Back to Journals » International Journal of Nanomedicine » Volume 20
Versatile Nanomaterials That Interfere with Ferroptosis in the Tumor Microenvironment
Authors Liu Y, Liu Y, Li X, Li S, Zhang X, Si L, Jiang S, Hu J, Chen J
Received 26 November 2024
Accepted for publication 17 February 2025
Published 25 February 2025 Volume 2025:20 Pages 2461—2473
DOI https://doi.org/10.2147/IJN.S508767
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Dong Wang
Yurong Liu,1,* Yunheng Liu,1,* Xinting Li,1,* Song Li,1 Xiaokang Zhang,1 Longqing Si,1 Shaojing Jiang,2 Jinghui Hu,2 Jing Chen1
1School of Pharmacy, The Key Laboratory of Prescription Effect and Clinical Evaluation of State Administration of Traditional Chinese Medicine of China, Binzhou Medical University, Yantai, 264003, People’s Republic of China; 2Yantai Engineering Research Center for Digital Technology of Stomatology, Characteristic Laboratories of Colleges and Universities in Shandong Province for Digital Stomatology, Institute of Stomatology, Binzhou Medical University, Yantai, 264003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinghui Hu, Yantai Engineering Research Center for Digital Technology of Stomatology, Characteristic Laboratories of Colleges and Universities in Shandong Province for Digital Stomatology, Institute of Stomatology, Binzhou Medical University, Yantai, 264003, People’s Republic of China, Email [email protected] Jing Chen, School of Pharmacy, the Key Laboratory of Prescription Effect and Clinical Evaluation of State Administration of Traditional Chinese Medicine of China, Binzhou Medical University, Yantai, 264003, People’s Republic of China, Email [email protected]
Abstract: Ferroptosis is a type of iron-dependent programmed cell death characterized by a depletion of glutathione. Although generally less harmful to normal cells, in tumor cells, the high demand for iron ions provides conditions conducive to ferroptosis. In this review, we provide an overview of recent progress in research on the regulation of ferroptosis in tumor cells, summarizing and assessing the current state, trends, and applications of nanomaterials in the regulation of ferroptosis in tumor cells. Given the advantages of nanomaterials in terms of targeting, safety, improved drug efficacy, and reduced side effects, these materials are considered to have potential therapeutic value in modulating ferroptosis in tumor cells via different mechanisms. In this respect, we describe methods for modifying the regulation of iron ions and interfering with glutathione activity and lipid peroxidation. The development of nanomaterials that can be applied to induce or inhibit ferroptosis is anticipated to provide new therapeutic options for the treatment of a diverse range of diseases.
Keywords: iron metabolism, tumor, ferroptosis, nanomaterial
Graphical Abstract:

Introduction
Cancers are life-threatening diseases that are often fatal,1–3 for which the traditional treatments include surgery, radiotherapy, and chemotherapy.4 Although these interventions are effective to varying extents, researchers are continually striving to identify more efficient, safer, and personalized treatment strategies.5–7 Among the therapeutic approaches that are currently attracting increasing attention is the treatment of tumors via a modification of ion metabolism,8 which compared with normal cells, differs considerably in tumor cells.9 In this regard, it has been established that ion channels and pumps in tumor cells are abnormally regulated, thereby resulting in an imbalance between intracellular and extracellular ion concentrations,10,11 and it is this imbalance that contributes to promoting the proliferation, growth, and metastasis of tumor cells, and heightens the risk of drug resistance.12 Consequently, iron ion metabolism in tumor cells has become an important focus of anti-tumor research.13
Ferroptosis is an iron-dependent type of programmed death, which essentially entails the cellular depletion of glutathione (GSH), and is characterized by an iron-dependent elevation of lipid peroxidation to lethal levels.14 The mechanisms underlying the induction of ferroptosis have been established to involve disordered iron metabolism, reduced GSH levels, a decline or loss of glutathione peroxidase 4 (GPX4) activity, and lipid peroxidation.15 Ferroptosis has also been found to have a substantial influence on the tumor microenvironment (TME).16 associated with the differentiation and function of immune cells, ultimately modifying the immune status. In addition, there is evidence to indicate that ferroptosis may also affect the interactions of other components in the TME, including cancer-associated fibroblasts (CAFs) and cytokines such as TGFβ1 and IL-1β, thereby ultimately regulating the process of ferroptosis.17 Moreover, given its effects on tumor angiogenesis, ferroptosis has been implicated in the efficacy of cancer treatments,18 with certain chemotherapy, radiotherapy, and immunotherapy treatments triggering ferroptosis, ultimately influencing the entire course of cancer treatment.19,20 A number of different aspects of the TME have been implicated in the occurrence of ferroptosis, including inflammatory responses, immunity, stromal cells, angiogenesis, and metabolism (Scheme 1), which could thus represent promising avenues for future tumor treatment. Accordingly, by examining ferroptosis in the context of the TME, it is anticipated that future research will provide further important insights into the effects of different factors within the TME on this process.
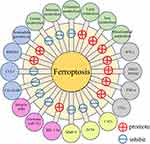 |
Scheme 1 The influence of different TME factors on ferroptosis. |
A notable feature of ferroptosis is that this process differs fundamentally from conventional apoptosis, necrosis, and autophagy in a number of respects, and has considerable potential in the treatment of tumors. Furthermore, it has been established that ferroptosis primarily affects tumor cells, which are highly dependent on iron ions, whilst being characterized by relatively low toxicity in normal cells, thereby indicating that targeting the ferroptotic process could represent a relatively safe therapeutic approach.21 Thus, ferroptosis is considered a potentially valuable novel target for cancer therapy, with significant research value and application prospects in the fields of biology and medicine.22
However, although targeting ferroptosis provides an alternative strategy for tumor treatment, there remain multiple challenges regarding the associated research and practical application.23,24 Numerous nanomedicines with specific functions that can accurately locate tumor cells have been designed and used to regulate tumor ferroptosis,25 and in this review, we describe some of the efficient targeting nanomedicines that modify cell ferroptosis by up- or downregulating iron ions or interfering with GSH metabolism and lipid peroxidation. The development strategies for nanomedicines targeting the upregulation or downregulation of iron ions primarily focus on enhancing the stability and bioavailability of nanoparticles, improving tumor targeting, controlling the release rate of iron ions, increasing the selectivity and affinity for iron ions, precise delivery of iron chelators, and reducing drug side effects and resistance. Moreover, the preparation of nanomedicines based on ferroptosis is a challenging and promising area of research, and the development of nanomedicines with high efficiency, safety, and specificity that interfere with the process of tumor ferroptosis can provide new insights and approaches for the treatment of diseases.
Nanomaterials That Interfere with Tumor Ferroptosis
The Biological Mechanisms of Ferroptosis
Ferroptosis is a type of iron-dependent programmed cell death, the biological mechanisms of which are characterized by a number of key aspects. The initial stages of this process are marked by a disruption of the normal regulation of iron ions and inhibition of related transport proteins.26 An imbalance in intracellular iron ion homeostasis, as a consequence of an increase in iron uptake or reduction in iron efflux, leads to an accumulation of intracellular iron ions. In this regard, transferrin mediates iron uptake via the transferrin receptor, whereas ferritin components promote increases iron levels via autophagic degradation.27 The cystine/glutamate antiporter System Xc- is inhibited, thereby influencing the synthesis of GSH, a reductive cofactor for GPX4, a reduction in the synthesis of which leads to either a reduction or complete loss of GPX4 activity.28
The progression stage of ferroptosis is characterized by an increase in the generation of reactive oxygen species (ROS) and, consequently, heightened levels of lipid peroxidation. Polyunsaturated fatty acids within the cell are converted to readily oxidizable phosphatidylethanolamines by enzymes such as acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3),29 and are oxidized to lipid peroxides by lipoxygenases.30 In addition, an accumulation of divalent iron ions within the cell results in the generation of hydroxyl radicals via the Fenton reaction, contributing to a further oxidization of lipids and hence the generation of further lipid peroxides and ROS, thereby leading to oxidative stress.31
The subsequent apoptotic stage is characterized by marked mitochondrial and cell membrane damage.32 The density of the mitochondrial membrane increases, with a reduction or disappearance of cristae, and the outer mitochondrial membrane ruptures, leading to mitochondrial dysfunction, evidenced by a reduction in adenosine triphosphate synthesis and impaired cellular respiratory chain activity, thereby further promoting cell death. The peroxidation of membrane lipids disrupts the integrity of the cell membrane, thereby increasing its permeability and thus leading to the leakage of intracellular substances and entry of extracellular substances, resulting in cell swelling and rupture.33
The final phase of the ferroptotic process is the immune response stage, during which cells undergoing ferroptosis release damage-associated molecular patterns34 that in turn activate the immune system within the TME, thereby promoting the recruitment and activation of immune cells, macrophages, and dendritic cells, inducing immunogenic cell death, and enhancing the body’s immune surveillance and immune killing effects against tumors35 (Figure 1).
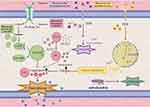 |
Figure 1 The interference mechanism of nanomaterials on tumor ferroptosis. |
The signaling pathways involved in the perturbation of iron ions are primarily divided into two types: GPX4-dependent and GPX4-independent. In the GPX4-dependent signaling pathway, iron-dependent cell death is primarily induced by excessive peroxidation of the phospholipid membrane. GSH-dependent lipid peroxides protect the cell membrane from peroxidation damage and regulate intracellular homeostasis. Inhibition of GPX4 or depletion of GSH enhances the accumulation of lipid peroxides, leading to abnormal signaling pathways and irreparable membrane damage, ultimately inhibiting the proliferation and migration of tumor cells. In the GPX4-independent signaling pathway, spontaneous lipid oxidation is an important mechanism that can cause phospholipid peroxidation through chain reactions such as the Fenton reaction, thereby triggering ferroptosis. Additionally, enzymes such as ACSL4 and LPCAT3 also participate in the regulation of ferroptosis by influencing the synthesis and metabolism of phospholipids, which in turn affects the occurrence of ferroptosis.
Nanomaterials That Interfere with Iron Ions
Iron is a metallic element that plays essential roles in the normal functioning of the body, in which it functions as a key cofactor of hemoglobin, myoglobin, and numerous other enzymes, thereby contributing to a diverse range of physiological processes, including oxygen transport, energy metabolism, and signaling.36 Given its indispensable contribution to human metabolism, either a deficiency or excess of iron can prove detrimental and lead to the development of a number of diseases. In this regard, recent advances in the study of iron metabolism have revealed the occurrence of certain complex pathways essential for the maintenance of iron homeostasis.37
Iron ions play pivotal roles in multiple cellular reactions, including the synthesis of heme and mitochondrial redox reactions.38 Iron deficiency can cause anemia, developmental delay, mental retardation, and compromised immunity.39,40 Notably, iron is of even greater importance in tumor cells, the metabolism and proliferation of which are generally higher than those of normal cells, and, accordingly, the iron requirements of tumor cells are generally considerably higher than those of normal cells.41,42 Tumor cells with a deficiency of iron ions have been found to be characterized DNA damage and shrinkage. Consequently, a deficiency of intracellular iron ions typically has a more pronounced impact on the viability and proliferation tumor cells. In addition, divalent and ferric iron ions can undergo mutual transformations, thereby facilitating their involvement in multiple intracellular redox reactions.43,44 Conversely, an excess of iron ions can contribute to increases in the generation of intracellular ROS, resulting in oxidative damage to intracellular organelles, and hence damage to different organs in the body. Consequently, a perturbation of the metabolism of iron ions in cells can have a substantial impact on cell viability, often leading to disease development and associated symptoms.
Combining the targeting of ferroptosis with photodynamic therapy (PDT) and sonodynamic therapy (SDT) has been demonstrated to have promising application prospects for the treatment of tumor, and research in this regard has provided evidence to indicate that PDT can serve as a source of H2O2 in the Fenton reaction and provide singlet oxygen for the process of lipid peroxidation. For example, for the purposes of synergistic therapy, Zhu et al constructed self-assembling nanoparticles loaded with Ce6 and ferroptosis inducers.45 Having been taken up by tumor cells, the internalized nanoparticles induce ferroptosis, leading to the accumulation of ROS within cells, thereby promoting an increase in oxygen concentrations at the tumor site via the Fenton reaction, and thus contributing to an enhancement of the oxidative damaging effect attributable to PDT. However, the efficacy of PDT is often limited by insufficient penetration, which can, nevertheless, be overcome by the use of SDT, and combining SDT with the targeting of ferroptosis has been established to have significant therapeutic advantages, as demonstrated by Zhou et al, who constructed a liposomal nanoplatform co-loaded with the sonosensitizer protoporphyrin and a clinically approved drug, nano-sized iron oxide.46 In response to ultrasonic exposure, the SDT induced by protoporphyrin not only has antitumor effects by inducing apoptosis but by promoting selective autophagy in cells, also enhances the sensitivity to nano-sized iron oxide-induced ferroptosis.
Disorders associated with iron metabolism are among the main causes of ferroptosis.47 The intake, storage, and utilization of iron in cells are stringently regulated, and the perturbation of these regulatory processes can lead to abnormal increases or reductions in the level of iron ions, which in turn adversely influences the normal metabolism and functioning of cells. When accumulating in large amounts, ferric ions are reduced to ferrous ions by the activity of metal reductases, and the highly oxidizing ferrous ions can readily react with hydrogen peroxide (H2O2) to generate hydroxyl radicals. This subsequently promotes lipid peroxidation reactions, damaging cell membranes, and leading to cell death. In the context of the association between abnormal iron metabolism and tumor development, a number of innovative treatment strategies are currently being evaluated, among which are approaches that aim to enhance iron uptake, the control of which can be employed to regulate iron metabolism and treat tumors. Additionally, by altering the regulation of iron metabolism, certain anti-tumor drugs can inhibit the growth and proliferation of tumors.48,49
Nanomaterials for Up-Regulating Iron Ions
Enhancing the levels of iron ions in tumor cells can trigger specific cell death mechanisms, thereby indicating the potential of alternative avenues for the treatment of tumors, and in this regard, the use of multi-functional biomaterials to facilitate an upregulation of iron ions has emerged as a promising therapeutic strategy. The adsorption of iron ions by nanomaterials can enhance the intracellular levels of these ions and thereby induce tumor ferroptosis. As an innovative approach for tumor imaging and targeting ferroptosis, Brabury et al used ultra-small, large-pore silica nanoparticles modified with polyethylene glycol and demonstrated that under conditions of amino acid deficiency, iron ions bound to silica nanoparticles can be endocytosed by tumor cells, resulting in an increase in cellular iron content, thereby promoting the ferroptosis of tumor cells.50 The controlled release of iron ions from iron-containing nanomaterials can also effectively enhance the stability of intracellular iron ions. Ferrocene, an organometallic compound containing iron, can be oxidized to generate large amounts of iron ions within acidic environments, and Kwon et al discovered that polyCAFe micelles, which self-assemble from ferrocene, benzoyloxy cinnamaldehyde (CBA), and amphiphilic polymers, can release iron ions and CBA under weakly acidic conditions, with the combination of iron ions and CBA effectively inducing tumor cell death.51 Furthermore, Zhang et al have synthesized amorphous Fe2O3 nanoparticles, which under acidic conditions rapidly ionize and release ferrous ion that enter into the Fenton reaction within cancer cells, thereby generating cytotoxic hydroxyl radicals, and thus facilitating specific cancer therapy.52 Cellular apoptosis induced by excessive levels of iron ions is dependent on the availability of large amounts of peroxide substrate, and hence enhancing the intracellular levels of hydrogen peroxide can effectively contribute to induction of cellular death. In this regard, Zhao et al proposed a multi-functional hybrid nanoparticle that combines cisplatin, lactoferrin, and Fe3O4/Gd2O3 nanomaterials. The ferrous and ferric ions released by these nanoparticles can augment the Fenton reaction, whereas cisplatin can indirectly promote the production of H2O2, thereby accelerating the Fenton reaction, which in turn induces oxidative stress in tumor cells, eventually leading to cell death.53 In addition, certain erastin analogs have been demonstrated to inhibit the excretion of iron ion transporters, thereby hindering intracellular iron ion transfer to the extracellular space, and subsequently triggering iron death via the intracellular accumulation of iron ions, hence inhibiting tumor growth.54,55
The levels of iron ions within tumor cells can thus be effectively enhanced via adsorption, delivery, and responsive release, and in combination with an elevation in the levels of peroxide substrates can contribute to an efficient triggering of cell death.56
Nanomaterials that can be used to upregulate iron ions provide a valuable supplementary approach in the treatment of cancer. In addition to directly promoting the death of tumor cells, these nanomaterials can contribute to modulating the redox state of the TME, and also have a profound influence on tumor growth and invasion.57 Elevated levels of iron both enhance the sensitivity of tumor cells to oxidative stress and disrupt the redox balance within the tumor, thereby generating an environment that is more conducive to treatment using other therapeutic approaches.58 Furthermore, by influencing immune cells within the TME, these nanomaterials may also further regulate immune responses, thereby enhancing anti-tumor efficacy.59 These strategies not only provide a new conceptual framework for the treatment of tumors but will also serve as a reference for future biomedical applications based on the regulation of iron ions.
Nanomaterials for Down-Regulating Iron Ions
The downregulation of iron ions using multifunctional biomaterials is a relatively recent treatment strategy. This approach is dependent upon the application iron chelators and other products that can be used to deplete the cellular levels of iron ions, resulting in an iron deficiency in tumor cells, thereby triggering cell death.60
Studies in this regard have found that iron chelators can selectively bind to, and thereby accumulate iron ions, thus leading to an iron deficiency within tumor cells and ultimately causing cell death. For example, Lv et al found that chitosan–deferoxamine nanomaterials can effectively chelate iron ions, promote iron efflux, and thus inhibit tumor growth, thereby providing new insights for cancer treatment,61 and Zhou et al have reported similar anti-tumor activity.62,63 Hepcidin is a peptide hormone produced by the liver that regulates the metabolism and transport of iron ions, and hepcidin-based composite nanomaterials have been developed that can inhibit iron ion transporters on the surface of tumor cells, thereby contributing to reductions in the uptake of iron ions by these cells,64 and thus effectively inhibiting tumor growth and spread.65
It has been established that iron ion levels are effectively downregulated within tumor cells, leading to iron deficiency and triggering ferroptosis, further confirming the important role of iron ions in tumor therapy.66 Biomaterials that downregulate iron ions are considered to have broad application prospects in anti-tumor treatments. By inducing a state of iron deficiency in tumor cells, these materials not only directly trigger the ferroptosis process, and thereby accelerate the apoptosis of tumor cells, but also have a significant influence on the overall homeostasis of the TME.67 By precisely regulating the availability of iron ions, nanomaterials can disrupt iron homeostasis within the TME, thus further inhibiting the growth and invasive capacity of tumors. Additionally, the iron-deficient state thus generated may also have an influence on the function of immune cells within the TME, thereby modulating anti-tumor immune responses.68 Furthermore, it is believed that these nanomaterials may interact with the tumor vascular system, thereby influencing angiogenesis and the blood supply, and, consequently, indirectly regulating the tumor growth environment.69 However, this is an emerging field of research, the continued progress of which faces multiple challenges, including the necessity for further improvements in drug targeting and efficacy, the reduction of side effects, and optimization of treatment strategies. Future research in this regard will necessitate an in-depth examination of the mechanisms underlying the associations between iron ions and tumor growth, and the development of safer and more effective biomaterials for the regulation of iron ions.
Nanomaterials That Interfere with GSH
GSH is an antioxidant that scavenges free radicals, promotes metabolism, and contributes to the normal functioning of the immune system,70 the depletion of GSH can lead to ferroptosis in tumor cells.71 Reductions in the levels of GSH leads to a corresponding reduction in the activity of GPX4, an important cellular antioxidant enzyme that scavenges the products of oxidative stress by reducing substrates and maintaining a redox balance within the cell. Suppression of GPX4 activity inhibits the metabolism of lipid peroxides via the GPX4 reaction,72 and, subsequently, ferrous ions oxidize lipids to yield ROS, thus promoting ferroptosis.73 However, the presence of iron may also inhibit the activity of GPX4, leading to disorders in the cellular redox balance, and ultimately triggering cell death.15 It has also been established that a deficiency in nicotinamide adenine dinucleotide phosphate (NADPH) can lead to the depletion of GSH in cells.74 In their innovative study, Zhao et al incorporated β-lapachone into calcium oxide nanocarriers, and used this system to promote a significant increase in ROS production and reduction in intracellular GSH levels.75 In addition, Lin et al further discovered that -Mn-O- bond breakage can consume two molecules of GSH, and on the basis of this finding, they designed manganese-doped silica nanoparticles that can be employed to deplete GSH in tumor cells, thereby inactivating GPX4 and inducing ferroptosis.76,77 Moreover, Gu et al have found that GSH-responsive hyaluronic acid nanocarriers can enhance the inhibitory effects of sulfasalazine on the stem cell-like properties of breast cancer cells. These findings thus indicate that nanocarriers of this type could serve as a promising platform for tumor-targeted drug delivery, thereby enhancing its therapeutic effect on tumors.78
In response to GPX4 catalysis, GSH mediates a reduction in lipid peroxidation and regulates ferroptosis via negative feedback. Accordingly, a depletion of GSH can lead to the accumulation of toxic peroxides, damage to proteins and cell membranes, and subsequent ferroptosis. In this regard, GSH-interfering nanomaterials can effectively promote tumor cell death by accelerating ferroptosis and attenuating the antioxidant defense of tumor cells, thereby enhancing their sensitivity to drugs. Additionally, these agents can regulate the function of immune cells in the TME, thereby enhancing treatment outcomes, and providing alternative strategies for tumor therapy.79 Consequently, the development of biomaterials that suppress the activity of GSH can potentially make important contributions to the treatment of tumors.
Nanomaterials That Interfere with Lipid Peroxidation
Within the membrane of tumor cells, the catalytic activities of divalent iron or ester oxygenases contribute to the oxidization of unsaturated fatty acids, leading to lipid peroxidation and the induction of cell death. Lipid peroxidation is an integral facet of ferroptosis that is closely associated with the occurrence and development of diverse diseases, and is accordingly a particular focus of current research.80
Lipid peroxidation is an important step in the iron death mechanism,81 in which iron ions contribute by promoting the production of free radicals. The subsequent propagation of fatty acid peroxyl radical chain reactions throughout the entire membrane induces changes in the lipid structure of the cell membrane, which in turn influences membrane function and permeability, leading to irreversible membrane damage and eventually cell death.82
In this context, it has been established that promoting increases in levels of the lipid peroxidation substrate H2O2 within tumor cells is among the most direct methods for enhancing the efficiency of lipid peroxidation, on the basis of which, Sucheta et al developed a therapeutic strategy involving the application of CaO2 nanoparticles loaded with the anticancer drug doxorubicin (DOX). The CaO2-DOX preparation is encapsulated with a Cu metal-organic framework (MOF) via polyethylene glycol (PEG) surface modification to generate a CaO2-DOX-CuMOF/PEG nanodrug. The production of H2O2 in this nanomedicine was established to be conducive to the generation of ROS, and combining the application of this synthesized nanocomposite with chemo-dynamic therapy was found to be more effective than the respective individual therapies in inhibiting tumors in an in vivo murine tumor model.83 Furthermore, Zhu et al have proposed an innovative strategy based on the design and synthesis a dual-responsive polypeptide complex for the selective eradication of cancer cells based on an inhibition of catalase activity using hydrogen sulfide, thereby promoting increases in the intracellular concentration of H2O2 and generating a large number of hydroxyl radicals via the Fenton reaction.84 In addition, hydroxycamptothecin–cinnamaldehyde-loaded nanoparticles have been found to effectively enhance drug accumulation at the tumor site and prolong the duration of drug action, thereby contributing to elevated levels of ROS production in cancer cells and ultimately leading to ferroptosis.85 In further studies, Daldrup-Link et al investigated the cytotoxic effects of ferumoxytol iron oxide nanoparticles, and accordingly found that rather than acting directly on cancer cells, these particles influence to activity of tumor-associated immune cells, specifically inducing the production of anti-tumor M1 macrophages,86 which under co-culture conditions without direct cell-to-cell contact, were induced to produce ROS that in turn promoted cancer cell death.
Moreover, it has been established that natural killer cells within the TME are characterized by elevated levels of proteins involved in lipid peroxidation and display morphologies similar to those of ferroptotic cells.87 Under conditions of oxidative stress, glucose metabolism within natural killer cells is inhibited, leading to their functional impairment within the TME, and the generation of lipid oxidation products has been shown to reduce the maturation of naive dendritic cells and thereby impairs normal dendritic cell function.88
In summary, the manipulation of lipid peroxidation, which plays an integral role in the process of ferroptosis, has significant application prospects in cancer therapy. By designing drug delivery systems that inhibit catalase activity and utilizing iron oxide nanoparticles, ROS production can be effectively promoted, thereby inducing ferroptosis in tumor cells. As a consequence of the increasing focus on nanomaterial-targeted ferroptosis, significant progress has been made in the design and application of anticancer therapies based on innovative nano-systems The ferroptosis-associated therapeutic strategies described in this review not only provide new ideas for cancer therapy but also offer new directions for research in the field of biomedicine.89
As research into nanomaterial-targeted ferroptosis deepens, significant progress has been made in anticancer therapies utilizing various nanomaterials targeting ferroptosis. Table 1 summarizes some of representative classes of nanomedicines.
 |
Table 1 The Design and Mechanisms of Strategies for Inducing Ferroptosis |
Conclusion
As an alternative approach to cancer therapy, targeting ferroptosis is considered to have considerable promise for clinical translation. The rapid development of nanotechnology has laid a solid foundation for the application of nanomaterials in cancer treatment, and research on the use of nanomaterials designed to induce ferroptosis is increasing. More importantly, utilizing nanomaterials for synergistic drug delivery can contribute to integrating multiple therapeutic modalities to gain more effective treatment outcomes. Moreover, nano-drugs that induce ferroptosis can play a valuable role in remodeling the TME, modulating tumor immunity, and in immune checkpoint blockade (ICB) therapy. With respect to the modulation of tumor immunity, these materials can promote macrophage polarization and dendritic cell maturation, enhance immunogenicity, regulate metabolite flow within the TME to reverse immunosuppressive conditions, and enhance the sensitivity of tumors to ICB therapy.
However, to enable effective clinical translation, it will initially be necessary to overcome a number of significant obstacles regarding the use of a synergistic anti-tumor approach based on nanomaterials for targeting ferroptosis.93,94
(1) At present, there is still a limited understanding of the detailed mechanisms and pathways involved in the ferroptosis induced by ferroptosis inducers. Moreover, studies on the therapeutic effects of utilizing the combined mechanisms of ferroptosis on different types/subtypes of cancer are still in their infancy, and further theoretical support is needed to evaluate the efficacy of these treatments among different types of tumor.
(2) Although iron-based nanomaterials can introduce large amounts of exogenous iron to trigger ferroptosis, the acidic nature of the TME and low H2O2 concentrations tend to be inconducive to the rapid and effective generation of iron/ferrous ions and ROS, potentially leading to suboptimal combined therapeutic effects. Accordingly, designing and developing iron-based nanomaterials that are not dependent on environmental pH and H2O2 concentrations may represent the promising future research directions. As an alternative to iron-based nanomaterials, non-iron-based nanomaterials can be used to induce ferroptosis via promoting a depletion of GSH, inactivation of GPX4, or enhanced generation of intracellular ROS, although these often lack tumor imaging capabilities. Thus, the development of non-iron-based nanomaterials with integrated diagnostic and therapeutic properties for achieving combined ferroptosis therapy could emerge as a particularly active area of future research.
(3) Studies assessing the efficacy of combining ferroptosis with radiotherapy, gene therapy, or other treatment modalities, or utilizing multiple combined treatment approaches to achieve synergistic anti-tumor effects, are still at a relative early exploratory stage. In this regard, key areas of future research might include the design of multi-functional nanomaterials that integrate imaging, treatment, and diagnosis, along with the formulation of appropriate combined treatment strategies.
(4) Importantly, evaluation of the biosafety of nanomaterials remains a foremost priority for any future clinical applications. It will accordingly be necessary to further determine the side effects of nanomaterials on normal cells, tissues, and organs, and to evaluate the toxicity of nanomaterials from multiple perspectives, including degradation and metabolism.
In summary, combining knowledge and research methodologies drawn from multiple disciplines will be necessary to elucidate the specific mechanisms of ferroptosis, determine efficient synergistic treatment strategies, and develop nanomaterials with good biocompatibility. It is, nevertheless, believed that with the joint efforts of researchers, combined ferroptosis therapy could have considerable application potential in the clinical treatment of cancer.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (NSFC) (grant no. 82102209), the Natural Science Foundation of Shandong (grant no. ZR2022QE230), Binzhou Medical College ”Stomatology + X” University Integration Innovation Project (KQRH2024ZD003, KQRH2024MS002).
Disclosure
The authors declare that they have no competing interests.
References
1. Jin HJ, Wang LQ, Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. 2023;22(3):213–234. doi:10.1038/s41573-022-00615-z
2. Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19(12):775–790. doi:10.1038/s41571-022-00689-z
3. Younis NK, Roumieh R, Bassil EP, Ghoubaira JA, Kobeissy F, Eid AH. Nanoparticles: attractive tools to treat colorectal cancer. Semi Cancer Biol. 2022;86:1–13. doi:10.1016/j.semcancer.2022.08.006
4. Jia ZL, Zhu XL, Zhou Y, et al. Polypeptides from traditional Chinese medicine: comprehensive review of perspective towards cancer management. Int J Biol Macromol. 2024;260:129423. doi:10.1016/j.ijbiomac.2024.129423
5. Wei GQ, Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11(13):6370–6392. doi:10.7150/thno.57828
6. Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. Ca-a Cancer J Clin. 2023;73(2):198–222. doi:10.3322/caac.21759
7. Besse B, Pons-Tostivint E, Park K, et al. Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: a Phase 2 umbrella trial. Nature Med. 2024;30(3):716–729. doi:10.1038/s41591-024-02808-y
8. Guo S, Li CH, Wang CR, et al. pH-Responsive polymer boosts cytosolic siRNA release for retinal neovascularization therapy. Acta Pharmaceutica Sinica B. 2024;14(2):781–794. doi:10.1016/j.apsb.2023.09.001
9. Fan RR, Cai LR, Liu H, et al. Enhancing metformin-induced tumor metabolism destruction by glucose oxidase for triple-combination therapy. J Pharm Anal. 2024;14(3):321–334. doi:10.1016/j.jpha.2023.09.015
10. Ma T, Ding Q, Liu CX, Wu H. Electromagnetic fields regulate calcium-mediated cell fate of stem cells: osteogenesis, chondrogenesis and apoptosis. Stem Cell Res Ther. 2023;14(1):133. doi:10.1186/s13287-023-03303-w
11. Harich OO, Gavriliuc OI, Ordodi VL, et al. In vitro study of the multimodal effect of Na+/K+ATPase blocker ouabain on the tumor microenvironment and malignant cells. Biomedicines. 2023;11(8):2205. doi:10.3390/biomedicines11082205
12. Zhang JY, Hu JH, Zhu WW, et al. Hypoxia-triggered tumor specific glutamine inhibition for reversing cisplatin resistance of chemotherapy. Chem Eng J. 2024;479:147692. doi:10.1016/j.cej.2023.147692
13. Yang HC, Yao XM, Liu YQ, Shen XK, Li MH, Luo Z. Ferroptosis nanomedicine: clinical challenges and opportunities for modulating tumor metabolic and immunological landscape. Acs Nano. 2023;17(16):15328–15353. doi:10.1021/acsnano.3c04632
14. Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401–2421. doi:10.1016/j.cell.2022.06.003
15. Tsuruta K, Matsuoka M, Harada S, et al. Slowly progressive cell death induced by GPx4-deficiency occurs via MEKUERK2 activation as a downstream signal after iron-independent lipid peroxidation. J Clin Biochem Nutr. 2023;74(2):97–107. doi:10.3164/jcbn.23-101
16. Lupica-Tondo GL, Arner EN, Mogilenko DA, Voss K. Immunometabolism of ferroptosis in the tumor microenvironment. Front Oncol. 2024;14:1441338. doi:10.3389/fonc.2024.1441338
17. Li YY, Ma ZY, Li WY, et al. PDPN+ CAFs facilitate the motility of OSCC cells by inhibiting ferroptosis via transferring exosomal lncRNA FTX. Cell Death Dis. 2023;14(11):759. doi:10.1038/s41419-023-06280-3
18. Zhao L, Zhou XX, Xie F, et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 2022;42(2):88–116. doi:10.1002/cac2.12250
19. Puylaert P, Roth L, Van Praet M, et al. Effect of erythrophagocytosis-induced ferroptosis during angiogenesis in atherosclerotic plaques. Angiogenesis. 2023;26(4):505–522. doi:10.1007/s10456-023-09877-6
20. Wang YM, Wu XR, Ren Z, et al. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist Updates. 2023;66:100916. doi:10.1016/j.drup.2022.100916
21. Guan Z, Hu J, Li S, et al. Self-enhanced targeted nanomedicines based on iron starvation acclimation for tumor-specific therapy. Chem Eng J. 2024;495:153371. doi:10.1016/j.cej.2024.153371
22. Wang C, Cheng TT, Lu QN, et al. Oxygen therapy accelerates apoptosis induced by selenium compounds via regulating Nrf2/MAPK signaling pathway in hepatocellular carcinoma. Pharmacol Res. 2023;187:106624. doi:10.1016/j.phrs.2022.106624
23. Chen S, Shi JL, Yu DZ, Dong SY. Advance on combination therapy strategies based on biomedical nanotechnology induced ferroptosis for cancer therapeutics. Biomed Pharmacother. 2024;176:116904. doi:10.1016/j.biopha.2024.116904
24. Ye YY, Yu HL, Chen BH, et al. Engineering nanoenzymes integrating Iron-based metal organic frameworks with Pt nanoparticles for enhanced Photodynamic-Ferroptosis therapy. J Colloid Interface Sci. 2023;645:882–894. doi:10.1016/j.jcis.2023.05.003
25. Graván P, Aguilera-Garrido A, Marchal JA, Navarro-Marchal SA, Galisteo-González F. Lipid-core nanoparticles: classification, preparation methods, routes of administration and recent advances in cancer treatment. Adv Colloid Interface Sci. 2023;314:102871. doi:10.1016/j.cis.2023.102871
26. Gao JP, Li YF, Song R. SIRT2 inhibition exacerbates p53-mediated ferroptosis in mice following experimental traumatic brain injury. Neuroreport. 2021;32(12):1001–1008. doi:10.1097/wnr.0000000000001679
27. Cheng ZY, Zhang XJ, Chen PS, Wang HT, Wang KJ, Shen YZ. Sarcoma protein kinase inhibition alleviates liver fibrosis by promoting hepatic stellate cells ferroptosis. Open Life Sci. 2023;18(1):20220781. doi:10.1515/biol-2022-0781
28. Liu JB, Yang GC, Zhang HN. Glyphosate-triggered hepatocyte ferroptosis via suppressing Nrf2/GSH/GPX4 axis exacerbates hepatotoxicity. Sci Total Environ. 2023;862:160839. doi:10.1016/j.scitotenv.2022.160839
29. Merkel M, Goebel B, Boll M, et al. Mitochondrial reactive oxygen species formation determines ACSL4/LPCAT2-mediated ferroptosis. Antioxidants. 2023;12(8):1590. doi:10.3390/antiox12081590
30. Chen ZX, Gan JF, Zhang M, Du Y, Zhao HB. Ferroptosis and its emerging role in pre-eclampsia. Antioxidants. 2022;11(7):1282. doi:10.3390/antiox11071282
31. Huang L, Feng J, Zhu JY, et al. A strategy of Fenton reaction cycloacceleration for high-performance ferroptosis therapy initiated by tumor microenvironment remodeling. Adv Healthcare Mater. 2023;12(18):2203362. doi:10.1002/adhm.202203362
32. Zhang L, Fu RQ, Duan DY, et al. Cyclovirobuxine D induces apoptosis and mitochondrial damage in glioblastoma cells through ROS-mediated mitochondrial translocation of cofilin. Front Oncol. 2021;11:656184. doi:10.3389/fonc.2021.656184
33. Zhao LY, Peng YM, He SX, et al. Apatinib induced ferroptosis by lipid peroxidation in gastric cancer. Gastric Cancer. 2021;24(3):642–654. doi:10.1007/s10120-021-01159-8
34. Wang KK, Wang JJ, Zhang JH, et al. Ferroptosis in glioma immune microenvironment: opportunity and challenge. Front Oncol. 2022;12:917634. doi:10.3389/fonc.2022.917634
35. Gao CL, Zhang HR, Wang XW. Current advances on the role of ferroptosis in tumor immune evasion. Discover Oncology. 2024;15(1):736. doi:10.1007/s12672-024-01573-1
36. Liu ZD, Liu SA, Liu B, et al. Fe(III)-naphthazarin metal-phenolic networks for glutathione-depleting enhanced ferroptosis-apoptosis combined cancer therapy. Small. 2023;19(19):2207825. doi:10.1002/smll.202207825
37. Man YX, Xu TT, Adhikari B, Zhou CS, Wang YC, Wang B. Iron supplementation and iron-fortified foods: a review. Crit Rev Food Sci Nutr. 2022;62(16):4504–4525. doi:10.1080/10408398.2021.1876623
38. Guan ZX, Li S, Liu YR, et al. A specific targeted enhanced nanotherapy strategy for inducing ferroptosis by regulating the iron pool levels in tumor cells. ACS Appl Mater Interfaces. 2024;16(42):56837–56849. doi:10.1021/acsami.4c13534
39. Xu HJ, Ye D, Ren ML, Zhang HY, Bi F. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol Med. 2021;27(9):856–867. doi:10.1016/j.molmed.2021.06.014
40. Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35(36):2468. doi:10.1093/eurheartj/ehu235
41. Chen MJ, Yang YX, Tang L, et al. Iron-rich semiconducting polymer dots for the combination of ferroptosis-starvation and phototherapeutic cancer therapy. Adv Healthcare Mater. 2023;12(26):2300839. doi:10.1002/adhm.202300839
42. Zhang ZW, Xiang JJ, Guan LJ, et al. Inducing tumor ferroptosis via a pH-responsive NIR-II photothermal agent initiating lysosomal dysfunction. Nanoscale. 2023;15(47):19074–19078. doi:10.1039/d3nr04124g
43. Liu CG, Guo LX, Wang Y, Zhang JT, Fu CY. Delivering metal ions by nanomaterials: turning metal ions into drug-like cancer theranostic agents. Coord Chem Rev. 2023;494:215332. doi:10.1016/j.ccr.2023.215332
44. Liu Y, Zhai SJ, Jiang XW, et al. Intracellular mutual promotion of redox homeostasis regulation and iron metabolism disruption for enduring chemodynamic therapy. Adv Funct Mater. 2021;31(17):2010390. doi:10.1002/adfm.202010390
45. Zhul T, Shi LL, Yu CY, et al. Ferroptosis promotes photodynamic therapy: supramolecular photosentizer-inducer nanodrug for enhanced cancer treatment. Theranostics. 2019;9(11):3293–3307. doi:10.7150/thno.32867
46. Wang YL, Yu J, Li D, et al. Paclitaxel derivative-based liposomal nanoplatform for potentiated chemo-immunotherapy. J Control Release. 2022;341:812–827. doi:10.1016/j.jconrel.2021.12.023
47. Fang A XX, H MJX, Wang FD, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20(1):7–23. doi:10.1038/s41569-022-00735-4
48. Zhao W, Zheng XD, Tang PYZ, et al. Advances of antitumor drug discovery in traditional Chinese medicine and natural active products by using multi-active components combination. Med Res Rev. 2023;43(5):1778–1808. doi:10.1002/med.21963
49. Zhang CY, Xu C, Gao XY, Yao QQ. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022;12(5):2115–2132. doi:10.7150/thno.69424
50. Janjua TI, Ahmed-Cox A, Meka AK, et al. Facile synthesis of lactoferrin conjugated ultra small large pore silica nanoparticles for the treatment of glioblastoma. Nanoscale. 2021;13(40):16909–16922. doi:10.1039/d1nr03553c
51. Kwon B, Han E, Cho W, et al. Nano-Fenton reactors as a new class of oxidative stress amplifying anticancer therapeutic agents. ACS Appl Mater Interfaces. 2016;8(9):5887–5897. doi:10.1021/acsami.5b12523
52. Zafar H, Raza F, Ma SY, Wei YW, Zhang J, Shen Q. Recent progress on nanomedicine-induced ferroptosis for cancer therapy. Biomater Sci. 2021;9(15):5092–5115. doi:10.1039/d1bm00721a
53. Zhao CH, Liu ZK, Chang CC, et al. Near-infrared phototheranostic iron pyrite nanocrystals simultaneously induce dual cell death pathways via enhanced Fenton reactions in triple-negative breast cancer. Acs Nano. 2023;17(5):4261–4278. doi:10.1021/acsnano.2c06629
54. Yoo W, Min SH, Zhang YS, Joo J, Kang HM, Kim DH. Photonic control of image-guided ferroptosis cancer nanomedicine. Coord Chem Rev. 2024;500:215532. doi:10.1016/j.ccr.2023.215532
55. Li YJ, Zeng XL, Lu DH, Yin MN, Shan MR, Gao Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum Reprod. 2021;36(4):951–964. doi:10.1093/humrep/deaa363
56. Xiong F, Zhou Q, Huang XB, Cao P, Wang Y. Ferroptosis plays a novel role in nonalcoholic steatohepatitis pathogenesis. Front Pharmacol. 2022;13:1055793. doi:10.3389/fphar.2022.1055793
57. Liu WN, Wang BD, Zhou MZ, et al. Redox dysregulation in the tumor microenvironment contributes to cancer metastasis. Antioxid Redox Signaling. 2023;39(7–9):472–490. doi:10.1089/ars.2023.0272
58. Aboelella NS, Brandle C, Kim T, Ding ZC, Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cancers. 2021;13(5):986. doi:10.3390/cancers13050986
59. Wu MM, Niu XM, Zhang R, Xu ZP. Two-dimensional nanomaterials for tumor microenvironment modulation and anticancer therapy. Adv Drug Delivery Rev. 2022;187:114360. doi:10.1016/j.addr.2022.114360
60. Zeng XY, An HD, Yu F, et al. Benefits of iron chelators in the treatment of Parkinson’s disease. Neurocheml Res. 2021;46(5):1239–1251. doi:10.1007/s11064-021-03262-9
61. Lv QB, Lin J, Huang H, et al. Nanosponge for iron chelation and efflux: a ferroptosis-inhibiting approach for myocardial infarction therapy. Adv Sci. 2024;11(25):2305895. doi:10.1002/advs.202305895
62. Zhou H, Chen JR, Fan MJ, et al. KLF14 regulates the growth of hepatocellular carcinoma cells via its modulation of iron homeostasis through the repression of iron-responsive element-binding protein 2. J Exp Clin Cancer Res. 2023;42(1):5. doi:10.1186/s13046-022-02562-4
63. Zhang M, Guo XL, Wang MF, Liu KH. Tumor microenvironment-induced structure changing drug/gene delivery system for overcoming delivery -associated challenges. J Control Release. 2020;323:203–224. doi:10.1016/j.jconrel.2020.04.026
64. Szabo R, Bodolea C, Iron MT. Copper, and zinc homeostasis: physiology, physiopathology, and nanomediated applications. Nanomaterials. 2021;11(11):2958. doi:10.3390/nano11112958
65. Meher MK, Naidu G, Mishra A, Poluri KM. A review on multifaceted biomedical applications of heparin nanocomposites: progress and prospects. Int J Biol Macromol. 2024;260(Pt 2):129379. doi:10.1016/j.ijbiomac.2024.129379
66. Zheng JZ, Du JJ, Ge HY, et al. Viscosity-dependent photocatalysis triggers ferroptosis and Type-I photodynamic therapy to kill drug-resistant tumors. Chem Eng J. 2022;449:136565. doi:10.1016/j.cej.2022.136565
67. Wang Y, Wei ZH, Pan KR, Li J, Chen QM. The function and mechanism of ferroptosis in cancer. Apoptosis. 2020;25(11–12):786–798. doi:10.1007/s10495-020-01638-w
68. Liu WY, Song XR, Jiang Q, et al. Transition metal oxide nanomaterials: new weapons to boost anti-tumor immunity cycle. Nanomaterials. 2024;14(13):1064. doi:10.3390/nano14131064
69. Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2020;9(1):84. doi:10.3390/jcm9010084
70. Yang TH, Zhou M, Gao M, et al. Carrier-free H2O2 self-supplier for amplified synergistic tumor therapy. Small. 2023;19(7):2205692. doi:10.1002/smll.202205692
71. Xu Y, Li YT, Li JX, Chen W. Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol. 2022;53:102349. doi:10.1016/j.redox.2022.102349
72. Zhou C, Zhao YH, Yang M, et al. Diselenide-containing polymer based on new antitumor mechanism as efficient GSH depletion agent for ferroptosis therapy. Adv Healthcare Mater. 2024;13(17):2303896. doi:10.1002/adhm.202303896
73. Chen MY, Tong XH, Sun YT, et al. A ferroptosis amplifier based on triple-enhanced lipid peroxides accumulation strategy for effective pancreatic cancer therapy. Biomaterials. 2024;309:122574. doi:10.1016/j.biomaterials.2024.122574
74. Niu BY, Liao KX, Zhou YX, et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110. doi:10.1016/j.biomaterials.2021.121110
75. Zhao P, Gong LY, Chang L, et al. Multifunctional Fe-based coordination polymer nano-bomb modified with β-lapachone and CaO2 for targeted tumor dual chemodynamic therapy with enhanced ferroptosis and H2O2 self-supply. J Nanobiotechnol. 2024;22(1):3. doi:10.1186/s12951-023-02287-2
76. Lin LS, Song JB, Song L, et al. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew Chem-Int Ed. 2018;57(18):4902–4906. doi:10.1002/anie.201712027
77. Liang JL, Jin XK, Zhang SM, et al. Specific activation of cGAS-STING pathway by nanotherapeutics- mediated ferroptosis evoked endogenous signaling for boosting systemic tumor immunotherapy. Sci Bull. 2023;68(6):622–636. doi:10.1016/j.scib.2023.02.027
78. Gu HF, Ren FZ, Mao XY, Du M. Mineralized and GSH-responsive hyaluronic acid based nano-carriers for potentiating repressive effects of sulforaphane on breast cancer stem cells-like properties. Carbohydr Polym. 2021;269:118294. doi:10.1016/j.carbpol.2021.118294
79. Wang YH, Huang K, Wang TY, et al. Nanosensors monitor intracellular GSH depletion: GSH triggers Cu(II) for tumor imaging and inhibition. Small. 2024;20(27):2310300. doi:10.1002/smll.202310300
80. Zhang YJ, Zhao JJ, Zhang LM, et al. A cascade nanoreactor for enhancing sonodynamic therapy on colorectal cancer via synergistic ROS augment and autophagy blockage. Nano Today. 2023;49:4825–4834. doi:10.1016/j.nantod.2023.101798
81. Suzuki S, Venkatesh D, Kanda H, et al. GLS2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma. Cancer Res. 2022;82(18):3209–3222. doi:10.1158/0008-5472.Can-21-3914
82. Yang SLX, Wu Y, Zhong WZ, Chen RE, Wang ML, Chen MW. GSH/pH dual activatable cross-linked and fluorinated PEI for cancer gene therapy through endogenous iron De-Hijacking and in situ ROS amplification. Adv Mater. 2024;36(2):2304098. doi:10.1002/adma.202304098
83. Zhang QY, Luo QH, Liu ZM, Sun MC, Dong X. Nano-ROS-generating approaches to cancer dynamic therapy: lessons from nanoparticles. Chem Eng J. 2023;457:497. doi:10.1016/j.cej.2022.141225
84. Zhu YM, Archer WR, Morales KF, Schulz MD, Wang Y, Matson JB. Enzyme-triggered chemodynamic therapy via a peptide-H2S donor conjugate with complexed Fe2+. Angew Chem-Int Ed. 2023;62(22):202302303. doi:10.1002/anie.202302303
85. Zhao CW, Cao WL, Zheng HL, et al. Acid-responsive nanoparticles as a novel oxidative stress-inducing anticancer therapeutic agent for colon cancer. Int J Nanomed. 2019;14:1597–1618. doi:10.2147/ijn.S189923
86. Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nature Nanotechnol. 2016;11(11):986–994. doi:10.1038/nnano.2016.168
87. Liu Y, Wang P, Hu WA, Chen D. New insights into the roles of peroxiredoxins in cancer. Biomed Pharmacother. 2023;164:114896. doi:10.1016/j.biopha.2023.114896
88. Yao LZ, Hou JY, Wu XY, et al. Cancer-associated fibroblasts impair the cytotoxic function of NK cells in gastric cancer by inducing ferroptosis via iron regulation. Redox Biol. 2023;67:102923. doi:10.1016/j.redox.2023.102923
89. Chatterjee S, Rai A, Patwardhan R, et al. GSH-responsive metal-organic framework-based nanoplatform for combined chemo-chemodynamic therapy. ACS Appl Nano Mater. 2024;7(7):8197–8211. doi:10.1021/acsanm.4c00787
90. Zhang DS, Cui P, Dai ZC, et al. Tumor microenvironment responsive FePt/MoS2 nanocomposites with chemotherapy and photothermal therapy for enhancing cancer immunotherapy. Nanoscale. 2019;11(42):19912–19922. doi:10.1039/c9nr05684j
91. Fu JK, Li T, Yang YZ, et al. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials. 2021;268:120537. doi:10.1016/j.biomaterials.2020.120537
92. Luo Y, Yan P, Li XY, Hou JW, Wang Y, Zhou SB. pH-sensitive polymeric vesicles for GOx/BSO delivery and synergetic starvation-ferroptosis therapy of tumor. Biomacromolecules. 2021;22(10):4383–4394. doi:10.1021/acs.biomac.1c00960
93. Xu ML, Zhong WL, Yang C, et al. Tiliroside disrupted iron homeostasis and induced ferroptosis via directly targeting calpain-2 in pancreatic cancer cells. Phytomedicine. 2024;127:155392. doi:10.1016/j.phymed.2024.155392
94. Li S, Liu YH, Zhang XK, et al. Multi-pathway oxidative stress amplification via controllably targeted nanomaterials for photoimmunotherapy of tumors. J Nanobiotechnol. 2024. doi:10.1186/s12951-025-03116-4
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

