that not hundreds of interventions are needed to achieve an adequate level of knowledge and skill in a structured training environment.26
Abbreviations
SCP, Laparoscopic sacrocolpopexy; GFPFQ, German translated validated female pelvic floor questionnaire; PGI-I, Patient Global Impression of Improvement; POP-Q, Pelvic Organ Prolapse Quantification system.
Acknowledgments
Special ackowledgements go to all involved surgical theater assistants documenting the timing of the surgical substeps.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaijser M, van Ramshorst G, van Wagensveld B, Pierie JP. Current techniques of teaching and learning in bariatric surgical procedures: a systematic review. J Surg Educ. 2018;75(3):730–738. doi:10.1016/j.jsurg.2017.09.023
2. Campo R, Wattiez A, Tanos V, et al. Gynaecological endoscopic surgical education and assessment. A diploma programme in gynaecological endoscopic surgery. Gynecol Surg. 2016;13(3):133–137. doi:10.1007/s10397-016-0957-1
3. Sleiman Z, Tanos V, Van Belle Y, Carvalho JL, Campo R. The European Academy laparoscopic “suturing training and testing” (SUTT) significantly improves surgeons’ performance. Facts Views Vision ObGyn. 2015;7(3):153–160.
4. van der Vaart LR, Vollebregt A, Milani AL, et al. Effect of pessary vs surgery on patient-reported improvement in patients with symptomatic pelvic organ prolapse: a randomized clinical trial. JAMA. 2022;328(23):2312–2323. doi:10.1001/jama.2022.22385
5. Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10(10):Cd012376. doi:10.1002/14651858.CD012376
6. Akladios CY, Dautun D, Saussine C, Baldauf JJ, Mathelin C, Wattiez A. Laparoscopic sacrocolpopexy for female genital organ prolapse: establishment of a learning curve. Eur J Obstet Gynecol Reprod Biol. 2010;149(2):218–221. doi:10.1016/j.ejogrb.2009.12.012
7. Claerhout F, Verguts J, Werbrouck E, Veldman J, Lewi P, Deprest J. Analysis of the learning process for laparoscopic sacrocolpopexy: identification of challenging steps. Int Urogynecol J. 2014;25(9):1185–1191. doi:10.1007/s00192-014-2412-z
8. Deprest J, Krofta L, Van der Aa F, et al. The challenge of implementing laparoscopic sacrocolpopexy. Int Urogynecol J. 2014;25(9):1153–1160. doi:10.1007/s00192-014-2398-6
9. Mowat A, Maher C, Pelecanos A. Can the learning curve of laparoscopic sacrocolpopexy be reduced by a structured training program? Female Pelvic Med Reconstr Surg. 2018;24(4):272–276. doi:10.1097/SPV.0000000000000441
10. Haylen BT, de Ridder D, Freeman RM, et al. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. doi:10.1007/s00192-009-0976-9
11. Baessler K, Kempkensteffen C. Validation of a comprehensive pelvic floor questionnaire for the hospital, private practice and research. Gynakol Geburtshilfliche Rundsch. 2009;49(4):299–307. doi:10.1159/000301098
12. Baessler K, O’Neill SM, Maher CF, Battistutta D. A validated self-administered female pelvic floor questionnaire. Int Urogynecol J. 2010;21(2):163–172. doi:10.1007/s00192-009-0997-4
13. Srikrishna S, Robinson D, Cardozo L. Validation of the patient global impression of improvement (PGI-I) for urogenital prolapse. Int Urogynecol J. 2010;21(5):523–528. doi:10.1007/s00192-009-1069-5
14. Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi:10.1016/S0002-9378(96)70243-0
15. Christmann-Schmid C, Koerting I, Ruess E, Faehnle I, Krebs J. Functional outcome after laparoscopic nerve-sparing sacrocolpopexy: a prospective cohort study. Acta Obstet Gynecol Scand. 2018;97(6):744–750. doi:10.1111/aogs.13337
16. Matthews CA. Minimally invasive sacrocolpopexy: how to avoid short- and long-term complications. Curr Urol Rep. 2016;17(11):81. doi:10.1007/s11934-016-0638-7
17. Muavha DA, Ras L, Jeffery S. Laparoscopic surgical anatomy for pelvic floor surgery. Best Pract Res Clin Obstet Gynaecol. 2019;54:89–102. doi:10.1016/j.bpobgyn.2018.11.005
18. Clavien PA, Barkun J, de Oliveira ML, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
19. Dell-Kuster S, Gomes NV, Gawria L, et al. Prospective validation of classification of intraoperative adverse events (ClassIntra): international, multicentre cohort study. BMJ. 2020;370:m2917. doi:10.1136/bmj.m2917
20. Dwyer L, Kumakech W, Ward K, Reid F, Smith A. Laparoscopic sacrocolpopexy (LSCP) using an ultra-lightweight polypropylene mesh. Eur J Obstet Gynecol Reprod Biol X. 2019;2:100008. doi:10.1016/j.eurox.2019.100008
21. Maher CM, Feiner B, Baessler K, Glazener CM. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22(11):1445–1457. doi:10.1007/s00192-011-1542-9
22. Sato H, Abe H, Ikeda A, Miyagawa T, Sato K. Complications and clinical outcomes of laparoscopic sacrocolpopexy for pelvic organ prolapse. J Obstet Gynaecol. 2021;41(1):128–132. doi:10.1080/01443615.2020.1724914
23. Carter-Brooks CM, Du AL, Bonidie MJ, Shepherd JP. The impact of fellowship surgical training on operative time and patient morbidity during robotics-assisted sacrocolpopexy. Int Urogynecol J. 2018;29(9):1317–1323. doi:10.1007/s00192-017-3468-3
24. Linder BJ, Anand M, Weaver AL, et al. Assessing the learning curve of robotic sacrocolpopexy. Int Urogynecol J. 2016;27(2):239–246. doi:10.1007/s00192-015-2816-4
25. Mustafa S, Amit A, Filmar S, et al. Implementation of laparoscopic sacrocolpopexy: establishment of a learning curve and short-term outcomes. Arch Gynecol Obstet. 2012;286(4):983–988. doi:10.1007/s00404-012-2391-6
26. Moore R, Moriarty C, Chinthakanan O, Miklos J. Laparoscopic sacrocolpopexy: operative times and efficiency in a high-volume female pelvic medicine and laparoscopic surgery practice. Int Urogynecol J. 2017;28(6):887–892. doi:10.1007/s00192-016-3179-1
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




