Back to Journals » International Journal of Nanomedicine » Volume 19
What is the Reason That the Pharmacological Future of Chemotherapeutics in the Treatment of Lung Cancer Could Be Most Closely Related to Nanostructures? Platinum Drugs in Therapy of Non-Small and Small Cell Lung Cancer and Their Unexpected, Possible Interactions. The Review
Authors Szupryczyński K, Czeleń P, Jeliński T, Szefler B
Received 26 April 2024
Accepted for publication 19 July 2024
Published 14 September 2024 Volume 2024:19 Pages 9503—9547
DOI https://doi.org/10.2147/IJN.S469217
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Kamil Szupryczyński,1 Przemysław Czeleń,2 Tomasz Jeliński,2 Beata Szefler2
1Doctoral School of Medical and Health Sciences, Faculty of Pharmacy, Collegium Medicum, Nicolaus, Copernicus University, Bydgoszcz, Poland; 2Department of Physical Chemistry, Faculty of Pharmacy, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland
Correspondence: Beata Szefler, Email [email protected]
Abstract: Over the course of several decades, anticancer treatment with chemotherapy drugs for lung cancer has not changed significantly. Unfortunately, this treatment prolongs the patient’s life only by a few months, causing many side effects in the human body. It has also been proven that drugs such as Cisplatin, Carboplatin, Oxaliplatin and others can react with other substances containing an aromatic ring in which the nitrogen atom has a free electron group in its structure. Thus, such structures may have a competitive effect on the nucleobases of DNA. Therefore, scientists are looking not only for new drugs, but also for new alternative ways of delivering the drug to the cancer site. Nanotechnology seems to be a great hope in this matter. Creating a new nanomedicine would reduce the dose of the drug to an absolute minimum, and thus limit the toxic effect of the drug; it would allow for the exclusion of interactions with competitive compounds with a structure similar to nucleobases; it would also permit using the so-called targeted treatment and bypassing healthy cells; it would allow for the introduction of other treatment options, such as radiotherapy directly to the cancer site; and it would provide diagnostic possibilities. This article is a review that aims to systematize the knowledge regarding the anticancer treatment of lung cancer, but not only. It shows the clear possibility of interactions of chemotherapeutics with compounds competitive to the nitrogenous bases of DNA. It also shows the possibilities of using nanostructures as potential Platinum drug carriers, and proves that nanomedicine can easily become a new medicinal product in personalized medicine.
Keywords: fullerenes, nanotube, nanoparticles, drug delivery, personalized medicine, platinum-based drugs, cisplatin, carboplatin, oxaliplatin, nedaplatin
Introduction
Cancer is defined by the WHO (World Health Organization) as a group of diseases caused by abnormal, uncontrolled cell growth, which can be located in almost any organ or tissue of the body.1 The primary cause of cancer-related deaths is metastasizing,2–7 which is the spread of cancer cells from the initial organ or tissue to another one in the host.7
Nowadays, cancer is one of the leading causes of death globally. The most common women’s cancers are breast, colorectal, lung, cervical, and thyroid, whereas among men, these are lung, prostate, colorectal, stomach, and liver cancers.1
Lung cancer is one of the most common and serious types of cancer worldwide, for which detailed treatment regimens are presented. In 2022, lung cancer had the most new cases among all cancers, as many as almost 2.5 million, which constituted 12.4% of all new cancer cases. Although this statistic was comparable to breast cancer, lung cancer had a twice as high mortality rate of over 1.8 million people, which accounted for 18.7% of all cancer deaths.8 The situation in Poland in 2019 is similar: among men it accounted for 16.1% and among women for 9.9% of new cases, while the mortality statistics were 27.4% and 17.9%, respectively.9 According to the American Cancer Society’s estimates of lung cancer in the USA, in 2024 there will be over 230 thousand new cases and over 125 thousand deaths.10
Lung cancer can be divided into two histologically different classes that grow and spread differently,11 Small Cell Lung Cancers (SCLC) and Non-Small Cell Lung Cancer (NSCLC).
Small Cell Lung Cancers (SCLC) - they constitute approximately 10–15% of all lung cancers. SCLC originate from hormonal cells of the lung and are characterized by rapid growth, a tendency to metastasize (even in over 60% of patients12), and susceptibility to chemotherapy. The survival rate is extremely low for 5 years - only 7%.13 Fortunately, they are highly susceptible to chemotherapy.12 Most often, they are located centrally in the lungs, and their presence is related to smoking.14
Non-Small Cell Lung Cancer (NSCLC) - they constitute approximately 80% of all lung cancers, and unlike SCLC, they most often develop slowly and may remain hidden until an advanced stage. They can originate from different types of cells.14 Non-Small Cell Lung Cancer (NSCLC) being divided into many types, the most common of which are squamous cell lung cancer, large cell lung cancer, and adenoma. Active and passive smoking are factors that increase the risk of developing the disease, but NSCLC can be detected in people who have never smoked. Unfortunately, NSCLC is more resistant to chemotherapy and radiotherapy than SCLC.10 The survival rate for the next 5 years is significantly higher - 30%.10
The likelihood of effective treatment increases with the early detection of cancer.11,15,16 The treatment method depends on the type of cancer cells, their location, and the stage of the disease. Nowadays, traditional therapeutic treatment methods include, first of all, surgical resection, radiation therapy, and chemotherapy, while newer forms of treatment include targeted therapy, immunotherapy, hormone therapy, gene therapy, and photodynamic therapy.3,11,17–20 Currently, however, combined therapy is used more and more often instead of monotherapy (Chapter 2, Subsection 2.4). The combination chemotherapy, including a Platinum derivative and drugs from other groups, is used in the treatment of many cancers (Chapter 2, Subsection 2.4 and Supplementary Materials: Tables S1 and S2). Nowadays, scientists are constantly looking for new anticancer drugs (Chapter 4, Subsections 4.3–4.5) that would provide a better therapeutic effect with minimal side effects (Chapter 2, Subsection 2.3), but also for new forms of treatment, which includes the creation of nanomedicine (Chapter 4). A significant development in chemotherapy is the combination of nanostructures with Platinum-derivative drugs (Chapter 4), which improves drug accumulation at the target site and weakens their side effects. Moreover, the creation of nanomedicine limits to an absolute minimum the possibility of combining the Platinum compounds with amino acids in the peripheral blood (Chapter 3, Subsection 3.2). Going further, at the cellular level, it limits the combination with compounds competing with nucleobases (Chapter 3, Subsection 3.1). There are many examples of combining a nanostructure with a chemotherapeutic agent in the literature (Chapter 4). Platinum compounds are often combined with organic nanostructures (protein nanotubes, liposomes, nanobodies, polymers), inorganic nanostructures (dendrimers) and carbon nanostructures represented by fullerenes, nanotubes, and rhombellans (Chapter 4, Subsection 4.4).
Platinum Compounds
Platinum compounds are among the first, most effective, and most frequently used chemotherapy drugs. The oldest of them is Cisplatin (formerly Peron Saltz21), discovered by Micheal Perone in 1844.22 However, its cell division-inhibiting properties remained hidden until 1965, when Bennett Rosenberg of the University of Michigan described the inhibition of sarcomas and leukemias in mice in the presence of Cisplatin.23 It focused the attention of the scientific world on Platinum compounds, which naturally led to the expansion of their family to include additional substances. Currently, the family of Platinum derivatives can be divided into compounds that have been approved for treatment around the world, such as Cisplatin, Carboplatin, Oxaliplatin, and those that are only used in selected countries, such as Nedaplatin, Heptaplatin and Lobaplatin, taken by patients in Japan, Korea and China, respectively.24–27 Over the years, thousands of Platinum-derived compounds have been tested, but most of them were rejected in subsequent stages, mainly due to their high toxicity.28
Cisplatin (used since 1978) is a drug with a very wide range of uses in solid cancers, such as testicular cancer, ovarian cancer, bladder cancer, lung cancer, head cancer, neck cancer, stomach cancer, mesothelioma, cervical cancer, prostate cancer, skin cancer, salivary gland cancer, and others (Figure 1a).
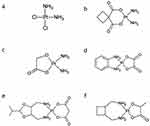 |
Figure 1 The structure of Pt drugs; (a) - Cisplatin, (b) - Carboplatin, (c) - Nedaplatin, (d) - Oxaliplatin, (e) – Heptaplatin, and (f) - Lobaplatin.29–47 |
Carboplatin (used since 1989) is a compound belonging to a second-generation of platinum drugs with weaker anticancer activity but, at the same time, a less destructive effect on the body, compared to cisplatin, which is used in ovarian and testicular cancers, small-molecule lung cancer, neck cancer, head cancer, bladder cancer, breast cancer, brain cancer, and neuroblastoma (Figure 1b).28,48–65
Nedaplatin (used since 1995 in Japan) is a second-generation platinum drug, developed to provide a treatment with effectiveness similar to that of cisplatin and better than Carboplatin but with decreased renal and gastrointestinal toxicities. Moreover, it is much more soluble in water than Cisplatin. Nedaplatin is used to treat cancers of the head, neck, a small cell lung cancer, as well as esophagus (Figure 1c).28,59,60,66–71
Oxaliplatin (used since 1996) is a third-generation platinum drug, developed to overcome the resistance against first- and second-generation platinum drugs. Oxaliplatin has a different mode of action than Cisplatin, it creates fewer cross-links, but induces ribosome biogenesis stress. The lipophilic ligand increases penetration through cell membranes. It is a substance used mainly in chemotherapy of the colorectal cancer and, to a lesser extent, in stomach and esophageal cancers (Figure 1d).42,58–60,65,72–110
Heptaplatin (used since 1999 in Korea) is currently used in the treatment of gastric cancer and has fewer hematologic toxicities than Cisplatin with this same combination. The important advantage is its high solubility in water (Figure 1e).59,111–114
Lobaplatin (used since 2010 in China)24,25,115–117 is used in patients suffering from chronic myelogenous leukemia, small cell lung cancer and metastatic breast cancer118 (Figure 1f).25–27,59,66,115–118
All the above-mentioned compounds are administered in monotherapy or combined therapy, in which, in addition to Platinum derivatives, other medicinal substances are used, and each one has its own patterns (Table S1 given in Supplementary Materials). In this work, we will focus mainly on complex therapies for Non-small cell lung cancer15,16 and Small cell lung cancer119,120 (Chapter 2, Subsection 2.4 and Table S2 given in Supplementary Materials).
The development strategies for these drugs focus on the combination therapy mentioned above (given in Supplementary Materials) and the therapy using nanostructures (for drug delivery), as well as other key issues: low selectivity towards cancer cells, limiting the phenomenon of resistance, and toxicity of chemotherapy.
Mode of Actions
Platinum compounds are administered intravenously in the form of a saline solution in specially prepared bags, to which a colored infusion device is connected. The bag contains all the necessary drug data (name, active substance, dose, solvent, infusion time), as well as the patient’s data and information to protect it from light. These solutions contain Platinum compounds in the form of a prodrug, which, due to the high concentration of chloride ions in the blood, remains inactive, but this state changes after the drug enters the cell.40 Compounds derived from Platinum penetrate the cell membrane by passive diffusion or active transport via the copper transporter CTR1106 organic cation transporters (OCTs),121 solute carriers (SLCs)121 and ATP-binding cassette (ABC) multidrug transporters.121 The details on these mechanisms for each Platinum drug are given in Table 1.
 |
Table 1 Mechanism of Platinum Drug Transport Across the Cell Membrane |
The choice of transport type depends on lipophilicity. More lipophilic substances, such as Oxaliplatin, will have dominant passive transport, whereas more hydrophilic substances, such as Heptaplatin and Nedaplatin, will cross copper receptors to a greater extent (Figure 2 and Table 1).
 |
Figure 2 Types of transport of chemotherapy drugs across the cell membrane and the blood/cancer cell barrier. Symbols +, -, and ? denote activity, inactivity, and no data in the literature, respectively.25,33,34,47,66,117,125–135 |
After passing through the membrane, these compounds are inactivated during the hydrolysis process and then show significant affinity for proteins (cysteine sulfhydryl groups)47,125–133 and nucleic acids (nitrogen atoms of purines, ie guanine and adenine).25,33,34,66,117,134,135, As a result of their action on DNA, the compounds generate a huge number of cross-links in nucleic acids, both within the same DNA strand and between strands, which distorts and damages the DNA. If these changes are not repaired by DNA polymerase, it leads to apoptosis (Figure 3).136–139
 |
Figure 3 The scheme of the mode of action of Cisplatin.123,136–139 |
The most preferred attachment site for Cisplatin is the N7 nitrogen atom in the nitrogenous base of guanine (Figure 4). Additionally, the N7 of adenine, N3 of cytosine and thymine, and N1 of guanine and adenine are less preferred sites. Cisplatin attaches to the greatest extent to the two guanine bases laying on the same strand. They may lie next to each other or be separated by another base.
 |
Figure 4 Binding sites of Cisplatin with nucleobases of DNA.40,62 |
The next most common connection is the attachment of Cisplatin to a guanine base and an adenine base, lying next to each other on the same strand. Bonds between DNA and monoadduct strands are relatively rare62 (Figure 5).
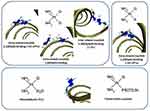 |
Figure 5 Intra- and inter-strand cross-linking of Platinum-derivative drugs with DNA bases. |
Additionally, Platinum drugs stimulate the production of reactive oxygen species (ROS), which indicate oxidative stress. This process is mainly responsible for drug toxicity.140–146 ROS also lead to cell death by damaging mitochondrial DNA, which causes mitochondrial leakage and the release of cytochrome C and caspase-9, which initiate the apoptotic pathway.135
Resistance
One of the most important reasons why the treatment with Platinum derivatives is abandoned or does not bring the expected results is the phenomenon of resistance. This phenomenon makes further treatment, eg with Cisplatin, ineffective and another effective therapy must be found. Such a solution may be, for example, the use of another Platinum-derivative drug, eg Nedaplatin, to which cancer cells are not resistant.67 Resistance to Platinum compounds can be divided into three types: pre-target, on-target and post-target. Pre-target resistance involves at least two processes. The first one involves reducing the cellular accumulation of Platinum-derivative drugs. This process occurs, for example, by reducing the absorption of Platinum-derivative drugs, eg by affecting copper transporters147 or also by increasing efflux from cells.148 The second one involves increasing the deactivation of Platinum during its journey to the cell and in the cell by binding it to other substances, such as B vitamins or proteins containing sulfur amino acids.148 As for the on-target resistance. cells acquire greater resistance to the action of Platinum-derivative drugs by improving the repair processes using DNA polymerase, which cuts out the nucleotide attacked by drugs. As a result, the changes caused by Platinum-derivative drugs that were supposed to lead to apoptosis are repaired, and the cell does not start the process of apoptosis.33,134 Post-target resistance to Platinum-derived drugs may involve inhibition of the reaction cascade leading to apoptosis. One of the most common examples of such resistance is TP53 inactivation, which occurs in approximately half of all human cancers.149 Unfortunately, the resistance that develops is most often multifactorial in nature, and therefore stopping one pathway of resistance development cannot bring any clear benefits. Additionally, the very low selectivity of Platinum-derivative drugs, and therefore the high administered dose, drives the development of resistance. This effect also increases with the administration of subsequent doses.150
Side Effects
The second most common reason that causes the treatment with Platinum derivatives to be discontinued is the resulting side effects. All Platinum-based drugs have dozens of different side effects, which are depicted in Figure 6. Their main reason is the poor selectivity of Platinum compounds. This means that apoptosis is not only induced in cancer cells but also in healthy ones.50,56,64,69,73,83,87,90,92,110,111,151–162 Each Platinum derivative has its own side effects, which occur relatively frequently after its use and are presented in Table 2. Side effects may become so intense that they require us to limit the dose of the drug or generally discontinue the treatment with a given substance. For Cisplatin, the most common limiting effect is nephrotoxicity, for Carboplatin it is myelosuppression, and for Oxaliplatin it is neurotoxicity.154
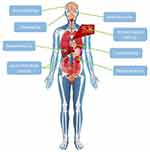 |
Figure 6 The side effects of Cisplatin.154 This picture has been designed using assets from Freepik.com.164 |
 |
Table 2 The Side Effects of Platinum Derivatives |
One of the most common side effects is gastrointestinal toxicity. The effect on the digestive system includes, in addition to the most common nausea, vomiting, reflux, diarrhea, and constipation, also gastrointestinal disorders, and, even though relatively rarely, it may lead to eating disorders. Platinum compounds release 5-hydroxytryptamine into the gut, which induces vomiting. Additionally, consequences may damage the villus.32 The effect of Platinum compounds on the bone marrow causes hematological toxicities such as leukopenia (low level of white platelets), especially neutropenia (low level of neutrophils), thrombocytopenia, and anemia (low level of red blood cells). The sum of all these phenomena is called myelosuppression, in which the production of all blood cells is decreased. Although this phenomenon is caused by all Platinum drugs from this group, it is most characteristic of Carboplatin for which it is a limiting factor.165 What’s more, for Loboplatin, the dose-limiting toxicity is thrombocytopenia.59 Platinum compounds are mainly excreted in the urine and therefore also cause nephrotoxicity, which is mainly manifested by acute renal failure, reduced reabsorption of magnesium and calcium (which may lead to hypomagnesemia and hypokalemia), reduced synthesis of Erythropoietin (the hormone responsible for the maturation of erythrocytes), and increased levels of uric acid in the blood (hyperuricemia). Cisplatin has the highest nephrotoxicity, affecting almost 90% of patients, for whom it is a limiting factor.154,166 A very common complication, especially among children, is ototoxicity, damage to the inner ear which may manifest itself in pain, loss of high-frequency hearing (4000–8000 Hz), or balance disorders.154,161,167,168 Ototoxicity may be low in the early stages of treatment, however, with more cycles, the risk increases significantly.154,165 The mechanism of ototoxicity is not clear, however it is assumed that the reason is reactive oxygen species (ROS). Platinum drugs enable the NADPH oxidase to lead ROS production.167 After infusion with Platinum compounds, severe neuropathies have been observed, which may include paresthesia, areflexia, loss of proprioceptive and vibration sensations, seizures, Lhermitte’s sign, and encephalopathy.169 Symptoms tend to be stronger in distal parts (such as hands and feet) and the perioral and pharyngolaryngeal areas. Peripheral sensory neuropathies are correlated with an increased cumulative dose administered.170 Nevertheless, an entire loss of motor function is extremely rare, and symptoms disappear after a few days and, in rare situations, last for a maximum of 4 years.154 Neuropathies are the main cause of limiting Oxaliplatin usage.154 Cardiotoxic effect is rarely observed and mainly manifests itself as arrhythmia, tachycardia (heart rate over 100 beats per minute) and bradycardia (heart rate under 60 beats per minute), cardiac ischemia (blood flow to your heart is reduced), myocardial infarction, diastolic disturbances of the ventricles, and pericarditis.171 The mechanism of action of Platinum compounds’ cardiotoxicity is unknown, however there is some evidence that it is linked to ROS.172 The liver is an important organ where several biochemical processes occur, for example, the metabolism of a majority of drugs. Oxidative stress is the main reason for Platinum compounds’ induced hepatotoxicity. These created ROS lead healthy liver cells to apoptosis.162 On the other hand, Cisplatin and Oxaliplatin have the ability to damage liver sinusoids (liver blood vessels). Consequently, these damages indicate liver vascular disorders.173 Side effects may become so intense that they can require limiting the dose of the drug or generally discontinuing the treatment with a given substance. For Cisplatin, the most common limiting effect is nephrotoxicity, for Carboplatin it is myelosuppression, and for Oxaliplatin it is neurotoxicity.154 To sum up, side effects may include delicate, reversible, and not very bothersome symptoms, such as hair loss,174 as well as irreversible, troublesome symptoms that will reduce the quality of life and may pose a threat to health and life.
For this reason, finding a solution that will significantly increase the selectivity and thus reduce the side effects of Platinum-based drugs is such a big challenge. Scientists are trying to modify existing drugs or replace them with others with similar effects and uses.175–177 One of the possible modifications that Platinum compounds can undergo in order to reduce side effects is their conjugation with nanostructures.178–189
General Schedules in Treatment of Lung Cancer in the Therapy with Platinum Drugs
The combination chemotherapy, including a Platinum derivative and drugs from other groups, is used in the treatment of many cancers (Table S1 and Table S2 given in Supplementary Materials). There are two main reasons for this phenomenon.
The first one is that we use different substances that produce a synergistic effect. This may be another cytostatic that acts in a different way, eg Vinorelbine, Gemcitabine, Etoposide, or immunotherapeutic drugs like a monoclonal antibody, eg Atezolizumab, Bevacizumab and Pembrolizumab. Monoclonal antibodies can act in multiple ways. One of them is to block the activity of abnormal proteins in cancer cells. The second way is to strengthen the immune system by inhibiting or stopping immune checkpoints. As a result, cancer cells cannot block these points and thus remain hidden from immune system cells. Such points include the PD-1/PD-L1 pathway190–194 and antibodies include Atezolizumab, Nivolumab, Pembrolizumab.195
What’s more, the administration of these substances helps to reduce the phenomenon of resistance to a specific drug, especially in the event of treatment relapses.28 In the case of Cisplatin, the phenomenon of resistance is described as a change in cellular uptake, efflux of Cisplatin from the cell, increased detoxification of molecules in the liver, or increased DNA repair and inhibition of apoptosis.28,196 All this makes the therapy with a given cytostatic less effective, and it is necessary to look for another alternative to the treatment or, if possible, increase the dose of the drug, which will further increase resistance and intensify side effects. Table 3 shows a regimen consisting of Carboplatin, Paclitaxel and Pembrolizumab, which was used in Stage IV Non-Small Cell Lung Cancer with PD-L1 expression lower than 50%, without the presence of EGFR gene mutations or rearrangements of the ALK and ROS1 genes. The dose of Carboplatin depends on the glomerular filtration rate (GFR) and the formula for the dose in milligrams is expressed as AUC x (GFR + 25), dissolved in 500 mL of a 5% glucose solution. However, the dose of Paclitaxel depends on the body surface and is 200 mg for each 1 m2 of the body dissolved in 500 mL of 0.9% NaCl. The monoclonal antibody Pembrolizumab has a fixed dose of 200 mg dissolved in 100 mL of 0.9% NaCl. The infusion time varies depending on the substance: Carboplatin is 1 hour, Paclitaxel is 3 hours, and Pembrolizumab is 0.5 hour. All three substances are always administered during the first day of a twenty-one-day treatment cycle. The number of repeated cycles in the treatment regimen for Carboplatin and Paclitaxel is 4 and for Pembrolizumab it is 35. However, these data may vary depending on the treatment regimens. All schemes are located in Supplementary Materials (Table S1) and a fragment of this table containing the above scheme is located below (Table 3).
 |
Table 3 Lung Cancer Treatment Regimens.197 |
Structures Competitive to Nucleobases of DNA
The mechanisms of action of Platinum-derived compounds have low selectivity, which makes them react with many different compounds. Based on in silico and experimental studies, it has been proven that Platinum-derived drugs can easily react and form complexes with other chemical compounds containing aromatic rings, especially nitrogen or sulfur, with a lone pair of electrons.58,102,198–200
B Vitamins
One of such groups are B vitamins, ie a group of eight water-soluble vitamins (B1, B2, B3, B5, B6, B7, B9, and B12), which play the role of cofactors for many cellular metabolic pathways and enzymes involved in the synthesis of nucleic acids.201–220 Only 5 out of the 8 are similar to purines, owing to the fact that these vitamins contain aromatic rings with a nitrogen atom (B1, B2, B3, B6, and B9). Based on in silico studies, it has been proven that Platinum-derivative drugs can easily react and form complexes with other chemical compounds with a structure similar to nucleobases.57,102,198–200 Such compounds include B vitamins, which can clearly compete with the nitrogenous bases of DNA.57,102,199,200 Ab initio research has been confirmed by experimental research.57 Physico-chemical characterization of interaction between vitamins and Platinum drugs was performed using UV-vis spectroscopic techniques.57
Thiamine (vitamin B1) is a coenzyme in the metabolism of carbohydrates, and additionally influences the synthesis of the neurotransmitter gamma-aminobutyric acid and catalyzes the conversion of pyruvate to acetyl-CoA. Deficiency may lead to disorders of the nervous system (beriberi disease), heart muscle, and skeletal muscles, as well as cardiovascular disorders.201,202,212,213,221–223 Riboflavin (vitamin B2) takes part in tissue respiration processes and is a component of many oxidation-reduction enzymes. Additionally, the vitamin takes part in the metabolism of carbohydrates, fats, and proteins, catalyzing oxidation processes. Deficiency may cause disorders in the functioning of the nervous system and inflammation of the mucous membranes.201,202,215,216,224 Niacin (vitamin B3) participates in the transfer of hydrogen and electrons in the processes of cellular respiration, glycolysis and lipid biosynthesis. Moreover, it is necessary for the proper functioning of the brain and the peripheral nervous system, and is also involved in the synthesis of the sex hormones, cortisol, thyroxine, and insulin. Deficiency may lead to pellagra, disorders of the nervous system, and the glycolysis process.201,202,217–219 Vitamin B6 is a group of six chemically similar compounds that can transform into each other and have a common active form, pyridoxal 5′-phosphate. This form is an extremely important cofactor in the metabolism of complex carbohydrates, fatty acids, phospholipids and cholesterol.201–204,220 It is also involved in heme synthesis.201,202,205,225 Vitamin B6 deficiencies cause inflammation of the skin and mucous membranes, neuropathies, and anemia.210 These vitamins are of key importance, eg in certain disease states like diabetes and Wernicke’s encephalopathy.206–208,211 However, it is worth noting and emphasizing that neuropathies are a common side effect of Platinum.209,212 It has been proven that B vitamins can weaken the effect of Platinum-derivative drugs.57,102,198–200
Based on previous research, it can be concluded that niacin (vitamin B3) has the greatest affinity for Cisplatin, both for its mono- and diaqua200 forms (Figure 7). In the case of thiamine (vitamin B1), Cisplatin forms complexes more easily with the nitrogen atom in the N1 position in the ring than with the N7 atom. Pyridoxal phosphate (vitamin B6) has a similar ease of complex formation as thiamine.200 On the other hand, the behavior of riboflavin (vitamin B2) is completely different.200 This vitamin reacts poorly with Carboplatin. A spontaneous reaction can be observed for pyridoxal phosphate (vitamin B6) and the value of Gibbs free energy of reaction, ΔGr, is negative. Niacin (vitamin B3) forms much worse complexes with Carboplatin,57 where the value of ΔGr is approximately zero. Oxaliplatin has the same affinity for B vitamins as Cisplatin.102 However, changing the calculation level from B3LYP/6-31G(d,p)/LANL2DZ226 to MN15/def2-TZVP changes this picture.102 The most reactive vitamins are thiamine (vitamin B1) and pyridoxal phosphate (vitamin B6), and the least reactive one is niacin (vitamin B3).
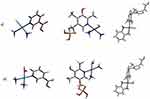 |
Figure 7 Exemplary structures of mono (a) and diaqua (b) complexes of Cisplatin with niacin (vitamin B3), Carboplatin with pyridoxal phosphate (vitamin B6) and Oxaliplatin with thiamine (vitamin B1).57,102,198,199 |
This means that a specially calculated therapy cannot be as effective as it could be expected, because some of the drug can react with vitamins instead of purines in DNA. This highlights another important problem, which is that a Platinum-derivative drug may react with competing molecules other than nucleobases before it reaches the target site, ie tumor DNA.
Proteins
Another group that may react with Platinum-derivative drugs are proteins.31,152,227–229 The main binding sites are sulfur-containing amino acids, such as methionine and histidine. Nitrogen atoms located on Lysine amino acids are also the site of binding to Platinum-derived drugs. Proteins can form adducts with more than one Pt-containing fragment or coordinating protein, and the number of bounds increases with time.31,152,227–229
Cisplatin, Carboplatin and Oxaliplatin were tested in this context, and the studies proved that they bind to proteins, mainly albumin, in the bloodstream by 98%, 25%, and 98%, respectively. Of which, 98% of the administered cisplatin and 87% of Oxaliplatin were irreversibly bound to proteins. This means that cisplatin binds practically irreversibly to proteins, while Oxaliplatin binds mostly irreversibly. However, in the case of Carboplatin, only 10% of the administered dose binds irreversibly, which means that if Carboplatin is already bound to proteins, it binds mainly reversibly.230
Other Structures
Drugs containing Platinum, due to their low selectivity towards nucleic acids, may interact with numerous other structures that contain a nitrogen or sulfur atom with a lone electron pair.57,198–200,231 These substances may come from medications, food, or be synthesized in the body.232 The influence of such substances may reduce therapeutic effects, as well as associated side effects.233 Understanding these interactions, that is which substances and to what extent bind with Platinum drugs, can help adjust medical doses to maintain their optimal effect.
The use of nanostructures in the anticancer treatment of lung cancers seems to be a good solution. For example, the creation of nanomedicine should reduce the high toxicity of Platinum-derivative compounds by not only repeatedly reducing the doses of the chemotherapy drug, but also by directing the drug directly to the cancer site, while bypassing healthy human cells. Moreover, nanomedicine solves the problem of the chemotherapy drug binding to amino acids during distribution in the peripheral blood and binding to other structures similar to nitrogenous bases at the cellular level.
Nanostructures
When Richard Feynman introduced the concept of nanotechnology in 1959,234–238 he did not think it would be one of the most promising technologies in medicine of the twenty-first century. Using nanotechnology improves diagnosis, treatment, and prevention and enables people to understand the human body.239–253 Nanostructures can also lead to the improvement of drugs. Application of nanostructures can remove the disadvantages of drugs and simultaneously highlight their advantages. To reduce the side effects of some drugs and increase the effectiveness of the treatment, nanostructures have been used for drug delivery. Because nanostructures are molecules whose external dimensions are in the range of 1 nm–100 nm151,254–256 (see Figure 8), they have a much larger surface area than their volume, which makes them perfect drug carriers.
 |
Figure 8 The 1 Å – 1 cm scale. The scale provides the size of exemplary objects, corresponding to a given dimension on the scale.151,254–256 |
This allows the dosage of the drug to be limited to an absolute minimum, and thus reduces the toxic effect.249 The insides of nanostructures, eg empty fullerene cages, can also protect drug molecules from inactivation before reaching the target site. As we know, Platinum-derived compounds have low selectivity and react with many different compounds, structurally similar to purines containing an aromatic ring with lone pairs of electrons on the N7 atom.57,102,198–200 One of such groups are B vitamins, which are supplied, for example, with food.57,102,198–200
General Division of Nanostructures
Nanostructures are a wide range of different systems which, due to various criteria, can be divided into many classes.257–276
The first criterion is the number of dimensions at the nanoscale. If there is only one dimension, such nanostructures are called nanoplates.277 Whereas, when two of the three dimensions are present at the nanoscale, these structures are called nanofibers.278 When all dimensions are at the nanoscale, we talk about nanoparticles (liposomes, quantum dots, and fullerenes).151,254,277,279,280
Secondly, the classification can be based on their chemical composition and include organic, inorganic, and carbon-based nanostructures, as shown in Figure 9.
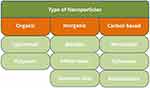 |
The third way of classifying nanostructures is the criterion of drug delivery technology, which includes biologic, polymeric, carbon-based, and metallic-based systems,286 as described in Table 4.
 |
Table 4 Nanostructure Classification by Drug Delivery Technology.286 |
Fate, Side Effects and Biodegradability of Nano Systems in vivo
Nanostructures with Platinum-derivative drugs are administered intravenously, increasing the accumulation of structures at the target site using targeting, which can be divided into active and passive.287 Passive targeting involves adding drugs to a specific area of nanostructures so that the drugs gain new pharmaceutical and physicochemical properties that result in increased accumulation at the tumor site.288,289 Active targeting, on the other hand, involves adding a nanostructure that is specifically targeted to a particular receptor or a biomarker located on target-specific cells on which drugs are to act. After reaching the target site, the drug is released, but the fate of the nanostructure does not end in this place. They have two most popular routes: intracellular degradation in endosomes and lysosomes, or they remain in the bloodstream and undergo opsonization and clearance in the process of phagocytosis.290 Phagocytosis most commonly takes place in the liver and spleen by macrophages, depending on individual properties such as surface properties or size.291,292 However, removal from the lungs takes place chemically and physically, eg in the area of the alveoli, absorbed by macrophages.293
The following side effects can be generated by nanoparticles:
- oxidative stress, which can cause inflammation, then genotoxicity, and finally apoptosis,
- genotoxicity, which may be limited to DNA strand breaks, chromosome fragmentation, the formation of oxidative adducts, and, consequently, changes in gene expression,
- enhancing cytokine production,
- damage to lysosomes and mitochondria,
- autophagy dysfunction.294
These effects depend on the properties (eg carbon nanotubes have negligible toxicity) and concentrations of specific compounds (eg lipid nanoparticles are toxic at a concentration of 500 μg/mL and nontoxic below 200 μg/mL.295,296
Nanomaterials as Carriers for Drugs in the Treatment of Lung Cancer
The use of nanocarriers in the treatment of lung cancer requires them to overcome the physiological and anatomical protective barrier of the lungs. Nanotechnology has changed the way in which nanocarriers are used in the treatment of lung cancer, which offers enormous opportunities for the future.289 Physicochemical properties such as size, shape, stiffness or surface properties are extremely important in determining the distribution capabilities of nanoparticles.297 Due to the fact that tumor blood vessels have a diameter of approximately 100–600 nm,190,194,298–305 nanoparticles with a size of 10–100 nm and a molecular weight above 50 kDa are ideal nanoparticle for drug delivery systems.289 Moreover, nanoparticles smaller than 150 nm can avoid uptake by macrophages in the RES.306 Because nanoparticles are larger than 10 nm, they can also avoid capillary leakage.307
A general distinction is made between organic and non-organic nanoparticles (NPs) in the treatment of lung cancer.289 In the first (organic) group, one can include:
- solid lipid NPs
- liposome-cholesterol and phospholipid-like biofilm-like NPs,
- nanostructured lipid carriers-NPs mixed with solid lipids and liquid lipids,
- polymeric micelles-colloidal NPs composed of amphiphilic block copolymers,
- polymeric NPs composed of polymers such as sodium alginate, chitosan, gelatin, polycaprolactone, polylactide, and polylactic acid,
- dendrimer-highly branched, symmetrical, radiating NPs.
The second one (non-organic) encompasses:
- carbon nanotubes-hydrophobic tubular structures made by carbon atoms with diameters between 4 nm and 100 mm,
- magnetic NPs-superparamagnetic materials with a size > 25 nm,
- quantum dots-colloidal NPs with atomic properties.308
There are many examples of clinically used nano- drugs in the treatment of lung cancer in the literature. These examples are presented in Tables 5 and 6. In the Table 5 nanomedicines contain Pt drugs, while nano-drugs built from other compounds than Platinum are presented in Table 6.
 |
Table 5 Nanodrugs Used in Lung Cancer Containing Platinum Compounds309,310,316 |
 |
Table 6 Nanodrugs Used in Lung Cancer Containing Other Than Platinum Compounds.312 |
Nanoparticles can have passive or active anti-cancer properties. Among the first, there are three techniques, but only two of them have been used in anti-cancer treatment in the human body. In the first case, the acidic microenvironment of tumors is used to limit the action of NPs to acidic conditions.289 In the second one, because NPs are positively charged, the tumor cells can be used to carry additional negative charges.313 Active targeting gives the effect of precise targeting by placing ligands on the surface of the nanoparticle, thanks to which it is possible to attach, for example, enzymes that recognize receptors in the blood vessels of the tumor.265,270,314,315 Bazak at al.,316 showed that in some cases, receptors and ligands help inhibit tumor drug resistance by facilitating endocytosis. Various types of ligands can be found in the literature, such as polymers,265,270,314,315 small molecules, fragments, antibodies and aptamers (APTs).311,317–319 In the treatment of lung cancer, numerous receptors have been used that have the ability to increase the specific binding of a nanoparticle containing a drug (Figure 10):320
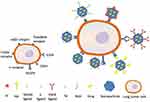 |
Figure 10 Lung cancer with cell receptors used as targets (right side of the Figure) and the nanoparticles with anticancer drugs and with a specific ligands structure (left side of the Figure and bottom of the drawing) used in targeted anticancer therapy.288,289,321 |
- Epidermal Growth Factor Receptor (EGFR) due to its structure322 plays a crucial role in the advancement of carcinoma. There is an external and internal fragment of tyrosine kinase activity. An extracellular section includes the ligand-binding area responsible for the regulation of tumor growth (invasion, angiogenesis, metastasis, and proliferation of cells). The binding of the ligand to the attachment region causes conformational modifications. Further, the binding of ligands and the activity of tyrosine kinase lead to autophosphorylation that triggers alterations in the signaling.323–328
- Growth Hormone Receptor (GHR) is a single-pass transmembrane receptor with at least one cytokine receptor homology domain (CRHD) in its extracellular portion. The CRHD has two fibronectin III (FNIII)-like folds. GHR overexpression has been identified in A549 NSCLC and other related cancers.288,329,330
- Folate acid (FA) Receptor, the folate receptors demonstrate a greater binding affinity for folic acid, which is composed of four forms of the receptor: Folate Receptor Alpha (FRA), Folate Receptor Beta (FRB), Folate Receptor Gamma (FRG) and Folate Receptor Delta (FRD). Folate Receptor Alpha (FRA) glycosylphosphatidylinositol-anchored glycoprotein placed on the cell surface, referred to as FOLR-1 or folate binding protein (FBP), assists in the delivery of 5-methyltetrahydrofolate (5-MTF). Overexpression of the FRA has been observed in lung-like solid tumor types, but several studies have reported elevated levels of overexpressed FRA in NSCLC.288,331–340
- Vascular Endothelial Growth Factor Receptors (VEGFR), vascular permeability factor, leadind to vascular leakage. There are three major types of tyrosine kinase receptors, namely FLT-1 (VEGFR1), FLK-1 (VEGFR2), and FLT-4 (VEGFR3), which show the ability to bind only with mammalian VEGFR. These receptors are frequently overexpressed in NSCLC and possess an acknowledged significance in the angiogenesis, proliferation, and metastasis of tumor cells.341–345
- Fibroblast Growth Factor Receptor (FGFR), the extracellular component, made up of immunoglobulin resembling domains with affinity for the FGF ligand, consists of a transmembrane region, and posses tyrosine kinase activity. Unusual signaling of FGF/FGFR, amplification or alterations of genes (oncogenic) may contribute to tumorigenesis and therapy resistance in various cancers such as solid tumors and malignant melanoma. Elevated expression of FGFR1 is reported in NSCLC with varying ratios in squamous cell carcinomas and lung adenocarcinomas.346–350
- σ receptor, membrane-bound protein sigma receptors, σ1 (sigma-1) and σ2 (sigma-2). Overexpression of σ2 receptors showed six out of 15 human adenocarcinoma samples and twelve out of 15 human SCLC samples.351–362
- Human transferrin (TF) Receptor, an iron-binding protein to transfer iron absorbed via the digestive tract and iron released by red blood cell degradation into bone marrow in the form of the TFR-Fe3+ complex to generate mature red blood cells and transporting iron into cells that express TFRs.363 TFR is expressed at a modest level in all normal nucleated cells. The ligature shows that expression level is higher in cells with a high proliferation rate, especially in tumor cells, such as chronic lymphocytic leukemia, liver cancer and lung cancer.364–371 Also, low molar concentration of artemisinin after pretreatment could kill the cells in SCLC. Thymoquinone-NP modified transferrin enhances the apoptosis and death cascade of NSCLC cells, and limits these migration cells without a toxicity effect.372
- αvβ3 Integrin, consists of non-covalently linked alpha and beta subunits and belong to the transmembrane heterodimeric glycoprotein family. There are 24 distinct integrin receptors, which depending on the orientation patterns, exhibit between of 18 α and 8 β subunits. Integrins are detected in almost 82% of all NSCLC patients, regardless of differentiation type or degree. However, 13% of SCLCs demonstrated an overexpressed α3β1. Downregulated levels of integrin expression have been related to SCLC, as well as the disease’s high severity and potential to spread.289,373–375
- CD44, membrane-bound glycoprotein, plays an important role in malignant tumor-related activities used as a cancer stem cell (CSC)/tumor-initiating cells (TIC) marker.376 CD44 has overexpressed in solid tumors, such as pancreatic cancer, breast cancer, and lung cancer.377–379 The receptor is involved in many signal cascades that mediate tumor enhancement and can indirectly activate cell proliferation pathways through ligands and activate anti-apoptotic pathways.380 In the case of SCLC, activation of CD44-MAPK-PI3K signal transduction results in increased invasiveness and multi-drug resistance phenotype of lung cancer cells.
A factor promoting the use of receptors in combination with a nanostructure in the treatment of lung cancer is the fact that the receptors themselves are significantly overexpressed only in cancer cells.381 Therefore, their use brings benefits in the form of reducing side effects and improving the effectiveness of treatment. For example, the σ receptor induces320 cell death only in cancer tissue, but never in unaffected (stem) cells. However, TFR, while showing high expression, also shows high histological specificity. Its expression in lung adenocarcinoma is much higher than in other histological types.289 Since TF plays, an important role in the abnormal iron metabolism of lung cancer cells, the iron uptake by lung cancer cells can be inhibited through this receptor, which will ultimately affect the proliferation of pathological cells. It has also been shown that FR activity is higher in diseased cells compared to physiological cells.289
In the case of nanomedicine treatment, the route of administration may be different, not only through injections into the peripheral blood. A new direction in the development of nanostructures for the treatment of lung cancer is the use of drug delivery by inhalation. This allows to reduce the dose of the drug without weakening the effect because the local concentration will be appropriate, and moreover, such a kind of application could limit the absorption into the bloodstream and thus reduce the side effects.374 The nanostructures used for inhalation are polymers and lipids, which increase bioavailability, stability and stay longer at the target site. The key requirements for a nanostructure to be used in inhalants are its size, from 10 to 100 nm, based on an animal model, and its surface-active properties, which are intended to prevent phagocytosis by macrophages.382 Animal studies have confirmed that in the case of inhalation, the accumulation of drugs in target sites is higher than when using the intravenous route.383,384 What is more, such application reduces concentrations in the liver, spleen and kidneys compared to intravenous administration.385
Examples of Applications of Nanostructures with Platinum Compounds
There are many examples of nanostructures combined with Platinum drugs in the literature. Platinum compounds, due to their low selectivity and increased resistance, are often combined with nanostructures. In the area of organic nanostructures, the following combinations with Platinum-derived drugs can be distinguished:
while inorganic nanostructures are represented by
- dendrimers,403–405
and among the representatives of the carbon-based ones, there are
The wide variety of nanostructures means that Platinum-based drugs can be located both on the surface of nanostructures, eg fullerenes and nanotubes, and inside, eg liposomes and nanotubes. These structures can, like liposomes, fuse with the cell membrane and release chemotherapeutics directly into the cell, or they can disintegrate in the acidic environment located near the tumor and be released there (polymer ProLindac403,422). Among the nanostructures transporting Platinum, both active (antibodies) and passive (nanotube (cis,cis,trans-Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H) and SWNCT406,407) targeting can be found.
This diversity makes it difficult to describe in detail all nanostructures combined with Platinum-derived drugs, apart from the general facts that nanostructures increase stability, protect Platinum-derived drugs against inactivation in the blood, increase accumulation in cancer tissues, and reduce side effects, while increasing selectivity.
These combinations are widely described in the literature, and in situ, in vitro, and in vivo studies, as well as clinical trials are very promising. Unfortunately, they have not yet been introduced to the market, but when they are, they will probably significantly improve the survival rate and quality of life of patients.
Protein Nanotubes
Protein nanotubes are nanotubes made of peptide and/or protein fragments that have been connected, for example, by self-assembly or by creating a pulsed electric field. Compared to classic nanotubes, they are more biocompatible and biodegradable. Moreover, they can undergo surface and internal modifications, enabling the effective and controlled release of substances.423 According to in vivo mouse studies, protein nanotubes from the plant virus TMV (tobacco mosaic virus) in combination with Cisplatin are an effective method in the treatment of ovarian cancer. The nanostructure consists of TMV monomers, in which each monomer is composed of 2130 identical copies of a coat protein, and each coat protein has two glutamic acids. TMVs were taken from Nicotiana benthamiana plants, multiplied, and formed into 300×18 nm nanotubes that can accommodate cisplatin molecules in a 4 nm wide empty channel. What is important to emphasize is that TMV is safe for humans because it does not infect mammals. The results of the study prove that highly distributed TMV-cisPt showed better effectiveness and accumulation within tumors than free Cisplatin.386 The plant virus TMV in combination with Cisplatin was tested in mice using Alanine Transaminase (ALT), Aspartate Aminotransferase (AST) (both are indicators of liver injury), and Kidney Injury Molecule 1 (KIM-1) assays. Mice were administered solutions containing Cisplatin alone, TMV- cisPt, TMV in Phosphate Buffered Saline (PBS) and a control containing only PBS in three injections on days 7, 10 and 14, and toxicity was examined on days 15 and 19 after tumor implantation. As shown by the results of the ALT test, all results were within the norm, unlike the AST tests, where on day 15, the TMV- cisPt, indicated potential hepatotoxicity, but on day 19, the results were normal. On the other hand, no renal toxicity was reported.386
Liposomes
Liposomes are bilayer delivery vehicles made of phospholipids and an aqueous core84,388,394–396 (Figure 11).
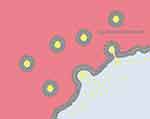 |
Figure 11 The scheme of liposomes.394 |
These nanostructures have a wide spectrum of use, because they can be applied to both hydrophilic and hydrophobic drugs. Liposomal delivery of small-molecule drugs has presented many advantages, such as the highest local drug concentration, reduced toxicity, and better LADME (liberation, absorption, distribution, metabolism, and excretion). Natural phospholipids possess low toxicity and weak immunogenicity. Disadvantages include rapid circulation clearance, a low half-life time, and worse penetration of such structures.
Currently, in clinical trials, there is Lipoplatin (9% Cisplatin and 91% lipid), the phospholipids’ bilayer of which contains dipalmitoylphos-phatidylglycerol, soy phosphatidylcholine-3, and DSPE-PEG 2000 cholesterol. However, Lipoplatin, instead of using copper receptors, delivers cisplatin by fusion with the cell membrane and releases cisplatin into the cell, which avoids membrane resistance. Inside the cell, Cisplatin activates mitochondria and other pathways leading to apoptosis. In preclinical studies on rodents, this combination had lower toxicity than normal Cisplatin. The main side effects reported were neutropenia and gastrointestinal toxicity, but unlike Cisplatin, hepatotoxicity, neuropathy, cardiotoxicity, ototoxicity, and hair loss were not reported. The use of Liposomal deserves special mention because of its significantly reduced nephrotoxicity, which is a major Cisplatin dose limit marker. Furthermore, compared with normal tissues, it was much higher than Cisplatin. The accumulation of lipoplatin in cancerous tissues was higher than in healthy tissues, up to 221 times higher for colon tumors; additionally, Lipoplatin reacted more often, causing cell damage 10 to 171 times more often in cancer cells than in healthy cells. Prospectively, it may significantly reduce side effects by improving selectivity. In the second phase of trials, Lipoplatin was combined with gemcitabine and used in the treatment of patients who were resistant to Platinum chemotherapy. The European Medicines Agency gave Lipoplatin an orphan drug status for treatment of pancreatic adenocarcinoma275,279–282,284,285,312,313.
Another liposomal drug with Cisplatin is SPI-77 (cisplatin: lipids ratio is circa 1:70), the bilayer of which consists of hydrogenated soy phosphatidylcholine, cholesterol, and DSPE-PEG200. The results of the first phase of clinical trials showed much fewer side effects compared with Cisplatin. Despite the fact that accumulation in tumor tissues was four times greater, antitumor activity was the same or less.388,398
The pH-sensitive liposomal formulation for Cisplatin is SpHL-CDDP, which consists of dioleoylphosphatidylethanolamine, unsaturated phosphatidylethanolamine, cholesteryl hemisuccinate, and DSPE-PEG 2000. Unsaturated phosphatidylethanolamine improves the release into the cell, whereas cholesteryl hemisuccinate gives properties of rapid release at acidic pH because, when the pH reaches acid values, it induces a destabilization formulation and releases Cisplatin. The survival rate in the ascitic tumor was much higher after using SpHL-CDDP than Cisplatin.389,395
The first combination of liposomes with Oxaliplatin is Aroplatin (drug: lipids ratio is 1:15), which consists of dimyristoylphosphatidylcholine and dimyristoyl phosphatidylglycerol. In the second clinical study, the response was good, though there was a problem with drug distribution and some tissues were not exposed to the Aroplatin.390
The next combination of Oxaliplatin is Lipoxal, which in the first clinical study phase had high effectiveness for gastrointestinal cancer and lower toxicity compared with Oxaliplatin.84
Nanobodies
In 1993, a heavy-chain antibody was discovered which, unlike regular antibodies, was naturally deficient in light chains.424 The structure of a heavy chain antibody includes two constant regions, ie a hinge region and a heavy chain variable domain, which, when isolated, give us nanobodies. Nanobodies are artificially obtained single-domain antibody molecules. They have an oval shape with dimensions of 4 nm × 2.5 nm × 3 nm and a molecular weight of 12–14 kDa (compared to ordinary antibodies (150 kDa) is approximately 1/10 of the molecular weight of conventional antibodies).
Compared to regular antibodies, a nanobody is characterized by lower immunogenicity, better tissue penetration, and higher stability. However, they have a shorter half-life. From a manufacturing standpoint, they are easier to modify and cheaper to produce. They also demonstrate high targeting ability.
Types of nanobodies include monovalent nanobodies, bivalent nanobodies, bispecific nanobodies, multivalent nanobodies, and nanobodies fused with other fragments, for instance Platinum drugs (Figure 12). Most nanobodies are currently in the clinical phase, but nanobodies are starting to enter the market and are used, for example, in the treatment of thrombotic thrombocytopenic purpura and multiple myeloma. Envafolimab is used in solid cancers, including non-small cell lung cancer, because, like Durvalumab and Atezolizumab (Supplementary Materials, Table S1-S2), it targets the protein programmed cell death-ligand 1 (PD-L1).425–429 An example of a combination of a nanobody with a Platinum drug is the combination of a biparatopic anti-EGFR nanobody (N, 7D12-9G8) synthesized by oxidizing cisplatin (maleimide-functionalized Pt(IV) prodrug [Pt(NH3)2Cl2(OH)(OAc)]). The drug accumulation is clearly higher in the EGFR-positive A431 cells than in the EGFR-negative A375 cells. Cisplatin showed strong toxicity, in contrast to the nanobodies themselves, which showed very low cytotoxicity; the survival of EGFR-positive cells was approximately 75%, and for EGFR-negative cells, it was over 90%. In the case of combining a nanobody with a Platinum-derivative drug, the cytotoxicity to EGFR-positive cells was high - survival rate was only 15%, while the cytotoxicity to EGFR-negative cells was very low and similar to the cytotoxicity of nanobodies alone (survival rate of over 90%), which proves that the drug conjugate nanobody with Platinum has much lower systemic toxicity than cisplatin. Moreover, nanobody conjugation improves pharmacokinetics and increases drug retention, which leads to an increased therapeutic effect.401
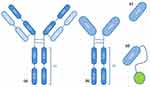 |
Figure 12 The scheme of (a) conventional, (b) heavy-chai antibodies, regular antibodies (c) and nanobodies fused with other fragments (d).424 |
Polymers
The third group of nanostructures connected with Platinum drugs are hydrophilic polymers. The most common polymer for anticancer drugs is N-methacrylamide (HPMA), which enhances therapeutic efficacy and decreases side effects. Furthermore, the connection of drugs with HPMA increases accumulation in tumor tissues.234
AP5280 is a combination of Cisplatin and HPMA (mass ratio 1:10). These results of these in vivo studies show a 19-fold higher accumulation in tumors and a 20-fold lower toxicity, as well as promising efficacy.430
ProLindac (AP5346) contains dichloro(1,2-diaminocyclohexane)Platinum(II) (DACH-Pt) and a hydrophilic polymer. This combination is stable at physiological pH and remains inactive, but at acidic pH, which is nearby the tumor surroundings (presumably the tumor extracellular fluid or the interior of the tumor cell), releases the Platinum drug. ProLindac presented a 16-fold better delivery to the tumor and, what is worth highlighting, a 14-fold one to tumor DNA. For this reason, the anticancer activity of this combination is much better, and the side effects are much lower in comparison with Cisplatin.429 In the first phase of clinical trials, dose-limiting neutropenia occurred only at the highest dose, and the main side effects included typical nausea and vomiting, and renal toxicity. However, these effects can be prevented by properly preparing the patient for chemotherapy by giving him appropriate antiemetics and hydrating him. These results correspond to the results of Phase 2 clinical trials and correspond to the frequency and severity of expected side effects of Platinum-based drugs.431
An innovative method is to combine Cisplatin with PLGA-block-polyethylene glycol and with the epidermal growth factor receptor. This method turned out to be effective in triple-negative breast cancer. Using epidermal growth factor receptors improves selectivity and therapeutic efficacy compared to untargeted and free Platinum drugs.43
AP5280 consists of a cytotoxic Pt complex combined with a water-soluble, biocompatible, non-toxic polymer backbone consisting of poly-N-(2-hydroxypropyl)methacrylamide. The platinum content by weight is approximately 8.5%. As indicated by Phase I studies, the use of nanostructure extends the residence time in the bloodstream and reduces renal toxicity and myelosuppression.430
Dendrimers
Another type of nanostructures are dendrimers. They contain in their center a small molecule or a polymer core, from which numerous side chains branch off, which sequentially branch again (Figure 13). Multiple end groups allow the conjugation of various molecules in the core or at the surface, for instance, Platinum drugs. Drugs connected with these structures are characterized by lengthened half-life, higher stability, selectivity, water solubility, tumor accumulation, as well as decreased immunogenicity, antigenicity, and toxicity.404,405,432 The preclinical research on complexes of Cisplatin and Dendrimers began as early as 1999.431,432
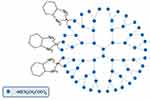 |
The complex of Cisplatin and poly(amidoamine) dendrimer containing a carboxylate surface group acted against cisplatin-resistant (squamous) cancer, causing a decrease in toxicity and an increase in accumulation in tumor tissues through increased permeability and retention effect.404,405,435,436
Carbon Nanotubes
Carbon Nanotubes (CNTs) were discovered by a Japanese physicist Sumio Iijiima in 1991.437 They are universal nanostructures with a wide range of applications resulting from unique properties, such as high resistance to stretching, high conductivity of heat and electricity, low weight, low toxicity, and a high aspect ratio, which increases the ability to penetrate into the cell. In fact, it is assumed that nanotubes pierce the cell membrane like needles. In addition, CNTs enter the cell through the membrane via endocytic pathways. Furthermore, they are characterized by greater accumulation in cancerous tissues than in normal tissues.25,34,116,117,161,162,395,438,439
Due to their structure and huge surface area, they have excellent abilities as drug transporters. This means that the drug can be placed outside the nanostructure using covalent and non-covalent bonds, as well as inside, where it does not require the modification of its structure by adding further bonds. The center has a higher and favorable binding energy in the direction of absorption, which means that the CNT-drug bonds are not needed. Importantly, hiding the drug inside protects it to a greater extent against interactions with external substances, other drugs, deactivating agents, or the unfavorable influence of the environment itself. Due to the type of structure, we divide them into single-walled (SWCNT) and multi-walled nanotubes (MWCNT).440,441
Various combinations of Platinum compounds and nanotubes can be found in the literature. Firstly, an in vitro study was presented in which Platinum(IV) complex cis,cis,trans-Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)] and SWNCT have been non-covalently functionalized with an amine-ended PL-PEG chain (Figure 14).
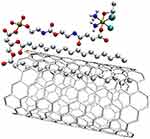 |
Figure 14 The structure of Platinum(IV) complex cis,cis,trans-Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)] with SWNCT, functionalized with PL-PEG.438 |
As indicated by studies, this complex enters the cell by clathrin-dependent endocytosis and, thanks to the reducing environment of endosomes, releases Platinum compounds inside the cell. Drug release at low pH is facilitated by the loss of the axial properties of the ligand by which it is linked to SWNCT. This is an extremely important property, considering that many cancer tumors have a low pH. As research confirms, the addition of a nanotube increased the concentration in cancer cells 6–8 times compared to the free complex, and was 2 times higher than the Cisplatin used. This proves the effectiveness of using SWNCTs as transporters for Platinum compounds.406 The second in vitro study is a modification and extension of the tumor cells that highly overexpress the folate receptor (Figure 15). In the studies, these complexes were 8.6 times more effective against cancer cells than Cisplatin.407
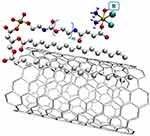 |
Figure 15 The structure being a modification and extension of the Platinum(IV) complex cis,cis,trans-Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)] with SWNCT, functionalized with PL-PEG, in which additional molecules were added to the complex.442 Symbol R means the targeted molecule containing folic acid (vitamin B9) with the structural formula (O2CCH2CH2CONH-PEG-FA). |
Another study describes a complex hydrophobic cisplatin drug, which is a derivative of cisplatin with two benzoic groups attached, and MWCNT (Figure 16). The in vitro studies confirmed the stable entrapment of Platinum derivative particles in the nanotube and effective therapy in ovarian carcinoma cells.411
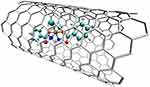 |
Figure 16 The structure of a complex of hydrophobic cisplatin drugs with two benzoic groups attached and MWCNT.411 |
Another example is a three-element complex consisting of: Cisplatin, SWCNT, and epidermal growth factor (EGF) (Figure 17).
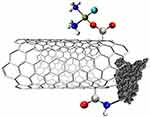 |
Figure 17 The structure of a complex of Cisplatin, SWCNT, and epidermal growth factor (EGF).443 |
To reduce the low selectivity and direct the complex towards squamous cancer, EGF was added to the CNT and Cisplatin complexes. Without reducing the effectiveness of the therapy, it targets cells overexpressing EGFR, such as squamous cancer cells. This results in greater accumulation in these cells and a longer duration of action, which is confirmed by both in vitro and in vivo studies.412,413 The next study describes Cisplatin molecules inside SWCNT (Figure 18). The results of this study demonstrated that the Cisplatin complex in the nanotube reduced side effects by inhibiting non-specific, non-selective absorption compared to free Cisplatin. However, the complex was not as effective as free Cisplatin.414,415
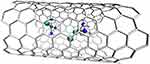 |
Figure 18 The structure of a complex of Cisplatin molecules inside SWCNT.444–446 |
An interesting form seems to be the “Carbon Nanotube Bottle” complex, consisting of Cisplatin with MWCNT and Gold Caps (Figure 19).440
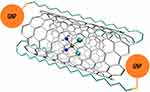 |
Figure 19 The structure of a complex of Cisplatin with MWCNT and Gold Caps.440 The abbreviation GNP represents gold nanoparticles. |
The study was designed so that the caps were made of alkanethiols combined with gold nanoparticles (GNP), protecting the open ends and protecting Cisplatin from a premature release. Only at low pH, ie the pH that occurs within the tumor, would it open and release the active substance. However, this significantly limited drug release once it reached the tumor, as in vitro studies showed that GNPs were end-blocked and approximately 40% of cisplatin was not released within the first hour.438 Another in vitro test was the complex of Cisplatin with ultrashort SWCNT and Pluronic F108 (Figure 20).416–418
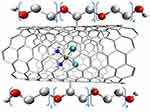 |
Figure 20 The structure of a complex of Cisplatin with ultrashort SWCNT and Pluronic F108.416–418 |
Pluronic-F108 is a neutrally charged, non-cytotoxic surfactant commonly used for release control in carbon nanotubes. This ternary complex showed increased cytotoxicity against breast cancer cell lines after 24 hours compared to free Cisplatin.416 The eighth and final in vitro study presents us this time with a complex consisting of Carboplatin and oxidized open-ended MWCN (Figure 21).
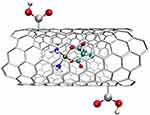 |
Figure 21 The structure of a complex of Carboplatin and oxidized open-ended MWCN.416,448,449 |
The results confirmed the lack of toxicity of the nanostructure and the effective, long-term release of Carboplatin particles, showing a maximum Platinum release of 68% on the fourteenth day. The results also showed higher cytotoxicity of compounds in nanostructures than free Carboplatin towards prostate, renal, and bladder cancer cell lines.450
Fullerenes
Fullerenes are allotropic forms of carbon, and their structure consists of sp2 carbons connected by single and double bonds. Their structure resembles a symmetrical cage of various sizes and shapes. The most popular fullerene is fullerene C60 (Figure 22), which consists of 12 pentagons and 20 hexagons, and resembles a sphere in shape.45
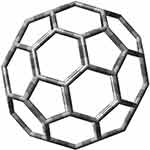 |
Figure 22 The structure of fullerene C60.452 |
Fullerenes properties resemble hydrophobic compounds, for which they have a high affinity. Additionally, thanks to these properties, they have good penetration through cell membranes and significant biological activity.453 Fullerenes also easily react with electron-rich molecules and are able to bind other compounds using physical and chemical bonds or electrostatic interactions.410,454,455 The structure of fullerenes can be modified to increase their binding capacity.453 The specific structure allows them to bypass traditional drug resistance mechanisms.456 As shown by in vivo studies (Figure 23), fullerenes do not exhibit toxicity while maintaining strong antioxidant properties as free radical scavengers.408 C60 fullerene derivatives penetrate plasma membranes and concentrate inside cancer cells, which makes them suitable for the transport of Platinum-derived drugs.119,120,132,185,239,258,409,415,419
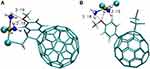 |
Figure 23 The examples of complex of functionalized fullerene C60 with Cisplatin.420 |
In vitro studies have shown that C60 fullerene nanoparticles in complexes do not cause DNA strand breaks while maintaining the cytotoxic properties of Cisplatin.408 Other studies have shown a greater cytotoxic effect of the complex compound on lung cancer cells compared to free Cisplatin, as demonstrated by IC 50 values, although lower after treatment for 48 and 72 hours.410 The nano complex also promotes cell penetration and accumulation inside cancer cells, and thus intensifies the toxic effect inside cancer cells, consequently leading to apoptosis.410 Studies on T-lymphoblastic leukemia cells showed that the fullerene complex with Cisplatin induces a statistically significant increase in the number of dead cells compared to Cisplatin alone.409
Rhombellans
Rhombellans are new theoretical nanostructures described for the first time in 2018 by Diudea, characterized by the fact that they are built in such a way that all strong rings are rhombuses, and some of them create propellant structures (tricyclo[1.1.1.01,3]pentane).457,458 They are synthesized from [1,1,1] propellane, which contains only triangles.265 As Diudea describes, Rhombellans have the following properties:
- all strong rings are rhombuses;
- vertex classes consist only of unconnected vertices;
- the Omega polynomial has one term: 1X^|E|;
- the line graph of the parent graph has a Hamiltonian perimeter;
- they contain at least one complete bipartite subgraph K2.3 or the smallest rbl.5 rbl.459
Quantum calculations at the B3LYP/6-31G level of theory confirmed that the syntheses are energetically feasible.459 Studies on the properties of ADME (absorption, distribution, metabolism, and excretion) confirmed the properties of Rhombellanes as nano transporters delivering to the target site.261,263,265,270,275,421
A comparative study on the structure of C60 fullerenes and Rhombellanes shows a significantly higher affinity of both Cisplatin420 and Carboplatin419 to fullerenes than to Rhombellanes (Figure 24). Even though Platinum-derived compounds formed more bonds with Rhombellanes, they were of lower quality.419 However, the affinity of Platinum derivatives is so high that they can form complexes with both fullerenes and Rhombellanes. Comparing Cisplatin and Carboplatin, the affinity values for Carboplatin are much higher. As a result, Carboplatin can easily form complexes with these nanostructures.421
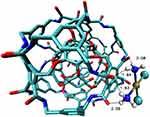 |
Figure 24 The structure of a complex of Rhombellane with Cisplatin.420 |
Others Nanostructures
Micelles
Micelles are colloidal nanoparticles that are formed in an aqueous environment using amphiphilic copolymers or surfactants when the critical micellar concentration is exceeded.289,458 Micelles are excellent drug carriers because, due to their smaller size below 100 nm in diameter, they actively avoid RES and renal exclusion. They can even passively increase the penetration of the endothelial barrier in the area dominated by the tumor. Hydrophobic drugs become trapped in the innermost central region, resulting in increased water solubility, reduced toxicity profile, tumor cell-specific aggregation, and reversal of drug resistance, ultimately improving the therapeutic efficacy and bioavailability of poorly water-soluble drugs. Additionally, circulation time can be extended by adding a hydrophilic coating that prevents vesicular uptake.461
Hydrogels
Hydrogels are a three-dimensional polymer mesh that retains a significant amount of water in its fibers.462,463 It is a system of a binary polymer solution that has a cross-linking agent. Hydrogels have gained much attention over the past three decades because they possess various unique features that are useful in biomedical applications. At room temperature, hydrogels exist as a solution, but when they reach body temperature, they transform into a gel. The main mechanism of the anticancer gel form is the creation of a drug reservoir from which the drug gradually penetrates. Hydrogels inhibit the formation of cancer, including lung cancer, and at the same time extend the life of the body.464
Nanoemulsions
Nanoemulsions are water-in-oil (w/o) or oil-in-water (o/w) droplets with an average radius of 10–100 nm that are translucent or transparent and exhibit a stable thermodynamic profile. This nanostructure is created by dispersing two immiscible phases: the water phase in the oil phase, and is maintained by reducing the interfacial tension using various surfactants and co-surfactants.465 This structure makes nanoemulsions excellent nanocarriers for hydrophobic and lipophilic chemotherapeutics. In addition, nanoemulsion helps in reducing hepatic bypass, inhibits drug degradation in an abnormal environment, eliminates P-glycoprotein efflux, facilitates penetration of mucous membranes, and thus improves the systemic availability of chemotherapy.466
Quantum Dots (QDs)
These are the smallest of all nanostructures (3–30 nm) made of semiconductor materials. QDs can be modified by coating with polymers that improve solubility and absorption.467 The biggest problem associated with the clinical use of QDs is the potential cytotoxicity.468 This problem is according with their structure which contains inducing cytotoxicity metal atom.469 QDs have been used in molecular profiling and cellular imaging of cancer.470 As proven by in vitro studies on lung cancer cell lines, conjugating Erlotinib with QD improves its effectiveness.471 Additionally, QDs can be modified to control drug release in an acidic pH environment.472
Metal NPs (Nanoparticles)
Between structures contain metal atoms, used in oncology are iron nanoparticles,321,473 titanium dioxide nanoparticles,329 zinc oxide nanoparticles,474 cerium nanoparticles,475 silver nanoparticles476 or gold nanoparticles. The latter, due to their unique optical and surface properties, high biocompatibility, are easy to modify during synthetize and can be coated with a large number of molecules, including drugs used in chemotherapy.477–479
Magnetic Nanostructures
Nanostructures that can be detected and manipulated using magnetic fields. Most often, they have sizes ranging from 10–200 nm and a neutral surface charge, which is needed to extend the circulation time.480,481 In recent years, they have been used in diagnosis and treatment of lung cancers.482–484 However, they exhibit high toxicity. Magnetic nanostructures can interfere with cellular metabolism and cause side effects by producing reactive oxygen species and increasing for example the concentration of free iron by metabolizing iron oxide nanoparticles.381,485
Conclusions
To sum up, Platinum compounds have been approved for treatment since 1978, and despite the fact that knowledge about these drugs has grown significantly and many newer anticancer drugs and various anticancer therapies have entered the market, virtually unchanged Platinum compounds have not fallen out of use and are still among the most popular compounds used in certain types of cancer. However, numerous problems associated with therapy with Platinum derivatives have been noticed. Firstly, increasing resistance to Platinum-derived compounds, and secondly, high toxicity resulting from low selectivity. The most popular solutions to these problems are complex therapy, targeted therapy, and conjugation of Platinum derivatives with nanostructures. Theoretical research, as well as in vitro and in vivo studies, on nanostructures are optimistic and prove that in the future we will be able to allow new, better forms of Platinum-derivative drugs for treatment, which will accumulate better at the site of the tumor, cause fewer side effects for the body, and limit the phenomenon of resistance. The presence of nanostructures can protect Platinum-derived drugs from reacting with substances in the bloodstream (eg originating from food and drugs, such as B vitamins or proteins), thus reducing the therapeutic effect. However, despite numerous studies on single complexes of nanostructures, there is still a lack of comparative studies that would compare different complexes of Platinum compounds and nanostructures under the same conditions.
Funding
Publication of this article (Article Processing Charge) was financially supported by the Doctoral School of Medical and Faculty of Pharmacy, Collegium Medicum, Nicolaus Copernicus University, Jagiellońska 13, 85-067 Bydgoszcz, Poland.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Available from: https://www.who.int/health-topics/cancer#tab=tab_1.
2. Available from: https://www.cancer.net/navigating-cancer-care/cancer-basics/what-metastasis.
3. Bartusik-Aebisher D, Serafin I, Dynarowicz K, Aebisher D. Photodynamic therapy and associated targeting methods for treatment of brain cancer. Front Pharmacol. 2023;14:1250699. doi:10.3389/fphar.2023.1250699
4. Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402–418. doi:10.1016/J.APSB.2015.07.005
5. Dillekås H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8(12):5574–5576. doi:10.1002/cam4.2474
6. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncogen. 2013;18(1–2):43–73. doi:10.1615/critrevoncog.v18.i1-2.40
7. Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduction and Targeted Therapy. 2020;5(1):28. doi:10.1038/s41392-020-0134-x
8. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
9. Didkowska J, Wojciechowska U, Michalek IM, Caetano dos santos FL. Cancer incidence and mortality in Poland in 2019. Sci Rep. 2022;12(1):10875. doi:10.1038/s41598-022-14779-6
10. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer Jr Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
11. Types of cancer treatment - NCI. Available from: https://www.cancer.gov/about-cancer/treatment/types.
12. Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: veterans administration lung study group versus international association for the study of lung cancer--what limits limited disease? Lung Cancer. 2002;37(3):271–276. doi:10.1016/s0169-5002(02)00072-7
13. Meijer -J-J, Leonetti A, Airò G, et al. Small cell lung cancer: novel treatments beyond immunotherapy. Seminars in Cancer Biology. 2022;86(Pt 2):376–385. doi:10.1016/j.semcancer.2022.05.004
14. Lemjabbar-Alaoui H, Hassan OU, Yang Y-W, Buchanan P. Lung cancer: biology and treatment options. Biochimi Biophysica Acta. 2015;1856(2):189–210. doi:10.1016/j.bbcan.2015.08.002
15. PDQ Adult Treatment Editorial Board. Non-small cell lung cancer treatment(PDQ®): Health Professional Version.; 2002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17873159.
16. Clark SB, Alsubait S Non–small cell lung cancer. StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31912902.
17. Debela DT, Muzazu SG, Heraro KD, et al. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med. 2021;9:20503121211034370. doi:10.1177/20503121211034366
18. Jin H, Liao S, Yao F, et al. Insight into the Crosstalk between Photodynamic Therapy and Immunotherapy in Breast Cancer. Cancers. 2023;15(5). doi:10.3390/cancers15051532
19. Fang T, Xiao J, Zhang Y, Hu H, Zhu Y, Cheng Y. Combined with interventional therapy, immunotherapy can create a new outlook for tumor treatment. Quantitative Imaging in Medicine and Surgery. 2021;11(6):2837–2860. doi:10.21037/qims-20-173
20. Dua K. Advanced Drug Delivery Systems in the Management of Cancer. Academic Press; 2021.
21. Kauffman GB, Pentimalli R, Doldi S, Hall MD. Michele Peyrone (1813–1883), Discoverer of Cisplatin. Plat Met Rev. 2010;54(4):250–256. doi:10.1595/147106710X534326
22. Peyrone M. Ueber die einwirkung des ammoniaks auf platinchlorür” [On the action of ammonia on platinum chloride]. Justus Liebigs Annalen der Chemie. 1844;51(1):1–29.
23. Rosenberg B, Van Camp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205(4972):698–699. doi:10.1038/205698a0
24. Ehrhart NP, Ryan SD, Fan TM. Tumors of the Skeletal System. In: Withrow and MacEwen’s Small Animal Clinical Oncology. Elsevier; 2013:463–503. doi:10.1016/B978-1-4377-2362-5.00024-4
25. Zhou -N-N, Zhao -Y-Y, Zhai L-Z, et al. The efficacy and toxicity of lobaplatin-contained chemotherapy in extensive-stage small-cell lung cancer. J Cancer. 2018;9(13):2232–2236. doi:10.7150/jca.24557
26. Lobaplatin (D-19466) | 99.23%(HPLC) | in Stock | DNA alkylator chemical. Available from: https://www.selleckchem.com/products/lobaplatin.html.
27. Chen B, Zhang H, Chen R, et al. Lobaplatin for the treatment of SK-MES-1 lung squamous cell line in vitro and in vivo. OncoTargets and Therapy. 2016;9:4215–4224. doi:10.2147/OTT.S108032
28. Tsvetkova D, Ivanova S. Application of approved cisplatin derivatives in combination therapy against different cancer diseases. Molecules. 2022;27(8):2466. doi:10.3390/molecules27082466
29. Lippert B, ed.. Cisplatin. Zürich: Verlag Helvetica Chimica Acta; 1999. doi:10.1002/9783906390420
30. Kopacz-Bednarska A, Król T. Cisplatin — properties and clinical application. Oncol Clin Pract. 2022;18(3):166–176. doi:10.5603/OCP.2022.0020
31. Balasco N, Ferraro G, Loreto D, Iacobucci I, Monti M, Merlino A. Cisplatin binding to β-lactoglobulin: a structural study. Dalton Transactions. 2020;49(35):12450–12457. doi:10.1039/D0DT02582H
32. Bearcroft CP, Domizio P, Mourad FH, André EA, Farthing MJ. Cisplatin impairs fluid and electrolyte absorption in rat small intestine: a role for 5-hydroxytryptamine. Gut. 1999;44(2):174–179. doi:10.1136/gut.44.2.174
33. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
34. Zoń A, Bednarek I. Cisplatin in ovarian cancer treatment—known limitations in therapy force new solutions. Int J Mol Sci. 2023;24(8):7585. doi:10.3390/ijms24087585
35. Goren MP. Cisplatin nephrotoxicity affects magnesium and calcium metabolism. Medical and Pediatric Oncology. 2003;41(3):186–189. doi:10.1002/mpo.10335
36. Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018. doi:10.1007/s40620-017-0392-z
37. Tsang RY, Al-Fayea T, Au H-J. Cisplatin Overdose. Drug Safety. 2009;32(12):1109–1122. doi:10.2165/11316640-000000000-00000
38. Ho GY, Woodward N, Coward JIG. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Criti Rev Oncol/Hematol. 2016. doi:10.1016/j.critrevonc.2016.03.014
39. Harstrick A, Schmoll HJ, Wilke H, et al. Cisplatin, etoposide, and ifosfamide salvage therapy for refractory or relapsing germ cell carcinoma. J Clin Oncol. 1991;9(9):1549–1555. doi:10.1200/JCO.1991.9.9.1549
40. Ghosh SC. The first metal based anticancer drug. Bioorganic Chemistry. 2019;88:102925. doi:10.1016/j.bioorg.2019.102925
41. Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiology and Oncology. 2019;53(2):148–158. doi:10.2478/raon-2019-0018
42. Riddell IA, Lippard SJ. Cisplatin and oxaliplatin: our current understanding of their actions. Metallo-Drugs. 2018. doi:10.1515/9783110470734-001
43. Florea A-M, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi:10.3390/cancers3011351
44. Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Translat Med. 2018;16(1):96. doi:10.1186/s12967-018-1471-1
45. Ivanov AI, Christodoulou J, Parkinson JA, et al. Cisplatin binding sites on human albumin. J Biol Chem. 1998;273(24):14721–14730.
46. Spiegel K, Rothlisberger U, Carloni P. Cisplatin binding to dna oligomers from hybrid car-parrinello/molecular dynamics simulations. J Phys Chem B. 2004;108(8):2699–2707. doi:10.1021/jp036230s
47. Messori L, Merlino A. Cisplatin binding to proteins: a structural perspective. Coord Chem Rev. 2016;315:67–89. doi:10.1016/J.CCR.2016.01.010
48. de Sousa GF, Wlodarczyk SR, Monteiro GC. Molecular mechanisms of action associated with chemoresistance. Braz J Pharma Sci. 2014;50(4):693–702. doi:10.1590/S1984-82502014000400004
49. Frizziero M, Spada F, Lamarca A, et al. Carboplatin in combination with oral or intravenous etoposide for extra-pulmonary, poorly-differentiated neuroendocrine carcinomas. Neuroendocrinology. 2019;109(2):100–112. doi:10.1159/000497336
50. Potolidis E, Mandros C. Carboplatin-induced hypersensitivity reaction. Case Rep Oncol. 2012;5(1):141–142. doi:10.1159/000337578
51. What is Carboplatin? Available from: https://www.news-medical.net/health/What-is-Carboplatin.aspx.
52. History of Carboplatin - Chemotherapy - Mesothelioma. Available from: https://www.usmesotheliomalaw.com/treatment-for-mesothelioma/carboplatin/history-of-carboplatin/.
53. PA V, GC J, G A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Nat Cancer Inst. 2004;96(22). doi:10.1093/JNCI/DJH323
54. Carboplatin. National institute of diabetes and digestive and kidney diseases; 2012. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3079484.
55. Lokich J, Anderson N. Carboplatin versus cisplatin in solid tumors: an analysis of the literature. Annal Oncol. 1998;9(1):13–21. doi:10.1023/a:1008215213739
56. Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol. 1993;50(2):147–158. doi:10.1006/gyno.1993.1184
57. Szefler B, Czeleń P, Krawczyk P. The affinity of carboplatin to b-vitamins and nucleobases. Int J Mol Sci. 2021;22(7):3634. doi:10.3390/ijms22073634
58. Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14(5):1291–1295. doi:10.1158/1078-0432.CCR-07-2238
59. Dilruba S, Kalayda GV. Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol. 2016;77(6):1103–1124. doi:10.1007/s00280-016-2976-z
60. Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev Oncol/Hematol. 2002;42(3):317–325.
61. Van Zyl B, Tang D, Bowden NA. Biomarkers of platinum resistance in ovarian cancer: what can we use to improve treatment. Endoc Cancer. 2018. doi:10.1530/ERC-17-0336
62. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–584. doi:10.1038/nrc2167
63. Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Prac. 2011;2011:1–5. doi:10.1155/2011/843019
64. Abu-Sbeih H, Mallepally N, Goldstein R, et al. Gastrointestinal toxic effects in patients with cancer receiving platinum-based therapy. Journal of Cancer. 2020;11(11):3144–3150. doi:10.7150/jca.37777
65. Farrell N, Kelland LR. Platinum-Based Drugs in Cancer Therapy. Humana Press; 2000.
66. Zhou J, Kang Y, Chen L, et al. The drug-resistance mechanisms of five platinum-based antitumor agents. Front Pharmacol. 2020;11:497006. doi:10.3389/fphar.2020.00343
67. Wang H, Zhu X, Huang J, Chen P, Han S, Yan X. Nedaplatin sensitization of cisplatin-resistant human non-small cell lung cancer cells. Oncol Lett. 2016;11(4):2566–2572. doi:10.3892/ol.2016.4276
68. Shimada M, Kigawa J, Itamochi. Nedaplatin: a cisplatin derivative in cancer chemotherapy. Cancer Manag Res. 2013;67. doi:10.2147/CMAR.S35785
69. Niioka T, Uno T, Yasui-Furukori N, et al. Pharmacokinetics of low-dose nedaplatin and validation of AUC prediction in patients with non-small-cell lung carcinoma. Cancer Chemother Pharmacol. 2007;59(5):575–580. doi:10.1007/s00280-006-0298-2
70. Kuwahara A, Yamamori M, Nishiguchi K, et al. Replacement of cisplatin with nedaplatin in a definitive 5-fluorouracil/cisplatin-based chemoradiotherapy in Japanese patients with esophageal squamous cell carcinoma. Int J Med Sci. 2009;6(6):305–311. doi:10.7150/ijms.6.305
71. Shimada M, Itamochi H, Kigawa J. CMAR-35785-nedaplatin----a-cisplatin-derviative-in-cancer-chemotherapy. Cancer Manage Res. 2013;5:67–76. doi:10.2147/CMAR.S35785
72. Baldwin J. FDA evaluating oxaliplatin for advanced colorectal cancer treatment. Cancer Spectrum Know Environ. 2002;94(16):1191–1193. doi:10.1093/jnci/94.16.1191
73. Cao Y, Xia Y, Wang Y, Shi H, Wu Y, Lu Y. MgIG attenuates oxaliplatin-induced hepatotoxicity through suppression of connexin 43 in hepatic stellate cells. J Clin Translat Hepatol. 2022;000(000):000–000. doi:10.14218/JCTH.2022.00048
74. Comella P, Casaretti R, Sandomenico C, Avallone A, Franco L. Role of oxaliplatin in the treatment of colorectal cancer. Ther Clin Risk Manage. 2009;5(1):229–238. doi:10.2147/tcrm.s3583
75. Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol. 2011;18(1):18–25. doi:10.3747/co.v18i1.708
76. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. New Eng J Med. 2008;358(1):36–46.
77. Pieck AC, Drescher A, Wiesmann KG, et al. Oxaliplatin-DNA adduct formation in white blood cells of cancer patients. Br J Cancer. 2008;98(12):1959–1965. doi:10.1038/sj.bjc.6604387
78. Seetharam RN. Oxaliplatin: preclinical perspectives on the mechanisms of action, response and resistance. ecancermedicalscience. 2010. doi:10.3332/ecancer.2009.153
79. Cvitkovic E. Ongoing and unsaid on oxaliplatin: the hope. Br J Cancer. 1998;77(Suppl 4):8–11. doi:10.1038/bjc.1998.429
80. Danyelle T. Oxaliplatin. In: XPharm: The Comprehensive Pharmacology Reference. Elsevier; 2007:1–4. doi:10.1016/B978-008055232-3.62973-3
81. Alian OM, Azmi AS, Mohammad RM. Network insights on oxaliplatin anti-cancer mechanisms. Clin Transl Med. 2012;1:1–26.
82. Woynarowski JM, Faivre S, Herzig MCS, et al. Oxaliplatin-induced damage of cellular DNA. Mol Pharmacol. 2000. doi:10.1124/mol.58.5.920
83. Moskovitz M, Wollner M, Haim N. Oxaliplatin-induced pulmonary toxicity in gastrointestinal malignancies: two case reports and review of the literature. Case Rep Oncol Med. 2015;2015:1–5. doi:10.1155/2015/341064
84. STATHOPOULOS GP, BOULIKAS T, KOURVETARIS A, STATHOPOULOS J. Liposomal oxaliplatin in the treatment of advanced cancer: a phase I study. Anti Res. 2006;26(2B). Available from: https://ar.iiarjournals.org/content/26/2B/1489.short.
85. Shimolina L, Gulin A, Ignatova N, et al. The role of plasma membrane viscosity in the response and resistance of cancer cells to oxaliplatin. Cancers. 2021;13(24). doi:10.3390/cancers13246165
86. Singh T, Kang DH, Kim TW, et al. Intracellular delivery of oxaliplatin conjugate via cell penetrating peptide for the treatment of colorectal carcinoma in vitro and in vivo. Int J Pharm. 2021;606:120904. doi:10.1016/J.IJPHARM.2021.120904
87. Joybari AY, Sarbaz S, Azadeh P, et al. Oxaliplatin-induced renal tubular vacuolization. Ann Pharmacother. 2014;48(6):796–800. doi:10.1177/1060028014526160
88. Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit Rev Oncol/Hematol. 2005. doi:10.1016/j.critrevonc.2004.08.008
89. Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. Br J Pharmacol. 2005. doi:10.1038/sj.bjp.0706407
90. Gebremedhn EG, Shortland PJ, Mahns DA. The incidence of acute oxaliplatin-induced neuropathy and its impact on treatment in the first cycle: a systematic review. BMC Cancer. 2018. doi:10.1186/s12885-018-4185-0
91. Culy CR, Clemett D, Wiseman LR. Oxaliplatin: a review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies. Drugs. 2000. doi:10.2165/00003495-200060040-00005
92. Yamada S, Yazawa M, Yamamoto M, et al. A case of biopsy-proven oxaliplatin-induced acute tubulointerstitial nephritis with thrombocytopenia and anemia. CEN Case Reports. 2019;8(3):188–193. doi:10.1007/s13730-019-00390-8
93. Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem Pharmacol. 1996. doi:10.1016/S0006-2952(97)81490-6
94. Faivre S, Chan D, Salinas R, Woynarowska B, Woynarowski JM. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem Pharmacol. 2003. doi:10.1016/S0006-2952(03)00260-0
95. Pasetto LM, D’Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol/Hematol. 2006. doi:10.1016/j.critrevonc.2006.01.001
96. Graham MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res. 2000; 6(4):1205–1218.
97. Bécouarn Y, Ychou M, Ducreux M, et al. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. digestive group of french federation of cancer centers. J Clin Oncol. 1998. doi:10.1200/jco.1998.16.8.2739
98. Lévi F, Metzger G, Massari C, Milano GO. Pharmacokinetics and chronopharmacological aspects. Clin Pharma. 2000. doi:10.2165/00003088-200038010-00001
99. Wiseman LR, Adkins JC, Plosker GL, Goa KL. Oxaliplatin: a review of its use in the management of metastatic colorectal cancer. Drugs Aging. 1999. doi:10.2165/00002512-199914060-00006
100. Riccardi A, Ferlini C, Meco D, Mastrangelo R, Scambia G, Riccardi R. Antitumour activity of oxaliplatin in neuroblastoma cell lines. Eur J Cancer. 1999. doi:10.1016/S0959-8049(98)00342-6
101. Martinez-Balibrea E, Martínez-Cardús A, Ginés A, et al. Tumor-related molecular mechanisms of oxaliplatin resistance. Mol Cancer Ther. 2015;14(8):1767–1776. doi:10.1158/1535-7163.MCT-14-0636
102. Szefler B, Czeleń P, Wojtkowiak K, Jezierska A. Affinities to oxaliplatin: vitamins from b group vs. Nucleobases. 2022;23(18):10567. doi:10.3390/ijms231810567
103. Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Semi Oncol. 2002. doi:10.1016/S0093-7754(02)90016-3
104. O’Dowd PD, Sutcliffe DF, Griffith DM. Oxaliplatin and its derivatives – an overview. Coord Chem Rev. 2023;497:215439. doi:10.1016/j.ccr.2023.215439
105. American Association for Cancer Research. MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E Clinical cancer research: an official journal of the american association for cancer research. Vol 6. Association for Cancer Research; 1995. Available from: https://aacrjournals.org/clincancerres/article/6/4/1205/288165/Clinical-Pharmacokinetics-of-Oxaliplatin-A.
106. Larson CA, Blair BG, Safaei R, Howell SB. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol. 2009;75(2):324–330. doi:10.1124/mol.108.052381
107. Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77(6):887–894. doi:10.1124/mol.109.063172
108. Extra JM, Marty M, Brienza S, Misset JL. Pharmacokinetics and safety profile of oxaliplatin. Semi Oncol. 1998;25(2 SUPPL. 5):13–22.
109. Di Francesco AM, Ruggiero A, Riccardi R. Cellular and molecular aspects of drugs of the future: oxaliplatin. Cell Mol Life Sci. 2002;59(11):1914–1927. doi:10.1007/PL00012514
110. Bruno PM, Liu Y, Park GY, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. 2017;23(4):461–471. doi:10.1038/nm.4291
111. Han’guk Imsang Yakhakhoe MS, Lim SC, Choi SO, Lee BK, Lee MK. Han’guk Imsang Yakhakhoe Chi. Vol 16. Han’guk Imsang Yakhakhoe; 2006. Available from: https://www.ekjcp.org/journal/view.html?spage=131&volume=16&number=2.
112. Ahn J-H, Kang Y-K, Kim T-W, et al. Nephrotoxicity of heptaplatin: a randomized comparison with cisplatin in advanced gastric cancer. Cancer Chemother Pharmacol. 2002;50(2):104–110. doi:10.1007/s00280-002-0483-x
113. Choi C-H, Cha Y-J, C-S A, et al. Molecular mechanisms of heptaplatin effective against cisplatin-resistant cancer cell lines: less involvement of metallothionein. Cancer Cell Int. 2004;4(1):6. doi:10.1186/1475-2867-4-6
114. Liu W, Jiang J, Xie C, et al. Synthesis, anticancer activity and toxicity of a water-soluble 4S,5S-derivative of heptaplatin, cis-{Pt(II)[(4S,5S)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane]·(3-hydroxyl-cyclobutane-1,1-dicarboxylate)}. J Inorg Biochem. 2014;140:126–130. doi:10.1016/J.JINORGBIO.2014.07.013
115. Jiang C, Zhang Y, Xu X, Su S, Pan H, Jiang A. The inhibitory effects of lobaplatin, or in combination with gemcitabine on triple-negative breast cancer cells in vitro and in vivo. Oncologie. 2023;25(1):81–91. doi:10.1515/oncologie-2023-0026
116. Yu Q, Lan T, Ma Z, et al. Lobaplatin induces apoptosis in T24 and 5637 bladder cancer cells by regulating Bcl-2 and Bax expression and inhibiting the PI3K/Akt signaling pathway. Translat Androl Urol. 2023;12(8):1296–1307. doi:10.21037/tau-23-376
117. Zhou Z, Jiang H, Xia J, Zhang J. Comparison of the therapeutic effects of lobaplatin and carboplatin on retinoblastoma in vitro and in vivo. Int J Oncol. 2020;57(3):697–706. doi:10.3892/ijo.2020.5085
118. Yu Q, Lan T, Ma Z, et al. Lobaplatin. Drugs in R & D. 2003;4(6):369–372. doi:10.2165/00126839-200304060-00008
119. Basumallik N, Agarwal M Small Cell Lung Cancer. StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22363907.
120. PDQ Adult Treatment Editorial Board. Small Cell Lung Cancer Treatment (PDQ®): patient Version; 2002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26389478.
121. Spreckelmeyer S, Orvig C, Casini A. Cellular transport mechanisms of cytotoxic metallodrugs: an overview beyond cisplatin. Molecules. 2014;19(10):15584–15610. doi:10.3390/molecules191015584
122. Kuo MT, Chen HHW, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer and Metastasis Reviews. 2007. doi:10.1007/s10555-007-9045-3
123. Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RHJ, Wiemer EAC. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011;14(1):22–34. doi:10.1016/j.drup.2010.12.002
124. Di Pasqua AJ, Goodisman J, Dabrowiak JC. Understanding how the platinum anticancer drug carboplatin works: from the bottle to the cell. Inorg Chim Acta. 2012;389:29–35. doi:10.1016/J.ICA.2012.01.028
125. Zheng Y-R, Suntharalingam K, Johnstone TC, et al. Pt(IV) prodrugs designed to bind non-covalently to human serum albumin for drug delivery. J Am Chem Soc. 2014;136(24):8790–8798. doi:10.1021/ja5038269
126. Xie X, Yu T, Li X, et al. Recent advances in targeting the “undruggable” proteins: from drug discovery to clinical trials. Sig Transd Targ Ther. 2023;8(1):335. doi:10.1038/s41392-023-01589-z
127. Kang M, Kim SY, An SSA, Ju YR. Characterizing affinity epitopes between prion protein and β-amyloid using an epitope mapping immunoassay. Experiment Mol Med. 2013;45(8):e34. doi:10.1038/emm.2013.63
128. Boike L, Henning NJ, Nomura DK. Advances in covalent drug discovery. Nat Rev Drug Discovery. 2022;21(12):881–898. doi:10.1038/s41573-022-00542-z
129. Potęga A. Glutathione-mediated conjugation of anticancer drugs: an overview of reaction mechanisms and biological significance for drug detoxification and bioactivation. Molecules. 2022;27(16):5252. doi:10.3390/molecules27165252
130. Anufrieva NV, Morozova EA, Kulikova VV, et al. Sulfoxides, analogues of l-methionine and l-cysteine as pro-drugs against gram-positive and gram-negative bacteria. Acta Nat. 2015;7(4):128–135.
131. Silaghi-Dumitrescu R, Bischin C. Platinum-containing anticancer drugs and proteins, interaction. In: Encyclopedia of Metalloproteins. New York: Springer New York;2013:1742–1748. doi:10.1007/978-1-4614-1533-6_532
132. Soldatović T, Bugarčić ŽD. Study of the reactions between platinum(II) complexes and l-methionine in the presence and absence of 5′-GMP. J Inorg Biochem. 2005;99(7):1472–1479. doi:10.1016/J.JINORGBIO.2005.04.005
133. Selçuki N A, Coşkun E, Biçer E. Combined computational and experimental studies on cysteine-sulfadiazine adduct formation. Turk J Chem. 2020;44(2):502–517. doi:10.3906/kim-1908-62
134. Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Design, Development and Therapy. 2016;10:1885–1895. doi:10.2147/DDDT.S106412
135. Dasari S, Njiki S, Mbemi A, Yedjou CG, Tchounwou PB. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int J Mol Sci. 2022;23(3):1532. doi:10.3390/ijms23031532
136. Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol. 2001;59(4):657–663. doi:10.1124/mol.59.4.657
137. Gibb RK, Taylor DD, Wan T, O’Connor DM, Doering DL, Gerçel-Taylor Ç. Apoptosis as a measure of chemosensitivity to cisplatin and taxol therapy in ovarian cancer cell lines. Gynecologic Oncology. 1997;65(1):13–22. doi:10.1006/GYNO.1997.4637
138. CREGAN IL, Dharmarajan AM, SA FOX. Mechanisms of cisplatin-induced cell death in malignant mesothelioma cells: role of inhibitor of apoptosis proteins (IAPs) and caspases. Int J Oncol. 2013;42(2):444–452. doi:10.3892/ijo.2012.1715
139. Rathinam R, Ghosh S, Neumann W, Jamesdaniel S. Cisplatin-induced apoptosis in auditory, renal, and neuronal cells is associated with nitration and downregulation of LMO4. Cell Death Discovery. 2015;1(1):15052. doi:10.1038/cddiscovery.2015.52
140. Nizami ZN, Aburawi HE, Semlali A, Muhammad K, Iratni R. Oxidative Stress Inducers in Cancer Therapy: preclinical and Clinical Evidence. Antioxidants. 2023;12(6):1159. doi:10.3390/antiox12061159
141. Mirzaei S, Hushmandi K, Zabolian A, et al. Elucidating role of reactive oxygen species (ros) in cisplatin chemotherapy: a focus on molecular pathways and possible therapeutic strategies. Molecules. 2021;26(8). doi:10.3390/molecules26082382
142. Coverdale JPC, Bridgewater HE, Song J-I, et al. In vivo selectivity and localization of reactive oxygen species (ros) induction by osmium anticancer complexes that circumvent platinum resistance. J Med Chem. 2018;61(20):9246–9255. doi:10.1021/acs.jmedchem.8b00958
143. Nakamura H, Takada K. Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 2021;112(10):3945–3952. doi:10.1111/cas.15068
144. Kleih M, Böpple K, Dong M, et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death & Disease. 2019;10(11):851. doi:10.1038/s41419-019-2081-4
145. Gunes S, He Z, van Acken D, Malone R, Cullen PJ, Curtin JF. Platinum nanoparticles inhibit intracellular ROS generation and protect against cold atmospheric plasma-induced cytotoxicity. Nanomedicine. 2021;36:102436. doi:10.1016/J.NANO.2021.102436
146. Hu X, Jiang Z, Teng L, et al. Platinum-induced peripheral neuropathy (pipn): ros-related mechanism, therapeutic agents, and nanosystems. Front Mol Biosci. 2021;8:770808. doi:10.3389/fmolb.2021.770808
147. Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17(6):574–583. doi:10.1016/j.ccr.2010.04.011
148. Yamasaki M, Makino T, Masuzawa T, et al. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br J Cancer. 2011;104(4):707–713. doi:10.1038/sj.bjc.6606071
149. Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275–283. doi:10.1038/nrm2147
150. Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi:10.1038/onc.2011.384
151. Joudeh N, Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol. 2022;20(1):262. doi:10.1186/s12951-022-01477-8
152. Hu G, Doruker P, Li H, Demet Akten E. Editorial: understanding protein dynamics, binding and allostery for drug design. Front Mol Biosci. 2021;8:681364. doi:10.3389/fmolb.2021.681364
153. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490–2518. doi:10.3390/toxins2112490
154. Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 2018;47(19):6645–6653. doi:10.1039/C8DT00838H
155. Waissbluth S, Daniel SJ. Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hearing Research. 2013;299:37–45. doi:10.1016/J.HEARES.2013.02.002
156. Solomon B, Colonna S. Recurrent cisplatin hypersensitivity reaction after first exposure: a case report. J Oncol Pharm Pract. 2019;25(2):481–483. doi:10.1177/1078155217735154
157. Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res. 2008;57(2):125–131. doi:10.1016/J.PHRS.2008.01.001
158. Rahman AA, Masango P, Stavely R, Bertrand P, Page A, Nurgali K. Oxaliplatin-induced damage to the gastric innervation: role in nausea and vomiting. Biomolecules. 2023;13(2):276. doi:10.3390/biom13020276
159. Lee CS, Ryan EJ, Doherty GA. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J Gastroenterol. 2014;20(14):3751–3761. doi:10.3748/wjg.v20.i14.3751
160. Hałka J, Spaleniak S, Kade G, Antosiewicz S, Sigorski D. The nephrotoxicity of drugs used in causal oncological therapies. Curr Oncol. 2022;29(12):9681–9694. doi:10.3390/curroncol29120760
161. Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin‐associated ototoxicity in pediatric oncology patients. Pediatric Blood & Cancer. 2012;59(1):144–148. doi:10.1002/pbc.24138
162. YILMAZ H, IRAZ M, SOGUT S, et al. The effects of erdosteine on the activities of some metabolic enzymes during cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2004;50(3):287–290. doi:10.1016/j.phrs.2004.03.003
163. Freepik | create great designs, faster. Available from: https://www.freepik.com/.
164. Chevreau C, Thomas F, Couteau C, Dalenc F, Mourey L, Chatelut E. Ototoxicity of high-dose carboplatin. J Clin Oncol. 2005;23(15):3649–3650. doi:10.1200/JCO.2005.05.348
165. Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 2019;32(8):1469–1486. doi:10.1021/acs.chemrestox.9b00204
166. Nematbakhsh M, Ashrafi F, Pezeshki Z, et al. A histopathological study of nephrotoxicity, hepatoxicity or testicular toxicity: which one is the first observation as side effect of Cisplatin-induced toxicity in animal model? J Nephropathol. 2012;1(3):190–193. doi:10.5812/nephropathol.8122
167. Tang Q, Wang X, Jin H, et al. Cisplatin-induced ototoxicity: updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm. 2021;163:60–71. doi:10.1016/J.EJPB.2021.03.008
168. Freyer DR, Brock PR, Chang KW, et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline. The Lancet Child & Adolescent Health. 2020;4(2):141–150. doi:10.1016/S2352-4642(19)30336-0
169. Tlili N, Feriani A, Allagui MS, Saadoui E, Khaldi A, Nasri N. Effects of Rhus tripartitum fruit extract on CCl 4 -induced hepatotoxicity and cisplatin-induced nephrotoxicity in rats. Canad J Physiol Pharmacol. 2016;94(8):801–807. doi:10.1139/cjpp-2016-0029
170. Park SB, Lin CS-Y, Kiernan MC. Nerve excitability assessment in chemotherapy-induced neurotoxicity. J JoVE. 2012. doi:10.3791/3439
171. Patanè S. Cardiotoxicity: cisplatin and long-term cancer survivors. Internat J Cardiol. 2014;175(1):201–202. doi:10.1016/j.ijcard.2014.04.238
172. Patanè S. Insights into cardio-oncology: the patient’s heavy cancer journey among doubts, controversies and pitfalls. The role of the cardiologist. Int J Cardiol. 2015;178:175–177. doi:10.1016/j.ijcard.2014.10.167
173. Choti MA. Chemotherapy-associated hepatotoxicity: do we need to be concerned? Annal Surg Oncol. 2009;16(9):2391–2394. doi:10.1245/s10434-009-0512-7
174. Schmitt NC, Rubel EW, Nathanson NM. Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29(12):3843–3851. doi:10.1523/JNEUROSCI.5842-08.2009
175. Kumar MNVR. Urolithin A nanoparticle therapy for cisplatin-induced acute kidney injury. Nephron. 2023;147(1):3–5. doi:10.1159/000524509
176. Ayyar P, Subramanian U. Repurposing – second life for drugs. Pharmacia. 2022;69(1):51–59. doi:10.3897/pharmacia.69.e72548
177. Low ZY, Farouk IA, Lal SK. Drug repositioning: new approaches and future prospects for life-debilitating diseases and the covid-19 pandemic outbreak. Viruses. 2020;12(9). doi:10.3390/v12091058
178. Alfonso-Triguero P, Lorenzo J, Candiota AP, Arús C, Ruiz-Molina D, Novio F. Platinum-based nanoformulations for glioblastoma treatment: the resurgence of platinum drugs? Nanomaterials. 2023;13(10). doi:10.3390/nano13101619
179. Zhang W, Taheri-Ledari R, Ganjali F, et al. Nanoscale bioconjugates: a review of the structural attributes of drug-loaded nanocarrier conjugates for selective cancer therapy. Heliyon. 2022;8(6):e09577. doi:10.1016/J.HELIYON.2022.E09577
180. Fang G, Zhang A, Zhu L, Wang Q, Sun F, Tang B. Nanocarriers containing platinum compounds for combination chemotherapy. Front Pharmacol. 2022;13:1050928. doi:10.3389/fphar.2022.1050928
181. Boulikas T. Clinical overview on Lipoplatin TM: a successful liposomal formulation of cisplatin. Expert Opinion on Investigational Drugs. 2009;18(8):1197–1218. doi:10.1517/13543780903114168
182. Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Materials. 2022;5(6):1593–1615. doi:10.1007/s42247-021-00335-x
183. Tian H, Zhang T, Qin S, et al. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J Hematol Oncol. 2022;15(1):132. doi:10.1186/s13045-022-01320-5
184. Song H, Kang X, Sun J, et al. Nanoparticle delivery of sterically hindered platinum(iv) prodrugs shows 100 times higher potency than that of cisplatin upon light activation. Chem Commun. 2016;52(11):2281–2283. doi:10.1039/C5CC09534D
185 Fronik P, Poetsch I, Kastner A, et al. Structure–activity relationships of triple-action platinum(iv) prodrugs with albumin-binding properties and immunomodulating ligands. J Med Chem. 2021;64(16):12132–12151. doi:10.1021/acs.jmedchem.1c00770
186. Chehelgerdi M, Chehelgerdi M, Allela OQB, et al. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22(1):169. doi:10.1186/s12943-023-01865-0
187. Zhong T, Yu J, Pan Y, Zhang N, Qi Y, Huang Y. Recent advances of platinum‐based anticancer complexes in combinational multimodal therapy. Adv Healthcare Mater. 2023;12(22):2300253. doi:10.1002/adhm.202300253
188. Zhang C, Xu C, Gao X, Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022;12(5):2115–2132. doi:10.7150/thno.69424
189. Abed A, Derakhshan M, Karimi M, et al. Platinum nanoparticles in biomedicine: preparation, anti-cancer activity, and drug delivery vehicles. Front Pharmacol. 2022;13:797804. doi:10.3389/fphar.2022.797804
190. J-X L, Huang J-M, Jiang Z-B, et al. Current clinical progress of pd-1/pd-l1 immunotherapy and potential combination treatment in non-small cell lung cancer. Int Cancer Ther. 2019;18:1534735419890020. doi:10.1177/1534735419890020
191. Liang J, Li M, Sui Q, et al. Compare the efficacy and safety of programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) inhibitors for advanced non-small cell lung cancer: a Bayesian analysis. Transl Lung Cancer Res. 2020;9(4):1302–1323. doi:10.21037/tlcr-20-192
192. Wu X, Gu Z, Chen Y, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–674. doi:10.1016/J.CSBJ.2019.03.006
193. Wu M, Huang Q, Xie Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15(1):24. doi:10.1186/s13045-022-01242-2
194. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:273409. doi:10.3389/fphar.2017.00561
195. Available from: https://www.mendeley.com/reference-manager/library/all-references/.
196. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi:10.1038/nrc706
197. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. New Eng J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
198. Szefler B, Czeleń P. Will the interactions of some platinum (ii)-based drugs with b-vitamins reduce their therapeutic effect in cancer patients? comparison of chemotherapeutic agents such as cisplatin, carboplatin and oxaliplatin—a review. Int J Mol Sci. 2023;24(2):1548. doi:10.3390/ijms24021548
199. Szefler B, Czeleń P, Kruszewski S, Siomek-Górecka A, Krawczyk P. The assessment of physicochemical properties of Cisplatin complexes with purines and vitamins B group. J Mol Graphics Modell. 2022;113:108144. doi:10.1016/J.JMGM.2022.108144
200. Szefler B, Czeleń P, Szczepanik A, Cysewski P. Does the affinity of cisplatin to b-vitamins impair the therapeutic effect in the case of patients with lung cancer-consuming carrot or beet juice? Anti-Cancer Agents in Medicinal Chemistry. 2019;19(14):1775–1783. doi:10.2174/1871520619666190325150624
201. HHL W, McDonnell T, Chinnadurai R. Physiological associations between vitamin b deficiency and diabetic kidney disease. Biomedicines. 2023;11(4):1153. doi:10.3390/biomedicines11041153
202. Tardy A-L, Pouteau E, Marquez D, Yilmaz C, Scholey A. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients. 2020;12(1):228. doi:10.3390/nu12010228
203. Parra M, Stahl S, Hellmann H. Vitamin B₆ and its role in cell metabolism and physiology. Cells. 2018;7(7). doi:10.3390/cells7070084
204. Blanco G, Blanco A Medical Biochemistry. Available from: https://www.sciencedirect.com/book/9780128035504/medical-biochemistry.
205. Schulman MP, Richert DA. HEME SYNTHESIS IN VITAMIN B6 AND PANTOTHENIC ACID DEFICIENCIES. J Biol Chem. 1957;226(1):181–189. doi:10.1016/S0021-9258(18)64819-7
206. Hammi C, Yeung B Neuropathy. StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24195230.
207. Ott M, Werneke U. Wernicke’s encephalopathy - from basic science to clinical practice. Part 1: understanding the role of thiamine. Ther Adv Psychopharmacol. 2020;10:2045125320978106. doi:10.1177/2045125320978106
208. Vasan S, Kumar A Wernicke Encephalopathy. StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30092713.
209. Smart M. The Vitamins: fundamental Aspects in Nutrition and Health
210. Vitamin B6 Deficiency and Dependency - Nutritional Disorders - MSD Manual Professional Edition. Available from: https://www.msdmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-b6-deficiency-and-dependency.
211. American Diabetes Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–69. doi:10.2337/dc10-S062
212. Combs GF The Vitamins: fundamental aspects in nutrition and health. Elsevier Academic Press; 2008. Available from: https://shop.elsevier.com/books/the-vitamins/combs-jr/978-0-12-802965-7.
213. Wilson RB. Pathophysiology, prevention, and treatment of beriberi after gastric surgery. Nutrition Reviews. 2020;78(12):1015–1029. doi:10.1093/nutrit/nuaa004
214. Fattal-Valevski A. Thiamine (Vitamin B 1). Journal of Evidence-Based Complementary & Alternative Medicine. 2011;16(1):12–20. doi:10.1177/1533210110392941
215. Shastak Y, Pelletier W. From metabolism to vitality: uncovering riboflavin’s importance in poultry nutrition. Ani. 2023;13(22). doi:10.3390/ani13223554
216. Rathee S, Nayak V, Singh KR, Ojha A. Nanofortification of vitamin B-complex in food matrix: need, regulations, and prospects. Food Chemistry: Molecular Sciences. 2022;4:100100. doi:10.1016/j.fochms.2022.100100
217. Perez-Castineira J. Chem Biochem Food. De Gruyter; 2020. doi:10.1515/9783110595482
218. Makarov MV, Trammell SAJ, Migaud ME. The chemistry of the vitamin B3 metabolome. Biochem Soc Transact. 2019;47(1):131–147. doi:10.1042/BST20180420
219. Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the central nervous system: an update of biological aspects and clinical applications. Int J Mol Sci. 2019;20(4). doi:10.3390/ijms20040974
220. Mascolo E, Vernì F. Vitamin B6 and diabetes: relationship and molecular mechanisms. Int J Mol Sci. 2020;21(10):3669. doi:10.3390/ijms21103669
221. Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98(7):5648–5652. doi:10.1063/1.464913
222. Kareem O, Nisar S, Tanvir M, Muzaffer U, Bader GN. Thiamine deficiency in pregnancy and lactation: implications and present perspectives. Front Nutrit. 2023;10:1080611. doi:10.3389/fnut.2023.1080611
223. Mrowicka M, Mrowicki J, Dragan G, Majsterek I. The importance of thiamine (vitamin B1) in humans. Biosci Rep. 2023;43(10). doi:10.1042/BSR20230374
224. Mahabadi N, Bhusal A, Banks SW Riboflavin Deficiency. StatPearls Publishing; 2024. Available from: https://pubmed.ncbi.nlm.nih.gov/29262062/.
225. Tisserand R, Young R. The digestive system. In: Essential Oil Safety. Elsevier; 2014:123–130. doi:10.1016/B978-0-443-06241-4.00009-6
226. Dhir S, Tarasenko M, Napoli E, Neurological GC. Psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Frontiers in Psychiatry. 2019;10:447129. doi:10.3389/fpsyt.2019.00207
227. Ju L, Dong J, Cruz MA, Zhu C. The N-terminal flanking region of the a1 domain regulates the force-dependent binding of von willebrand factor to platelet glycoprotein Ibα. Jo Biol Chem. 2013;288(45):32289–32301. doi:10.1074/jbc.M113.504001
228 Russo Krauss I, Ferraro G, Merlino A. Cisplatin–protein interactions: unexpected drug binding to n-terminal amine and lysine side chains. Inorg Chem. 2016;55(16):7814–7816. doi:10.1021/acs.inorgchem.6b01234
229. Monti DM, Loreto D, Iacobucci I, et al. Protein-based delivery systems for anticancer metallodrugs: structure and biological activity of the oxaliplatin/β-lactoglobulin adduct. Pharmaceuticals. 2022;15(4). doi:10.3390/ph15040425
230. Kato R, Sato T, Iwamoto A, et al. Interaction of platinum agents, cisplatin, carboplatin and oxaliplatin against albumin in vivo rats and in vitro study using inductively coupled plasma-mass spectrometory. Biopharmaceutics & Drug Disposition. 2019;40(7):242–249. doi:10.1002/bdd.2197
231. Hodgkinson E, Neville-Webbe HL, Coleman RE. Magnesium depletion in patients receiving cisplatin-based chemotherapy. Clin Oncol. 2006;18(9):710–718. doi:10.1016/J.CLON.2006.06.011
232. Zalecenia postępowania diagnostyczno-terapeutycznego w nowotworach złośliwych 2021 rok. Available from: http://onkologia.zalecenia.med.pl/.
233. Benoehr P, Krueth P, Bokemeyer C, Grenz A, Osswald H, Hartmann JT. Nephroprotection by theophylline in patients with cisplatin chemotherapy: a randomized, single-blinded, placebo-controlled trial. J Am Soc Nephrol. 2005;16(2):452–458. doi:10.1681/ASN.2004030225
234. Bensaude-Vincent B, Simon J. Introduction. nanotechnoscience: the end of the beginning. Philosophia Scientae. 2019;23(1):5–17. doi:10.4000/philosophiascientiae.1723
235. Toumey CP. Reading Feynman into nanotechnology. Techné: Research in Philosophy and Technology. 2008;12(3):133–168. doi:10.5840/techne20081231
236. Drexler KE. Nanotechnology: from Feynman to funding. Bull Sci Technol Soc. 2004;24(1):21–27. doi:10.1177/0270467604263113
237. Richard Feynman introduces the world to nanotechnology with two seminal lectures | open culture; 1984. Available from: https://www.openculture.com/2013/04/richard_feynman_introduces_the_world_to_nanotechnology.html.
238. Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The History of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25(1). doi:10.3390/molecules25010112
239. Ngowi EE, Wang Y-Z, Qian L, et al. The application of nanotechnology for the diagnosis and treatment of brain diseases and disorders. Front Bioeng Biotechnol. 2021;9:629832. doi:10.3389/fbioe.2021.629832
240. Thwala LN, Ndlovu SC, Mpofu KT, Lugongolo MY, Mthunzi-Kufa P. Nanotechnology-based diagnostics for diseases prevalent in developing countries: current advances in point-of-care tests. Nanomaterials. 2023;13(7). doi:10.3390/nano13071247
241. Azzawi M, Seifalian A, Ahmed W. Nanotechnology for the diagnosis and treatment of diseases. Nanomedicine. 2016;11(16):2025–2027. doi:10.2217/nnm-2016-8000
242. Malik S, Muhammad K, Waheed Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules. 2023;28(18):6624. doi:10.3390/molecules28186624
243. Kemp JA, Kwon YJ. Cancer nanotechnology: current status and perspectives. Nano Convergence. 2021;8(1):34. doi:10.1186/s40580-021-00282-7
244. Morales CS, Grodzinski P. Current landscape of treating different cancers using nanomedicines: trends and perspectives. WIREs Nanomedicine and Nanobiotechnology. 2024;16(1). doi:10.1002/wnan.1927
245. Kher C, Kumar S, Kher C, Kumar S. The Application of nanotechnology and nanomaterials in cancer diagnosis and treatment: a review. Cureus. 2022;14(9). doi:10.7759/cureus.29059
246. Alrushaid N, Khan FA, Al-Suhaimi EA, Elaissari A. nanotechnology in cancer diagnosis and treatment. Pharmaceutics. 2023;15(3):1025. doi:10.3390/pharmaceutics15031025
247. Dessale M, Mengistu G, Mengist HM. Nanotechnology: a promising approach for cancer diagnosis, therapeutics and theragnosis. Int J Nanomed. 2022;17:3735–3749. doi:10.2147/IJN.S378074
248 Jackson TC, Patani BO, Ekpa DE, Jackson TC, Patani BO, Ekpa DE. Nanotechnology in diagnosis: a review. Adv Nanopart. 2017;06(03):93–102. doi:10.4236/anp.2017.63008
249. Haleem A, Javaid M, Singh RP, Rab S, Suman R. Applications of nanotechnology in medical field: a brief review. Glob Health J. 2023;7(2):70–77. doi:10.1016/J.GLOHJ.2023.02.008
250. Xu Y, Luo C, Wang J, et al. Application of nanotechnology in the diagnosis and treatment of bladder cancer. J Nanobiotechnol. 2021;19(1):393. doi:10.1186/s12951-021-01104-y
251. Sim S, Wong N. Nanotechnology and its use in imaging and drug delivery (Review). Biomed Rep. 2021;14(5):42. doi:10.3892/br.2021.1418
252. Li J, Yao M, Shao Y, Yao D. The application of bio-nanotechnology in tumor diagnosis and treatment: a view. Nanotechnol Rev. 2018;7(3):257–266. doi:10.1515/ntrev-2018-0011
253. Deng S, Gu J, Jiang Z, et al. Application of nanotechnology in the early diagnosis and comprehensive treatment of gastrointestinal cancer. J Nanobiotechnol. 2022;20(1):415. doi:10.1186/s12951-022-01613-4
254. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi:10.1016/J.ARABJC.2017.05.011
255. Mekuye B, Abera B. Nanomaterials: an overview of synthesis, classification, characterization, and applications. Nano Select. 2023;4(8):486–501. doi:10.1002/nano.202300038
256. Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein Journal of Nanotechnology. 2018;9:1050–1074. doi:10.3762/bjnano.9.98
257. Czeleń P, Szefler B. The Immobilization of oxindole derivatives with use of cube rhombellane homeomorphs. Symmetry. 2019;11(7):900. doi:10.3390/sym11070900
258. Szefler B, Diudea MV. Spongy Nanostructures. J Nanosci Nanotechnol. 2017;17(1):323–328. doi:10.1166/jnn.2017.10859
259. Czeleń P. Investigation of the inhibition potential of new oxindole derivatives and assessment of their usefulness for targeted therapy. Symmetry. 2019;11(8):974. doi:10.3390/sym11080974
260. Szefler B. Docking linear ligands to glucose oxidase. Symmetry. 2019;11(7):901. doi:10.3390/sym11070901
261. Szefler B, Czeleń P. Docking of polyethylenimines derivatives on cube rhombellane functionalized homeomorphs. Symmetry. 2019;11(8):1048. doi:10.3390/sym11081048
262. Czeleń P, Szefler B. The Immobilization of chembl474807 molecules using different classes of nanostructures. Symmetry. 2019;11(8):980. doi:10.3390/sym11080980
263. Szefler B, Diudea MV. Quantum-mechanical calculations on molecular substructures involved in nanosystems. Molecules. 2014;19(10):15468–15506. doi:10.3390/molecules191015468
264. Diudea MV, Szefler B. Nanotube junctions and the genus of multi-tori. Phys Chem Chem Phys. 2012;14(22):8111–8115. doi:10.1039/c2cp40696a
265. Szefler B. Nano-structures as materials in biosciences. J Mol Struct. 2021;1224:129186. doi:10.1016/J.MOLSTRUC.2020.129186
266. Saheli M, Nagy K, Szefler B, Bucila V, Diudea MV. P-Type and related networks: design, energetics, and topology. Diamond and Related Nanostructures. 2013;141–170. doi:10.1007/978-94-007-6371-5_8
267. Nagy CL, Diudea MV. Diamond D5. Diam Rel Nanostruct; 2013:91–105. doi:10.1007/978-94-007-6371-5_5
268. Szefler B. On molecular dynamics of the diamond d5 substructures. Diamon Rel Nanostruc; 2013:121–139. doi:10.1007/978-94-007-6371-5_7
269. Szefler B, Diudea MV. Polybenzene revisited. Acta chim Slovenica. 2012;59(4):795–802.
270. Szefler B. Nanotechnology, from quantum mechanical calculations up to drug delivery. Int J Nanomed. 2018;13:6143–6176. doi:10.2147/IJN.S172907
271. Szefler B, Diudea M. Modeling tetrapodal nanotube junctions. Comput Methods Sci Technol. 2012;18(2):111–115. doi:10.12921/cmst.2012.18.02.111-115
272. Distance, symmetry, and topology in carbon nanomaterials - google książki. Available from: https://books.google.pl/books?id=IP7cDAAAQBAJ&pg=PR8&lpg=PR8&dq=nano+Szefler+Beata&source=bl&ots=TVgslc_eqc&sig=ACfU3U0WV65nqPxQNr-ATiFfD37BRrKjBQ&hl=pl&sa=X&ved=2ahUKEwjx65buibEAxUOX_EDHb3SCSc4FBDoAXoECAIQAw#v=onepage&q=nanoSzeflerBeata&f=false.
273. Diudea MV, Szefler B. Topology of C20 based spongy nanostructures. Comput Methods Sci Technol. 2015;21(2):65–68. doi:10.12921/cmst.2015.21.02.002
274. Szefler B, Diudea M. Polybenzene multitori. Open Chemistry. 2012;10(6):1779–1785. doi:10.2478/s11532-012-0113-3
275. Szefler B, Czeleń P, Diudea MV. Docking of Indolizine Derivatives on Cube Rhombellane Functionalized Homeomorphs. Studia Universitatis Babeș-Bolyai Chemia. 2018;63(2):7–18. doi:10.24193/subbchem.2018.2.01
276. Jäntschi L, Szefler B. Applied Designs in Chemical Structures with High Symmetry. MDPI; 2020. doi:10.3390/books978-3-03936-572-2
277. Njuguna J, Ansari F, Sachse S, Zhu H, Rodriguez VM. Nanomaterials, nanofillers, and nanocomposites: types and properties. Health and Environmental Safety of Nanomaterials. 2014; 2014:3–27. doi:10.1533/9780857096678.1.3
278. Garg T, Rath G, Goyal AK. Biomaterials-based nanofiber scaffold: targeted and controlled carrier for cell and drug delivery. J Drug Target. 2015;23(3):202–221. doi:10.3109/1061186X.2014.992899
279. Baig N, Kammakakam I, Falath W. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv. 2021;2(6):1821–1871. doi:10.1039/D0MA00807A
280. Nanotechnology. Available from: https://education.nationalgeographic.org/resource/nanotechnology/.
281. Huertas J-D, Fuentes Y-V, Garcia J-C, Bustos R-H. The role of education in nanomedicine as a current need for academic programs related to the healthcare field: a scoping review. Adv Med Educ Pract. 2024;15:65–74. doi:10.2147/AMEP.S431359
282. Jahanmahin A, Borji H. Nanotechnology-based Approaches for the Treatment of Toxocariasis: a Prospective Review. J Vet Physiol Pathol. 2023;2(2):12–19. doi:10.58803/jvpp.v2i2.24
283. Abaszadeh F, Ashoub MH, Khajouie G, Amiri M. Nanotechnology development in surgical applications: recent trends and developments. Eur J Med Res. 2023;28(1):537. doi:10.1186/s40001-023-01429-4
284. Nanotechnology and nanomedicine | oxford University Department for Continuing Education. Available from: https://www.conted.ox.ac.uk/about/nanotechnology-and-nanomedicine?utm_source=ads&utm_medium=cpc&utm_campaign=2402-pg-nanotechnology-courses&gad_source=1&gclid=EAIaIQobChMIwrW8jYe_hAMVhE5BAh1K2Qd3EAAYASAAEgL2HfD_BwE.
285. Cheon J, Chan W, Zuhorn I. The Future of Nanotechnology: cross-disciplined Progress to Improve Health and Medicine. Acc Chem Res. 2019;52(9):2405–2405. doi:10.1021/acs.accounts.9b00423
286. GA H. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1(1). doi:10.1016/J.NANO.2004.11.009
287. Cho K, Wang X, Nie S, Chen Z, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi:10.1158/1078-0432.CCR-07-1441
288. Carrasco-Esteban E, Domínguez-Rullán JA, Barrionuevo-Castillo P, et al. Current role of nanoparticles in the treatment of lung cancer. J Clin Transl Res. 2021;7(2):140–155.
289. Wang J, Zhou T, Liu Y, Chen S, Yu Z. Application of nanoparticles in the treatment of lung cancer with emphasis on receptors. Front Pharmacol. 2022;12:781425. doi:10.3389/fphar.2021.781425
290. Xia Q, Li H, Xiao K. Factors affecting the pharmacokinetics, biodistribution and toxicity of gold nanoparticles in drug delivery. Curr Drug Metabol. 2016;17(9):849–861. doi:10.2174/1389200217666160629114941
291. Li Y, Wan J, Wang F, Guo J, Wang C. Effect of increasing liver blood flow on nanodrug clearance by the liver for enhanced antitumor therapy. Biomater Sci. 2019;7(4):1507–1515. doi:10.1039/C8BM01371C
292. Li X, Wang B, Zhou S, et al. Surface chemistry governs the sub-organ transfer, clearance and toxicity of functional gold nanoparticles in the liver and kidney. J Nanobiotechnol. 2020;18(1):45. doi:10.1186/s12951-020-00599-1
293. Garnett MC, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occupat Med. 2006;56(5):307–311. doi:10.1093/occmed/kql052
294. Sarma A, Bania R, Devi JR, Deka S. Therapeutic nanostructures and nanotoxicity. J Appl Toxicol. 2021;41(10):1494–1517. doi:10.1002/jat.4157
295. Nassimi M, Schleh C, Lauenstein HD, et al. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur J Pharm Biopharm. 2010;75(2):107–116. doi:10.1016/j.ejpb.2010.02.014
296. Yildirimer L, Thanh NTK, Loizidou M, Seifalian AM. Toxicology and clinical potential of nanoparticles. Nano Today. 2011;6(6):585–607. doi:10.1016/J.NANTOD.2011.10.001
297. Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers. 2019;11(5):640. doi:10.3390/cancers11050640
298. Tavares MR, Islam R, Šubr V, et al. Polymer theranostics with multiple stimuli-based activation of photodynamic therapy and tumor imaging. Theranostics. 2023;13(14):4952–4973. doi:10.7150/thno.86211
299. Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik eV. 2009;71(3):409–419. doi:10.1016/j.ejpb.2008.11.010
300. Juhász Á, Gombár G, Várkonyi EF, Wojnicki M, Ungor D, Csapó E. Thermodynamic characterization of the interaction of biofunctionalized gold nanoclusters with serum albumin using two- and three-dimensional methods. Int J Mol Sci. 2023;24(23):16760. doi:10.3390/ijms242316760
301. Narwade M, Shaikh A, Gajbhiye KR, Kesharwani P, Gajbhiye V. Advanced cancer targeting using aptamer functionalized nanocarriers for site-specific cargo delivery. Biomater Res. 2023;27(1):42. doi:10.1186/s40824-023-00365-y
302. Elsafy S, Metselaar J, Lammers T. Nanomedicine - Immune System Interactions: limitations and Opportunities for the Treatment of Cancer. Handbook of Experimental Pharmacology. 2024;284:231–265. doi:10.1007/164_2023_685
303. Seymour LW. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit Rev Ther Drug Carrier Syst1992;9(2):135–187.
304. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392.:.
305. Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Controll Rel. 2012;164(2):138–144. doi:10.1016/j.jconrel.2012.04.038
306. Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Ann Rev Immunol. 1999;17:593–623. doi:10.1146/annurev.immunol.17.1.593
307. Gaucher G, Dufresne M-H, Sant VP, Kang N, Maysinger D, Leroux J-C. Block copolymer micelles: preparation, characterization and application in drug delivery. J Controll Rel. 2005;109(1–3):169–188. doi:10.1016/j.jconrel.2005.09.034
308. Sharma P, Mehta M, Dhanjal DS, et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem Biol Interact. 2019;309:108720. doi:10.1016/j.cbi.2019.06.033
309. Mylonakis N, Athanasiou A, Ziras N, et al. Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer. 2010;68(2):240–247. doi:10.1016/j.lungcan.2009.06.017
310. Stathopoulos GP, Antoniou D, Dimitroulis J, et al. Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol. 2010;21(11):2227–2232. doi:10.1093/annonc/mdq234
311. Hussain S. Nanomedicine for Treatment of Lung Cancer. Adv Exp Med Biol. 2016;890:137–147. doi:10.1007/978-3-319-24932-2_8
312. eRegulatory and esource capability checklist EU - Florence. Available from: https://florencehc.com/campaign/eregulatory-and-esource-capability-checklist-eu/?utm_campaign=paid-gl-2024-02-12-ereg-checklist-euversion&utm_medium=Paid&utm_source=Google&LatestCampaign=701Rn000007KxpZ&LatestCampaignStatus=Clicked&gad_source=1&.
313. Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomed. 2015;10:1001–1018. doi:10.2147/IJN.S56932
314. Szefler B, Diudea MV, Putz MV, Grudzinski IP. Molecular dynamic studies of the complex polyethylenimine and glucose oxidase. Int J Mol Sci. 2016;17(11). doi:10.3390/ijms17111796
315. Szefler B, Diudea MV, Grudzinski IP. Nature of polyethyleneimine-glucose oxidase interactions. Studia Universitatis Babes-Bolyai Chemia. 2016;61(1):260
316. Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141(5):769–784. doi:10.1007/s00432-014-1767-3
317. Crintea A, Dutu AG, Samasca G, Florian IA, Lupan I, Craciun AM. The Nanosystems Involved in Treating Lung Cancer. Life. 2021;11(7). doi:10.3390/life11070682
318. S SM, Naveen NR, Rao GK, et al. A spotlight on alkaloid nanoformulations for the treatment of lung cancer. Front Oncol. 2022;12:994155. doi:10.3389/fonc.2022.994155
319. Mangal S, Gao W, Li T, Zhou QT. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharmacol Sin. 2017;38(6):782–797. doi:10.1038/aps.2017.34
320. Georgiadis M-O, Karoutzou O, Foscolos A-S, Papanastasiou I. Sigma receptor (σr) ligands with antiproliferative and anticancer activity. Molecules. 2017;22(9). doi:10.3390/molecules22091408
321. Silva AC, Oliveira TR, Mamani JB, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomed. 2011;6:591–603. doi:10.2147/IJN.S14737
322. Master AM, Sen Gupta A. EGF Receptor-Targeted Nanocarriers for Enhanced Cancer Treatment. Nanomedicine. 2012;7(12):1895–1906. doi:10.2217/nnm.12.160
323. Duval KEA, Vernice NA, Wagner RJ, et al. Immunogenetic effects of low dose (CEM43 30) magnetic nanoparticle hyperthermia and radiation in melanoma cells. Int J Hyperther. 2019;36(sup1):37–46. doi:10.1080/02656736.2019.1627433
324. Cędrowska E, Pruszyński M, Gawęda W, et al. Trastuzumab conjugated superparamagnetic iron oxide nanoparticles labeled with 225ac as a perspective tool for combined α-radioimmunotherapy and magnetic hyperthermia of her2-positive breast cancer. Molecules. 2020;25(5). doi:10.3390/molecules25051025
325. Brero F, Albino M, Antoccia A, et al. Hadron therapy, magnetic nanoparticles and hyperthermia: a promising combined tool for pancreatic cancer treatment. Nanomaterials. 2020;10(10):1919. doi:10.3390/nano10101919
326. Liang B, Zuo D, Yu K, et al. Multifunctional bone cement for synergistic magnetic hyperthermia ablation and chemotherapy of osteosarcoma. Mater Sci Eng C. 2020;108:110460. doi:10.1016/j.msec.2019.110460
327. Gupta R, Sharma D. Evolution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem Neurosci. 2019;10(3):1157–1172. doi:10.1021/acschemneuro.8b00652
328. Legge CJ, Colley HE, Lawson MA, Rawlings AE. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J Oral Pathol Med. 2019;48(9):803–809. doi:10.1111/jop.12921
329. Hou Z, Zhang Y, Deng K, et al. UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano. 2015;9(3):2584–2599. doi:10.1021/nn506107c
330. Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3(3):319–328. doi:10.1016/S1567-5769(02)00271-0
331. Tseng C-L, Chang K-C, Yeh M-C, Yang K-C, Tang T-P, Lin F-H. Development of a dual-functional Pt–Fe-HAP magnetic nanoparticles application for chemo-hyperthermia treatment of cancer. Ceram Int. 2014;40(4):5117–5127. doi:10.1016/j.ceramint.2013.09.137
332. Kang MK, Mao W, Lee JB, Yoo HS. Epidermal growth factor (EGF) fragment-guided anticancer theranostic particles for pH-responsive release of doxorubicin. Int J Pharm. 2017;519(1–2):104–112. doi:10.1016/j.ijpharm.2017.01.017
333. Ma J, Zhang Z, Zhang Z, et al. Magnetic nanoparticle clusters radiosensitise human nasopharyngeal and lung cancer cells after alternating magnetic field treatment. Int J Hyperther. 2015;31(7):800–812. doi:10.3109/02656736.2015.1063168
334. Baskar G, Ravi M, Panda JJ, et al. Efficacy of dipeptide-coated magnetic nanoparticles in lung cancer models under pulsed electromagnetic field. Cancer Invest. 2017;35(6):431–442. doi:10.1080/07357907.2017.1318894
335. Barenholz Y. Doxil®--The first FDA-approved nano-drug: lessons learned. J Controll Rel. 2012;160(2):117–134. doi:10.1016/j.jconrel.2012.03.020
336. Adrianzen Herrera D, Ashai N, Perez-Soler R, Cheng H. Nanoparticle albumin bound-paclitaxel for treatment of advanced non-small cell lung cancer: an evaluation of the clinical evidence. Expert Opinion on Pharmacotherapy. 2019;20(1):95–102. doi:10.1080/14656566.2018.1546290
337. Araya T, Kasahara K, Nishikawa S, et al. Antitumor effects of inductive hyperthermia using magnetic ferucarbotran nanoparticles on human lung cancer xenografts in nude mice. Oncol Targ Ther. 2013;6:237–242. doi:10.2147/OTT.S42815
338. Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9(2):E128–147. doi:10.1208/aapsj0902015
339. Dabbagh A, Hedayatnasab Z, Karimian H, et al. Polyethylene glycol-coated porous magnetic nanoparticles for targeted delivery of chemotherapeutics under magnetic hyperthermia condition. Int J Hyperther. 2019;36(1):104–114. doi:10.1080/02656736.2018.1536809
340. Zhang H. Onivyde for the therapy of multiple solid tumors. OncoTargets and Therapy. 2016;9:3001–3007. doi:10.2147/OTT.S105587
341. Zeng F, Xu B, Zhu H, et al. A cascade dual-targeted nanocarrier for enhanced alectinib delivery to ALK-positive lung cancer. Biomaterials Science. 2020;8(22):6404–6413. doi:10.1039/D0BM00970A
342. Ak G, Aksu D, Çapkın E, Sarı Ö, Kımız Geboloğlu I, Şanlıer ŞH. Delivery of pemetrexed by magnetic nanoparticles: design, characterization, in vitro and in vivo assessment. Preparative Biochemistry & Biotechnology. 2020;50(3):215–225. doi:10.1080/10826068.2019.1692220
343. Taylor A, Krupskaya Y, Krämer K, et al. Cisplatin-loaded carbon-encapsulated iron nanoparticles and their in vitro effects in magnetic fluid hyperthermia. Carbon. 2010;48(8):2327–2334. doi:10.1016/j.carbon.2010.03.009
344. Taratula O, Garbuzenko O, Savla R, Wang YA, He H, Minko T. Multifunctional nanomedicine platform for cancer specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr Drug Delivery. 2011;8(1):59–69. doi:10.2174/156720111793663642
345. Ramasamy S, Enoch IVMV, Rex jeya rajkumar S. Polymeric cyclodextrin-dextran spooled nickel ferrite nanoparticles: expanded anticancer efficacy of loaded camptothecin. Mater Lett. 2020;261:127114. doi:10.1016/j.matlet.2019.127114
346. Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Controll Rel. 2008;132(3):153–163. doi:10.1016/j.jconrel.2008.08.012
347. Guthi JS, Yang S-G, Huang G, et al. MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells. Mol Pharm. 2010;7(1):32–40. doi:10.1021/mp9001393
348. Kong W-H, Sung D-K, Shim Y-H, et al. Efficient intracellular siRNA delivery strategy through rapid and simple two steps mixing involving noncovalent post-PEGylation. J Controll Rel. 2009;138(2):141–147. doi:10.1016/j.jconrel.2009.04.034
349. Chan JM, Valencia PM, Zhang L, Langer R, Farokhzad OC. Polymeric nanoparticles for drug delivery. Method Mol Biol. 2010;624:163–175. doi:10.1007/978-1-60761-609-2_11
350. Liu J, Liu J, Chu L, et al. Novel peptide-dendrimer conjugates as drug carriers for targeting nonsmall cell lung cancer. Int J Nanomed. 2010;6:59–69. doi:10.2147/IJN.S14601
351. Castro RI, Forero-Doria O, Guzmán L. Perspectives of dendrimer-based nanoparticles in cancer therapy. Anais da Academia Brasileira de Ciencias. 2018;90(2 suppl 1):2331–2346. doi:10.1590/0001-3765201820170387
352. Offermann M. The era of targeted therapies. Am Family Phys. 2008;77(3):294–296.:.
353. Widakowich C, de Castro G, de Azambuja E, Dinh P, Awada A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007;12(12):1443–1455. doi:10.1634/theoncologist.12-12-1443
354. Grünwald V, Soltau J, Ivanyi P, Rentschler J, Reuter C, Drevs J. Molecular targeted therapies for solid tumors: management of side effects. Onkologie. 2009;32(3):129–138. doi:10.1159/000194949
355. Naufal M, Hermawati E, Syah YM, Hidayat AT, Hidayat IW, Al-Anshori J. Structure-activity relationship study and design strategies of hydantoin, thiazolidinedione, and rhodanine-based kinase inhibitors: a two-decade review. ACS Omega. 2024;9(4):4186–4209. doi:10.1021/acsomega.3c04749
356. Ebrahimpour M, Hosseinzadeh H, Abedi F, et al. Enhancing treatment strategies for small bowel cancer: a clinical review of targeted therapy and immunotherapy approaches. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2024;397(7):4601–4614. doi:10.1007/s00210-024-02992-1
357. Khan A, Waheed Y, Kuttikrishnan S, et al. Network pharmacology, molecular simulation, and binding free energy calculation-based investigation of Neosetophomone B revealed key targets for the treatment of cancer. Front Pharmacol. 2024;15:1352907. doi:10.3389/fphar.2024.1352907
358. Duranti E, Cordani N, Villa C. Edaravone: a Novel Possible Drug for Cancer Treatment? Int J Mol Sciences. 2024;25(3):1633. doi:10.3390/ijms25031633
359. Esper P, Gale D, Muehlbauer P. What kind of rash is it?: deciphering the dermatologic toxicities of biologic and targeted therapies. Clin J Oncol Nur. 2007;11(5):659–666. doi:10.1188/07.CJON.659-666
360. Doostmohammadi A, Jooya H, Ghorbanian K, Gohari S, Dadashpour M. Potentials and future perspectives of multi-target drugs in cancer treatment: the next generation anti-cancer agents. Cell Commun Signal. 2024;22(1):228. doi:10.1186/s12964-024-01607-9
361. Rowinsky EK. Challenges of developing therapeutics that target signal transduction in patients with gynecologic and other malignancies. J Clin Oncol. 2003;21(10 Suppl):175s–186s. doi:10.1200/JCO.2003.01.146
362. Ricciardi S, Tomao S, de Marinis F. Toxicity of targeted therapy in non-small-cell lung cancer management. Clin Lung Cancer. 2009;10(1):28–35. doi:10.3816/CLC.2009.n.004
363. Vaidya B, Vyas SP. Transferrin coupled vesicular system for intracellular drug delivery for the treatment of cancer: development and characterization. J Drug Target. 2012;20(4):372–380. doi:10.3109/1061186X.2012.662687
364. Sadava D, Phillips T, Lin C, Kane SE. Transferrin overcomes drug resistance to artemisinin in human small-cell lung carcinoma cells. Cancer Lett. 2002;179(2):151–156. doi:10.1016/s0304-3835(02)00005-8
365. Z X, Z H, L Y, et al. Mechanisms of gambogic acid-induced apoptosis in non-small cell lung cancer cells in relation to transferrin receptors. J Chemother. 2009;21(6). doi:10.1179/JOC.2009.21.6.666
366. de I CT, D N. Delivery of therapeutic nucleic acids via transferrin and transferrin receptors: lipoplexes and other carriers. Exp Opin Drug Delivery. 2013;10(11). doi:10.1517/17425247.2013.837447
367. Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV. Iron and cancer: recent insights. Annals of the New York Academy of Sciences. 2016;1368(1):149–161. doi:10.1111/nyas.13008
368. Feelders RA, Kuiper-Kramer EP, van Eijk HG. Structure, function and clinical significance of transferrin receptors. Clin Chem Lab Med. 1999;37(1):1–10. doi:10.1515/CCLM.1999.001
369. Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodialysis International International Symposium on Home Hemodialysis. 2017;21(Suppl 1):S6–S20. doi:10.1111/hdi.12542
370. Cheng Z, Dai LL, Song YN, et al. Regulatory effect of iron regulatory protein-2 on iron metabolism in lung cancer. Gene Mol Res. 2014;13(3):5514–5522. doi:10.4238/2014.July.25.5
371. Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121(2):144–158. doi:10.1016/j.clim.2006.06.010
372. Upadhyay P, Sarker S, Ghosh A, et al. Transferrin-decorated thymoquinone-loaded PEG-PLGA nanoparticles exhibit anticarcinogenic effect in non-small cell lung carcinoma via the modulation of miR-34a and miR-16. Biomater Sci. 2019;7(10):4325–4344. doi:10.1039/C9BM00912D
373. Zhang Z, Wang J, Nie X, et al. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J Am Chem Soc. 2014;136(20):7317–7326. doi:10.1021/ja412735p
374. Wang X, Chen H, Zeng X, et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin B. 2019;9(1):167–176. doi:10.1016/j.apsb.2018.08.006
375. Tseng C-L, W-Y S, Yen K-C, Yang K-C, Lin F-H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30(20):3476–3485. doi:10.1016/j.biomaterials.2009.03.010
376. Nguyen VN, Mirejovský T, Melinová L, Mandys V. CD44 and its v6 spliced variant in lung carcinomas: relation to NCAM, CEA, EMA and UP1 and prognostic significance. Neoplasma. 2000;47(6):400–408.:.
377. Mattheolabakis G, Milane L, Singh A, Amiji MM. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target. 2015;23(7–8):605–618. doi:10.3109/1061186X.2015.1052072
378. Wintzell M, Hjerpe E, Åvall Lundqvist E, Shoshan M. Protein markers of cancer-associated fibroblasts and tumor-initiating cells reveal subpopulations in freshly isolated ovarian cancer ascites. BMC Cancer. 2012;12:359. doi:10.1186/1471-2407-12-359
379. Marhaba R, Klingbeil P, Nuebel T, Nazarenko I, Buechler M, Zoeller M. cd44 and Epcam: cancer-initiating cell markers. Curr Mol Med. 2008;8(8):784–804. doi:10.2174/156652408786733667
380. Bourguignon LYW, Gilad E, Peyrollier K. Heregulin-mediated ErbB2-ERK signaling activates hyaluronan synthases leading to CD44-dependent ovarian tumor cell growth and migration. J Biol Chem. 2007;282(27):19426–19441. doi:10.1074/jbc.M610054200
381. Sharma A, Shambhwani D, Pandey S, et al. Advances in lung cancer treatment using nanomedicines. ACS Omega. 2023;8(1):10–41. doi:10.1021/acsomega.2c04078
382. Singh A, Bhatia S, Rana V. Inhalable nanostructures for lung cancer treatment: progress and challenges. Curr Nanomed. 2019;9(1):4–29. doi:10.2174/2468187308666180307152049
383. Patlolla RR, Chougule M, Patel AR, Jackson T, PNV T, Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Controll Rel. 2010;144(2):233–241. doi:10.1016/j.jconrel.2010.02.006
384. Videira M, Almeida AJ, Fabra A. Preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effect. Nanomedicine. 2012;8(7):1208–1215. doi:10.1016/j.nano.2011.12.007
385. Garbuzenko OB, Mainelis G, Taratula O, Minko T. Inhalation treatment of lung cancer: the influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biol Med. 2014;11(1):44–55. doi:10.7497/j.issn.2095-3941.2014.01.004
386. Zhao Z, Simms A, Steinmetz NF. Cisplatin-loaded tobacco mosaic virus for ovarian cancer treatment. Biomacromolecules. 2022;23(10):4379–4387. doi:10.1021/acs.biomac.2c00831
387. Stathopoulos GP, Boulikas T, Vougiouka M, et al. Pharmacokinetics and adverse reactions of a new liposomal cisplatin (Lipoplatin): phase I study. Oncol Rep. 2005;13(4):589–595.:.
388. Newman MS, Colbern GT, Working PK, Engbers C, Amantea MA. Comparative pharmacokinetics, tissue distribution, and therapeutic effectiveness of cisplatin encapsulated in long-circulating, pegylated liposomes (SPI-077) in tumor-bearing mice. Cancer Chemother Pharmacol. 1999;43(1):1–7. doi:10.1007/s002800050855
389. Araújo JGC, Das G ML, Leite EA, et al. Biodistribution and antitumoral effect of long-circulating and pH-sensitive liposomal cisplatin administered in Ehrlich tumor-bearing mice. Experiment Biol Med. 2011;236(7):808–815. doi:10.1258/ebm.2011.011038
390. Oberoi HS, Nukolova NV, Kabanov AV, Bronich TK. Nanocarriers for delivery of platinum anticancer drugs. Adv Drug Delivery Rev. 2013;65(13–14):1667–1685. doi:10.1016/j.addr.2013.09.014
391. Jehn CF, Boulikas T, Kourvetaris A, Kofla G, Possinger K, Lüftner D. First safety and response results of a randomized phase III study with liposomal platin in the treatment of advanced squamous cell carcinoma of the head and neck (SCCHN). Anticancer Res. 2008;28(6B):3961–3964.
392. Kantauskaite M, Hucke A, Snieder B, Ciarimboli G. Exacerbation of cisplatin cellular toxicity by regulation of the human organic cation transporter 2 through angiotensin II. Int J Mol Sci. 2022;23(24):15866. doi:10.3390/ijms232415866
393. Pavelić K, Kraljević Pavelić S, Bulog A, et al. Nanoparticles in medicine: current status in cancer treatment. Int J Mol Sci. 2023;24(16):12827. doi:10.3390/ijms241612827
394. Fulton MD, Najahi-Missaoui W. Liposomes in cancer therapy: how did we start and where are we now. Int J Mol Sci. 2023;24(7):6615. doi:10.3390/ijms24076615
395. Zalba S, Garrido MJ. Liposomes, a promising strategy for clinical application of platinum derivatives. Exper Opin Drug Delivery. 2013;10(6):829–844. doi:10.1517/17425247.2013.778240
396. Stathopoulos GP. Liposomal cisplatin: a new cisplatin formulation. Anti-Cancer Drugs. 2010;21(8):732–736. doi:10.1097/CAD.0b013e32833d9adf
397. Specenier PM, Ciuleanu T, Latz JE, Musib LC, Darstein CLS, Vermorken JB. Pharmacokinetic evaluation of platinum derived from cisplatin administered alone and with pemetrexed in head and neck cancer patients. Cancer Chemother Pharmacol. 2009;64(2):233–241. doi:10.1007/s00280-008-0853-0
398. Veal GJ, Griffin MJ, Price E, et al. A phase I study in paediatric patients to evaluate the safety and pharmacokinetics of SPI-77, a liposome encapsulated formulation of cisplatin. Br J Cancer. 2001;84(8):1029–1035. doi:10.1054/bjoc.2001.1723
399. De Vita A, Vanni S, Fausti V, et al. Deciphering the genomic landscape and pharmacological profile of uncommon entities of adult rhabdomyosarcomas. Int J Mol Sci. 2021;22(21):11564. doi:10.3390/ijms222111564
400. Miserocchi G, Cocchi C, De Vita A, et al. Three-dimensional collagen-based scaffold model to study the microenvironment and drug-resistance mechanisms of oropharyngeal squamous cell carcinomas. Cancer Biol Med. 2021;18(2):502–516. doi:10.20892/j.issn.2095-3941.2020.0482
401. Huang H, Wu T, Shi H, et al. Modular design of nanobody–drug conjugates for targeted-delivery of platinum anticancer drugs with an MRI contrast agent. Chem Commun. 2019;55(35):5175–5178. doi:10.1039/C9CC01391A
402. Kang JH, Loomis SJ, Wiggs JL, Willett WC, Pasquale LR. A prospective study of folate, vitamin B6, and vitamin B12 intake in relation to exfoliation glaucoma or suspected exfoliation glaucoma. JAMA Ophthalmol. 2014;132(5):549–559. doi:10.1001/jamaophthalmol.2014.100
403. Agnello L, Tortorella S, d’Argenio A, et al. Optimizing cisplatin delivery to triple-negative breast cancer through novel EGFR aptamer-conjugated polymeric nanovectors. J Experiment Clin Cancer Res. 2021;40(1):239. doi:10.1186/s13046-021-02039-w
404. Abd-El-Aziz AS, Youssef AM, Abd-El-Aziz A. Transition Metal-Containing Dendrimers in Biomedicine: Current Trends. Roy Societ Chemistry; 2023. doi:10.1039/9781837671441
405. Crintea A, Motofelea AC, Șovrea AS, et al. Dendrimers: advancements and Potential Applications in Cancer Diagnosis and Treatment-An Overview. Pharmaceutics. 2023;15(5):1406. doi:10.3390/pharmaceutics15051406
406. Feazell RP, Nakayama-Ratchford N, Dai H, Stephen JL. Soluble Single-Walled Carbon Nanotubes as Longboat Delivery Systems for Platinum(IV) Anticancer Drug Design. 2007. doi:10.1021/JA073231F
407. Dhar S, Liu Z, Thomale J, Dai H, Lippard SJ. Targeted single-wall carbon nanotube-mediated pt(iv) prodrug delivery using folate as a homing device. J Am Chem Soc. 2008;130(34):11467–11476. doi:10.1021/ja803036e
408. Prylutska S, Politenkova S, Afanasieva K, et al. A nanocomplex of C60 fullerene with cisplatin: design, characterization and toxicity. Beilst J Nanotechnol. 2017;8. doi:10.3762/BJNANO.8.149
409. Dzhemilev UM, Khuzin AA, Akhmetov AR, et al. Synthesis of C 60 fullerene–quadricyclane hybrid compound and its preliminary in vitro antitumor activity in combination with cisplatin. ACS Omega. 2019;4(14):15929–15934. doi:10.1021/acsomega.9b01982
410. Prylutska S, Grynyuk I, Skaterna T, et al. Toxicity of C60 fullerene–cisplatin nanocomplex against Lewis lung carcinoma cells. Archives of Toxicology. 2019;93(5):1213–1226. doi:10.1007/s00204-019-02441-6
411. Li J, Yap SQ, Chin CF, et al. Platinum(iv) prodrugs entrapped within multiwalled carbon nanotubes: selective release by chemical reduction and hydrophobicity reversal. Chem Sci. 2012;3(6):2083. doi:10.1039/c2sc01086k
412. Bhirde AA, Patel V, Gavard J, et al. Targeted killing of cancer cells in vivo and in vitro with egf-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3(2):307–316. doi:10.1021/nn800551s
413. Bhirde AA, Sousa AA, Patel V, et al. Imaging the distribution of individual platinum-based anticancer drug molecules attached to single-wall carbon nanotubes. Nanomedicine. 2009;4(7):763–772. doi:10.2217/nnm.09.56
414. Wong BS, Yoong SL, Jagusiak A, et al. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Delivery Rev. 2013;65(15):1964–2015. doi:10.1016/J.ADDR.2013.08.005
415. Tripisciano C, Kraemer K, Taylor A, Borowiak-Palen E. Single-wall carbon nanotubes based anticancer drug delivery system. Chem Phys Lett. 2009;478(4–6):200–205. doi:10.1016/J.CPLETT.2009.07.071
416. Guven A, Rusakova IA, Lewis MT, Wilson LJ. Cisplatin@US-tube carbon nanocapsules for enhanced chemotherapeutic delivery. Biomaterials. 2012;33(5):1455–1461. doi:10.1016/J.BIOMATERIALS.2011.10.060
417. Guven A, Villares GJ, Hilsenbeck SG, et al. Carbon nanotube capsules enhance the in vivo efficacy of cisplatin. Acta Biomaterialia. 2017;58:466–478. doi:10.1016/J.ACTBIO.2017.04.035
418. Kirkpatrick DL, Weiss M, Naumov A, Bartholomeusz G, Weisman RB, Gliko O. Carbon nanotubes: solution for the therapeutic delivery of siRNA? Materials. 2012;5(12):278–301. doi:10.3390/ma5020278
419. Szefler B. Docking of carboplatin towards chosen nanostructures. Biointerface Research in Applied Chemistry. 2022;13(2):109. doi:10.33263/BRIAC132.109
420. Szefler B, Czeleń P. Docking of cisplatin on fullerene derivatives and some cube rhombellane functionalized homeomorphs. Symmetry. 2019;11(7):874. doi:10.3390/sym11070874
421. Szefler B, Czeleń P. Docking of platinum compounds on cube rhombellane functionalized homeomorphs. Symmetry. 2020;12(5):749. doi:10.3390/sym12050749
422. Gareth Williams JA, Develay S, Rochester DL, Murphy L. Optimising the luminescence of platinum(II) complexes and their application in organic light emitting devices (OLEDs). Coord Chem Rev. 2008;252(23–24):2596–2611. doi:10.1016/j.ccr.2008.03.014
423. Katouzian I, Jafari SM. Protein nanotubes as state-of-The-art nanocarriers: synthesis methods, simulation and applications. J Controll Rel. 2019;303:302–318. doi:10.1016/j.jconrel.2019.04.026
424. Ghaderi H, Alipour A, Mohammadi Zadeh Holagh A, et al. Recombinant antibody fragment therapeutics: current status and future prospects of scFv, nanobody, and mimotopes. J Drug Delivery Sci Technol. 2023;89:105009. doi:10.1016/J.JDDST.2023.105009
425. Liu X, Balligand T, Carpenet C, Ploegh HL. An armed anti-immunoglobulin light chain nanobody protects mice against influenza A and B infections. Sci Immunol. 2023;8(84):eadg9459. doi:10.1126/sciimmunol.adg9459
426. Panikar SS, Banu N, Haramati J, Del Toro-Arreola S, Riera Leal A, Salas P. Nanobodies as efficient drug-carriers: progress and trends in chemotherapy. J Controll Rel. 2021;334:389–412. doi:10.1016/j.jconrel.2021.05.004
427. Van Audenhove I, Gettemans J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine. 2016;8:40–48. doi:10.1016/j.ebiom.2016.04.028
428. Naidoo DB, Chuturgoon AA. Nanobodies Enhancing Cancer Visualization, Diagnosis and Therapeutics. Int J Mol Sci. 2021;22(18):9778. doi:10.3390/ijms22189778
429. Jovčevska I, Muyldermans S. The therapeutic potential of nanobodies. bioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2020;34(1):11–26. doi:10.1007/s40259-019-00392-z
430. Rademaker-Lakhai JM, Terret C, Howell SB, et al. a phase i and pharmacological study of the platinum polymer ap5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin Cancer Res. 2004;10(10):3386–3395. doi:10.1158/1078-0432.CCR-03-0315
431. Nowotnik DP, Cvitkovic E. ProLindac (AP5346): a review of the development of an HPMA DACH platinum polymer therapeutic. Adv Drug Delivery Rev. 2009;61(13):1214–1219. doi:10.1016/j.addr.2009.06.004
432. Abbasi E, Aval SF, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. doi:10.1186/1556-276X-9-247
433. February 1999 - Volume 10 - issue 2: anti-cancer drugs. Available from: https://journals.lww.com/anti-cancerdrugs/toc/1999/02000.
434. Cancer Research Campaign (Great Britain). Anti-Cancer Drug Design. Macmillan; 1985.
435. Trollsås M, Hedrick JL. Hyperbranched Poly(ε-caprolactone) Derived from Intrinsically Branched AB2 Macromonomers. 1998. doi:10.1021/MA980151A
436. Howell BA, Fan D. Poly(amidoamine) dendrimer-supported organoplatinum antitumour agents. Proceedings of the Royal Society A. 2010;466(2117):1515–1526. doi:10.1098/rspa.2009.0359
437. Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:6348):56–58. doi:10.1038/354056a0
438. Zhao Y, Woods JA, Farrer NJ, et al. Diazido mixed‐amine platinum(iv) anticancer complexes activatable by visible‐light form novel dna adducts. Chem Eur J. 2013;19(29):9578–9591. doi:10.1002/chem.201300374
439. Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzy Regulat. 2001;41(1):189–207. doi:10.1016/S0065-2571(00)00013-3
440. Li J, Yap SQ, Yoong SL, et al. Carbon nanotube bottles for incorporation, release and enhanced cytotoxic effect of cisplatin. Carbon. 2012;50(4):1625–1634. doi:10.1016/J.CARBON.2011.11.043
441. Mirzaei M, Meskinfam M, Yousefi M. Covalent hybridizations of carbon nanotubes through peptide linkages: a density functional approach. Comput Theoret Chem. 2012;981:47–51. doi:10.1016/J.COMPTC.2011.11.043
442. Johnstone T, S K, L S. The next generation of platinum drugs: targeted pt(ii) agents, nanoparticle delivery, and pt(iv) prodrugs. Chem Rev. 2016;2016:3436–3486.
443. Patel S, Bhirde AA, Rusling JF, Chen X, Gutkind JS, Patel V. Nano delivers big: designing molecular missiles for cancer therapeutics. Pharmaceutics. 2011;3(1):34–52. doi:10.3390/pharmaceutics3010034
444. Mejri A, Tangour B, Herlem G, Picaud F. Confinement of the antitumoral drug cisplatin inside edge-functionalized carbon nanotubes and its release near lipid membrane. Eur Phys J D. 2021;75(3):99. doi:10.1140/epjd/s10053-021-00114-7
445. Li R, Bao Z, Wang P, et al. Gelatin-functionalized carbon nanotubes loaded with cisplatin for anti-cancer therapy. Polymers. 2023;15(16):3333. doi:10.3390/polym15163333
446. Kazemi-Beydokhti A, Zeinali Heris S, Jaafari MR. Investigation of different methods for cisplatin loading using single-walled carbon nanotube. Chem Eng Res Design. 2016;112:56–63. doi:10.1016/J.CHERD.2016.06.006
447. Dehaghani MZ, Yousefi F, Seidi F, et al. Encapsulation of an anticancer drug Isatin inside a host nano-vehicle SWCNT: a molecular dynamics simulation. Sci Rep. 2021;11(1):18753. doi:10.1038/s41598-021-98222-2
448. Seifalian A. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomed. 2011;2963. doi:10.2147/IJN.S16923
449. Elgamal HA, Mohamed SA, Farghali AA, Hassan AME. PEG@ carbon nanotubes composite as an effective nanocarrier of ixazomib for myeloma cancer therapy. Nanoscale Res Lett. 2022;17(1):72. doi:10.1186/s11671-022-03707-2
450. Arlt M, Haase D, Hampel S, et al. Delivery of carboplatin by carbon-based nanocontainers mediates increased cancer cell death. Nanotechnology. 2010;21(33):335101. doi:10.1088/0957-4484/21/33/335101
451. Kazemzadeh H, Mozafari M. Fullerene-based delivery systems. Drug Discovery Today. 2019;24(3):898–905. doi:10.1016/j.drudis.2019.01.013
452. Powell WH, Cozzi F, Moss GP, Thilgen C, Hwu -RJ-R, Yerin A. Nomenclature for the C60-Ih and C70-D5h(6) fullerenes (IUPAC Recommendations 2002). Pure and Applied Chemistry. 2002;74(4):629–695. doi:10.1351/pac200274040629
453. Czeleń P, Szefler B, Skotnicka A. A computational study of the immobilization of new 5-nitroisatine derivatives with the use of c60-based functionalized nanocarriers. Symmetry. 2023;15(1):226. doi:10.3390/sym15010226
454. Fernandes NB, Shenoy RUK, Kajampady MK, et al. Fullerenes for the treatment of cancer: an emerging tool. Environ Sci Pollut Res Int. 2022;29(39):58607–58627. doi:10.1007/s11356-022-21449-7
455. Horak I, Prylutska S, Krysiuk I, et al. Nanocomplex of berberine with c60 fullerene is a potent suppressor of lewis lung carcinoma cells invasion in vitro and metastatic activity in vivo. Materials. 2021;14(20). doi:10.3390/ma14206114
456. Franskevych D, Prylutska S, Grynyuk I, et al. Mode of photoexcited C60 fullerene involvement in potentiating cisplatin toxicity against drug-resistant L1210 cells. BioImpacts. 2019;9(4):211–217. doi:10.15171/bi.2019.26
457. Medeleanu M, Khalaj Z, Diudea MV. Rhombellane-related Crystal Networks. Iran J Mathemat Chem. 2020;11(2):73–81. doi:10.22052/IJMC.2020.144902.1384
458. Diudea MV, Lungu CN, Nagy CL. Cube-rhombellane related structures: a drug perspective. Molecules. 2018;23(10):2533. doi:10.3390/molecules23102533
459. Diudea MV. Rhombellanic diamond. Fullerenes, Nanotubes and Carbon Nanostructures. 2019;27(2):137–140. doi:10.1080/1536383X.2018.1524375
460. Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16. doi:10.1007/s11095-006-9132-0
461. Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci. 2016;83:184–202. doi:10.1016/j.ejps.2015.12.031
462. Xu F, Dawson C, Lamb M, et al. Hydrogels for tissue engineering: addressing key design needs toward clinical translation. Front Bioeng Biotechnol. 2022;10:849831. doi:10.3389/fbioe.2022.849831
463. Davari N, Bakhtiary N, Khajehmohammadi M, et al. Protein-based hydrogels: promising materials for tissue engineering. Polymers. 2022;14(5):986. doi:10.3390/polym14050986
464. Wu Z, Zou X, Yang L, et al. Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer. Colloids and Surfaces B, Biointerfaces. 2014;122:90–98. doi:10.1016/j.colsurfb.2014.06.052
465. Meghani N, Patel P, Kansara K, et al. Formulation of vitamin D encapsulated cinnamon oil nanoemulsion: its potential anti-cancerous activity in human alveolar carcinoma cells. Colloids and Surfaces B, Biointerfaces. 2018;166:349–357. doi:10.1016/j.colsurfb.2018.03.041
466. Choudhury H, Gorain B, Chatterjee B, Mandal UK, Sengupta P, Tekade RK. Pharmacokinetic and Pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr Pharm Design. 2017;23(17):2504–2531. doi:10.2174/1381612822666161201143600
467. Jin S, Hu Y, Gu Z, Liu L, Wu H-C. Application of quantum dots in biological imaging. J Nanomater. 2011;2011(1):1–13. doi:10.1155/2011/834139
468. Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114(2):165–172. doi:10.1289/ehp.8284
469. Smith AM, Nie S. Next-generation quantum dots. Nat Biotechnol. 2009;27(8):732–733. doi:10.1038/nbt0809-732
470. Singh RD, Shandilya R, Bhargava A, et al. Quantum dot based nano-biosensors for detection of circulating cell free mirnas in lung carcinogenesis: from biology to clinical translation. Front Gene. 2018;9:616. doi:10.3389/fgene.2018.00616
471. Kulkarni NS, Parvathaneni V, Shukla SK, et al. Tyrosine kinase inhibitor conjugated quantum dots for non-small cell lung cancer (NSCLC) treatment. Eur J Pharm Sci. 2019;133:145–159. doi:10.1016/j.ejps.2019.03.026
472. Cai X, Luo Y, Zhang W, Du D, Lin Y. pH-Sensitive ZnO quantum dots-doxorubicin nanoparticles for lung cancer targeted drug delivery. ACS Appl Mater Interfaces. 2016;8(34):22442–22450. doi:10.1021/acsami.6b04933
473. Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. Journal of Neuro-Oncology. 2011;103(2):317–324. doi:10.1007/s11060-010-0389-0
474. Baskar G, Chandhuru J, Sheraz Fahad K, Praveen AS, Chamundeeswari M, Muthukumar T. Anticancer activity of fungal L-asparaginase conjugated with zinc oxide nanoparticles. J Mater Sci Mater Med. 2015;26(1):5380. doi:10.1007/s10856-015-5380-z
475. Ali D, Alarifi S, Alkahtani S, AlKahtane AA, Almalik A. Cerium oxide nanoparticles induce oxidative stress and genotoxicity in human skin melanoma cells. Cell Biochem Biophys. 2015;71(3):1643–1651. doi:10.1007/s12013-014-0386-6
476. Zhang X-F, Liu Z-G, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi:10.3390/ijms17091534
477. Zhao N, Pan Y, Cheng Z, Liu H. Gold nanoparticles for cancer theranostics — a brief update. J Innov Opt Health Sci. 2016;09(04):1630004. doi:10.1142/S1793545816300044
478. Haume K, Rosa S, Grellet S, et al. Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnol. 2016;7(1):8. doi:10.1186/s12645-016-0021-x
479. Lopez-Campos F, Candini D, Carrasco E, Berenguer Francés MA. Nanoparticles applied to cancer immunoregulation. Rep Pract Oncol Radiother. 2019;24(1):47–55. doi:10.1016/j.rpor.2018.10.001
480. Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Delivery Rev. 2009;61(6):428–437. doi:10.1016/j.addr.2009.03.009
481. Kandasamy G, Maity D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int J Pharm. 2015;496(2):191–218. doi:10.1016/j.ijpharm.2015.10.058
482. Avval ZM, Malekpour L, Raeisi F, et al. Introduction of magnetic and supermagnetic nanoparticles in new approach of targeting drug delivery and cancer therapy application. Drug Metab Rev. 2020;52(1):157–184. doi:10.1080/03602532.2019.1697282
483. Wu K, Su D, Liu J, Saha R, Wang J-P. Magnetic nanoparticles in nanomedicine: a review of recent advances. Nanotechnology. 2019;30(50):502003. doi:10.1088/1361-6528/ab4241
484. Woodman C, Vundu G, George A, Wilson CM. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin Cancer Biol. 2021;69:349–364. doi:10.1016/j.semcancer.2020.02.009
485. Soenen SJH, De Cuyper M. Assessing cytotoxicity of (iron oxide-based) nanoparticles: an overview of different methods exemplified with cationic magnetoliposomes. Contrast Media & Molecular Imaging. 2009;4(5):207–219. doi:10.1002/cmmi.282
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

