Back to Journals » Nature and Science of Sleep » Volume 16
Waiting for in-Lab Polysomnography May Unnecessarily Prolong Treatment Start in Patients with Moderate or Severe OSA at Home Sleep Apnea Testing
Authors Pordzik J, Seifen C , Ludwig K , Ruckes C, Huppertz T , Matthias C, Gouveris H
Received 13 June 2024
Accepted for publication 23 November 2024
Published 5 December 2024 Volume 2024:16 Pages 1881—1889
DOI https://doi.org/10.2147/NSS.S482614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Johannes Pordzik,1 Christopher Seifen,1 Katharina Ludwig,1 Christian Ruckes,2 Tilman Huppertz,1 Christoph Matthias,1 Haralampos Gouveris1
1Department of Otolaryngology, Head and Neck Surgery & Sleep Medicine Center, University Medical Center Mainz, Mainz, 55131, Germany; 2Interdisciplinary Center for Clinical Trials (IZKS), University Medical Center Mainz, Mainz, 55131, Germany
Correspondence: Johannes Pordzik, Department of Otolaryngology, Head and Neck Surgery & Sleep Medicine Center, University Medical Center Mainz, Mainz, 55131, Germany, Email [email protected]
Purpose: The gold standard in obstructive sleep apnea (OSA) diagnostics is nocturnal full-night polysomnography (PSG). Due to high costs and high time effort portable respiratory polygraphy (PG or home sleep apnea testing-HSAT) has been developed. In contrast to PG the PSG gains relevant further information concerning sleep stages, arousals and leg movements. However, the role of PG in the diagnostic of OSA remains largely undefined. The aim of this study was to investigate the difference of PG- and PSG- related metrics in OSA, to understand if there is a difference in PG and PSG-based treatment decision and show up the time between performed PG and PSG.
Patients and Methods: 99 consecutive patients with existing outpatient performed PG and followed PSG in our tertiary care otorhinolaryngology department between February 2020 and December 2023 were retrospectively assessed. All patients were treatment-naive at the time of consultation. The time between performed outpatient PG and PSG was calculated. Furthermore, clinical baseline parameter and PG as well as PSG data were evaluated. All data were then blinded presented with relevant comorbid diseases to two experts in sleep medicine in our tertiary care centre to decide whether PAP therapy was indicated or not.
Results: Mean AHI was significantly higher in PSG (32.32 ± 22.78/h) compared to PG (22.60 ± 15.12/h) (p< 0.001). Mean duration between performed PG and PSG was 194.99 ± 131.96 days (range between 37 and 842 days). Only in two patients PAP-therapy was indicated with PG results but not with PSG results. Only in one case PAP-therapy was not indicated with PG results but with PSG results.
Conclusion: These data suggest initiating OSA therapy based on PG results for patients with at least moderate OSA on PG, followed by a confirming PSG and a control PSG under treatment to avoid unnecessary prolongation of treatment start.
Keywords: obstructive sleep apnea, portable respiratory polygraphy, polysomnography, PAP
Introduction
The prevalence of obstructive sleep apnea (OSA) is very high, affecting nearly 1 billion people.1 The gold standard for diagnosing OSA is overnight polysomnography (PSG).2 Due to the high cost and increased time associated with PSG, portable respiratory polygraphy (PG) has been proposed as a viable alternative in the clinical setting. In contrast to PG, PSG additionally records electroencephalogram (EEG), electrooculogram (EOG), and chin and leg electromyogram (EMG). These additional recording channels provide relevant additional biosignals related to sleep stages, arousals and leg movements. Regarding the role of PG in the diagnosis of OSA, it is well known that AHI is underestimated in PG compared to PSG.3 In the management of OSA patients, a study showed that a full PG protocol was non-inferior to a PSG protocol based on the measurement of daytime sleepiness using the Epworth Sleepiness Scale.4 The German S3 sleep-disordered breathing guideline recommends using PG to diagnose OSA only in cases with a high pretest probability.5 As a result, the use of PG in clinical practice is rather limited. The routine clinical pathway in Germany for patients with suspected OSA is first to see a general practitioner, followed by referral to a sleep medicine specialist. An outpatient PG is then performed, followed by a PSG. This process usually takes several months, especially since the number of medical facilities offering PSG is limited. As a result, the time from PG screening to initiation of OSA treatment can be several months. However, starting OSA treatment is very important because OSA is associated with an increased risk of coronary heart disease,6 hypertension,7 diabetes mellitus,8 non-alcoholic hepatic steatosis,9 and stroke.10 CPAP can reduce and eliminate not only the breathing disorder, but also daytime sleepiness.11,12 In addition, OSA is thought to increase healthcare costs. Therefore, early effective treatment of obstructive sleep apnea is a cost-effective solution.13–17 It is well known that PG is less expensive than PSG. One study revealed the costs of home sleep apnea testing (HSAT) and in-laboratory polysomnography (iPSG) participants were $199.94 CAD (SD=194.83) and $492.71 CAD (SD=0.48), respectively.18 Therefore, the question arises as to whether delaying treatment initiation in patients with a PG-based diagnosis of OSA is responsible and correct in all patients who have had a PG.
Although OSA is a highly prevalent disease, a search of the Pubmed database using different search terms such as “OSA time from diagnosis to treatment”, “OSA time from polygraphy to treatment” and “OSA time from polygraphy to polysomnography” did not reveal any precise data on the time between PG and PSG. One study showed a time to initiate medical therapy (continuous positive airway pressure) of 11.6 months.19 However, the time between PG and PSG has not been investigated. Therefore, the aim of this study was to investigate the difference of PG and PSG related parameters in OSA, to understand the accuracy of PG for correct OSA diagnosis and correct treatment indication (PAP) compared to gold standard PSG, and to provide evidence on the time period between performed PG and PSG from real-world clinical data.
Materials and Methods
All consecutive patients with existing ambulatory PG who underwent PSG in our tertiary care otolaryngology department between February 2020 and December 2023 were retrospectively reviewed. All externally performed and previously evaluated PGs were included. The current guideline requires manual scoring,20 so it can be assumed that all outpatient PGs were scored manually. Only patients over 18 years of age with a first diagnosis of OSA were included. All patients were treatment-naive at the time of consultation. After consultation at our tertiary center, PSG was performed according to the standard guidelines of the American Academy of Sleep Medicine (AASM). All PSGs were then analyzed by experts in the field of sleep medicine. The time between outpatient PG and PSG was calculated. In addition, the following baseline clinical parameters and PG and PSG data were evaluated Age at PG in years, body mass index (BMI) (kg/m2), sex, total sleep time (TST) for PSG and time in bed (TIB) for PG, apnea-hypopnea index (AHI) (n/h), total number of apneic events per hour (apnea index (AI)), total number of hypoxic events per hour (hypopnea index (HI)), cumulative time of apneic and hypopneic events in minutes of total sleep time (TST), total number of snoring events per hour (snoring index), total number of oxygen desaturation events (≥4%) per hour (oxygen desaturation index (ODI)), percentage of oxygen desaturation less than 90% (t90), mean oxygen saturation (%), minimum oxygen saturation (%), pulse variance index, time in bed (TIB), AHI in supine and non-supine position. No OSA was defined as AHI <5/h, mild OSA as AHI 5–15/h, moderate OSA as AHI 15–30/h and severe OSA as AHI > 30/h. All data were then blinded and presented with relevant comorbidities to two experts in sleep medicine at our tertiary care center to decide whether or not aPAP therapy was indicated. aPAP therapy was indicated in symptomatic patients with AHI>5/h.
Ethical Statement
In this study, only health data that is collected in the clinical routine was analyzed retrospectively. So-called “third parties” did not have access to the data and publication occurs exclusively in anonymized form. The Ethics Committee of the Rhineland-Palatinate Medical Association clearly states, that patient informed consent as well as ethical approval can be waved in these kind of studies and furthermore refrains from providing advice in such cases, citing the State Hospital Act (§36 and §37) (see also: https://www.laek-rlp.de/ausschuesse-kommissionen/ethikkommission/). Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Statistical Analysis
SPSS 27 (IBM, Armonk, NY, USA) was used for statistical analysis. All parameters were expressed as mean ± standard deviation. Normal distribution of samples was analyzed by Kolmogorov–Smirnov test. As all data were nonnormally distributed the Wilcoxon signed rank test was used. Results with p<0.05 were considered statistically significant.
Results
The patients (67 male, 32 female) were 51.59 ± 13.86 years old at the time of PG. Figure 1, Figure 2 and Table 1 show the respective clinical PSG and PG metrics.
 |
Table 1 PG and PSG Parameters |
 |
Figure 1 Differences in mean apnea-hypopnea-index (AHI) between polygraphy (PG): 22.60 ± 15.12 and polysomnography and PSG: 32.32 ± 22.78; * statistically significant with p <0.001. |
The mean time between PG and PSG was 194.99 ± 131.96 days (ranging from 37 to 842 days).
Statistically significant differences between PG and PSG results were observed for TST/TIB, AHI, HI, snoring index, minimum oxygen saturation, and ODI. No statistically significant differences were observed for BMI, AI, cumulative apnea and hypopnea time, mean oxygen saturation, t90, pulse variance index, and AHI in supine position/not supine position (s. Table 1, Figures 1 and 2).
Differentiating between no OSA (PG: 3.90 ± 0.57; PSG 5.55 ± 5.02; p=0.66), mild OSA (PG: 10.30 ± 3.08; PSG: 23.41 ± 18.00; p<0.001), moderate OSA (PG: 21.32 ± 4.67; PSG 29.71 ± 17.99; p<0. 01) and severe OSA (PG 46.65 ± 16.55; PSG 55.47 ± 25.78; p=0.03) based on PG measurement mean AHI was statistically significantly higher in each subgroup with diagnosed OSA regarding PSG vs PG (PG measurement was responsible for the classification into different groups such as no OSA, mild OSA, moderate OSA and severe OSA) (see Table 2).
Differentiation into no OSA (PG: 0.00 ± 0.00; PSG: 0.05 ± 0.07; p=0.32), mild OSA (PG: 3.12 ± 6.90; PSG: 1.81 ± 3.79; p=0.40), moderate OSA (PG: 5.87 ± 9.04; PSG: 6.06 ± 11. 23; p=0.80) and severe OSA (PG 20.44 ± 26.05; PSG 24.26 ± 27.03; p=0.14) based on PG measurement mean t90 did not differ significantly in each subgroup regarding PSG vs PG (Categorization into different groups such as no OSA, mild OSA, moderate OSA and severe OSA was based on the PG independent of PSG) (see Table 2).
AHI ≥ 5/h was detected in 97 patients (97.98%) on PG and in 96 patients (96.97%) on PSG. PG correctly identified 95 of these 96 patients as having OSA. This results in a sensitivity of the PG of 98.96% (s. Table 3).
 |
Table 3 Sensitivity and Specificity of PG for OSA Diagnosis |
AHI < 5/h was detected by PG in 2 patients (2.02%) and by PSG in 3 patients (3.03%). Of these 3 patients, 1 was correctly classified as not having OSA by the PG for a specificity of 33.33% (s. Table 3).
Based on PG-based values, more patients were classified as having mild and moderate OSA, while based on PSG-based values, more patients were classified as having severe OSA (see Figure 3 and Table 4).
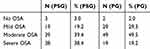 |
Table 4 Classification of OSA Severity Based on PG and PSG Parameters (N and %) |
Of the 99 patients, only two would have been indicated for aPAP therapy based on PG results, but not based on PSG results. The first patient underwent PSG 625 days after PG. The other patient had a relatively low AHI on PG, 5.8/h (s. Table 5). In only one case would the PG results not have indicated aPAP therapy, whereas the PSG results would have (s. Table 5). Based on current guidelines the treatment decision for aPAP therapy have been exactly the same for both sleep experts.
 |
Table 5 Wrong Three a-PAP Therapy Indications Based on PG |
Discussion
In this study, we provide evidence that standard device-based nocturnal respiratory metrics (such as AHI, HI, snoring index, minimum oxygen saturation, and ODI) are significantly higher based on PSG than compared to PG. However, other metrics are not significantly different between PSG and PG, such as AI, cumulative apnea and hypopnea time, mean oxygen saturation, t90, pulse variance index, and AHI in supine position/not supine position.
There is evidence that PG underestimates AHI compared to PSG in OSA patients.21–24 We show that PG significantly underestimates AHI in each OSA severity group. This finding is not surprising because, unlike PG, PSG includes EEG and hypopneas associated with arousals (even without accompanying desaturations) tend to inflate AHI values. PSG allows differentiation between total sleep time and measured time. Therefore, awake phases are excluded in PSG, which is not possible in PG. However, the severity of OSA is associated with comorbidities, especially in severe OSA.25 Therefore, the underdiagnosis of patients with PG could have a significant impact on comorbidities. The cumulative time of apnea and hypopnea was not significantly different between PG and PSG. However, the cumulative time of apnea and hypopnea was lower in PG compared to PSG. It is not surprising that HI (in contrast to AI) is significantly higher in PSG compared to PG. In particular, the detection of hypopnea events is complex and difficult in PG, with several possible events defining hypopnea.2
Across all OSA severity levels, t90 showed no significant difference between PG and PSG measurements. Pulse oximetry is almost identical for PG and PSG. It is not surprising that no significant difference was found between PG and PSG. On the other hand, t90 values in PG are based on time in bed (TIB), whereas in PSG they are based on total sleep time (TST). Consequently, t90 in PG may have been underestimated. Therefore, an important finding of our study is that t90 is not significantly different between PG and PSG. A value of t90 > 20% is associated with a higher risk of hypertension, T2DM, more severe sleepiness (as measured by the ESS), and an increased risk of mortality at 5 years of follow-up.26 It is well known that higher t90 is associated with increased severity of OSA.26 With this in mind, and in the absence of significant differences in t90 between PG and PSG, t90 should continue to be included in the therapeutic decision-making process.
In contrast to t90, ODI showed a significant difference between PG and PSG measurements. The ODI was significantly underestimated in the PG measurements. This could be due to the ratio of desaturation events to total sleep time in PSG and measured time in bed in PG.
In conclusion, relevant parameters are underestimated in PG compared to PSG. PG-based therapy decisions should take this into account.
However, the mean time difference between PG and PSG was 194.99 ± 131.96 days (range between 37 and 842 days). The sensitivity of the PG was very high (98.96%). Out of 99 patients, only two would have been indicated for aPAP therapy with PG results but not with PSG results. The first patient underwent PSG almost two years after PG. The other patient already had a low AHI of 5.6/h on PG. Only in one case aPAP therapy would not have been indicated on the basis of PG results but on the basis of PSG results. Considering that waiting times for PSG may have delayed the start of OSA treatment in our patient cohort by 194.99 ± 131.96 days, it should be carefully discussed whether aPAP therapy should be regularly initiated on the basis of PG results alone. To date, one study has attempted to compare measurements from ambulatory PG devices with inpatient PSG in routine clinical practice.24 These authors found that a treatment decision based on PG findings alone would have been incorrect in a total of 52 cases (13%). In 35 cases (9%) an indicated PAP therapy would have been omitted and in 17 patients (4%) an unnecessary CPAP therapy would have been initiated. The authors concluded that outpatient PG was not able to reliably assess OSA severity in routine clinical practice and that confirmation by PSG should be mandatory.24 In our study, the rate of inappropriate treatment initiation based on PG results alone would have been 3%. Our study confirms that PG is not able to reliably assess the severity of OSA, as it mostly underestimates the severity of OSA. However, early treatment of OSA is of paramount importance. Among other negative outcomes, OSA is associated with an increased risk of coronary artery disease,6 hypertension,7 diabetes mellitus,8 non-alcoholic hepatic steatosis,9 and stroke,10 among others. PAP-treatment improves ESS score, improves mental and physical quality of life, and reduces motor vehicle crashes.27
Limitations
The retrospective study design is a potential limitation of the study. In addition, PSG and PG results were inconsistently analyzed by different experts and evaluation methods. In particular, the evaluation of ambulatory PGs remains unclear due to the lack of raw data. Information about how the outpatient PGs were evaluated are missing. However, the current guideline requires manual scoring,20 so it can be assumed that all PG's were scored manually by different experts. That is how ambulatory PG is usually performed in the real world, with all the possible factors that could complicate things.24 Information on daytime sleepiness, as measured by the ESS, was not available at the time of the outpatient PGs. Therefore, we must assume that all patients who underwent PG were symptomatic (with or without sleepiness) and therefore received PG. Furthermore, home PAP titration was not performed at all. Therefore, aPAP therapy should be indicated by the sleep specialists in our study. aPAP has been developed and established as an alternative to fixed continuous PAP (cPAP) therapy. aPAP and cPAP therapy can be considered as equivalent therapeutic options.20 One of the advantages of aPAP therapy is that it does not necessarily need to be titrated like cPAP therapy.20
Conclusion
The mean time between PG and PSG was 194.99 ± 131.96 days (range 37 to 842 days). Despite significant differences between PG and PSG for AHI, HI, snoring index, minimum oxygen saturation, and ODI, only three (out of 99) divergent therapy indications based on PG- and PSG- results were made. Out of 99 patients, only in two cases PAP therapy was indicated with PG results but not with PSG results. In only one case was PAP therapy indicated with PSG results rather than PG results. Both experts gave exactly the same indications for PAP therapy. Considering this and the low rate of wrong / divergent therapy decisions based on PG results, we recommend using PG at least as a screening tool and prioritizing PSG in patients with high AHI. In countries with very few PSG facilities and very long waiting times, we recommend initiating aPAP therapy based on PG results in patients with at least moderate OSA on PG, followed by a confirmatory PSG (after initiation of PAP-treatment) and a control PSG under treatment to reduce the waiting time and avoid unnecessary prolongation of therapy initiation.
Abbreviations
OSA, Obstructive sleep apnoea; PSG, Polysomnography; PG, Polygraphy; EEG, Electroencephalogram; EOG, Electrooculogram; EMG, Electromyogram; BMI, Body-mass-index; AHI, Apnea-hypnoea-index; AI, Apnea index; HI, Hypopnea index; TST, Total sleep time; ODI, Oxygen desaturation index; TIB, Time in bed in min; PAP, Positive airway pressure.
Data Sharing Statement
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding authors.
Ethics Approval and Consent to Participate
In this study, only health data that is collected in the clinical routine was analyzed retrospectively. So-called “third parties” did not have access to the data and publication occurs exclusively in anonymized form. The Ethics Committee of the Rhineland-Palatinate Medical Association clearly states, that patient informed consent as well as ethical approval can be waved in these kind of studies and furthermore refrains from providing advice in such cases, citing the State Hospital Act (§36 and §37) (see also: https://www.laek-rlp.de/ausschuesse-kommissionen/ethikkommission/). Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Funding
No funding was received for this study.
Disclosure
Prof. Dr. Haralampos Gouveris reports grants from Inspire Medical Systems, Inc., outside the submitted work. The authors have no other conflict of interest to declare.
References
1. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
2. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
3. Masa JF, Corral J, Pereira R, et al. Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax. 2011;66(7):567–573. doi:10.1136/thx.2010.152272
4. Corral J, Sanchez-Quiroga MA, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196(9):1181–1190. doi:10.1164/rccm.201612-2497OC
5. Stuck BA, Arzt M, Fietze I, et al. Teil-Aktualisierung S3-Leitlinie Schlafbezogene Atmungsstörungen bei Erwachsenen [Partial update of the S3 guideline on sleep-related breathing disorders in adults]. Somnologie. 2020;24(3):176–208. German. doi:10.1007/s11818-020-00257-6
6. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
7. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. New Engl J Med. 2000;342(19):1378–1384. doi:10.1056/NEJM200005113421901
8. Seifen C, Pordzik J, Ludwig K, et al. Obstructive sleep apnea disrupts glycemic control in obese individuals. Medicina. 2022;58(11):1602. doi:10.3390/medicina58111602
9. Bahr K, Simon P, Leggewie B, Gouveris H, Schattenberg J. The Snoring index identifies risk of non-alcoholic fatty liver disease in patients with obstructive sleep apnea syndrome. Biology. 2021;11(1):10. doi:10.3390/biology11010010
10. Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. doi:10.1161/CIRCOUTCOMES.111.964783
11. Bratton DJ, Gaisl T, Schlatzer C, Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3(11):869–878. doi:10.1016/S2213-2600(15)00416-6
12. Giles TL, Lasserson TJ, Smith B, White J, Wright JJ, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;1.
13. Ayas NT, FitzGerald JM, Fleetham JA, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Archiv Intern Med. 2006;166(9):977–984. doi:10.1001/archinte.166.9.977
14. Fischer J, Raschke F. Economic and medical significance of sleep-related breathing disorders. Respiration. 1997;64(1):39. doi:10.1159/000196735
15. Mar J, Rueda J-R, Durán-Cantolla J, Schechter C, Chilcott J. The cost-effectiveness of nCPAP treatment in patients with moderate-to-severe obstructive sleep apnoea. Eur Respir J. 2003;21(3):515–522. doi:10.1183/09031936.03.00040903
16. Tan M, Ayas N, Mulgrew A, et al. Cost‐effectiveness of continuous positive airway pressure therapy in patients with obstructive sleep apnea‐hypopnea in British Columbia. Canadian Respiratory J. 2008;15(3):159–165. doi:10.1155/2008/719231
17. Weatherly HL, Griffin SC, Mc Daid C, et al. An economic analysis of continuous positive airway pressure for the treatment of obstructive sleep apnea-hypopnea syndrome. Int J Technol Asses Health Care. 2009;25(1):26–34. doi:10.1017/S0266462309090047
18. Boulos MI, Kamra M, Colelli DR, et al. SLEAP SMART (Sleep Apnea Screening Using Mobile Ambulatory Recorders After TIA/Stroke): a randomized controlled trial. Stroke. 2022;53(3):710–718. doi:10.1161/STROKEAHA.120.033753
19. Rotenberg B, George C, Sullivan K, Wong E. Wait times for sleep apnea care in Ontario: a multidisciplinary assessment. Canadian Respiratory J. 2010;17(4):170–174. doi:10.1155/2010/420275
20. Mayer G. S3-Leitlinie Nicht erholsamer Schlaf/Schlafstörungen-Kapitel” Schlafbezogene Atmungsstörungen”. Springer Medizin; 2017.
21. Abdelghani A, Roisman G, Escourrou P. Evaluation of a home respiratory polygraphy system in the diagnosis of the obstructive sleep apnea syndrome. Revue Des Maladies Respiratoires. 2007;24(3 Pt 1):331–338. doi:10.1016/S0761-8425(07)91065-7
22. Candela A, Hernandez L, Asensio S, et al. Validation of a respiratory polygraphy system in the diagnosis of sleep apnea syndrome. Archivos de bronconeumologia. 2005;41(2):71–77. doi:10.1016/S1579-2129(06)60400-X
23. Driver HS, Pereira EJ, Bjerring K, et al. Validation of the MediByte(R) type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Canadian Respiratory J. 2011;18(3):137–143. doi:10.1155/2011/760958
24. Lindemann J, Augenstein B, Stupp F, et al. Diagnostic accuracy of outpatient polygraphy devices: a comparison with inpatient polysomnography in clinical routine. Hno. 2017;65(2):134–140. doi:10.1007/s00106-016-0308-6
25. Silva M, Poyares D, Silva LO, et al. Associations of the severity of obstructive sleep apnea with age-related comorbidities: a population-based study. Front Neurol. 2022;13:802554. doi:10.3389/fneur.2022.802554
26. Labarca G, Campos J, Thibaut K, Dreyse J, Jorquera J. Do T90 and SaO(2) nadir identify a different phenotype in obstructive sleep apnea? Sleep Breathing = Schlaf Atmung. 2019;23(3):1007–1010. doi:10.1007/s11325-019-01860-0
27. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–1400. doi:10.1001/jama.2020.3514
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Comparison of Ring Pulse Oximetry Using Reflective Photoplethysmography and PSG in the Detection of OSA in Chinese Adults: A Pilot Study
Zhao R, Xue J, Zhang X, Peng M, Li J, Zhou B, Zhao L, Penzel T, Kryger M, Dong XS, Gao Z, Han F
Nature and Science of Sleep 2022, 14:1427-1436
Published Date: 18 August 2022
New Metrics from Polysomnography: Precision Medicine for OSA Interventions
Guo J, Xiao Y
Nature and Science of Sleep 2023, 15:69-77
Published Date: 9 March 2023
Association Between Sleep Efficiency and Hypertension in Chinese Obstructive Sleep Apnea Patients
Xia N, Wang H, Chen Y, Fan XJ, Nie XH
Nature and Science of Sleep 2023, 15:79-88
Published Date: 10 March 2023
Association Between Nocturnal Hypoxemia Parameters and Coronary Microvascular Dysfunction: A Cross-Sectional Study
Feng L, Zhao X, Song J, Yang S, Xiang J, Zhang M, Tu C, Song X
Nature and Science of Sleep 2024, 16:2279-2288
Published Date: 28 December 2024
Performance of Four Screening Tools for Identifying Obstructive Sleep Apnea Among Patients with Insomnia
Shi C, Wang Y, Luo J, Huang R, Xiao Y
Nature and Science of Sleep 2025, 17:379-390
Published Date: 3 March 2025




